Abstract
Patient: Female, 83
Final Diagnosis: Angioedema
Symptoms: Edema
Medication: Ramipril
Clinical Procedure: —
Specialty: Otolaryngology
Objective:
Unusual clinical course
Background:
Bradykinin is an underestimated mediator of angioedema. One subgroup of bradykinin induced angioedema is angioedema triggered by treatment with angiotensin converting enzyme (ACE) inhibitors. Due to its localization in the head and neck region and its unpredictable course, it is a possibly life-threatening condition. There is not an officially approved treatment for ACE inhibitor induced angioedema.
Case Report:
We present a case of an 83-year-old woman, who presented to our ENT department because of acute swelling of the tongue. On admission, there was no pharyngeal or laryngeal edema and no dyspnea. Treatment with glucocorticoids and antihistamines had no response. The patient had ramipril as regular medication, so we assumed ACE inhibitor induced angioedema and treated consequently with C1-inhibitor (human) 1,500 IU. Nevertheless, swelling was progressive and required intubation. Even after the second specific treatment with icatibant, her angioedema subsided extremely slowly. The patient also had regular treatment with saxagliptin, a dipeptidyl peptidase 4 inhibitor, so we assumed that the simultaneous inhibition of two bradykinin degrading enzymes led to a treatment-refractory course of angioedema.
Conclusions:
General awareness for bradykinin induced angioedema due to regular medication is limited. Our case demonstrated the importance of improving awareness and knowledge about this side effect. We need a better understanding of the pathomechanism to aid in more precise clinical diagnosis. Securing the patient’s airway as well as administration of an officially approved therapy is of utmost importance. As the number of patients simultaneously treated with antihypertensive and antidiabetic drugs is likely to increase, the incidence of bradykinin mediated drug induced angioedema is likely to increase as well.
MeSH Keywords: Angioedema, Bradykinin, Cholinesterase Inhibitors, Dipeptidyl-Peptidase IV Inhibitors, Drug-Related Side Effects and Adverse Reactions
Background
Angiotensin converting enzyme (ACE) is not only important for the maintenance of normal blood pressure, but also for degradation of bradykinin, a vasoactive inflammatory mediator [1]. ACE inhibitors like ramipril or enalapril are effective and widely used for the treatment of hypertension or congestive heart failure. ACE is not the only degrading enzyme of bradykinin: Dipeptidyl peptidase 4 (DPP4), carboxypeptidase N, aminopeptidase P, and neprilysin are also involved in the degradation process [2–5]. According to the present state of knowledge, ACE inhibitor induced angioedema (ACEI-AE) results from accumulation of bradykinin [6]. In the formation of bradykinin, C1-inhibitor (C1-INH) plays an important role in slowing down its formation – mutations affecting C1-INH are considered the reason for the occurrence of bradykinin mediated hereditary angioedema (HAE) [7]. Based on current knowledge, bradykinin mediates its effects mainly via activation of bradykinin 2 receptor [8].
ACEI-AE is a rare side effect (0.2%–0.5% of patients with regular ACE inhibition) but because of the large number of patients treated with ACE inhibitors, the incidence should not be underestimated [9]. Dark-skinned people are three-times more likely to develop ACEI-AE [10]. ACE inhibitor induced swellings are nearly always located in the head and neck region – though the cause for this finding is unknown [11]. ACEI-AE often affects the upper airway, leading to dyspnea and resulting in a potentially life-threatening condition. Due to the unpredictable course, patients with ACEI-AE should be monitored intensely; with securing and maintaining a patent airway of up-most importance [12]. The diagnosis of ACEI-AE is not simple – there is no reliable laboratory value and the clinical presentation is often similar to histamine induced angioedema. The most important indicators for ACEI-AE are the patient’s medication history combined with therapy-resistance to antihistamines and glucocorticoids and the absence of urticaria and pruritus. ACEI-AE may occur shortly after the initial ACE inhibitor treatment, but there are patients with a latency period of more than 10 years from the beginning of ACE inhibitor therapy and the occurrence of angioedema [13].
Up until now, there has been no approved treatment for ACEIAE available. Recent studies have shown that treatment with icatibant, a selective antagonist of bradykinin 2 receptor, is effective, well tolerated and significantly superior to antihistamines and glucocorticoids [14]. However, due to the relatively small number of study patients, there has not been definitive approval for icatibant therapy. IV therapy with C1-INH is another potentially effective off-label treatment possibility for ACEI-AE [15].
Angioedema is also a possible side effect of treatment with angiotensin II type 1 receptor blockers (ARBs) like losartan or valsartan [16]. The underlying pathophysiology is still unknown and its occurrence is significantly less common than ACEI-AE. Evidence suggests that this side effect is also mediated by bradykinin. Patients with ACEI-AE who were switched to an ARB afterwards had a distinct increase of risk for recurrent angioedema (up to 17%) [17].
Case Report
An 83-year-old woman was taken in an ambulance to our ENT department late in the evening because of acute swelling of her tongue (Figure 1). At this point, she had no dyspnea and was able to speak. The patient had no known allergies. Due to hypertension and coronary heart disease, she had been taking ramipril 5 mg (an ACE inhibitor) as regular medication for more than five years. For treatment of diabetes mellitus type II she had been taking saxagliptin 5 mg, an inhibitor of DPP4. Clinical examination revealed left-side swelling of her tongue, while her laryngeal and pharyngeal region were not affected. There was no urticaria and no pruritus. Her family medical history showed no indication of hereditary disease. IV treatment with 250 mg prednisolone and 4 mg of dimethindene maleate (a H1-receptor antagonist) by the emergency physician had no effect. We monitored the patient in the ICU for another 15 hours, gave her 250 mg prednisolone IV again as well as inhalations with epinephrine (8 drops in 10 mL NaCl). Due to the lack of improvement and the patient’s medication history, we presumed bradykinin mediated angioedema and started treatment with plasma-derived C1-INH 1,500 IU IV. This dose was correctly adapted to the patient’s body weight (60 kg) according to the I.M.P.A.C.T. study [18]. Additionally, we stopped therapy with ramipril. We did not stop the treatment with saxagliptin, as recent studies of patients with regular DPP4 inhibitor treatment show that inhibition of DPP4 alone is not able to trigger angioedema [19]. Three hours later, the patient’s angioedema was progressive, swelling affected the whole tongue and occlusion was no longer possible so we intubated the patient via the nasal route for airway protection. The next morning, her angioedema affected even the hypopharyngeal and supraglottical region. Subsequently, we treated her with icatibant 30 mg SC, a selective and specific bradykinin 2 receptor antagonist and again with 250 mg SDH IV. The swelling persisted and showed first improvement eight hours later.
Figure 1.
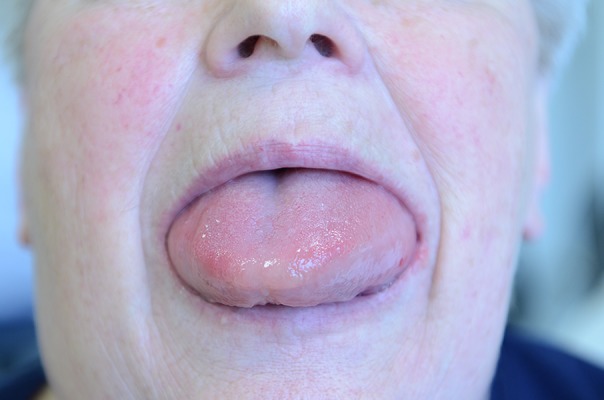
Female patient with acute swelling of the tongue due to treatment with ACE inhibitors.
Outcome and follow-up
It took nearly 24 hours until extubation was possible. Determination of laboratory parameters revealed normal concentration of complement factor C4 (0.24 g/L; range: 0.1–0.4 g/L) and normal concentration of C1-INH (0.49 g/L; range: 0.17–0.44 g/L). Activity of C1-INH was drastically increased (>150%; range: 70–130%) and ACE was decreased (<12 U/L; range: 20–70 U/L). Five days after extubation, angioedema resolved completely, so the patient could be released. Treatment with ramipril was stopped immediately after the patient’s presentation to the ENT department and replaced with amlodipine. Until now – one and a half year later – no further swelling attack has occurred.
Discussion
Although there is no officially approved treatment for patients with ACEI-AE, recent studies show that therapy with icatibant 30 mg SC. is effective and leads to a comparatively rapid onset of symptom relief (median time: two hours versus 12 hours with antihistamine and glucocorticoid) [14]. Our patient had nearly no regression within more than 30 hours, despite treatment with C1-INH, icatibant, and glucocorticoids. Additionally, the time to complete resolution of angioedema was comparably long: after more than two days, swelling had completely resolved. In a recent study with nearly 30 patients with acute ACEI-AE attack, the median time to complete symptom disappearance after icatibant treatment was eight hours [14]. Altogether, our patient showed an unusual therapy-resistant course of ACEI-AE. We presume that the reason for this finding was the double inhibition of two bradykinin degradation enzymes: while ACE is well known as the most important enzyme for degradation of bradykinin but the proteolytic enzyme DPP4 also plays an essential role via cleaving dipeptides resulting in inactivation of bradykinin [3]. Our patient had saxagliptin as regular medication for treatment of diabetes mellitus type II as DPP4 also degrades glucagon-like peptide 1 (GLP1) and therefore leads to increased release of insulin. Recent studies of patients with regular DPP4 inhibitor treatment have shown that inhibition of DPP4 alone was not enough to trigger angioedema, but simultaneous inhibition of two bradykinin degrading enzymes lead to markedly increased risk of developing bradykinin mediated angioedema [19]. It was also found that patients receiving vildagliptin plus ACE inhibitors had a greater than 4-fold increased risk for developing angioedema. Angioedema caused by simultaneous DPP4 and ACE inhibition seem to have the same clinical presentation as ACEI-AE: they nearly always affect the head and neck region, especially the face and the upper airway region [1]. As there is not yet an officially approved treatment for ACEI-AE available, there is also no licensed therapy for patients with angioedema triggered by any other bradykinin antagonists or inhibitors alone or in combination (like DPP4 inhibitors and ACE inhibitors in this case). However, with regard to the similar underlying pathomechanism, inhibition of bradykinin 2 receptor and application of C1-INH could be an effective treatment option. So far, there is no data available regarding whether angioedema due to double inhibition leads not only to increased risk for developing angioedema but also to a longer and nearly therapy-resistant course, similar to our patient.
A case-control study with 145 cases of ACEI-AE and 280 ACE inhibitor exposed controls revealed that the increased risk of developing ACEI-AE in combination with decreased DPP4 activity involves not only patients with DPP4 inhibitor treatment but also transplant patients: Byrd et al. hypothesized that immunosuppressive agents increased the risk of ACEI-AE by decreasing the activity of DPP4 [20].
In a recent review about angioedema induced by cardiovascular drugs, Bas et al. refer not only to the aforementioned mentioned medicaments, but also to neprilysin inhibitors like sacubitril [21]. Recent studies have demonstrated their beneficial effect on chronic heart failure and at the same time shown a significant increased risk for patients developing angioedema: Nearly twice as many cases of this side effect occurred in patients with sacubitril/valsartan therapy (without additional ACE inhibitor treatment) compared to patients treated with enalapril [22]. Neprilysin, also called neutral endopeptidase 24.11, is an unspecific metallopeptidase and degrades many peptides like atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP) as well as bradykinin [21]. In this context, it is important to mention that the combination of valsartan with sacubitril (termed LCZ696) specifically shows positive effects with cardiovascular patients [23]. ARBs, like valsartan, induce angioedema markedly less frequently than ACE inhibitors. Yet, treatment with ARB and neprilysin inhibition is a combination of two bradykinin influencing drugs. Thus, the increased potential risk for the occurrence of angioedema, in addition to the missing knowledge about the resulting courses of angioedema, should not be overlooked.
Conclusions
Bradykinin mediated angioedema is not only induced by renin-angiotensin-aldosterone-system (RAAS) blockers like ACE inhibitors or ARBs. New cardiovascular drug classes like DPP4 inhibitors can also potentiate the risk for this life-threatening side effect.
Concerning ACEI-AE, general knowledge is limited, leading to misdiagnosis and improper treatment. Knowledge about the connection between DPP4, ACE, and bradykinin induced angioedema is even more limited, but patients who need both diabetes mellitus type II and cardiovascular disease therapy are common.
With the regard to the potentially severe course of angioedema affecting the head and neck region, knowledge about angioedema caused by simultaneous inhibition of DPP4 and ACE is important and possibly lifesaving.
Footnotes
Disclosure of potential conflict of interest
JH and ST have received travel grants for attending a scientific congress from Shire GmbH and received financial support for a congress speech from Behring CSL. TKH reports grant support from Shire GmbH. JG has been investigator in a company-sponsored scientific study for ViroPharma GmbH and has received travel grants for presenting at a scientific congress from Shire and ViroPharma GmbH; he has lectured at a company-sponsored meeting for Shire and received honoraria from CLS Behring, Shire, and ViroPharma GmbH.
References:
- 1.Bas M, Adams V, Suvorava T, et al. Nonallergic Angioedema: Role of Bradykinin. Allergy. 2007;62:842–56. doi: 10.1111/j.1398-9995.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 2.Dalzell JR, Seed A, Berry C, et al. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: Studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014;32:13–18. doi: 10.1111/1755-5922.12053. [DOI] [PubMed] [Google Scholar]
- 3.Devin JK, Pretorius M, Nian H, et al. Substance P Increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. Hypertension. 2014;63:951–57. doi: 10.1161/HYPERTENSIONAHA.113.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy J, Rivard GE, Wagner E, et al. Examination of genetic variants involved in generation and biodisposition of kinins in patients with angioedema. Allergy Asthma Clin Immunol. 2014;10:60. doi: 10.1186/s13223-014-0060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skidgel RA, Erdos EG. Structure and function of human plasma carboxypeptidase N, the anaphylatoxin inactivator. Int Immunopharmacol. 2007;7:1888–99. doi: 10.1016/j.intimp.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosravani F, Suvorava T, Dao VT, et al. Stability of murine bradykinin type 2 receptor despite treatment with NO, bradykinin, icatibant, Or C1-INH. Allergy. 2015;70:285–94. doi: 10.1111/all.12556. [DOI] [PubMed] [Google Scholar]
- 7.Bas M, Greve J. FAST-2 open-label study. Allergy. 2013;68:1452–59. doi: 10.1111/all.12244. [DOI] [PubMed] [Google Scholar]
- 8.Maurer M, Bader M, Bas M, et al. New topics in bradykinin research. Allergy. 2011;66:1397–406. doi: 10.1111/j.1398-9995.2011.02686.x. [DOI] [PubMed] [Google Scholar]
- 9.Bas M, Hoffmann TK, Tiemann B, et al. Potential genetic risk factors in angiotensin-converting enzyme-inhibitor-induced angiooedema. Br J Clin Pharmacol. 2010;69:179–86. doi: 10.1111/j.1365-2125.2009.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006;332:1177–81. doi: 10.1136/bmj.38803.528113.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bas M, Hoffmann TK, Kojda G, et al. ACE-inhibitor induced angioedema. Laryngorhinootologie. 2007;86:804–8. doi: 10.1055/s-2007-966932. quiz 809–13. [DOI] [PubMed] [Google Scholar]
- 12.Cicardi M, Bellis P, Bertazzoni G, et al. Guidance for diagnosis and treatment of acute angioedema in the Emergency Department: Consensus Statement by a Panel of Italian Experts. Intern Emerg Med. 2014;9:85–92. doi: 10.1007/s11739-013-0993-z. [DOI] [PubMed] [Google Scholar]
- 13.Hellebrand MC, Kojda G, Hoffmann TK, et al. Angioedema due to ACE inhibitors and AT(1) receptor antagonists. Hautarzt. 2006;57:808–10. doi: 10.1007/s00105-005-1046-y. [DOI] [PubMed] [Google Scholar]
- 14.Bas M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372:418–25. doi: 10.1056/NEJMoa1312524. [DOI] [PubMed] [Google Scholar]
- 15.Greve J, Bas M, Hoffmann TK, et al. Effect of C1-esterase-inhibitor in angiotensin-converting enzyme inhibitor-induced angioedema. Laryngoscope. 2015;125:E198–202. doi: 10.1002/lary.25113. [DOI] [PubMed] [Google Scholar]
- 16.Strassen U, Bas M, Hoffmann TK, et al. Treatment of angiotensin receptor blocker-induced angioedema: A case series. Laryngoscope. 2015;125:1619–23. doi: 10.1002/lary.25163. [DOI] [PubMed] [Google Scholar]
- 17.Haymore BR, Yoon J, Mikita CP, et al. Risk of angioedema with angiotensin receptor blockers in patients with prior angioedema associated with angiotensin-converting enzyme inhibitors: A meta-analysis. Ann Allergy Asthma Immunol. 2008;101:495–99. doi: 10.1016/S1081-1206(10)60288-8. [DOI] [PubMed] [Google Scholar]
- 18.Craig TJ, Wasserman RL, Levy RJ, et al. Prospective study of rapid relief provided by C1 esterase inhibitor in emergency treatment of acute laryngeal attacks in hereditary angioedema. J Clin Immunol. 2010;30:823–29. doi: 10.1007/s10875-010-9442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown NJ, Byiers S, Carr D, et al. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension. 2009;54:516–23. doi: 10.1161/HYPERTENSIONAHA.109.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd JB, Woodard-Grice A, Stone E, et al. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use. Allergy. 2010;65:1381–87. doi: 10.1111/j.1398-9995.2010.02398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bas M, Greve J, Strassen U, et al. Angioedema induced by cardiovascular drugs: New players join old friends. Allergy. 2015;70(10):1196–200. doi: 10.1111/all.12680. [DOI] [PubMed] [Google Scholar]
- 22.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 23.Packer M, McMurray JJ, Desai AS, et al. Angiotensin Receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]


