Abstract
Three-dimensional Arterial Spin Labeling (ASL) MRI was performed before surgery in a cohort of 146 prospectively enrolled subjects ≥ 70 years old scheduled to undergo elective surgery. We investigated the prospective association between ASL-derived measures of cerebral blood flow (CBF) before surgery with postoperative delirium incidence and severity using whole-brain and globally normalized voxel-wise analysis. We also investigated the cross-sectional association of CBF with patients’ baseline performance on specific neuropsychological tests, and with a composite general cognitive performance measure (GCP). Out of 146 subjects, 32 (22%) developed delirium. We found no significant association between global and voxel-wise CBF with delirium incidence or severity. We found the most significant positive associations between CBF of the posterior cingulate and precuneus and the Hopkins Verbal Learning Test – Revised total score, Visual Search and Attention Test (VSAT) score and the GCP composite. VSAT score was also strongly associated with right parietal lobe CBF. ASL can be employed in a large, well-characterized older cohort to examine associations between CBF and age-related cognitive performance. Although ASL CBF measures in regions previously associated with preclinical Alzheimer’s Disease were correlated with cognition, they were not found to be indicators of baseline pathology that may increase risk for delirium.
Keywords: Cerebral blood flow, delirium, cognition, aging, arterial spin labeling
Introduction
Delirium is an acute confusional state marked by global impairments in cognition and attention.1 Post-operative delirium occurs in 11–53% of hospitalized older patients, yet it is frequently underdiagnosed.1 It conveys significant mortality and morbidity, increasing risk of institutionalization, caregiver burden, cognitive impairment and death.2 The pathogenesis of delirium, however, and the mechanisms underlying its complications and sequelae, remain unclear.
It has been hypothesized that underlying brain damage or degeneration may increase vulnerability to delirium. Dementia, stroke and traumatic brain injury are known risk factors for delirium.3 A number of neuroimaging studies have suggested pre-existing structural and functional abnormalities in patients with delirium.4–13 CBF is an important quantitative physiologic indicator that typically correlates with brain metabolism at rest14 but can also reflect cerebrovascular dysfunction.15 Global and regional CBF abnormalities have been found on brain imaging of patients during delirium16–18 and altered functional connectivity during and after delirium has been reported.19 However, there is a lack of studies examining larger, well-characterized cohorts. In most studies, sample sizes have been small and baseline pre-delirium imaging was not available (see Alsop et al. for a review17). As neuroimaging technology has advanced, techniques have become available to quantitatively characterize CBF abnormalities noninvasively with MRI, and thus it may be useful to investigate their role in delirium.
Arterial spin labeling (ASL) is a non-invasive technique for measuring CBF by using magnetic labeling of endogenous arterial water as a tracer that diffuses into the brain tissue.20 As the tracer circulates and replaces normal tissue water, total brain tissue magnetization changes and thus alters MRI signal intensity. These changes are directly proportional to regional blood flow and can readily be converted into a quantitative measure of CBF.21,22
Our study aimed to address the potential association of presurgical ASL-derived CBF abnormalities with postoperative delirium incidence and severity. Previous studies have documented that cognitive function is the strongest risk factor for delirium,23,24 and have also documented the association between blood flow and cognitive function in the elderly.25,26 Thus, we also explored the cross-sectional association between baseline regional changes in CBF and performance in different cognitive domains in this large, well-characterized cohort of older adults without dementia.
Materials and methods
Study design and cohort assembly
Our study population is a subsample of the Successful AGing after Elective Surgery (SAGES) study, an ongoing prospective cohort study of older adults undergoing major elective surgery. The study design and methods have been described in detail previously.27 In brief, eligible participants were age 70 years and older, English speaking and able to communicate verbally, scheduled to undergo elective surgery at two Harvard-affiliated academic medical centers with an anticipated length of stay of at least three days, and available for in-person follow-up interviews. Eligible surgical procedures were: total hip or knee replacement, lumbar, cervical, or sacral laminectomy, lower extremity arterial bypass surgery, open abdominal aortic aneurysm repair, and open or laparoscopic colectomy. Exclusion criteria included evidence of dementia, delirium, prior hospitalization within three months, legal blindness, severe deafness, terminal condition, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. A total of 566 patients met all eligibility criteria and were enrolled between 18 June 2010 and 8 August 2013.
Approximately one-third of the enrolled SAGES participants were recruited to undergo MRI approximately one month prior to surgery. Additional exclusion criteria for the nested cohort MRI study included contraindications to three Tesla MRI, such as pacemakers, certain stents, and metallic implants. Written informed consent for study participation was obtained from all participants according to procedures approved by the Committee on Clinical Investigations of Beth Israel Deaconess Medical Center and the Partners Human Research Committee of Brigham and Women’s Hospital, the two study hospitals, and the Institutional Review Board of Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts. Procedures and their review were guided by the ethical principles in the Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research.
Baseline Measures
After study enrollment, all participants completed a 75-minute interview in their homes prior to surgery, which included a standardized battery of neuropsychological tests, as well as assessments of demographics and functional status. Demographic information such as age, gender, vascular comorbidities, ethnicity, years of education and type of surgery planned (orthopedic, vascular, gastrointestinal) were collected at this time. During the baseline interview, the Modified Mini-Mental State (3MS) and a complete neuropsychological test battery (described below) were administered, and the Confusion Assessment Method (CAM)28 was rated. MRI scans, as described below, were performed within three months prior to surgery, with most within two weeks.
The neuropsychological test battery was administered to all subjects prior to surgery. The test battery assessed attention, memory, learning, and executive functioning.29 It included the Hopkins Verbal Learning Test – Revised (HVLT-R), Visual Search and Attention Task (VSAT), Trail Making Tests A and B, the Repeatable Battery for the Assessment of Neuropsychological Status Digit Symbol Substitution Test, Category (animal naming) and Phonemic (F-A-S) Fluency Tasks, Digit Spans forward and backward, the 15-item Boston Naming Test, and the Wechsler Test of Adult Reading. All scores were converted to Z-scores (mean zero and standard deviation 1) prior to inclusion in regression analyses. A composite global cognitive performance score (GCP) was derived for each patient with a higher score indicating better cognitive performance. The GCP scores in SAGES were scaled to reflect population-based norms30 with a mean value of 50 and standard deviation of 10. Thus, a score of 40 on the GCP corresponds to cognitive functioning one standard deviation below the U.S. population mean.
Outcome Measures
The primary outcomes of interest were post-operative delirium incidence and severity during the hospital stay. Trained interviewers assessed patients daily during hospitalization for the development of delirium with brief cognitive screening tests and the CAM,28 a standardized method for delirium identification that has high sensitivity, specificity and inter-rater reliability.31 The CAM diagnostic algorithm requires the presence of acute change or fluctuating course, inattention, and either disorganized thinking or an altered level of consciousness to fulfill criteria for delirium. A validated chart review method32,33 was performed to improve detection of all delirium episodes. Delirium severity was measured by the CAM-S Long Form, with scores ranging from 0 to 19 (19 = most severe)34; peak (maximum scores during hospitalization) CAM-S scores were used in our analysis. The CAM-S provides an additive score for delirium severity, has predictive validity for clinical complications of delirium, and was used in our earlier work on global structural measures.35
MRI acquisition
All subjects received neuroimaging at the Beth Israel Deaconess Medical Center Radiology Department on a 3 Tesla HDxt MRI (General Electric Medical Systems, Milwaukee, WI) scanner using a standard 8-channel head coil. All participants completed a standard clinical screening procedure for MRI safety and contraindications before the MRI scan.
The MRI acquisition protocol included:
High-resolution 3D anatomic imaging – A magnetization prepared fast gradient echo (MPRAGE) 3D T1-weighted sequence with a TR of 7.9 ms, a TE of 3.2 ms, a 15° flip angle and 32 kHz bandwidth, a coronal acquisition plane with 24 × 19 cm field of view, 0.94 mm in-plane resolution, 1.4 mm slices, a preparation time of 1100 ms with repeated saturation at the beginning of the SAT period, and an adiabatic inversion pulse 500 ms before imaging.
ASL – A 3D pseudocontinuous ASL sequence was used to acquire whole-brain CBF images.22 This utilized a pseudocontinuous labeling technique and fast spin echo acquisition with interleaved stack of spiral readout and centric ordering in the slice-encoding direction.36 Background suppression was performed using presaturation, four optimally timed inversion pulses, and inferior saturation pulses to suppress late inflowing blood.37 Labeling was performed for 3.5 s with a post-labeling delay of 1.5 s.21 An axial slice thickness of 4 mm and an in-plane resolution of 3.7 mm were selected. Eight spiral interleaves and four averages were acquired. In addition, a reference proton density weighted image was acquired to enable absolute blood flow quantification. The entire ASL acquisition required approximately 8 min, 34 s.
Other sequences collected but not used in the present analyses were fluid attenuated inversion recovery, three-dimensional T2, and diffusion imaging.
MRI analysis
A CBF map for each subject was calculated by dividing the CBF weighted difference images by the reference images followed by appropriate scaling.22,36 CBF maps were then normalized to the standard Montreal Neurological Institute (MNI) space within SPM8 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology) using customized batch scripts implemented in MATLAB (Mathworks, Natick, MA, USA). The structural T1 images were first segmented into different tissue classes of white matter, gray matter, and cerebrospinal fluid using the “New Segment” function of SPM8.38 The resulting segmented gray matter probability map was used as a target for registration of the ASL CBF weighted difference images using the SPM co-registration function. The same registration parameters were then used to realign the CBF map. A brain mask was constructed by addition of the gray matter, white matter, and cerebrospinal fluid probability maps and applied to the CBF map. Spatial transformation to standard space was then achieved by SPM “normalization” of the gray matter map to the gray matter a priori probability map of SPM8 and application of the normalization parameters to the masked CBF map. The CBF maps were also transformed to the standard space but using the “preserve total” option. This option avoids partial correction for brain size or atrophy that can occur by spatial transformation.
Statistical analysis
Image analysis was performed using multiple linear regression models within SPM8. Global brain blood flow values were extracted from image data in standard MNI space using a region defined to contain the entire supratentorial brain. Global brain blood flow was assessed for significant relationship to delirium incidence, delirium severity, each of the individual cognitive measures described above and the GCP composite score, both alone and after controlling for age, sex, and vascular comorbidities as in our prior analyses.35 Subjects with missing test scores were excluded from the global analysis. Voxel-wise regression analysis was performed to quantify the same relationships after controlling for age, sex and vascular comorbidities. For compatibility with processing batch scripts, missing z transformed test scores were replaced with zeros. Proportional scaling (division by each subject’s mean brain CBF) was used to remove global effects from the voxel-wise analysis. The global mean for proportional scaling was automatically calculated by the SPM software using an algorithm that includes all brain regions with CBF above a low threshold that should include gray and white matter. A threshold of p < 0.01 uncorrected for voxel significance and a p < 0.05 cluster threshold after correcting for multiple comparisons were employed. Cluster locations were summarized using the Automatic Anatomic Labeling toolbox.39 All regions with greater than 5% of volume in the cluster and/or representing greater than 5% of the cluster volume were reported.
SAS software version 9.3 and JMP software version 12.0 were used for all non-imaging statistical analyses in this paper. The Student’s t-test or the Mann–Whitney test was used to assess differences in continuous demographic and clinical variables between subjects who developed delirium and subjects who did not. Chi-square or Fisher’s exact tests were used to assess differences in categorical demographic and clinical variables between subjects who developed delirium and subjects who did not, as appropriate.
Results
Demographic and clinical characteristics of the 146 subjects included in this study are summarized in Table 1. Of the 146 subjects, 32 (22%) developed delirium during hospitalization. Delirium was diagnosed in 23 subjects by CAM assessment and in nine subjects by the validated chart review method. No significant differences were found between subjects with and without delirium with regards to age, gender, and 3MS score at baseline (Table 1). Subjects with delirium had lower GCP scores at baseline (55 ± 7 vs. 59 ± 7, p = 0.002, Student’s t-test) (Table 1), and also significantly lower scores for the individual tests including HVLT-R total recall (p < 0.0001), HVLT-R delayed recall (p = 0.0018), Trails B (p = 0.012), and the Boston Naming Test (p = 0.0032). Five individual test scores were missing as detailed in Table 1. Missing values represented less than 0.3% of the scores.
Table 1.
Cohort characteristics.
| All subjects | No delirium | Delirium | P-value | |
|---|---|---|---|---|
| Number of subjects (n, %) | 146 | 114 | 32 | – |
| Age (years, mean ± SD) | 76 ± 4 | 76 ± 5 | 77 ± 4 | 0.467a |
| Female Gender (n, %) | 87 (60%) | 65 (57%) | 22 (69%) | 0.232c |
| Non-white or Hispanic (n, %) | 14 (10%) | 11 (10%) | 3 (9%) | 1.000d |
| Education (years, mean ± SD) | 15 ± 3 | 15 ± 3 | 14 ± 3 | 0.188a |
| Vascular comorbidity (n, %) | 58 (40%) | 45 (29%) | 13 (41%) | 0.906c |
| • Cardiovascular disease | 28 (19%) | 18 (16%) | 10 (31%) | 0.049c |
| • Congestive heart failure | 3 (2%) | 2 (2%) | 1 (3%) | 0.527d |
| • Peripheral vascular disease | 11 (8%) | 8 (7%) | 3 (9%) | 0.706d |
| • Hemiplegia | 0 | – | – | – |
| • Diabetes | 28 (19%) | 20 (18%) | 8 (25%) | 0.344c |
| • Diabetes w/ end organ damage | 5 (3%) | 4 (4%) | 1 (3%) | 1.000d |
| Surgery (n, %) | ||||
| • Orthopedic | 120 (82%) | 92 (81%) | 28 (88%) | |
| • Vascular | 8 (5%) | 7 (6%) | 1 (3%) | 0.782d |
| • Gastrointestinal | 18 (12%) | 15 (13%) | 3 (9%) | |
| Delirium severity (mean ± SD) | ||||
| • CAM-S Long (0–19, 19 severe) | 3.59 ± 2.2 | 2.37 ± 1.6 | 7.94 ± 3.8 | <0.0001b |
| Baseline Cognitive tests (mean ± SD) | ||||
| • 3MS | 26 ± 2 | 26 ± 1 | 26 ± 2 | 0.26b |
| • GCP (externally scaled) | 58 ± 7 | 59 ± 7 | 55 ± 7 | 0.0036b |
| • HVLT-R Total Recall | 22.7 ± 5.2 | 23.6 ± 5.4 | 19.4 ± 4.6 | <0.0001b |
| • HVLT-R Delayed Recall | 7.5 ± 2.7 | 8.0 ± 2.6 | 6.1 ± 3.0 | 0.0018b |
| • HVLT-R Discrimination | 10.1 ± 1.8 | 10.3 ± 1.6 | 9.6 ± 2.2 | 0.14b |
| • Visual Search and Attention Test | 45 ± 9.9 | 46 ± 9.3 | 42 ± 12 | 0.15b |
| • Trails A (s) | 40 ± 16 | 39 ± 14 | 45 ± 22 | 0.069b |
| • Trails B (s)e | 110 ± 54 | 104 ± 49 | 133 ± 66 | 0.012b |
| • Phonemic fluency | 37 ± 12 | 38 ± 12 | 33 ± 11 | 0.11b |
| • Category fluencye | 22 ± 5.9 | 23 ± 6.1 | 21 ± 5.2 | 0.089b |
| • Digit Span – Forward | 10.0 ± 2.3 | 10.2 ± 2.5 | 9.3 ± 1.9 | 0.081b |
| • Digit Span – Backward | 6.3 ± 2.0 | 6.5 ± 2.1 | 5.7 ± 1.7 | 0.057b |
| • Boston Naming Teste | 13.5 ± 2.1 | 13.7 ± 2.1 | 12.8 ± 2.1 | 0.0032b |
| • Digit Symbol Substitutione,f | 37 ± 9.9 | 38 ± 9.6 | 35 ± 11.0 | 0.13b |
| • Wechsler Test of Adult Reading | 37.6 ± 11 | 38.2 ± 11 | 35.5 ± 12 | 0.23b |
Note: P-values refer to group comparison no delirium vs. delirium by (a) Student’s t-test, (b) Mann–Whitney test, (c) Chi-squared test or (d) Fisher’s exact test. (e) One nondelirious subject with missing value. (f) One nondelirious subject with missing value. 3MS, Modified Mini-Mental State Examination; GCP, Global Cognitive Performance; HVLT, Hopkins Visual Learning Test-Revised.
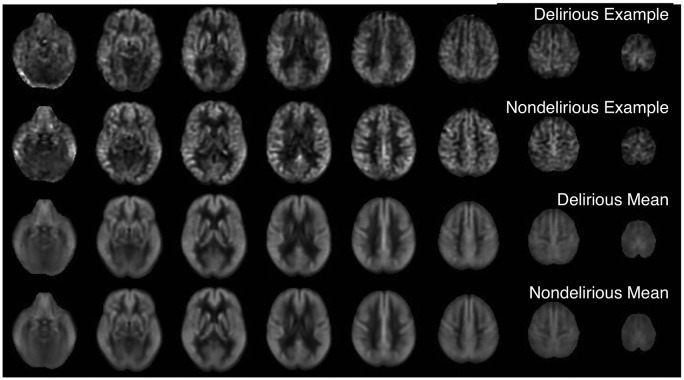
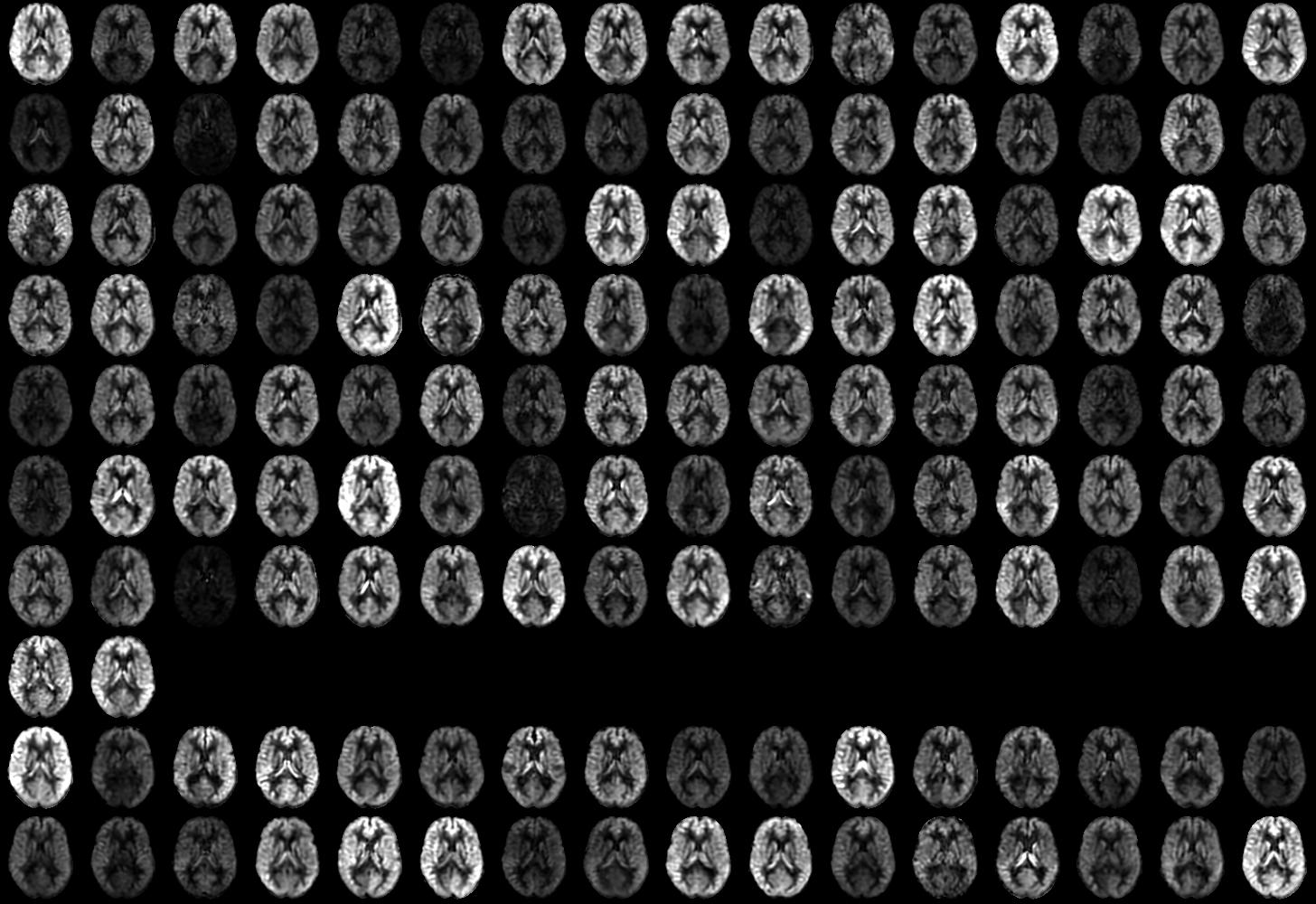
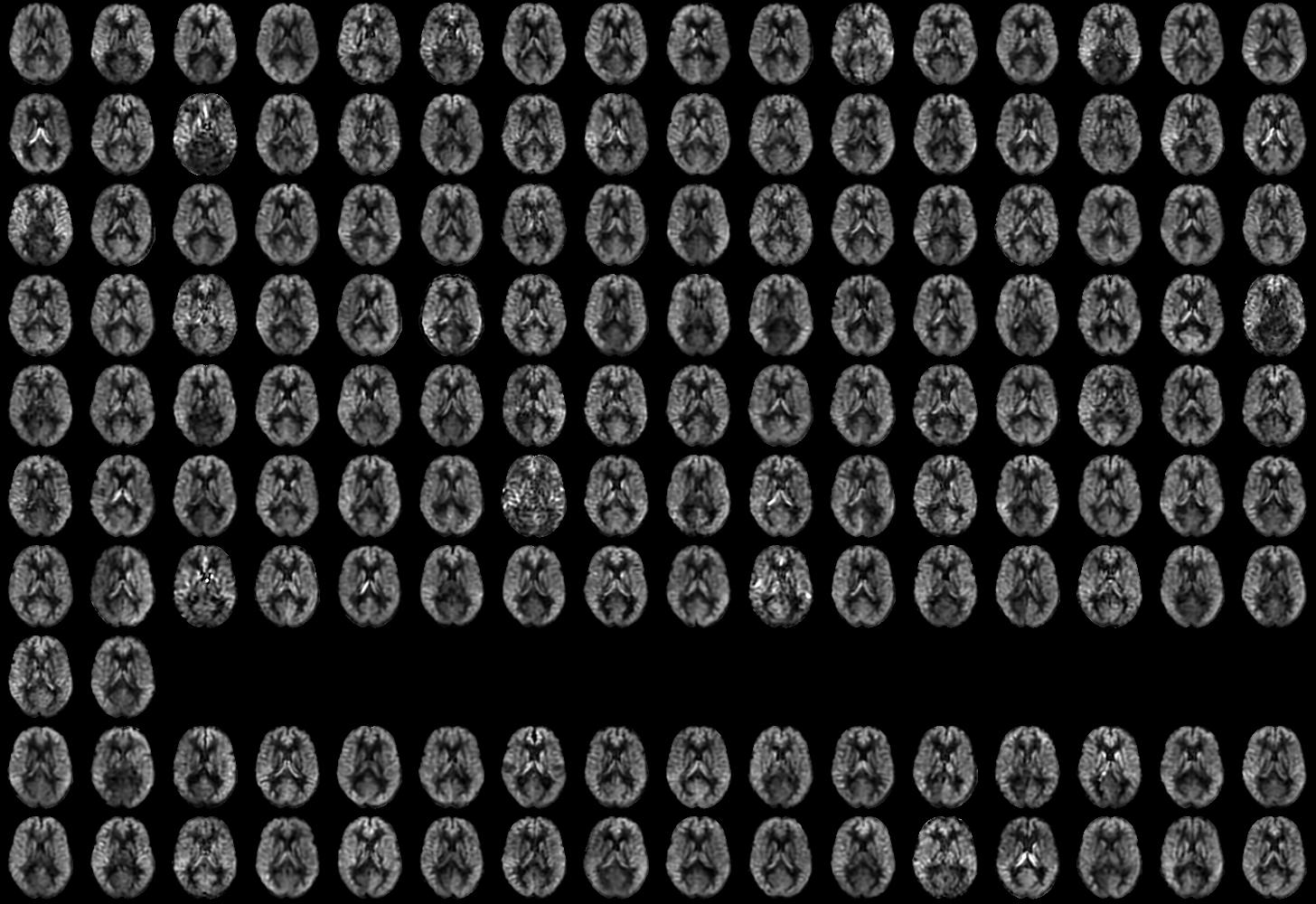
CBF images were generally of good quality and free from motion artifact. Representative images from individual subjects with and without delirium along with group averages are shown in Figure 1. Individual subject images showed considerable variability in global and regional flow (Supplementary Figures S1 and S2). Some subjects with low flow showed signs of bright vascular signal suggesting delayed arrival. Reduced flow in vascular territories was occasionally observed, especially in the posterior circulation. Such variability is greater than that observed with these methods in healthy younger subjects.
Figure 1.
Cerebral blood flow images from representative subjects with and without delirium and group means after transformation to standard MNI space.
We found no significant association between global or regional CBF with delirium incidence or delirium severity. This absence of association was robust with inclusion of other covariates, such as vascular comorbidities and years of education.
Since prior studies have documented that cognitive function is the strongest risk factor for delirium,23,26 and have also documented the association between blood flow and cognitive function in the elderly,25,26 we also investigated the association between cognitive measures and blood flow. Global and regional analysis of blood flow demonstrated significant associations with a number of neuropsychological measures (Table 2, Supplementary Tables 1 and 2). In regression models for test performance with age, sex, vascular comorbidities, and global CBF as dependent variables, CBF was significantly associated with performance on the Trails B and Digit Symbol Substitution tests and close to significant (p = 0.06) for the VSAT test. Age was a significant predictor of GCP, HVLT-R discrimination, and Trails A scores. Women performed significantly better than men on HVLT-R total recall, and both phonemic and category fluency.
Table 2.
Global and regional CBF covariates of cognitive performance.
| Global regression |
Voxel-based regional analysis |
||||
|---|---|---|---|---|---|
| Positive association |
Negative association |
||||
| Cognitive measure | Significance | No. of Voxels | Peak Z score | No. of Voxels | Peak Z score |
| GCP | 0.107 | 4332 | 4.17 | 5971 | 4.3 |
| HVLT-R total recall | 0.956 | 4316 | 5.9 | 8303 | 4.74 |
| HVLT-R delayed recall | 0.543 | 0 | – | 3953 | 4.03 |
| HVLT-R discrimination | 0.962 | 6777 | 3.52 | 4511 | 4.35 |
| Visual search and attention | 0.061 | 15,378 | 4.54 | 12,204 | 4.68 |
| Trails A | 0.126 | 1926 | 3.64 | 2186 | 4.11 |
| Trails B | 0.034 | 0 | – | 8632 | 4.49 |
| Phonemic fluency | 0.232 | 0 | – | 0 | – |
| Category fluency | 0.636 | 0 | – | 2465 | 3.57 |
| Digit span forward | 0.681 | 0 | – | 0 | – |
| Digit span backward | 0.645 | 0 | – | 0 | – |
| Boston naming test | 0.837 | 3735 | 3.64 | 0 | – |
| Digit symbol substitution | 0.049 | 5216 | 3.94 | 7993 | 4.67 |
| Wechsler adult reading | 0.694 | 7627 | 3.6 | 0 | – |
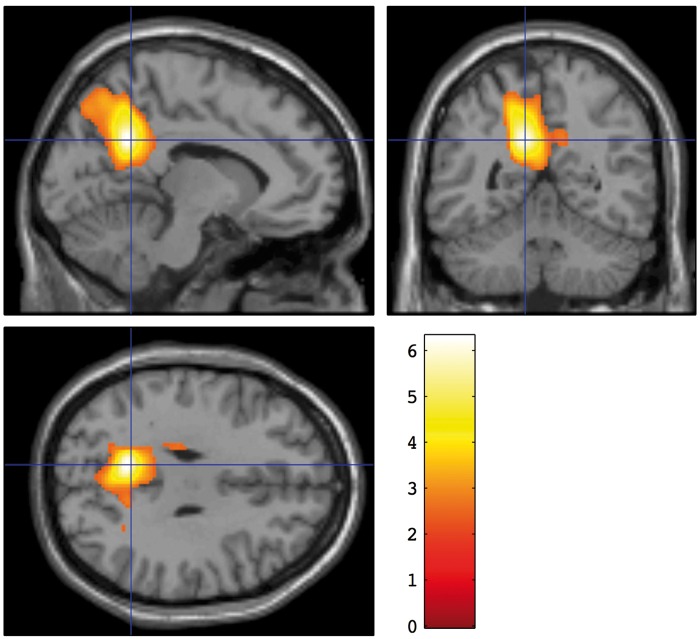
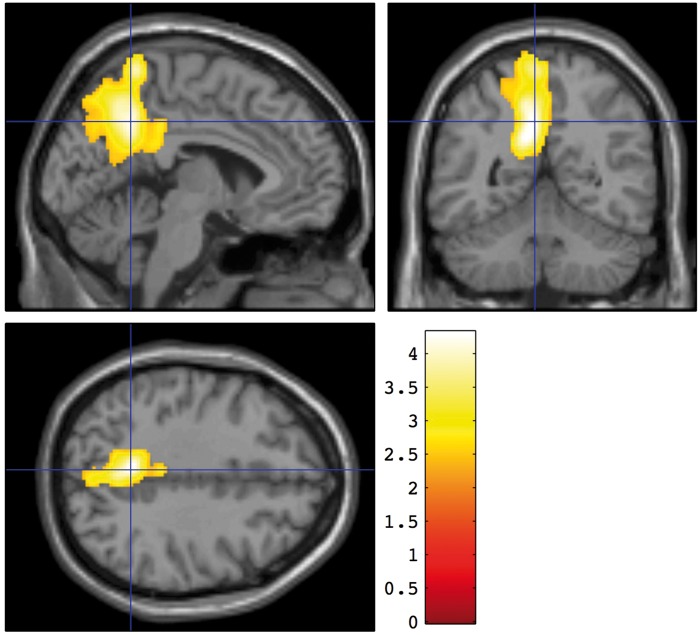
After controlling for global CBF differences by proportional scaling, significant positive association was found between regional blood flow and a number of neuropsychological measures. Two measures of the strength of the association are provided in Table 2. The peak z statistic for the association within the cluster is a measure of the strength of correlation while the number of voxels reflects the spatial extent of the association. The highest peak z statistic was found for the association between the HVLT-R total recall score and blood flow (Figure 2). The association was centered in the posterior cingulate and precuneus region. The regional CBF correlate of the GCP composite score was localized similarly (Figure 3).
Figure 2.
Brain regions with CBF significantly associated with the Hopkins Verbal Learning Test – Revised (HVLT-R) total scores. A voxel threshold of p < 0.01 and a corrected cluster threshold of p < 0.05 were applied. Colors indicate the t statistic for the suprathreshold cluster overlaid on the SPM canonical T1 image.
Figure 3.
Brain regions with CBF significantly associated with the General Cognitive Performance composite measure. A voxel threshold of p < 0.01 and a corrected cluster threshold of p < 0.05 were applied. Colors indicate the t statistic for the suprathreshold cluster overlaid on the SPM canonical T1 image.
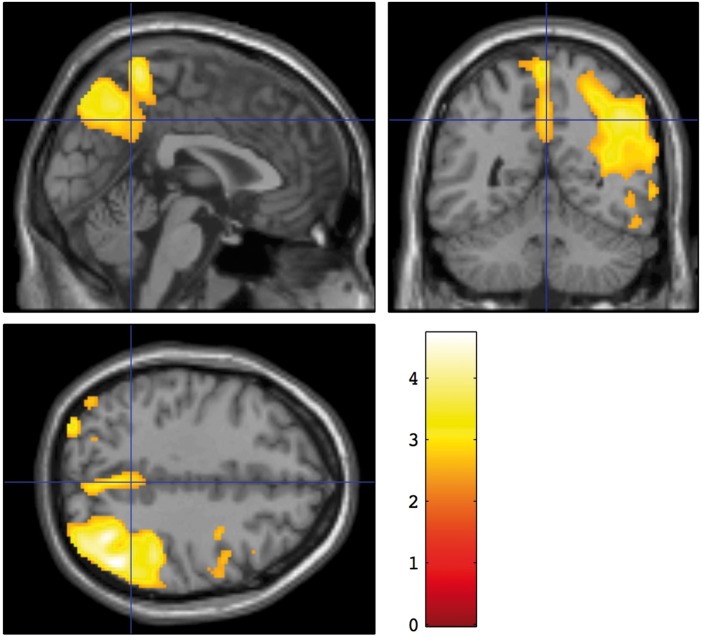
This region was also significantly but more weakly associated with VSAT, Digit Symbol Substitution, the Boston Naming Test, Trails A, Wechsler’s Test of Adult Reading, and the HVLT-R discrimination score. For the VSAT and Digit Symbol Substitution tests, the association extended substantially into the right parietal cortex (Figure 4), resulting in a larger total number of associated voxels.
Figure 4.
Brain regions with CBF significantly associated with the Visual Search and Attention Test scores. A voxel threshold of p < 0.01 and a corrected cluster threshold of p < 0.05 were applied. Colors indicate the t statistic for the suprathreshold cluster overlaid on the SPM canonical T1 image.
Significant negative associations between blood flow and performance were also observed, after controlling for global CBF differences by proportional scaling (Supplementary Table 2). Negative associations in the frontal lobes were found for all 3 HVLT-R scores, VSAT, Digit Symbol Substitution, Category Fluency, and Trails A and B.
Discussion
The primary goal of this study was to investigate the association between ASL-derived measures of brain CBF and delirium incidence and severity, within a prospective study of older subjects undergoing elective surgery, who were cognitively normal and without dementia at baseline. We found no significant association between CBF measures with delirium incidence or severity in both unadjusted and adjusted analyses in our study.
There is minimal previous research on the relationship between pre-surgical brain CBF and delirium.17 Most of the literature has been limited to cardiovascular surgeries, performed in patients with a number of vascular risk factors for cognitive, functional and other complications. Recently, Xu et al.40 found in a retrospective study that cerebral blood flow abnormalities on head CT scans of patients prior to undergoing CABG surgery was associated with increased neurological complications, including delirium. However, delirium incidence in this study was very low (1.5%) and the finding does not refer to delirium alone, but to pooled neurological complications. Thus, our present study contributes an advance in the investigation of the association between pre-operative CBF and post-operative delirium in older persons with normal cognitive aging. Our null findings may suggest that ASL-derived measures of CBF are not sensitive enough to detect brain perfusion-related predictors of delirium or that the pathophysiology leading to delirium is reflected in measures other than CBF. It is known that a number of factors can affect global and local baseline CBF, including mood, alertness, time of day, and arterial pCO2, and variance from these factors may have obscured subtle relationships between baseline CBF and delirium risk. The related factor of vascular reserve, the ability to augment flow in response to challenges, was not examined in the present study and could play a role in delirium risk. Alternatively, relatively unrelated factors such as white matter structure or neuronal connectivity may mediate delirium risk. Indeed, analysis of presurgical diffusion MRI in this same cohort found that diffusivity and fractional anisotropy in white matter were associated with delirium incidence and severity.13
Although the substantial modulations of attention and cognition during delirium are likely to be reflected in CBF, we were unable to scan patients during the delirium episode for this study. Clinical management of delirious patients is often complex and the logistics of scanner scheduling, transport, consent, and controlling motion during imaging are difficult, but smaller functional studies during delirium have been reported.16,19 Interpretation of such studies also requires attention to delayed surgery effects, inter-subject variability, and separation of potentially pathogenic from symptomatic functional alterations. Our study design focused on identifying predictive factors from baseline imaging, but studies during the acute phase of delirium are also certainly worth pursuing.
Given prior studies suggesting associations between cognitive function and delirium,23 we also investigated the cross-sectional association between ASL-derived measures of brain CBF and neuropsychological test performance. Overall, we found that in this cohort of nondemented individuals, CBF globally and regionally was correlated with performance on several neuropsychological tests. Most notably, CBF in the posterior cingulate and precuneus region was associated with most of the tests examined. In addition, performance on the VSAT test was strongly associated with CBF in the right parietal lobe, a region known to be involved in visual attention.41 These two regions have been consistently implicated in CBF and metabolism studies of Alzheimer’s disease (AD) and mild cognitive impairment (MCI) conversion to AD. These findings support the sensitivity of our flow measures to detect age-related cognitive decline. The absence of significant delirium associations of CBF, however, suggests that other factors not reflected in CBF play an important role in delirium risk.
ASL studies of AD42–44 and MCI44,45 have demonstrated a spatial distribution of CBF decrease consistent with studies of CBF using H2O15 positron emission tomography (PET)46 and studies of glucose metabolism using 18F-deoxyglucose (FDG) PET.47 Posterior cingulate and precuneus hypometabolism or hypoperfusion is more prominent in people with MCI that convert to AD within a few years,48 and even in long-term monitoring of normal elderly subjects who progress to AD dementia.49 One recent ASL study examined CBF changes in cognitively intact older adults in a sample of 148 patients. Posterior cingulate perfusion was found to predict subsequent cognitive decline.26 The authors did not report on the cross-sectional association between regional CBF and neuropsychological performance.
Significant negative associations between a number of cognitive measures and CBF in the frontal lobes were also observed in our study. Interpretation of these results requires some caution. If substantial positive associations between global flow and cognitive variables are present, then global proportional scaling can introduce negative correlations in regions that are actually independent of the cognitive variables.50 Such positive associations between global CBF and Trails B, Digit Symbol Substitution and VSAT scores were observed. For other tests, especially the HVLT, negative associations were present despite the absence of global CBF associations. Negative associations suggest an increase in CBF with cognitive impairment. Increases in CBF have been reported in the frontal lobes and other brain regions in ASL studies of AD and MCI,42,45 although such increases are not consistently reported in other studies.
Although the observed regional CBF covariates of cognition are suggestive of preclinical AD pathology, our study design cannot exclude the contributions of other age-related effects, or of intersubject variations in cognition and brain function unrelated to aging. Studies of CBF and metabolism in normal aging51–56 generally emphasize changes in the frontal lobes rather than the posterior regions observed here. Both decreases and, in some regions, increases52 of blood flow with age have been reported. In some reports, however, the precuneus and posterior cingulate regions are among a large number of regions noted to decrease.53,56 High intersubject variability of this region in cognitively normal aging has also been reported.57 Few studies have assessed intersubject variability in CBF and cognition within younger cohorts. One study58 did find a relationship between precuneus CBF and a measure of creative thinking in young adults. Another study used covariance analysis of PET CBF imaging to identify patterns predicting memory test performance in young and older cohorts.59 The precuneus posterior cingulate region was not identified as a significant predictor.
Our results also highlight considerable variability in magnitude and anatomic distribution of ASL CBF in this elderly cohort. Variability of signal in particular arterial territories could represent technical errors in labeling efficiency. Off-resonance errors in pseudo-continuous labeling can reduce efficiency of labeling and consequently of measured CBF in specific arterial territories. Such variability has not been observed in studies of younger, healthier subjects with our methods, however. Alternatively, such ASL CBF variability could reflect the underlying variability of vascular supply in participants of this age.60 Transit time errors secondary to very low CBF in a minority of subjects is also apparent in our data. Such errors can be reduced by longer post-labeling delays or transit time mapping methods. Transit time mapping61 performed in a subset of the subjects has been analyzed for its contribution to reducing CBF variability but the effects were modest.
Limitations to our study include its generalizability as our patient population was from two tertiary care hospitals in the Boston area. The strength of our study lies in the fact that it addresses important questions regarding the feasible role of ASL analysis and CBF in assessing cognitive variability. Our study provides quantitative CBF data on older cognitively normal individuals, as well as their association with cognitive performance. It provides a regional brain map of cerebral blood flow in normal cognitive aging. Our study involves the largest sample size known to date to investigate the relationship between cutting edge MRI and neuropsychological performance and delirium in older adults without dementia. Other strengths of our study include its prospective study design, comprehensive pre-surgical data collection, well-established neuropsychological measures, and robust assessment of delirium within a nested cohort population that is part of the larger SAGES study.27
Although our findings do not suggest the utility of baseline ASL perfusion measures for predicting delirium incidence and severity, we have not yet completed analysis of longitudinal neuroimaging data. MRI perfusion changes may prove useful in long-term monitoring of patients who develop delirium. Furthermore, other neuroimaging analysis techniques such as functional MRI and diffusion tensor imaging may reveal pathophysiological associations with delirium and remain important areas for future research.
Supplementary Material
Acknowledgements
The authors acknowledge the tireless efforts and contributions of the entire SAGES Study Group. The participants are listed in the online supplementary information.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is supported by Grant nos P01AG031720 (SKI) and K07AG041835 (SKI) from the National Institute on Aging and (DCA) by the National Institute of Mental Health (grant no. R01MH080729). Dr Hshieh is supported by a T32 Training Grant (T32AG000158) from the National Institute on Aging. Dr Marcantonio is supported in part by the National Institute on Aging (grant no. K24AG035075). Dr Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct, or reporting of this study.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCA is the inventor on patents related to the pseudocontinuous ASL technique employed for this study and receives postmarket royalties through licenses between his institution and GE Healthcare, Philips Healthcare, and Hitachi Medical.
Authors’ contributions
TTH drafted the manuscript. WD, DZP, ERM, RNJ, TGF, LN, SKI, and DCA designed the protocol. WD, EMS, DZP, ERM, RNJ, YRG, TGT, TGF, SKI, and DCA, acquired the data. TTH, WD, MC, CRGG, DSM, BCD, RNJ, YRG, TGT, TGF, LN, and DCA analyzed the data. All authors contributed to the interpretation of the data and revision of the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014; 383: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010; 304: 443–451. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK. Delirium in older persons. N Engl J Med 2006; 354: 1157–1165. [DOI] [PubMed] [Google Scholar]
- 4.Figiel GS, Coffey CE, Djang WT, et al. Brain magnetic resonance imaging findings in ECT-induced delirium. J Neuropsychiatry Clin Neurosci 1990; 2: 53–58. [DOI] [PubMed] [Google Scholar]
- 5.Hatano Y, Narumoto J, Shibata K, et al. White-matter hyperintensities predict delirium after cardiac surgery. Am J Geriatr Psychiatr 2013; 21: 938–945. [DOI] [PubMed] [Google Scholar]
- 6.Hufschmidt A, Shabarin V. Diagnostic yield of cerebral imaging in patients with acute confusion. Acta Neurol Scand 2008; 118: 245–250. [DOI] [PubMed] [Google Scholar]
- 7.Hughes CG, Patel MB, Pandharipande PP. Pathophysiology of acute brain dysfunction: what's the cause of all this confusion? Curr Opin Crit Care 2012; 18: 518–526. [DOI] [PubMed] [Google Scholar]
- 8.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med 2012; 40: 2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otomo S, Maekawa K, Goto T, et al. Pre-existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2013; 17: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Root JC, Pryor KO, Downey R, et al. Association of pre-operative brain pathology with post-operative delirium in a cohort of non-small cell lung cancer patients undergoing surgical resection. Psychooncology 2013; 22: 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shioiri A, Kurumaji A, Takeuchi T, et al. White matter abnormalities as a risk factor for postoperative delirium revealed by diffusion tensor imaging. Am J Geriatr Psychiatr 2010; 18: 743–753. [DOI] [PubMed] [Google Scholar]
- 12.Soiza RL, Sharma V, Ferguson K, et al. Neuroimaging studies of delirium: a systematic review. J Psychosom Res 2008; 65: 239–248. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari M, Dai W, Guttmann CRG, et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain 2016; 139: 1282–1294. [DOI] [PMC free article] [PubMed]
- 14.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A 1986; 83: 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detre JA, Alsop DC, Vives LR, et al. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology 1998; 50: 633–641. [DOI] [PubMed] [Google Scholar]
- 16.Fong TG, Bogardus ST, Jr., Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci 2006; 61: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 17.Alsop DC, Fearing MA, Johnson K, et al. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci 2006; 61: 1287–1293. [DOI] [PubMed] [Google Scholar]
- 18.Yokota H, Ogawa S, Kurokawa A, et al. Regional cerebral blood flow in delirium patients. Psychiatr Clin Neurosci 2003; 57: 337–339. [DOI] [PubMed] [Google Scholar]
- 19.Choi SH, Lee H, Chung TS, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry 2012; 169: 498–507. [DOI] [PubMed] [Google Scholar]
- 20.Williams DS, Detre JA, Leigh JS, et al. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A 1992; 89: 212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsop DC, Detre JA. Reduced transit-time sensitivity in noninvasive magnetic resonance imaging of human cerebral blood flow. J Cereb Blood Flow Metab 1996; 16: 1236–1249. [DOI] [PubMed] [Google Scholar]
- 22.Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 2015; 73: 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fong TG, Hshieh TT, Wong B, et al. Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc 2015; 63: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Meenen LC, van Meenen DM, de Rooij SE, et al. Risk prediction models for postoperative delirium: a systematic review and meta-analysis. J Am Geriatr Soc 2014; 62: 2383–2390. [DOI] [PubMed] [Google Scholar]
- 25.Beason-Held LL, Goh JO, An Y, et al. Changes in brain function occur years before the onset of cognitive impairment. J Neurosci 2013; 33: 18008–18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xekardaki A, Rodriguez C, Montandon ML, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology 2015; 274: 490–499. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 2012; 13: 818 e1–818 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–948. [DOI] [PubMed] [Google Scholar]
- 29.Jones RN, Rudolph JL, Inouye SK, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol 2010; 32: 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross AL, Jones RN, Fong TG, et al. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology 2014; 42: 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei LA, Fearing MA, Sternberg EJ, et al. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 2008; 56: 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inouye SK, Leo-Summers L, Zhang Y, et al. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 2005; 53: 312–318. [DOI] [PubMed] [Google Scholar]
- 33.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc 2014; 62: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014; 160: 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavallari M, Hshieh TT, Guttmann CR, et al. Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging 2015; 36: 2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 2008; 60: 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maleki N, Dai W, Alsop DC. Optimization of background suppression for arterial spin labeling perfusion imaging. MAGMA 2012; 25: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 39.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 40.Xu B, Qiao Q, Chen M, et al. Relationship between neurological complications, cerebrovascular and cerebral perfusion following off-pump coronary artery bypass grafting. Neurol Res 2015; 37: 421–426. [DOI] [PubMed] [Google Scholar]
- 41.Corbetta M, Shulman GL, Miezin FM, et al. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science 1995; 270: 802–805. [DOI] [PubMed] [Google Scholar]
- 42.Alsop DC, Casement M, de Bazelaire C, et al. Hippocampal hyperperfusion in Alzheimer's disease. Neuroimage 2008; 42: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000; 47: 93–100. [PubMed] [Google Scholar]
- 44.Johnson NA, Jahng GH, Weiner MW, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 2005; 234: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 2009; 250: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishii K, Sasaki M, Yamaji S, et al. Demonstration of decreased posterior cingulate perfusion in mild Alzheimer's disease by means of H215O positron emission tomography. Eur J Nucl Med 1997; 24: 670–673. [DOI] [PubMed] [Google Scholar]
- 47.Minoshima S, Giordani B, Berent S, et al. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol 1997; 42: 85–94. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Y, Gu ZX, Wei WS. Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer disease in patients with mild cognitive impairment: a meta-analysis. AJNR Am J Neuroradiol 2009; 30: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer's disease. Eur J Nucl Med Mol Imag 2009; 36: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchert R, Wilke F, Chakrabarti B, et al. Adjusted scaling of FDG positron emission tomography images for statistical evaluation in patients with suspected Alzheimer's disease. J Neuroimaging 2005; 15: 348–355. [DOI] [PubMed] [Google Scholar]
- 51.Beason-Held LL, Kraut MA, Resnick SM. II. Temporal patterns of longitudinal change in aging brain function. Neurobiol Aging 2008; 29: 497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beason-Held LL, Kraut MA, Resnick SM. I. Longitudinal changes in aging brain function. Neurobiol Aging 2008; 29: 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage 2011; 55: 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chetelat G, Landeau B, Salmon E, et al. Relationships between brain metabolism decrease in normal aging and changes in structural and functional connectivity. Neuroimage 2013; 76: 167–177. [DOI] [PubMed] [Google Scholar]
- 55.Lu H, Xu F, Rodrigue KM, Kennedy KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 2011; 21: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asllani I, Habeck C, Borogovac A, et al. Separating function from structure in perfusion imaging of the aging brain. Hum Brain Mapp 2009; 30: 2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee C, Lopez OL, Becker JT, et al. Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: implications for imaging of neurodegenerative disease. J Neuroimaging 2009; 19: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi H, Taki Y, Hashizume H, et al. Cerebral blood flow during rest associates with general intelligence and creativity. PLoS One 2011; 6: e25532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siedlecki KL, Habeck CG, Brickman AM, et al. Examining the multifactorial nature of cognitive aging with covariance analysis of positron emission tomography data. J Int Neuropsychol Soc 2009; 15: 973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tegeler CH, Crutchfield K, Katsnelson M, et al. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging 2013; 23: 466–472. [DOI] [PubMed] [Google Scholar]
- 61.Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012; 67: 1252–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials