Abstract
Recent evidence suggests an extensive exchange of fluid and solutes between the subarachnoid space and the brain interstitium, involving preferential pathways along blood vessels. We studied the anatomical relations between brain vasculature, cerebrospinal fluid compartments, and paravascular spaces in male Wistar rats. A fluorescent tracer was infused into the cisterna magna, without affecting intracranial pressure. Tracer distribution was analyzed using a 3D imaging cryomicrotome, confocal microscopy, and correlative light and electron microscopy. We found a strong 3D colocalization of tracer with major arteries and veins in the subarachnoid space and large cisterns, attributed to relatively large subarachnoid space volumes around the vessels. Confocal imaging confirmed this colocalization and also revealed novel cisternal connections between the subarachnoid space and ventricles. Unlike the vessels in the subarachnoid space, penetrating arteries but not veins were surrounded by tracer. Correlative light and electron microscopy images indicated that this paravascular space was located outside of the endothelial layer in capillaries and just outside of the smooth muscle cells in arteries. In conclusion, the cerebrospinal fluid compartment, consisting of the subarachnoid space, cisterns, ventricles, and para-arteriolar spaces, forms a continuous and extensive network that surrounds and penetrates the rat brain, in which mixing may facilitate exchange between interstitial fluid and cerebrospinal fluid.
Keywords: Cerebrospinal fluid, interstitial fluid, subarachnoid space, paravascular space, glymphatic pathway
Introduction
The cerebrospinal fluid (CSF) is mainly produced by the choroid plexuses and flows from the ventricular system into the subarachnoid space (SAS), from where it leaves the brain via multiple exit pathways. Apart from well-known structures such as the cribriform plate and arachnoid granulations, recently discovered dural lymphatic vessels1,2 may contribute to CSF outflow. Interestingly, work from the Nedergaard group3 suggested that part of the CSF is recirculated along paravascular routes along arteries into the brain, where it mixes with brain interstitial fluid (ISF) and leaves the brain along veins. This mechanism is referred to as the glymphatic pathway and could be important for clearance of the brain, washing out waste products, and toxic substances such as amyloid β. Several features of this pathway, however, are unclear as these are described as a “physically and functionally distinct subcompartment” at the level of the SAS. In addition, the paravascular route seems at variance with the perivascular route described earlier by Carare et al.,4 which describes the drainage of ISF along basement membranes of small vessels and arteries out of the brain. An excellent review by Hladky and Barrand5 recently evaluated the evidence for both views. These authors pointed out several caveats and theoretical concerns for both views, among others related to the anatomical base for proposed transport channels and the presence or not of compartments and barriers for bulk fluid transport around blood vessels. Hladky and Barrand outline several possible routes for fluid drainage, based on the apparently conflicting experimental data indicated above.
The purpose of the current study, therefore, is to better identify the nature of transport routes surrounding leptomeningeal and parenchymal blood vessels. We set out to elucidate the distribution of fluorescent tracers injected into the cisterna magna (CM) of rats, focusing on the spatial relation with the brain vasculature. For this purpose, we imaged the rat brain vasculature and CSF compartments with a combination of three imaging modalities. At the whole brain level, a custom-built automated imaging cryomicrotome was used.6,7 This instrument, based on sequential episcopic fluorescence imaging of the tissue block rather than the individual sections, enables detailed 3D reconstruction of large structures. At the microscopic level, immunostaining and confocal microscopy were used to study paravascular structures and tracer distribution in brain sections. Lastly, correlative light electron microscopy (CLEM) was employed to gain insight into the anatomy of the PVS at the sub-micron level.
Materials and methods
Animals
For this study, 12 Male Wistar Kyoto rats (WKY/NCrl), 4 months old, mean weight 326 ± 2 g, were purchased from Charles River. Rats were housed in groups under a 12 h light-dark cycle and fed ad libitum with standard laboratory food and free access to water. All experimental protocols were approved by the Committee for Animal Experiments of the Academic Medical Center Amsterdam, according to the ARRIVE and the European directive 2010/63/EU.
Anesthesia
During the experiments, rats were anesthetized by intraperitoneal injection of a combination of ketamine (75 mg/kg, Nimatek, Eurovet, Bladen, The Netherlands), dexmedetomidine (0.5 mg/kg, Dexdor, Orion Pharma, Mechelen, Belgium), and atropine (0.05 mg/kg, atropinesulfaat, Eurovet) dissolved in phosphate-buffered saline (PBS, Lonza, Basel, Switzerland). Experiments were carried in the laboratory during the sleeping phase of the rat diurnal rhythm, i.e. during daytime hours.
Reagents
Fluorescein-labeled dextran (500 kD, Ex. 494 nm/Em. 521) tracer, lysine-fixable, was purchased from Molecular Probes-Life Technologies (Eugene, OR, USA). The tracer was dissolved in artificial cerebrospinal fluid (aCSF) at a concentration of 20 mg/ml. For immunofluorescence, before freezing and sectioning, brains were embedded in Tissue-Tek (Sakura, Leiden, The Netherlands). Cell nuclei were stained with Vectashield mounting medium with DAPI (4, 6-diamidino-2-phenylindole, Burlingame, CA, USA). Anti-laminin antibody (Sigma-Aldrich) was used for visualization of the vasculature and pial sheets. To discriminate between veins and arteries, we used an anti-smooth muscle myosin heavy chain 11 antibody (Abcam).
CM infusion
After anesthesia, animals were placed on a heating pad to maintain body temperature at 37℃, as monitored by a rectal thermometer. Additional oxygen was provided to prevent hypoxia. Ocular lubricant ointment (Duratears, Alcon, Breda, The Netherlands) was applied to keep the eyes hydrated. The surgery was performed according to the protocol previously described for mice.8 For the rat, the subcutaneous tissues and two layers of neck muscles were separated along the midline in order to reach the CM. Then, a 29-gauge needle connected to a polyethylene tube was inserted. A total volume of 10 μl of the 500 kD fluorescein dextran (F-500) solution (20 mg/ml) was infused at a controlled flow rate of 0.34 μl/min using a syringe pump (Harvard Apparatus, Holliston, MA, USA) over a 30-min period. At the end of infusion, the animals were euthanized with an overdose of anesthetic and rapidly decapitated.
Intracranial pressure measurement
Two rats were used in this group. To measure intracranial pressure (ICP) during different rates of infusion, a second 29-gauge needle was inserted into the CM. This needle was connected to a pressure transducer by stiff polyethylene tubing to prevent damping of pulsations. Data were recorded with a PowerLab acquisition system and analyzed with Chart data analysis software (AD instruments).
3D cryomicrotome imaging and image processing
In this group, three animals were included to obtain a representative picture of the vasculature and outline of tracer distribution. After infusion of tracer and just before sacrifice of the animals, 100 μl of heparin solution was injected IP to prevent clotting of blood in the vasculature. Immediately after death, animals were flushed for 5 min with PBS via the abdominal aorta to remove the blood from the vasculature before filling. The vasculature was filled with replica material (Batson’s #17, Polysciences) that was fluorescently labeled (UV blue, VasQtec) at 80 mmHg to maintain physiological pressure. After polymerization (45 min), we carefully dissected the brains and stored these at −20℃. Once frozen, the brains were embedded in a black gel (carboxymethylcellulose sodium solvent 5% – Brunschwig Chemie – mixed with Indian ink 5% – Royal Talens) and placed back at −20℃. Using an imaging cryomicrotome, equipped with multispectral power LEDs and selectable filters (custom-built, AMC, Amsterdam, The Netherlands),6,7 the brains were automatically cut into sequential coronal slices of 10 µm thickness. After each cut, 4096 × 4096 pixel en face images of the remaining tissue block were taken at 16 bit gray scale resolution by a digital camera (Apogee Alta U-16) equipped with a variable-focus lens (Nikon 70–180 mm). These images included a white light reflection image and fluorescence images for the 500 kD fluorescein dextran tracer (excitation 480 nm, emission 535 nm) and vascular cast (excitation 365 nm, emission 505 nm). This resulted in co-registered stacks of about 2200 images (per color) with an in-plane resolution of 8 µm. For reconstruction, all images were converted into 8 bit gray scale. The brain was then reconstructed in 3D using Amira (FEI Europe B.V., Eindhoven, Netherlands) to obtain a detailed virtual representation of the brain, vasculature, and tracer distribution.
Immunofluorescence and confocal microscopy
In this set of experiments, three rats were used. After infusion of tracer and sacrifice of the animals, brains were carefully dissected and cut into three coronal blocks using an adult rat brain slicer matrix (Zinc instruments). The blocks were separately embedded in Tissue-Tek, snap-frozen in liquid nitrogen and stored at −80℃. Subsequently, 5 µm coronal sections were cut using a cryostat (Microm HM 560) and then stored at −80℃ until use. Before imaging, sections were fixed in 3.7% paraformaldehyde (PFA) for 30 min and then coverslipped with fluorescent mounting medium (DAPI). To visualize vascular structures and pial sheets, some sections were stained with anti-laminin. Staining for myosin heavy chain was done to discriminate arteries and veins. A confocal laser scanning microscope (Leica TCS SP8) was used to acquire fluorescent images, with a 10 × objective for overviews and a 20 × objective for details.
CLEM
Four animals were used in this protocol. After infusion of tracer and immediately before sacrifice of the animals, 100 μl of heparin solution was injected IP to prevent blood clotting. Immediately after death, rats were flushed via the abdominal aorta with PBS for 5 min to remove blood from the vasculature before perfusion fixation with PFA (4%). After fixation for 10 min at 80 mmHg, the brains were carefully dissected and cut into several coronal blocks using an adult rat brain slicer matrix (Zinc instruments). The blocks were quickly observed under a fluorescent microscope to confirm the presence of the tracer in PVS, placed in PFA (4%) and fixed for two days. Then, regions of interest were selected for CLEM and incubated in 2% gelatine for 30 min. Then small blocks of 2–3 mm2 were cut and embedded in increasing amounts of gelatine (2, 5, 12% in PBS). Blocks were incubated overnight in 2 M sucrose and snap-frozen on a pin in liquid nitrogen. Semi and ultrathin sections were cut as described by van der Wel et al.9 and semi-thin sections (300–400 nm) were first analyzed using confocal microscopy (Leica SP8), using FITC detection and DAPI for counterstaining of nuclei in the tissue. When vessels positive for FITC were detected, 70–100 nm thin sections were cut, transferred to a 150 mesh cupper grid. For TEM analysis, grids with ultrathin sections were washed and stained using a Uranyl acetate/Tylose mixture and imaged using Tecnai T12 at 120 kV. Combining the Light and Electron Microscopy images was performed at numbered grids (Electron Microscopy Science, Alphabetic Finders) to be able to image the identical vessel by both microscope techniques. In addition, serial sections of blocks of maximal 0.1 × 0.1 mm were imaged first in FM and the next section in TEM. The position of the nuclei stained with DAPI, which is visible in both FM and EM, was used to align and overlay the images using rigid registration.
Results
Impact of infusion rate on ICP
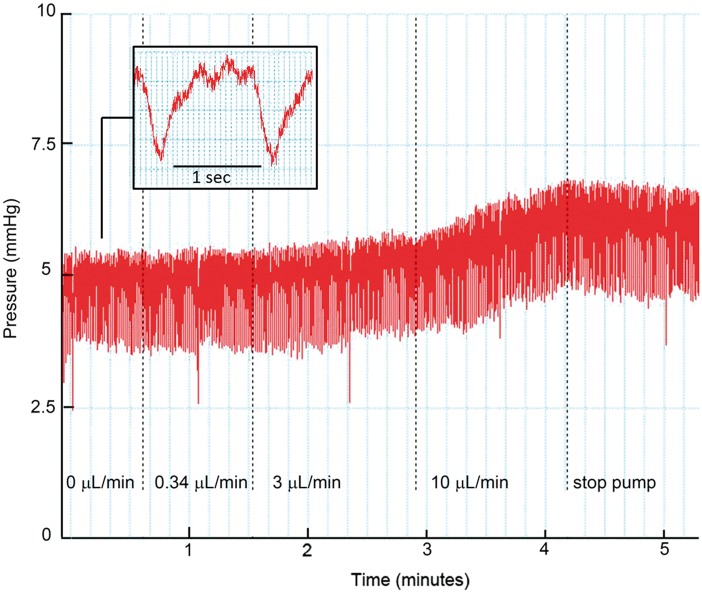
To study the anatomy of the paravascular system and its connection to the CSF compartment, we infused fluorescently labeled dextran as a tracer molecule. We used a high-molecular weight tracer to avoid diffusion of tracer into the parenchyma, which would obscure the anatomical outline of these structures. Distribution of tracers, however, may be affected by the infusion rate. To avoid artifacts due to increased ICP generated by the infusion pump, we measured ICP at different infusion rates into the CM. Before starting infusion, we recorded the baseline physiological ICP, which averaged 5.7 ± 1.3 mmHg (SEM, n = 2). Figure 1 shows a tracing of the pressure profile in the CM. The pressure profile was found to be highly oscillatory, which was attributable to the heartbeat and respiration. Herein, respiration had the larger effect (see insert). The infusion rate used in the current manuscript (0.34 µl/min) did not increase ICP. In the example shown in Figure 1, ICP slightly increased, from 4.8 to 5.1 mmHg, when the infusion rate was raised up to 3 µl/min. More obvious changes in ICP were observed when the infusion rate was increased up to 10 µl/min. After stopping the pump, ICP decreased gradually.
Figure 1.
Changes in ICP during infusion of aCSF in the cisterna magna. Pressure (mmHg) as a function of time (min). The dotted lines indicate a stepwise increase in infusion rate (µl/min). At baseline (0 µl/min), the mean ICP was 4.8 mmHg in this example. An infusion rate of 0.34 µl/min did not change the ICP, while a further stepwise increase in infusion rate to 10 µl/min clearly raised ICP. Insert: the pressure profile was affected by the heartbeat, but more importantly, by respiration. Three heartbeats are visible between two large pressure drops induced by inspiration.
3D analysis of rat brain vasculature and tracer distribution
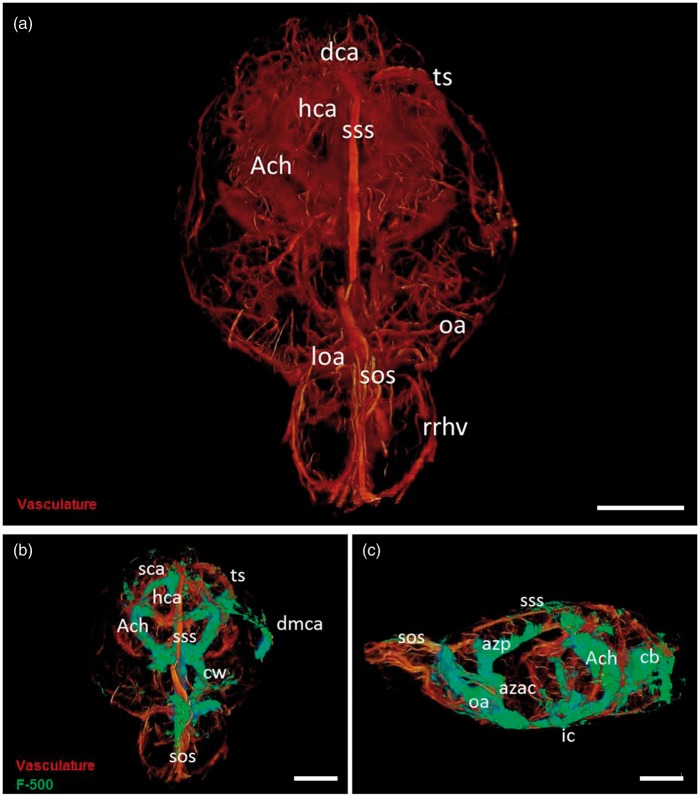
To obtain a combined 3D representation of the vasculature and CSF compartments of the whole brain, we first evaluated vascular and CSF tracer distribution at 8 µm resolution with an imaging cryomicrotome. Figure 2(a) shows the major arteries, including the circle of Willis, hippocampal arteries, dorsocerebellar arteries, anterior choroidal arteries, as well as the major veins such as the transverse sinus, superior sagittal sinus, and superior olfactory sinus. Tracer signal was strong at the base of the brain around the circle of Willis, and in several of the large cisterns (Figure 2(b) and(c)). The tracer was particularly present in the ambient cistern, where it co-localized with the anterior choroidal arteries. Tracer was also observed along the dorsal middle cerebral artery, as well as around the azygos anterior cerebral artery, and olfactory arteries. Moreover, the tracer was found around the superior sagittal sinus, the superior olfactory sinus, and to some extent around the transverse sinus. Thus, tracer spreading was evident around all major vessels supplying and draining the brain. Anatomically, these vessels are embedded in the SAS, after which some follow the major cisterns to deeper brain parts (striatum, hippocampus).
Figure 3.
Tracer distribution over the SAS and cisterns of the rat brain. Sections were stained for laminin (pink) and cell nuclei (blue). Tracer is shown in green. Panel A: Coronal section of a rat brain at approximately 5.6 mm posterior to the bregma showing some of the major cerebral cisterns. In the left upper corner a miniature of the whole section. Tracer was present along the cleft (*) between the hippocampus and the medial geniculate nucleus (MG). This space connected the interpeduncular cistern (IPCi) at the base of the brain, with the ambient cistern (ACi) and the quadrigeminal cistern (QCi). Arteries and veins followed the outline of these cisterns and were embedded in tracer. Panels B and C: horizontal sections of the rat brain at approximately −7 mm from the bregma. The stars show the presence of an extension of the interpeduncular cistern that connects the SAS with the lateral ventricle (LV). The tracer was observed along this tract, which followed the outline of the hippocampus (Hi) up to the lateral ventricle. Scale bar represents 1 mm.
Figure 2.
3D visualization of the rat cerebral vasculature and CSF tracer distribution after infusion in the cisterna magna. Panel A: 3D reconstruction of the rat brain vasculature. Vessels were filled with fluorescent cast material (orange). Panel B: Frontal view of the rat brain. Tracer (F500, green) is visible around the circle of Willis (cw), and the anterior choroidal arteries (ach); along the dorsal portion of the middle cerebral artery (dmca), in the region of the hippocampal arteries (hca), and along the major sinuses-transverse sinus (ts) superior sagittal sinus (sss), and superior olfactory sinus (sos). Panel C: Lateral view of the rat brain. Tracer is present around the azygos anterior cerebral artery (azac) that dorsally becomes the azygos pericallosal artey (azp). Signal was present also caudally along the vasculature of the cerebellum (cb) and the around the superior sagittal sinus in the SAS. Scale bar represents 5 mm.
A 3D representation of the vasculature and tracer distribution is provided in the supplemental movie. This movie shows that the SAS, cisterns, and spaces surrounding the major arteries and veins form a functional continuum without barriers.
Continuity between the SAS, cisterns, and ventricular system
Next, we analyzed the distribution of the CSF tracer by confocal fluorescence imaging on longitudinal and coronal sections of the brain. Sections were stained with an anti-laminin antibody to visualize the arachnoid, pial membranes, and basement membranes of blood vessels. Control sections were negative (data not shown).
The tracer had spread from the infusion site over the SAS. Similar to the 3D approach, tracer signal was strong on the ventral side of the brain. The tracer found its way along the deep clefts between the major brain structures that connect the cerebral cisterns (quadrigeminal, ambience and interpeduncular cistern (Figure 3(a)). Virtually all major arteries were found to be embedded in the SAS and followed these cisterns further into the brain. Thus, the SAS, cisterns, and vessels were part of a continuous compartment filled with CSF. Remarkably, we observed that tracer reached the lateral ventricles (Figure 3(b) and (c)) via one of these cisterns in which the longitudinal hippocampal arteries run. Thus, these images show the presence of a connection between the ventricular system and the SAS via the interpeduncular cistern.
Figure 4.
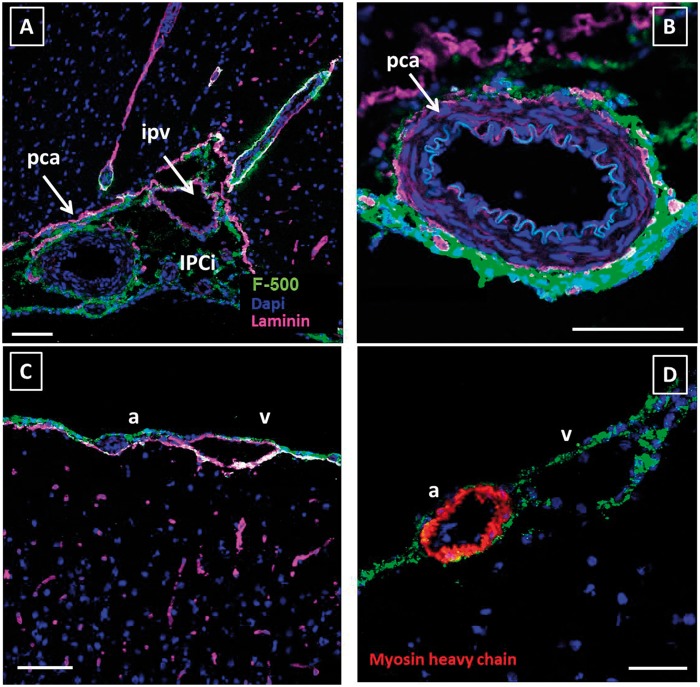
Anatomical details of tracer distribution around blood vessels. Sections were stained for laminin (pink) and cell nuclei (blue). Tracer is shown in green. Panel A shows the interpeduncular cistern (IPCi) filled with CSF, which embedded the posterior cerebral artery (pca) and the intrapeduncular vein (ipv) Panel B: close-up of the posterior cerebral artery. Panel C: Tracer surrounded a leptomeningeal artery (a) and vein (v) but did penetrate along vessels into the parenchyma in this area. Panel D: Myosin heavy chain staining was used to discriminate arteries from veins. Both artery and vein in the SAS showed tracer signal. In all pictures, the tracer is present around the leptomeningeal vessels, in the SAS, and cisterns. Scale bar in panels A–C represents 100 µm, panel D 50 µm.
Paravascular space
After making a general outline of tracer distribution over the brain, we focused on paravascular spaces along parenchymal and leptomeningeal vessels. Figure 4(a) shows the posterior cerebral artery and the interpeduncular vein located in the interpeduncular cistern at the base of the brain. Both artery and vein are embedded in the SAS and bathed by the CSF. There was no visible anatomical boundary between these vessels that would suggest different compartments. Panel B shows the paravascular localization of the tracer around the posterior cerebral artery in detail. A particularly strong signal was observed along the outside of this and other arteries, continuous with the signal along the arachnoid mater.
All vessels in the SAS were surrounded by tracer, indicating direct contact with the CSF in this compartment. Additional staining for myosin heavy chain confirmed that this was the case for both arteries and veins (Figure 4(c) and (d)). As anticipated for this high-molecular weight tracer, it did not cross the pia mater or the PVS to enter the brain tissue.
Selective tracer distribution along arteries in the brain parenchyma
While in the cisterns and SAS both arteries and veins were surrounded by tracer, a different pattern appeared within the parenchyma. While the dorsal and lateral part of the brain showed minimal presence of tracer along penetrating arteries, several vessels at the ventral side of the brain showed strong tracer signal. Here, we observed tracer around the arteries but not the veins (Figure 5(a) and (b)). Tracer surrounding the parenchymal arteries was found to adjoin the smooth muscle cell layer (Figure 5(c) and (d)).
Figure 5.
Paravascular distribution of CSF tracer within the brain parenchyma. In contrast to the cortex, tracer was found to penetrate into the parenchyma along vessels on the ventral side of the brain. Sections were stained for laminin (panel A; pink) or myosin heavy chain (panel B; red) and cell nuclei (blue). Tracer is shown in green. Panels A, B: adjacent slides from the same specimen. Panel A was stained for laminin, while panel B was stained for myosin heavy chain, to discriminate arteries (a) from veins (v). This revealed that tracer signal was confined to arteries only. Panels C and D show a longitudinal section of a small artery. The tracer is present around the artery and is located just outside the SMC layer. EC: endothelial cell; SMC: smooth muscle cell. Panels A and B: scale bar 100 µm; Panels C and D: scale bar 10 µm.
Correlative light and electron microscopic imaging of the paravascular space
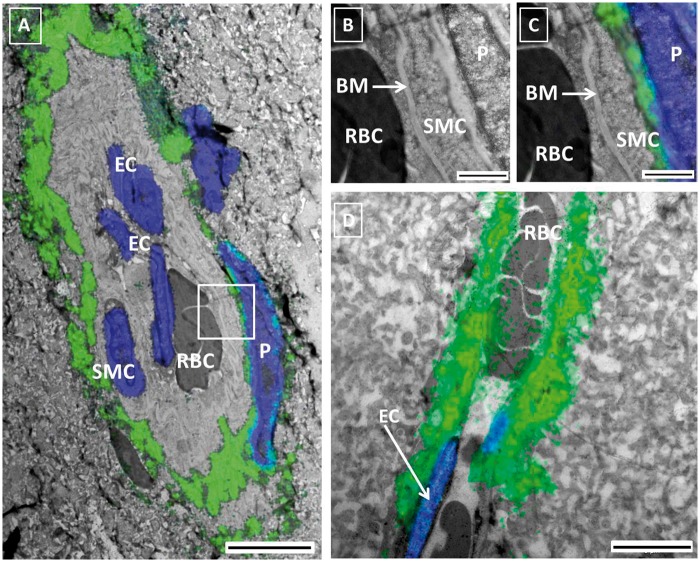
To further study the subcellular distribution of tracer around blood vessels in more detail, we used correlative light and electron microscopy (CLEM). This technique is based on merging of images from two consecutive slices of the same specimen acquired with the two techniques. Figure 6, panel A, shows a parenchymal artery with tracer signal superimposed. The tracer encircled the vessel, just outside the SMC layer. A pericyte was also surrounded by a thin layer of tracer. Panel B shows a detail of the same arteriole, in panel C the fluorescent signal is superimposed. These images show tracer between a smooth muscle cell and a pericyte. The basement membrane (BM) of the smooth muscle cells is well fixed and preserved. When using the Uranyl stained EM preparation technique, membranes will appear white and electron lucent. Indeed, the membranes surrounding the extracellular matrix of the BM are visible (Figure 6(b)). The lumen of the vessel and the first layer of BM did not show tracer. Panel D depicts a parenchymal capillary surrounded by tracer.
Figure 6.
Correlative light Electron Microscopy (CLEM) of the PVS. Panel A: CLEM image of a small artery obtained after merging fluorescent (green tracer, blue nuclei) and EM images of two consecutive slices of the same brain. Red blood cells (RBC) are present in the lumen. Endothelial cells (EC) and smooth muscle cells (SMC) were identified based on location and morphology. The tracer was found in the extracellular space immediately surrounding the smooth muscle layer of the artery and in between the latter and the pericyte (P). Panels B and C are higher magnifications of the vessel wall, without the fluorescent (B) and with the fluorescent (C) signal superimposed. The basement membrane between the EC and the SMC did not present signal. Panel D shows a capillary with tracer immediately below the endothelium. Panel A: scale bar 5 µm Panels B, C: scale bar 1 µm; Panel D: scale bar 5 µm.
Discussion
The most important results from the present study are: (1) the vasculature of the brain has an intricate relationship with the CSF compartment. The main feeding arteries are embedded in the SAS, follow the cisterns and outlines of the major brain structures, and are surrounded by CSF throughout their course; (2) Preferential pathways for CSF transport within the SAS exist around arteries, due to a local widening of the SAS; (3) A novel functional connection between the lateral ventricle and the SAS has been identified; (4) Paravascular spaces within the parenchyma are continuous with and extensions of the CSF compartment, which under low rate tracer injection are mainly observed at the ventral part of the brain; (5) Paravascular spaces are located just outside the endothelial layer at the capillary level and immediately outside of the smooth muscle layer in arteries.
Slow infusion in the CM does not increase ICP
A high rate of tracer infusion may alter the pattern of CSF flow and leave a false impression of tracer penetration along paravascular spaces. We therefore first established that infusion at the chosen low rates did not appreciably alter pressure. Therefore, we are confident that the results obtained in this study are not obscured by the infusion rate. Kress et al.10 observed an increase in ICP of about 2.5 mmHg, using a pump rate of 2 μl/min in mice. These authors reported that the effect persisted during the infusion and upon stopping the pump, the ICP returned to normal values. Such an increase in pressure may seem insignificant, but given the low ICP in rat of approximately 5 mmHg, this represents an increase in pressure of about 50%. It is therefore quite conceivable that such pressures may affect the normal CSF flow and tracer distribution.
One large CSF compartment
In our experiments, we observed that tracer signal was prominent along the ventral side of the rat brain up to the olfactory bulb. Arteries of the circle of Willis were clearly embedded in tracer. This probably reflects the relatively large size of the SAS at these locations. From the base of the brain, tracer spread into the cisterns. The major vessels feeding the brain followed the outline of these structures. It thus appears that these major vessels are embedded in a connected network of cisterns that separate the main anatomical structures of the brain. An initial study in mice pointed at a similar distribution of tracer.8 Thus, also there the CSF tracer followed the outline of the major arteries and cisterns. The large tracer similarly penetrated the brain along arteries, particularly at the base of the brain, but not along veins.
Within the SAS, tracer was visible around both arteries and veins. The lack of paravascular sheets or other structures that would allow physical separation between arteries and veins suggests that CSF surrounding these vessels forms a single compartment. This view seems to contradict the notion of Iliff et al3 who proposed a “physically and functionally distinct subcompartment” within the SAS that surrounds arteries. Their view is based on the observation that 5 min after the infusion, the tracer is present in the PVS only, but in the course of 30 min spread all over the cortical surface. An alternative view that we propose is that tracers appear first around arteries because the PVS is a widening of the SAS. Due to lower resistance, tracers would travel much faster in the wider PVS portion of the same compartment, as compared to the narrower SAS. Thus, based on differences in local resistance, tracer is expected to appear first in the PVS and later in the SAS, when injected in the CM. We therefore propose that at least in rats, the SAS is one compartment, which widens around arteries because of their rigidity and round shape. This creates a functional, rather than a physically separated compartment.
Our data provide evidence for an additional connection between the ventricular system and SAS via the interpeduncular cistern. The importance of this alternative pathway is unclear, and flow along these tracts may be negligible under physiological conditions. However, some studies showed that in an animal model of hydrocephalus with obstructed fourth ventricular outlets, CSF “leaked” from the third ventricle to the quadrigeminal cistern and from the lateral ventricle to the ambient cistern, via ‘compensatory CSF pathways’.11,12 Also, Ghersi-Egea et al.13 described in rat an alternative spread of sucrose infused into the lateral ventricles to the CSF cisterns via the velum interpositum and superior medullary velum. Thus, these and our observations extent current knowledge on the ventricular system and CSF flow with pathways that may become important under conditions where normal outflow is obstructed.
Peri- and paravascular tracts, one and the same?
The exact location, namely along the basement membranes of endothelial cells and smooth muscle cells (i.e. perivascular) or outside the vessel wall (i.e. paravascular), is subject of discussion. Alternatively, two pathways might exist along arteries, carrying flow in opposite directions. Here, we investigated tracer localization after infusion in the CM. The source location of the tracer may be important for the exact pathway and perceived direction of tracer distribution (and thus flow). Thus, in most cases the paravascular compartment described by Iliff et al.3 was identified after injection of tracers in the CM. On the other hand, Weller and Carare4 mostly injected tracers into the parenchyma, from where they describe perivascular outflow along basement membranes of capillaries and arterial smooth muscle cells. Our data show that tracer, injected into the CM, follows the space just outside the layer of smooth muscle cells and further downstream along the basement membrane of capillaries. The basement membranes of endothelial cells and smooth muscle cells were continuous with the extracellular matrix and space surrounding the vessels, in agreement with recent work from Morris et al.14 Thus, a physical separation between the paravascular space and basement membranes was not found. In a previous work, Zhang, et al.15 found a thin layer of pial cells around arteries in the SAS of human brain. Here, we found no evidence for pial sheets along arteries within the tissue using EM. It is therefore possible that species differences and the exact localization play a role here. Taken together, we believe that the most probable anatomical outline for the rat is therefore that one compartment is present, rather than the combination of a peri- and paravascular compartment that would facilitate bulk flow in opposite directions. Not only would two pathways running in opposite directions make little sense physiologically, but the physical separation into compartments that is needed for this scenario was not found. Whether this space should be referred to as peri- or paravascular remains a semantic issue.
Despite the continuity between the paravascular space and the basement membrane of smooth muscle cells and endothelial cells, there was limited penetration into the vessel wall. We anticipate that this relates to the relatively large size of the tracer (500 kD).
CSF flow and solute transport
According to textbook knowledge, CSF flows from the ventricles, where it is produced, via the foramen of Magendie into the SAS from where it is cleared via various outflow paths. We add to this that the spaces surrounding cortical and cisternal vessels provide preferential pathways. While net flow depends on production rate, we found strong pulsatility of ICP, suggesting that CSF velocity patterns are dynamic. Bulk oscillating movement between ventricles and the spinal lumbar space has been observed in MRI.16 Yet, considering the available space, dynamic CSF velocity patterns may also exist along the cortical and cisternal vessels. Respiration was a much larger source for ICP oscillations than cardiac contraction, indicating coupling of intrathoracic pressure and ICP via veins. Additional effects may have come from arterial pulsations related to the aortic pressure. Such pulsations cause a sloshing and mixing of CSF that may help transport of solutes. This dynamic regime around cortical and cisternal vessels may extend into the paravascular space of parenchymal vessels, explaining tracer penetration here without a net forward flow. This would simultaneously allow waste removal from these paravascular spaces.
Limitations
The data provided in the current study rely on tracer distribution that is analyzed by ex-vivo methods. Thus, caution needs to be taken interpreting the data, as tissue dissection and processing may obscure analysis. Here we used a high-molecular weight, fixable tracer to minimize tracer spread after sacrifice of the animal. Brain tissue is notoriously difficult to fix for EM purposes and fixation with aldehydes can cause shrinkage of tissues. Still, since we used perfusion fixation followed by on block fixation (all using EM-grade fixatives), the vessels and the surrounding tissues appear well fixed. The basement membrane and the membranes surrounding it were intact and visible. In addition, as currently most researchers in the field agree that CSF and paravascular flow is dependent on vital functions,17,18 we expect that tracer spreading after sacrifice was limited. Another limitation of the study is that we only used male rats. Future studies should therefore include both genders.
Conclusions
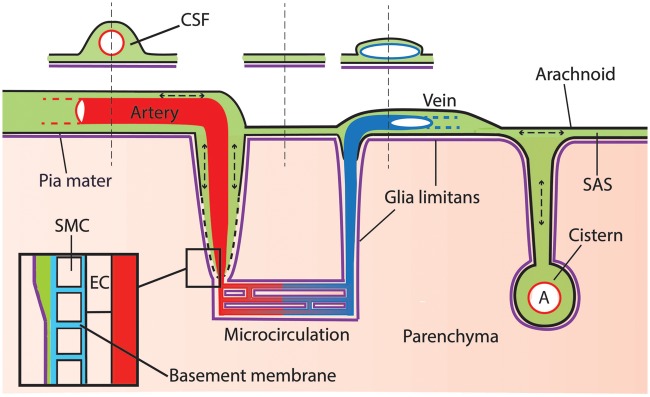
Our results reveal a continuous and extensive CSF compartment, consisting of the SAS, cisterns, and paravascular spaces, embedding the main vasculature of the brain. We found a new connection between the SAS and the ventricular system, the importance of which remains to be established. Paravascular spaces extend from the SAS and cisterns into the parenchyma and provide a possibility for waste removal that could be facilitated by a mixing action generated by pressure pulsations in the CSF (Figure 7).
Figure 7.
Schematic representation of the CSF compartment. Tracer injected in the cisterna magna distributes via the CSF and reaches the SAS and paravascular space, which extents along arteries into the parenchyma. Leptomeningeal vessels are bathed by the CSF. However, the space around artery and vein may be different due to the particular shape of these vessels: bigger around the circular arteries and smaller around the flattened veins (see sketched cross-section). Cerebral cisterns connect to the subarachnoid space and embed major branches of leptomeningeal arteries. Pressure oscillations produced by respiration and heart beat cause mixing within the CSF compartment. Insert: at the level of the microcirculation, the PVS disappears and fuses with the basement membrane of smooth muscle cells, pericytes, and endothelial cells. Green: CSF; red: artery; blue: vein; purple: glia limitans; light blue: basement membrane; pink: parenchyma.
Supplementary Material
Acknowledgements
We thank Duy Ha Ly for his help with the cryomicrotome data.
Funding
This project has received funding from the Internationale Stichting Alzheimer Onderzoek (ISAO) and from the European Union’s Seventh Framework Programme for research technological development and demonstration under Grant agreement no 606998.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
BB, EvB, and EB designed the study and wrote the manuscript. MS critically reviewed the manuscript. BB, EB, JdeV, NNvderW, and HvanV performed the experiments.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 2015; 212: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012; 4: 147ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 2008; 34: 131–144. [DOI] [PubMed] [Google Scholar]
- 5.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 2014; 11: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaan JA, ter Wee R, van Teeffelen JW, et al. Visualisation of intramural coronary vasculature by an imaging cryomicrotome suggests compartmentalisation of myocardial perfusion areas. Med Biol Eng Comput 2005; 43: 431–435. [DOI] [PubMed] [Google Scholar]
- 7.van den Wijngaard JP, Schwarz JC, van Horssen P, et al. 3D Imaging of vascular networks for biophysical modeling of perfusion distribution within the heart. J Biomech 2013; 46: 229–239. [DOI] [PubMed] [Google Scholar]
- 8.Bedussi B, van Lier MG, Bartstra JW, et al. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 2015; 12: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Wel NN, Fluitsma DM, Dascher CC, et al. Subcellular localization of mycobacteria in tissues and detection of lipid antigens in organelles using cryo-techniques for light and electron microscopy. Curr Opin Microbiol 2005; 8: 323–330. [DOI] [PubMed] [Google Scholar]
- 10.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014; 76: 845–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Park YS, Suk JS, et al. Cerebrospinal fluid pathways from cisterns to ventricles in N-butyl cyanoacrylate-induced hydrocephalic rats. J Neurosurg Pediatr 2011; 8: 640–646. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JS, Nam TK, Kwon JT, et al. CSF flow pathways through the ventricle-cistern interfaces in kaolin-induced hydrocephalus rats-laboratory investigation. Childs Nerv Syst 2015; 31: 2277–2281. [DOI] [PubMed] [Google Scholar]
- 13.Ghersi-Egea JF, Finnegan W, Chen JL, et al. Rapid distribution of intraventricularly administered sucrose into cerebrospinal fluid cisterns via subarachnoid velae in rat. Neuroscience 1996; 75: 1271–1288. [DOI] [PubMed] [Google Scholar]
- 14.Morris AW, Carare RO, Schreiber S, et al. The cerebrovascular basement membrane: role in the clearance of beta-amyloid and cerebral amyloid angiopathy. Front Aging Neurosci 2014; 6: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anatomy 1990; 170: 111–123. [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton R, Baldwin K, Fuller J, et al. Intracranial pressure pulse waveform correlates with aqueductal cerebrospinal fluid stroke volume. J Appl Physiol 2012; 113: 1560–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci 2013; 33: 18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker EN, Bacskai BJ, Arbel-Ornath M, et al. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 2016; 36: 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.