Abstract
Background
Atypical antipsychotic agents (AAP) alleviate the symptoms of severe mental health disorders, such as schizophrenia, by antagonizing dopamine and serotonin receptors. Recently, AAP have also been shown to exhibit immunomodulatory properties in the central nervous system (CNS).
Objective
Building on research which demonstrated the ability of the AAP risperidone and clozapine to modify the disease course of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), we aimed to more fully investigate the potential of clozapine as a possible treatment for MS.
Results
We report that orally administered clozapine significantly reduced the disease severity of EAE in a dose-dependent manner and was effective when administered prophylactically and therapeutically. In comparison to risperidone, quetiapine, and olanzapine, clozapine was the best at reducing disease severity. While clozapine-treated mice had only modest changes to peripheral leukocytes and cytokine responses, these animals had significantly fewer CNS-infiltrating CD4 T cells and myeloid cells. Furthermore, the CNS myeloid cells displayed a less activated phenotype in mice treated with clozapine. Finally, we found that co-administration of clozapine with glatiramer acetate enhanced disease protection compared to either treatment alone.
Conclusion
These studies indicate that clozapine is an effective immunomodulatory agent with the potential to treat immune-mediated diseases such as MS.
Keywords: Experimental autoimmune encephalomyelitis, multiple sclerosis, clozapine, atypical antipsychotic agents, glatiramer acetate, immune modulation
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system (CNS) characterized by immune-mediated demyelination and axonal death.1 An estimated 2.3 million people worldwide live with the debilitating symptoms of MS.2 Since the etiology of MS is unknown, current MS therapeutic plans comprise of first- and second-line immune-modulation strategies to reduce the frequency of relapse and to slow the progression of disease.3 However, these therapeutic strategies have so far proven ineffective at treating the progressive forms of MS. The ineffectiveness of these therapeutics during progressive MS is believed to be because the chronic inflammatory events driving demyelination and neurodegeneration during progressive MS are localized in the CNS and not the periphery,4 and current therapies are unable to cross the blood–brain barrier to modify chronic neuroinflammatory processes.5,6
Atypical antipsychotic agents (AAP) such as clozapine readily cross the blood–brain barrier to antagonize neuroreceptors (e.g. dopamine and serotonin receptors).7 Recent research has also revealed that an immune component contributes to an inflammatory environment within the CNS in schizophrenic individuals,8,9 and treatment with AAP suppresses pro-inflammatory cytokine production in both in vitro and in vivo models and clinical patients.9–12 From this growing evidence that AAP modify the inflammatory environment within the CNS, key studies have shown that several AAP (quetiapine, risperidone, and clozapine) also attenuate immune-driven demyelination and paralysis in experimental autoimmune encephalomyelitis (EAE).13,14 While these preliminary reports are encouraging, further work is needed to optimize and prepare these compounds for therapeutic use in humans. Using the EAE model, this study aimed to optimize and assess the suitability of clozapine as a new therapeutic approach for treating MS.
Materials and methods
Animals and treatment administration
Female C57BL/6 mice were purchased from the Malaghan Institute (Wellington, New Zealand) and used between 8 and 12 weeks of age. Food and water were available ad libitum, and all experimental procedures were approved by the VUW Animal Ethics Committee (approval 2008-R12 and 2014-R23). Clozapine, risperidone, and quetiapine were kindly supplied by Douglas Pharmaceuticals Ltd. (Auckland, New Zealand) and olanzapine by Eli Lilly and Company (NZ) Ltd. (Auckland, New Zealand). Clozapine, risperidone, and quetiapine were dissolved in 0.1 M acetic acid at a concentration of 6 mg/mL before dilution in drinking water to deliver the doses indicated in the figure text to healthy mice or mice with EAE. Olanzapine, due to its insolubility, was mixed with ground feed and reformed into pellets to deliver 3 mg/kg/day. Mice treated with glatiramer acetate (GA; Teva Pharmaceutical Industries Ltd., Petach Tikva, Israel) received 100 and 500 µg/mouse in the MOG/CFA emulsion on day 0 as described.15 Daily water or food consumption was recorded to ensure correct dosing, and all treatments were maintained until euthanasia.
Experimental autoimmune encephalomyelitis
Mice were immunized in the rear flanks by sub-cutaneous injection of 50 µg/mouse myelin oligodendrocyte glycoprotein (MOG)35–55 peptide (Genscript, Piscataway, NJ, USA) emulsified in Freund’s Adjuvant (Sigma-Aldrich, St. Louis, MO, USA) containing 500 µg/mouse M. tuberculosis (Difco, Lawrence, KS, USA). On days 0 and 2 post-immunization, animals received an intraperitoneal injection of 200 ng/mouse pertussis toxin (List Biochemical, Campbell, CA, USA). Mice were weighed and scored daily for disease as follows: 0, normal; 1, partial tail paralysis; 2, full tail paralysis; 3, full paralysis of one hind limb; 4, full paralysis of both hind limbs; and 5, moribund.
Splenocyte restimulation and cytokine production
Following euthanasia, isolated spleens were dissociated into a single-cell suspension and prepared for restimulation as previously described.13 The total number of live cells was determined by trypan dye exclusion. Cytokine levels in the supernatant were evaluated using the Th1/Th2/Th17/Th22 13-plex cytometric bead array kit (Bender MedSystems, Burlingame, CA, USA).
Flow cytometric analysis
Splenocytes and spinal cord mononuclear cells were isolated and characterized as described (see Supplementary methods online for antibody details).13,16 All flow data was collected on a FACSCanto II (Becton Dickinson, Franklin Lakes, NJ, USA) and analyzed using FlowJo v6.1 & 10.1r7 (Tree Star Inc., Ashland, OR, USA).
In vitro culture of RAW264.7
Low passage RAW264.7 cells (5 × 104/well; gifted from the Malaghan Institute) were cultured in complete culture medium, as described,13 and primed overnight with IFN-γ (20 U/mL; Peprotech, Rocky Hill, NJ) before stimulating with or without LPS (200 ng/mL), clozapine and GA as indicated. Viability was assessed by the MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide) cellular metabolism colorimetric assay.17
Quantification of serum clozapine and N-desmethyl clozapine (norclozapine) levels
Rapid detection of clozapine and norclozapine by liquid chromatography/tandem mass spectrometry (LCMS) technology has been previously described.18 Full details are available in the Supplementary methods.
Statistical analyses
All data were graphed and analyzed using GraphPad Prism version 5 or 7 (La Jolla, CA, USA). In general, for two group comparisons, unpaired or paired Student’s t-test was used for parametric data and Mann–Whitney for non-parametric data. For >3 groups, one-way or two-way analysis of variance (ANOVA) was used with the recommended multiple comparison tests as indicated in the figure legend and as recommended by GraphPad Prism. Differences of p < 0.05 were considered significant.
Results and discussion
Oral administration of clozapine lessened EAE disease severity and promoted a sustained reduction in peak disease severity
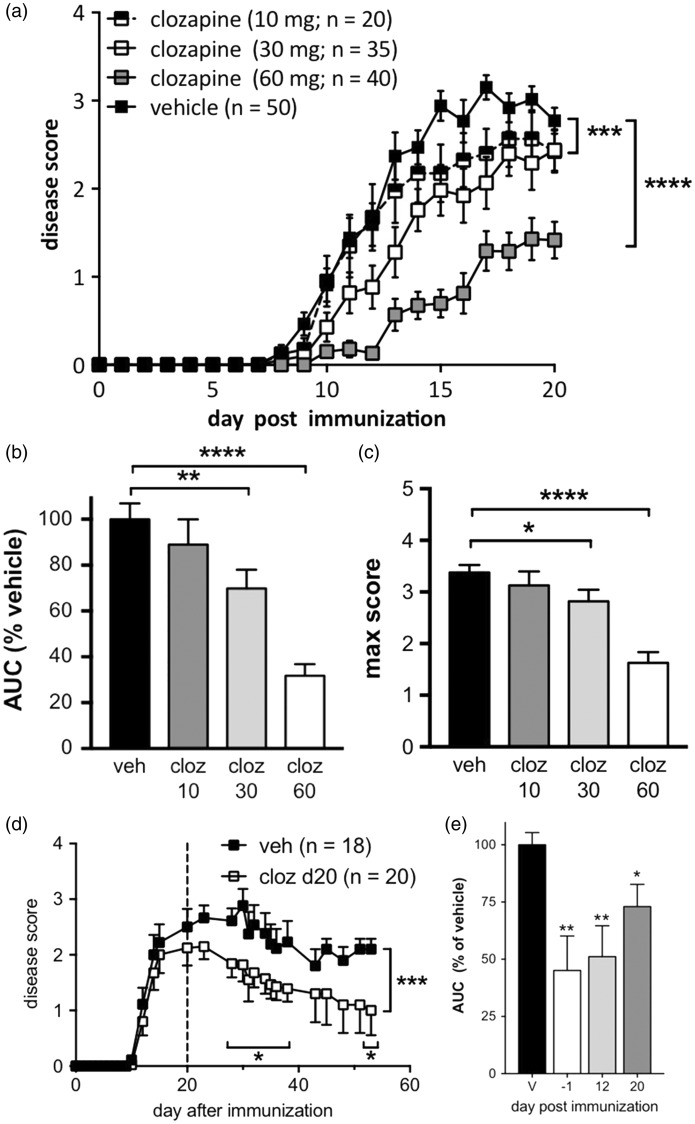
The ability of clozapine to ameliorate symptom severity during the murine EAE model has previously been demonstrated by O’Sullivan et al.13 Daily oral administration of clozapine had a dose-dependent reduction in disease symptoms as assessed by ascending paralysis (Figure 1(a)). The maximum and intermediate doses (60 and 30 mg/kg/day) were calculated to correspond to the human equivalent doses of 292 and 146 mg/day, respectively.19,20 At 60 mg/kg/day, treated animals exhibited a 75.6% decrease in cumulative disease when compared to the vehicle-treated animals as represented by area under the curve (AUC; Figure 1(b)) and even at 30 mg/kg/day, a significant effect was observed (Figure 1(b)). While clozapine treatment did not alter the onset of EAE with doses of 10 and 30 mg/kg/day, 60 mg/kg/day was effective (Figure 1(a) and Table 1), and both 30 and 60 mg/kg/day reduced the maximum disease score (Figure 1(c) and Table 1). Furthermore, clozapine was also effective when administered after disease onset (day 12 post-immunization; Figure 1(e)) or at peak disease (day 20; Figures 1(d) and (e)). Together, these studies indicate that at 60 mg/kg/day, clozapine treatment significantly reduced EAE severity even after disease onset.
Figure 1.
Clozapine reduced EAE disease severity prophylactically (a–c) and therapeutically (d, e). (a) C57BL/6 female mice were treated with clozapine (10, 30, and 60 mg/kg/day) or vehicle control in their drinking water commencing one day prior to immunization and were scored daily. Shown are the means and SEM of scores from individual mice (n = 20–50 mice as indicated) from 10 independent experiments. ***p < 0.001 and ****p < 0.0001 compared to vehicle control by 2-way ANOVA. (b, c) Clozapine treatment led to a dose-dependent reduction in overall disease burden as assessed by area under the curve (AUC) compared to the vehicle control and reduced peak disease score. Shown are the means and SEM of values from individual mice (n = 20–50/group as indicated in (a)). *p < 0.05, **p < 0.01, and ****p < 0.0001 compared to vehicle control by 1-way ANOVA with Holm–Sidak’s multiple comparison test (b) or Kruskal–Wallis with Dunn’s multiple comparison test (c). (d, e) Clozapine reduced EAE severity when administered before or 12 or 20 days after immunization. Shown are the means and SEM of values from individual mice (n = 18–20/group, veh and d20; n = 10/group d-1 and d12) from three independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by 2-way ANOVA with Fisher’s LSD test (d) and compared to vehicle control by 1-way ANOVA with Holm-Sidak’s multiple comparison test (e).
Table 1.
Clozapine dose-dependently reduced EAE parameters.
| Vehicle | 10 mg/kg | 30 mg/kg | 60 mg/kg | |
|---|---|---|---|---|
| % incidence (n) | 96% (50) | 95% (20) | 89% (35) | 60% (40) |
| Max disease score (SEM) | 3.7 (0.1) | 3.0 (0.3) | 3.0 (0.2) | 2.1 (0.4)** |
| Disease score @d20 (SEM) | 3.1 (0.1) | 2.3 (0.4) | 2.8 (0.3) | 1.5 (0.5)** |
| Day of onset (SEM) | 10.1 (1.7) | 11.5 (1.1) | 12.1 (1.1) | 16.8 (0.9)** |
p < 0.01 by one-way ANOVA with Dunnett’s multiple comparison test (max score & score @ d20) and Mantel–Cox log-rank test (day of onset) compared to vehicle control.
Not all AAP protected against EAE
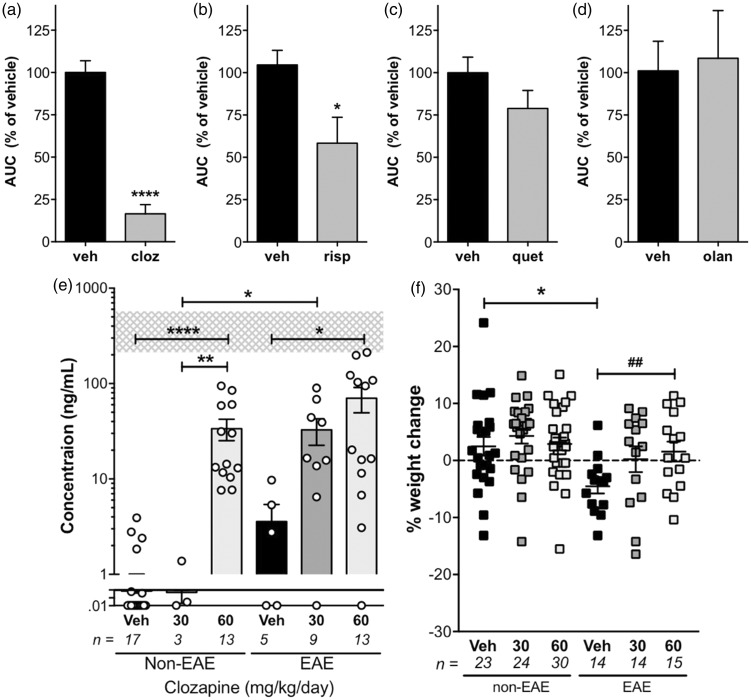
While selective antagonism of the dopamine type 2 receptor (D2) is traditionally considered to be the primary mechanism by which AAP provide psychiatric benefit,7 in the context of EAE, O’Sullivan et al. found that symptom amelioration was not due to D2 antagonism alone.13 Next generation AAPs have unique receptorome profiles pertaining their molecular targets,21 and the chemical structure–activity relationship of each compound can have a profound impact on biological response. Thus, we compared the ability of several commonly used AAP to that of clozapine in the EAE model when used prophylactically. As with clozapine, each compound was delivered orally to mice at doses which were calculated to be equivalent to human therapeutic doses: 3 mg/kg/day (olanzapine) and 60 mg/kg/day (quetiapine).19 Risperidone was also included at a dose of 3 mg/kg/day, which was the optimal dose tested previously.13 Analysis of the overall reduction in disease showed that while clozapine and risperidone effectively reduced disease, neither quetiapine or olanzapine achieved effective disease modification in this model (Figure 2(a) to (d)).
Figure 2.
Clozapine treatment (a) resulted in a greater reduction in disease than risperidone (b), quetiapine (c), or olanzapine (d) and at doses below the mean human dose as assessed by plasma concentrations (e) and weight gain (f). (a–d) C57BL/6 female mice were treated with clozapine (60 mg/kg/day; n = 20/group), risperidone (3 mg/kg/day; n = 5/group), quetiapine (60 mg/kg/day; n = 10/group), olanzapine (3 mg/kg/day; n = 5/group), or vehicle control commencing one day prior to immunization and were scored daily. (a–d) Shown are the means and SEM of AUC values from individual mice. *p < 0.05 and ****p < 0.0001 compared to vehicle control by Mann–Whitney test. (e, f) Clozapine concentrations were determined in plasma by mass spectrometry and % weight change at 31 days post-immunization. Shown are the means and SEM of values from individual mice combined from four independent experiments (n/group as indicated). *p < 0.05, **p < 0.01, and ****p < 0.0001 by 1-way ANOVA with Tukey’s multiple comparison test and ##p < 0.01 by unpaired Student’s t-test.
This study is the first to compare four different AAP in the same EAE model and demonstrates that of the four, clozapine showed the greatest effect on disease reduction. The effect of risperidone on disease parameters is similar to the study by O’Sullivan et al.,13 and there are no previous reports of the use of olanzapine in EAE. However, in contrast to our findings, a previous study by Mei et al. demonstrated that quetiapine was effective at delaying disease onset and reducing disease severity in EAE.14 Interestingly, this study used a lower oral dose of quetiapine than our studies (10 compared to 60 mg/kg/day, respectively) and dissolved the quetiapine in distilled water. While it is unlikely that a higher dose would be less effective or that the weakly acidic diluent would affect the action of orally-administered quetiapine, these possibilities cannot be discounted.
Serum clozapine levels and weight gain
Because the clozapine dose was initially calculated based upon a dose translation equation developed by Reagan-Shaw et al.,19 serum clozapine was directly measured by mass spectrometry to verify that the 60 mg/kg/day dose fell within the average dose range used in humans (350 mg/day; 228–425 interquartile).22 Interestingly, unimmunized and immunized mice treated with 60 mg/kg/day had an average serum clozapine level of 72 ng/mL, which is well below the level reported in humans (352 ng/mL, 230–614 interquartile; shaded area in Figure 2(e)).22 In addition, at this dose there was no overall weight gain in clozapine-treated unimmunized animals when compared to vehicle-treated animals. A modest weight gain was observed in clozapine-treated EAE animals compared to vehicle controls; however, given that EAE induced a significant weight loss in vehicle-treated animals, this weight gain is likely related to the reduced disease experienced by clozapine-treated mice (Figure 2(f)). These results suggest that clozapine provides a greater benefit than other AAP in this model at plasma levels below the average levels in clozapine-treated schizophrenic patients.
One explanation for the lower than expected concentration of clozapine in the plasma in mice compared to humans when administered comparable doses, is a difference in the metabolism of the drug in mice compared to humans.23,24 In support of this possibility, clozapine has been reported to be metabolized up to 6 times faster in rodents than in humans with a half-life in the serum of 1.5 h in the rat compared to 8–10 h in humans.24,25 For mice, clozapine was virtually eliminated from the serum by 4 h post i.v. injection.25 Because of this short half-life in mice, we administered clozapine in the drinking water to enable a more stable plasma concentration to be maintained throughout the study. However, given the individual variability in the plasma levels of clozapine in our study, it is likely that this fluctuation was due to individual drinking patterns. Finally, clozapine is known to cause weight gain when administered to humans at doses greater than 100 mg/day,26 and as we did not observe significant weight gain even in unimmunized animals compared to vehicle controls, this lack of weight gain supports the low plasma concentrations measured by mass spectrometry.
Clozapine treatment only modestly altered peripheral immune subsets and responses
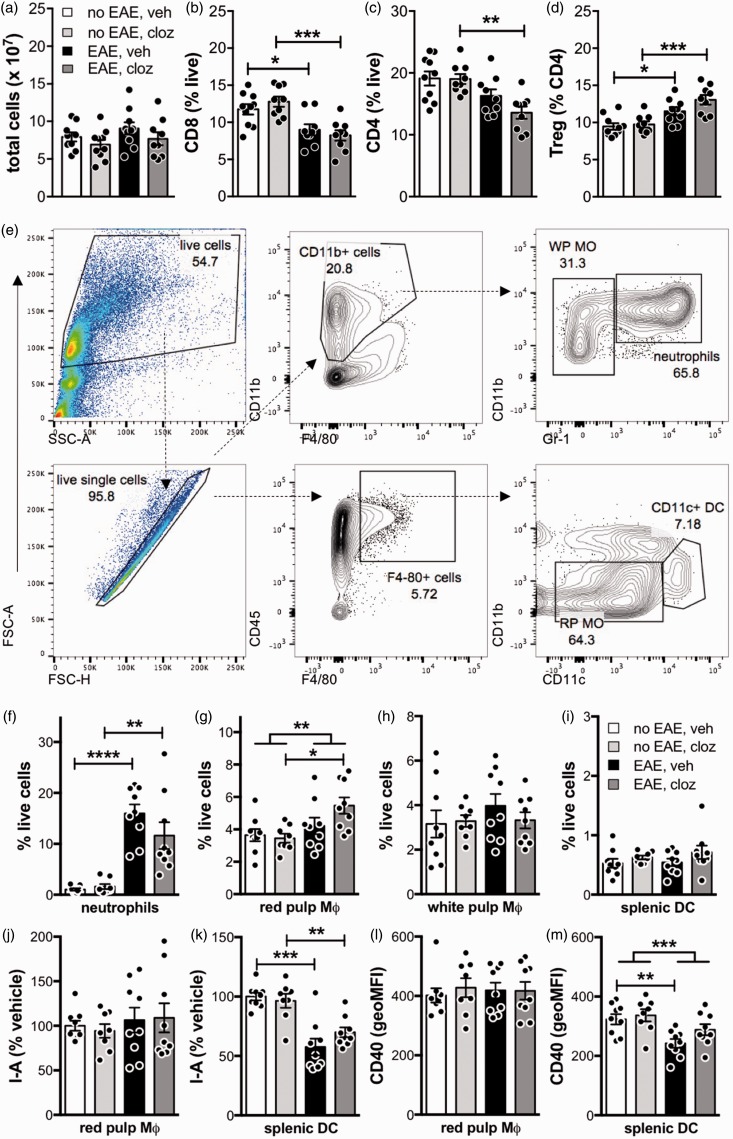
Given the effectiveness of clozapine at ameliorating disease when administered prophylactically, we investigated if clozapine were mediating its effects by modifying the peripheral immune system. To identify if the immune phenotype was altered, we analyzed splenic T cell and myeloid populations at peak disease (day 15 post-immunization) and found that neither EAE nor clozapine significantly altered the total number of live splenocytes (Figure 3(a)). EAE treatment led to a reduction in CD8+ and CD4+ T cells and an increase in Tregs (Figure 3(b) to (d) and Supplementary figure 1), but clozapine did not alter splenic T cells compared to vehicle in mice with or without EAE (Figure 3(b) to (d)).
Figure 3.
Clozapine did not alter the number or phenotype of peripheral adaptive (a–d) or innate (e–m) immune cells 15 days post-immunization. C57BL/6 mice were immunized and treated prophylactically with clozapine (60 mg/kg/day) or vehicle as in Figure 1(a). Fifteen days post-immunization, splenocytes were isolated and analyzed by flow cytometry. (a–d) The total number of live splenocytes and % of CD8 T cells, CD4 T cells and Tregs. Gating strategy for lymphocyte populations is illustrated in Supplementary figure 1. Shown are the means and SEM of individual mice (n = 9–10/group) from two independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by 1-way ANOVA with Tukey’s multiple comparison test. Gating strategy (e) for splenic myeloid populations identifies neutrophils (f), F4/80+CD11b− red pulp macrophages (g), F4/80−CD11b+ white pulp macrophages (h), and CD11b−CD11c+ dendritic cells (i). I-Ab and CD40 expression was analyzed on red pulp macrophages (j, l) and dendritic cells (k, m) and expressed as either % vehicle (I-Ab) or geoMFI (CD40). Shown are the means and SEM of individual mice (n = 9–10/group) from two independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 by 1-way ANOVA with Tukey’s multiple comparison test. (g, m) The effect of immunization independent of drug treatment was analyzed by 2-way ANOVA.
The major splenic myeloid populations were analyzed using the gating strategy in Figure 3(e).13 Using this strategy, we found that EAE increased CD11b+Gr-1+ neutrophils and F4/80+CD11b− red pulp macrophages (Figure 3(f) and (g)), but did not alter F4/80−CD11b+ white pulp macrophages or CD11c+ DC (Figure 3(h) and (i)). In immunized animals, clozapine appeared to enhance, but not significantly, the number of red pulp macrophages present over vehicle (Figure 3(g)) and had little effect on other myeloid populations (Figure 3(f), (h), and (i)). In addition, we found that while EAE altered the expression of I-Ab (i.e. MHCII) and CD40 on splenic DC, the activation of these myeloid subpopulations remained unchanged with clozapine compared to vehicle (Figure 3(j) to (m) and Supplementary figure 2). These findings are similar to the report by O’Sullivan et al.,13 who found no difference in splenic lymphocyte or myeloid populations in animals treated with risperidone 15 days post-immunization although they did report an increase in CD4+ T cells and CD4+CD25+Tregs at 40 days post-immunization. In contrast, treatment with quetiapine was found to reduce the number of CD4+ and CD8+ T cells in the spleens and lymph nodes of mice 10 days post-immunization.14
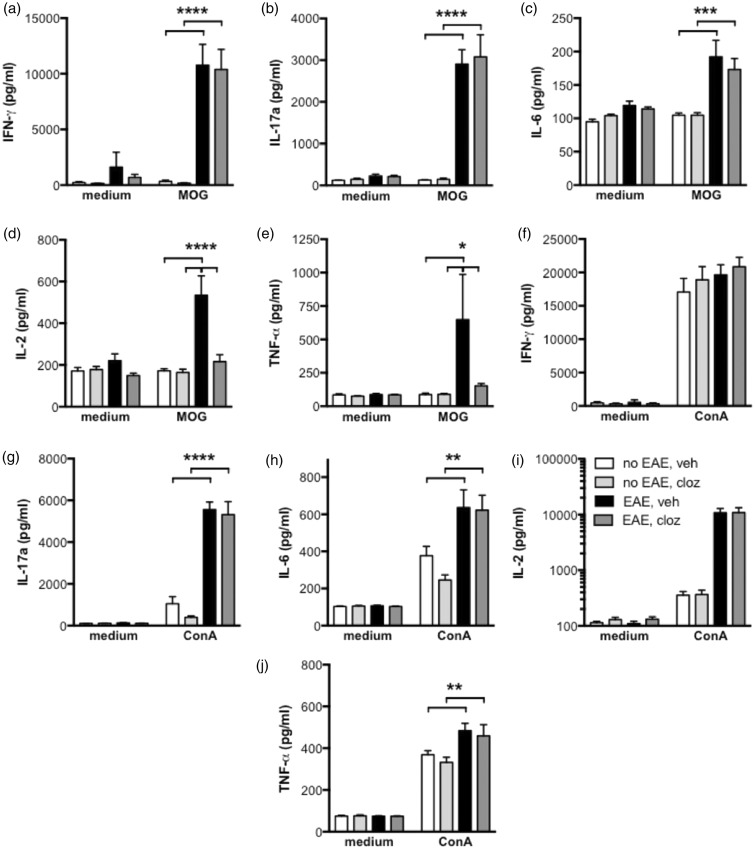
Although the splenocyte lineages remained largely unaltered following clozapine treatment, we identified a subtly altered cytokine profile within the antigen-specific lymphocytes of the periphery. Splenocytes from clozapine- and vehicle-treated, immunized and unimmunized mice were stimulated ex vivo at peak disease with either the MOG peptide (Figure 4(a) to (e)) or the polyclonal T cell mitogen, concanavalin A (ConA; Figure 4(f) and (g)). While MOG-specific IFN-γ, IL-17, and IL-6 production remained unchanged by clozapine (Figure 4(a) to (c)), there was a significant reduction in MOG-specific IL-2 and TNF-α production with clozapine treatment. In contrast to antigen-specific responses, no difference in cytokine production was observed after ConA stimulation (Figure 4(f) to (j)). The reduction in IL-2 production by T cells from immunized mice treated with AAP is consistent with previous reports as is the absence of an effect on antigen-specific IFN-γ production.13,14 These data taken together suggest that clozapine, while not affecting the ability to develop an antigen-specific Th1 or Th17 response, is perhaps modestly modifying the Th cytokine balance within the periphery during EAE.
Figure 4.
Antigen-specific cytokine responses were only modestly altered by clozapine treatment 15 days post-immunization. C57BL/6 mice were immunized and treated with clozapine (60 mg/kg/day) or vehicle as in Figure 1(a). Fifteen days post-immunization, splenocytes were isolated and cultured in the presence of MOG (a–e; 27 µg/mL) or ConA (f–j; 3 µg/mL) for 72 or 48 hours, respectively. IFN-γ (a, f), IL-17 a (b, g), IL-6 (c, h), IL-2 (d, i), and TNF-α (e, j) were assessed by cytometric bead array. Shown are the means and SEM of individual mice (n = 13-15/group) from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by 2-way ANOVA with Sidak’s multiple comparison test.
Clozapine treatment reduced leukocyte infiltration and activation of myeloid cell lineages in the CNS
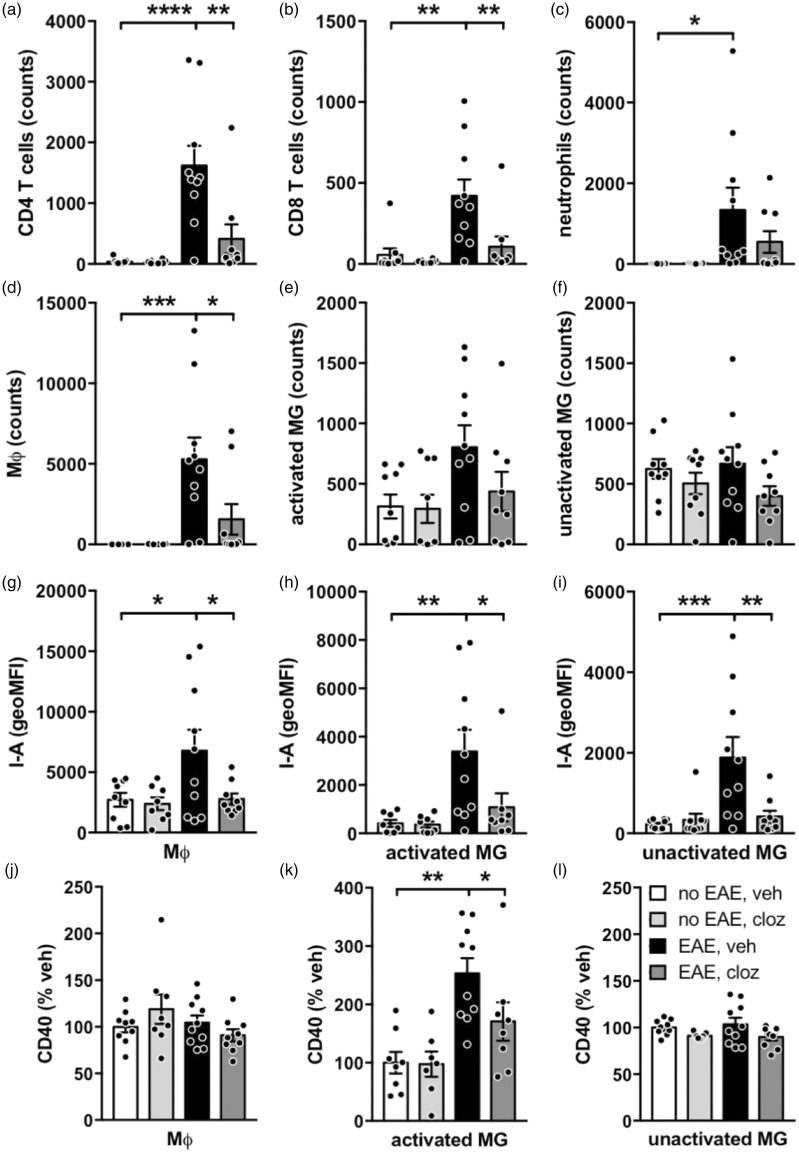
Given clozapine’s reported immunomodulatory activity in the CNS,13 we investigated the impact of clozapine treatment on CNS inflammation in EAE. At peak disease, vehicle-treated, immunized animals had a significant increase in CD4 T cells, CD8 T cells, neutrophils, and macrophages in the spinal cord but no effect on microglia compared to unimmunized animals (Figure 5(a) to (f) and Supplementary figure 3). Using the level of CD45 expression (high versus intermediate) to distinguish activated and unactivated microglia, we did not find any difference in the number of unactivated microglia in any group (Figure 5(f) and Supplementary figure 3). While there appeared to be an increase in activated microglia between the vehicle-treated immunized compared to unimmunized groups, this difference did not reach statistical significance (Figure 5(e); p = 0.0993 by one-way ANOVA with Tukey’s post-test.) Treatment with clozapine had a profound impact on the number of CD4 T cells, CD8 T cells, and macrophages while the numbers of activated and unactivated microglia were unaffected (Figure 5(a) to (f)). This reduction in CNS-infiltrating leukocytes is supported by a previous analysis of spinal cord lesions in tissue sections (see Supplementary figure 4).13
Figure 5.
CNS inflammation was significantly reduced by clozapine administration at 15 days post-immunization. C57BL/6 mice were immunized and treated with clozapine (60 mg/kg/day) or vehicle as in Figure 1(a). Fifteen days post-immunization, cells were isolated from spinal cords and analyzed by flow cytometry. The gating strategy for all immune populations is illustrated in Supplementary figure 3. Clozapine treatment reduced the number of CD4 T cells (a), CD8 T cells (b), and macrophages (d) but did not alter the number of neutrophils (c), activated microglia (e), or unactivated microglia (f). (g–i) I-Ab expression (geoMFI) is reduced on macrophages, activated microglia and unactivated microglia and CD40 expression (% vehicle) on activated macrophages by clozapine treatment. Shown are the means and SEM of individual mice (n = 9–10/group) from two independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by 1-way ANOVA with Tukey’s multiple comparison test.
To assess myeloid cell activation, we evaluated I-Ab and CD40 on macrophages and microglia and found that immunization significantly upregulated I-Ab on macrophages and microglia in vehicle-treated animals (Figure 5(g) to (i)) and CD40 on activated microglia (Figure 5(k)). Importantly, the EAE-induced upregulation of I-Ab and CD40 on macrophages and microglia was completely abolished by clozapine treatment (Figure 5(g) to (l)). Therefore, these results indicate that clozapine is potentiating an anti-inflammatory environment specifically within the CNS through alterations in the number of infiltrating leukocytes and tempered activation of infiltrating macrophages and resident microglia as measured by I-Ab and CD40 expression.
Previous studies using EAE have reported similar effects on leukocyte infiltration and spinal cord lesions after treatment with risperidone or quetiapine.13,14 Furthermore, Mei et al. found that there was less demyelination and oligodendrocyte loss in quetiapine-treated EAE animals while O’Sullivan et al. reported Iba-1 was significantly reduced in the CNS after risperidone treatment.13,14 In the past decade, there have been many reports highlighting the ability of AAP to modify neuroinflammation in other disease settings, including the cuprizone model of demyelination, LPS-induced neuroinflammation, and several psychiatric disorders such as schizophrenia,27–29 and indeed it is now widely believed that one explanation for the enhanced efficacy of AAP compared to typical antipsychotics is their immunomodulatory effects.27 In support of this idea, studies have shown that AAP can normalize neuroinflammation in mice and humans and that the reduction in inflammatory parameters correlated to clinical improvement in symptoms in schizophrenic patients.12,28,30 More targeted studies looking at the ability of AAP to directly modulate immune cells (especially microglia and macrophages) in vitro further indicate that not only are AAP directly immunomodulatory but also that the signals antagonized by these agents are essential components of the immune signaling network.11,13,31,32 Together this body of research indicates that AAP have distinct anti-inflammatory effects within the CNS (esp. microglia) and supports the use of AAP for wide-ranging neuroinflammatory applications beyond psychiatric disorders.
Enhanced disease modification with GA co-administration
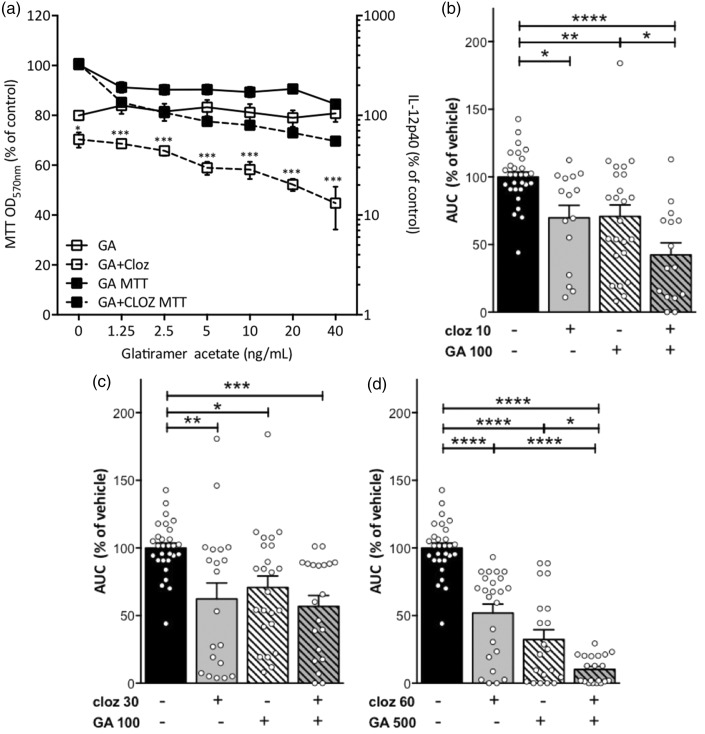
GA is a commonly prescribed therapy for relapsing–remitting MS;1,33 thus relapsing–remitting MS patients who are transitioning into secondary progressive MS may still be receiving GA treatment. Therefore, we investigated potential positive or negative drug interactions that could occur with prophylactic GA and clozapine co-administration. Because of the known ability of clozapine and GA to modify macrophage responses in vitro,13,32,34,35 we investigated the effect of clozapine and GA co-administration on pro-inflammatory cytokine production by LPS-stimulated RAW 264.7 macrophages. In accordance with previous reports,13 clozapine (10 µM) significantly reduced IL-12 while GA alone did not attenuate IL-12 (Figure 6(a)). In contrast, when combined with 10 µM clozapine, GA dose-dependently enhanced the reduction in IL-12 caused by clozapine without impacting viability (Figure 6(a)) indicating that in vitro clozapine and GA have additive immunomodulatory effects on macrophages.
Figure 6.
Combination therapy with suboptimal or optimal doses of clozapine and glatiramer acetate provided enhanced disease reduction compared to monotherapy. (a) RAW macrophages were primed with IFN-γ for 18 hours prior to stimulation with LPS (200 ng/mL) in the presence or absence of clozapine (10 µM) and increasing concentrations of glatiramer acetate for 24 h. Viability was assessed by MTT assay and IL-12p40 by ELISA. Shown are the means and SEM from two or three independent experiments. *p < 0.05 and ***p < 0.001 by 2-way ANOVA with Bonferroni’s post-test. (b–d) C57BL/6 mice were immunized and treated with clozapine (10, 30, or 60 mg/kg/day in drinking water from day -1) or vehicle as in Figure 1(a) and glatiramer acetate (100 or 500 µg/mouse) was included in the EAE immunization at d0. Mice were weighed and scored daily and cumulative disease assessed by AUC and expressed as % vehicle. Shown are the means and SEM from individual mice (n = 15–29/group) from at least three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 by 1-way ANOVA with Tukey’s multiple comparison test.
To determine if this complementary immunomodulatory activity occurred in vivo, we compared the effect of a suboptimal dose of GA (100 µg) and suboptimal doses of clozapine (10 and 30 mg/kg, respectively) on EAE disease. As with the in vitro studies, low dose GA significantly improved the ability of sub-optimal doses of clozapine (10 mg/kg) to reduce EAE compared to GA alone (Figure 6(b)), and while low dose GA alone, clozapine alone (30 mg/kg), and both together significantly reduced disease, the combination of GA and clozapine (30 mg/kg/day) was not significantly different from either agent alone (Figure 6(c)). Using the optimal doses of GA and clozapine, we found that co-administration did not cause any overt adverse effects but significantly improved the disease modifying effects in comparison to the optimal doses of GA or clozapine alone (Figure 6(d)). Overall, these results support the complementary effects of clozapine and GA on disease reduction in EAE.
Although the precise mode of action of glatiramer acetate in MS is unclear, previous studies have elegantly shown that GA alters Th biasing, enhances thymic Treg number and function, and causes type II activation of monocytes and macrophages.34–36 Thus, GA induces a wide ranging of immunomodulatory effects on the peripheral immune system, but given its inability to enter the CNS, there is no evidence that it can directly modify the CNS immune environment. In contrast, our study highlights that clozapine modified the CNS but not the peripheral immune environment in this model of neuroinflammation. Therefore, while both GA and clozapine have the ability to regulate myeloid-mediated inflammatory responses by downregulating pro-inflammatory and upregulating anti-inflammatory cytokines, the location of their optimal effects appears to differ.
In summary, this study is the first to compare directly the ability of several AAP to reduce disease in the EAE model of MS and to show that clozapine, which was the most effective AAP, combined with GA to provide complementary disease reduction at suboptimal and optimal doses. Together these results provide a solid foundation for the design and realization of a clinical trial to assess the use of the atypical anti-psychotic, clozapine, in MS patients.
Supplementary Material
Conflicts of interest.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ACL and BC have a patent for the use of clozapine during MS. The authors declare no other financial interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/ or publication of this article: This work was supported by the Neurological Foundation of New Zealand (grant numbers 1130-PG and 1320-PG) and the Ministry of Business, Innovation, and Employment (grant number RTVU1503).
References
- 1.Compston A. The pathogenesis and basis for treatment in multiple sclerosis. Clin Neurol Neurosurg 2004; 106: 246–248. [DOI] [PubMed] [Google Scholar]
- 2.Browne P, Chandraratna D, Angood C, et al. Atlas of multiple sclerosis 2013: a growing global problem with widespread inequity. Neurology 2014; 83: 1022–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart TM, Tran ZV. Injectable multiple sclerosis medications: a patient survey of factors associated with injection-site reactions. Int J MS Care 2012; 14: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 2012; 8: 647–656. [DOI] [PubMed] [Google Scholar]
- 5.Palmer AM. Multiple sclerosis and the blood–central nervous system barrier. Cardiovasc Psychiatry Neurol 2013; 2013: 530356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardridge WM. The blood–brain barrier: bottleneck in brain drug development. NeuroRx 2005; 2: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med 2001; 52: 503–517. [DOI] [PubMed] [Google Scholar]
- 8.Rothermundt M, Arolt V, Leadbeater J, et al. Cytokine production in unmedicated and treated schizophrenic patients. Neuroreport 2000; 11: 3385–3388. [DOI] [PubMed] [Google Scholar]
- 9.Al-Amin MM, Nasir Uddin MM, Mahmud Reza H. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci 2013; 11: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YK, Myint AM, Lee BH, et al. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 11.Kato T, Monji A, Hashioka S, et al. Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res 2007; 92: 108–115. [DOI] [PubMed] [Google Scholar]
- 12.Sugino H, Futamura T, Mitsumoto Y, et al. Atypical antipsychotics suppress production of proinflammatory cytokines and up-regulate interleukin-10 in lipopolysaccharide-treated mice. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 303–307. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan D, Green L, Stone S, et al. Treatment with the antipsychotic agent, risperidone, reduces disease severity in experimental autoimmune encephalomyelitis. PLoS One 2014; 9: e104430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei F, Guo S, He Y, et al. Quetiapine, an atypical antipsychotic, is protective against autoimmune-mediated demyelination by inhibiting effector T cell proliferation. PLoS One 2012; 7: e42746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toker A, Slaney CY, Backstrom BT, et al. Glatiramer acetate treatment directly targets CD11b(+)Ly6G(−) monocytes and enhances the suppression of autoreactive T cells in experimental autoimmune encephalomyelitis. Scand J Immunol 2011; 74: 235–243. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan D, Miller JH, Northcote PT, et al. Microtubule-stabilizing agents delay the onset of EAE through inhibition of migration. Immunol Cell Biol 2013; 91: 583–592. [DOI] [PubMed] [Google Scholar]
- 17.Crume KP, Miller JH, La Flamme AC. Peloruside A, an antimitotic agent, specifically decreases tumor necrosis factor-alpha production by lipopolysaccharide-stimulated murine macrophages. Exp Biol Med 2007; 232: 607–613. [PubMed] [Google Scholar]
- 18.Rao LV, Snyder ML, Vallaro GM. Rapid liquid chromatography/tandem mass spectrometer (LCMS) method for clozapine and its metabolite N-desmethyl clozapine (norclozapine) in human serum. J Clin Lab Anal 2009; 23: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22: 659–661. [DOI] [PubMed] [Google Scholar]
- 20.Freudenreich O and McEvoy J. Guidelines for prescribing clozapine in schizophrenia, www.uptodate.com/contents/guidelines-for-prescribing-clozapine-in-schizophrenia (2017, accessed 15 October 2014).
- 21.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 2004; 3: 353–359. [DOI] [PubMed] [Google Scholar]
- 22.Olesen OV, Thomsen K, Jensen PN, et al. Clozapine serum levels and side effects during steady state treatment of schizophrenic patients: a cross-sectional study. Psychopharmacology 1995; 117: 371–378. [DOI] [PubMed] [Google Scholar]
- 23.Bun H, Disdier B, Aubert C, et al. Interspecies variability and drug interactions of clozapine metabolism by microsomes. Fundam Clin Pharmacol 1999; 13: 577–581. [DOI] [PubMed] [Google Scholar]
- 24.Baldessarini RJ, Centorrino F, Flood JG, et al. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology 1993; 9: 117–124. [DOI] [PubMed] [Google Scholar]
- 25.Gauch R, Michaelis W. The metabolism of 8-chloro-11-(4-methyl-1-piperazinyl)-5H-dibenzo(b,e) (1,4)diazepine (clozapine) in mice, dogs and human subjects. Farmaco Prat 1971; 26: 667–681. [PubMed] [Google Scholar]
- 26.Nielsen J, Damkier P, Lublin H, et al. Optimizing clozapine treatment. Acta Psychiatr Scand 2011; 123: 411–422. [DOI] [PubMed] [Google Scholar]
- 27.Mondelli V, Howes O. Inflammation: its role in schizophrenia and the potential anti-inflammatory effects of antipsychotics. Psychopharmacology 2014; 231: 317–318. [DOI] [PubMed] [Google Scholar]
- 28.Macdowell KS, Garcia-Bueno B, Madrigal JL, et al. Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol 2013; 16: 121–135. [DOI] [PubMed] [Google Scholar]
- 29.Xiao L, Xu H, Zhang Y, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry 2008; 13: 697–708. [DOI] [PubMed] [Google Scholar]
- 30.Cazzullo CL, Sacchetti E, Galluzzo A, et al. Cytokine profiles in schizophrenic patients treated with risperidone: a 3-month follow-up study. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 33–39. [DOI] [PubMed] [Google Scholar]
- 31.Basta-Kaim A, Budziszewska B, Jagla G, et al. Inhibitory effect of antipsychotic drugs on the Con A- and LPS-induced proliferative activity of mouse splenocytes: a possible mechanism of action. J Physiol Pharmacol 2006; 57: 247–264. [PubMed] [Google Scholar]
- 32.Chen ML, Wu S, Tsai TC, et al. Regulation of macrophage immune responses by antipsychotic drugs. Immunopharmacol Immunotoxicol 2013; 35: 573–580. [DOI] [PubMed] [Google Scholar]
- 33.Kieseier BC, Jeffery DR. Chemotherapeutics in the treatment of multiple sclerosis. Ther Adv Neurol Disord 2010; 3: 277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber MS, Prod'homme T, Youssef S, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med 2007; 13: 935–943. [DOI] [PubMed] [Google Scholar]
- 35.Chuluundorj D, Harding SA, Abernethy D, et al. Glatiramer acetate treatment normalized the monocyte activation profile in MS patients to that of healthy controls. Immunol Cell Biol. Epub ahead of print 3 October 2016. DOI: 10.1038/icb.2016.99. [DOI] [PubMed]
- 36.Haas J, Korporal M, Balint B, et al. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+)FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J Neuroimmunol 2009; 216: 113–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.