Abstract
Magnesium sulfate is now widely recommended for neuroprotection for preterm birth; however, this has been controversial because there is little evidence that magnesium sulfate is neuroprotective. Preterm fetal sheep (104 days gestation; term is 147 days) were randomly assigned to receive sham occlusion (n = 7), i.v. magnesium sulfate (n = 10) or saline (n = 8) starting 24 h before asphyxia until 24 h after asphyxia. Sheep were killed 72 h after asphyxia. Magnesium sulfate infusion reduced electroencephalograph power and fetal movements before asphyxia. Magnesium sulfate infusion did not affect electroencephalograph power during recovery, but was associated with marked reduction of the post-asphyxial seizure burden (mean ± SD: 34 ± 18 min vs. 107 ± 74 min, P < 0.05). Magnesium sulfate infusion did not affect subcortical neuronal loss. In the intragyral and periventricular white matter, magnesium sulfate was associated with reduced numbers of all (Olig−2+ve) oligodendrocytes in the intragyral (125 ± 23 vs. 163 ± 38 cells/field) and periventricular white matter (162 ± 39 vs. 209 ± 44 cells/field) compared to saline-treated controls (P < 0.05), but no effect on microglial induction or astrogliosis. In conclusion, a clinically comparable dose of magnesium sulfate showed significant anticonvulsant effects after asphyxia in preterm fetal sheep, but did not reduce asphyxia-induced brain injury and exacerbated loss of oligodendrocytes.
Keywords: Magnesium sulfate, asphyxia, brain, neuroprotection, preterm fetus
Introduction
Magnesium sulfate (MgSO4) is now widely recommended for neuroprotection for preterm birth in many countries.1,2 This recommendation arises directly from meta-analysis of randomized controlled trials of antenatal administration of MgSO4 to women at risk of preterm birth that found that this intervention is associated with a small but significant reduction in the risk of cerebral palsy and motor dysfunction in early childhood.3 However, there was no significant effect on the combination of death and disability, raising the possibility that exposure to MgSO4 may have been associated with a shift between outcomes rather than overall improvement. Further, follow-up to school age suggests no significant improvement in neurodevelopmental outcomes,4,5 although these studies are relatively small.
The most likely neuroprotective mechanism for magnesium is that it is a key physiological anti-excitatory factor, inhibiting N-methyl-D-aspartate-glutamate (NMDA) receptor activity through a specific site on the receptor.6 There is some evidence for other mechanisms for neuroprotection, including inhibition of free radical production,7 reduced inflammation,8 stabilization of the neuronal cell membrane,9 and improved cardiovascular stability.10
Despite these potentially beneficial effects, systematic review of the preclinical literature found that the effects of MgSO4 treatment during or after hypoxia-ischemia on neural outcomes were highly inconsistent between studies.11 There was no apparent benefit in large animal studies at term equivalent,11 and we have been unable to identify any studies in preterm-equivalent large animals. In part, the inconsistent effects in small animal studies likely reflected confounding with induced hypothermia. More generally, it is unclear whether systemic infusions can achieve significant anti-excitotoxic effects,6 at levels that do not induce hypotension.12 Collectively, these data indicate a need for rigorous testing of the efficacy of MgSO4 for perinatal encephalopathy in the very immature brain.
Preterm brain injury is multifactorial. Asphyxia, as shown by metabolic acidosis and need for resuscitation at birth, remains common among preterm babies and is associated with increased risk of death, subcortical brain injury, and disability.13–16 Excessive glutaminergic excitation during hypoxia-ischemia and during post-hypoxic-ischemic seizures is widely implicated in preterm white and gray matter injury.17
Thus, the aim of this study was to test whether MgSO4 can improve neurophysiological recovery from acute severe asphyxia and reduce white and gray matter damage in preterm fetal sheep at 0.7 of gestation. At this gestational age, neural maturation is broadly equivalent to 28–32 weeks of human development.18 Because MgSO4 may have beneficial effects on events both during and after asphyxia, in this proof of concept study, the infusion was started 24 h before asphyxia, and then continued for 24 h afterwards. This enabled us to characterize the effects of elevated circulating MgSO4 levels on post insult recovery of electroencephalograph (EEG) activity, neural excitation, and cell survival.
Materials and methods
All procedures were approved by the Animal Ethics Committee of The University of Auckland under the New Zealand Animal Welfare Act, and the Code of Ethical Conduct for animals in research established by the Ministry of Primary Industries, Government of New Zealand. This manuscript complies with the ARRIVE guidelines for reporting animal research.19 Eighteen Romney/Suffolk fetal sheep underwent aseptic surgery between 97 and 99 days’ gestation (term = 147 days). Food but not water was withdrawn 18 h before surgery. Ewes were given long acting oxytetracycline (20 mg/kg, Phoenix Pharm, Auckland, New Zealand) i.m. 30 min before the start of surgery. Anesthesia was induced by i.v. injection of propofol (5 mg/kg; AstraZeneca Limited, Auckland, New Zealand) and maintained using 2–3% isoflurane in O2 (Bomac Animal Health, NSW, Australia). During surgery, ewes received an i.v. infusion of isotonic saline (250 mL/h) to maintain fluid balance. Depth of anesthesia, maternal heart rate, and respiration were continuously monitored by trained anesthetic staff.
Instrumentation
In brief, following a maternal midline abdominal incision, the fetus was exposed, and polyvinyl catheters were inserted in the left femoral and brachial arteries, brachial vein, and amniotic cavity. Vascular flow probes (Transonic systems, Ithaca, NY, USA) were placed around the right femoral artery (2.5 mm) and right carotid artery (3 mm) to monitor femoral blood flow (FBF) and carotid blood flow (CaBF). A pair of electrodes was sewn over the fetal chest to measure the fetal electrocardiogram (ECG). An inflatable silicone rubber occluder (OC16HD; In Vivo Metric, Healdsburg, CA, USA) was loosely secured around the umbilical cord. Two pairs of EEG electrodes (AS633-7SSF; Cooner Wire, Chatsworth, CA, USA) were placed through burr holes onto the dura over the parasagittal parietal cortex (5 and 10 mm anterior to bregma and 5 mm lateral) and secured with cyanoacrylate glue. To measure cortical impedance, a pair of electrodes (AS633-3SSF; Cooner Wire) was placed over the dura 5 mm lateral to the EEG electrodes. A pair of electrodes was sewn into the nuchal muscle to record electromyographic (EMG) activity to measure fetal movement and a reference electrode was sewn over the occiput. All fetal leads were exteriorized through the maternal flank. Antibiotics (Gentamicin; 80 mg; Rousell Ltd, Auckland, New Zealand) were administered into the amniotic sac before closure of the uterus. A maternal long saphenous vein was catheterized to provide access for postoperative care. The maternal laparotomy incision was repaired and the skin infiltrated with 10 mL of 0.25% bupivacaine plus adrenaline (AstraZeneca Limited).
Sheep were housed in separate metabolic cages with access to water and food ad libitum in a temperature-controlled room (16 ± 1℃, humidity 50 ± 10%) with a 12 h light dark cycle. Five days of postoperative recovery were allowed before experiments commenced. During this time, ewes received intravenous antibiotics daily for four days (benzylpenicillin sodium; 600 mg; Novartis, Auckland, New Zealand and gentamycin; 80 mg). Fetal catheters were maintained patent by continuous infusion of heparinized saline (20 IU/mL) at a rate of 0.2 mL/h.
At 104 days, fetuses were randomly allocated to receive an intravenous infusion of normal saline (n = 8) or magnesium sulfate heptahydrate dissolved in saline (MgSO4 ċ 7H2O, 500 mg/mL; Phebra, NSW, Australia; n = 10), or sham occlusion with saline infusion (n = 7). Randomization was stratified by cohort to control for time of year, with a small over-allocation to MgSO4 to increase power to show any effect of MgSO4. Post hoc power analysis for neuronal survival suggested 80% power to detect a minimum difference of 36 cells/field, with alpha 0.05. Similarly, for oligodendrocyte survival, the study had 80% power to detect a minimum difference of 40 cells /field.
Twenty-four hours before umbilical cord occlusion, the MgSO4 group received a 160 mg loading dose over 5 min followed by a 48 mg/h maintenance infusion over 24 h before asphyxia (104–105 days) and 24 mg/h for 24 h (105–106 days) after asphyxia. This regime was adapted from a randomized controlled trial of antenatal MgSO4.20
Experimental recordings
Fetal mean arterial blood pressure (MAP), corrected for maternal movement by subtraction of amniotic pressure, FBF, CaBF, ECG, EEG, and nuchal EMG were recorded continuously for off-line analysis using custom data acquisition software (LabVIEW for Windows, National Instruments, Texas, USA). The blood pressure signal was collected at 64 Hz and low pass filtered at 30 Hz. The fetal ECG was analog filtered between 0.05 and 100 Hz and digitized at 512 Hz, and used to derive fetal heart rate (FHR). The analog fetal EEG signal was low pass filtered with a cut off frequency set with the −3 dB point at 30 Hz, and digitized at a sampling rate of 512 Hz. The intensity (power) was derived from the intensity spectrum signal between 0.5 and 20 Hz, while spectral edge was calculated as the frequency below which 90% of the intensity was present.21 For data presentation, total EEG power was normalized by log transformation (dB, 20 × log intensity). Cortical impedance was measured as an index of cytotoxic edema.22 The nuchal EMG signal was band pass filtered between 100 Hz and 1 kHz, the signal was then integrated using a time constant of 1 s and digitized at 512 Hz.
Experimental protocol
Experiments were conducted between 103 and 107 days of gestation. Fetal MAP, FBF, CaBF and FHR, EEG, cortical impedance, and nuchal EMG were recorded continuously from 48 h before umbilical cord occlusion, until 72 h after occlusion. Fetal asphyxia was induced at 10 a.m. by rapid, complete inflation of the umbilical cord occluder for 25 min. Successful occlusion was confirmed by the rapid onset of bradycardia, a rise in MAP and changes in pH and blood gas measurements. Samples of fetal arterial blood were collected at 60 min before occlusion and 1, 2, 4, 6, 24, 48, and 72 h after the end of occlusion for pre-ductal pH, blood gas (ABL 800, Radiometer, Copenhagen, Denmark) glucose and lactate measurements (model 2300, YSI, OH, USA). Fetal serum magnesium levels were measured at the baseline (1 h before the start of the i.v. infusion), 1 h before asphyxia (i.e. 24 h after starting infusion) and 1, 2, 4, 6, 24, 48, and 72 h after asphyxia (Roche/Hitachi 902 clinical chemistry analyzer, Hoffman-La Roche, Basel, Switzerland). At the end of the experiment, ewes and fetuses were killed by an overdose of pentobarbitone sodium to the ewe (9 g, Pentobarb 300; Chemstock International, Christchurch, New Zealand).
Histopathology
At post mortem, three days after umbilical cord occlusion, the fetal brains were perfusion fixed in situ with 10% phosphate-buffered formalin. Following removal from the skull, tissue was fixed for a further five days before processing and embedding using a standard paraffin tissue preparation. Brain slices were cut (10 µm thick) using a microtome (Leica Jung RM2035, Leica Microsystems, Albany, New Zealand). The cornu ammonis of the dorsal horn of the anterior hippocampus (CA 1-2, 3, 4 and dentate gyrus) and thalamus were examined on sections taken 17 mm anterior to stereotaxic zero. Brain regions of the forebrain used for analysis included the caudate nucleus and putamen at the level of the mid-striatum, and periventricular and intragyral white matter from sections taken 23 mm anterior to stereotaxic zero. Slides were dewaxed in xylene and rehydrated in decreasing concentrations of ethanol and then washed in 0.1 mol/L phosphate-buffered saline (PBS). Antigen retrieval was performed in citrate buffer using the pressure cooker technique in an antigen retrieval system (EMS Antigen 200 Retriever, Emgrid, Australia). Endogenous peroxidase quenching was performed by incubation in 0.1% H2O2 in methanol. Non-specific antigens were blocked using 3% normal goat serum. The sections were labeled with 1:200 mouse anti-NeuN (Chemicon International, Temelcula, CA, USA), 1:200 rabbit anti-Olig-2 (Chemicon International; a marker of oligodendrocytes at all stages of the lineage),23 1:200 rabbit anti-Iba1 (Abcam, Hamilton, New Zealand), 1:200 mouse anti-GFAP (Abcam), 1:200 rabbit anti-CNPase (Abcam), and 1:200 mouse anti-Ki-67 (Dako, NSW, Australia) overnight, at 4℃. Sections were incubated in biotin-conjugated IgG (1:200, goat anti rabbit or mouse; Vector Laboratories, Burlingame, CA, USA) for 3 h at room temperature. Sections were incubated in ExtrAvidin® (1:200, Sigma Aldrich, Auckland, New Zealand) for 2 h at room temperature and allowed to react with 3,3′-diaminobenzidine tetrahydrochloride (Sigma Aldrich). The reaction was stopped by washing in phosphate-buffered saline prior to being dehydrated and mounted.
Changes in neurons (NeuN), oligodendrocytes (Olig-2 and CNPase), proliferating cells (Ki-67) and astrocytes (GFAP) were quantified by light microscopy at × 20 magnification, while microglia (Iba-1) were quantified at × 40 magnification using a Nikon 80i microscope and NIS elements Br4.0 software (Nikon Instruments Inc., Melville, NY, USA). NeuN-positive cells were counted only if they were morphologically normal; cells displaying condensed or fragmented nuclei were not counted.24 Microglia (Iba-1-positive cells) showing ramified and amoeboid morphology were included in our assessment. The area fraction of GFAP immunoreactivity was determined with a standard intensity threshold using ImageJ software (NIH, Bethesda, MD, USA). Average scores from both hemispheres from two sections were calculated for each region. All analyses were performed by an assessor who was blinded to the treatment groups by independent coding of slides and data files. The proportion of immature/mature oligodendrocytes within the intragyral and periventricular white matter was calculated as the ratio of CNPase to Olig-2 positive cells.
Data analysis and statistics
Off-line physiological data analysis was performed using LabVIEW based customized programs (LabVIEW for Windows, National instruments Inc.). The relative change in nuchal EMG, CaBF, and FBF was calculated as the percentage change from the 12 h baseline period before fetal MgSO4 infusion. Carotid and femoral vascular conductance were calculated as mean blood flow/MAP. The raw EEG was assessed for seizure activity. Seizures were identified visually and defined as sudden repetitive and evolving waveforms in the EEG signal lasting more than 10 s and of an amplitude greater than 20 μV.25 Total seizure burden was calculated as the total duration of seizure activity in minutes during the recovery period.
Statistical analysis was undertaken using SPSS (v22 SPSS, Chicago, IL, USA) and Sigmaplot software (v12 Systat software, San Jose, CA, USA). Between- and within-group comparisons of fetal blood gases, glucose, lactate, serum magnesium levels, and physiological data were performed by two-way repeated measures ANOVA. Physiological data for the baseline and infusion and recovery periods were analyzed as separate time periods. When statistical significance was found between groups or between group and time, post hoc comparisons were made using a Fisher’s Least Significant Difference test. Between groups comparison of neuropathological, data were performed using a two-way ANOVA, followed by the Fisher’s Least Significant Difference post hoc test when significance was found between groups or region and group. If there was an effect of region and group, the effect of group was assessed for each region separately. Mann–Whitney U-tests were used for testing non-parametric data. Physiological and neuropathological data are presented as means ± SEM and means ± SD, respectively. Statistical significance was accepted when P < 0.05.
Results
The baseline period
Before the saline or MgSO4 infusions, baseline MAP, FHR, carotid, and FBFs, EEG activity, nuchal EMG (Figure 1), spectral edge frequency and extradural temperature (Supplementary Figure 1), blood gases and glucose and lactate concentrations (Supplementary table 1) did not differ between groups and were within the normal range by our laboratory standards.
Figure 1.
Mean arterial pressure (MAP), carotid artery blood flow (CaBF), fetal heart rate (FHR), carotid artery vascular conductance (CaVC), EEG power, femoral artery blood flow (FBF), nuchal electromyography (EMG), and femoral artery vascular conductance (FVC) in saline + occlusion (open circles) and MgSO4 + occlusion (closed circles) groups. The gray box indicates the period of fetal i.v. saline/ MgSO4 infusion. Data are hourly means ± SEM. *P < 0.05 versus saline + occlusion.
Physiological changes during baseline MgSO4 infusion before occlusion
In MgSO4-treated fetuses, serum magnesium levels increased to a peak of 1.89 ± 0.08 mmol/L (mean ± SEM) versus 0.88 ± 0.07 mmol/L in saline controls 1 h before occlusion (P < 0.05) and remained elevated compared to saline controls until the end of the experiment (Supplementary table 1). EEG power and nuchal EMG activity were reduced before occlusion in the MgSO4 + occlusion group compared to Saline + occlusion (P < 0.05; Figure 1). There was no effect of MgSO4 infusion on spectral edge frequency, extradural temperature (Supplementary Figure 1), MAP or CaBF (Figure 1). MgSO4 infusion was associated with reduced FHR (Figure 1) and increased FBF and FVC before occlusion (P < 0.05; Figure 1). Blood gases, acid base status, glucose, and lactate levels did not differ between groups (Supplementary table 1).
Umbilical cord occlusion
Cardiovascular, cerebrovascular, and neurophysiological adaptations during asphyxia have been documented in Galinsky et al.26 Briefly, umbilical cord occlusion was associated with profound bradycardia, with initial hypertension, followed by progressive hypotension and central and peripheral hypoperfusion. There were no differences in MAP, FHR, and neurophysiological adaptations between groups. There were no differences between groups in either the nadir of the fall in blood pressure (mean ± SEM; Saline + occlusion: 13.1 ± 1.1 mmHg vs. MgSO4 + occlusion: 12.4 ± 1.0 mmHg) or the time to reach terminal hypotension during umbilical cord occlusion (Saline + occlusion: 23.0 ± 0.8 vs. MgSO4 + occlusion: 23.1 ± 0.8 min).
Physiological changes after umbilical cord occlusion
After umbilical cord occlusion, there was a transient suppression of EEG power, nuchal EMG activity (Figure 1) and spectral edge frequency (Supplementary Figure 1) that did not differ between groups. Additionally, there was a transient increase in MAP and a reduction in CaBF, FBF, and vascular conductance that did not differ between groups (Figure 1; P < 0.05). In the MgSO4 + occlusion group, FHR was reduced during the first 5 h of the recovery period (P < 0.05 vs. Saline + occlusion; Figure 1).
Immediately after asphyxia, MgSO4 treatment did not affect the peak rise in cortical impedance (mean ± SEM; Saline + occlusion: 109 ± 3% vs. MgSO4 + occlusion: 110 ± 3%) and the subsequent recovery of cortical impedance relative to baseline values did not differ between groups (mean cortical impedance during recovery; Saline + occlusion = 99 ± 3 vs. MgSO4 + occlusion: 104 ± 3%, data not shown). Furthermore, there was no effect of MgSO4 treatment on extradural brain temperature during the recovery period (Supplementary Figure 1). In contrast, MgSO4 treatment was associated with a reduction in the number of seizures (82 ± 21 vs. 33 ± 6, P < 0.05) and total seizure burden (107 ± 24 vs. 34 ± 7 min, P < 0.05) after asphyxia (Figure 2). There were no premature deaths in any of the experimental groups. All subjects were studied until the end of the experimental protocol.
Figure 2.

Representative continuous EEG data (1-min averages) from a fetus in the saline + occlusion group (top) shows continued suppression of EEG power after asphyxia (0 h) followed by a large prolonged rise in EEG activity between 10 and 48 h, reflecting electrographic seizures. In the example of an animal that received MgSO4 + occlusion (bottom), a delayed period of discrete seizures occurred between 36 and 48 h, reflecting the reduction in the number of seizures and total seizure burden in the MgSO4 + occlusion group compared to saline + occlusion. The gray box indicates the period of fetal i.v. saline / MgSO4 infusion.
Post-mortem findings
There were no significant differences between groups for body weight, brain weight, and the ratio of males to females (Supplementary table 2).
Histopathology
The number of NeuN-positive cells was reduced in the CA1/2, CA3, CA4 regions of the hippocampus, and the caudate and putamen after umbilical cord occlusion compared to sham occlusion (P < 0.05; Figure 3). There were no significant differences in the number of surviving NeuN-positive cells between the Saline + occlusion and MgSO4 + occlusion groups in all subcortical regions assessed.
Figure 3.
Top: Anti-neuronal nuclei (NeuN) monoclonal antibody cell counts in cornu ammonis (CA) 1–2, 3, and 4, dentate gyrus (DG), thalamus (Thal), caudate nucleus (Caud) and putamen (Put) in sham occlusion (open bars), saline + occlusion (black bars) and MgSO4 + occlusion (grey bars) groups. Data are mean ± SD. *P < 0.05 versus sham occlusion. Bottom: representative photomicrographs showing NeuN staining. Scale bar is 200 µm.
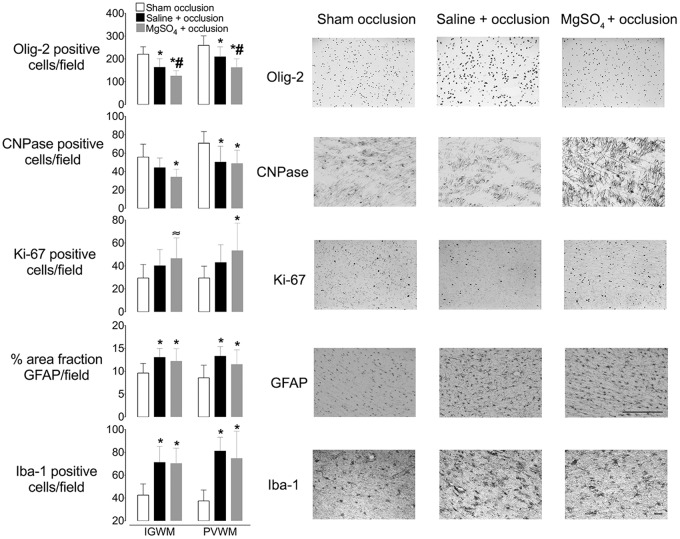
In the intragyral and periventricular white matter tracts, the number of Olig-2 positive cells was reduced in both occlusion groups compared to sham occlusion (P < 0.05; Figure 4). Critically, there were fewer Olig-2 positive cells after MgSO4 + occlusion compared to Saline + occlusion or sham occlusion (P < 0.05). The number of CNPase-positive cells in intragyral white matter was also reduced in the MgSO4 + occlusion, but not Saline + occlusion group, compared to sham occlusion (P < 0.05; Figure 4). In the periventricular white matter, the number of CNPase-positive cells was reduced in both the MgSO4 + occlusion and Saline + occlusion groups compared to sham occlusion (P < 0.05). The percentage of immature and mature (i.e. CNPase + ve / Olig-2 + ve) oligodendrocytes was not different between groups in the intragyral and periventricular white matter tracts (mean ± SD; intragyral: sham occlusion = 26 ± 8%, Saline + occlusion = 29 ± 9%, MgSO4 + occlusion = 28 ± 8%; periventricular: sham occlusion = 28 ± 8%, Saline + occlusion = 27 ± 16%, MgSO4 + occlusion = 33 ± 19%; Supplementary Figure 2).
Figure 4.
Left: Olig-2, CNPase, Ki-67, and Iba-1-positive cell counts and the percentage area of GFAP-positive staining in intragyral and periventricular white matter (IGWM and PVWM, respectively) in sham occlusion (open bars), saline + occlusion (black bars) and MgSO4 + occlusion (grey bars) groups. Data are mean ± SD. *P < 0.05 versus sham occlusion, #P < 0.05 MgSO4 + occlusion versus saline + occlusion,≈P = 0.07 versus sham occlusion. Right: representative photomicrographs showing Olig-2, CNPase, Ki-67, GFAP, and Iba-1 staining in the periventricular white matter tracts. Scale bar is 200 µm. GFAP: Glial fibrillary acidic protein; CNPase: 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase; Iba-1: ionized calcium-binding adapter molecule-1.
The number of Ki-67-positive cells in the white matter tracts was increased overall in the MgSO4 + occlusion group compared to sham occlusion (P < 0.05; Figure 4). Post hoc analysis suggested that the difference was statistically borderline in intragyral white matter (P = 0.07) and significant in periventricular white matter compared to sham occlusion (P < 0.05). The Saline + occlusion group showed intermediate values that were not significantly different from either the sham occlusion or MgSO4 + occlusion groups.
The number of Iba-1-positive cells in the white matter tracts was increased in both occlusion groups compared to sham occlusion (P < 0.05; Figure 4), with no difference between Saline + occlusion and MgSO4 + occlusion. Similarly, the % area fraction of GFAP positive staining in the white matter tracts was increased in both occlusion groups compared to sham occlusion (P < 0.05), with no difference between the Saline + occlusion and MgSO4 + occlusion groups.
Discussion
This study demonstrates that a clinically comparable increase in fetal plasma magnesium concentration was associated with reduced EEG power and fetal movements before asphyxia, and a substantial reduction in overall seizure burden after asphyxia. However, MgSO4 infusion did not improve recovery of EEG power or frequency, or survival of subcortical neurons after three days recovery from acute severe asphyxia in preterm fetal sheep. In contrast, MgSO4 infusion was associated with greater loss of oligodendrocytes.
In the present study, the fetal MgSO4 infusion achieved comparable serum magnesium levels to those observed clinically using current guidelines.27 Magnesium is a key, physiological inhibitor of neural glutamatergic activity.6 We show for the first time that MgSO4 infusion before occlusion was associated with a modest but significant suppression of normal EEG power and then, after asphyxia, a marked reduction in the total seizure burden. Furthermore, MgSO4-induced suppression of fetal electrographic activity was not associated with hypotension. Pragmatically, these data strongly infer that a clinically comparable increase in serum magnesium levels is sufficient to provide a significant central anti-excitatory effect.
During recovery from umbilical cord occlusion, there was sustained reduction of CaBF and EEG power, reflecting actively mediated suppression of cerebral metabolism and activity.28–30 This suppression was not affected by MgSO4 infusion and progressively recovered towards baseline levels after 48–72 h. Suppression of EEG activity in the first few hours after asphyxia is an active process mediated by inhibitory neuromodulators, such as adenosine28 and neurosteroids.31 The present observation that MgSO4 treatment did not modulate this background EEG suppression after asphyxia is consistent with previous data showing no effect of postnatal MgSO4 treatment on amplitude integrated or continuous EEG activity in asphyxiated near term / term neonates.12,32
From approximately 10 h after the insult, we observed large amplitude stereotypical seizures, similar to previous studies.33,34 These stereotypical evolving seizures are preceded by secondary loss of mitochondrial function30 and are strongly associated with adverse neurological outcome.35 The total number of seizures and overall seizure burden were markedly reduced in MgSO4-treated fetuses, strongly supporting a significant central effect on the NMDA receptor.
Pathologically, the pattern of injury in this paradigm of preterm hypoxic-ischemic encephalopathy is consistent with the clinical pattern of moderate-to-severe damage to subcortical neurons, diffuse loss of periventricular white matter, and sparing of the cerebral cortex after acute asphyxia at birth.14 Despite the significant anti-excitatory effects of MgSO4, in the present study MgSO4 was not associated with improved survival of subcortical neurons after severe asphyxia. It is important to appreciate that the present study was powered to detect moderate changes; thus, we cannot exclude the possibility that MgSO4 could be associated with a minor change in neuronal survival. Nevertheless, there was no overall trend to altered neuronal survival after MgSO4 infusion, and in several regions the mean values after MgSO4 infusion were less than in saline controls. Thus, it is improbable that we failed to detect a clinically significant improvement.
These data are consistent with previous findings of no effect of antenatal MgSO4 treatment on neuronal damage after asphyxia in term-equivalent fetal sheep36 and postnatal treatment after hypoxia-ischemia in the piglet.37,38 Furthermore, it is consistent with previous reports that intravenous infusion of the non-competitive NMDA-glutamate receptor antagonist, dizocilpine maleate, during the first 4 or 36 h after asphyxia or cerebral ischemia in preterm and near-term fetal sheep suppressed abnormal brain activity, but was associated with only a small improvement in neuronal survival in some less injured regions.39,40 Collectively, these findings suggest that electrographic seizure activity during the secondary phase of recovery from an asphyxial insult is primarily a marker of cellular injury rather than a major cause of neuronal loss.
Intriguingly, there is both in vivo and in vitro evidence that increasing systemic and extracellular magnesium levels can trigger physiological apoptosis in developing neurons by raising the threshold for action potential generation and causing alterations in sodium channel gating, causing depression of spontaneous neuronal activity.41,42 In contrast, in the present study we did not observe an increase in neuronal loss in MgSO4-treated fetuses. Speculatively, reduced local metabolic stress associated with reduced seizure burden during MgSO4 infusion43 may have offset any adverse effect of magnesium-induced suppression of neuronal activity.
An advantage of studies in the fetal sheep is that the thermal environment is highly stable.44 Consistent with this, in the present study there was no difference in fetal extradural brain temperature between groups. In turn, the lack of neuroprotection with MgSO4 infusion after asphyxia in the present study supports the previous suggestion that apparent protective effects of magnesium in small animal studies of perinatal hypoxic ischemic encephalopathy were confounded by lack of temperature control.11 Moreover, studies in adult rodents have reported that beneficial effects of MgSO4 were associated with confounding mild hypothermia and that maintenance of normothermia abolished neuroprotection, as previously reviewed.11
Asphyxia was associated with a marked loss of oligodendrocytes from the intragyral and periventricular white matter in the present study, similar to previous reports.44,45 Of concern, MgSO4 treatment was associated with significantly greater loss of oligodendrocytes. This reduction in the total number oligodendrocytes was paralleled by an apparent increase in cell proliferation in the MgSO4 + occlusion group compared with occlusion alone. Furthermore, there was no difference in the proportion of immature and mature (CNPase positive) oligodendrocytes between groups, suggesting the reduction in oligodendrocyte number was not unique to a specific stage of development of oligodendrocytes but rather reflects a reduction in oligodendrocytes at all stages of development.
The specific mechanism of this loss is not known. It is well established that NMDA receptors are present on oligodendrocytes and are activated during hypoxia-ischemia.46 Both immature and mature oligodendrocytes exhibit glutamate-evoked currents, which can be inhibited by magnesium.46 Recent advances have shown that neurons send synaptic input to oligodendrocytes residing within cerebral grey and white matter structures.46,47 This form of neuronal-oligodendrocyte signaling may contribute to oligodendrocyte differentiation and stimulation of axonal myelination.48 This suggests that prolonged NMDA-glutamate receptor blockade by magnesium cannot salvage the acute loss of oligodendrocytes after acute severe asphyxia. It is important to appreciate that several studies have shown that recovery from asphyxia is associated with initial loss of immature oligodendrocytes followed by intense proliferation of oligodendrocyte progenitors, resulting in restoration of total cell numbers but impaired lineage maturation and reduced myelination.33,49 The present study examined recovery to three days after asphyxia. In future studies, it will be important to incorporate a longer survival time to enable detailed characterization of the effect of antenatal MgSO4 exposure on cerebral myelination.
There have been suggestions that magnesium’s neuroprotective effects may be associated with inhibition of inflammation, possibly mediated by down regulating nuclear factor kappa B activation.8 It is well established that inflammation is an important extrinsic mechanism involved in the activation of pro-apoptotic pathways involved in the evolution of hypoxic ischemic encephalopathy, as previously reviewed.50 However, the present study demonstrates a similar increase in microglial infiltration and astrogliosis, as measured by the area fraction of GFAP-positive staining, in both occlusion groups compared to the sham occlusion group. These findings strongly suggest that MgSO4 did not suppress post-asphyxial inflammation within the intragyral and periventricular white matter after 72 h of recovery.
In the present study, MgSO4 was associated with a reduction in the fetal biophysical profile, as shown by reduced fetal body movement and heart rate during MgSO4 infusion. This correlated with the period during which peak serum magnesium levels were observed. Supporting this, clinical and preclinical observations have shown that antenatal MgSO4 administration was associated with reduced fetal breathing movements, FHR and FHR accelerations.26,51 This is likely mediated through both neuroinhibition and a direct electrophysiological effect of magnesium on the heart.26,52 Furthermore, MgSO4 administration was associated with peripheral vasodilation, as shown by increased FVC and FBF in the baseline period, although interestingly, not after asphyxia. These data are consistent with previous reports in fetal sheep26 and adult dogs,53 and likely reflect magnesium’s actions as a competitive antagonist of the Ca2 + channel on vascular smooth muscle.54 In contrast, we did not observe an effect of MgSO4 infusion on carotid artery perfusion before or after umbilical cord occlusion. This is consistent with previous reports in preterm fetal sheep26 and humans.55,56 Finally, the occlusion groups tended to be heavier at post-mortem than sham controls, with no apparent effect of magnesium after occlusion. Although we did not measure fetal extracellular volume in this study, this small apparent difference consistent with our previous finding that asphyxia in preterm fetal sheep was associated with significant tissue edema and increased total body weight.57
In conclusion, administration of MgSO4 to preterm fetal sheep suppressed EEG power before asphyxia and markedly reduced electrographic seizure burden after asphyxia, consistent with a significant central anti-excitatory effect. Despite this, MgSO4 treatment did not improve survival of subcortical neurons and was associated with increased loss of oligodendrocytes. These data suggest a possible depressive effect of prolonged MgSO4 administration on developing oligodendroglia that may trigger oligodendrocyte loss during recovery from asphyxia. Based on these data, further careful investigation into the long-term effects of antenatal MgSO4 exposure on the pathogenesis of cerebral white matter injury after asphyxia is essential.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the technical assistance of Mrs Rani Wilson and Mr Vaho Maisashvilli.
Funding
This study was supported by the Health Research Council of New Zealand, the Auckland Medical Research Foundation, the Lottery Health Grants Board of New Zealand, the CJ Martin Postdoctoral Fellowship from the National Health and Medical Research Council of Australia (R.G.; 1090890) and the Victorian Government’s Operational Infrastructure Support Program.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
RG, LB, and AJG conceptualized and designed the study. RG, JD, GW, PD, and CAL undertook experiments and analyzed data. RG and VD undertook immunohistochemistry, cell quantification, analysis, and preparation of figures. LB provided overall oversight of the research. All authors critically reviewed the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Committee on Obstetric Practice and the Society for Maternal-Fetal Medicine. Committee opinion No. 455: magnesium sulfate before anticipated preterm birth for neuroprotection. Obstet Gynecol 2010; 115: 669–671. [DOI] [PubMed] [Google Scholar]
- 2.Magee L, Sawchuck D, Synnes A, et al. SOGC clinical practice guideline. magnesium sulphate for fetal neuroprotection. J Obstetr Gynaecol Canada 2011; 33: 516–529. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009, pp. CD004661. [DOI] [PubMed] [Google Scholar]
- 4.Doyle LW, Anderson PJ, Haslam R, et al. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 2014; 312: 1105–1113. [DOI] [PubMed] [Google Scholar]
- 5.Chollat C, Enser M, Houivet E, et al. School-age outcomes following a randomized controlled trial of magnesium sulfate for neuroprotection of preterm infants. J Pediatr 2014; 165: 398–400 e393. [DOI] [PubMed] [Google Scholar]
- 6.Zeevalk GD, Nicklas WJ. Evidence that the loss of the voltage-dependent Mg2 + block at the N-methyl-D-aspartate receptor underlies receptor activation during inhibition of neuronal metabolism. J Neurochem 1992; 59: 1211–1220. [DOI] [PubMed] [Google Scholar]
- 7.Maulik D, Zanelli S, Numagami Y, et al. Oxygen free radical generation during in-utero hypoxia in the fetal guinea pig brain: the effects of maturity and of magnesium sulfate administration. Brain Res 1999; 817: 117–122. [DOI] [PubMed] [Google Scholar]
- 8.Sugimoto J, Romani AM, Valentin-Torres AM, et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J Immunol 2012; 188: 6338–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman DJ, Marro PJ, McGowan JE, et al. Protective effect of MgSO4 infusion on nmda receptor binding characteristics during cerebral cortical hypoxia in the newborn piglet. Brain Res 1994; 644: 144–149. [DOI] [PubMed] [Google Scholar]
- 10.Shokry M, Elsedfy GO, Bassiouny MM, et al. Effects of antenatal magnesium sulfate therapy on cerebral and systemic hemodynamics in preterm newborns. Acta Obstet Gynecol Scand 2010; 89: 801–806. [DOI] [PubMed] [Google Scholar]
- 11.Galinsky R, Bennet L, Groenendaal F, et al. Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: a systematic review. Dev Neurosci 2014; 36: 73–82. [DOI] [PubMed] [Google Scholar]
- 12.Levene M, Blennow M, Whitelaw A, et al. Acute effects of two different doses of magnesium sulphate in infants with birth asphyxia. Arch Dis Child Fetal Neonatal Ed 1995; 73: F174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid SM, Dagia CD, Ditchfield MR, et al. An Australian population study of factors associated with MRI patterns in cerebral palsy. Dev Med Child Neurol 2014; 56: 178–184. [DOI] [PubMed] [Google Scholar]
- 14.Barkovich AJ, Sargent SK. Profound asphyxia in the premature infant: imaging findings. AJNR Am J Neuroradiol 1995; 16: 1837–1846. [PMC free article] [PubMed] [Google Scholar]
- 15.Sukhov A, Wu Y, Xing G, et al. Risk factors associated with cerebral palsy in preterm infants. J Matern Fetal Neonatal Med 2012; 25: 53–57. [DOI] [PubMed] [Google Scholar]
- 16.Randolph DA, Nolen TL, Ambalavanan N, et al. Outcomes of extremely low birthweight infants with acidosis at birth. Arch Dis Child Fetal Neonatal Ed 2014; 99: F263–F268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009; 8: 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. J Comp Neurol 1969; 135: 249–262. [DOI] [PubMed] [Google Scholar]
- 19.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magpie Trial Follow-Up Study Collaborative Group. The magpie trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG 2007; 114: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams CE, Gluckman PD. Real-time spectral intensity analysis of the EEG on a common microcomputer. J Neurosci Methods 1990; 32: 9–13. [DOI] [PubMed] [Google Scholar]
- 22.Williams CE, Gunn A, Gluckman PD. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991; 22: 516–521. [DOI] [PubMed] [Google Scholar]
- 23.Jakovcevski I, Filipovic R, Mo Z, et al. Oligodendrocyte development and the onset of myelination in the human fetal brain. Front Neuroanat 2009; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozo Devoto VM, Chavez JC, Fiszer de Plazas S. Acute hypoxia and programmed cell death in developing CNS: Differential vulnerability of chick optic tectum layers. Neuroscience 2006; 142: 645–653. [DOI] [PubMed] [Google Scholar]
- 25.Scher MS, Hamid MY, Steppe DA, et al. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia 1993; 34: 284–288. [DOI] [PubMed] [Google Scholar]
- 26.Galinsky R, Davidson JO, Drury PP, et al. Magnesium sulphate and cardiovascular and cerebrovascular adaptations to asphyxia in preterm fetal sheep. J Physiol 2016; 594: 1281–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borja-Del-Rosario P, Basu SK, Haberman S, et al. Neonatal serum magnesium concentrations are determined by total maternal dose of magnesium sulfate administered for neuroprotection. J Perinat Med 2014; 42: 207–211. [DOI] [PubMed] [Google Scholar]
- 28.Hunter CJ, Bennet L, Power GG, et al. Key neuroprotective role for endogenous adenosine A1 receptor activation during asphyxia in the fetal sheep. Stroke 2003; 34: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 29.Dean JM, Gunn AJ, Wassink G, et al. Endogenous alpha(2)-adrenergic receptor-mediated neuroprotection after severe hypoxia in preterm fetal sheep. Neuroscience 2006; 142: 615–628. [DOI] [PubMed] [Google Scholar]
- 30.Bennet L, Roelfsema V, Pathipati P, et al. Relationship between evolving epileptiform activity and delayed loss of mitochondrial activity after asphyxia measured by near-infrared spectroscopy in preterm fetal sheep. J Physiol 2006; 572: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirst JJ, Palliser HK, Yates DM, et al. Neurosteroids in the fetus and neonate: potential protective role in compromised pregnancies. Neurochem Int 2008; 52: 602–610. [DOI] [PubMed] [Google Scholar]
- 32.Groenendaal F, Rademaker CM, Toet MC, et al. Effects of magnesium sulphate on amplitude-integrated continuous EEG in asphyxiated term neonates. Acta Paediatr 2002; 91: 1073–1077. [DOI] [PubMed] [Google Scholar]
- 33.Drury PP, Davidson JO, Bennet L, et al. Partial neural protection with prophylactic low-dose melatonin after asphyxia in preterm fetal sheep. J Cereb Blood Flow Metab 2014; 34: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennet L, Dean JM, Wassink G, et al. Differential effects of hypothermia on early and late epileptiform events after severe hypoxia in preterm fetal sheep. J Neurophysiol 2007; 97: 572–578. [DOI] [PubMed] [Google Scholar]
- 35.Glass HC. Neonatal seizures: advances in mechanisms and management. Clin Perinatol 2014; 41: 177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Haan HH, Gunn AJ, Williams CE, et al. Magnesium sulfate therapy during asphyxia in near-term fetal lambs does not compromise the fetus but does not reduce cerebral injury. Am J Obstet Gynecol 1997; 176: 18–27. [DOI] [PubMed] [Google Scholar]
- 37.Penrice J, Amess PN, Punwani S, et al. Magnesium sulfate after transient hypoxia-ischemia fails to prevent delayed cerebral energy failure in the newborn piglet. Pediatr Res 1997; 41: 443–447. [DOI] [PubMed] [Google Scholar]
- 38.Greenwood K, Cox P, Mehmet H, et al. Magnesium sulfate treatment after transient hypoxia-ischemia in the newborn piglet does not protect against cerebral damage. Pediatr Res 2000; 48: 346–350. [DOI] [PubMed] [Google Scholar]
- 39.Dean JM, George SA, Wassink G, et al. Suppression of post hypoxic-ischemic EEG transients with dizocilpine is associated with partial striatal protection in the preterm fetal sheep. Neuropharmacology 2006; 50: 491–503. [DOI] [PubMed] [Google Scholar]
- 40.Tan WK, Williams CE, Gunn AJ, et al. Suppression of postischemic epileptiform activity with MK-801 improves neural outcome in fetal sheep. Ann Neurol 1992; 32: 677–682. [DOI] [PubMed] [Google Scholar]
- 41.Dribben WH, Creeley CE, Wang HH, et al. High dose magnesium sulfate exposure induces apoptotic cell death in the developing neonatal mouse brain. Neonatology 2009; 96: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dribben WH, Eisenman LN, Mennerick S. Magnesium induces neuronal apoptosis by suppressing excitability. Cell Death Dis 2010; 1: e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez H, Hunter CJ, Bennet L, et al. Cerebral oxygenation during post-asphyxial seizures in near-term fetal sheep. J Cereb Blood Flow Metab 2005; 25: 911–918. [DOI] [PubMed] [Google Scholar]
- 44.Bennet L, Roelfsema V, George S, et al. The effect of cerebral hypothermia on white and grey matter injury induced by severe hypoxia in preterm fetal sheep. J Physiol 2007; 578: 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barrett RD, Bennet L, Naylor A, et al. Effect of cerebral hypothermia and asphyxia on the subventricular zone and white matter tracts in preterm fetal sheep. Brain Res 2012; 1469: 35–42. [DOI] [PubMed] [Google Scholar]
- 46.Karadottir R, Cavelier P, Bergersen LH, et al. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005; 438: 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci 2007; 10: 311–320. [DOI] [PubMed] [Google Scholar]
- 48.Kolodziejczyk K, Saab AS, Nave KA, et al. Why do oligodendrocyte lineage cells express glutamate receptors? F1000 Biol Rep 2010; 2: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buser JR, Maire J, Riddle A, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 2012; 71: 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallard C, Davidson JO, Tan S, et al. Astrocytes and microglia in acute cerebral injury underlying cerebral palsy associated with preterm birth. Pediatr Res 2014; 75: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fujimori K, Ishida T, Yamada J, et al. The effect of magnesium sulfate on the behavioral activities of fetal goats. Obstet Gynecol 2004; 103: 137–142. [DOI] [PubMed] [Google Scholar]
- 52.Kolte D, Vijayaraghavan K, Khera S, et al. Role of magnesium in cardiovascular diseases. Cardiol Rev 2014; 22: 182–192. [DOI] [PubMed] [Google Scholar]
- 53.Nakaigawa Y, Akazawa S, Shimizu R, et al. Effects of magnesium sulphate on the cardiovascular system, coronary circulation and myocardial metabolism in anaesthetized dogs. Br J Anaesth 1997; 79: 363–368. [DOI] [PubMed] [Google Scholar]
- 54.Altura BM, Altura BT, Carella A, et al. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol 1987; 65: 729–745. [DOI] [PubMed] [Google Scholar]
- 55.Twickler DM, McIntire DD, Alexander JM, et al. Effects of magnesium sulfate on preterm fetal cerebral blood flow using Doppler analysis: a randomized controlled trial. Obstet Gynecol 2010; 115: 21–25. [DOI] [PubMed] [Google Scholar]
- 56.Sayin NC, Arda S, Varol FG, et al. The effects of ritodrine and magnesium sulfate on maternal and fetal Doppler blood flow patterns in women with preterm labor. Eur J Obstet Gynecol Reprod Biol 2010; 152: 50–54. [DOI] [PubMed] [Google Scholar]
- 57.Lumbers ER, Gunn AJ, Zhang DY, et al. Nonimmune hydrops fetalis and activation of the renin-angiotensin system after asphyxia in preterm fetal sheep. Am J Physiol Regul Integr Comp Physiol 2001; 280: R1045–R1051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.