Abstract
Magnetic resonance imaging (MRI) has developed into the most important tool for the diagnosis and monitoring of multiple sclerosis (MS). Its high sensitivity for the evaluation of inflammatory and neurodegenerative processes in the brain and spinal cord has made it the most commonly used technique for the evaluation of patients with MS. Moreover, MRI has become a powerful tool for treatment monitoring, safety assessment as well as for the prognostication of disease progression. Clinically, the use of MRI has increased in the past couple decades as a result of improved technology and increased availability that now extends well beyond academic centers. Consequently, there are numerous studies supporting the role of MRI in the management of patients with MS. The aim of this review is to summarize the latest insights into the utility of MRI in MS.
Keywords: lesion, McDonald criteria, magnetic resonance imaging, multiple sclerosis, no evidence of disease activity, treatment efficacy
Introduction
The diagnosis of multiple sclerosis (MS) is based upon a clinical assessment; despite significant effort, no single biomarker has been found to independently confirm the diagnosis. In an attempt to assure the highest sensitivity and specificity, a set of guidelines, referred to as the McDonald criteria,1 utilizes magnetic resonance imaging (MRI) to provide supportive data to facilitate the diagnosis of MS. MRI has been part of the International Panel criteria for the diagnosis of MS since 2001, and its use has become increasingly vital as reflected in the last changes by the committee guidelines in 2010.2,3 MRI has been elevated to an essential nonclinical tool for the detection of early MS, for which it may provide concomitant criteria of dissemination in time and space at an initial clinical event, facilitating an established diagnosis of relapsing–remitting MS (RRMS) early within the disease course.
Aside from being an invaluable tool for the diagnosis of MS, MRI has also become a fundamental part of the routine medical management of an individual patient with MS. The concept of predicting disease progression has gained more importance, and the impact of T1-hypointense lesions, T2-hyperintense lesion load as well as regional and general atrophy will be discussed.
Moreover, MRI has developed a crucial role for the assessment of treatment response, especially in an era with a multitude of new medications with varying levels of efficacy. With the introduction of different and often very potent immunosuppressive medications, MRI has found a role in monitoring for potential safety concerns associated with treatment. Lastly, more advanced MRI modalities may provide insight into the pathogenesis of the disease, novel treatment targets and improved diagnostics; the potential applications of nonconventional MRI for MS will be briefly discussed.
The aim of this review is to summarize the latest insights into the use of MRI in MS, especially with respect to diagnosis and monitoring of disease activity. Special focus is also put on the evaluation of treatment efficacy and safety.
MRI as diagnostic tool in MS
Utilization of MRI has facilitated the diagnosis of MS, and has simplified the decision making as to when disease-modifying treatment should be initiated. However, there remains a need for standardization of MRI acquisition methods to advance patient care and to reach comparable results between centers. Improved and updated MRI protocols have been proposed by various expert panels4,5 and the most recent guidelines were created by the MRI in MS (MAGNIMS) consortium in 2015.6–8 The following paragraphs will summarize the technical recommendations for the diagnostic workup for MS, given by the MAGNIMS steering committee.
Various MRI platforms with different magnetic field strengths are in use for the diagnosis of MS, and the most frequently applied magnet strengths are 1.5 Tesla (1.5 T) or 3 T. The latter has been shown to have increased sensitivity for the detection of MS lesions due to improved resolution and signal-to-noise ratio;9 however, the use of high-field 3 T MRI in comparison to 1.5 T has not been shown to improve early diagnosis of MS.10 Despite these findings, 3 T MRI is the preferred magnet strength in the current MAGNIMS criteria, however both field strengths are included in the recommendations.6,7 Specific MRI sequences have been recommended by MAGNIMS as the most appropriate for the diagnosis of MS. As per the guidelines, the following sequences are mandatory: axial proton density or T2-weighted/T2- fluid attenuated inversion recovery (FLAIR) spin echo or turbo spin echo, sagittal two-dimensional (2D) or three-dimensional (3D) T2-FLAIR and axial 2D or 3D post-contrast T1-weighted spin echo or turbo spin echo.7 Optional sequences include unenhanced 2D or high-resolution isotropic 3D T1-weighted, 2D or 3D dual inversion recovery, and axial diffusion weighted imaging (DWI).
The new McDonald guidelines highlight the importance of lesion location [periventricular, juxtacortical (JC), posterior fossa and spinal cord] rather than the amount of lesions, and lesions are best visualized with T2 and T2-FLAIR sequences. T2-weighted sequence has a high utility to identify chronic lesions in the posterior fossa,11 yet lesions close to the ventricles are not easily depicted, given the increased signal of cerebrospinal fluid (CSF).12 For this reason, axial and sagittal T2-FLAIR sequences are the preferred method for the diagnosis of white matter lesions adjacent to the ventricles.6,7 The other mandatory sequence for initial diagnostic evaluation of MS is T1-weighted imaging, following contrast application. A single dose of gadolinium-based contrast should be administered (0.1 mmol/kg), and a minimum of a 5-min delay until T1 sequence acquisition is recommended.13 T1 contrast-enhanced lesions (T1+) indicate blood–brain barrier breakdown, representing active lesions,14 and are an important parameter for the assessment of dissemination in time (the presence of asymptomatic T1+ and T1 lesions on an initial MRI) to allow for the early diagnosis of MS. This sequence is also commonly used in clinical practice for subsequent disease monitoring, and has been used as a biomarker for phase II studies to evaluate the response to disease-modifying therapy (DMT).15,16
The diagnostic value of spinal cord lesions has become increasingly more evident and is apparent in the most recent changes in the diagnostic criteria. Approximately 50–90% of patients with MS have spinal cord lesions,5,7,17 and imaging of the spine has evolved to a more important part of the diagnostic workup. The value of spinal cord imaging is not as well established as brain imaging, but emphasis is put on spine imaging in certain clinical circumstances and depending on the results from brain MRI. For example, spine imaging is important at disease onset to detect not only symptomatic but also clinically silent lesions and to exclude other pathological processes causing a spinal cord syndrome.7 The presence of an asymptomatic spinal cord lesion in patients with asymptomatic white matter changes, referred to as radiologically isolated syndrome (RIS), increases the likelihood of future progression to clinical isolated syndrome (CIS) or MS.17,18
The recommended magnet strength for spinal cord imaging is at least 1.5 T,19 and mandatory sequences include sagittal dual-echo (proton density and T2 weighted) conventional or fast-spin echo, short T1 inversion recovery (STIR) (as an alternative to a proton-density-weighted sequence) and post-contrast T1-weighted spin echo. Optional sagittal sequences are phase-sensitive inversion recovery (PSIR) (as an alternative to STIR). In addition, optional axial sequences are recommended such as 2D or 3D T2-weighted fast spin echo. MRI of the spinal cord is more challenging, given its thin structure, low volume and its anatomic localization, surrounded by CSF and vasculature,20 which can limit the detection of clinically silent lesions. Breathing motions as well as CSF and blood vessel pulsation increase the occurrence of artifacts. Spin echo sequences can decrease vessel pulsation, however given the longer acquisition time, there is an increased likelihood of motion artifacts.21 T2-hyperintense lesions are more common in the cervical than the thoracic cord, and classically span the length of two or fewer vertebrae.5,22–25
The increased use of MRI has led to a number of patients diagnosed with incidental white matter lesions. These findings, when suggestive of MS in an asymptomatic patient, are referred to as RIS. Diagnostic criteria for RIS were proposed in 2009 and include the number, shape and location of the brain lesions.26 Lesions are ovoid and well circumscribed with a size greater than 3 mm, show dissemination in space, and can be juxtaposed to the corpus callosum. Lesions should not follow a vascular distribution and do not account for any other pathologic processes. Within the first documented cohort, radiologic progression was identified in 59% of all patients over a median time period of 2.7 years, but only a quarter of the followed patients converted to either CIS or definite MS, with a median time of 5.4 years.26 Subsequently, a larger cohort was evaluated to assess the 5-year risk for developing a clinical event.27 Involvement of spinal cord, younger age and male sex at RIS identification were associated with a higher risk of developing neurological symptoms. In cases when MS is suspected and the brain MRI is suggestive but not diagnostic, the presence of spinal cord lesions can be helpful in confirming suspicion of MS. Although these patients are identified with MRI, there is a need for standardized treatment guidelines at that stage. Discovery of patients with RIS may present an opportunity to treat at the earliest stages of the disease and potentially to alter the disease course, however we lack the evidence to confirm this.27 Given the adverse events associated with DMTs, the benefits of exposing patients to medications might not outweigh the risks. More and especially predictive studies are warranted to assess the conversion from RIS into MS as well as to evaluate the benefits from treatment initiation at the time point of RIS detection.
Despite the significant improvements in the most recent changes to the McDonald criteria, there are a few aspects that have provoked criticism. Importantly, simplification of the criteria has made them less restrictive, which may lead to an overdiagnosis of MS.7,28 In addition, collection and interpretation of CSF is not required according to the most recent guidelines. Caution is therefore needed when McDonald criteria are used to differentiate MS from other potential central nervous system (CNS) pathologies. In unclear cases, CSF might be required to increase diagnostic specificity. The most common condition that mimics MS lesions is neuromyelitis optica (NMO), which can present with brain lesions in up to 70% of cases.29,30 The diagnosis of NMO can be supported by antibody findings against aquaporin 4; however, the relatively low sensitivity of this test can make a definite diagnosis of NMO or NMO spectrum disorder more difficult than that of MS.31 Furthermore, microvascular disease also needs to be considered and excluded as a potential differential diagnosis; age, risk factors and spinal cord images are particularly important. Other autoimmune, genetic or infectious disorders with a demyelinating component can also cause inflammatory changes on MRI and need to be ruled out.
One of the commonly experienced differential diagnostic dilemmas is the differentiation of white matter lesions from other white matter pathologies such as vascular lesions. More recent in vivo and ex vivo studies have established that MS lesions are oriented around a central vein and these findings are referred to as central vein sign (CVS).32 These lesions are present periventricularly and in deep grey matter and are seen in all forms of MS;33 however, further investigations are needed to evaluate the presence of the CVS in cortical, subcortical and spinal cord lesions. Susceptibility-weighted imaging (SWI) has been proposed as a sequence for better diagnostic values and SWI at 7 T has shown remarkable detection of central veins.34,35 Other studies have shown that the utilization of FLAIR in combination with T2* at 3 T also has diagnostic accuracy to depict perivenular inflammatory lesions. Once validated, this would present a highly useful tool in the clinical setting to distinguish MS from other white matter pathology.36 These new measures would facilitate the differential diagnosis of MS and support the depiction of demyelinating lesions associated with MS.
The 2010 McDonald criteria are based on imaging studies that were obtained in mostly European and North American white adults. Expanded studies within the past 5 years, evaluating the specificity and sensitivity in Asian and South American countries, have demonstrated that the guidelines can be applied to patients from these continents.37–39 Published data on the applicability of the McDonald criteria for the African American population are missing. However, a study in an African American pediatric cohort comparing 2005 with 2010 McDonald criteria showed a higher diagnostic rate of MS when the parameters from 2010 were used.40 African American children with MS were also diagnosed faster than European children, suggesting that the new McDonald criteria is an appropriate tool for the diagnosis of MS in African American pediatric patients. In regards to the general pediatric population, the 2010 McDonald criteria are most appropriate for children above the age of 11.41
Moreover, it needs to be stressed that the above presented measures are predominantly used for the diagnosis of RRMS and have limitations for the diagnosis of primary progressive MS (PPMS). The committee agreed that a similar set of criteria can be applied for PPMS to facilitate diagnosis. These criteria focus on dissemination of lesions in space within the brain as well as in the spinal cord, where at least two focal lesions are warranted. In addition, contrary to the guidelines for RRMS, analysis of CSF is recommended. Thus, the diagnosis of PPMS can be challenging, particularly in patients with normal brain MRI and inconclusive spinal cord findings.
Overall, the use of MRI has become a well established tool for diagnostic purposes and facilitates the early diagnosis of MS. This offers the opportunity to start immune-modulatory treatment early. Yet different pathologies need to be carefully assessed and excluded before a patient is committed to long-term treatment. In summary, the McDonald criteria have a high sensitivity but are not as specific for the diagnosis of MS and caution is still required when confirming the MS diagnosis.
MRI as a prognostic tool in MS
MRI plays an important role for the prognosis of disease development and monitoring of disease progression. Several studies have put special focus on the predictive value of T2-hyperintense lesions, T1-hypointense lesions, so-called black holes, as well as the implication of overall atrophy seen on MRI on the progression of disease. These individual modalities were used for the prediction of developing MS from CIS, RIS, as well as for the general prediction of long-term disability.
T2-hyperintense lesions
Initial studies on T2-hyperintense lesions showed minimal clinical correlation with disease burden, seen on MRI, leading to the term ‘clinico-radiological paradox’.12,42,43 High lesion load was associated with neither disease duration nor functional status. Longitudinal studies, however, were able to demonstrate that an increased number of T2-hyperintense lesions and the higher lesion volume were associated with increased disability.44 The number of new T2-hyperintense lesions within the first 5 years was the strongest predictor of increased Expanded Disability Status Scale (EDSS) at 14 years and the follow-up study confirmed an association between early lesion accumulation and subsequent 20-year disability.45 Moreover, T2-hyperintense lesions can also be used to predict short-term disability,46 wherein baseline T2-hyperintense lesion volume is predictive for worsening EDSS.47,48 Despite the commonly observed lower lesion load of patients with PPMS, the number of new T2-hyperintense lesions is also modestly predictive for the disease outcome in these patients.49
Lesion location has been associated with disability and in particular periventricular, brainstem and spinal cord lesions correlate with progression of disease;50–58 interestingly, the relationship has been reported to be stronger in patients with PPMS than in patients with RRMS.59 The impact of infratentorial lesions in long-term prognosis has been evaluated in patients with CIS and brainstem rather than cerebellar lesions were responsible for increased disability. Recent studies, quantifying cord lesions, have found that especially cervical lesions were associated with disability in both relapsing and progressive forms of MS and a higher lesion load was seen in patients with progressive MS.60 As in RIS, spinal lesions are predictive of developing clinically definite MS from CIS, wherein two thirds of patients with initial nonspinal CIS, having concomitant spinal cord lesions, developed MS after 5 years.50
T1-hypointense lesions
T1-hypointense lesions, so-called ’black holes’, are hypointensities that are persistent for 6 months after the initial enhancement61 and show significant demyelination and axonal loss.62 Chronic T1-hypointense lesions are closely linked to neurodegeneration and are known to correlate with disability in patients with MS.63 There is increased interest in the predictive value of T1-hypointense lesions, which are utilized more often as an endpoint for clinical studies.64 A 10-year follow-up study showed that the number of T1 hypointensities at baseline and the increase in T1-hypointense lesion volumes predicted worsening EDSS. New or enlarging T1-hypointense lesion number and total lesion volume also correlated with EDSS change.65 It is important to note that the comparison of newer 3D gradient echo (GE) sequences with previously used spin-echo T1-hypointense lesions demonstrates an increased number of detected T1 hypointesities.66 Therefore, the 3D GE sequences increase the sensitivity for detection of T1-hypointense lesions with a potential sacrifice of specificity for pathological damage.
Brain atrophy
Brain atrophy during the course of MS has become a well recognized phenomenon.45, 67–71 Brain atrophy has the strongest correlation with clinical disease progression and increased atrophy over time is thought to predict worsening ambulatory and cognitive function.72 Brain atrophy is known to occur in normal aging with an annual loss of brain volume of approximately 0.2–0.5%.73 In patients with MS, the atrophy rate is estimated to be 0.5–1.3% per year,74 probably driven by predominantly gray matter loss. Commonly the dimension of brain atrophy appears to be more prominent in patients with progressive MS than in those with RRMS. Yet several studies have shown that significant volume loss can already occur in patients with early RRMS68,75 and in patients with CIS.76 More recently, a parenchymal loss of 0.4% per year was proposed as the ‘pathological atrophy rate’ to define patients with MS72 and had a high correlation with increase in EDSS. Given the better understanding and interpretation of brain volume loss and its correlation with disability, more consistent use of brain atrophy measures has been suggested as a parameter for clinical studies. However, it needs to be taken into consideration that initiation of treatment acutely reduces CNS inflammation. This can mimic a decrease in brain volume and regular stabilization thereafter and has been described as ‘pseudoatrophy’.72
Besides the loss of whole-brain volume, regional cerebral atrophy is now known to contribute to disease progression.77,78 Regional white matter atrophy, specifically, the involvement of white matter tracts and decreased volume of the corpus callosum at baseline, has been found to be predictive for developing MS.79 Similarly, regional cortical and deep gray matter atrophy has been associated with a conversion to clinically defined MS and suggestive of early ongoing neurodegenerative process.65 Multiple studies have determined that thalamic volume loss has a strong correlation with disease progression.77,78,80 Interestingly, thalamic atrophy has been consistently detected early in the disease course and closely linked to cognitive dysfunction, depression and fatigue.81,82 Other gray matter regions, including the precentral gyrus, superior frontal gyrus and putamen have shown volume loss in patients with MS compared with healthy age-matched controls, however the significance of atrophy in these particular regions has yet to be established.65,83
MRI has traditionally been used as a potent tool for the monitoring of lesion load and new disease activity in clinical practice, as discussed in the next section. Despite promising results from the aforementioned brain atrophy studies, the implementation of a standardized postprocessing protocol for the evaluation of brain volume is currently not clinically feasible. However, a future goal for the MS field would be the development of validated and efficient volumetric tools to be used in conjunction with the standard clinic read.
The use of MRI in the assessment of optimal treatment response
Most clinical trials investigating treatment efficacy use MRI findings as a secondary outcome measure and focus on changes in the amount and size of T2-hyperintense and contrast-enhanced T1-hypointense lesions. One recent meta-analysis of various trials evaluated the effect of treatment on lesion burden in treatment studies. It showed that treatment effects on MRI lesions over short time periods (6–9 months) can also predict the effects on relapses over longer follow-up periods (12–24 months).84 The overall analysis of these 31 studies demonstrated that new or enlarging T2-hyperintense lesions and contrast-enhanced T1-hypointense lesions were associated with the number of relapses and the use of MRI was proposed as a primary endpoint for treatment trials.
MRI has been used in a number of observational studies to identify patients at high risk for treatment failure as measured by clinical disease progression. The most common evaluated treatment in these studies was interferon β (Table 1).44,85–91 Overall disease activity was lower in interferon β treated patients, however patients with new T2-hyperintense lesions at 1-year follow up had much higher risk of poor response to interferon treatment.86 Long-term results revealed that persistent disease activity, measured again in increased lesion burden, predicted disability.92,93 More specifically, three or more new T2-hyperintense lesions or new enhanced lesion within the first 2 years predicted worse disease progression and follow-up after 15 years confirmed these findings.92 Given the availability of more effective therapeutic options, an emphasis has been made on achieving multi-metric disease stability or ‘no evidence of disease activity’ (NEDA). The definition of NEDA is based on the absence of new activity on MRI, as well as on absence of relapses and disability, and has been utilized to assess positive treatment response for patients with RRMS after 2 years.94 The original criteria are now referred to as NEDA3, given the recent proposed expansion to NEDA4, which includes brain atrophy and has been suggested as an improved metric for disease stability.95 It needs to be taken into consideration that NEDA is still an evolving measure and there are conflicting studies regarding the prognostic potential of NEDA3 for long-term disease stability.96,97 However, the availability of new treatment modalities offers a more aggressive ‘treatment to target’ approach and might provide an opportunity to achieve NEDA.
Table 1.
Summary of prospective studies evaluating the use of MRI after initiation of treatment.
| Authors (study publication year) | Investigated medication | Number of patients | Disease type | Study duration (years) | Responder classification |
|---|---|---|---|---|---|
| Rudick et al.93
Bermel et al.92 |
Intramuscular IFNβ-1a | 172 | RRMS | 2 | ⩾3 new T2 lesions or new enhanced lesion (at year 1 and 2) predicted worse disease progression over 2 years; follow up at 15 years confirmed findings |
| Kinkel et al. (2014) |
IFNβ | 383 | CIS | 2 | Active Gd+ or new T2 lesions at 6 months predicted CDMS in IFNβ-1a but not placebo patients |
| Pozzilli et al.88 | IFNβ | 242 | RRMS | 4 | 101 of 242 patients had MRI data. Gd+ lesions or new T2 lesions 1 year after beginning IFNβ with higher likelihood of relapses in the 4-year observation period |
| Tomassini et al.89 | IFNβ | 68 | RRMS | 6 | Gd+ lesions at 1 year after beginning IFNβ therapy predicted relapse or disability at 6 years |
| Rio et al.90 | IFNβ | 152 | RRMS | 2 | >2 active lesions at 1 year was the primary factor predicting sustained EDSS progression at 2 years |
| Durelli et al.91 | IFNβ | 147 | RRMS | 2 | Gd+ or T2 lesions 6 months after starting treatment predicted relapse or sustained EDSS increase in the next 18 months |
| Prosperini et al.86 | IFNβ | 394 | RRMS | 4.8 | ⩾1 new T2 lesion after 1 year of starting IFNβ was best predictor of sustained disability at 5 years |
CDMS, clinically defined multiple sclerosis; EDSS, Expanded Disability Status Scale; Gd, gadolinium; IFNβ, interferon β; MRI, magnetic resonance imaging; RRMS, relapsing–remitting multiple sclerosis.
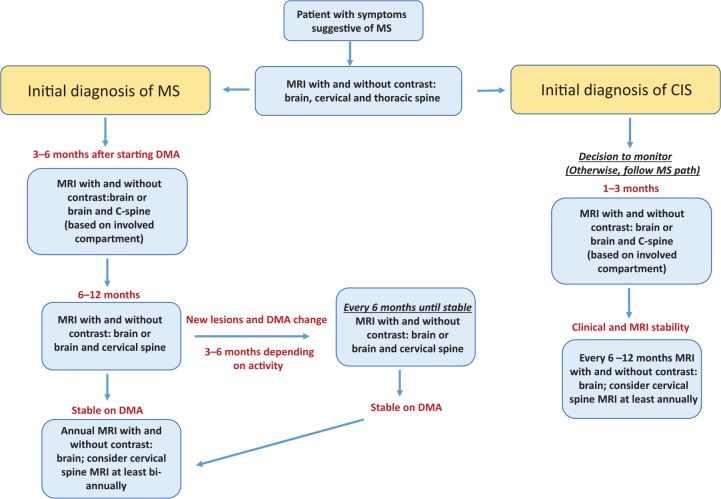
The presence of new activity on MRI is an important marker for the clinical setting, which can be interpreted as a suboptimal treatment response and a change of treatment should be considered on a case-by-case basis. No current guidelines are available as to when imaging should be obtained for best objective assessment and the following recommendations are based on the literature as well as protocols used in our MS center (Figure 1). A baseline MRI (with and without gadolinium) of the entire CNS axis (brain, cervical and thoracic spine) should be completed prior to the initiation of DMT. Follow-up scans, including brain and potentially spine imaging if patients have initial active spine disease, are recommended 3–6 months after starting therapy to ensure an early response is achieved. Further MRIs should then be obtained in 6–12 months; subsequently, in the case of stable disease, an annual MRI is warranted to monitor disease activity and optimal treatment response.8,42,98 A brain MRI with and without contrast is recommended every year and a cervical spine MRI should be considered at least biannually. In patients with disease present within the spine, we recommend obtaining both brain and cervical spine MRIs on an annual basis.
Figure 1.
Flowchart for suggested magnetic resonance imaging (MRI) to monitor patients with multiple sclerosis (MS) or clinically isolated syndrome (CIS).
DMA, disease-modifying agent.
If a patient presents with new clinical symptoms, MRI should be obtained to determine the extent of disease activity and based upon the imaging result a change in DMT may be considered. Alternatively, if a patient is clinically stable and new lesions are seen on a routine MRI, closer follow up with repeat imaging, generally obtained at 3–6 months, is recommended to ensure disease stability. Depending on the extent of disease activity found on the routine MRI (or follow-up MRI), a change in DMT may be discussed with the patient. In both of the aforementioned cases, subsequent MRIs would be obtained at 6–12 months to ensure treatment response and then once again move to annual scans.
In patients with RRMS on DMT with long disease durations who are clinically and radiographically stable or patients with longstanding progressive MS, further imaging should be tailored on the basis of individual circumstances. A new MRI can be indicated every 2–5 years and more frequent imaging is especially recommended for younger patients with progressive disease. New lesions might occur in patients with progressive MS and adjusting therapy can be considered. Patients with untreated CIS should be scanned every 1–3 months for the initial 6 months and if stable repeating MRIs every 6–12 months is recommended, unless new clinical symptoms occur. Overall these imaging recommendations allow close monitoring in order to assess disease activity and treatment response to achieve NEDA.
MRI in the monitoring of adverse effects associated with treatment
In recent years, the use of MRI has gained more relevance for the detection of adverse effects associated with available newer treatment options. Important is the monitoring of immune-suppressive medication and the associated disease progressive multifocal leukoencephalopathy (PML).42,99–103 Among MS treatments, the incidence of PML is greatest with natalizumab, but there have been cases reported with newer oral medications, such as dimethyl fumarate and fingolimod. Obtaining regular MRIs for patients on these types of treatments is crucial as it may be possible to detect PML on imaging prior to fulminant clinical symptoms. Differentiation between PML and new MS lesions can be difficult, especially if clinical signs are lacking; however, early detection of PML has been shown to increase survival.100 One recent study evaluated survival of patients with PML after natalizumab treatment; poor functional outcome was associated with higher age, higher JC titers in CSF, and more extensive PML lesions on initial MRI.100 Thus regular monitoring with MRI, to ensure early detection of pathological changes attributed to PML, is important.
Distinguishing MS lesions from PML can be challenging, however there are specific features that may aid in differentiating the two disease entities on MRI. Cortical involvement can be seen in about half of the cases of PML and these lesions tend to involve U fibers and often extend into the gyrus compared with MS lesions. Another typical finding of PML are punctate T2-hyperintense lesions in close proximity to the main lesion.104 Recent MRI criteria were proposed to have a strong predictive value to differentiate between MS disease progression and PML. The presence of punctate T2-hyperintense lesions, cortical grey matter involvement, JC white matter involvement, the pattern of contrast enhancement, ill-defined lesion borders and lesion size of more than 3 cm were all associated with PML rather than MS lesions.103 Interestingly, the classic understanding is that the presence of contrast enhancement is not found in PML,104 yet gadolinium enhancement can be detected in PML lesions associated with natalizumab-treated patients. This finding was incorporated into guidelines, proposed as consensus-based criteria by McGuigan and colleagues. Typically, contrast enhancement is homogenous or rim enhancing in MS lesions, whereas the pattern of contrast uptake in PML in patients with MS is patchy or punctate in appearance. However, given the known accumulation of gadolinium contrast within the CNS,105 postcontrast T1 scans are not recommended for frequent surveillance MRIs and are recommended only for the further evaluation of suspected disease. PML has a higher likelihood of being detected on DWI than are MS lesions; therefore, DWI is recommended as an additional sequence for the assessment of acute PML lesions as well as surveillance for subclinical disease.106
Patients treated with natalizumab, despite a positive JC titer (antibody titer above 0.4), should undergo surveillance scans on a regular basis. It has been recommended that patients with a JC antibody positive titer of less than 1.5 should undergo the regular 12-month scan and then start surveillance scans every 6 months. With a JC titer higher than 1.5, surveillance scans should also be started after 12 months of treatment, but should be obtained every 3–4 months (Table 2). All PML monitoring scans should include T2, FLAIR and DWI sequences. It is important to mention that absence of new findings on MRI does not exclude PML. If the clinical suspicion for PML is high, CSF should be obtained and the patient should be monitored closely.104
Table 2.
MRI recommendations for patients starting natalizumab.
| Time | Initial 12 months | After 12 months |
|---|---|---|
| Patient with continuously negative JC antibody | No additional safety monitoring within the first 12 months. Obtain regular MRIs to monitor treatment effects | Obtain MRIs every 12 months |
| Patient becomes positive for JC antibody (titer <1.5) | No additional safety monitoring within the first 12 months required. Obtain regular MRIs to monitor treatment effects | Obtain MRI surveillance scans every 6 months (minimum) (minimum sequences: T2, DWI and FLAIR) |
| Patient becomes positive for JC antibody (titer >1.5) | No additional safety monitoring within the first 12 months required. Obtain regular MRIs to monitor treatment effects | Obtain MRI scans every 3–4 months (minimum sequences: T2, DWI and FLAIR) |
These recommendations do not take into consideration the prior use of immune-suppressant medications. More data are required for the use of natalizumab beyond 2 years. These tests are recommended in addition to close clinical monitoring.
DWI, diffusion weighted imaging; MRI, magnetic resonance imaging; FLAIR, fluid attenuated inversion recovery.
Introduction of new MRI sequences and future utilization
Gray matter lesions have so far not been included in the latest McDonald criteria, yet the presence of gray matter pathology is well known and important for the assessment of long-term cognitive decline.107 Additional sequences such as double inversion recovery or PSIR can help with the detection of gray matter lesions.108,109 Yet the sensitivity is not ideal and therefore caution is still required before the presence of gray matter lesions can be reliably assessed and added to the diagnostic guidelines.
Newer drugs, targeting remyelination, require advanced imaging modalities to assess longitudinal myelin changes. The modalities that have been most extensively studied to assess myelin content include myelin water fraction,110–120 magnetization transfer ratio (MTR)121–123 and diffusion tensor imaging.124,125 There are several more advanced modalities that show significant promise, however they still remain in clinical development; these include quantitative MTR (qMT),126,127 diffusion basis spectrum imaging128 and g-ratio weighted imaging,129 just to name a few. Quantitative susceptibility mapping (QSM) can be used to visualize iron within the basal ganglia and MS lesions.112,130 The presence of iron is suggestive of microglia activity, and positive signal has been associated with chronic active MS lesions.34,131–141 Longitudinal QSM studies, conducted in our center, showed that lesion susceptibility increased as the lesion evolved from contrast enhanced to nonenhanced, indicating that it could be used as a biomarker.142–144 All of these advanced magnetic resonance modalities require further validation in larger cohorts of patients and over a longitudinal time span. In addition, many of these sequences remain too cumbersome for clinical MRI protocols, given the long acquisition times, or require extensive postprocessing. However, these techniques are highly promising measures to investigate mechanisms of MS disease pathogenesis, facilitate the development of novel treatments as well as potentially improving diagnostic certainty and prognostication.
Conclusion
MRI has become an established tool for the diagnosis and monitoring of MS and has advanced the field of MS significantly. The great potential of MRI for atrophy measures and cortical lesions has been increasingly appreciated, yet there remains a need for refined MRI sequences and new techniques. With respect to treatment efficacy and treatment safety monitoring, MRI has gained more importance and is crucial for the assessment of optimal treatment response, or treatment failure, and is invaluable for the early diagnosis of PML. In regards to new sequences, there has been substantial advancement of knowledge in the field of imaging, which has generated great research interest in the utilization of these modalities to advance our knowledge of MS and potentially foster in a new generation of treatment targets.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Conflict of interest statement: UK has received grant support from Biogen. SG has received grant support from Biogen, Novartis Pharmaceuticals, Mallinckrodt and Genzyme.
Contributor Information
Ulrike W. Kaunzner, Judith Jaffe Multiple Sclerosis Center, Weill Cornell Medicine, New York, NY, USA
Susan A. Gauthier, Judith Jaffe Multiple Sclerosis Center, Weill Cornell Medicine, 1305 York Avenue, New York, NY 10021, USA.
References
- 1. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol 2001; 50: 121–127. [DOI] [PubMed] [Google Scholar]
- 2. Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 2005; 58: 840–846. [DOI] [PubMed] [Google Scholar]
- 3. Selchen D, Bhan V, Blevins G, et al. MS, MRI, and the 2010 McDonald criteria: a Canadian expert commentary. Neurology 2012; 79(23 Suppl. 2): S1–15. [DOI] [PubMed] [Google Scholar]
- 4. Lovblad KO, Anzalone N, Dorfler A, et al. MR imaging in multiple sclerosis: review and recommendations for current practice. AJNR Am J Neuroradiol 2010; 31: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon JH, Li D, Traboulsee A, et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am J Neuroradiol 2006; 27: 455–461. [PMC free article] [PubMed] [Google Scholar]
- 6. Filippi M, Rocca MA, Ciccarelli O, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 2016; 15: 292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rovira A, Wattjes MP, Tintore M, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nat Rev Neurol 2015; 11: 471–482. [DOI] [PubMed] [Google Scholar]
- 8. Wattjes MP, Rovira A, Miller D, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis–establishing disease prognosis and monitoring patients. Nat Rev Neurol 2015; 11: 597–606. [DOI] [PubMed] [Google Scholar]
- 9. Stankiewicz JM, Glanz BI, Healy BC, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging 2011; 21: e50–e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wattjes MP, Harzheim M, Lutterbey GG, et al. Does high field MRI allow an earlier diagnosis of multiple sclerosis? J Neurol 2008; 255: 1159–1163. [DOI] [PubMed] [Google Scholar]
- 11. Gawne-Cain ML, O’Riordan JI, Thompson AJ, et al. Multiple sclerosis lesion detection in the brain: a comparison of fast fluid-attenuated inversion recovery and conventional T2-weighted dual spin echo. Neurology 1997; 49: 364–370. [DOI] [PubMed] [Google Scholar]
- 12. Barkhof F, Scheltens P. Imaging of white matter lesions. Cerebrovasc Dis 2002; 13(Suppl. 2): 21–30. [DOI] [PubMed] [Google Scholar]
- 13. Uysal E, Erturk SM, Yildirim H, et al. Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 2007; 188: 697–702. [DOI] [PubMed] [Google Scholar]
- 14. Grossman RI, Gonzalez-Scarano F, Atlas SW, et al. Multiple sclerosis: gadolinium enhancement in MR imaging. Radiology 1986; 161: 721–725. [DOI] [PubMed] [Google Scholar]
- 15. O’Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 2006; 66: 894–900. [DOI] [PubMed] [Google Scholar]
- 16. Nagtegaal GJ, Pohl C, Wattjes MP, et al. Interferon beta-1b reduces black holes in a randomised trial of clinically isolated syndrome. Mult Scler 2014; 20: 234–242. [DOI] [PubMed] [Google Scholar]
- 17. Rovira A, Auger C. Spinal cord in multiple sclerosis: magnetic resonance imaging features and differential diagnosis. Semin Ultrasound CT MR 2016; 37: 396–410. [DOI] [PubMed] [Google Scholar]
- 18. Okuda DT, Mowry EM, Cree BA, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology 2011; 76: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stankiewicz JM, Neema M, Alsop DC, et al. Spinal cord lesions and clinical status in multiple sclerosis: a 1.5 T and 3 T MRI study. J Neurol Sci 2009; 279: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lycklama G, Thompson A, Filippi M, et al. Spinal-cord MRI in multiple sclerosis. Lancet Neurol 2003; 2: 555–562. [DOI] [PubMed] [Google Scholar]
- 21. Gass A, Rocca MA, Agosta F, et al. MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol 2015; 14: 443–454. [DOI] [PubMed] [Google Scholar]
- 22. Honig LS, Sheremata WA. Magnetic resonance imaging of spinal cord lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 1989; 52: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kidd D, Thorpe JW, Thompson AJ, et al. Spinal cord MRI using multi-array coils and fast spin echo. II. Findings in multiple sclerosis. Neurology 1993; 43: 2632–2637. [DOI] [PubMed] [Google Scholar]
- 24. Wiebe S, Lee DH, Karlik SJ, et al. Serial cranial and spinal cord magnetic resonance imaging in multiple sclerosis. Ann Neurol 1992; 32: 643–650. [DOI] [PubMed] [Google Scholar]
- 25. Hua LH, Donlon SL, Sobhanian MJ, et al. Thoracic spinal cord lesions are influenced by the degree of cervical spine involvement in multiple sclerosis. Spinal Cord 2015; 53: 520–525. [DOI] [PubMed] [Google Scholar]
- 26. Okuda DT, Mowry EM, Beheshtian A, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 2009; 72: 800–805. [DOI] [PubMed] [Google Scholar]
- 27. Okuda DT, Siva A, Kantarci O, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One 2014; 9: e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology 2016; 87(13): 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huh SY, Min JH, Kim W, et al. The usefulness of brain MRI at onset in the differentiation of multiple sclerosis and seropositive neuromyelitis optica spectrum disorders. Mult Scler 2014; 20: 695–704. [DOI] [PubMed] [Google Scholar]
- 30. Kim W, Kim SH, Huh SY, et al. Brain abnormalities in neuromyelitis optica spectrum disorder. Mult Scler Int 2012; 2012: 735486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kilsdonk ID, Lopez-Soriano A, Kuijer JP, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol 2014; 261: 1356–1364. [DOI] [PubMed] [Google Scholar]
- 33. Sati P, Oh J, Constable RT, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12: 714–722. [DOI] [PubMed] [Google Scholar]
- 34. Absinta M, Sati P, Gaitan MI, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol 2013; 74: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Louapre C, Govindarajan ST, Gianni C, et al. Beyond focal cortical lesions in MS: an in vivo quantitative and spatial imaging study at 7T. Neurology 2015; 85: 1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. George IC, Sati P, Absinta M, et al. Clinical 3-tesla FLAIR* MRI improves diagnostic accuracy in multiple sclerosis. Mult Scler 2016; 22: 1578–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SH, Huh SY, Kim W, et al. Clinical characteristics and outcome of multiple sclerosis in Korea: does multiple sclerosis in Korea really differ from that in the Caucasian populations? Mult Scler 2013; 19: 1493–1498. [DOI] [PubMed] [Google Scholar]
- 38. Lai C, Chang Q, Tian G, et al. Lesion activity on brain MRI in a Chinese population with unilateral optic neuritis. PLoS One 2015; 10: e0141005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patrucco L, Rojas JI, Miguez JS, et al. Application of the McDonald 2010 criteria for the diagnosis of multiple sclerosis in an Argentinean cohort of patients with clinically isolated syndromes. Mult Scler 2013; 19: 1297–1301. [DOI] [PubMed] [Google Scholar]
- 40. Altmann DR, Button T, Schmierer K, et al. Sample sizes for lesion magnetisation transfer ratio outcomes in remyelination trials for multiple sclerosis. Mult Scler Relat Disord 2014; 3: 237–243. [DOI] [PubMed] [Google Scholar]
- 41. Sadaka Y, Verhey LH, Shroff MM, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Ann Neurol 2012; 72: 211–223. [DOI] [PubMed] [Google Scholar]
- 42. Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol 2015; 25(Suppl. 2): 157–165. [DOI] [PubMed] [Google Scholar]
- 43. Barkhof F. The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 2002; 15: 239–245. [DOI] [PubMed] [Google Scholar]
- 44. Rudick RA, Lee JC, Simon J, et al. Significance of T2 lesions in multiple sclerosis: a 13-year longitudinal study. Ann Neurol 2006; 60: 236–242. [DOI] [PubMed] [Google Scholar]
- 45. Fisniku LK, Chard DT, Jackson JS, et al. Gray matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol 2008; 64: 247–254. [DOI] [PubMed] [Google Scholar]
- 46. Gauthier SA, Mandel M, Guttmann CR, et al. Predicting short-term disability in multiple sclerosis. Neurology 2007; 68: 2059–2065. [DOI] [PubMed] [Google Scholar]
- 47. Minneboo A, Jasperse B, Barkhof F, et al. Predicting short-term disability progression in early multiple sclerosis: added value of MRI parameters. J Neurol Neurosurg Psychiatry 2008; 79: 917–923. [DOI] [PubMed] [Google Scholar]
- 48. Scott TF, Schramke CJ, Novero J, et al. Short-term prognosis in early relapsing-remitting multiple sclerosis. Neurology 2000; 55: 689–693. [DOI] [PubMed] [Google Scholar]
- 49. Stevenson VL, Ingle GT, Miller DH, et al. Magnetic resonance imaging predictors of disability in primary progressive multiple sclerosis: a 5-year study. Mult Scler 2004; 10: 398–401. [DOI] [PubMed] [Google Scholar]
- 50. Brownlee WJ, Altmann DR, Alves Da, Mota P, et al. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult Scler. Epub ahead of print 6 August 2016. DOI: 10.1177/1352458516663034. [DOI] [PubMed] [Google Scholar]
- 51. Patti F, De Stefano M, Lavorgna L, et al. Lesion load may predict long-term cognitive dysfunction in multiple sclerosis patients. PLoS One 2015; 10: e0120754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Popescu V, Agosta F, Hulst HE, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84: 1082–1091. [DOI] [PubMed] [Google Scholar]
- 53. Kincses ZT, Ropele S, Jenkinson M, et al. Lesion probability mapping to explain clinical deficits and cognitive performance in multiple sclerosis. Mult Scler 2011; 17: 681–689. [DOI] [PubMed] [Google Scholar]
- 54. Rovaris M, Rocca MA, Barkhof F, et al. Relationship between brain MRI lesion load and short-term disease evolution in non-disabling MS: a large-scale, multicentre study. Mult Scler 2011; 17: 319–326. [DOI] [PubMed] [Google Scholar]
- 55. Mostert JP, Koch MW, Steen C, et al. T2 lesions and rate of progression of disability in multiple sclerosis. Eur J Neurol 2010; 17: 1471–1475. [DOI] [PubMed] [Google Scholar]
- 56. Di Filippo M, Anderson VM, Altmann DR, et al. Brain atrophy and lesion load measures over 1 year relate to clinical status after 6 years in patients with clinically isolated syndromes. J Neurol Neurosurg Psychiatry 2010; 81: 204–208. [DOI] [PubMed] [Google Scholar]
- 57. Tedeschi G, Lavorgna L, Russo P, et al. Brain atrophy and lesion load in a large population of patients with multiple sclerosis. Neurology 2005; 65: 280–285. [DOI] [PubMed] [Google Scholar]
- 58. Giorgio A, Battaglini M, Rocca MA, et al. Location of brain lesions predicts conversion of clinically isolated syndromes to multiple sclerosis. Neurology 2013; 80: 234–241. [DOI] [PubMed] [Google Scholar]
- 59. Vellinga MM, Geurts JJ, Rostrup E, et al. Clinical correlations of brain lesion distribution in multiple sclerosis. J Magn Reson Imaging 2009; 29: 768–773. [DOI] [PubMed] [Google Scholar]
- 60. Kearney H, Altmann DR, Samson RS, et al. Cervical cord lesion load is associated with disability independently from atrophy in MS. Neurology 2015; 84: 367–373. [DOI] [PubMed] [Google Scholar]
- 61. Zivadinov R, Dwyer M, Barkay H, et al. Effect of glatiramer acetate three-times weekly on the evolution of new, active multiple sclerosis lesions into T1-hypointense ‘black holes’: a post hoc magnetic resonance imaging analysis. J Neurol 2015; 262: 648–653. [DOI] [PubMed] [Google Scholar]
- 62. Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol 2014; 122: 15–58. [DOI] [PubMed] [Google Scholar]
- 63. Tam RC, Traboulsee A, Riddehough A, et al. The impact of intensity variations in T1-hypointense lesions on clinical correlations in multiple sclerosis. Mult Scler 2011; 17: 949–957. [DOI] [PubMed] [Google Scholar]
- 64. Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol 2009; 5: 256–266. [DOI] [PubMed] [Google Scholar]
- 65. Jacobsen C, Hagemeier J, Myhr KM, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry 2014; 85: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 66. Dupuy SL, Tauhid S, Kim G, et al. MRI detection of hypointense brain lesions in patients with multiple sclerosis: T1 spin-echo vs. gradient-echo. Eur J Radiol 2015; 84: 1564–1568. [DOI] [PubMed] [Google Scholar]
- 67. Azevedo CJ, Pelletier D. Whole-brain atrophy: ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol 2016; 29: 237–242. [DOI] [PubMed] [Google Scholar]
- 68. Chard DT, Griffin CM, Rashid W, et al. Progressive grey matter atrophy in clinically early relapsing-remitting multiple sclerosis. Mult Scler 2004; 10: 387–391. [DOI] [PubMed] [Google Scholar]
- 69. Chard D, Miller D. Grey matter pathology in clinically early multiple sclerosis: evidence from magnetic resonance imaging. J Neurol Sci 2009; 282: 5–11. [DOI] [PubMed] [Google Scholar]
- 70. Nourbakhsh B, Azevedo C, Nunan-Saah J, et al. Longitudinal associations between brain structural changes and fatigue in early MS. Mult Scler Relat Disord 2016; 5: 29–33. [DOI] [PubMed] [Google Scholar]
- 71. Zivadinov R, Uher T, Hagemeier J, et al. A serial 10-year follow-up study of brain atrophy and disability progression in RRMS patients. Mult Scler. Epub ahead of print 16 February 2016. [DOI] [PubMed] [Google Scholar]
- 72. De Stefano N, Stromillo ML, Giorgio A, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci 2009; 29: 15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Vagberg M, Lindqvist T, Ambarki K, et al. Automated determination of brain parenchymal fraction in multiple sclerosis. AJNR Am J Neuroradiol 2013; 34: 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Stefano N, Iannucci G, Sormani MP, et al. MR correlates of cerebral atrophy in patients with multiple sclerosis. J Neurol 2002; 249: 1072–1077. [DOI] [PubMed] [Google Scholar]
- 76. Dalton CM, Brex PA, Jenkins R, et al. Progressive ventricular enlargement in patients with clinically isolated syndromes is associated with the early development of multiple sclerosis. J Neurol Neurosurg Psychiatry 2002; 73: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology 2013; 80: 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zivadinov R, Bergsland N, Dolezal O, et al. Evolution of cortical and thalamus atrophy and disability progression in early relapsing-remitting MS during 5 years. AJNR Am J Neuroradiol 2013; 34: 1931–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kalincik T, Vaneckova M, Tyblova M, et al. Volumetric MRI markers and predictors of disease activity in early multiple sclerosis: a longitudinal cohort study. PLoS One 2012; 7: e50101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Datta S, Staewen TD, Cofield SS, et al. Regional gray matter atrophy in relapsing remitting multiple sclerosis: baseline analysis of multi-center data. Mult Scler Relat Disord 2015; 4: 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Debernard L, Melzer TR, Alla S, et al. Deep grey matter MRI abnormalities and cognitive function in relapsing-remitting multiple sclerosis. Psychiatry Res 2015; 234: 352–361. [DOI] [PubMed] [Google Scholar]
- 82. Wilting J, Rolfsnes HO, Zimmermann H, et al. Structural correlates for fatigue in early relapsing remitting multiple sclerosis. Eur Radiol 2016; 26: 515–523. [DOI] [PubMed] [Google Scholar]
- 83. Hofstetter L, Naegelin Y, Filli L, et al. Progression in disability and regional grey matter atrophy in relapsing-remitting multiple sclerosis. Mult Scler 2014; 20: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol 2013; 12: 669–676. [DOI] [PubMed] [Google Scholar]
- 85. Simon JH, Lull J, Jacobs LD, et al. A longitudinal study of T1 hypointense lesions in relapsing MS: MSCRG trial of interferon beta-1a. Multiple Sclerosis Collaborative Research Group. Neurology 2000; 55: 185–192. [DOI] [PubMed] [Google Scholar]
- 86. Prosperini L, Gallo V, Petsas N, et al. One-year MRI scan predicts clinical response to interferon beta in multiple sclerosis. Eur J Neurol 2009; 16: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 87. Kinkel RP, Simon JH, O’Connor P, et al. Early MRI activity predicts treatment nonresponse with intramuscular interferon beta-1a in clinically isolated syndrome. Mult Scler Relat Disord 2014; 3: 712–719. [DOI] [PubMed] [Google Scholar]
- 88. Pozzilli C, Prosperini L, Sbardella E, et al. Post-marketing survey on clinical response to interferon beta in relapsing multiple sclerosis: the Roman experience. Neurol Sci 2005; 26(Suppl. 4): S174–S178. [DOI] [PubMed] [Google Scholar]
- 89. Tomassini V, Paolillo A, Russo P, et al. Predictors of long-term clinical response to interferon beta therapy in relapsing multiple sclerosis. J Neurol 2006; 253: 287–293. [DOI] [PubMed] [Google Scholar]
- 90. Rio J, Castillo J, Rovira A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 2009; 15: 848–853. [DOI] [PubMed] [Google Scholar]
- 91. Durelli L, Barbero P, Bergui M, et al. MRI activity and neutralising antibody as predictors of response to interferon beta treatment in multiple sclerosis. J Neurol Neurosurg Psychiatry 2008; 79: 646–651. [DOI] [PubMed] [Google Scholar]
- 92. Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 2013; 73: 95–103. [DOI] [PubMed] [Google Scholar]
- 93. Rudick RA, Lee JC, Simon J, et al. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol 2004; 56: 548–555. [DOI] [PubMed] [Google Scholar]
- 94. Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 2015; 4: 329–333. [DOI] [PubMed] [Google Scholar]
- 95. Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016; 22: 1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cree BA, Gourraud PA, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016; 80: 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72: 152–158. [DOI] [PubMed] [Google Scholar]
- 98. Stangel M, Penner IK, Kallmann BA, et al. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord 2015; 8: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol 2014; 1: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hoepner R, Kolb EM, Dahlhaus S, et al. Predictors of severity and functional outcome in natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler. Epub ahead of print 6 September 2016. DOI: 10.1177/1352458516667241. [DOI] [PubMed] [Google Scholar]
- 101. Wattjes MP, Barkhof F. Diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy using MRI. Curr Opin Neurol 2014; 27: 260–270. [DOI] [PubMed] [Google Scholar]
- 102. Wattjes MP, Warnke C. Guidelines on PML risk stratification and diagnosis in patients with MS treated with natalizumab: so far so good? J Neurol Neurosurg Psychiatry 2016; 87: 115. [DOI] [PubMed] [Google Scholar]
- 103. Wijburg MT, Witte BI, Vennegoor A, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry 2016; 87: 1138–1145. [DOI] [PubMed] [Google Scholar]
- 104. McGuigan C, Craner M, Guadagno J, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016; 87: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276: 228–232. [DOI] [PubMed] [Google Scholar]
- 106. Honce JM, Nagae L, Nyberg E. Neuroimaging of natalizumab complications in multiple sclerosis: PML and other associated entities. Mult Scler Int 2015; 2015: 809252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Geurts JJ, Calabrese M, Fisher E, et al. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol 2012; 11: 1082–1092. [DOI] [PubMed] [Google Scholar]
- 108. Harel A, Ceccarelli A, Farrell C, et al. Phase-sensitive inversion-recovery MRI improves longitudinal cortical lesion detection in progressive MS. PLoS One 2016; 11: e0152180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Favaretto A, Poggiali D, Lazzarotto A, et al. The parallel analysis of phase sensitive inversion recovery (PSIR) and double inversion recovery (DIR) images significantly improves the detection of cortical lesions in multiple sclerosis (MS) since clinical onset. PLoS One 2015; 10: e0127805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Dayan M, Monohan E, Pandya S, et al. Profilometry: a new statistical framework for the characterization of white matter pathways, with application to multiple sclerosis. Hum Brain Mapp 2016; 37: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Nguyen TD, Deh K, Monohan E, et al. Feasibility and reproducibility of whole brain myelin water mapping in 4 minutes using fast acquisition with spiral trajectory and adiabatic T2prep (FAST-T2) at 3T. Magn Reson Med 2016; 76: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nguyen TD, Wisnieff C, Cooper MA, et al. T2 prep three-dimensional spiral imaging with efficient whole brain coverage for myelin water quantification at 1.5 tesla. Magn Reson Med 2012; 67: 614–621. [DOI] [PubMed] [Google Scholar]
- 113. Vargas WS, Monohan E, Pandya S, et al. Measuring longitudinal myelin water fraction in new multiple sclerosis lesions. Neuroimage Clin 2015; 9: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Laule C, Vavasour IM, Kolind SH, et al. Long T2 water in multiple sclerosis: what else can we learn from multi-echo T2 relaxation? J Neurol 2007; 254: 1579–1587. [DOI] [PubMed] [Google Scholar]
- 115. Meyers SM, Vavasour IM, Madler B, et al. Multicenter measurements of myelin water fraction and geometric mean T2: intra- and intersite reproducibility. J Magn Reson Imaging 2013; 38: 1445–1453. [DOI] [PubMed] [Google Scholar]
- 116. Minty EP, Bjarnason TA, Laule C, et al. Myelin water measurement in the spinal cord. Magn Reson Med 2009; 61: 883–892. [DOI] [PubMed] [Google Scholar]
- 117. Vavasour IM, Laule C, Li DK, et al. Longitudinal changes in myelin water fraction in two MS patients with active disease. J Neurol Sci 2009; 276: 49–53. [DOI] [PubMed] [Google Scholar]
- 118. Kolind S, Matthews L, Johansen-Berg H, et al. Myelin water imaging reflects clinical variability in multiple sclerosis. NeuroImage 2012; 60: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kolind SH, Madler B, Fischer S, et al. Myelin water imaging: implementation and development at 3.0T and comparison to 1.5T measurements. Magn Reson Med 2009; 62: 106–115. [DOI] [PubMed] [Google Scholar]
- 120. Kitzler HH, Su J, Zeineh M, et al. Deficient MWF mapping in multiple sclerosis using 3D whole-brain multi-component relaxation MRI. NeuroImage 2012; 59: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lema A, Bishop C, Malik O, et al. A Comparison of magnetization transfer methods to assess brain and cervical cord microstructure in multiple sclerosis. J Neuroimaging. Epub ahead of print 5 August 2016. DOI: 10.1111/jon.12377. [DOI] [PubMed] [Google Scholar]
- 122. Kearney H, Yiannakas MC, Samson RS, et al. Investigation of magnetization transfer ratio-derived pial and subpial abnormalities in the multiple sclerosis spinal cord. Brain 2014; 137: 2456–2468. [DOI] [PubMed] [Google Scholar]
- 123. Amann M, Papadopoulou A, Andelova M, et al. Magnetization transfer ratio in lesions rather than normal-appearing brain relates to disability in patients with multiple sclerosis. J Neurol 2015; 262: 1909–1917. [DOI] [PubMed] [Google Scholar]
- 124. Kolasa M, Hakulinen U, Helminen M, et al. Longitudinal assessment of clinically isolated syndrome with diffusion tensor imaging and volumetric MRI. Clin Imaging 2015; 39: 207–212. [DOI] [PubMed] [Google Scholar]
- 125. Koenig KA, Sakaie KE, Lowe MJ, et al. The relationship between cognitive function and high-resolution diffusion tensor MRI of the cingulum bundle in multiple sclerosis. Mult Scler 2015; 21: 1794–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Reitz SC, Hof SM, Fleischer V, et al. Multi-parametric quantitative MRI of normal appearing white matter in multiple sclerosis, and the effect of disease activity on T2. Brain Imaging Behav. Epub ahead of print 2 May 2016. [DOI] [PubMed] [Google Scholar]
- 127. Baron K, Neumayer B, Widek T, et al. Quantitative MR imaging in fracture dating–Initial results. Forensic Sci Int 2016; 261: 61–69. [DOI] [PubMed] [Google Scholar]
- 128. Donos C, Maliia MD, Mindruta I, et al. A connectomics approach combining structural and effective connectivity assessed by intracranial electrical stimulation. NeuroImage 2016; 132: 344–358. [DOI] [PubMed] [Google Scholar]
- 129. Duval T, Levy S, Stikov N, et al. g-Ratio weighted imaging of the human spinal cord in vivo. NeuroImage. Epub ahead of print 22 September 2016. DOI: 10.1016/j.neuroimage.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wang Y, Liu T. Quantitative susceptibility mapping (QSM): decoding MRI data for a tissue magnetic biomarker. Magn Reson Med 2015; 73: 82–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mehta V, Pei W, Yang G, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS One 2013; 8: e57573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain 2011; 134: 3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yao B, Bagnato F, Matsuura E, et al. Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 2012; 262: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hametner S, Wimmer I, Haider L, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol 2013; 74: 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Craelius W, Migdal MW, Luessenhop CP, et al. Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med 1982; 106: 397–399. [PubMed] [Google Scholar]
- 136. Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126: 2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. de Rochefort L, Brown R, Prince MR, et al. Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med 2008; 60: 1003–1009. [DOI] [PubMed] [Google Scholar]
- 138. Langkammer C, Liu T, Khalil M, et al. Quantitative susceptibility mapping in multiple sclerosis. Radiology 2013; 267: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Li W, Wu B, Liu C. Quantitative susceptibility mapping of human brain reflects spatial variation in tissue composition. NeuroImage 2011; 55: 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li X, Vikram DS, Lim IA, et al. Mapping magnetic susceptibility anisotropies of white matter in vivo in the human brain at 7 T. NeuroImage 2012; 62: 314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee J, Shmueli K, Fukunaga M, et al. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proc Natl Acad Sci U S A 2010; 107: 5130–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang Y, Gauthier SA, Gupta A, et al. Quantitative susceptibility mapping and R2* measured changes during white matter lesion development in multiple sclerosis: myelin breakdown, myelin debris degradation and removal, and iron accumulation. AJNR Am J Neuroradiol 2016; 37: 1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zhang Y, Gauthier SA, Gupta A, et al. Longitudinal change in magnetic susceptibility of new enhanced multiple sclerosis (MS) lesions measured on serial quantitative susceptibility mapping (QSM). J Magn Reson Imaging 2016; 44: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang Y, Gauthier SA, Gupta A, et al. Magnetic susceptibility from quantitative susceptibility mapping can differentiate new enhancing from nonenhancing multiple sclerosis lesions without gadolinium injection. AJNR Am J Neuroradiol. Epub ahead of print 30 June 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]