Abstract
Introduction
Selective reporting bias occurs when chance or selective outcome reporting rather than the intervention contributes to group differences. The prevailing concern about selective reporting bias is the possibility of results being modified towards specific conclusions. In this study, we evaluate randomized controlled trials (RCTs) published in hematology journals, a group in which selective outcome reporting has not yet been explored.
Methods
Our primary goal was to examine discrepancies between the reported primary and secondary outcomes in registered and published RCTs concerning hematological malignancies reported in hematology journals with a high impact factor. The secondary goals were to address whether outcome reporting discrepancies favored statistically significant outcomes, whether a pattern existed between the funding source and likelihood of outcome reporting bias, and whether temporal trends were present in outcome reporting bias. For trials with major outcome discrepancies, we contacted trialists to determine reasons for these discrepancies. Trials published between January 1, 2010 and December 31, 2015 in Blood; British Journal of Haematology; American Journal of Hematology; Leukemia; and Haematologica were included.
Results
Of 499 RCTs screened, 109 RCTs were included. Our analysis revealed 118 major discrepancies and 629 total discrepancies. Among the 118 discrepancies, 30 (25.4%) primary outcomes were demoted, 47 (39.8%) primary outcomes were omitted, and 30 (25.4%) primary outcomes were added. Three (2.5%) secondary outcomes were upgraded to a primary outcome. The timing of assessment for a primary outcome changed eight (6.8%) times. Thirty-one major discrepancies were published with a P-value and twenty-five (80.6%) favored statistical significance. A majority of authors whom we contacted cited a pre-planned subgroup analysis as a reason for outcome changes.
Conclusion
Our results suggest that outcome changes occur frequently in hematology trials. Because RCTs ultimately underpin clinical judgment and guide policy implementation, selective reporting could pose a threat to medical decision making.
Introduction
Selective outcome reporting leading to bias in clinical trial results is a significant methodological concern in medicine [1–4]. This bias occurs when chance or selective outcome reporting rather than the intervention contributes to group differences [5]. Comparisons between outcomes reported in clinical trial registrations and those published later allow investigators to understand the extent of selective reporting bias among trialists. Thus, trial registration has played a significant role in understanding this form of bias and its effect on clinical outcomes [6].
Since 2005, the International Committee of Medical Journal Editors (ICMJE) has required that all journals within its network publish clinical trials registered in a public clinical trials registry prior to enrollment of the first patient [7–9]. Previously, only 13,153 trials were registered with ClinicalTrials.gov. This number increased to 22,714, one month after the policy took effect. Less than two years after implementation, ClinicalTrials.gov contained more than 40,000 trials [10], and to date, it contains more than 243,000 studies from 200 countries [11]. According to ICMJE, trial registration should comprise 20 items, including a description of the primary and secondary endpoints, assessment period, and funding sources, to enable stakeholders to fully investigate the validity and accuracy underlying how a trial is conducted and ultimately published [9, 12]. Despite ICMJE requirements, completeness and quality of trial registry information remain obstacles [13]. Nonetheless, information contained in trial registries allows for examining both publication and selective reporting bias [2].
The prevailing concern about selective reporting bias is the possibility of results being modified towards specific conclusions. A recent evaluation of Cochrane systematic reviews found that one-third of the included reviews contained at least 1 study for which a high suspicion of outcome reporting bias existed. Accounting for such bias changed the estimated treatment effect by 20% or more in a large number of reviews and nullified the treatment effects in others [14]. Inconsistent outcome reporting occurs when trialists add or omit outcomes, downgrade primary outcomes to secondary outcomes based on a lack of statistical significance, upgrade secondary outcomes to primary outcomes based on statistical significance, or change the outcome’s definition [15]. A systematic review of studies that evaluated the discrepancies between registered and published outcomes reported substantial variability across medical specialties in the prevalence and nature of such discrepancies, highlighting the need for further study of this issue across clinical specialties [5].
The Center for Evidence Based Medicine Outcome Monitoring Project (COMPare) currently monitors outcome reporting discrepancies for all trials published in New England Journal of Medicine, Journal of the American Medical Association, The Lancet, Annals of Internal Medicine, and British Medical Journal [16], notifies the journal that published the trial, and makes these discrepancies publically available. In this study, we evaluate randomized controlled trials (RCTs) published in hematology journals, a group in which selective outcome reporting has not yet been explored. Additionally, we include information from correspondence with authors of trials with major discrepancies detailing reasons that explain the changes in outcomes.
Materials and methods
Our primary goal was to examine discrepancies between the reported primary and secondary outcomes in registered and published RCTs concerning hematological malignancies reported in hematology journals with a high impact factor. The secondary goals were to address whether outcome reporting discrepancies favored statistically significant outcomes, whether a pattern existed between the funding source and likelihood of outcome reporting bias, and whether temporal trends were present in outcome reporting bias. We also catalogued incidental findings during data extraction and analysis that warranted further examination. Following extraction and analysis of data, we emailed the authors of RCTs with major discrepancies to determine the reason for outcome changes from registration to publication. This study did not meet the regulatory definition of human subjects research according to 45 CFR 46.102(d) and (f) of the Department of Health and Human Services’ Code of Federal Regulations [17], and it was not subject to Institutional Review Board oversight.
We consulted Li et al [18] the Cochrane Handbook for Systematic Reviews of Interventions [19]; and the National Academies of Science, Engineering, and Medicine’s Standards for Systematic Reviews [20] to ensure best practices regarding data extraction and management. We applied relevant PRISMA guidelines [21] to ensure reporting quality for systematic reviews and SAMPL guidelines [22] for reporting descriptive statistics. This study was registered with the University hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) prior to commencement (R000025787UMIN000022374). Data for this study are available on figshare (https://doi.org/10.6084/m9.figshare.4968476). A PRISMA Checklist is available for this study as a supplemental file (S1 Checklist).
Eligibility criteria for considering studies for this review
We used the Google H-5 power index to identify relevant hematology journals. Journals were chosen based off whether or not they publish RCTs concerning hematological malignancies, according to their “About” section on their respective websites. RCTs published in Blood; British Journal of Haematology; American Journal of Hematology; Leukemia; and Haematologica were included. We searched for RCTs indexed in PubMed between January 1, 2010, and December 31, 2015. This time period was several years after the ICMJE trial registration policy and allowed sufficient time to observe reporting trends. We used the National Institutes of Health definition of clinical trial: “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes [23].” We included RCTs, RCTs that used a crossover method, and follow-up studies if the listed trial registration number was for the follow up only, rather than for the primary analysis also. This ensured that the outcomes were properly evaluated for changes.
Search strategy for identifying relevant studies
Our search was performed with assistance from a medical research librarian with the following search string: (("Br J Haematol"[Journal] OR "Haematologica"[Journal]) OR "Leukemia"[Journal] OR "Blood"[Journal]) OR "Am J Hematol"[Journal] AND (Randomized Controlled Trial[ptyp] AND ("2010/01/01"[PDAT]: "2015/12/31"[PDAT])). The search occurred on September 26, 2016.
Study selection and data extraction
Citations retrieved during the search were uploaded to Endnote X7.5. Two investigators independently screened the title and abstract of each citation for possible inclusion after completing an internally developed training exercise. Any disagreement about inclusion was resolved by consensus. Excluded citations were copied into Excel and coded for the reason for exclusion. Investigators were blinded to a trial’s registration status during screening to minimize observer bias.
After screening, citations were imported into the Agency for Healthcare Research and Quality’s Systematic Review Data Repository (SRDR) [24] for data extraction. For calibration and for minimizing discrepancies in extraction, each investigator underwent SRDR and data extraction training following an internally developed protocol.
Two investigators independently reviewed the full-text articles. Once per day, these investigators met to resolve disagreements. A third investigator was available for adjudication but was not needed. We extracted the following items from the published RCT: primary outcome(s), secondary outcome(s), subject enrollment date, trial registry database and registration number, timing of assessment in primary outcomes, sample size, any discrepancies between publication and registry disclosed by the author, and funding source. We classified funding source into the following categories: private, public, industry/corporate, mixed funding, or undisclosed. For RCTs that reported multiple primary and secondary outcomes, we recorded each explicitly stated outcome. If authors failed to differentiate between primary and secondary outcomes in the publication, these non-delineated outcomes were coded as “unspecified,” set aside for individual analysis, and excluded from selective reporting analysis.
If a publication did not discuss registration, we emailed authors and asked about registration status. If we did not receive a reply after 1 week, a second email was sent. If trialists did not respond to email attempts, we designed search queries for each trial with unknown registration status. For ClinicalTrials.gov queries, we used every author's last name listed on the publication, separated them using the Boolean operator "OR", and placed the string in parentheses. We next selected key words from each title (such as the intervention and condition) that were more likely to generate accurate search returns and used the same Boolean operator and parenthetical organization. The author and keyword stings were next joined by the Boolean operator "AND". For the WHO ICTRP, each search was modified to accommodate the particular search capabilities of this registry. We consulted Glanville et al. [25] to appropriately translate our search queries. After both sets of search queries were built, the search was performed.
Trial data were extracted from registries by two independent investigators. The following data were extracted using SRDR: date of trial registration, date range of subject enrollment, original primary registered outcome(s), final primary registered outcome(s), date of initial primary outcome registration, secondary registered outcome(s), sample size, and funding source using previously defined categories. Trials lacking registration of primary or secondary outcomes and those registered after the completion of the trial (retrospectively) were excluded from our primary analysis. Per the International Standards for Clinical Trial Registries section 2.4, [26] WHO-approved registries are required to time-stamp registry-approved changes to any registered trial, including data additions, deletions, and revisions. Therefore, if a WHO-approved trial registry did not display a history of changes, we recorded the date the registry application was approved as the date of initial primary outcome registration. We used the same methodology for other non-WHO registries with the same conditions.
Three investigators next compared primary and secondary outcome(s) listed in the publication to the initial registered primary outcome(s) for consistency. Decisions were made by consensus. We catalogued 5 major discrepancies according to the classification system described by Chan et al [27] and refined by Mathieu et al [15]:
A registered primary outcome was demoted to secondary in the publication.
A registered primary outcome was omitted from the publication.
A new primary outcome was silently added to the publication.
A registered secondary outcome was promoted to primary in the publication.
The timing of assessment of the registered and published primary outcomes differed.
We also noted other discrepancies. These included instances of a demoted or omitted secondary outcome and a silently added unspecified outcome.
Articles with discrepancies were also assessed to determine whether the discrepancies favored statistically significant results. Examples of a statistically significant discrepancy include a silently added primary outcome or a promoted secondary outcome, each with a P-value less than 0.05. Additionally, if a primary outcome was omitted or demoted with a P-value greater than 0.05, this was also considered significant.
Data were exported from SRDR and analyzed using Excel 2013. We used STATA 13.1 (StataCorp, College Station, Texas) for statistical analysis. We performed a Fisher’s exact test to evaluate the relationship between funding source and selective outcome reporting for trials that reported P-values.
Following data analysis, we emailed the corresponding author of all trials for which a major discrepancy was found. In the email, we first listed all changes between the registry-listed outcomes and the published outcomes. We asked authors to verify that our data were accurate. We next asked authors to disclose the reasons for outcome changes. The response options were based on previous literature [28–30]. This email also contained a link to a Google Form which also listed the potential reasons for outcome changes in case authors preferred to answer in this manner. We employed Dillman’s method of contacting trialists three times over 1 week intervals to improve response rates [31]. The email template is available on figshare. (https://doi.org/10.6084/m9.figshare.4968476).
Results
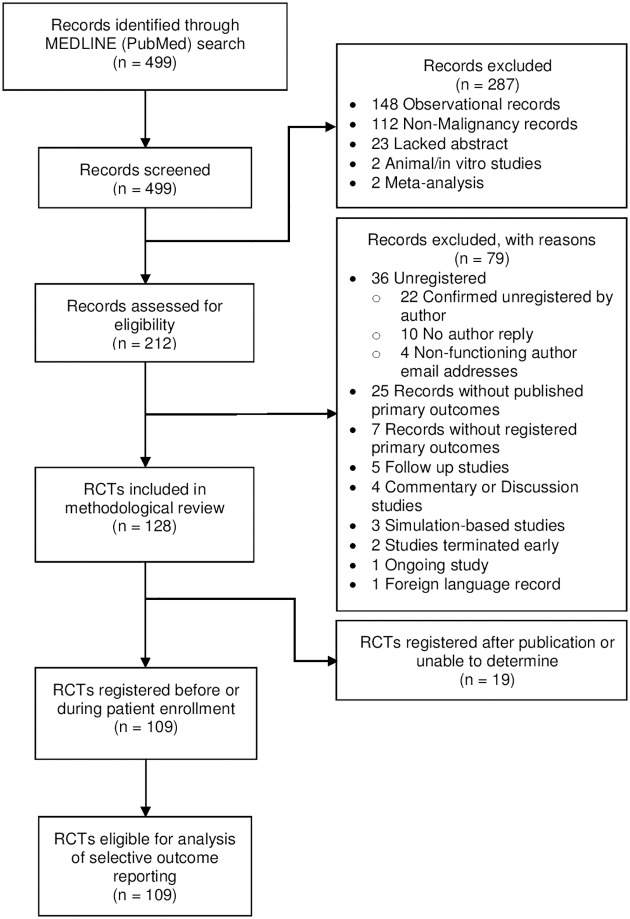
Our search yielded 499 records. Excluded records and the reason for exclusion are shown in the PRISMA flow diagram (Fig 1). One hundred twenty-eight RCTs were eligible for inclusion. One hundred nine (85.2%) RCTs were registered before completion of the trial and constituted our final sample.
Fig 1. PRISMA flow diagram.
Details regarding exclusions at each level of analysis.
The demographics of included RCTs are listed in Table 1. We encountered 6 different registries in our analysis. The majority of trials were registered in ClinicalTrials.gov followed by ISRCTN (Table 1).
Table 1. Demographic information.
Characteristics of included RCTs published between January 1, 2010 and December 31, 2015.
| n = 109 | |
|---|---|
| Journal | |
| Blood | 51 |
| American Journal of Hematology | 5 |
| British Journal of Haematology | 23 |
| Leukemia | 6 |
| Haematologica | 24 |
| Funding Source | |
| Industry/Corporate | 45 |
| Private | 8 |
| Public | 20 |
| Mixed | 33 |
| None Disclosed | 3 |
| Registry | |
| Australian New Zealand Clinical Trials Registry | 1 |
| ClinicalTrials.gov | 90 |
| EU Clinical Trials Register | 3 |
| ISRCTN | 10 |
| Netherlands Trial Registry | 3 |
| UMIN | 2 |
| Time of Registration | |
| Prospective | 83 |
| During Patient Enrollment | 26 |
| Publication Year | |
| 2010 | 15 |
| 2011 | 11 |
| 2012 | 18 |
| 2013 | 19 |
| 2014 | 20 |
| 2015 | 26 |
| Total RCTs Stratified by Number of Major Discrepancies | |
| 0 | 56 |
| 1 | 20 |
| 2 | 21 |
| 3 | 3 |
| 4+ | 9 |
The primary outcome was the number of major discrepancies present in our sample. The 109 RCTs evaluated included 118 major discrepancies, and 629 discrepancies overall. Among the 118 major discrepancies, 30 (25.4%) primary outcomes were demoted, 47 (39.8%) primary outcomes were omitted, and 30 (25.4%) primary outcomes were added. Three (2.5%) secondary outcomes were upgraded to a primary outcome. The timing of assessment for a primary outcome changed eight (6.8%) times. Fifty-six RCTs (51.4%) were reported without major discrepancy and 11 (10.1%) were reported without discrepancy whatsoever. Two (3.6%) of the RCTs without a major discrepancy made changes to their registered outcomes after publication. The frequency of major discrepancies over time shows no detectable trend (Table 2).
Table 2. Number of major discrepancies per year.
Frequency of major discrepancies between registered and published outcomes of included RCTs (n = 109) and whether discrepancies favored statistical significance, by year.
| Publication Year | Number of major discrepancies | Number of discrepancies with a reported P-value | Of evaluable discrepancies, number of discrepancies favoring statistically significant results | ||
|---|---|---|---|---|---|
| 2010 | 18 | 3 | 17% | 3 | 100% |
| 2011 | 19 | 4 | 21% | 3 | 75% |
| 2012 | 13 | 4 | 31% | 3 | 75% |
| 2013 | 27 | 5 | 19% | 5 | 100% |
| 2014 | 16 | 5 | 31% | 3 | 60% |
| 2015 | 25 | 10 | 40% | 8 | 80% |
| Total | 118 | 31 | 26% | 25 | 81% |
Thirty-one major discrepancies were published with a P-value; twenty-five (80.6%) favored statistical significance. Results from Fisher’s exact test support a relationship between funding source and selective outcome reporting among trials with reported P-values (p = .023). Detailed information regarding discrepancies by funding source is shown in Table 3.
Table 3. Number of major discrepancies per funding source.
Published RCTs that were registered before or during trial completion and have major discrepancies with their trial registries and the effect of these discrepancies on the statistical significance of published outcomes, by funding source.
| Number of published RCTs | Total | Private | Public | Industry | Mixed | Undisclosed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 109 | 8 | 20 | 45 | 33 | 3 | |||||||
| Number of published RCTs with major discrepancies between registry and publication | 53 | 49% | 2 | 25% | 12 | 60% | 17 | 38% | 19 | 58% | 3 | 100% |
| Number of major discrepancies between registry and publication | 118 | 3 | 32 | 36 | 40 | 7 | ||||||
| Registered primary outcomes demoted in publication | 30 | 25% | 0 | 0% | 12 | 38% | 6 | 29% | 12 | 30% | 0 | 0% |
| Registered primary outcomes omitted from publication | 47 | 40% | 1 | 33% | 12 | 38% | 14 | 39% | 14 | 35% | 6 | 86% |
| Unregistered primary outcomes added to publication | 30 | 25% | 1 | 33% | 6 | 19% | 13 | 36% | 9 | 23% | 1 | 14% |
| Registered secondary outcomes promoted in publication | 3 | 3% | 0 | 0% | 1 | 3% | 2 | 6% | 0 | 0% | 0 | 0% |
| Timing of assessment of primary outcomes differs | 8 | 7% | 1 | 33% | 1 | 3% | 1 | 3% | 5 | 13% | 0 | 0% |
| Number of major discrepancies between registry and publication | 118 | 3 | 32 | 36 | 40 | 7 | ||||||
| Did not report p-values | 87 | 74% | 1 | 33% | 21 | 66% | 27 | 75% | 32 | 80% | 6 | 86% |
| Reported p-values | 31 | 26% | 2 | 67% | 11 | 34% | 9 | 25% | 8 | 20% | 1 | 14% |
| Major discrepancy favors statistical significance | 25 | 81% | 2 | 100% | 11 | 100% | 5 | 56% | 7 | 88% | 0 | 0% |
| Major discrepancy does not favor statistical significance | 6 | 19% | 0 | 0% | 0 | 0% | 4 | 44% | 1 | 13% | 1 | 100% |
| Number of RCTs containing major discrepancies favoring statistical significance | 19 | 2 | 6 | 5 | 6 | 0 | ||||||
Our final analysis included an email to the authors of the 53 RCTs with a major outcome discrepancy between registry and publication. One RCT [32] already mentioned a protocol change in their publication; therefore, 52 authors were emailed. The response rate was 23.1% (12/52). Two authors had non-functioning email addresses. The most common reason for a change in outcomes was a pre-planned extension study or subgroup analysis (5/12, 41.7%). Two authors (16.7%) denied a change in outcomes, despite our findings, but did not elaborate further. One author admitted to a change occurring after discussion with peer reviewers. One author reported a change in outcome following the publication of new clinical evidence which resulted in a change to best practices, and one author cited insufficient power to analyze the outcome. One author replied that our study revealed a mistake in trial registration that resulted in a different timing of assessment in the registry compared to the original protocol. This error was corrected in the registry on April 18, 2017.
Discussion
Our results suggest that selective outcome reporting bias may occur frequently in hematology journals. Across trials, we found 118 major discrepancies and 629 discrepancies overall. Furthermore, 80.6% of major discrepancies with a reported P-value (n = 31) favored statistically significant results. In addition to major discrepancies, authors often contributed to other discrepancies, seemingly treating non-primary outcomes as malleable. These results indicate a need within hematology for heightened attention to timely and consistent registration of outcomes by both authors and journals alike, as has been indicated in other medical specialties [15, 33]. Because RCTs ultimately underpin clinical judgment and guide policy implementation, selective reporting of outcomes threatens medical decision making.
Other studies of selective outcome reporting across various fields of medicine support our findings [15, 27, 34–37]. The vast majority (85.2%) of hematology RCTs we evaluated were registered before the end of patient enrollment. Hematology RCTs were properly registered more so than has been reported in other specialties [15, 36, 37]. However, the rates of selective reporting bias found in this investigation are cause for concern and appears to be a significant issue in many medical specialties.
Adequate trial registration and adherence to reporting guidelines can potentially limit selective outcome reporting; however, it must be the joint effort of authors, editors, peer reviewers, and other stakeholders. Authors must adhere to guidelines and use best practices to improve the quality of their studies, which includes prospectively registering trials, clearly defining primary and secondary outcomes, detailing ethical changes to the registry during their study period, and addressing discrepancies in their published reports. Editors and peer reviewers should use registries to evaluate manuscripts for accuracy of data and consistency of outcomes and to verify adequate registration prior to publication. Another option would be to design registry databases so that trial registration could not be completed without first listing pertinent information like methodology, primary and secondary outcome(s), and date of participant enrollment. Some methods to improve outcome reporting have already been instituted, such as a declaration of transparency by the lead author, which has been adopted by The BMJ and BMJ Open, stating that the manuscript shows an honest and accurate account of the study. Finally, funding agencies should take responsibility by auditing the consistency and completeness of trial results and making authors accountable for discrepancies with regard to funding for subsequent research [38].
Prior studies have not identified a correlation between frequency of discrepancies and funding source [5, 13, 27]. In our sample, publicly funded RCTs had a higher frequency of major discrepancies that favored statistical significance; however, the reasons for this finding are not clear. Action is being taken to improve aspects of trial registration and reporting. Effective January 18, 2017, the National Institutes of Health (NIH) began requiring all RCTs funded, in any part, by the NIH to be registered in ClinicalTrials.gov and to report summary results and adverse events [39]. This new requirement will likely improve rates of reporting and potentially reduce rates of selective reporting bias.
Trial registration aims to enhance transparency and accountability in planning, conducting, and reporting clinical trials by making details about a trial available to the public [9]. In 2005, ICMJE instituted a policy requiring prior registration as a condition for publication. The Food and Drug Administration later mandated that all applicable clinical trials be prospectively registered. Our data reflect this policy shift; however, we still found evidence of retrospective registration. Overall, the rate of trial registration has improved, with a 5-fold increase in global trial registration from 2004 to 2013 [40]. According to their Instructions for Authors sections, Blood, Leukemia, and Haematologica all require registration of clinical trials prior to patient enrollment, adherence to the ICMJE’s Uniform Requirements for Manuscripts (URM), and CONSORT guidelines. British Journal of Haematology and American Journal of Hematology require adherence to the ICMJE’s URM but do not mention adherence to CONSORT guidelines or trial registration. Item 6a on the CONSORT checklist and the ICMJE’s URM section III.L requires authors to completely define pre-specified outcomes in their publication.
The most frequent major discrepancy encountered was the omission of a registered primary outcome from the publication. We found that, on average, there were more than four publications associated with each trial registration number in our sample. These two findings must be interpreted together. We understand that many trialists report preliminary data or subgroup analyses which are dispersed over multiple publications, and this is supported by the results of our survey. This multiplicity might account for some of the omitted primary outcomes in our sample. We were unable to determine which specific outcomes were omitted completely and which were described in other publications. We, therefore, recommend that hematology trialists fully describe the extent to which outcomes are reported elsewhere. Duplicate publication and “salami slicing” [41–48] are noted concerns in medicine and would be an interesting line of future research in hematology.
The difficulty in determining which omitted outcomes were published elsewhere and which were truly omitted emphasizes a potential shortcoming in trial registries. This issue is also a limitation of our study. These registry shortcomings include, but are not limited to, permitting retrospective registration and not providing dedicated fields in the registries for authors to detail pre-planned subgroup analyses. A recent Special Report in the New England Journal of Medicine highlights these concerns [49]. The authors of this report correctly state that allowing retrospective trial registration fundamentally undermines the primary goal of public trial registrations by interfering with the ability to see the evolution of trial outcomes and the amount of unpublished data that were generated. They further note the difficulty in determining which major discrepancies are benign (e.g., an outcome was omitted from publication because it was published in the subgroup report) with respect to pre-planned subgroup analyses. We encountered this problem when we emailed authors. We found five confirmed discrepancies due to pre-planned subgroup analyses. In our study, outcome discrepancies due to subgroup analyses should be considered when interpreting our results. Concerns regarding subgroup analyses are widespread [50–54] and revolve around statistical methods, statistical power, and credibility of subgroup analysis claims. Mandatory reporting of subgroup analyses outcomes and methodology would allow for the proper evaluation of selective reporting bias and improve transparency. The standardization of study protocols [55] and the ability to clarify pre-planned subgroup outcomes in trial registries are reasonable steps toward reducing selective reporting bias.
We acknowledge that during the course of a clinical trial, adaptations may need to be made due to unforeseen toxicities or changes in study design. For example, one RCT measured progression-free survival in patients with CD-20-positive diffuse large B-cell lymphoma randomized to receive rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone plus either placebo (R-CHOP) or bevacizumab (RA-CHOP). Early analysis revealed poor risk/benefit ratios of RA-CHOP compared to R-CHOP alone, with elevated rates of cardiotoxicity in the group receiving RA-CHOP. The trial was terminated early, and safety with 12 month follow up became the primary end point. This RCT provides an example of the need to alter the trial due to unforeseen circumstances, and in such cases, changes to the registry record are needed [56]. For trials that continue despite changes in protocol, proper disclosure of protocol changes should coincide with an immediate update of the trial registry. This includes updating the registry to reflect changes to outcomes, including the timing of assessment.
To conclude, selective outcome reporting continues to be prevalent. In many cases, authors are inhibited by shortcomings in trial registries and are unable to clarify how their outcomes are allocated (e.g., as primary or subgroup analysis). Joint efforts to ensure publication quality and unbiased results require authors, editors, and reviewers to all participate in the process. Such collaboration will bolster the accuracy and reliability of outcomes and therefore that of trials, clinical decision making, and health policy.
Supporting information
List of relevant PRISMA Items with corresponding page number denotation.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Dwan K, Altman DG, Clarke M, Gamble C, Higgins JPT, Sterne JAC, et al. Evidence for the selective reporting of analyses and discrepancies in clinical trials: a systematic review of cohort studies of clinical trials. PLoS Med. 2014;11(6):e1001666 10.1371/journal.pmed.1001666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Oliveira GS Jr., Jung MJ, McCarthy RJ. Discrepancies Between Randomized Controlled Trial Registry Entries and Content of Corresponding Manuscripts Reported in Anesthesiology Journals. Anesth Analg. 2015;121(4):1030–3. 10.1213/ANE.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen N, Lee K, Bero L. Association of trial registration with the results and conclusions of published trials of new oncology drugs. Trials. 2009;10:116 10.1186/1745-6215-10-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghav KPS, Mahajan S, Yao JC, Hobbs BP, Berry DA, Pentz RD, et al. From Protocols to Publications: A Study in Selective Reporting of Outcomes in Randomized Trials in Oncology. J Clin Oncol. 2015;33(31):3583–90. 10.1200/JCO.2015.62.4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CW, Keil LG, Holland WC, Caughey MC, Platts-Mills TF. Comparison of registered and published outcomes in randomized controlled trials: a systematic review. BMC Med. 2015;13:282 10.1186/s12916-015-0520-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto RZ, Elkins MR, Moseley AM, Sherrington C, Herbert RD, Maher CG, et al. Many randomized trials of physical therapy interventions are not adequately registered: a survey of 200 published trials. Phys Ther. 2013;93(3):299–309. 10.2522/ptj.20120206 [DOI] [PubMed] [Google Scholar]
- 7.Besselink MGH, Gooszen HG, Buskens E. Clinical trial registration and the ICMJE. JAMA. 2005;293(2):157–8; author reply 8. 10.1001/jama.293.2.157-c [DOI] [PubMed] [Google Scholar]
- 8.Khalil O, Govindarajan R, Safar M, Hutchins L, Mehta P. Clinical trial registration and the ICMJE. JAMA. 2005;293(2):157; author reply 8. 10.1001/jama.293.2.157-b [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004;292(11):1363–4. 10.1001/jama.292.11.1363 [DOI] [PubMed] [Google Scholar]
- 10.Laine C, Horton R, De Angelis C, Drazen JM, Frizelle FA, Godlee F, et al. Clinical trial registration: looking back and moving ahead. Med J Aust. 2007;186(12):612–3. [DOI] [PubMed] [Google Scholar]
- 11.Trends, Charts, and Maps. [Internet]. Revised May 2017. Accessed May 3, 2017. https://clinicaltrials.gov/ct2/resources/trends.
- 12.De Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Is this clinical trial fully registered? A statement from the International Committee of Medical Journal Editors. Ann Intern Med. 2005;143(2):146–8. [DOI] [PubMed] [Google Scholar]
- 13.Dwan K, Altman DG, Cresswell L, Blundell M, Gamble CL, Williamson PR. Comparison of protocols and registry entries to published reports for randomised controlled trials. Cochrane Database Syst Rev. 2011;(1):MR000031 10.1002/14651858.MR000031.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ. 2010;340:c365 10.1136/bmj.c365 [DOI] [PubMed] [Google Scholar]
- 15.Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA. 2009;302(9):977–84. 10.1001/jama.2009.1242 [DOI] [PubMed] [Google Scholar]
- 16.Goldacre B, Drysdale H, Powell-Smith A, Dale A, Milosevic I, Slade E, Hartley P, Marston C, Mahtani K, Heneghan C. The COMPare Trials Project www.COMPare-trials.org, 2016.
- 17.Code of Federal Regulations, Department of Health and Human Services: Title 45 Public Welfare, Part 46 Protection of Human Subjects. [Internet]. Revised January 15, 2009. Effective July 14, 2009. Accessed July 1, 2016.
- 18.Li T, Vedula SS, Hadar N, Parkin C, Lau J, Dickersin K. Innovations in data collection, management, and archiving for systematic reviews. Ann Intern Med. 2015;162(4):287–94. 10.7326/M14-1603 [DOI] [PubMed] [Google Scholar]
- 19.Cochrane Handbook for Systematic Reviews of Interventions. [Internet]. Published November 19, 2014. Accessed November 19, 2014. http://handbook.cochrane.org/. [Google Scholar]
- 20.Standards for Systematic Reviews: Health and Medicine Division. [Internet]. Published June 21, 2016. Accessed June 21, 2016. http://www.nationalacademies.org/hmd/Reports/2011/Finding-What-Works-in-Health-Care-Standards-for-Systematic-Reviews/Standards.aspx.
- 21.The PRISMA Statement Checklist. [Internet]. Published June 22, 2016. Accessed June 22, 2016. http://www.prisma-statement.org/documents/PRISMA2009checklist.pdf.
- 22.SAMPL Guidelines. [Internet]. Published June 21, 2016. Accessed June 21, 2016. http://www.equator-network.org/wp-content/uploads/2013/07/SAMPL-Guidelines-6-27-13.pdf.
- 23.An NIH Outreach Toolkit: NIH Definitions. http://orwh.od.nih.gov/toolkit/nih-policies-inclusion/definitions.html. Published June 22, 2016. Accessed June 22, 2016.
- 24.SRDR—Systematic Review Data Repository | Home. http://srdr.ahrq.gov/. Published June 20, 2016. Accessed June 20, 2016.
- 25.Glanville JM, Duffy S, McCool R, Varley D. Searching ClinicalTrials.gov and the International Clinical Trials Registry Platform to inform systematic reviews: what are the optimal search approaches? J Med Libr Assoc. 2014;102(3):177–83. 10.3163/1536-5050.102.3.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO International Clinical Trials Registry Platform (ICTRP). [Internet]. Published June 22, 2016. Accessed June 22, 2016.
- 27.Chan A-W, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA. 2004;291(20):2457–65. 10.1001/jama.291.20.2457 [DOI] [PubMed] [Google Scholar]
- 28.Smyth RMD, Kirkham JJ, Jacoby A, Altman DG, Gamble C, Williamson PR. Frequency and reasons for outcome reporting bias in clinical trials: interviews with trialists. BMJ. 2011;342:c7153 10.1136/bmj.c7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pocock SJ, Stone GW. The Primary Outcome Fails—What Next? N Engl J Med. 2016;375(9):861–70. 10.1056/NEJMra1510064 [DOI] [PubMed] [Google Scholar]
- 30.Chan A-W, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ. 2005;330(7494):753 10.1136/bmj.38356.424606.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dillman DA, Smyth JD, Christian LM. Internet, Phone, Mail and Mixed-Mode Surveys: The Tailored Design Method, 4th edn Hoboken, NJ: John Wiley, 2014. [Google Scholar]
- 32.Seymour JF, Pfreundschuh M, Trnĕný M, Sehn LH, Catalano J, Csinady E, et al. R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: final MAIN study outcomes. Haematologica. 2014;99(8):1343–9. 10.3324/haematol.2013.100818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You B, Gan HK, Pond G, Chen EX. Consistency in the analysis and reporting of primary end points in oncology randomized controlled trials from registration to publication: a systematic review. J Clin Oncol. 2012;30(2):210–6. 10.1200/JCO.2011.37.0890 [DOI] [PubMed] [Google Scholar]
- 34.Li X-Q, Yang G-L, Tao K-M, Zhang H-Q, Zhou Q-H, Ling C-Q. Comparison of registered and published primary outcomes in randomized controlled trials of gastroenterology and hepatology. Scand J Gastroenterol. 2013;48(12):1474–83. 10.3109/00365521.2013.845909 [DOI] [PubMed] [Google Scholar]
- 35.Killeen S, Sourallous P, Hunter IA, Hartley JE, Grady HLO. Registration Rates, Adequacy of Registration, and a Comparison of Registered and Published Primary Outcomes in Randomized Controlled Trials Published in Surgery Journals. Ann Surg. 2014;259(1):193–6. 10.1097/SLA.0b013e318299d00b [DOI] [PubMed] [Google Scholar]
- 36.Rongen JJ, Hannink G. Comparison of Registered and Published Primary Outcomes in Randomized Controlled Trials of Orthopaedic Surgical Interventions. J Bone Joint Surg Am. 2016;98(5):403–9. 10.2106/JBJS.15.00400 [DOI] [PubMed] [Google Scholar]
- 37.Hannink G, Gooszen HG, Rovers MM. Comparison of registered and published primary outcomes in randomized clinical trials of surgical interventions. Ann Surg. 2013;257(5):818–23. 10.1097/SLA.0b013e3182864fa3 [DOI] [PubMed] [Google Scholar]
- 38.Dal-Ré R, Caplan AL. Journal editors impasse with outcome reporting bias. Eur J Clin Invest. 2015;45(9):895–8. 10.1111/eci.12484 [DOI] [PubMed] [Google Scholar]
- 39.FDAAA for NIH Grantees: The Basics. https://grants.nih.gov/clinicaltrials_fdaaa/the-basics.htm#whatisFDAAA. [Internet]. Updated September 16, 2016. Accessed May 3, 2017.
- 40.Viergever RF, Li K. Trends in global clinical trial registration: an analysis of numbers of registered clinical trials in different parts of the world from 2004 to 2013. BMJ Open. 2015;5(9):e008932 10.1136/bmjopen-2015-008932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Talbott JA. Salami Slicing, Duplicative Publication, and Data Set Utilization: Where to Draw the Line. J Nerv Ment Dis. 2016;204(11):868 10.1097/NMD.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 42.Cheung VWF, Lam GOA, Wang YF, Chadha NK. Current incidence of duplicate publication in otolaryngology. Laryngoscope. 2014;124(3):655–8. 10.1002/lary.24294 [DOI] [PubMed] [Google Scholar]
- 43.Durani P. Duplicate publications: redundancy in plastic surgery literature. J Plast Reconstr Aesthet Surg. 2006;59(9):975–7. 10.1016/j.bjps.2005.11.039 [DOI] [PubMed] [Google Scholar]
- 44.Schein M, Paladugu R. Redundant surgical publications: tip of the iceberg? Surgery. 2001;129(6):655–61. 10.1067/msy.2001.114549 [DOI] [PubMed] [Google Scholar]
- 45.Spielmans GI, Biehn TL, Sawrey DL. A case study of salami slicing: pooled analyses of duloxetine for depression. Psychother Psychosom. 2010;79(2):97–106. 10.1159/000270917 [DOI] [PubMed] [Google Scholar]
- 46.Tugwell P, Knottnerus JA. Are triallists guilty of 'imbalanced salami slicing'- by favouring positive results in secondary publications? J Clin Epidemiol. 2016;79:1–2. 10.1016/j.jclinepi.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 47.Klein AA, Pozniak A, Pandit JJ. Salami slicing or living off the fat? Justifying multiple publications from a single HIV dataset. Anaesthesia. 2014;69(3):195–8. 10.1111/anae.12603 [DOI] [PubMed] [Google Scholar]
- 48.Beaufils P, Karlsson J. Legitimate division of large datasets, salami slicing and dual publication. Where does a fraud begin? Orthop Traumatol Surg Res. 2013;99(2):121–2. 10.1016/j.otsr.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 49.Zarin DA, Tse T, Williams RJ, Rajakannan T. Update on Trial Registration 11 Years after the ICMJE Policy Was Established. N Engl J Med. 2017;376(4):383–91. 10.1056/NEJMsr1601330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barraclough H, Govindan R. Biostatistics primer: what a clinician ought to know: subgroup analyses. J Thorac Oncol. 2010;5(5):741–6. 10.1097/JTO.0b013e3181d9009e [DOI] [PubMed] [Google Scholar]
- 51.Guillemin F. Primer: the fallacy of subgroup analysis. Nat Clin Pract Rheumatol. 2007;3(7):407–13. 10.1038/ncprheum0528 [DOI] [PubMed] [Google Scholar]
- 52.Kasenda B, Schandelmaier S, Sun X, von Elm E, You J, Blümle A, et al. Subgroup analyses in randomised controlled trials: cohort study on trial protocols and journal publications. BMJ. 2014;349:g4539 10.1136/bmj.g4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Venekamp RP, Rovers MM, Hoes AW, Knol MJ. Subgroup analysis in randomized controlled trials appeared to be dependent on whether relative or absolute effect measures were used. J Clin Epidemiol. 2014;67(4):410–5. 10.1016/j.jclinepi.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 54.Zhang S, Liang F, Li W, Hu X. Subgroup Analyses in Reporting of Phase III Clinical Trials in Solid Tumors. J Clin Oncol. 2015;33(15):1697–702. 10.1200/JCO.2014.59.8862 [DOI] [PubMed] [Google Scholar]
- 55.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baron F, Suciu S, Amadori S, Muus P, Zwierzina H, Denzlinger C, et al. Value of infliximab (Remicade®) in patients with low-risk myelodysplastic syndrome: final results of a randomized phase II trial (EORTC trial 06023) of the EORTC Leukemia Group. Haematologica. 2012;97(4):529–33. 10.3324/haematol.2011.044347 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of relevant PRISMA Items with corresponding page number denotation.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.