Abstract
Transforming growth factor β (TGF-β) is a pleiotropic cytokine involved in both suppressive and inflammatory immune responses. After 30 years of intense study, we have only begun to elucidate how TGF-β alters immunity under various conditions. Under steady-state conditions, TGF-β regulates thymic T-cell selection and maintains homeostasis of the naïve T-cell pool. TGF-β inhibits cytotoxic T lymphocyte (CTL), Th1-, and Th2-cell differentiation while promoting peripheral (p)Treg-, Th17-, Th9-, and Tfh-cell generation, and T-cell tissue residence in response to immune challenges. Similarly, TGF-β controls the proliferation, survival, activation, and differentiation of B cells, as well as the development and functions of innate cells, including natural killer (NK) cells, macrophages, dendritic cells, and granulocytes. Collectively, TGF-β plays a pivotal role in maintaining peripheral tolerance against self- and innocuous antigens, such as food, commensal bacteria, and fetal alloantigens, and in controlling immune responses to pathogens.
TGF-β regulates the differentiation and function of different classes of leukocytes, controls the magnitude and type of immune responses against microbes, and helps maintain tolerance against self and benign antigens.
In mammals, the innate and adaptive arms of the immune system orchestrate host–defense and inflammatory responses. For example, leukocytes of the myeloid cell lineage use germline-encoded receptors to detect conserved molecular patterns associated with pathogens, which allows them to alert and activate the rest of the immune system, including adaptive immunity. Alternatively, lymphocytes of the adaptive immune system express antigen-specific receptors that distinguish small differences in macromolecules and establish long-term immunity by forming immunological memory. The coupling of these innate and adaptive recognition pathways, and their precise modes of communication provide a robust mechanism that stimulates immunity and protects the host against pathogens. Nevertheless, the immune system tolerates antigens originating from self-, commensal organisms, and the allogeneic fetus. By maintaining this balance between immunity and tolerance, the immune system can promote the physiological well-being of an individual.
A pivotal and pleiotropic regulator of immune responses is transforming growth factor β (TGF-β), which was first reported to control immune cell function three decades ago (Kehrl et al. 1986b). TGF-β controls the magnitude and type of immune responses against microbes, and has fundamentally important roles in maintaining immune tolerance and homeostasis against self- and benign antigens at steady-state (Li et al. 2006b; Oh and Li 2013; Travis and Sheppard 2014). In this review, we discuss how TGF-β regulates the differentiation and function of different classes of leukocytes, and how it modulates immune activities, from conception to autoimmunity and infection.
TGF-β IN THE IMMUNE SYSTEM
T Cells
Thymic Development
T cells arise from bone marrow–derived precursors that traffic to the thymus, where their developmental process is completed. In the thymus, T-cell precursors are exposed to a variety of extrinsic signals, for example, peptides presented by major histocompatibility complexes (MHCs), costimulation, and cytokines, which stimulate molecular changes that cause differentiation into distinct T-cell lineages. The differentiation of conventional CD4+ and CD8+ αβ T cells requires T-cell receptor (TCR) engagement that follows the Goldilocks principle, in which both too little and too much TCR signaling are detrimental to the successful development of mature T cells. T-cell precursors require appropriate TCR signaling to trigger their survival and maturation, a process termed positive selection. “Too little” signaling results in death of the developing T cells. Yet “too much” TCR signaling, which reflects strong reactivity to self-peptide:MHC complexes, can also cause death of the developing T cell. This process of negative selection, a key aspect of central tolerance, eliminates autoreactive T cells from the T-cell repertoire. However, this process is not complete, and some autoreactive T cells mature in the thymus and exit to the periphery, where they must be kept in check to prevent the development of autoimmunity. The immunosuppressive functions of TGF-β have long been appreciated, and TGF-β signaling is one mechanism by which such “escaped” autoreactive T cells can be controlled in the periphery, a process called peripheral tolerance. Although TGF-β is well known for its tolerance-inducing activities in the periphery, its contributions to T-cell biology clearly extend beyond its role as an immunosuppressive cytokine. Indeed, TGF-β also has important functions in the development of several T-cell lineages.
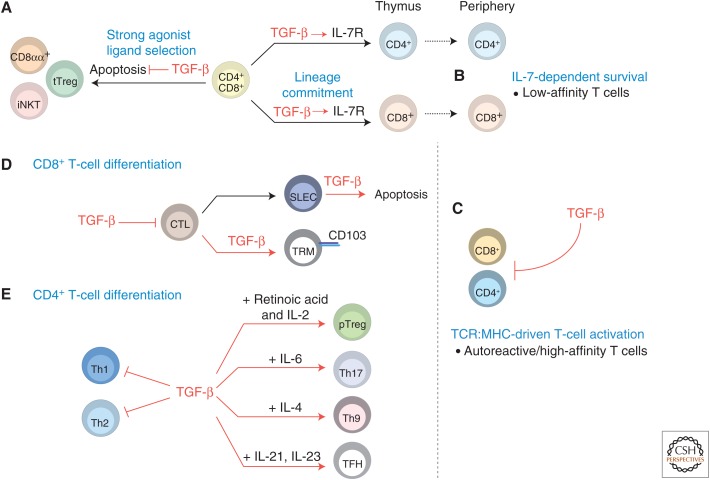
In the thymus, the differentiation of conventional CD8+ T cells requires both TCR engagement and signaling through the common γ-chain family cytokine interleukin 7 (IL-7) (Park et al. 2010). Consequently, maintaining expression of the IL-7 receptor on CD8+ T-cell precursors is critical given the role of IL-7 signaling in the specification of the CD8+ T-cell fate. TGF-β regulates the expression of the IL-7 receptor α-chain (IL-7Rα) in developing CD8+ T cells (Ouyang et al. 2013), thus supporting IL-7 signaling, and therefore CD8+ T-cell lineage commitment. Mechanistically, TGF-β signaling promotes IL-7Rα expression on CD8+ thymocytes by suppressing the expression of the transcriptional repressor Gfi-1, a known inhibitor of Il7ra expression in CD8+ T cells (Park et al. 2004). This cross talk between TGF-β and IL-7 signaling pathways is an essential aspect of conventional CD8+ T-cell development (Fig. 1A).
Figure 1.
Regulation of T cells by transforming growth factor β (TGF-β). (A) TGF-β promotes the thymic development of multiple T-cell lineages. TGF-β supports the survival of thymus-derived Treg (tTreg), invariant natural killer T (iNKT), and CD8αα+ T-cell precursors, and thus promotes the development of T-cell populations that are induced by strong agonist ligands. TGF-β also supports the development of conventional CD8+ T cells by promoting thymocyte expression of interleukin (IL)-7Rα. (B) TGF-β regulates peripheral T-cell homeostasis by promoting IL-7-dependent survival of low-affinity T cells, through its control of thymocyte IL-7Rα expression, and by (C) inhibiting T-cell receptor (TCR)-driven activation of autoreactive or high-affinity T cells. (D) In early stages of CD8+ T-cell differentiation, TGF-β inhibits cytotoxic T lymphocyte (CTL) development. However, TGF-β also promotes the apoptosis of short-lived effector cells (SLECs) and the differentiation of CD103-expressing tissue resident memory (TRM) cells. (E) Whereas TGF-β inhibits T helper 1 (Th1)- and Th2-cell differentiation, TGF-β (in concert with other factors) promotes the development of peripheral Treg (pTreg), Th17, Th9, and T follicular helper (Tfh) cells.
TGF-β also regulates the development of multiple subsets of regulatory and innate-like T cells. Thymus-derived CD4+CD25+Foxp3+ regulatory T (tTreg) cells, invariant natural killer T (iNKT) cells, and CD8αα+TCRαβ+ intraepithelial lymphocytes (IELs) share a common thread: their development requires high-affinity interactions with MHC-presenting self-ligands, which are referred to as agonist ligands (Stritesky et al. 2012). Because TGF-β promotes the survival of precursor populations for each of these lineages (discussed in more detail below), it functions as a unifying molecule to promote the differentiation of T-cell populations that require strong agonist ligands for their development (Fig. 1A).
Treg cells are essential for the maintenance of immune tolerance (Josefowicz et al. 2012). Although Treg cells can be generated in the periphery by the conversion of conventional naïve CD4+ T cells, the thymus gives rise to the majority of Treg cells, which are referred to as thymus-derived Treg (tTreg) cells (Shevach and Thornton 2014). The development of tTreg cells is driven by a combination of stringent TCR interactions, costimulation, and cytokine signals. The TGF-β signaling pathway plays a role in the early development of tTreg cells, and 3- to 5-day old mice lacking the TGF-β type I receptor (TβRI) show a dramatic reduction in the frequency of Foxp3+ thymocytes (Liu et al. 2008). Although a conserved DNA sequence for Smad3 binding is present in Foxp3 gene regulatory sequences (Tone et al. 2008), TGF-β signaling is dispensable for the induction of Foxp3 expression in tTreg cells (Zheng et al. 2010; Schlenner et al. 2012), showing that TGF-β does not promote tTreg-cell development by directly regulating Foxp3 expression. Instead, TGF-β signaling promotes tTreg-cell development by antagonizing thymic-negative selection, and therefore promoting the survival of tTreg-cell precursors (Ouyang et al. 2010).
iNKT cells recognize lipids presented by the MHC class I–like molecule CD1d, and possess qualities that are reminiscent of both the innate and adaptive immune responses. This subset of lipid-sensing T cells has been shown to play both beneficial and pathogenic roles in a variety of inflammatory and disease conditions (Brennan et al. 2013). Strong agonist ligand interactions are also thought to promote iNKT-cell development (Stritesky et al. 2012), and, as observed with tTreg cells, TGF-β signaling appears to play a critical role in promoting the survival of iNKT-cell precursors. T-cell-specific deletion of the TGF-β type II receptor (TβRII) leads to a reduction of both thymic and peripheral iNKT cells (Li et al. 2006a; Marie et al. 2006; Doisne et al. 2009), which results, in part, from increased apoptosis of immature precursor cells in the absence of TGF-β signaling (Doisne et al. 2009).
CD8αα+TCRαβ+ IELs are innate-like T cells that play important roles in intestinal homeostasis (Cheroutre et al. 2011). The development of these innate-like T cells is thought to occur both in and outside the thymus, and also appears to be driven by high-affinity TCR interactions with their selection ligands (Pobezinsky et al. 2012). In the absence of TGF-β signaling, the CD8αα+TCRαβ+ IEL population is decreased, which is partially driven by a reduction in numbers of thymic precursors of these innate-like T cells (Konkel et al. 2011).
Collectively, the studies of tTreg cells, iNKT cells, and CD8αα+TCRαβ+ IEL development highlight the importance of TGF-β signaling in mediating the survival of a precursor population in the ontogeny of multiple T-cell lineages.
Peripheral Homeostasis
An effective immune system must maintain a diverse pool of naïve T cells within the confines of a relatively constant number of peripheral T cells. TGF-β critically contributes to the maintenance of an effective naïve T-cell population by regulating T-cell proliferation, homeostasis, and repertoire diversity.
TGF-β was first shown to immunoregulate and reduce proliferation of human T cells through studies in cell culture (Kehrl et al. 1986b). During priming, TGF-β inhibits T-cell proliferation by inhibiting transcription of the Il2 gene and suppressing IL-2 production (Brabletz et al. 1993; Tzachanis et al. 2001). At the molecular level, Smad3 mediates this suppression, as both Smad3-deficient CD4+ and CD8+ T cells are not sensitive to IL-2 inhibition mediated by TGF-β (McKarns et al. 2004; McKarns and Schwartz 2005). TGF-β also inhibits the expression of several cell-cycle regulators in primary T cells and T-cell lines (Ruegemer et al. 1990; Genestier et al. 1999; Nelson et al. 2003; Wolfraim et al. 2004). However, whether TGF-β inhibits T-cell priming under inflammatory conditions in vivo and the exact mechanism by which this regulation may occur are unclear.
Befitting the pleiotropic nature of TGF-β, its ability to regulate T-cell proliferation depends on the status of T-cell differentiation and the cumulative signaling pathways involved in cell activation. For example, TGF-β can inhibit the proliferation of naïve but not activated T cells, an effect associated with decreased TβRII expression on activated T cells (Cottrez and Groux 2001; Sanjabi et al. 2009). Additionally, in naïve T cells, CD28 engagement sends a costimulatory signal that abrogates TGF-β inhibition of proliferation (Sung et al. 2003), ensuring that TGF-β does not inhibit the ability of activated antigen-presenting cells (APCs) to prime naïve T cells.
T-cell proliferation must be properly regulated to maintain homeostasis under steady-state conditions and during immune challenges. The absence of TGF-β signaling alters homeostasis of both CD4+ and CD8+ T cells. For example, loss of TGF-β signaling in mice, in which CD4+ T cells are engineered to express a single TCR, results in a dramatic reduction of the peripheral T-cell population (Li et al. 2006a; Ouyang et al. 2013). TGF-β supports the homeostasis of peripheral CD4+ T cells by promoting IL-7Rα expression in the thymus during T-cell development, which allows naïve peripheral CD4+ T cells to sense IL-7 for their survival (Ouyang et al. 2013). Interestingly, this regulation is particularly important for the survival of low-affinity CD4+ T cells (Fig. 1B). This phenomenon was shown in studies using transgenic expression of the AND TCR that possesses a higher affinity for its positive selection ligand in the MHC H-2k background than that in the H-2b background (Smith et al. 2001; Ouyang et al. 2013). In both genetic backgrounds, regardless of high or low affinity for the positive selection ligand, TβRII-deficient AND T cells express lower levels of IL-7Rα than their wild-type counterparts. However, comparison of the transgenic T cells between the two genetic backgrounds showed that TβRII-deficient AND T cells in the high-affinity background (i.e., H-2k) express greater levels of IL-7Rα than even wild-type AND T cells in the low-affinity background (i.e., H-2b), showing that TGF-β signaling and TCR signal strength both contribute to IL-7Rα expression. Thus, the absence of TGF-β signaling causes a more profound defect in IL-7Rα expression and correspondingly results in poor peripheral homeostasis in T cells bearing low-affinity TCRs. Notably, Tgfb1−/− mice show altered diversity of CD4+ TCRs in the periphery, but not in the thymus (Robinson and Gorham 2007), which likely reflects repertoire changes caused by the preferential loss of low-affinity CD4+ T cells.
TGF-β signaling is also important for the regulation of peripheral CD8+ T cells. Defects in TGF-β signaling result in altered homeostasis and aberrant activation of CD8+ T cells (Lucas et al. 2000; Johnson and Jameson 2012; Zhang and Bevan 2012). However, mice expressing certain transgenic TCRs do not show changes in CD8+ T-cell homeostasis on loss of TGF-β signaling (Lucas et al. 2006). These differences may be explained by differences in the affinity of each of these transgenic TCRs for self-peptide MHC complexes. As such, T cells expressing high-affinity TCRs undergo greater homeostatic proliferation than T cells expressing low-affinity TCRs (Kieper et al. 2004). Indeed, homeostatic proliferation of CD8+ T cells with defective TGF-β signaling depends on TCR and MHC class I (Fig. 1C) (Johnson and Jameson 2012). At its extreme, loss of control of CD8+ T-cell homeostasis by TGF-β can lead to cell transformation, as expression of a dominant-negative form of TβRII (dnTβRII) in T cells causes mice to develop lymphoma (Lucas et al. 2004). Interestingly, expression of dnTβRII, but not deletion of TβRII, causes a CD8+ T-cell lymphoproliferative disorder (Ishigame et al. 2013a). These findings suggest that either the dnTβRII exerts a dominant function independent of its inhibiting TGF-β signaling, or that T-cell homeostasis is regulated by TGF-β signaling in a dose-dependent manner (Ishigame et al. 2013a).
CD8+ T cells undergo massive clonal expansion after becoming activated in response to pathogens, followed by apoptosis-mediated contraction on pathogen clearance. TGF-β plasma levels increase in response to acute Listeria monocytogenes (LM) infection. Mice expressing OTI TCR transgenic T cells, specific for MHC I–restricted ovalbumin SIINFEKL peptide, were crossed to dnTβRII animals and further crossed to RAG1−/− animals to eliminate V(D)J recombination of endogenous TCR locus. Naïve T cells from corresponding OTI-dnTβRII-RAG1−/− and OTI-RAG1−/− animals were adoptively cotransferred into wild-type animals that were then infected with a recombinant LM-expressing chicken ovalbumin (LM-OVA). Using this system, it was shown that TGF-β plays a major role in maintaining T-cell homeostasis during CD8+ T-cell clonal expansion by promoting apoptosis of short-lived effector CD8+ T cells that are enriched among the cells expressing the killer-cell lectin-like receptor subfamily G member 1 (KLRG1) (Fig. 1D) (Sanjabi et al. 2009). Similarly, adding TGF-β to cultures of human T cells at 72 h postactivation induces T-cell death (Sillett et al. 2001; Hernandez-Garay and Mendez-Samperio 2003). However, using a model in which TβRII expression is specifically inactivated in CD8+ T cells, it was shown that higher proliferation rather than less apoptosis was responsible for expansion of Tgfbr2−/− CD8+ T cells after pathogenic challenge (Hu et al. 2015). Although both dnTβRII-expressing and Tgfbr2−/− mouse models show an increase in the ratio of KLRG1+ short-lived effector T cells compared with KLRG1− memory precursor T cells, the exact mechanism of this increase during effector CD8+ T-cell expansion remains unclear.
Differentiation
TGF-β broadly inhibits T-cell activation by interfering with TCR signaling (Chen et al. 2003a). It also specifically suppresses cytotoxic T lymphocytes (CTL) and T helper 1 (Th1) and T helper 2 (Th2) lymphocyte subset differentiation by inhibiting the expression of lineage-defining transcription factors such as T-bet and GATA-3, respectively (Fig. 1D,E) (Gorelik et al. 2000, 2002; Heath et al. 2000). TGF-β was also shown to suppress Th1 differentiation by inhibiting the expression of Stat4 (Lin et al. 2005), a transcription factor whose expression is activated in response to IL-12 signaling. TGF-β regulates T-bet and Stat4 expression to control Th1-cell differentiation in culture at distinct stages. Indeed, repressing Stat4 activation inhibits interferon γ (IFN-γ) production during the priming phase, whereas loss of T-bet expression impairs IFN-γ production during the recall response, that is, the restimulation of T cells after initial priming (Lin et al. 2005). Mechanistically, Smad2 and Smad3 transcription factors may have a redundant role in TGF-β-mediated inhibition of Th1-cell differentiation (Takimoto et al. 2010; Gu et al. 2012). The Smad pathway also contributes to the inhibition of Th2-cell differentiation by TGF-β, by inducing the expression of Sox4, a transcription factor that can bind to GATA-3 (Kuwahara et al. 2012). Retroviral expression of wild-type Sox4, but not Sox4 mutants lacking the ability to interact with GATA-3, inhibits Th2 cytokine production in CD4+ T cells (Kuwahara et al. 2012).
In contrast to its role in inhibiting Th1 and Th2-cell differentiation, TGF-β promotes the development of peripheral regulatory T (pTreg), Th17, Th9, and Tfh (follicular helper T) cells (Fig. 1E). Suboptimal stimulation of T cells, for example, using an altered peptide ligand (Windhagen et al. 1995), low-dose antigen (Gunnlaugsdottir et al. 2005; Kohyama et al. 2005), or absence of complement signaling (Strainic et al. 2012), stimulates TGF-β production and converts CD4+ T cells into Treg cells. Indeed, TGF-β promotes regulatory activity in naïve CD4+ T cells (Yamagiwa et al. 2001) by inducing Foxp3 expression (Chen et al. 2003b; Selvaraj and Geiger 2007) and pTreg-cell differentiation. Foxp3 then provides a positive feedback loop in TGF-β signaling by down-regulating TGF-β-induced expression of the inhibitory Smad7 (Fantini et al. 2004).
TGF-β plays both direct and indirect roles in Foxp3 expression. The Foxp3 gene contains an enhancer element that allows for direct binding of Smad3 and nuclear factor of activated T (NFAT) cells (Tone et al. 2008) to a conserved DNA sequence (Xu et al. 2010; Zheng et al. 2010). TGF-β also promotes locus activation by opposing the recruitment of a DNA methyltranferase to the Foxp3 locus (Josefowicz et al. 2009). As an indirect mechanism, TGF-β induces expression of the adaptor Nedd4 family interacting protein 1 (Ndfip1), which promotes JunB degradation through the E3 ubiquitin ligase Itch, and suppresses IL-4 production to support pTreg-cell differentiation (Beal et al. 2011). However, the presence of inflammatory cytokines and strong costimulatory signals potently inhibits Foxp3 induction by TGF-β (Wei et al. 2007; Molinero et al. 2011; Battaglia et al. 2013). These findings show that pTreg-cell differentiation is modulated by the microenvironment, with highly inflammatory conditions favoring effector over Treg-cell generation.
TGF-β also regulates Th17-cell differentiation (Bettelli et al. 2006; Veldhoen et al. 2006a; Li et al. 2007; Manel et al. 2008; Yang et al. 2008; Gutcher et al. 2011). Th17 cells express the lineage-specific transcription factor RAR (retinoic acid receptor)-related orphan receptor C (RORC) in humans (and RORγt in mice) and produce many cytokines, including IL-17A, IL-17F, IL-21, IL-22, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Ivanov et al. 2006; Korn et al. 2009). RORγt expression is induced by IL-21 or IL-23, and TGF-β together with IL-6 amplifies RORγt-dependent Th17-cell differentiation (Zhou et al. 2007). Although RORγt expression is activated independently of Smad2 and Smad3 (Takimoto et al. 2010), Smad2 has been shown to directly associate with RORγt to enhance Th17-cell differentiation (Martinez et al. 2010). In addition, TGF-β promotes Th17-cell differentiation by inhibiting the expression of the transcription factors Stat4 and GATA-3 (Das et al. 2009), Eomesodermin (Eomes) (Ichiyama et al. 2011), and growth factor independent 1 (Gfi-1) (Zhu et al. 2009), thus preventing Th1- and Th2-cell differentiation. TGF-β also represses the expression of Blimp-1, a transcription factor that limits Th17-cell differentiation (Salehi et al. 2012). Thus, TGF-β promotes the differentiation of Th17 cells both directly and indirectly by inhibiting T-cell differentiation into other cell lineages.
Th17 cells can be both immunoregulatory and pathogenic (Sharma et al. 2013). Generation of regulatory Th17 cells is promoted by the combination of TGF-β and IL-6 (Esplugues et al. 2011; Chalmin et al. 2012; Zhao et al. 2012). Pathogenic Th17 cells, however, require further stimulation with IL-23 (McGeachy et al. 2007; Chikuma et al. 2012; Lee et al. 2012). Pathogenic Th17 cells can also be induced in cell culture without TGF-β, in the presence of IL-6, IL-1β, and IL-23 (Ghoreschi et al. 2010; Lee et al. 2012). At low concentrations, TGF-β synergizes with IL-6 and IL-21 to promote IL-23 receptor expression and Th17-cell differentiation, whereas high TGF-β concentrations repress IL-23 receptor expression and promote Treg-cell differentiation (Zhou et al. 2008). In Th17 cells, TGF-β also differentially regulates IL-22 and IL-17 expression. In the absence of TGF-β, IL-6 induces IL-22 (Basu et al. 2012); however, in the presence of TGF-β, expression of cMaf, a repressor of Il22 gene expression, is induced (Rutz et al. 2011). Such opposing effects of TGF-β on Th17-associated cytokines may contribute to the regulatory or pathogenic functions of these cells. Intriguingly, Th17 cells transdifferentiate into regulatory T cells in a TGF-β- and aryl hydrocarbon receptor (AhR)-dependent manner at the resolution of inflammation (Gagliani et al. 2015), thus further showing the role of TGF-β in the plasticity and switch between immunity and tolerance.
TGF-β cooperates with the Notch pathway and IL-4 to induce IL-9+IL-10+ Th9 cells. These cells have effector rather than regulatory function, despite their ability to produce abundant levels of IL-10 (Dardalhon et al. 2008; Elyaman et al. 2012). They also play a critical and non-redundant role in host-protective type 2 immunity against gastrointestinal infection with parasitic worms (Licona-Limón et al. 2013). The ability of TGF-β and IL-4 to promote Th9-cell differentiation is enhanced by OX40 costimulation, which activates TRAF6 (the ubiquitin ligase tumor necrosis factor [TNF] receptor-associated factor 6) and, in turn, the noncanonical nuclear factor (NF)-κB pathway (Xiao et al. 2012). Additionally, Smad2 and Smad3 cooperate with IL-4-induced interferon regulatory factor 4 (IRF4) at the Il9 locus, where they displace binding of enhancer of zeste homolog 2 (EZH2), causing derepression of chromatin modification in the locus (Tamiya et al. 2013; Wang et al. 2013). The exact contribution of TGF-β signaling to the various biological functions of Th9 cells remains to be further explored.
Tfh cells are an important component of humoral immunity as they help B cells generate antigen-specific antibody responses, and their differentiation depends on the transcription repressor Bcl6 (Crotty 2011). Together with IL-12 and IL-23, TGF-β is an important cofactor for the early differentiation of human Tfh cells in cell culture (Schmitt et al. 2014). Interestingly, TGF-β signaling in mouse CD4+ T cells was also shown to be required for Tfh-cell development, although by using a different mechanism than what was shown in human cells. In response to influenza infection, TGF-β suppressed the expression of the high-affinity IL-2 receptor on virus-specific CD4+ T cells, and dampened IL-2-induced Stat5 signaling and mammalian target of rapamycin (mTOR) activation in Tfh precursor cells (Marshall et al. 2015).
TGF-β plays an active role in the development and maintenance of IELs that reside in the epithelial layer of the mucosal lining and have immediate effector functions. As already discussed, TGF-β signaling is required for the thymic development of CD8αα+TCRαβ+ IELs, and also maintains CD8α expression on peripheral T cells (Konkel et al. 2011). Once in the epithelium, IELs are maintained by the interaction between the cell-surface proteins CD103 (αEβ7) and epithelial (E)-cadherin (Cepek et al. 1993; Schon et al. 1999). CD103 shares the β7 subunit with α4β7, an integrin required for lymphocyte migration to the gut. TGF-β induces the expression of αE and enhances the constitutive expression of β7, leading to increased CD103 expression at the surface of IELs (Suzuki et al. 2002; Kang et al. 2011).
One subset of IELs, tissue-resident memory T cells (TRMs), are noncirculating memory cells that are maintained in the mucosal tissue, near the site of the first antigen encounter (Cauley and Lefrancois 2013; Schenkel and Masopust 2014). Most TRMs express CD69 and CD103 (Casey et al. 2012; Mackay et al. 2013; Skon et al. 2013). Environmental cytokines, including TGF-β, IL-33, and TNF, induce repression of Krüppel-like factor 2 (KLF2) expression and its target gene S1pr1. Thus, repressing sphingosine-1-phosphate receptor 1 (S1P1) and enhancing CD69 and CD103 expression allows the maintenance of TRMs in the tissue (Mackay et al. 2013; Skon et al. 2013). TGF-β is a potent inducer of CD103 expression by CD8+ T cells (Fig. 1D) (El-Asady et al. 2005; Casey et al. 2012). Inactivation of TβRII expression in CD8+ T cells results in a defect in the retention of intestinal TRMs in the IELs, most likely a result of the lack of CD103 expression (Zhang and Bevan 2013). In an oral model of LM infection, TGF-β signaling in CD8+ T cells was required for the rapid generation of memory precursor cells that give rise to TRMs in the gut (Sheridan et al. 2014). In another oral infection study with Yersinia pseudotuberculosis, TGF-β signaling was required for the generation of the CD103+ TRMs, but dispensable for the generation of CD103− TRMs that reside in the lamina propria and cluster with CD4+ T and CX3CR1+ myeloid cells (Bergsbaken and Bevan 2015). Additionally, the development of TRMs in the skin depends on TGF-β-mediated repression of T-bet and loss of Eomes expression, while forced expression of these transcription factors reduces TβRII and CD103 expression. A residual amount of T-bet is required for expression of IL-15R and IFN-γ, thus promoting TRM survival and effector function, respectively (Mackay et al. 2015). A deeper understanding of how TRMs are developed and maintained long term in various tissues will shed more light on the exact contribution of TGF-β signaling to the development and function of these memory cells.
Tolerance
Mice with impaired or total loss of TGF-β signaling in T cells develop severe autoimmunity, which shows the importance of TGF-β in controlling T-cell tolerance (Gorelik and Flavell 2000; Li et al. 2006a; Marie et al. 2006). The breach of tolerance that occurs without TGF-β signaling is not solely caused by altered activity of Treg cells (Li et al. 2006a; Marie et al. 2006), suggesting that a major mechanism by which TGF-β maintains tolerance is by directly regulating autoreactive T cells. This direct regulation is evident in a transgenic diabetes mouse model in which loss of TGF-β signaling in activated diabetogenic CD4+ T cells, but not Treg cells, induces disease (Ishigame et al. 2013b). In addition, in the intestine, TGF-β signaling limits tissue damage by diverting pathogenic CD4+ T cells to a nonpathogenic phenotype (Reis et al. 2013). Moreover, the control of autoreactive T cells by Treg cells in vivo may also occur through TGF-β signaling. Activated human and murine Treg cells express the glycoprotein A repetitions predominant (GARP) protein, which associates with the latent form of TGF-β, resulting in cell-surface expression of TGF-β on Treg cells (Stockis et al. 2009; Tran et al. 2009; Wang et al. 2009; Edwards et al. 2013). Indeed, active TGF-β can be generated from the membrane-bound complex of GARP and latent TGF-β (Wang et al. 2012). The activation of latent complexes of TGF-β has been reviewed (Robertson and Rifkin 2016).
Interestingly, recent studies indicate that loss of TGF-β signaling in mature T cells is not sufficient to induce autoimmunity. In stark contrast to mice in which Tgfbr2 inactivation occurs during the double-positive thymocyte stage of T-cell maturation (Li et al. 2006a; Marie et al. 2006), Tgfbr2 deletion at later stages, using the distal Lck-Cre or a tamoxifen-induced Cre in mature CD4+ T cells, does not induce development of overt autoimmunity (Zhang and Bevan 2012; Sledzinska et al. 2013). However, in both models, autoimmunity can be induced in Rag-deficient animals, suggesting that an added insult of extreme lymphopenia in combination with the absence of TGF-β signaling is required for the loss of tolerance. Notably, TGF-β signaling in double-positive thymocytes induces IL-7Rα expression, which serves a particularly important role in promoting the homeostatic survival of low-affinity TCR CD4+ T cells (Ouyang et al. 2013). Thus, in the CD4-Cre model of Tgfbr2 deletion, the absence of TGF-β signaling in developing T cells may create a lymphopenic environment, with preferential loss of low-affinity T cells, which favors activation of higher affinity autoreactive T cells. In addition, TGF-β-supported survival of low-affinity CD4+ T cells may play an essential role in the maintenance of a novel regulatory population of CD4+ T cells, termed “deletor” T cells, which contributes to the control of T-cell repertoire diversity and homeostasis. These deletor T cells limit the expansion of T-cell clones in a TCR-specific manner by outcompeting other T cells for subthreshold TCR ligands (e.g., positive selection ligands) that likely promote survival signals without causing overt T-cell activation (Singh et al. 2012).
B Cells
Proliferation and Survival
Early studies showed that TGF-β inhibits proliferation of mature human and immature murine B cells (Kehrl et al. 1986a; Petit-Koskas et al. 1988; Warner et al. 1992). Mechanistically, TGF-β induces growth arrest of B cells, which has been associated with decreased expression of the cell-cycle regulator cyclin A and inactivation of the cell-cycle-dependent kinase Cdk2 (Bouchard et al. 1997). TGF-β also regulates B-cell survival, as TGF-β signaling induces apoptosis in the murine B-cell line WEHI (Warner et al. 1992). Furthermore, TGF-β can promote B-cell death by inducing expression of the transcriptional E protein antagonist Id3 to stimulate apoptosis in B-cell progenitors (Kee et al. 2001).
Studies in mice with B-cell-specific loss of TGF-β signaling verified the cell-culture observations that TGF-β regulates B-cell proliferation and survival. Splenic B cells deficient in TβRII expression show increased BrdU incorporation when compared with wild-type B cells, confirming an important role for TGF-β in controlling B-cell proliferation in vivo (Cazac and Roes 2000). Mice with B-cell-specific deficiency in TGF-β signaling also show an expanded population of innate-like B cells, termed B1 cells (Cazac and Roes 2000). Interestingly, TβRII-deficient B1 cells are not characterized by increased BrdU incorporation, suggesting that their accumulation may be caused by enhanced survival in the absence of TGF-β signaling.
Activation and Differentiation
TGF-β regulates B-cell activation by inhibiting immunoglobulin synthesis and class switching to the majority of IgG isotypes (Kehrl et al. 1986a, 1991). Although mice with a B-cell-specific deficiency in TGF-β signaling do not show signs of overt autoimmunity, these cells show a more activated phenotype, are hyperresponsive to normally weak immunogens, and produce anti-dsDNA antibodies (Cazac and Roes 2000).
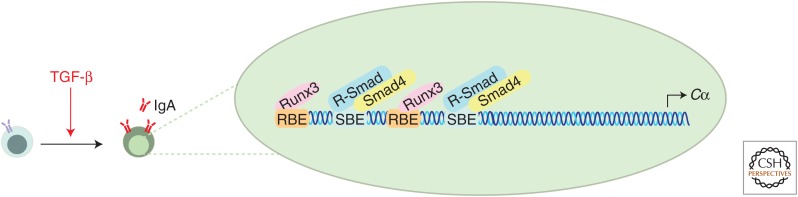
In contrast to TGF-β inhibiting IgG class switching, TGF-β promotes B-cell production of IgA antibodies (Coffman et al. 1989; Sonoda et al. 1989; Kim and Kagnoff 1990; Lebman et al. 1990; Ehrhardt et al. 1992; van Vlasselaer et al. 1992), which provide an important defense mechanism at mucosal barriers (Fig. 2) (Cerutti et al. 2011). Induction of IgA class switching by TGF-β is associated with increased transcription of α-germline transcripts (Lebman et al. 1990; Shockett and Stavnezer 1991) because of binding of activated Smads and Runx3 to a tandem-repeat element in the α-germline transcript promoter (Lin and Stavnezer 1992; Shi and Stavnezer 1998; Hanai et al. 1999; Pardali et al. 2000; Zhang and Derynck 2000; Park et al. 2001). Mice with B-cell-specific loss of TGF-β signaling also show dramatic reductions in serum and mucosal IgA levels (Cazac and Roes 2000; Borsutzky et al. 2004). Interestingly, whereas Smad3-deficient mice show relatively normal IgA production (Yang et al. 1999), B-cell-specific inactivation of Smad2 expression recapitulates the reduction in IgA observed in mice with TβRII-deficient B cells. This finding indicates that Smad2 has a nonredundant role in controlling responses of TGF-β-regulated IgA (Klein et al. 2006).
Figure 2.
Regulation of IgA class switching by transforming growth factor β (TGF-β). TGF-β promotes the production of IgA antibodies by increasing the transcription of α-germline transcripts. Activated Smad3 in complex with Smad4, and Runx3 bind to Smad-binding elements (SBEs) and Runx-binding elements (RBEs), respectively, which are found in the promoter of the constant heavy chain α (Cα).
Dendritic Cells
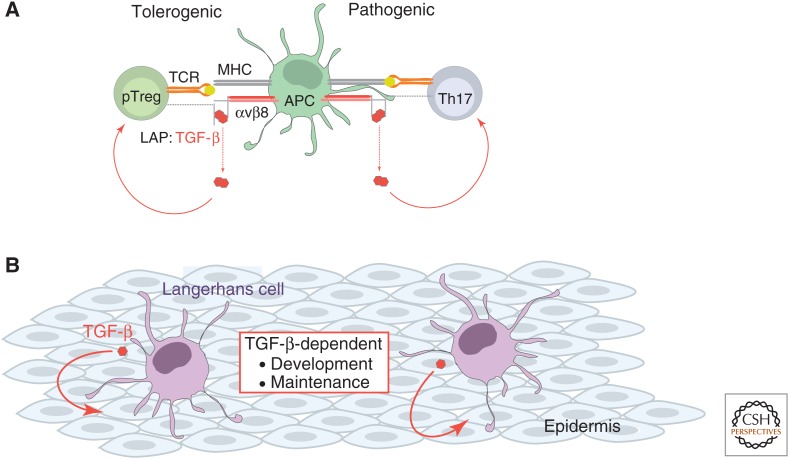
Dendritic cells (DCs) are important effectors of both tolerogenic and pathogenic functions of TGF-β activity (Fig. 3A). Mice with myeloid or DC-specific loss of the αv integrins, which play key roles in integrin-mediated activation of TGF-β, develop colitis, showing that DCs play an important role in T-cell tolerance mediated by TGF-β (Lacy-Hulbert et al. 2007; Travis et al. 2007). A subset of mucosal CD103+ DCs also specifically promote tolerance by inducing pTreg-cell differentiation (Coombes et al. 2007; Sun et al. 2007) likely by enabling integrin-mediated activation of TGF-β (Paidassi et al. 2011; Worthington et al. 2011). From a pathogenic perspective, DC-mediated activation of latent TGF-β also contributes to Th17-cell differentiation in vivo and the development of experimental autoimmune encephalomyelitis (EAE) (Acharya et al. 2010; Melton et al. 2010).
Figure 3.
Regulation of dendritic cells by TGF-β. (A) Dendritic cells (DCs) are key effectors of TGF-β activity. TGF-β is produced in a latent form in which the mature TGF-β is noncovalently associated with the latency-associated peptide (LAP). Release of TGF-β from its association with LAP is a critical step in activation of the cytokine. Integrin-mediated activation of TGF-β by DCs promotes the generation of peripheral Treg (pTreg) cells that possess important tolerogenic functions, and induces the differentiation of pathogenic Th17 cells. (B) Langerhans cells are specialized DCs of the epithelia that depend on TGF-β for their development and maintenance. Autocrine TGF-β signaling is required for the maintenance of this cell population.
In addition to a role for DCs in maintaining T-cell tolerance through integrin-mediated activation of TGF-β, studies show that DCs themselves can acquire a tolerogenic phenotype on exposure to TGF-β in cell culture. TGF-β promotes the tolerogenic properties of plasmacytoid DCs (pDCs) by inducing pDC expression of the tryptophan-catabolizing enzyme indoleamine 2,3 dioxygenase (IDO) (Pallotta et al. 2011). TGF-β appears to induce expression of this enzyme in pDCs through activation of noncanonical NF-κB signaling, which promotes IDO expression in DCs (Tas et al. 2007). Besides TGF-β, culture of pDCs with IFN-γ also induces IDO expression (Mellor and Munn 2004). However, although pDCs treated with IFN-γ and TGF-β together show short-term tolerogenic functions, only pDCs cultured with TGF-β alone show long-term IDO-dependent tolerogenic activity (Pallotta et al. 2011).
TGF-β also has an inhibitory effect on DC function. Indeed, exposure of DCs to TGF-β in culture represses the antigen presentation capabilities and maturation status of developing DCs (Nandan and Reiner 1997; Yamaguchi et al. 1997; Piskurich et al. 1998; Geissmann et al. 1999; Zhang et al. 1999b). TGF-β may also exert control over DC function in part by regulating DC responses to inflammatory stimuli. Whereas TGF-β treatment of human monocyte-derived DCs that are not exposed to other inflammatory stimuli has no effect on DC-induced T-cell proliferation, human monocyte–derived DCs exposed to TGF-β before lipopolysaccharide (LPS) stimulation induce a lower degree of T-cell proliferation than LPS-only-treated counterparts (Fogel-Petrovic et al. 2007). Furthermore, human monocyte-derived DCs pretreated with TGF-β also produce reduced levels of a variety of inflammatory mediators in response to Toll-like receptor (TLR) or cytokine stimulation (Fogel-Petrovic et al. 2007). Notably, the ability of TGF-β to modulate the activation of a DC is determined by the stimulation conditions. Although TGF-β can inhibit DC maturation induced by cytokines, TLR ligands, or Fc-receptor engagement, engagement of the costimulatory CD40 receptor overrides the suppressive effect of TGF-β on DC maturation (Geissmann et al. 1999).
Efforts to unravel how TGF-β signaling regulates DC function in vivo have yielded less clear conclusions. Despite evidence supporting the inhibitory effects of TGF-β on DC function in culture, TβRII-deficient splenic DCs showed no difference in their activation status, based on the expression of MHC class II and costimulatory molecules when compared with their wild-type counterparts (Ramalingam et al. 2012). Yet, mice with CD11c-Cre-mediated deletion of Tgfbr2 in DCs succumbed to a systemic autoimmune disorder, the major manifestation of which is gastritis. Gene-expression analyses of TβRII-deficient splenic and mesenteric lymph node DCs indicated some changes in cytokine, chemokine, and chemokine receptor expression patterns, but whether and how these phenotypic alterations contribute to the manifestation of autoimmunity remains unknown. In addition, how TGF-β signaling affects DCs in nonlymphoid tissues, such as the stomach or intestines, has not been examined, and a major question that remains is whether the sensitivity of DCs to TGF-β regulation is determined by the identity and/or location of DCs.
TGF-β plays an important role is the biology of Langerhans cells, which are specialized DCs of the epithelia that possess important immunological and tolerogenic functions (Romani et al. 2010). TGF-β was first implicated as a regulator of Langerhans cells by the observation that mice with global inactivation of Tgfb1 expression lack this specific DC population (Borkowski et al. 1996). Cell-culture studies subsequently showed that TGF-β induces Langerhans cell differentiation of a variety of human-derived precursor cells (Strobl et al. 1996; Geissmann et al. 1998; Zhang et al. 1999b). The role of TGF-β in Langerhans cell biology is further supported by studies using mouse lines with conditional deficiency of TβRI or TβRII, which established that TGF-β directly regulates the development and maintenance of Langerhans cells (Kaplan et al. 2007; Kel et al. 2010; Zahner et al. 2011). Additionally, mice in which TβRII or TGF-β1 were deleted specifically in Langerhans cells phenocopied each other, showing that this pathway, and specifically autocrine TGF-β signaling, is critical for the development or maintenance of Langerhans cells (Fig. 3B) (Kaplan et al. 2007). In addition, TGF-β signaling increases recruitment of the transcription factor PU.1 to the promoter and intronic regions of the Runx3 gene (Chopin et al. 2013), which encodes a critical transcription factor in Langerhans cell development (Fainaru et al. 2004).
It has been proposed that, in the intestinal lamina propria, a combination of TGF-β and bacterial sensing regulates the tolerogenic properties of gut DCs, largely by controlling DC production of TGF-β, which is suggested to direct pTreg-cell generation (Kashiwagi et al. 2015). Notably, although autocrine TGF-β signaling confers enhanced TGF-β expression in DCs, the TGF-β-activated Smads appear to play opposing roles in this regulation with Smad3 promoting and Smad2 inhibiting TGF-β production. DCs that lack only Smad2 express higher levels of mRNA for TGF-β1 and IL-10, and lower levels of mRNA for inflammatory cytokines, for example, TNF-α, IL-6, and IL-12, and show tolerogenic activity. However, despite the indication that autocrine TGF-β signaling promotes TGF-β production in DCs, whether DCs represent the critical source of TGF-β for pTreg-cell differentiation, as proposed (Kashiwagi et al. 2015), remains to be validated by genetic methods, for example, using CD11c-Cre-mediated deletion of floxed Tgfb1. Indeed, it has been shown that T cells themselves are the essential source of TGF-β for Th17 differentiation (Li et al. 2007; Gutcher et al. 2011).
NK Cells
TGF-β has a general inhibitory effect on the development and function of NK cells. In neonates, blocking TGF-β signaling on NK cells promotes faster NK maturation and reduces susceptibility of neonates to viral infection (Marcoe et al. 2012). In adults, TGF-β inhibits IFN-γ and T-bet expression in NK cells, thus inhibiting type 1 immunity (Laouar et al. 2005; Yu et al. 2006). Reciprocally, proinflammatory cytokines can down-regulate TβRII expression and inhibit TGF-β signaling in NK cells (Yu et al. 2006). Additionally, TGF-β expressed by T cells may inhibit proliferation of NK cells in vivo after they become activated by infection with acute lymphocytic choriomeningitis virus (LCMV) (Su et al. 1991, 1993), or by hepatitis B virus (HBV) infection in humans (Sun et al. 2012). NK cells express activating receptors at their surface, including NKG2D and NKp30, whose expression is suppressed by TGF-β (Castriconi et al. 2003; Lee et al. 2004; Crane et al. 2010). The expression of NKG2D at the cell surface requires its association with the intracellular adaptors DAP10 or DAP12 to stabilize the complex. Although IL-2 signaling stabilizes the cell-surface expression of activating NKG2D–DAP10 receptor complexes, TGF-β prevents this interaction by inhibiting the expression of DAP10 (Park et al. 2011; Sun et al. 2012). TGF-β also induces miR-183 expression to repress DAP12 expression, which destabilizes the NKG2D receptors at the surface of NK cells and inhibits their downstream signals (Donatelli et al. 2014).
Monocytes and Macrophages
Early work examining TGF-β regulation of myeloid cells showed that TGF-β largely inhibits the proinflammatory response of macrophages activated by TLR ligands or cytokine stimulation (Li et al. 2006b). However, stimulation with TGF-β alone, in the absence of TLR ligands or other cytokines, promotes myeloid cell production of several inflammatory cytokines (Wahl et al. 1987; Chantry et al. 1989; Musso et al. 1990; Turner et al. 1990). TGF-β also induces migration of monocytes and macrophages isolated from human peripheral blood (Wahl et al. 1987), and enhances the adherent properties of monocytes (Bauvois et al. 1992; Wahl et al. 1993b). The distinct effects of TGF-β on myeloid cell function, which depend on the specific nature of the activating conditions, reflect the complex and pleiotropic nature of this cytokine. Furthermore, the regulation of myeloid cells by TGF-β appears to be influenced by the identity of the cell. For example, depending on their anatomic origin, some subsets of macrophages show more sensitivity to TGF-β signaling than others (Fan et al. 1992). In addition, in chemotaxis studies, blood monocytes, but not intestinal macrophages, were found to traffic in response to TGF-β signaling (Smythies et al. 2006). Nevertheless, determining whether and how TGF-β regulates distinct myeloid cell populations in vivo remains elusive.
The intestine may be an anatomic location where regulation of myeloid cells by TGF-β is of particular importance. The intestine is a unique tissue in which the maintenance of resident macrophages relies on continuous input from circulating monocytes (Ginhoux and Jung 2014). In this context, TGF-β may induce trafficking of monocytes and promote their differentiation into noninflammatory macrophages that reside in the tissue. Indeed, after prolonged exposure to TGF-β, human blood monocytes begin to acquire a less activated phenotype, illustrated by down-regulation of innate response receptor expression and reduced cytokine production (Smythies et al. 2005). Notably, this altered phenotype resembles the less inflammatory profile that characterizes human intestinal macrophages. These macrophages produce no or only limited amounts of inflammatory cytokines in response to a variety of stimuli, despite maintaining their phagocytic and bacteriocidal functions (Smythies et al. 2005). The ability of these macrophages to perform the functions needed for tissue health, while tightly regulating inflammatory cytokine secretion, is likely an essential feature in maintaining intestinal tolerance and homeostasis. Indeed, mice with expression of dnTβRII from the CD68 promoter, which is primarily expressed in monocytes and macrophages, show loss of TGF-β inhibition of LPS-induced cytokine production. These mice are also more susceptible to dextran sulfate sodium (DSS)-induced colitis, showing that TGF-β controls monocytes and/or macrophages to regulate intestinal inflammation (Rani et al. 2011).
The exact mechanism by which TGF-β regulates the inflammatory response of myeloid cells in vivo remains largely unknown. However, TGF-β may promote suppression of TLR signaling. For example, in myeloid cells, TGF-β induces and maintains expression of Axl (Bauer et al. 2012), a member of the Tyro3, Axl, and Mer (TAM) receptor tyrosine kinase family that inhibits innate immune inflammatory responses (Sharif et al. 2006; Rothlin et al. 2007). Indeed, blocking Axl increased cytokine production after TLR stimulation (Bauer et al. 2012).
In addition, several cell-culture studies indicate that TGF-β may also control myeloid cell activation by directly inhibiting NF-κB signaling activated by innate receptors or cytokines (Naiki et al. 2005; Choi et al. 2006; Hong et al. 2007; Lee et al. 2011). TLR engagement is a major pathway of microbial recognition, and multiple adaptor proteins, including MyD88 (myeloid differentiation primary response protein 88), TRIF (Toll/IL-1R [TIR] domain-containing, adaptor-inducing interferon-β), and TRAM (TRIF-related adaptor molecule) mediate signal transduction downstream from these innate receptors. Studies using the RAW macrophage cell line show that TGF-β represses MyD88-dependent, but not TRIF- or TRAM-dependent, TLR signaling (Naiki et al. 2005). TGF-β inhibits this pathway by promoting the ubiquitylation and degradation of MyD88 (Naiki et al. 2005; Lee et al. 2011), which involves Smad6 and the E3 ubiquitin ligases Smurf1 and Smurf2 (Lee et al. 2011). TGF-β also impedes NF-κB activation by sequestering the adaptor protein pellino-1 and, consequently, disrupting the formation of a signaling complex containing IRAK1 (IL-1-receptor-associated kinase 1), TRAF6, and MyD88 downstream from IL-1R/TLR activation (Choi et al. 2006). Both Smad6 and Smad7 can interact with pellino-1 through discrete sequences in their respective MH2 domains, which allows simultaneous interactions with the adaptor protein (Choi et al. 2006; Lee et al. 2010). In addition to controlling the responses of receptors that recognize microbial patterns, TGF-β inhibits NF-κB activation downstream from TNF-α signaling by promoting interactions between Smad7 and the adaptor proteins TAB2 (TAK1 binding protein 2) and TAB3. These components are part of the signaling complex that forms after activation of the TNF-α pathway (Hong et al. 2007). These findings indicate important cross talk between TGF-β and NF-κB signaling. How these interactions shape myeloid cell biology in vivo remains to be determined.
Granulocytes
Granulocytes are innate immune cells that are identified by the presence of dense granules in their cytoplasm, and are also termed polymorphonuclear leukocytes for their distinctly shaped nuclei. This subset of innate immune cells has important functions in infection and inflammation, and includes neutrophils, eosinophils, and basophils. TGF-β induces chemotaxis of both neutrophils (Brandes et al. 1991; Fava et al. 1991; Reibman et al. 1991) and eosinophils (Luttmann et al. 1998). At the molecular level, Smad3 may be required for TGF-β-induced neutrophil migration, as Smad3−/− neutrophils show impaired chemotactic responses (Yang et al. 1999). However, TGF-β can also inhibit neutrophil migration by suppressing TNF-α–induced endothelial cell production of IL-8, a known neutrophil chemoattractant (Smith et al. 1996). Under some conditions, TGF-β may also promote neutrophil oxidant production (Brandes et al. 1991; Balazovich et al. 1996). Alternatively, TGF-β can act as a negative regulator of granulocytes, and inhibits the survival of human eosinophils by promoting apoptosis and inhibiting cytokine production (Alam et al. 1994). Despite these observations, the extent to which TGF-β regulates the granulocytic arm of the innate immune system remains poorly understood.
Mast Cells
Mast cells have been predominantly associated with allergy responses, but a growing understanding of mast cell functions has identified additional roles for these cells in wound healing, tissue repair, and infections. Similar to other innate immune cells, TGF-β can induce chemotaxis and enhance the adherent properties of mast cells (Gruber et al. 1994; Olsson et al. 2000; Rosbottom et al. 2002). TGF-β has been reported to promote or suppress mast cell function. As part of its negative regulatory functions, TGF-β inhibits the expression of the high-affinity IgE receptor FcɛRI, a mechanism that activates mast cells (Gomez et al. 2005). TGF-β was also reported to inhibit mast cell proliferation, degranulation, and production of several effector molecules (Broide et al. 1989; Bissonnette et al. 1997; Gebhardt et al. 2005; Gomez et al. 2005). However, in mast cells, TGF-β can also promote expression of inflammatory mediators, such as IL-6 and lymphotactin (Rumsaeng et al. 1997; Miller et al. 1999; Ganeshan and Bryce 2012).
TGF-β CONTROLS IMMUNE RESPONSES
Fetal–Maternal Tolerance
Treg cells are essential for suppressing destructive alloantigenic immunity during pregnancy (Zenclussen 2006; Munoz-Suano et al. 2011; Robertson et al. 2013). These cells are peripherally induced, as their differentiation depends on both paternal antigens and the conserved noncoding sequence-1 (CNS1) enhancer element that contains a Smad-binding site at the Foxp3 locus (Zheng et al. 2010; Rowe et al. 2012; Samstein et al. 2012). Female mice that lack CNS1 have higher rates of embryo resorption when mated with allogeneic, but not syngeneic, males, confirming that pTreg cells modulate maternal immune responses to paternal alloantigens during pregnancy (Samstein et al. 2012). In addition, during secondary pregnancy, these fetal-specific Treg cells are maintained as a memory pool with accelerated expansion, which provides more resistance to embryo resorption if Treg cells are partially ablated (Rowe et al. 2012). The importance of pTreg-cell generation in fetal–maternal tolerance has prompted much interest in understanding the biological source of TGF-β in this process.
The female reproductive tract (FRT) is a rich environment for TGF-β production and responsiveness (Zhao et al. 1994; Polli et al. 1996). TGF-β production is regulated by ovarian sex hormones and enables several aspects of immunosuppression in the FRT at different stages of the menstrual cycle (Chegini et al. 1994; Takahashi et al. 1994; Wira and Rossoll 2003; Kim et al. 2005; Maurya et al. 2013). Accordingly, hormone-regulated fluctuations occur in systemic and uterine Treg-cell populations, with an estrogen-regulated increase at the time of ovulation (Arruvito et al. 2007). Additionally, in vaginal cells, estradiol regulates tolerance induction and antigen presentation by mediating the local production of TGF-β (Wira et al. 2002; Wira and Rossoll 2003). Furthermore, endogenous TGF-β in the human endometrium suppresses the activity of uterine NK cells (Eriksson et al. 2004, 2006).
Several studies have linked Treg-cell expansion in early pregnancy with exposure to male seminal fluid. Semen provides both male alloantigens and immunomodulatory factors that sufficiently exert biological influences in the FRT, such as activating cytokine gene expression and eliciting changes in the abundance and behavior of infiltrating leukocyte populations. These responses promote tolerance and receptivity for embryo implantation (Robertson 2005; Robertson et al. 2013). The mechanisms underlying immunological suppression by semen are not clearly defined but appear related, at least in part, to extremely high concentrations of TGF-β and prostaglandin (PG)E2 (Robertson et al. 2002, 2009b). Seminal plasma contains high concentrations of TGF-β, nearly 500 ng/ml, which is approximately fivefold higher than that of serum (Saito et al. 1993; Nocera and Chu 1995; Loras et al. 1999). In seminal fluid, TGF-β induces Treg-cell expansion and promotes tolerance to paternal alloantigens in mice (Robertson et al. 2009a). Exogenous TGF-β delivered at conception also boosts the numbers of vaginal Treg cells and helps reduce fetal loss in the CBA/J × DBA/2J spontaneous abortion model (Clark et al. 2008). Thus, seminal TGF-β has been implicated as a key factor in initiating the remodeling events and immunological changes that occur in the uterus during the preimplantation period of pregnancy (Robertson et al. 2002, 2013).
Mucosal Immune Responses
Development of the Gut Barrier
After leaving the sterile intrauterine environment, neonates enter a world full of innocuous environmental antigens, as well as harmful pathogens. Gradual and age-dependent maturation of the immune system fulfills several demands, such as preparing the skin and intestine for colonization by commensal bacteria, tolerizing the host for exposure to food and environmental antigens, and protecting against pathogenic infections (PrabhuDas et al. 2011). TGF-β preserves the intestinal barrier function (Planchon et al. 1994, 1999; Jarry et al. 2008), and its production in the intestine is age-dependent (Zhang et al. 1999a; Maheshwari et al. 2011). For example, rodent pups initially produce low levels of endogenous intestinal TGF-β, which increase during the weaning period (Penttila et al. 1998).
Although still controversial, mammalian milk is thought to provide an important exogenous source of TGF-β to the infant until it can fully produce endogenous TGF-β (Prokesova et al. 2006; Oddy and Rosales 2010; Penttila 2010). In mammalian milk, TGF-β is present at high concentrations and may be a key immunoregulatory factor for promoting intestinal maturation (Rautava et al. 2012), IgA production, and tolerance induction (Letterio et al. 1994; Hawkes et al. 1999; Kalliomaki et al. 1999; Lebman and Edmiston 1999; Donnet-Hughes et al. 2000; Saarinen et al. 2000; Ogawa et al. 2004; Verhasselt et al. 2008; Verhasselt 2010; Arnold et al. 2011).
Microbiome
The composition of the intestinal microbiota regulates the balance between Th17 and Treg cells in the lamina propria and influences intestinal homeostasis (Honda and Littman 2012). Treg-cell numbers are increased in the colonic lamina propria compared with other organs, and these numbers are reduced in germ-free or antibiotics-treated mice, suggesting that the nature of the microbiota affects colonic pTreg-cell differentiation (Atarashi et al. 2011; Honda and Littman 2012). A cocktail of 17 strains of bacteria, belonging to the clusters of Clostridium species, isolated from the stool of a healthy human provided bacterial antigens and a TGF-β-rich environment to support the expansion of Treg cells in germ-free mice (Atarashi et al. 2013). Th17 cells are also induced in the small intestinal lamina propria in the presence of members of the cytophaga–flavobacter–bacteroides phylum, which requires TGF-β activity (Ivanov et al. 2008).
Inflammatory Bowel Disease
IL-10 and TGF-β play nonredundant roles in maintaining intestinal homeostasis (Fiocchi 2001; Izcue et al. 2009; Feagins 2010; Jarry et al. 2011; Biancheri et al. 2014). IL-10 functions both upstream and downstream in TGF-β signaling (Fuss et al. 2002; Kitani et al. 2003). For example, IL-10 can induce TGF-β expression and secretion in T cells of the lamina propria (Zhou et al. 1998; Fuss et al. 2002). Additionally, it cooperates with TGF-β to promote differentiation of Treg cells (Weiner 2001; Di Giacinto et al. 2005), which produce more TGF-β and IL-10 (Harrison and Powrie 2013). Mutations in genes encoding components of TGF-β and IL-10 signaling pathways have been implicated in human inflammatory bowel disease (IBD) (Glocker et al. 2009; Franke et al. 2010; McGovern et al. 2010; Naviglio et al. 2014). Indeed, when both of these pathways are simultaneously genetically blocked, mice develop severe fulminant ulcerative colitis caused by the microbially induced proinflammatory cytokines IFN-γ and TNF-α (Kang et al. 2008).
Similarly, in IBD patients, Smad7 overexpression in mucosal T cells inhibits TGF-β signaling, causing uncontrolled production of inflammatory cytokines (Fiocchi 2001; Monteleone et al. 2001). In IBD patients, Smad7 protein is more highly stabilized by p300-mediated posttranslational acetylation compared with healthy controls (Monteleone et al. 2005), potentially making the cells more resistant to Treg-cell-mediated suppression (Fantini et al. 2009). Indeed, overproduction of TGF-β has been reported (Feagins 2010), which may cause the loss of protective cells that produce IL-22 (Leung et al. 2014). Regardless of the abundance of environmental TGF-β, high Smad7 levels block TGF-β signaling in pathogenic T cells (Fiocchi 2001). IL-25 can limit proinflammatory cytokine production and chronic intestinal inflammation (Owyang et al. 2006). TGF-β induces, whereas TNF-α inhibits, IL-25 production in the human gut, and knockdown of Smad7 increases IL-25 production (Fina et al. 2011). Oral administration of Smad7 antisense oligonucleotides can restore TGF-β signaling and ameliorate inflammation in hapten-induced colitis (Boirivant et al. 2006), suggesting that blocking Smad7 may be a promising and safe method to dampen inflammation in IBD patients (Monteleone et al. 2008, 2012; Marafini et al. 2013).
Autoimmune Diseases
Arthritis
Rheumatoid arthritis (RA) is an inflammatory disorder that targets the joints and is driven by aberrant responses in T and B cells. The effects of TGF-β on RA development appear to be determined by the anatomical context of cytokine signaling, as local versus systemic modulation of TGF-β activity has opposing effects on disease development in rodent models of RA. For example, injecting TGF-β into the joints of Lewis rats induces synovial inflammation and joint swelling associated with macrophage infiltration and increased expression of IL-1β (Allen et al. 1990). Correspondingly, administering a TGF-β blocking antibody into a joint ameliorates group A streptococci–induced arthritis (Wahl et al. 1993a). In contrast, studies in the collagen-induced arthritis model indicate that systemic TGF-β signaling protects against disease development (Kuruvilla et al. 1991; Thorbecke et al. 1992). These protective effects are, in part, mediated by direct regulation of T cells, as mice with T-cell-specific expression of the dnTβRII subunit develop more severe arthritis (Schramm et al. 2004).
Diabetes
Type 1 diabetes mellitus (T1D) is a chronic autoimmune disease driven by immune-mediated destruction of pancreatic islet β cells. Multiple models of pancreas-specific overexpression of active TGF-β show that TGF-β inhibits diabetes development (King et al. 1998; Moritani et al. 1998; Grewal et al. 2002). Protection against T1D is associated with the induction of tolerogenic T-cell responses, suggesting that TGF-β regulates diabetogenic T cells to prevent disease (King et al. 1998; Moritani et al. 1998). Indeed, coadministering a TGF-β blocking antibody reverses the protective effects of CD3-specific antibody treatment in the nonobese diabetic (NOD) model (Belghith et al. 2003). Studies using a model of diabetes in which CD4+ T cells express a transgenic TCR that recognizes a pancreas-specific peptide clearly show the importance of TGF-β in directly regulating the response of effector T cells to prevent diabetes (Ishigame et al. 2013b). Whereas deleting TβRII expression in activated effector T cells induces diabetes development, Treg-cell-specific loss of TGF-β signaling has no effect on disease pathogenesis (Ishigame et al. 2013b).
Multiple Sclerosis
EAE is the animal model system that is commonly used to study the central nervous system disorder multiple sclerosis (MS). Administering TGF-β has a protective effect in several murine models of EAE (Johns et al. 1991; Kuruvilla et al. 1991; Racke et al. 1991), suggesting that TGF-β inhibits disease development. Indeed, two models of EAE showed that suppression of disease by tolerogenic CD4+ T cells depends on TGF-β activity (Chen et al. 1994, 2008). In accordance, T-cell lines derived from patients with stable MS produce more TGF-β than those from patients with active MS, suggesting that disease severity may associate inversely with levels of TGF-β production (Mokhtarian et al. 1994).
Interestingly, TGF-β signaling also promotes differentiation of Th17 cells that induce EAE development. CD4+ T cells that express a dnTβRII do not differentiate into Th17 cells, and mice expressing a dnTβRII under the control of the CD4 promoter are resistant to EAE, indicating that TGF-β signaling is critical for the in vivo generation of pathogenic Th17 cells and EAE development (Veldhoen et al. 2006b). Furthermore, genetic studies show that autocrine TGF-β signaling is required for in vivo Th17 differentiation, as mice with T-cell-specific deletion of Tgfb1 do not develop Th17 cells and are resistant to EAE (Li et al. 2007; Gutcher et al. 2011). In contrast, cell-culture studies suggest that treatment of CD4+ T cells with TGF-β1 produces nonpathogenic Th17 cells that fail to induce EAE (McGeachy et al. 2007; Ghoreschi et al. 2010), whereas treatment with TGF-β3 induces a pathogenic Th17 population that causes disease (Lee et al. 2012). However, the ability of TGF-β3 to induce pathogenic Th17 cells in vivo (e.g., by using T-cell-specific Tgfb3 knockouts) has not been explored.
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by autoantibody production that affects multiple organs. Administering a vector that encodes TGF-β1 enhances survival and ameliorates disease severity in a murine model of lupus, suggesting that TGF-β plays a protective role in disease (Raz et al. 1995). In general, SLE patients produce lower levels of TGF-β when compared with healthy individuals (Ohtsuka et al. 1998, 1999; Becker-Merok et al. 2010). Total TGF-β levels were lowest in hospitalized patients with active disease, suggesting that TGF-β production may be associated with disease severity (Ohtsuka et al. 1999). This is supported by a survey of 102 SLE patients in which TGF-β levels associate inversely with disease severity (Becker-Merok et al. 2010).
Infection
Bacterial
The route by which a pathogen infects its host can determine the arm of protective immunity that the host elicits against that pathogen. For example, oral infection with Yersinia enterocolitica promotes Th17-mediated immunity, whereas systemic infection promotes Th1-mediated immunity (DePaolo et al. 2012). Vaginal Neisseria gonorrhoeae induces TGF-β production, which inhibits a protective Th1-cell response while promoting a Th17 response (Liu et al. 2012). Other mucosal pathogens, such as Citrobacter rodentium, induce Th17-cell responses by promoting apoptosis of intestinal epithelium, and producing TLR-containing apoptotic cells that induce both IL-6 and TGF-β production (Torchinsky et al. 2009; Brereton and Blander 2010). However, without normal production of inflammatory cytokines, high TGF-β levels produced by infected intestinal epithelium can also lead to defects in proper activation of T cells against mucosal pathogens, as apparent during Helicobacter pylori infection (Beswick et al. 2011).
Viral
Influenza virus neuraminidase activates latent TGF-β (Schultz-Cherry and Hinshaw 1996), which protects the host from influenza pathogenesis and virus-mediated pathology in the lung (Carlson et al. 2010). Although TGF-β protects against excessive pathology during acute viral infections, it can have a detrimental effect on T-cell immunity during chronic viral infections. For example, during LCMV infection, sustained TGF-β expression and Smad2 activation cause apoptosis of virus-specific CD8+ T cells, and genetic blockade of TGF-β signaling using dnTβRII rapidly eradicates the virus (Tinoco et al. 2009). However, blocking the TGF-β receptor during the early memory phase failed to substantially enhance the antiviral T-cell response or reduce viral titers in vivo (Boettler et al. 2012), suggesting that TGF-β may exert its apoptotic effect on clonally expanding effector cells rather than on exhausted T cells (Sanjabi et al. 2009; Tinoco et al. 2009). Therapeutically blocking TGF-β signaling before viral infection significantly increases viral-specific T cells; however, it also fails to improve T-cell function or the ability of T cells to clear chronic LCMV infection. These findings suggest that the inflammatory environment and the potential difference in T-cell repertoire in mice expressing a dnTβRII in T cells may contribute to their ability to clear chronic viral infection, as originally reported by Tinoco et al. (2009). In a fourth study of chronic LCMV infection, TβRII expression was increased in CD8+ T cells, but conditionally inactivating Tgfbr2 expression in peripheral T cells decreased the expansion of CD8+ T cells yet did not affect their function or exhaustion (Zhang and Bevan 2013). Thus, the exact role of TGF-β signaling on the expansion, function, and exhaustion of CD8+ T cells during chronic viral infection remains unclear.
In human studies, serum TGF-β levels are increased in patients with HBV or hepatitis C virus (HCV), which contributes to the liver fibrosis often seen with these chronic viral infections (Alatrakchi et al. 2007; Khorramdelazad et al. 2012; Karimi-Googheri et al. 2014). Furthermore, TGFB1 genetic polymorphisms have been associated with higher systemic TGF-β levels and worse outcome in hepatic viral infections (Dai et al. 2008; Pereira et al. 2008). Viral-specific proteins have also been shown to enhance TGF-β production and its cellular activity. The HBV-encoded pX oncoprotein enhances transcriptional activity of TGF-β by stabilizing the Smad complex on the transcriptional machinery (Lee et al. 2001), whereas the HCV nonstructural protein 4 (NS4) induces TGF-β expression in monocytes (Rowan et al. 2008). The HCV core protein can also activate TGF-β, and mice that overexpress HCV core protein through transgenic expression in the liver, show deregulated expression of TGF-β target genes (Benzoubir et al. 2013). Finally, TGF-β produced by hepatic cells can induce Treg-cell generation, which can further dampen antiviral immunity and contribute to chronic hepatic viral infections (Dunham et al. 2013; Karimi-Googheri et al. 2014).
TGF-β1 expression is also increased in HIV-infected patients and correlates with disease progression (Lotz and Seth 1993; Wiercinska-Drapalo et al. 2004). HIV tat protein has been linked to high TGF-β secretion in infected cells (Wahl et al. 1991; Zauli et al. 1992; Lotz et al. 1994; Sawaya et al. 1998; Reinhold et al. 1999). The association of viral gp160 with CD4 on monocytes can also induce TGF-β production (Hu et al. 1996). TGF-β induces C-X-C chemokine receptor type 4 (CXCR4) expression on macrophages, contributing to enhanced tropism of HIV for both CD4 T cells and macrophages (Chen et al. 2005). TGF-β1 production by monocytes may also cause HIV-induced apoptosis of CD4+ T cells and consequent depletion in vivo (Wang et al. 2001). Conversely, TGF-β represses CD4 and C-C chemokine receptor type 5 (CCR5) expression, and inhibits NF-κB activation, thus limiting HIV replication in intestinal macrophages (Shen et al. 2011). Interestingly, TGF-β induces the expression of CD169, which is a main HIV-1 receptor expressed on mucosal DCs that captures virus and transmits it to target cells; thus, TGF-β found in semen may contribute to sexual transmission of the virus (De Saint Jean et al. 2014). Infecting human CD4+ T cells with HIV induces TGF-β production that further promotes Treg-cell generation specific to the gp120 surface viral antigen (Amarnath et al. 2007; Stevceva et al. 2008). However, infection of Treg cells with HIV represses Foxp3 expression, reduces the generation of TGF-β, and increases IL-4 production, thus limiting Treg-cell function (Pion et al. 2013) and likely contributing to chronic inflammation seen in HIV-infected patients. TGF-β produced in the mucosal lymph nodes during HIV infection can also induce apoptosis of activated CD8+ T cells (Cumont et al. 2007), whereas promoting the generation of NKT cells, which share properties of both T cells and NK cells, which produce IL-17 (Campillo-Gimenez et al. 2010). Furthermore, the HIV envelope protein gp120 can bind to α4β7 integrins on naïve B cells to induce TGF-β and Fc receptor-like 4 (FcRL4) expression, which causes B-cell dysfunction and inhibits their proliferation (Jelicic et al. 2013). Furthermore, TGF-β is an important mediator of pathological fibrosis, and promotes collagen deposition in lymphoid organs in response to HIV- or SIV (simian immunodeficiency virus)-induced inflammation. This effect disrupts IL-7 production in lymph nodes, which can eventually contribute to depletion of CD4+ T cells (Estes et al. 2007; Zeng et al. 2011).
When rhesus macaques are infected with SIV, they develop AIDS-like syndromes that are accompanied by massive inflammation, similar to HIV-infected patients; however, African green monkeys (AGM) infected with SIV remain healthy and do not show this chronic inflammation (Chahroudi et al. 2012). SIV-infected AGMs show early and strong increases in IL-10, TGF-β, and Foxp3 expression, which oppose findings in infected macaques that show diminished sensitivity to TGF-β signaling in T cells (Kornfeld et al. 2005; Ploquin et al. 2006).
One of the hallmarks of AIDS is HIV-mediated immunodeficiency against other pathogenic and nonpathogenic organisms. TGF-β may play an active role in this process. In HIV and HCV coinfections, HIV-induced TGF-β expression promotes both HCV replication and advanced liver fibrosis (Lin et al. 2008). Similarly, HIV-infected macrophages produce high levels of TGF-β and permit the survival and multiplication of otherwise nonpathogenic parasites (further discussed below) (Barreto-de-Souza et al. 2008).
Parasitic
During acute Trypanosoma cruzi and Leishmania infection, TGF-β production inhibits macrophage function, including IFN-γ production and increased pathogen replication (Silva et al. 1991; Barral-Netto et al. 1992; Barral et al. 1993). Conversely, malaria infection leads to activation of latent TGF-β, whose production correlates with protective immune responses. This effect results in slowed parasite growth early on, and less pathology late in infection (Omer and Riley 1998; Omer et al. 2003). TGF-β-mediated induction of Treg-cell differentiation is also associated with higher rates of parasite growth after malaria infection in humans (Walther et al. 2005; Scholzen et al. 2009), and inhibiting TGF-β activity results in more robust CD8+ T-cell responses and protection against reinfection in mice (Ocana-Morgner et al. 2007). As helminth parasites stimulate TGF-β production and Treg-cell induction, they may, in fact, protect the host from allergic diseases (Dittrich et al. 2008; Grainger et al. 2010).
CONCLUDING REMARKS
Studies in the past three decades have revealed the remarkably diverse and important functions of TGF-β in the immune system, and its penetrating control of immune responses under pathophysiological conditions. These discoveries support the notion that immune regulatory mechanisms, established by coopting cell signaling pathways that are evolutionarily conserved, work in concert with mechanisms of both innate and adaptive immune recognition to ensure well-ordered immune activities. Future investigations will define the precise cellular and molecular mechanisms of immune regulation by TGF-β, and will explore targeting this pleiotropic cell signaling pathway for therapies to treat pathogenic immune disorders.
ACKNOWLEDGMENTS
Studies are supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (RO1 AR060723 to M.O.L.), National Institute of Allergy and Infectious Diseases (NIAID) (RO1 AI122264 to M.O.L. and R21 AI108953 to S.S.), the Rita Allen Foundation (M.O.L.), Office of the Director (DP2 AI112244 to S.S.), UC Hellman Award (S.S.), and National Institutes of Health (NIH) (T32-CA9149-35 to S.A.O.). Research in the Li laboratory is also supported by the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). The Gladstone Institutes received support from a National Center for Research Resources Grant RR18928.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Acharya M, Mukhopadhyay S, Paidassi H, Jamil T, Chow C, Kissler S, Stuart LM, Hynes RO, Lacy-Hulbert A. 2010. αv Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest 120: 4445–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam R, Forsythe P, Stafford S, Fukuda Y. 1994. Transforming growth factor β abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med 179: 1041–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatrakchi N, Graham CS, van der Vliet HJ, Sherman KE, Exley MA, Koziel MJ. 2007. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor β that can suppress HCV-specific T-cell responses. J Virol 81: 5882–5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JB, Manthey CL, Hand AR, Ohura K, Ellingsworth L, Wahl SM. 1990. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor β. J Exp Med 171: 231–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarnath S, Dong L, Li J, Wu Y, Chen W. 2007. Endogenous TGF-β activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology 4: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Muller A. 2011. Tolerance rather than immunity protects from Helicobacter pylori–induced gastric preneoplasia. Gastroenterology 140: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruvito L, Sanz M, Banham AH, Fainboim L. 2007. Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the follicular phase of the menstrual cycle: Implications for human reproduction. J Immunol 178: 2572–2578. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500: 232–236. [DOI] [PubMed] [Google Scholar]
- Balazovich KJ, Fernandez R, Hinkovska-Galcheva V, Suchard SJ, Boxer LA. 1996. Transforming growth factor-β1 stimulates degranulation and oxidant release by adherent human neutrophils. J Leukoc Biol 60: 772–777. [DOI] [PubMed] [Google Scholar]
- Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. 1993. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci 90: 3442–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral-Netto M, Barral A, Brownell CE, Skeiky YA, Ellingsworth LR, Twardzik DR, Reed SG. 1992. Transforming growth factor-β in leishmanial infection: A parasite escape mechanism. Science 257: 545–548. [DOI] [PubMed] [Google Scholar]
- Barreto-de-Souza V, Xavier Medeiros T, Machado Motta MC, Bou-Habib DC, Saraiva EM. 2008. HIV-1 infection and HIV-1 Tat protein permit the survival and replication of a non-pathogenic trypanosomatid in macrophages through TGF-β1 production. Microbes Infect 10: 642–649. [DOI] [PubMed] [Google Scholar]
- Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia A, Buzzonetti A, Baranello C, Fanelli M, Fossati M, Catzola V, Scambia G, Fattorossi A. 2013. Interleukin-21 (IL-21) synergizes with IL-2 to enhance T-cell receptor-induced human T-cell proliferation and counteracts IL-2/transforming growth factor-β-induced regulatory T-cell development. Immunology 139: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer T, Zagorska A, Jurkin J, Yasmin N, Koffel R, Richter S, Gesslbauer B, Lemke G, Strobl H. 2012. Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis. J Exp Med 209: 2033–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B, Rouillard D, Sanceau J, Wietzerbin J. 1992. IFN-γ and transforming growth factor-β1 differently regulate fibronectin and laminin receptors of human differentiating monocytic cells. J Immunol 148: 3912–3919. [PubMed] [Google Scholar]
- Beal AM, Ramos-Hernandez N, Riling CR, Nowelsky EA, Oliver PM. 2011. TGF-β induces the expression of the adaptor Ndfip1 to silence IL-4 production during iTreg cell differentiation. Nat Immunol 13: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Merok A, Eilertsen GO, Nossent JC. 2010. Levels of transforming growth factor-β are low in systemic lupus erythematosus patients with active disease. J Rheumatol 37: 2039–2045. [DOI] [PubMed] [Google Scholar]
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. 2003. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 9: 1202–1208. [DOI] [PubMed] [Google Scholar]
- Benzoubir N, Lejamtel C, Battaglia S, Testoni B, Benassi B, Gondeau C, Perrin-Cocon L, Desterke C, Thiers V, Samuel D, et al. 2013. HCV core-mediated activation of latent TGF-β via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J Hepatol 59: 1160–1168. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Bevan MJ. 2015. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8+ T cells responding to infection. Nat Immunol 16: 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick EJ, Pinchuk IV, Earley RB, Schmitt DA, Reyes VE. 2011. Role of gastric epithelial cell-derived transforming growth factor β in reduced CD4+ T cell proliferation and development of regulatory T cells during Helicobacter pylori infection. Infect Immun 79: 2737–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. 2014. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev 25: 45–55. [DOI] [PubMed] [Google Scholar]
- Bissonnette EY, Enciso JA, Befus AD. 1997. TGF-β1 inhibits the release of histamine and tumor necrosis factor-α from mast cells through an autocrine pathway. Am J Respir Cell Mol Biol 16: 275–282. [DOI] [PubMed] [Google Scholar]
- Boettler T, Cheng Y, Ehrhardt K, von Herrath M. 2012. TGF-β blockade does not improve control of an established persistent viral infection. Viral Immunol 25: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M, Pallone F, Di Giacinto C, Fina D, Monteleone I, Marinaro M, Caruso R, Colantoni A, Palmieri G, Sanchez M, et al. 2006. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-β1-mediated suppression of colitis. Gastroenterology 131: 1786–1798. [DOI] [PubMed] [Google Scholar]
- Borkowski TA, Letterio JJ, Farr AG, Udey MC. 1996. A role for endogenous transforming growth factor β1 in Langerhans cell biology: The skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J Exp Med 184: 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsutzky S, Cazac BB, Roes J, Guzman CA. 2004. TGF-β receptor signaling is critical for mucosal IgA responses. J Immunol 173: 3305–3309. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Fridman WH, Sautes C. 1997. Effect of TGF-β1 on cell cycle regulatory proteins in LPS-stimulated normal mouse B lymphocytes. J Immunol 159: 4155–4164. [PubMed] [Google Scholar]
- Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. 1993. Transforming growth factor β and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol Cell Biol 13: 1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes ME, Mai UE, Ohura K, Wahl SM. 1991. Type I transforming growth factor-β receptors on neutrophils mediate chemotaxis to transforming growth factor-β. J Immunol 147: 1600–1606. [PubMed] [Google Scholar]
- Brennan PJ, Brigl M, Brenner MB. 2013. Invariant natural killer T cells: An innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13: 101–117. [DOI] [PubMed] [Google Scholar]
- Brereton CF, Blander JM. 2010. Responding to infection and apoptosis—A task for TH17 cells. Ann NY Acad Sci 1209: 56–67. [DOI] [PubMed] [Google Scholar]
- Broide DH, Wasserman SI, Alvaro-Gracia J, Zvaifler NJ, Firestein GS. 1989. Transforming growth factor-β1 selectively inhibits IL-3-dependent mast cell proliferation without affecting mast cell function or differentiation. J Immunol 143: 1591–1597. [PubMed] [Google Scholar]
- Campillo-Gimenez L, Cumont MC, Fay M, Kared H, Monceaux V, Diop O, Muller-Trutwin M, Hurtrel B, Levy Y, Zaunders J, et al. 2010. AIDS progression is associated with the emergence of IL-17-producing cells early after simian immunodeficiency virus infection. J Immunol 184: 984–992. [DOI] [PubMed] [Google Scholar]
- Carlson CM, Turpin EA, Moser LA, O’Brien KB, Cline TD, Jones JC, Tumpey TM, Katz JM, Kelley LA, Gauldie J, et al. 2010. Transforming growth factor-β: Activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Path 6: e1001136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. 2012. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol 188: 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castriconi R, Cantoni C, Della Chiesa M, Vitale M, Marcenaro E, Conte R, Biassoni R, Bottino C, Moretta L, Moretta A. 2003. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci 100: 4120–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley LS, Lefrancois L. 2013. Guarding the perimeter: Protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol 6: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazac BB, Roes J. 2000. TGF-β receptor controls B cell responsiveness and induction of IgA in vivo. Immunity 13: 443–451. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Parker CM, Madara JL, Brenner MB. 1993. Integrin αEβ7 mediates adhesion of T lymphocytes to epithelial cells. J Immunol 150: 3459–3470. [PubMed] [Google Scholar]
- Cerutti A, Chen K, Chorny A. 2011. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol 29: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: Showing AIDS the door. Science 335: 1188–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, et al. 2012. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36: 362–373. [DOI] [PubMed] [Google Scholar]
- Chantry D, Turner M, Abney E, Feldmann M. 1989. Modulation of cytokine production by transforming growth factor-β. J Immunol 142: 4295–4300. [PubMed] [Google Scholar]
- Chegini N, Zhao Y, Williams RS, Flanders KC. 1994. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-β1 (TGFβ1), TGFβ2, TGFβ3, and TGFβ type II receptor messenger ribonucleic acid and protein and contains [125I]TGFβ1-binding sites. Endocrinology 135: 439–449. [DOI] [PubMed] [Google Scholar]
- Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. 1994. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science 265: 1237–1240. [DOI] [PubMed] [Google Scholar]
- Chen CH, Seguin-Devaux C, Burke NA, Oriss TB, Watkins SC, Clipstone N, Ray A. 2003a. Transforming growth factor β blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med 197: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. 2003b. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Tuttle DL, Oshier JT, Knot HJ, Streit WJ, Goodenow MM, Harrison JK. 2005. Transforming growth factor-β1 increases CXCR4 expression, stromal-derived factor-1α-stimulated signalling and human immunodeficiency virus-1 entry in human monocyte-derived macrophages. Immunology 114: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. 2008. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFβ-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol 180: 7327–7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheroutre H, Lambolez F, Mucida D. 2011. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikuma S, Suita N, Okazaki IM, Shibayama S, Honjo T. 2012. TRIM28 prevents autoinflammatory T cell development in vivo. Nat Immunol 13: 596–603. [DOI] [PubMed] [Google Scholar]
- Choi KC, Lee YS, Lim S, Choi HK, Lee CH, Lee EK, Hong S, Kim IH, Kim SJ, Park SH. 2006. Smad6 negatively regulates interleukin 1-receptor-Toll-like receptor signaling through direct interaction with the adaptor Pellino-1. Nat Immunol 7: 1057–1065. [DOI] [PubMed] [Google Scholar]
- Chopin M, Seillet C, Chevrier S, Wu L, Wang H, Morse HC III, Belz GT, Nutt SL. 2013. Langerhans cells are generated by two distinct PU.1-dependent transcriptional networks. J Exp Med 210: 2967–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Fernandes J, Banwatt D. 2008. Prevention of spontaneous abortion in the CBA × DBA/2 mouse model by intravaginal TGF-β and local recruitment of CD4+ 8+ FOXP3+ cells. Am J Reprod Immunol 59: 525–534. [DOI] [PubMed] [Google Scholar]
- Coffman RL, Lebman DA, Shrader B. 1989. Transforming growth factor β specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med 170: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrez F, Groux H. 2001. Regulation of TGF-β response during T cell activation is modulated by IL-10. J Immunol 167: 773–778. [DOI] [PubMed] [Google Scholar]
- Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. 2010. TGF-β downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol 12: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29: 621–663. [DOI] [PubMed] [Google Scholar]
- Cumont MC, Monceaux V, Viollet L, Lay S, Parker R, Hurtrel B, Estaquier J. 2007. TGF-β in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus. Cell Death Differ 14: 1747–1758. [DOI] [PubMed] [Google Scholar]
- Dai CY, Chuang WL, Lee LP, Pan WC, Huang JF, Hsieh MY, Hou NJ, Lin ZY, Chen SC, Wang LY, et al. 2008. Association between transforming growth factor-β1 polymorphism and virologic characteristics of chronic hepatitis C. Transl Res 152: 151–156. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, Mitsdoerffer M, Strom TB, Elyaman W, Ho IC, et al. 2008. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3-effector T cells. Nat Immunol 9: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. 2009. Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med 206: 2407–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaolo RW, Kamdar K, Khakpour S, Sugiura Y, Wang W, Jabri B. 2012. A specific role for TLR1 in protective TH17 immunity during mucosal infection. J Exp Med 209: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint Jean A, Lucht F, Bourlet T, Delezay O. 2014. Transforming growth factor β1 up-regulates CD169 (sialoadhesin) expression on monocyte-derived dendritic cells: Role in HIV sexual transmission. AIDS 28: 2375–2380. [DOI] [PubMed] [Google Scholar]
- Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. 2005. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-β-bearing regulatory cells. J Immunol 174: 3237–3246. [DOI] [PubMed] [Google Scholar]
- Dittrich AM, Erbacher A, Specht S, Diesner F, Krokowski M, Avagyan A, Stock P, Ahrens B, Hoffmann WH, Hoerauf A, et al. 2008. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J Immunol 180: 1792–1799. [DOI] [PubMed] [Google Scholar]
- Doisne JM, Bartholin L, Yan KP, Garcia CN, Duarte N, Le Luduec JB, Vincent D, Cyprian F, Horvat B, Martel S, et al. 2009. iNKT cell development is orchestrated by different branches of TGF-β signaling. J Exp Med 206: 1365–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatelli SS, Zhou JM, Gilvary DL, Eksioglu EA, Chen X, Cress WD, Haura EB, Schabath MB, Coppola D, Wei S, et al. 2014. TGF-β-inducible microRNA-183 silences tumor-associated natural killer cells. Proc Natl Acad Sci 111: 4203–4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnet-Hughes A, Duc N, Serrant P, Vidal K, Schiffrin EJ. 2000. Bioactive molecules in milk and their role in health and disease: The role of transforming growth factor-β. Immunol Cell Biol 78: 74–79. [DOI] [PubMed] [Google Scholar]
- Dunham RM, Thapa M, Velazquez VM, Elrod EJ, Denning TL, Pulendran B, Grakoui A. 2013. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol 190: 2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP, Fujii H, Zhou AX, Creemers J, Unutmaz D, Shevach EM. 2013. Regulation of the expression of GARP/latent TGF-β1 complexes on mouse T cells and their role in regulatory T cell and Th17 differentiation. J Immunol 190: 5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt RO, Strober W, Harriman GR. 1992. Effect of transforming growth factor (TGF)-β1 on IgA isotype expression. TGF-β1 induces a small increase in sIgA+ B cells regardless of the method of B cell activation. J Immunol 148: 3830–3836. [PubMed] [Google Scholar]
- El-Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. 2005. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med 201: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyaman W, Bassil R, Bradshaw EM, Orent W, Lahoud Y, Zhu B, Radtke F, Yagita H, Khoury SJ. 2012. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity 36: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Meadows SK, Wira CR, Sentman CL. 2004. Unique phenotype of human uterine NK cells and their regulation by endogenous TGF-β. J Leukocyte Biol 76: 667–675. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Meadows SK, Wira CR, Sentman CL. 2006. Endogenous transforming growth factor-β inhibits toll-like receptor mediated activation of human uterine natural killer cells. Am J Reprod Immunol 56: 321–328. [DOI] [PubMed] [Google Scholar]
- Esplugues E, Huber S, Gagliani N, Hauser AE, Town T, Wan YY, O’Connor W Jr, Rongvaux A, Van Rooijen N, Haberman AM, et al. 2011. Control of TH17 cells occurs in the small intestine. Nature 475: 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, Milush JM, Lifson JD, Sodora DL, Carlis JV, et al. 2007. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor β1-positive regulatory T cells and begins in early infection. J Infect Dis 195: 551–561. [DOI] [PubMed] [Google Scholar]
- Fainaru O, Woolf E, Lotem J, Yarmus M, Brenner O, Goldenberg D, Negreanu V, Bernstein Y, Levanon D, Jung S, et al. 2004. Runx3 regulates mouse TGF-β-mediated dendritic cell function and its absence results in airway inflammation. EMBO J 23: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan K, Ruan Q, Sensenbrenner L, Chen B. 1992. Transforming growth factor-β1 bifunctionally regulates murine macrophage proliferation. Blood 79: 1679–1685. [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. 2004. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol 172: 5149–5153. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C, Becker C, Macdonald TT, Pallone F, Neurath MF, et al. 2009. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 136: 1308–1316, e1301–e1303. [DOI] [PubMed] [Google Scholar]
- Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, Lucas C, Townes AS. 1991. Transforming growth factor β1 (TGF-β1) induced neutrophil recruitment to synovial tissues: Implications for TGF-β-driven synovial inflammation and hyperplasia. J Exp Med 173: 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagins LA. 2010. Role of transforming growth factor-β in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis 16: 1963–1968. [DOI] [PubMed] [Google Scholar]
- Fina D, Franze E, Rovedatti L, Corazza GR, Biancone L, Sileri PP, Sica G, MacDonald TT, Pallone F, Di Sabatino A, et al. 2011. Interleukin-25 production is differently regulated by TNF-α and TGF-β1 in the human gut. Mucosal immunol 4: 239–244. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. 2001. TGF-β/Smad signaling defects in inflammatory bowel disease: Mechanisms and possible novel therapies for chronic inflammation. J Clin Invest 108: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel-Petrovic M, Long JA, Misso NL, Foster PS, Bhoola KD, Thompson PJ. 2007. Physiological concentrations of transforming growth factor β1 selectively inhibit human dendritic cell function. Int Immunopharmacol 7: 1924–1933. [DOI] [PubMed] [Google Scholar]
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. 2010. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42: 1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Boirivant M, Lacy B, Strober W. 2002. The interrelated roles of TGF-β and IL-10 in the regulation of experimental colitis. J Immunol 168: 900–908. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. 2015. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523: 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Bryce PJ. 2012. Regulatory T cells enhance mast cell production of IL-6 via surface-bound TGF-β. J Immunol 188: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Lorentz A, Detmer F, Trautwein C, Bektas H, Manns MP, Bischoff SC. 2005. Growth, phenotype, and function of human intestinal mast cells are tightly regulated by transforming growth factor β1. Gut 54: 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. 1998. Transforming growth factor β1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J Exp Med 187: 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. 1999. TGF-β1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol 162: 4567–4575. [PubMed] [Google Scholar]
- Genestier L, Kasibhatla S, Brunner T, Green DR. 1999. Transforming growth factor β1 inhibits Fas ligand expression and subsequent activation-induced cell death in T cells via downregulation of c-Myc. J Exp Med 189: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. 2010. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Jung S. 2014. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol 14: 392–404. [DOI] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. 2009. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 361: 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez G, Ramirez CD, Rivera J, Patel M, Norozian F, Wright HV, Kashyap MV, Barnstein BO, Fischer-Stenger K, Schwartz LB, et al. 2005. TGF-β1 inhibits mast cell FcɛRI expression. J Immunol 174: 5987–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik L, Flavell RA. 2000. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12: 171–181. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Fields PE, Flavell RA. 2000. Cutting edge: TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 165: 4773–4777. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Constant S, Flavell RA. 2002. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J Exp Med 195: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, Finney CA, Greenwood EJ, Knox DP, Wilson MS, et al. 2010. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med 207: 2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA Jr, Flavell RA. 2002. Expression of transgene encoded TGF-β in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun 19: 9–22. [DOI] [PubMed] [Google Scholar]
- Gruber BL, Marchese MJ, Kew RR. 1994. Transforming growth factor-β1 mediates mast cell chemotaxis. J Immunol 152: 5860–5867. [PubMed] [Google Scholar]
- Gu AD, Wang Y, Lin L, Zhang SS, Wan YY. 2012. Requirements of transcription factor Smad-dependent and -independent TGF-β signaling to control discrete T-cell functions. Proc Natl Acad Sci 109: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnlaugsdottir B, Maggadottir SM, Ludviksson BR. 2005. Anti-CD28-induced co-stimulation and TCR avidity regulates the differential effect of TGF-β1 on CD4+ and CD8+ naive human T-cells. Int Immunol 17: 35–44. [DOI] [PubMed] [Google Scholar]
- Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. 2011. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity 34: 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanai J, Chen LF, Kanno T, Ohtani-Fujita N, Kim WY, Guo WH, Imamura T, Ishidou Y, Fukuchi M, Shi MJ, et al. 1999. Interaction and functional cooperation of PEBP2/CBF with Smads. Synergistic induction of the immunoglobulin germline Cα promoter. J Biol Chem 274: 31577–31582. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Powrie FM. 2013. Regulatory T cells and immune tolerance in the intestine. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JS, Bryan DL, James MJ, Gibson RA. 1999. Cytokines (IL-1β, IL-6, TNF-α, TGF-β1, and TGF-β2) and prostaglandin E2 in human milk during the first three months postpartum. Pediatr Res 46: 194–199. [DOI] [PubMed] [Google Scholar]
- Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. 2000. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol 30: 2639–2649. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garay M, Mendez-Samperio P. 2003. Transforming growth factor-β decreases survival of Mycobacterium bovis–activated T cells. Arch Med Res 34: 20–25. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. 2012. The microbiome in infectious disease and inflammation. Annu Rev Immunol 30: 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Lim S, Li AG, Lee C, Lee YS, Lee EK, Park SH, Wang XJ, Kim SJ. 2007. Smad7 binds to the adaptors TAB2 and TAB3 to block recruitment of the kinase TAK1 to the adaptor TRAF2. Nat Immunol 8: 504–513. [DOI] [PubMed] [Google Scholar]
- Hu R, Oyaizu N, Than S, Kalyanaraman VS, Wang XP, Pahwa S. 1996. HIV-1 gp160 induces transforming growth factor-β production in human PBMC. Clin Immunol Immunopathol 80: 283–289. [DOI] [PubMed] [Google Scholar]
- Hu Y, Lee YT, Kaech SM, Garvy B, Cauley LS. 2015. Smad4 promotes differentiation of effector and circulating memory CD8 T cells but is dispensable for tissue-resident memory CD8 T cells. J Immunol 194: 2407–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama K, Sekiya T, Inoue N, Tamiya T, Kashiwagi I, Kimura A, Morita R, Muto G, Shichita T, Takahashi R, et al. 2011. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity 34: 741–754. [DOI] [PubMed] [Google Scholar]
- Ishigame H, Mosaheb MM, Sanjabi S, Flavell RA. 2013a. Truncated form of TGF-βRII, but not its absence, induces memory CD8+ T cell expansion and lymphoproliferative disorder in mice. J Immunol 190: 6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Zenewicz LA, Sanjabi S, Licona-Limon P, Nakayama M, Leonard WJ, Flavell RA. 2013b. Excessive Th1 responses due to the absence of TGF-β signaling cause autoimmune diabetes and dysregulated Treg cell homeostasis. Proc Natl Acad Sci 110: 6961–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. 2009. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27: 313–338. [DOI] [PubMed] [Google Scholar]
- Jarry A, Bossard C, Bou-Hanna C, Masson D, Espaze E, Denis MG, Laboisse CL. 2008. Mucosal IL-10 and TGF-β play crucial roles in preventing LPS-driven, IFN-γ-mediated epithelial damage in human colon explants. J Clin Invest 118: 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarry A, Bossard C, Sarrabayrouse G, Mosnier JF, Laboisse CL. 2011. Loss of interleukin-10 or transforming growth factor β signaling in the human colon initiates a T-helper 1 response via distinct pathways. Gastroenterology 141: 1887–1896, e1–e2. [DOI] [PubMed] [Google Scholar]
- Jelicic K, Cimbro R, Nawaz F, Huang da W, Zheng X, Yang J, Lempicki RA, Pascuccio M, Van Ryk D, Schwing C, et al. 2013. The HIV-1 envelope protein gp120 impairs B cell proliferation by inducing TGF-β1 production and FcRL4 expression. Nat Immunol 14: 1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LD, Flanders KC, Ranges GE, Sriram S. 1991. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-β1. J Immunol 147: 1792–1796. [PubMed] [Google Scholar]
- Johnson LD, Jameson SC. 2012. TGF-β sensitivity restrains CD8+ T cell homeostatic proliferation by enforcing sensitivity to IL-7 and IL-15. PLoS ONE 7: e42268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Wilson CB, Rudensky AY. 2009. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J Immunol 182: 6648–6652. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. 2012. Regulatory T cells: Mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Ouwehand A, Arvilommi H, Kero P, Isolauri E. 1999. Transforming growth factor-β in breast milk: A potential regulator of atopic disease at an early age. J Allergy Clin Immunol 104: 1251–1257. [DOI] [PubMed] [Google Scholar]
- Kang SS, Bloom SM, Norian LA, Geske MJ, Flavell RA, Stappenbeck TS, Allen PM. 2008. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med 5: e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SG, Park J, Cho JY, Ulrich B, Kim CH. 2011. Complementary roles of retinoic acid and TGF-β1 in coordinated expression of mucosal integrins by T cells. Mucosal Immunol 4: 66–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. 2007. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J Exp Med 204: 2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Googheri M, Daneshvar H, Nosratabadi R, Zare-Bidaki M, Hassanshahi G, Ebrahim M, Arababadi MK, Kennedy D. 2014. Important roles played by TGF-β in hepatitis B infection. J Med Virol 86: 102–108. [DOI] [PubMed] [Google Scholar]
- Kashiwagi I, Morita R, Schichita T, Komai K, Saeki K, Matsumoto M, Takeda K, Nomura M, Hayashi A, Kanai T, et al. 2015. Smad2 and Smad3 inversely regulate TGF-β autoinduction in Clostridium butyricum-activated dendritic cells. Immunity 43: 65–79. [DOI] [PubMed] [Google Scholar]
- Kee BL, Rivera RR, Murre C. 2001. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-β. Nat Immunol 2: 242–247. [DOI] [PubMed] [Google Scholar]
- Kehrl JH, Roberts AB, Wakefield LM, Jakowlew S, Sporn MB, Fauci AS. 1986a. Transforming growth factor β is an important immunomodulatory protein for human B lymphocytes. J Immunol 137: 3855–3860. [PubMed] [Google Scholar]
- Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. 1986b. Production of transforming growth factor β by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med 163: 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl JH, Thevenin C, Rieckmann P, Fauci AS. 1991. Transforming growth factor-β suppresses human B lymphocyte Ig production by inhibiting synthesis and the switch from the membrane form to the secreted form of Ig mRNA. J Immunol 146: 4016–4023. [PubMed] [Google Scholar]
- Kel JM, Girard-Madoux MJ, Reizis B, Clausen BE. 2010. TGF-β is required to maintain the pool of immature Langerhans cells in the epidermis. J Immunol 185: 3248–3255. [DOI] [PubMed] [Google Scholar]
- Khorramdelazad H, Hassanshahi G, Nasiri Ahmadabadi B, Kazemi Arababadi M. 2012. High serum levels of TGF-β in Iranians with chronic HBV infection. Hepat Mon 12: e7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieper WC, Burghardt JT, Surh CD. 2004. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol 172: 40–44. [DOI] [PubMed] [Google Scholar]
- Kim PH, Kagnoff MF. 1990. Transforming growth factor β1 increases IgA isotype switching at the clonal level. J Immunol 145: 3773–3778. [PubMed] [Google Scholar]
- Kim MR, Park DW, Lee JH, Choi DS, Hwang KJ, Ryu HS, Min CK. 2005. Progesterone-dependent release of transforming growth factor-β1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol Human Reprod 11: 801–808. [DOI] [PubMed] [Google Scholar]
- King C, Davies J, Mueller R, Lee MS, Krahl T, Yeung B, O’Connor E, Sarvetnick N. 1998. TGF-β1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 8: 601–613. [DOI] [PubMed] [Google Scholar]
- Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. 2003. Transforming growth factor (TGF)-β1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-β1-mediated fibrosis. J Exp Med 198: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J, Ju W, Heyer J, Wittek B, Haneke T, Knaus P, Kucherlapati R, Bottinger EP, Nitschke L, Kneitz B. 2006. B cell-specific deficiency for Smad2 in vivo leads to defects in TGF-β-directed IgA switching and changes in B cell fate. J Immunol 176: 2389–2396. [DOI] [PubMed] [Google Scholar]
- Kohyama M, Yasogi Y, Nakano N, Ise W, Kaminogawa S, Hozumi N. 2005. Ca2+ signaling down-regulates TGF-β1 gene expression in CD4+ T cells. Biochem Biophys Res Commun 327: 494–499. [DOI] [PubMed] [Google Scholar]
- Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, Chen W. 2011. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol 12: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu Rev Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. 2005. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest 115: 1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino MA, Thorbecke GJ. 1991. Protective effect of transforming growth factor β1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci 88: 2918–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara M, Yamashita M, Shinoda K, Tofukuji S, Onodera A, Shinnakasu R, Motohashi S, Hosokawa H, Tumes D, Iwamura C, et al. 2012. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-β and suppresses TH2 differentiation. Nat Immunol 13: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. 2007. Ulcerative colitis and autoimmunity induced by loss of myeloid αv integrins. Proc Natl Acad Sci 104: 15823–15828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. 2005. Transforming growth factor-β controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nat Immunol 6: 600–607. [DOI] [PubMed] [Google Scholar]
- Lebman DA, Edmiston JS. 1999. The role of TGF-β in growth, differentiation, and maturation of B lymphocytes. Microbes Infect 1: 1297–1304. [DOI] [PubMed] [Google Scholar]
- Lebman DA, Lee FD, Coffman RL. 1990. Mechanism for transforming growth factor β and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J Immunol 144: 952–959. [PubMed] [Google Scholar]
- Lee DK, Park SH, Yi Y, Choi SG, Lee C, Parks WT, Cho H, de Caestecker MP, Shaul Y, Roberts AB, et al. 2001. The hepatitis B virus encoded oncoprotein pX amplifies TGF-β family signaling through direct interaction with Smad4: Potential mechanism of hepatitis B virus-induced liver fibrosis. Genes Dev 15: 455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Lee KM, Kim DW, Heo DS. 2004. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 172: 7335–7340. [DOI] [PubMed] [Google Scholar]
- Lee YS, Kim JH, Kim ST, Kwon JY, Hong S, Kim SJ, Park SH. 2010. Smad7 and Smad6 bind to discrete regions of Pellino-1 via their MH2 domains to mediate TGF-β1-induced negative regulation of IL-1R/TLR signaling. Biochem Biophys Res Commun 393: 836–843. [DOI] [PubMed] [Google Scholar]
- Lee YS, Park JS, Kim JH, Jung SM, Lee JY, Kim SJ, Park SH. 2011. Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat Commun 2: 460. [DOI] [PubMed] [Google Scholar]
- Lee Y, Awasthi A, Yosef N, Quintana FJ, Xiao S, Peters A, Wu C, Kleinewietfeld M, Kunder S, Hafler DA, et al. 2012. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 13: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. 1994. Maternal rescue of transforming growth factor-β1 null mice. Science 264: 1936–1938. [DOI] [PubMed] [Google Scholar]
- Leung JM, Davenport M, Wolff MJ, Wiens KE, Abidi WM, Poles MA, Cho I, Ullman T, Mayer L, Loke P. 2014. IL-22-producing CD4+ cells are depleted in actively inflamed colitis tissue. Mucosal Immunol 7: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. 2006a. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25: 455–471. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. 2006b. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol 24: 99–146. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Flavell RA. 2007. T cell-produced transforming growth factor-β1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity 26: 579–591. [DOI] [PubMed] [Google Scholar]
- Licona-Limón P, Henao-Mejia J, Temann Angela U, Gagliani N, Licona-Limón I, Ishigame H, Hao L, Herbert DBroski R, Flavell Richard A. 2013. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity 39: 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Stavnezer J. 1992. Regulation of transcription of the germ-line Igα constant region gene by an ATF element and by novel transforming growth factor-β1-responsive elements. J Immunol 149: 2914–2925. [PubMed] [Google Scholar]
- Lin JT, Martin SL, Xia L, Gorham JD. 2005. TGF-β1 uses distinct mechanisms to inhibit IFN-γ expression in CD4+ T cells at priming and at recall: Differential involvement of Stat4 and T-bet. J Immunol 174: 5950–5958. [DOI] [PubMed] [Google Scholar]
- Lin W, Weinberg EM, Tai AW, Peng LF, Brockman MA, Kim KA, Kim SS, Borges CB, Shao RX, Chung RT. 2008. HIV increases HCV replication in a TGF-β1-dependent manner. Gastroenterology 134: 803–811. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. 2008. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 9: 632–640. [DOI] [PubMed] [Google Scholar]
- Liu Y, Islam EA, Jarvis GA, Gray-Owen SD, Russell MW. 2012. Neisseria gonorrhoeae selectively suppresses the development of Th1 and Th2 cells, and enhances Th17 cell responses, through TGF-β-dependent mechanisms. Mucosal Immunol 5: 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loras B, Vetele F, El Malki A, Rollet J, Soufir JC, Benahmed M. 1999. Seminal transforming growth factor-β in normal and infertile men. Hum Reprod 14: 1534–1539. [DOI] [PubMed] [Google Scholar]
- Lotz M, Seth P. 1993. TGFβ and HIV infection. Ann NY Acad Sci 685: 501–511. [DOI] [PubMed] [Google Scholar]
- Lotz M, Clark-Lewis I, Ganu V. 1994. HIV-1 transactivator protein Tat induces proliferation and TGFβ expression in human articular chondrocytes. J Cell Biol 124: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PJ, Kim SJ, Melby SJ, Gress RE. 2000. Disruption of T cell homeostasis in mice expressing a T-cell-specific dominant negative transforming growth factor β II receptor. J Exp Med 191: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas PJ, McNeil N, Hilgenfeld E, Choudhury B, Kim SJ, Eckhaus MA, Ried T, Gress RE. 2004. Transforming growth factor-β pathway serves as a primary tumor suppressor in CD8+ T cell tumorigenesis. Cancer Res 64: 6524–6529. [DOI] [PubMed] [Google Scholar]
- Lucas PJ, Kim SJ, Mackall CL, Telford WG, Chu YW, Hakim FT, Gress RE. 2006. Dysregulation of IL-15-mediated T-cell homeostasis in TGF-β dominant-negative receptor transgenic mice. Blood 108: 2789–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttmann W, Franz P, Matthys H, Virchow JC Jr. 1998. Effects of TGF-β on eosinophil chemotaxis. Scand J Immunol 47: 127–130. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, et al. 2015. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity 43: 1101–1111. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Kelly DR, Nicola T, Ambalavanan N, Jain SK, Murphy-Ullrich J, Athar M, Shimamura M, Bhandari V, Aprahamian C, et al. 2011. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 140: 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. 2008. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat Immunol 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marafini I, Zorzi F, Codazza S, Pallone F, Monteleone G. 2013. TGF-β signaling manipulation as potential therapy for IBD. Curr Drug Targets 14: 1400–1404. [DOI] [PubMed] [Google Scholar]
- Marcoe JP, Lim JR, Schaubert KL, Fodil-Cornu N, Matka M, McCubbrey AL, Farr AR, Vidal SM, Laouar Y. 2012. TGF-β is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat Immunol 13: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. 2006. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25: 441–454. [DOI] [PubMed] [Google Scholar]
- Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM. 2015. The transforming growth factor β signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. eLife 4: e04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez GJ, Zhang Z, Reynolds JM, Tanaka S, Chung Y, Liu T, Robertson E, Lin X, Feng XH, Dong C. 2010. Smad2 positively regulates the generation of Th17 cells. J Biol Chem 285: 29039–29043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya VK, Jha RK, Kumar V, Joshi A, Chadchan S, Mohan JJ, Laloraya M. 2013. Transforming growth factor-β1 (TGF-β1) liberation from its latent complex during embryo implantation and its regulation by estradiol in mouse. Biol Reprod 89: 84. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. 2007. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol 8: 1390–1397. [DOI] [PubMed] [Google Scholar]
- McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagace C, Li C, et al. 2010. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 42: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKarns SC, Schwartz RH. 2005. Distinct effects of TGF-β1 on CD4+ and CD8+ T cell survival, division, and IL-2 production: A role for T cell intrinsic Smad3. J Immunol 174: 2071–2083. [DOI] [PubMed] [Google Scholar]
- McKarns SC, Schwartz RH, Kaminski NE. 2004. Smad3 is essential for TGF-β1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol 172: 4275–4284. [DOI] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. 2004. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol 4: 762–774. [DOI] [PubMed] [Google Scholar]
- Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. 2010. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 120: 4436–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller HR, Wright SH, Knight PA, Thornton EM. 1999. A novel function for transforming growth factor-β1: Upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific β-chymase, mouse mast cell protease-1. Blood 93: 3473–3486. [PubMed] [Google Scholar]
- Mokhtarian F, Shi Y, Shirazian D, Morgante L, Miller A, Grob D. 1994. Defective production of anti-inflammatory cytokine, TGF-β by T cell lines of patients with active multiple sclerosis. J Immunol 152: 6003–6010. [PubMed] [Google Scholar]
- Molinero LL, Miller ML, Evaristo C, Alegre ML. 2011. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner. J Immunol 186: 4609–4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. 2001. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest 108: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Del Vecchio Blanco G, Monteleone I, Fina D, Caruso R, Gioia V, Ballerini S, Federici G, Bernardini S, Pallone F, et al. 2005. Post-transcriptional regulation of Smad7 in the gut of patients with inflammatory bowel disease. Gastroenterology 129: 1420–1429. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Boirivant M, Pallone F, MacDonald TT. 2008. TGF-β1 and Smad7 in the regulation of IBD. Mucosal Immunol 1: S50–53. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Fantini MC, Onali S, Zorzi F, Sancesario G, Bernardini S, Calabrese E, Viti F, Monteleone I, Biancone L, et al. 2012. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther 20: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani M, Yoshimoto K, Wong SF, Tanaka C, Yamaoka T, Sano T, Komagata Y, Miyazaki J, Kikutani H, Itakura M. 1998. Abrogation of autoimmune diabetes in nonobese diabetic mice and protection against effector lymphocytes by transgenic paracrine TGF-β1. J Clin Invest 102: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Suano A, Hamilton AB, Betz AG. 2011. Gimme shelter: The immune system during pregnancy. Immunol Rev 241: 20–38. [DOI] [PubMed] [Google Scholar]
- Musso T, Espinoza-Delgado I, Pulkki K, Gusella GL, Longo DL, Varesio L. 1990. Transforming growth factor β downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood 76: 2466–2469. [PubMed] [Google Scholar]
- Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. 2005. Transforming growth factor-β differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J Biol Chem 280: 5491–5495. [DOI] [PubMed] [Google Scholar]
- Nandan D, Reiner NE. 1997. TGF-β attenuates the class II transactivator and reveals an accessory pathway of IFN-γ action. J Immunol 158: 1095–1101. [PubMed] [Google Scholar]
- Naviglio S, Arrigo S, Martelossi S, Villanacci V, Tommasini A, Loganes C, Fabretto A, Vignola S, Lonardi S, Ventura A. 2014. Severe inflammatory bowel disease associated with congenital alteration of transforming growth factor β signaling. J Crohns Colitis 8: 770–774. [DOI] [PubMed] [Google Scholar]
- Nelson BH, Martyak TP, Thompson LJ, Moon JJ, Wang T. 2003. Uncoupling of promitogenic and antiapoptotic functions of IL-2 by Smad-dependent TGF-β signaling. J Immunol 170: 5563–5570. [DOI] [PubMed] [Google Scholar]
- Nocera M, Chu TM. 1995. Characterization of latent transforming growth factor-β from human seminal plasma. Am J Reprod Immunol 33: 282–291. [DOI] [PubMed] [Google Scholar]
- Ocana-Morgner C, Wong KA, Lega F, Dotor J, Borras-Cuesta F, Rodriguez A. 2007. Role of TGF-β and PGE2 in T cell responses during Plasmodium yoelii infection. Eur J Immunol 37: 1562–1574. [DOI] [PubMed] [Google Scholar]
- Oddy WH, Rosales F. 2010. A systematic review of the importance of milk TGF-β on immunological outcomes in the infant and young child. Pediatr Allergy Immunol 21: 47–59. [DOI] [PubMed] [Google Scholar]
- Ogawa J, Sasahara A, Yoshida T, Sira MM, Futatani T, Kanegane H, Miyawaki T. 2004. Role of transforming growth factor-β in breast milk for initiation of IgA production in newborn infants. Early Hum Dev 77: 67–75. [DOI] [PubMed] [Google Scholar]
- Oh SA, Li MO. 2013. TGF-β: Guardian of T cell function. J Immunol 191: 3973–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K, Gray JD, Stimmler MM, Toro B, Horwitz DA. 1998. Decreased production of TGF-β by lymphocytes from patients with systemic lupus erythematosus. J Immunol 160: 2539–2545. [PubMed] [Google Scholar]
- Ohtsuka K, Gray JD, Stimmler MM, Horwitz DA. 1999. The relationship between defects in lymphocyte production of transforming growth factor-β1 in systemic lupus erythematosus and disease activity or severity. Lupus 8: 90–94. [DOI] [PubMed] [Google Scholar]
- Olsson N, Piek E, ten Dijke P, Nilsson G. 2000. Human mast cell migration in response to members of the transforming growth factor-β family. J Leukoc Biol 67: 350–356. [DOI] [PubMed] [Google Scholar]
- Omer FM, Riley EM. 1998. Transforming growth factor β production is inversely correlated with severity of murine malaria infection. J Exp Med 188: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer FM, de Souza JB, Corran PH, Sultan AA, Riley EM. 2003. Activation of transforming growth factor β by malaria parasite-derived metalloproteinases and a thrombospondin-like molecule. J Exp Med 198: 1817–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Li MO. 2010. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Oh SA, Ma Q, Bivona MR, Zhu J, Li MO. 2013. TGF-β signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor α expression. Immunity 39: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, et al. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C, Stuart LM, Savill J, Lacy-Hulbert A. 2011. Preferential expression of integrin αvβ8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 141: 1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S, et al. 2011. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12: 870–878. [DOI] [PubMed] [Google Scholar]
- Pardali E, Xie XQ, Tsapogas P, Itoh S, Arvanitidis K, Heldin CH, ten Dijke P, Grundstrom T, Sideras P. 2000. Smad and AML proteins synergistically confer transforming growth factor β1 responsiveness to human germ-line IgA genes. J Biol Chem 275: 3552–3560. [DOI] [PubMed] [Google Scholar]
- Park SR, Lee JH, Kim PH. 2001. Smad3 and Smad4 mediate transforming growth factor-β1-induced IgA expression in murine B lymphocytes. Eur J Immunol 31: 1706–1715. [DOI] [PubMed] [Google Scholar]
- Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. 2004. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: A novel mechanism for maximizing IL-7-dependent T cell survival. Immunity 21: 289–302. [DOI] [PubMed] [Google Scholar]
- Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, et al. 2010. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol 11: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YP, Choi SC, Kiesler P, Gil-Krzewska A, Borrego F, Weck J, Krzewski K, Coligan JE. 2011. Complex regulation of human NKG2D-DAP10 cell surface expression: Opposing roles of the γc cytokines and TGF-β1. Blood 118: 3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila IA. 2010. Milk-derived transforming growth factor-β and the infant immune response. J Pediatr 156: S21–S25. [DOI] [PubMed] [Google Scholar]
- Penttila IA, van Spriel AB, Zhang MF, Xian CJ, Steeb CB, Cummins AG, Zola H, Read LC. 1998. Transforming growth factor-β levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr Res 44: 524–531. [DOI] [PubMed] [Google Scholar]
- Pereira FA, Pinheiro da Silva NN, Rodart IF, Carmo TM, Lemaire DC, Reis MG. 2008. Association of TGF-β1 codon 25 (G915C) polymorphism with hepatitis C virus infection. J Med Virol 80: 58–64. [DOI] [PubMed] [Google Scholar]
- Petit-Koskas E, Genot E, Lawrence D, Kolb JP. 1988. Inhibition of the proliferative response of human B lymphocytes to B cell growth factor by transforming growth factor-β. Eur J Immunol 18: 111–116. [DOI] [PubMed] [Google Scholar]
- Pion M, Jaramillo-Ruiz D, Martinez A, Munoz-Fernandez MA, Correa-Rocha R. 2013. HIV infection of human regulatory T cells downregulates Foxp3 expression by increasing DNMT3b levels and DNA methylation in the FOXP3 gene. AIDS 27: 2019–2029. [DOI] [PubMed] [Google Scholar]
- Piskurich JF, Wang Y, Linhoff MW, White LC, Ting JP. 1998. Identification of distinct regions of 5′ flanking DNA that mediate constitutive, IFN-γ, STAT1, and TGF-β-regulated expression of the class II transactivator gene. J Immunol 160: 233–240. [PubMed] [Google Scholar]
- Planchon SM, Martins CA, Guerrant RL, Roche JK. 1994. Regulation of intestinal epithelial barrier function by TGF-β1. Evidence for its role in abrogating the effect of a T cell cytokine. J Immunol 153: 5730–5739. [PubMed] [Google Scholar]
- Planchon S, Fiocchi C, Takafuji V, Roche JK. 1999. Transforming growth factor-β1 preserves epithelial barrier function: Identification of receptors, biochemical intermediates, and cytokine antagonists. J Cell Physiol 181: 55–66. [DOI] [PubMed] [Google Scholar]
- Ploquin MJ, Desoutter JF, Santos PR, Pandrea I, Diop OM, Hosmalin A, Butor C, Barre-Sinoussi F, Muller-Trutwin MC. 2006. Distinct expression profiles of TGF-β1 signaling mediators in pathogenic SIVmac and non-pathogenic SIVagm infections. Retrovirology 3: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. 2012. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol 13: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli V, Bulletti C, Galassi A, Borini A, Ciotti PM, Seracchioli R, Alfieri S, Flamigni C. 1996. Transforming growth factor-β1 in the human endometrium. Gynecol Endocrinol 10: 297–302. [DOI] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. 2011. Challenges in infant immunity: Implications for responses to infection and vaccines. Nat Immunol 12: 189–194. [DOI] [PubMed] [Google Scholar]
- Prokesova L, Lodinova-Zadnikova R, Zizka J, Kocourkova I, Novotna O, Petraskova P, Sterzl I. 2006. Cytokine levels in healthy and allergic mothers and their children during the first year of life. Pediatr Allergy Immunol 17: 175–183. [DOI] [PubMed] [Google Scholar]
- Racke MK, Dhib-Jalbut S, Cannella B, Albert PS, Raine CS, McFarlin DE. 1991. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-β1. J Immunol 146: 3012–3017. [PubMed] [Google Scholar]
- Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, Kiela PR. 2012. Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol 189: 3878–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani R, Smulian AG, Greaves DR, Hogan SP, Herbert DR. 2011. TGF-β limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol 41: 2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautava S, Lu L, Nanthakumar NN, Dubert-Ferrandon A, Walker WA. 2012. TGF-β2 induces maturation of immature human intestinal epithelial cells and inhibits inflammatory cytokine responses induced via the NF-κB pathway. J Pediatr Gastroenterol Nutr 54: 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz E, Dudler J, Lotz M, Baird SM, Berry CC, Eisenberg RA, Carson DA. 1995. Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus 4: 286–292. [DOI] [PubMed] [Google Scholar]
- Reibman J, Meixler S, Lee TC, Gold LI, Cronstein BN, Haines KA, Kolasinski SL, Weissmann G. 1991. Transforming growth factor β1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc Natl Acad Sci 88: 6805–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold D, Wrenger S, Kahne T, Ansorge S. 1999. HIV-1 Tat: Immunosuppression via TGF-β1 induction. Immunol Today 20: 384–385. [DOI] [PubMed] [Google Scholar]
- Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. 2013. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4+ T cell immunity. Nat Immunol 14: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA. 2005. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res 322: 43–52. [DOI] [PubMed] [Google Scholar]
- *.Robertson IB, Rifkin DB. 2016. Regulation of the bioavailability of TGF-β and TGF-β-related proteins. Cold Spring Harb Perspect Biol 8: a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Ingman WV, O’Leary S, Sharkey DJ, Tremellen KP. 2002. Transforming growth factor β—A mediator of immune deviation in seminal plasma. J Reprod Immunol 57: 109–128. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Bromfield JJ, Branson KM, Ahlstrom AC, Care AS. 2009a. Seminal fluid drives expansion of the CD4+CD25+ T regulatory cell pool and induces tolerance to paternal alloantigens in mice. Biol Reprod 80: 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. 2009b. Activating T regulatory cells for tolerance in early pregnancy—The contribution of seminal fluid. J Reprod Immunol 83: 109–116. [DOI] [PubMed] [Google Scholar]
- Robertson SA, Prins JR, Sharkey DJ, Moldenhauer LM. 2013. Seminal fluid and the generation of regulatory T cells for embryo implantation. Am J Reprod Immunol 69: 315–330. [DOI] [PubMed] [Google Scholar]
- Robinson RT, Gorham JD. 2007. TGF-β1 regulates antigen-specific CD4+ T cell responses in the periphery. J Immunol 179: 71–79. [DOI] [PubMed] [Google Scholar]
- Romani N, Clausen BE, Stoitzner P. 2010. Langerhans cells and more: Langerin-expressing dendritic cell subsets in the skin. Immunol Rev 234: 120–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbottom A, Scudamore CL, von der Mark H, Thornton EM, Wright SH, Miller HR. 2002. TGF-β1 regulates adhesion of mucosal mast cell homologues to laminin-1 through expression of integrin α7. J Immunol 169: 5689–5695. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. 2007. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131: 1124–1136. [DOI] [PubMed] [Google Scholar]
- Rowan AG, Fletcher JM, Ryan EJ, Moran B, Hegarty JE, O’Farrelly C, Mills KH. 2008. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-β. J Immunol 181: 4485–4494. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Ertelt JM, Xin L, Way SS. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegemer JJ, Ho SN, Augustine JA, Schlager JW, Bell MP, McKean DJ, Abraham RT. 1990. Regulatory effects of transforming growth factor-β on IL-2- and IL-4-dependent T cell-cycle progression. J Immunol 144: 1767–1776. [PubMed] [Google Scholar]
- Rumsaeng V, Vliagoftis H, Oh CK, Metcalfe DD. 1997. Lymphotactin gene expression in mast cells following Fcɛ receptor I aggregation: Modulation by TGF-β, IL-4, dexamethasone, and cyclosporin A. J Immunol 158: 1353–1360. [PubMed] [Google Scholar]
- Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. 2011. Transcription factor c-Maf mediates the TGF-β-dependent suppression of IL-22 production in TH17 cells. Nat Immunol 12: 1238–1245. [DOI] [PubMed] [Google Scholar]
- Saarinen KM, Juntunen-Backman K, Jarvenpaa AL, Klemetti P, Kuitunen P, Lope L, Renlund M, Siivola M, Vaarala O, Savilahti E. 2000. Breast-feeding and the development of cows’ milk protein allergy. Adv Exp Med Biol 478: 121–130. [DOI] [PubMed] [Google Scholar]
- Saito S, Yoshida M, Ichijo M, Ishizaka S, Tsujii T. 1993. Transforming growth factor-β (TGF-β) in human milk. Clin Exp Immunol 94: 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi S, Bankoti R, Benevides L, Willen J, Couse M, Silva JS, Dhall D, Meffre E, Targan S, Martins GA. 2012. B lymphocyte-induced maturation protein-1 contributes to intestinal mucosa homeostasis by limiting the number of IL-17-producing CD4+ T cells. J Immunol 189: 5682–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjabi S, Mosaheb MM, Flavell RA. 2009. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity 31: 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya BE, Thatikunta P, Denisova L, Brady J, Khalili K, Amini S. 1998. Regulation of TNFα and TGFβ-1 gene transcription by HIV-1 Tat in CNS cells. J Neuroimmunol 87: 33–42. [DOI] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. 2012. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 209: 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt N, Liu Y, Bentebibel SE, Munagala I, Bourdery L, Venuprasad K, Banchereau J, Ueno H. 2014. The cytokine TGF-β co-opts signaling via STAT3-STAT4 to promote the differentiation of human TFH cells. Nat Immunol 15: 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. 2009. Plasmodium falciparum-mediated induction of human CD25hiFoxp3hi CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFβ. PLoS Path 5: e1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. 1999. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol 162: 6641–6649. [PubMed] [Google Scholar]
- Schramm C, Kriegsmann J, Protschka M, Huber S, Hansen T, Schmitt E, Galle PR, Blessing M. 2004. Susceptibility to collagen-induced arthritis is modulated by TGFβ responsiveness of T cells. Arthritis Res Ther 6: R114–R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz-Cherry S, Hinshaw VS. 1996. Influenza virus neuraminidase activates latent transforming growth factor β. J Virol 70: 8624–8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj RK, Geiger TL. 2007. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. J Immunol 179: 11. [PubMed] [Google Scholar]
- Sharif MN, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. 2006. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med 203: 1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Kaveri SV, Bayry J. 2013. Th17 cells, pathogenic or not? TGF-β3 imposes the embargo. Cell Mol Immunol 10: 101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R, Meng G, Ochsenbauer C, Clapham PR, Grams J, Novak L, Kappes JC, Smythies LE, Smith PD. 2011. Stromal down-regulation of macrophage CD4/CCR5 expression and NF-κB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Path 7: e1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. 2014. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 40: 747–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM, Thornton AM. 2014. tTregs, pTregs, and iTregs: Similarities and differences. Immunol Rev 259: 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi MJ, Stavnezer J. 1998. CBFα3 (AML2) is induced by TGF-β1 to bind and activate the mouse germline Igα promoter. J Immunol 161: 6751–6760. [PubMed] [Google Scholar]
- Shockett P, Stavnezer J. 1991. Effect of cytokines on switching to IgA and α germline transcripts in the B lymphoma I.29µ. Transforming growth factor-β activates transcription of the unrearranged Cα gene. J Immunol 147: 4374–4383. [PubMed] [Google Scholar]
- Sillett HK, Cruickshank SM, Southgate J, Trejdosiewicz LK. 2001. Transforming growth factor-β promotes “death by neglect” in post-activated human T cells. Immunology 102: 310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JS, Twardzik DR, Reed SG. 1991. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor β (TGF-β). J Exp Med 174: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Bando JK, Schwartz RH. 2012. Subsets of nonclonal neighboring CD4+ T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity 37: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledzinska A, Hemmers S, Mair F, Gorka O, Ruland J, Fairbairn L, Nissler A, Muller W, Waisman A, Becher B, et al. 2013. TGF-β signalling is required for CD4+ T cell homeostasis but dispensable for regulatory T cell function. PLoS Biol 11: e1001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. 1996. Transforming growth factor-β1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol 157: 360–368. [PubMed] [Google Scholar]
- Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. 2001. Sensory adaptation in naïve peripheral CD4 T cells. J Exp Med 194: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. 2005. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest 115: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, Vu HL, Mosteller-Barnum LM, Sellers M, Smith PD. 2006. Mucosal IL-8 and TGF-β recruit blood monocytes: Evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol 80: 492–499. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. 1989. Transforming growth factor β induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med 170: 1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevceva L, Yoon V, Carville A, Pacheco B, Santosuosso M, Korioth-Schmitz B, Mansfield K, Poznansky MC. 2008. The efficacy of T cell-mediated immune responses is reduced by the envelope protein of the chimeric HIV-1/SIV-KB9 virus in vivo. J Immunol 181: 5510–5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockis J, Colau D, Coulie PG, Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-β on the surface of activated human Treg. Eur J Immunol 39: 3315–3322. [DOI] [PubMed] [Google Scholar]
- Strainic MG, Shevach EM, An F, Lin F, Medof ME. 2012. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol 14: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA. 2012. Selection of self-reactive T cells in the thymus. Annu Rev Immunol 30: 95–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. 1996. TGF-β1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J Immunol 157: 1499–1507. [PubMed] [Google Scholar]
- Su HC, Leite-Morris KA, Braun L, Biron CA. 1991. A role for transforming growth factor-β1 in regulating natural killer cell and T lymphocyte proliferative responses during acute infection with lymphocytic choriomeningitis virus. J Immunol 147: 2717–2727. [PubMed] [Google Scholar]
- Su HC, Ishikawa R, Biron CA. 1993. Transforming growth factor-β expression and natural killer cell responses during virus infection of normal, nude, and SCID mice. J Immunol 151: 4874–4890. [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. 2007. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Fu B, Gao Y, Liao X, Sun R, Tian Z, Wei H. 2012. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 8: e1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JL, Lin JT, Gorham JD. 2003. CD28 co-stimulation regulates the effect of transforming growth factor-β1 on the proliferation of naive CD4+ T cells. Int Immunopharmacol 3: 233–245. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Nakao A, Kanamaru Y, Okumura K, Ogawa H, Ra C. 2002. Localization of intestinal intraepithelial T lymphocytes involves regulation of αEβ7 expression by transforming growth factor-β. Int Immunol 14: 339–345. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Eitzman B, Bossert NL, Walmer D, Sparrow K, Flanders KC, McLachlan J, Nelson KG. 1994. Transforming growth factors β1, β2, and β3 messenger RNA and protein expression in mouse uterus and vagina during estrogen-induced growth: A comparison to other estrogen-regulated genes. Cell Growth Differ 5: 919–935. [PubMed] [Google Scholar]
- Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, Takahashi R, Asakawa M, Muto G, Mori T, et al. 2010. Smad2 and Smad3 are redundantly essential for the TGF-β-mediated regulation of regulatory T plasticity and Th1 development. J Immunol 185: 842–855. [DOI] [PubMed] [Google Scholar]
- Tamiya T, Ichiyama K, Kotani H, Fukaya T, Sekiya T, Shichita T, Honma K, Yui K, Matsuyama T, Nakao T, et al. 2013. Smad2/3 and IRF4 play a cooperative role in IL-9-producing T cell induction. J Immunol 191: 2360–2371. [DOI] [PubMed] [Google Scholar]
- Tas SW, Vervoordeldonk MJ, Hajji N, Schuitemaker JH, van der Sluijs KF, May MJ, Ghosh S, Kapsenberg ML, Tak PP, de Jong EC. 2007. Noncanonical NF-κB signaling in dendritic cells is required for indoleamine 2,3-dioxygenase (IDO) induction and immune regulation. Blood 110: 1540–1549. [DOI] [PubMed] [Google Scholar]
- Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM, Palladino MA. 1992. Involvement of endogenous tumor necrosis factor α and transforming growth factor β during induction of collagen type II arthritis in mice. Proc Natl Acad Sci 89: 7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. 2009. Cell-intrinsic transforming growth factor-β signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity 31: 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. 2008. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol 9: 194–202. [DOI] [PubMed] [Google Scholar]
- Torchinsky MB, Garaude J, Martin AP, Blander JM. 2009. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458: 78–82. [DOI] [PubMed] [Google Scholar]
- Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. 2009. GARP (LRRC32) is essential for the surface expression of latent TGF-β on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci 106: 13445–13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Sheppard D. 2014. TGF-β activation and function in immunity. Annu Rev Immunol 32: 51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, et al. 2007. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449: 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Chantry D, Feldmann M. 1990. Transforming growth factor β induces the production of interleukin 6 by human peripheral blood mononuclear cells. Cytokine 2: 211–216. [DOI] [PubMed] [Google Scholar]
- Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Berezovskaya A, Nadler LM, Boussiotis VA. 2001. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat Immunol 2: 1174–1182. [DOI] [PubMed] [Google Scholar]
- van Vlasselaer P, Punnonen J, de Vries JE. 1992. Transforming growth factor-β directs IgA switching in human B cells. J Immunol 148: 2062–2067. [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. 2006a. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24: 179–189. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. 2006b. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol 7: 1151–1156. [DOI] [PubMed] [Google Scholar]
- Verhasselt V. 2010. Neonatal tolerance under breastfeeding influence. Curr Opin Immunol 22: 623–630. [DOI] [PubMed] [Google Scholar]
- Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, Glaichenhaus N, Julia V. 2008. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med 14: 170–175. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB. 1987. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci 84: 5788–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Allen JB, McCartney-Francis N, Morganti-Kossmann MC, Kossmann T, Ellingsworth L, Mai UE, Mergenhagen SE, Orenstein JM. 1991. Macrophage- and astrocyte-derived transforming growth factor β as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med 173: 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Allen JB, Costa GL, Wong HL, Dasch JR. 1993a. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor β. J Exp Med 177: 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl SM, Allen JB, Weeks BS, Wong HL, Klotman PE. 1993b. Transforming growth factor β enhances integrin expression and type IV collagenase secretion in human monocytes. Proc Natl Acad Sci 90: 4577–4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, et al. 2005. Upregulation of TGF-β, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23: 287–296. [DOI] [PubMed] [Google Scholar]
- Wang J, Guan E, Roderiquez G, Norcross MA. 2001. Synergistic induction of apoptosis in primary CD4+ T cells by macrophage-tropic HIV-1 and TGF-β1. J Immunol 167: 3360–3366. [DOI] [PubMed] [Google Scholar]
- Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. 2009. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci 106: 13439–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. 2012. GARP regulates the bioavailability and activation of TGFβ. Mol Biol Cell 23: 1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Pan D, Lee YH, Martinez GJ, Feng XH, Dong C. 2013. Cutting edge: Smad2 and Smad4 regulate TGF-β-mediated Il9 gene expression via EZH2 displacement. J Immunol 191: 4908–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner GL, Ludlow JW, Nelson DA, Gaur A, Scott DW. 1992. Anti-immunoglobulin treatment of murine B-cell lymphomas induces active transforming growth factor β but pRB hypophosphorylation is transforming growth factor β independent. Cell Growth Differ 3: 175–181. [PubMed] [Google Scholar]
- Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. 2007. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci 104: 18169–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner HL. 2001. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev 182: 207–214. [DOI] [PubMed] [Google Scholar]
- Wiercinska-Drapalo A, Flisiak R, Jaroszewicz J, Prokopowicz D. 2004. Increased plasma transforming growth factor-β1 is associated with disease progression in HIV-1-infected patients. Viral Immunol 17: 109–113. [DOI] [PubMed] [Google Scholar]
- Windhagen A, Scholz C, Hollsberg P, Fukaura H, Sette A, Hafler DA. 1995. Modulation of cytokine patterns of human autoreactive T cell clones by a single amino acid substitution of their peptide ligand. Immunity 2: 373–380. [DOI] [PubMed] [Google Scholar]
- Wira CR, Rossoll RM. 2003. Oestradiol regulation of antigen presentation by uterine stromal cells: role of transforming growth factor-β production by epithelial cells in mediating antigen-presenting cell function. Immunology 109: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wira CR, Roche MA, Rossoll RM. 2002. Antigen presentation by vaginal cells: Role of TGFβ as a mediator of estradiol inhibition of antigen presentation. Endocrinology 143: 2872–2879. [DOI] [PubMed] [Google Scholar]
- Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. 2004. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-β-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol 173: 3093–3102. [DOI] [PubMed] [Google Scholar]
- Worthington JJ, Czajkowska BI, Melton AC, Travis MA. 2011. Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology 141: 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Balasubramanian S, Liu W, Chu X, Wang H, Taparowsky EJ, Fu YX, Choi Y, Walsh MC, Li XC. 2012. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat Immunol 13: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Kitani A, Stuelten C, McGrady G, Fuss I, Strober W. 2010. Positive and negative transcriptional regulation of the Foxp3 gene is mediated by access and binding of the Smad3 protein to enhancer I. Immunity 33: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. 2001. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol 166: 7282–7289. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Tsumura H, Miwa M, Inaba K. 1997. Contrasting effects of TGF-β1 and TNF-α on the development of dendritic cells from progenitors in mouse bone marrow. Stem Cells 15: 144–153. [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. 1999. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J 18: 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. 2008. IL-21 and TGF-β are required for differentiation of human TH17 cells. Nature 454: 350–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wei M, Becknell B, Trotta R, Liu S, Boyd Z, Jaung MS, Blaser BW, Sun J, Benson DM Jr, et al. 2006. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-γ production by human natural killer cells. Immunity 24: 575–590. [DOI] [PubMed] [Google Scholar]
- Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. 2011. Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol 187: 5069–5076. [DOI] [PubMed] [Google Scholar]
- Zauli G, Davis BR, Re MC, Visani G, Furlini G, La Placa M. 1992. tat protein stimulates production of transforming growth factor-β1 by marrow macrophages: A potential mechanism for human immunodeficiency virus-1-induced hematopoietic suppression. Blood 80: 3036–3043. [PubMed] [Google Scholar]
- Zenclussen AC. 2006. Regulatory T cells in pregnancy. Springer Semin Immunopathol 28: 31–39. [DOI] [PubMed] [Google Scholar]
- Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, et al. 2011. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest 121: 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2012. TGF-β signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol 13: 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. 2013. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Derynck R. 2000. Transcriptional regulation of the transforming growth factor-β-inducible mouse germ line Igα constant region gene by functional cooperation of Smad, CREB, and AML family members. J Biol Chem 275: 16979–16985. [DOI] [PubMed] [Google Scholar]
- Zhang MF, Zola H, Read LC, Penttila IA. 1999a. Localization of transforming growth factor-β receptor types I, II, and III in the postnatal rat small intestine. Pediatr Res 46: 657–665. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang YY, Ogata M, Chen P, Harada A, Hashimoto S, Matsushima K. 1999b. Transforming growth factor-β1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood 93: 1208–1220. [PubMed] [Google Scholar]
- Zhao Y, Chegini N, Flanders KC. 1994. Human fallopian tube expresses transforming growth factor (TGFβ) isoforms, TGFβ type I-III receptor messenger ribonucleic acid and protein, and contains [125I]TGFβ-binding sites. J Clin Endocrinol Metab 79: 1177–1184. [DOI] [PubMed] [Google Scholar]
- Zhao F, Hoechst B, Gamrekelashvili J, Ormandy LA, Voigtlander T, Wedemeyer H, Ylaya K, Wang XW, Hewitt SM, Manns MP, et al. 2012. Human CCR4+ CCR6+ Th17 cells suppress autologous CD8+ T cell responses. J Immunol 188: 6055–6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Miller G, Seder RA. 1998. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J Immunol 160: 1359–1368. [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. 2007. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 8: 967–974. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. 2008. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, Guo L, Zhao K, Shevach EM, Paul WE. 2009. Down-regulation of Gfi-1 expression by TGF-β is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med 206: 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]