Abstract
Hypoxic-ischemic encephalopathy is a condition caused by reduced oxygen and cerebral blood flow to the brain resulting in neurological impairments. Effective therapeutic treatments to ameliorate these disabilities are still lacking. We sought to investigate the role of sestrin2, a highly conserved stress-inducible protein, in a neonatal rat hypoxic-ischemic encephalopathy model. Ten-day-old rat pups underwent right common carotid artery ligation followed by 2.5 h hypoxia. At 1 h post hypoxic-ischemic encephalopathy, rats were intranasally administered with recombinant human sestrin2 and sacrificed for brain infarct area measurement, Fluoro-Jade C, immunofluorescence staining, Western blot, and neurological function testing. rh-sestrin2 reduced brain infarct area, brain atrophy, apoptosis, ventricular area enlargement, and improved neurological function. Western blot showed that sestrin2 expression levels were increased after treatment with rh-sestrin2, and sestrin2 exerts neuroprotective effects via activation of the adenosine monophosphate-activated protein kinase pathway which in turn inhibits mammalian target of rapamycin signaling resulting in the attenuation of apoptosis. In conclusions: Sestrin2 plays an important neuroprotective role after hypoxic-ischemic encephalopathy via adenosine monophosphate-activated protein kinase signaling pathway and serves as a negative feedback regulator of mammalian target of rapamycin. Administration of rh-sestrin2 not only reduced infarct area and brain atrophy, but also significantly improved neurological function.
Keywords: Sestrins, mammalian target of rapamycin, adenosine monophosphate-activated protein kinase, apoptosis, neonatal hypoxic-ischemic encephalopathy
Introduction
Hypoxic-ischemic encephalopathy (HIE) is the leading cause of morbidity and mortality in infants affecting two to four out of 1000 full term births and about 60% of premature births.1 HIE results in severe brain damage, caused due to the lack of oxygen and blood supply to the brain, which leads to the development of neurological impairments such as epilepsy, mental retardation, cerebral palsy, seizures, and behavioral difficulties.2–7 Current clinical treatment plans include hypothermia, anticonvulsants, fluid and electrolytes management, and a selection of drugs such as atropine and epinephrine.8 However, effective treatment avenues are still lacking, mainly due to the lack of understanding HIE pathophysiology and the mechanisms involved, thus requiring to examine alternative strategies to either replace or amplify current therapeutic protocols.
Sestrins are a family of stress-inducible proteins that have been shown to be highly conserved among species.9,10 Sestrins are able to maintain homeostasis, help in cellular repair, and eliminate toxic metabolites as a result of various insults.11 In mammals, Sestrins have three isoforms: sestrin1, sestrin2, and sestrin3. Sestrin1, which is also known as p53-activated gene 26, is a gene that responds as a result of stress such as, UV-light and γ-irradiation, and is activated in a p53-dependant manner. Sestrin2, also known as hypoxia-inducible gene 95 (Hi95), is an important member of the family and has been shown to be up regulated by various insults such as DNA damage, oxidative stress, and hypoxia. Sestrin3, the last one in the Sestrin family of proteins is directly activated by Fork head Box O transcriptional factors. Sestrin2 plays a crucial role in antioxidant defenses through regeneration of peroxiredoxins and through regulation of the adenosine monophosphate-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway which controls cells growth and metabolism.
Increasing evidence has shown that sestrins can protect cells by reducing oxidative stress and apoptosis in models of diabetes, cancer, and neurodegenerative disorders, most of which target mTOR as a downstream factor.12–14 In this study, we aim to investigate the neuroprotective effects of sestrin2, and whether the mechanism via which sestrin2 exerts its neuroprotective effects is via activation of AMPK and subsequent inhibition of mTOR signaling pathway in neonatal hypoxic-ischemic injury model. To test this, we divided rat pups into three groups: sham, vehicle, and treatment (recombinant human sestrin2: rh-sestrin2). Three different doses of rh-sestrin2 were administered intranasally in order to determine best dose and outcomes were measured at 24 h and four weeks post HIE using most effective dose. In addition, we investigated whether sestrin2 acts on the AMPK/mTOR pathway. To do that we used selective agonists and antagonists to up regulate or knock down mTOR, AMPK, or sestrin2.
Methods
This report is conducted according to the ARRIVE guidelines.
Animals
All protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University. The animals were cared for in accordance with the Guidelines of the Committee. Sprague Dawley rat mothers, with litters of 10–12 pups, were purchased from Harlan Labs (Livermore, CA). A total of 201 10-day-old unsexed Sprague Dawley rat pups were used.
All experiments are done in a blinded fashion, one investigator (not blinded) assigned animal into different groups and administrated drugs to animals throughout study. For neurobehavioral tests (Righting reflex, Geotaxis, Foot-fault test, Rotarod test, Water maze test): two investigators (blinded to drugs) performed behavioral tests, collected data, and analyzed data. For other experiments, two investigators (blinded to drugs) performed all the procedures such as triphenyltetrazolium chloride (TTC) staining, Western blot, immunofluorescence staining, Fluoro-Jade C, Nissl’s staining, brain weight, and collected and analyzed data.
Neonatal HIE model
The model that is used for this study is the standard neonatal Hypoxia-ischemia model (Rice-Vannucci Model15). Briefly, rat pups were anesthetized with 3% isoflurane and maintained throughout the surgery with 2%. A small midline neck incision on the anterior neck was made with a No. 11 blade surgical knife and common carotid artery was isolated and ligated. After the surgical procedure was completed, the rats were allowed to recover for 1 h on a heated blanket. Thereafter, they were placed in a 500 ml airtight jar partially submerged in a 37℃ water bath. A gas mixture of 8% oxygen and 92% nitrogen was delivered into the jars for 2 h and 30 min. Thereafter, animals were returned to their mothers.
Drug administration
Rat pups were allowed to rest for 1 h on a warm blanket before initiating therapy. rh-sestrin2 (0.3 µg, 1 µg, and 3 µg) (Sigma-Aldrich, USA) or saline was administered intranasally16 at 2 μl per drop every 2 min in alternating nostrils, while pups were under anesthesia. siRNA sestrin2 (300 pmol/μl, mixed by three different rat-derived siRNA with siRNA ID: SASI_Rn02_00247651, SASI_Rn02_00247652, SASI_Rn02_00247645, Sigma-Aldrich, USA) and scramble siRNA (300 pmol/μl) were administered via intracerebral ventricular injection17 at 1.5 mm posterior, 1.5 mm lateral to the bregma and 1.7 mm deep on the ipsilateral hemisphere at 24 h pre HIE. A total of 1 μl of the siRNA was injected per pup over 5 min. AICAR (30 mg/kg, Sigma-Aldrich, USA), dorsomorphin (10 mg/kg, Sigma-Aldrich, USA) and rapamycin (3 mg/kg, Cayman Chemical) were administered intraperitoneally18 at 1 h, 0.5 h, and 0.5 h after HIE respectively.
Infarct area measurements
As previously described,19 animals were anesthetized and euthanized at 24 h post HIE. Brains were then removed and sectioned into 2 mm slices. A solution of 2% 2,3,5-triphenyltetrazolium chloride monohydrate was prepared and brains were immersed in it (Fisher scientific, Waltham, MA, USA) for 5 min, followed by a 10% formaldehyde solution. The infarct area was traced and analyzed by Image J software (NIH).
Western blot
Western Blot was performed as described previously.19 Animals were euthanized at 24 h post HIE. Animals were perfused with ice cold PBS solution (pH 7.4), after which brains were removed and instantly divided into ipsilateral and contralateral cerebrums. Before placing the samples in −80℃ freezer for long-term storage, they were snap frozen in liquid nitrogen.
Before running Western blot, whole-cell lysates were obtained by homogenizing the tissue in RIPA lysis buffer (sc-24948, Santa Cruz Biotechnology, Inc., TX, USA) and further centrifuged at 14,000g at 4℃ for 30 min. The supernatant was collected and aliquoted which was later used for measuring protein concentration by using a detergent compatible assay (Bio-Rad, Dc protein assay). Equal amounts of protein (30 µg) were loaded on a 8%–12% SDS-PAGE gel. Once samples were loaded and electrophoresed, they were transferred to a nitrocellulose membrane (0.2 µm), which was then blocked with 5% non-fat blocking grade milk (Bio-Rad, Hercules, CA, USA) and incubated with primary antibodies with sestrin2 (1:2000), DYDDDDK Tag (1:1000, Cell Signaling Technology, USA), p-mTOR (1:1000, Millipore, USA), p-AMPK (1:1000, Abcam, USA), AMPK (1:1000, Abcam, USA), Bcl2 (1:1000, Cell Signaling Technology, USA), Bax (1:1000, NOVUS Biologicals, USA), caspase3 (1:1000, Cell Signaling Technology, USA), Actin (1:3000, Santa Cruz Biotechnology, USA). The following day, nitrocellulose membranes were incubated with secondary antibodies (1:2000, Santa Cruz Biotechnology, USA) for 1 h at room temperature. Immunoblots were then probed via ECL Plus chemiluminescence reagent kit (American Bioscience, Arlington Heights, IL) and analyzed using Image J (4.0, Media Cybernetics, Silver Springs, MD).
Histological analysis
Pups were anesthetized and transcardially perfused with 0.1 M PBS followed by 4% formaldehyde solution (PFA) at 24 h post HIE. The brains were removed and postfixed (4% PFA, 4℃, 24 h), then transferred into a 30% sucrose solution for two days. The brains were then sectioned at 10 µm thickness with a cryostat (Leica LM3050S) for Fluoro-Jade C, immunofluorescence staining, and 16 µm for Nissl’s staining. Detailed Fluoro-Jade C staining,20 immunofluorescence staining,19 and Nissl’s staining21 are shown as following.
Fluoro-Jade C staining: 10 µm brain sections were cut with cryostat (Leica LM3050S) for Fluoro-Jade C. Slides were immersed in 1% sodium hydroxide in 80% ethanol for 5 min, followed by being rinsed for 2 min in 70% ethanol, then 2 min in distilled water. Slides were incubated in 0.06% potassium permanganate solution for 10 min, after 2 min in distilled water, transferred into 0.0001% solution of Fluoro-Jade C (Millipore, USA) which was dissolved in 0.1% acetic acid. Slides were rinsed with distilled water for 1 min, three times. The water was then drained and slides were dried, finally cleared in xylene for 1 min and cover slipped with DPX (Sigma-Aldrich, USA).
Immunofluorescence staining: The cryoprotected sections were washed with 0.1 M PBS three times for 5 min then incubated in 0.3% Triton X-100 in 0.1 M PBS for half an hour at room temperature. They were then washed with 0.1 M PBS for 5 min, three times and incubated with primary antibodies: sestrin2 (1:50, Proteintech Group, USA), cleaved caspase3 (1:400, Cell Signaling Technology, USA), LC3 A/B (1:400, Cell Signaling Technology, USA), DYDDDDK Tag (1:1000, Cell Signaling Technology, USA), NeuN (1:1000, Abcam, USA), respectively (4℃, overnight). After washing with 0.1 M PBS (5 min, three times), the slides were then incubated with secondary antibodies which are from Santa Cruz Biotechnology: anti-rabbit IgG-TR, anti-mouse IgG-FITC, anti-goat IgG-FITC, anti-rabbit IgG-FITC (1:200) for 1 h under room temperature, then washed again with 0.1 M PBS for 5 min, three times. Finally, slides were covered with DAPI (Vector Laboratories, Inc.). Fluorescent microscope and Magna Fire SP system (Olympus) were used to analyze microphotographs.
Nissl’s staining and evaluation of brain tissue loss and ventricular area: The prepared slides (16 µm) were dehydrated in 95% and 70% ethanol for 1 min, respectively, rinsed in tap water and distilled water for 30 s. Slides were stained with 0.5% cresyl violet (Sigma-Aldrich, USA) for 2 min and washed in distilled water for 10 s and 30 s; 100% ethanol and xylene were used to dehydrate for 1.5 min, two times before a coverslip with permount was placed. Brain tissue loss was measured with Image J.
Evaluation of brain tissue loss and ventricular area
The percentage of brain tissue loss = (contralateral hemisphere – ipsilateral hemisphere)/contralateral hemisphere × 100%. The ratio of ipsilateral ventricular area = (ipsilateral ventricular area/whole brain area) × 100%.
Neurobehavioral tests
The following neurobehavioral tests were performed in a blinded setup at either 24 h or four weeks post HIE, there were two independent researchers involved, one was in charge of recording, the other was in charge of implementing, and both of them were blinded to these neurobehavioral tests. To evaluate short-term neurological function, righting reflex and negative geotaxis tests22 were performed before and 24 h after HIE. To evaluate long-term neurological function, water maze, rotarod, and foot-fault were performed at four weeks post HIE.18
Righting reflex: pups were placed in supine position and the time taken for the pups to flip to prone position was recorded.
Geotaxis: pups were placed head downward onto an inclined board (40°), and the time it took for the pups to rotate to head upward position was recorded; the maximum testing time was 20 s.
Foot-fault test: rats were placed on a horizontal grid floor (square size 20 cm × 40 cm with a mesh size of 4 cm2) elevated 1 m above ground for 1 min. Foot-fault was defined as when the animal inaccurately placed a fore- or hindlimb and fell through one of the openings in the grid. The number of foot-faults for each animal was recorded.
Rotarod test: assessed motor impairment using an accelerating rotarod (Columbus Instruments Rotamex, OH, USA). A total of three rotarod trials were performed and the average duration (in seconds) was recorded and analyzed.
Water maze test: is a learning and memory test that evaluated the ability of rats to learn and remember. This test is set up by submerging a platform in a pool of water and testing the rats’ ability to find the hidden platform using visual cues around the room. It is a four-day test where rats are tested in both cued and hidden tests with all trials lasting no more than 60 s. In the cued test if the rats had not discovered the platform in 60 s they were manually guided to the platform. A video recording system traced all of the animals’ activities and the swim paths were measured for quantification of distance, latency, and swimming speed by the Video Tracking System SMART-2000 (San Diego Instruments Inc., CA).
Statistical analysis
Prism 6 software was used for statistical analysis. All the data were expressed as mean ± SD. Statistical difference between groups were analyzed using one-way ANOVA or two-way ANOVA, followed by Tukey multiple-comparison post hoc analysis or Student–Newman–Keuls test on ranks. A ‘P’ value of 0.5 (p < 0.05) was considered statistically significant. The specific p value was marked in each figure.
Results
Expression levels of endogenous Sestrin2 and AMPK increased in time-dependent manner post HIE
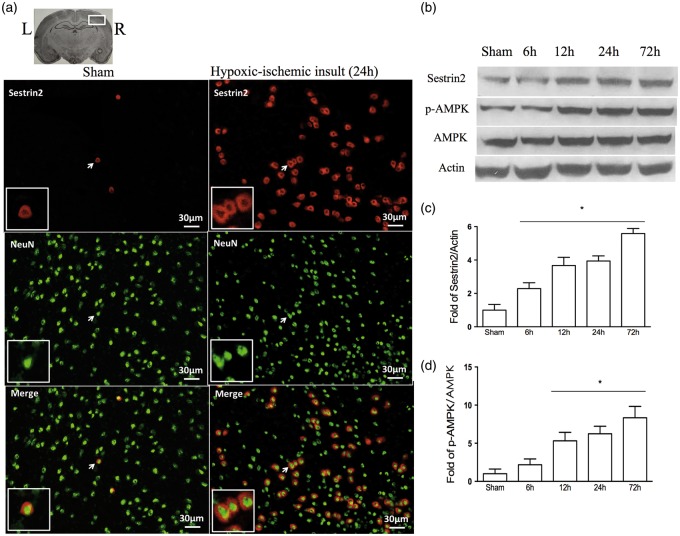
Immunofluorescence staining showed minimal expression of endogenous sestrin2 in neurons in sham-operated animals at 24 h post HIE. However, sestrin2 expression in neurons was significantly increased in vehicle-operated animals at 24 h post HIE (Figure 1(a)). Furthermore, sestrin2 expression levels significantly increased in a time-dependent manner, reaching peak at 72 h post HIE (Figure 1(b) and (c)). The ratio of p-AMPK to AMPK followed a similar expression pattern to sestrin2 (Figure 1(d)).
Figure 1.
Representative picture of endogenous sestrin2 expression in the brain and temporal expression of endogenous sestrin2 and AMPK at 24 h post HIE. (a) Immunofluorescence staining showed a significantly higher expression of sestrin2 on neurons in vehicle-operated animals compared to sham (arrowhead part was amplified. Red staining showed sestrin2 and green was NeuN staining). (b–c) Representative Western blot data showed sestrin2 expression levels significantly increased in a time-dependent manner from 6 h to 72 h reaching peak at 72 h post HIE. (d) Ratio of p-AMPK to AMPK significantly increased from 6 h to 72 h, reaches highest at the 72 h time point. All the data were expressed as mean ± SD. Statistical difference between groups were analyzed using one-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham, n = 1/group in A, n = 6/group in B–D (all sham samples in Western blot were from the same animals which were euthanized after short-term neurobehavioral tests).
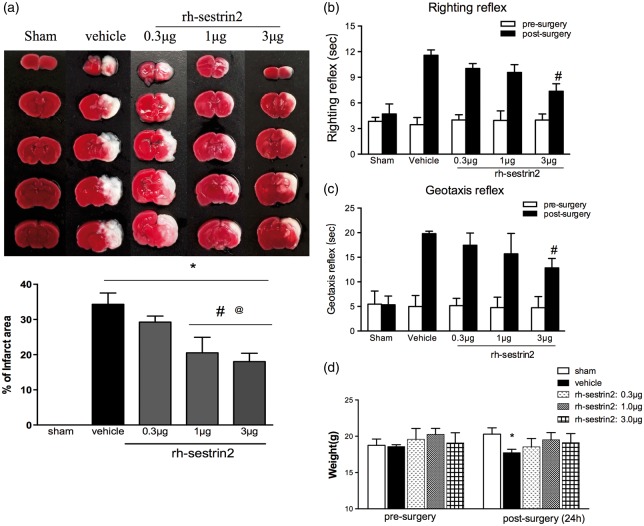
rh-sestrin2 reduced infarct area and improved short-term neurological function at 24 h post HIE
rh-sestrin2 treatment group medium and high dose (1 µg and 3 µg) showed to significantly reduce infarct area in the right hemisphere compared to vehicle-treated group (Figure 2(a)). No significant reduction in infarct area was observed with low treatment dose (0.3 µg). Furthermore, rh-sestrin2-treated group showed no significant weight loss when compared to sham, while vehicle group showed to significantly lose weight (Figure 2(d)).
Figure 2.
Effect of intranasal administration of rh-sestrin2 on brain infarct area (a) and short-term neurological function (b–c) at 24 h post HIE. (a) TTC staining showed that medium (1 µg) and high (3 µg) doses of rh-sestrin2 treatment significantly reduced infarct area when compared to vehicle. (b–c) Righting reflex and Geotaxis reflex showed that high dose (3 µg) of rh-sestrin2 significantly improved neurological function compared to vehicle animals. (d) Animals’ weight change after HIE. Statistical differences between groups were analyzed using one-way or two way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle; @ versus rh-sestrin2 (0.3 µg). n = 6/group.
In order to test the effects of rh-sestrin2 on short-term neurological impairments, righting reflex and geotaxis were performed. In both behavioral tests, vehicle group performed significantly worse compared to sham. In both tests, although low and medium doses of rh-sestrin2 treatment showed a tendency to improve neurological function only, the high dose sestrin2 treatment group (3 µg) reached significance when compared to vehicle (Figure 2(b) and (c)).
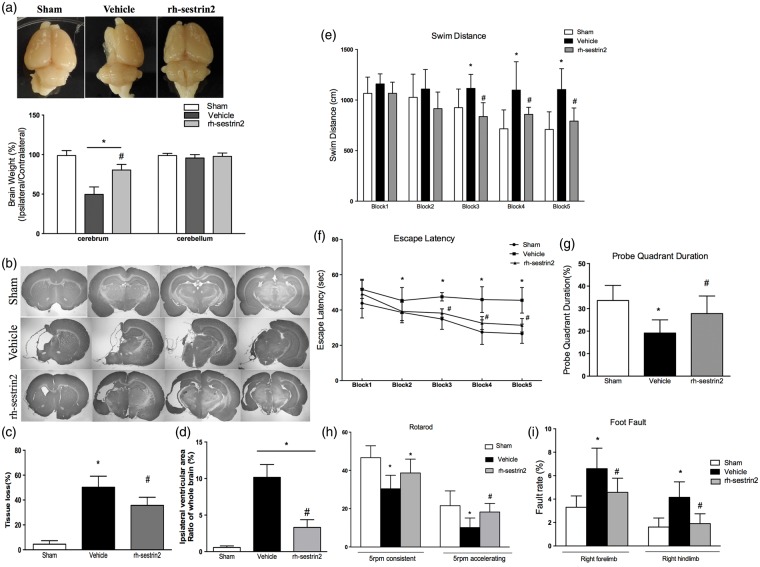
rh-sestrin2 reduced brain atrophy and improved long-term neurological function at four weeks post HIE
rh-sestrin2 treatment showed to significantly reduce brain atrophy of the injured hemisphere caused by hypoxic-ischemic injury (Figure 3(a), p < 0.0001, vehicle vs. rh-sestrin2 treatment) as assessed by measuring brain weights. There was no difference in weight in cerebellum. Nissl’s staining showed that rh-sestrin2 treatment can attenuate brain tissue loss (Figure 3(b) and (c)). In addition, rh-sestrin2 treatment significantly reduced ipsilateral ventricular area at four weeks after hypoxic-ischemic injury when compared to vehicle (Figure 3(d)).
Figure 3.
Effects of rh-sestrin2 on brain atrophy, tissue loss, ventricular area and neurological function at four weeks post HIE. (a) Significant loss of right-to-left cerebrum (ipsilateral/contralateral) weight ratio is evident in vehicle rats, and which was significantly improved by rh-sestrin2 treatment at four weeks post HIE. There was no substantially change in cerebellum in each group. (b) Representative picture of Nissl’s stained brain slices showing tissue loss in ipsilateral hemisphere. (c) rh-sestrin2 treatment significantly reduced the percent of tissue loss when compared to vehicle (Vehicle: 50.33% ± 3.593%, rh-sestrin2 treatment: 35.67% ± 2.679%). (d) Significant reduction in ipsilateral ventricular area in rh-sestrin2 treatment group compared to vehicle. (e) rh-sestrin2 treatment group showed a significant improvement in spatial memory loss in terms of swimming more distance to find the platform, and improved memory and learning compared to vehicle as seen from latency time to escape (f) and probe quadrant duration (g) (h–i) rh-sestrin2 treatment group significantly improved animals’ motor function shown by rotarod and foot-fault tests. Statistical differences between groups were analyzed using one-way ANOVA or two-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle. n = 6/group (a–d), n = 12/group (e–i). (after long-term behavioral tests, each group animals (n = 12/group) were divided into two groups randomly (n = 6/group) and used to test brain weight and Nissl’s staining, respectively).
To test the effects of rh-sestrin2 treatment on the long-term neurological impairments induced by neonatal HIE, neurological function was assessed by water maze, rotarod, and foot-fault at four weeks post HIE. In all three behavioral tests, vehicle group performed significantly worse compared to sham group. In water maze test, compared to sham group, vehicle group demonstrated substantial spatial memory loss in terms of swimming more distance to find the platform (Figure 3(e)), more latency time to escape (Figure 3(f)), and less time to spend during probe quadrant (Figure 3(g)). rh-sestrin2 treatment showed significant memory function recovery compared to vehicle group in reduced swimming distance to platform, reduced escape latency, and spent more time during target quadrant. In addition, sestrin2 treatment group significantly improved sensorimotor coordination as assessed by rotarod (Figure 3(h)) and foot-fault tests (Figure 3(i)). rh-sestrin2 group showed to significantly improve rotarod latency at the 5rpm acceleration compared to vehicle. Furthermore, rh-sestrin2 treatment group also showed to significantly reduce both right forelimb and hindlimb foot-faults compared to vehicle group.
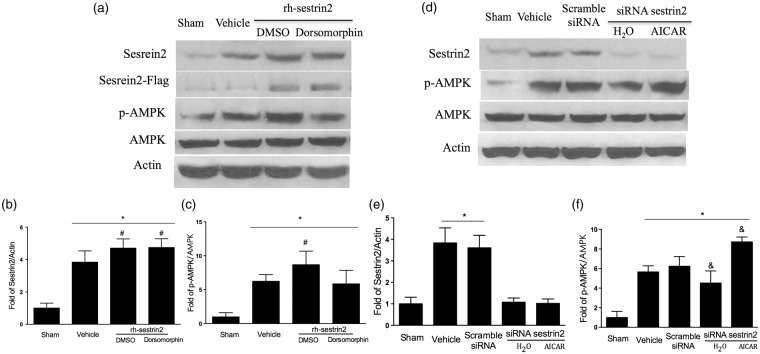
rh-sestrin2 significantly up regulated endogenous sestrin2 and p-AMPK at 24 h post HIE
In order to test the underlying mechanism of sestrin2 and whether rh-sestrin2 treatment can up regulate AMPK, pups were divided into the following groups: rh-sestrin2 + DMSO, rh-sestrin2 + AMPK inhibitor (Dorsomorphin), scramble siRNA, siRNA sestrin2 + H2O, siRNA sestrin2 + AMPK activator (AICAR). Western blot results showed that rh-sestrin2 treatment group was successfully transfected with the flag-tagged rh-sestrin2 and it significantly increased sestrin2 expression in the brain (Figure 4(a) and (b)) as well as significantly up regulated p-AMPK when compared to vehicle group (Figure 4(c)). Furthermore, when endogenous sestrin2 was inhibited with siRNA sestrin2, it showed a reduction in sestrin2 expression (Figure 4(d) and (e)) and down regulation of p-AMPK expression (Figure 4(f)). We further showed that we could reverse p-AMPK up regulation by administering Dorsomorphin and vice versa, reverse p-AMPK down regulation by administering AICAR (Figure 4(b) and (c)).
Figure 4.
Representative pictures of modulation of sestrin2 on AMPK at 24 h post HIE. (a) Representative picture of Western blot data showed bands of the expression levels of proteins of interest with or without AMPK inhibitor (Dorsomorphin). (b–c) Western blot data quantification showed that animals treated with rh-sestrin2 significantly increased sestrin2 and p-AMPK expression in the brain compared to vehicle, while animal groups treated with rh-sestrin + AMPK inhibitor (Dorsomorphin) did not affect sestrin2 expression levels (b) however, it reduced p-AMPK levels (c) when compared to rh-sestrin2 treatment group animals. (d) Representative picture of Western blot data showed bands of the expression levels of proteins of interest with or without AMPK activator (AICAR). (e–f), Western blot data showed that silencing sestrin2 with siRNA sestrin2 reduced sestrin2 and p-AMPK expression levels in the brain compared to vehicle and scramble siRNA, while animal groups treated with siRNA sestrin2 + AMPK activator (AICAR) did not change sestrin2 expression levels when compared to sham (e) however, it significantly increased p-AMPK expression levels when compared to siRNA sestrin2 animal group (f). Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle; & versus scramble siRNA. n = 6/group. (Western blot samples were from those animals euthanized after short-term neurobehavioral tests.)
AMPK mimicked the effects of sestrin2 at 24 h post HIE
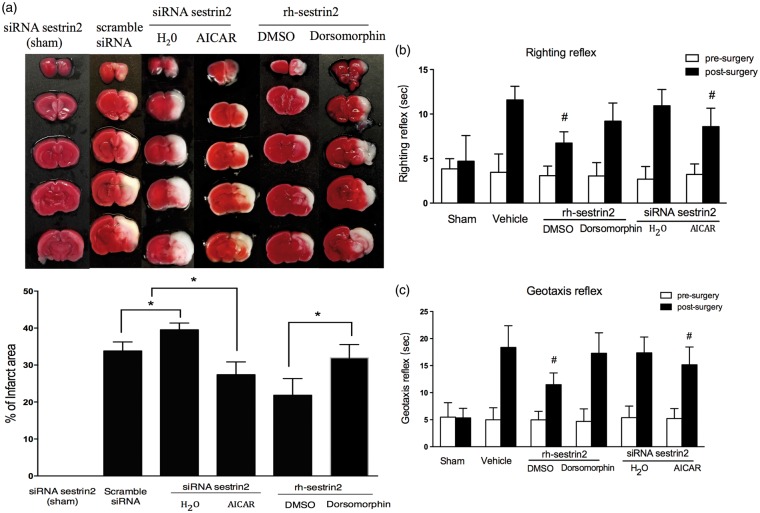
In order to test whether AMPK activator, AICAR, can reduce infarct area and improve neurological function after inhibiting sestrin2, and whether AMPK inhibitor (Dorsomorphin) can reverse sestrin2 neuroprotective effects, we divided animals into the following groups: scramble siRNA, siRNA sestrin2 + H2O, siRNA sestrin2 + AMPK activator (AICAR), rh-sestrin2 + DMSO, rh-sestrin2 + AMPK inhibitor (Dorsomorphin). Firstly, TTC staining showed that AMPK activator (AICAR) substantially reduced brain infarct area after silencing sestrin2; on the contrary, the effect which sestrin2 alleviated infarct area was reversed by AMPK inhibitor (Dorsomorphin) (Figure 5(a)). Secondly, neurological impairment was significantly decreased in the siRNA sestrin2 + AICAR group compared to control (siRNA sestrin2 + H2O), while the AMPK inhibitor (Dorsomorphin) reversed those protective effects (Figure 5(b) and (c)).
Figure 5.
The effects of AMPK activator (AICAR) and inhibitor (Dorsomorphin) on infarct area and neurological function at 24 h post HIE. (a) The infarct area was significantly reduced in the siRNA sestrin2 + AICAR group compared to only siRNA sestrin2. rh-sestrin2 treatment with Dorsomorphin significantly increased infarct area when compared to rh-sestrin2 treatment group alone. Silencing sestrin2 with siRNA sestrin2 showed no effect to brain infarct area, while after hypoxic-ischemic injury, knockout sestrin2 exacerbated brain infarct area. (b–c), Righting reflex and Geotaxis showed that animal group treated with rh-sestrin2 + Dorsomorphine significantly impaired neurological function compared to rh-sestrin2 treatment group, whereas siRNA sestrin2 + AICAR showed to significantly improve neurological function when compared to siRNA sestrin2 group alone. Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle or scramble siRNA. n = 6/group.
Moreover, TTC staining also showed that silencing sestrin2 in sham group, siRNA sestrin2 (sham) had no significant increased infarct area (Figure 5(a)), whereas silencing sestrin2 with siRNA sestrin2 showed to have a significant increase in infarct area compared to scramble siRNA (Figure 5(a)). However, neurological outcomes showed no significant difference when compared to control group (scramble siRNA) at 24 h post HIE (Figure 5(b) and (c)).
rh-sestrin2 attenuated neuronal apoptosis at 24 h post HIE
Since hypoxic-ischemic injury results in neuronal degeneration and apoptosis, to test whether rh-sestrin2 treatment can attenuate those adverse effects, we used Fluoro-Jade C and immunofluorescence staining. In Figure 6(a), we found that there was a strong green contrast Fluoro-Jade C positive staining in ipsilateral cortex section of vehicle, located in degenerating soma, neuropil, and terminals but not in surrounding intact tissue when compared to sham. rh-sestrin2 treatment group reduced Fluoro-Jade C positive degenerated neurons in ipsilateral lesion compared to vehicle. Furthermore, immunofluorescence staining showed that animal in vehicle group had an increased staining for cleaved caspase3 (pro-apoptotic marker) compared to sham, whereas animal in rh-sestrin2 treatment group showed to have a reduced staining for cleaved caspase3 when compared to vehicle (Supplement Figure 1). In addition, double immunolabeling against cleaved caspase3 and sestrin2-flag showed that increased cleaved caspase3 was co-localized with sestrin2-flag. There was co-localization between neuron and sestrin2-flag showed in supplement Figure 2.
Figure 6.
The effects of rh-sestrin2 treatment on neuronal apoptosis, and via activation of the AMPK signaling pathway at 24 h post HIE. (a) Fluoro-Jade C staining showed massive positively stained neurons undergoing apoptosis in vehicle group compared to sham. rh-sestrin2 treatment significantly reduced positive staining neurons. Arrowhead part was amplified. Strong green: Fluoro-Jade C staining positive. (b) Representative picture of Western blot data showing bands of the expression levels of Bcl2, Bax, caspase3, cleaved caspase3 either with rh-sestrin2 treatment alone, rh-sestrin2 treatment + Dorsomorphin or siRNA sestrin2 + AICAR. (c) Western blot data quantification of bands showed that Bcl-2 expression levels were significantly reduced in vehicle group compared to sham. In addition, rh-sestrin2 + Dorsomorphin significantly reduced Bcl-2 expression when compared to rh-sestrin2 treatment alone group. siRNA sestrin2 + AICAR significantly increased Bcl-2 expression levels when compared to siRNA sestrin2 group. (d–e) Western blot data showed that vehicle group significantly increased Bax and cleaved caspase3 expression levels when compared rh-sestrin2 treatment group. Dorsomorphin inhibited rh-sestrin2’s protective effects and showed to significantly up regulate Bax and cleaved caspase3 expression levels compared to rh-sestrin2 treatment group. siRNA sestirn2 + AICAR group reduced expression of Bax and cleaved caspase3 when compared to siRNA sestrin2 group. Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle; & versus scramble siRNA. n = 1/group (A), n = 6/group (b–e). (brain slices of sham and vehicle in Fluoro-Jade C were from the same animals involved in Figure 1(a))
rh-sestrin2 suppressed apoptotic proteins via activation of AMPK signaling at 24 h post HIE
Western blot data showed that hypoxic-ischemic injury significantly reduced B-cell lymphoma 2 levels (Bcl-2, an anti-apoptotic marker) compared to sham, while treatment with rh-sestrin2 significantly increased Bcl-2 levels compared to vehicle and AMPK inhibitor (Dorsomorphin) which abolished sestrin2 protective effects. In addition, administration of AMPK activator (AICAR) showed to increase Bcl-2 expression levels compared to siRNA sestrin2 + H2O group (Figure 6(b) and (c)).
Conversely, the pro-apoptotic marker, Bcl-2-associated X protein (Bax) was significantly increased after hypoxic-ischemic injury compared to sham, but reversed by rh-sestrin2 and AICAR. Both Dorsomorphin and siRNA sestrin2 increased levels of Bax in comparison with sham after HIE (Figure 6(d)). Cleaved caspase3, another pro-apoptotic marker, was significantly up regulated in all groups compared to sham; however, rh-sestrin2 treatment and AICAR showed to significantly reduce cleaved caspase3 expression levels compared to vehicle or scramble siRNA groups (Figure 6(e)). There was no significant difference in total caspase3 expression levels in all groups (data not shown).
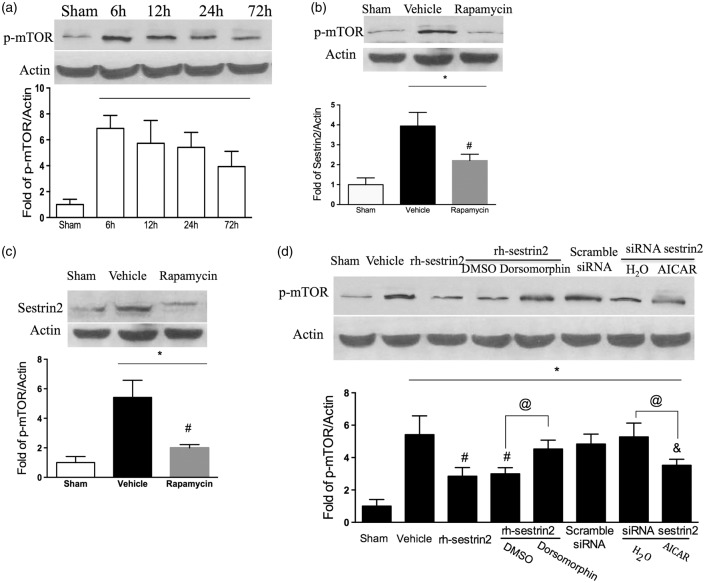
Sestrin2 inhibited phosphorylated mTOR as a negative feedback regulator at 24 h post HIE
Compared with basal level, phosphorylated mTOR (p-mTOR) was significantly increased after HIE in a time-dependent manner (Figure 7(a)). p-mTOR was mainly suppressed by rapamycin (Figure 7(b)), accordingly, the increased sestrin2 level followed HIE was reduced by rapamycin (Figure 7(c)). Moreover, rh-sestrin2 administration downregulated p-mTOR level but was partly reversed by Dorsomorphin. When sestrin2 was silenced, p-mTOR level showed no change in comparison with vehicle, whereas there was significant change by AICAR treatment (Figure 7(d)).
Figure 7.
The effects of sestrin2 on mTOR signaling at 24 h post HIE. (a) Western blot data showed time course expression of p-mTOR levels, with p-mTOR peaking at 6 h and slowly decreasing by 72 h. (b–c) Significant decrease in both sestrin2 and p-mTOR expression levels after rapamycin treatment compared to vehicle at 24 h post hypoxic-ischemic injury. (d) rh-sestrin2 treatment significantly reduced p-mTOR expression levels in the brain compared to vehicle, while rh-sestrin2 + Dorsomorphin reversed that effect. AICAR significantly reduced p-mTOR expression even after sestrin2 was silenced with siRNA sestrin2. Statistical differences between groups were analyzed using one-way ANOVA followed by Tukey multiple-comparison post hoc analysis. * versus sham; # versus vehicle; & versus scramble siRNA. n = 6/group.
Discussion
This study focused on sestrin2 and its role on apoptosis after hypoxic-ischemic injury. There is increasing evidence to show that sestrin2 has pro-survival properties. In an in vitro experiment, it was shown that 1-methyl-4-pgenylpyridinium up regulated sestrin2 via p53, while knocking it down promoted 1-methyl-4-pgenylpyridinium-induced apoptosis.23 Another in vitro study showed that an increase in sestrin2 expression as a result of brain-derived neurotrophic factor administration confers neuronal resistance against oxidative stress in primary rat cortical cultures.24 Similar results were observed in a study by Budanov et al.,25 where over expression of sestrin2 in MCF7-tet-off cells resulted in their protection against cell death which was induced by hypoxia/glucose deprivation. Furthermore, sestrin2 induced by accumulation of amyloid β-peptide plays a protective role against amyloid neurotoxicity in primary cortical neurons possibly through the autophagy signaling pathway.14 Additionally, it was reported that sestrin2 can protect hippocampal CA1 neurons against transient global ischemia-induced apoptosis by regulating ribosomal protein S6 phosphorylation in rats.26 Based on the above studies and the role of sestrins’ diverse incidents, including oxidative stress, hypoxia, and nutrient deficiency, it is of a particular interest to us to study the effects of sestrin2 on brain injury and neurological function as well as the possible underlying mechanism via which sestrin2 exerts its neuroprotective effects.
Firstly, we showed that sestrin2 was expressed in the neonatal rat brain, specifically in neurons using immunofluorescence staining. Since sestrin2 is a stress-inducible protein,10 and hence is only up regulated after stress-inducible conditions, we saw minimal expression of sestrin2 in non-injured rats.
Secondly, we tested whether exogenous administration of rh-sestrin2 could play a neuroprotective role by reducing brain atrophy and improving neurological function via the AMPK signaling pathway in a rat neonatal HIE model. These findings will help to provide clinically relevant evidence for the potential development of new therapeutic strategies against hypoxic-ischemic-induced brain injury in neonates. As the main outcome of HIE is brain damage and irreversible tissue loss which leads to neurological impairments, we administered three doses of rh-sestrin2 intranasally at 1 h post HIE and measured infarct area and neurological function at 24 h post HIE in order to determine the most effective dose. Although medium and high doses showed to significantly reduce infarct area, only high dose rh-sestrin2 treatment showed to significantly improve short-term neurological function as assessed by righting reflex and negative geotaxis; therefore, we chose high dose rh-sestrin2 for subsequent experiments. Similar results were seen in our long-term experiments (four weeks), where rh-sestrin2 reduced brain atrophy as measured by Nissl’s staining and also improve long-term neurological function as assessed by water maze, rotarod, and foot-fault.
As neuronal apoptosis is one of the main outcomes of hypoxic-ischemic injury, our data showed that rh-sestrin2 treatment can significantly reduce apoptosis as measured by Fluoro-Jade C staining and Western blot. In the Western blot data, pro-apoptotic markers such as cleaved caspase3 were significantly reduced while anti-apoptotic markers like Bcl-2 were increased.
We then wanted to test whether sestrin2 exhibits these protective properties via the AMPK signaling pathway. AMPK is a conserved serine/threonine protein kinase that regulates the intracellular ratio of AMP to ATP. It is generally activated under conditions that deplete cellular ATP and increase AMP levels such as oxidative stress, DNA damage, glucose deprivation, hypoxia, and ischemia.9,10 Furthermore, AMPK is also known to inhibit phosphorylation of mTOR, thereby shutting down protein synthesis, an ATP-consuming process as the main role of mTOR is to promote proliferation and growth. Wide arrays of studies have shown that inhibition of mTOR exhibits neuroprotective properties in the cerebral ischemia model27–31 by reducing infarct area. In addition, sestrin2 can suppress mTOR by activating AMPK via either direct binding or via indirect transcriptional regulation and hence stop ATP consumption and apoptosis.
Furthermore, overexpression of sestrins can up regulate the phosphorylation of AMPK and lead to the down regulation of mTOR.12,32 Interestingly, sestrin not only act as inhibitors of mTOR but also as negative feedback regulator of mTOR pathway. Studies have shown where sestrin2-induced AMPK activation inhibited mTOR independently of redox-regulating activities.33 Conversely, mutations or inhibitions of sestrins result in the activation of mTOR through translocation to the lysosome by Rag GTPases. Studies done in in vivo models have demonstrated that lack of sestrin is associated with increased activation of mTOR resulting in increased postnatal mortality in multiple organs during fasting, while activation of sestrin can suppress the mTOR-p70 S6K signaling pathway while mildly up regulating mTOR/AKT signaling.10
From the above evidence that suppression of mTOR can protect against neuronal apoptosis, together with that overexpression of sestrin can induce suppression of mTOR via AMPK activation, we believe that sestrin may serve as an endogenous agents to protect neuronal cells from apoptosis induced by ischemia or hypoxia via the AMPK/mTOR signaling pathway. Our Western blot data and immunohistochemistry pictures showed that sestrin2 acted as a negative feedback regulator of mTOR during HIE. Activation of mTOR signaling was hindered by either rh-sestrin2 or pharmacological activation of AMPK using AICAR. While when sestrin2 was silenced with siRNA sestrin2 or AMPK was inhibited with Dorsomorphin, mTOR expression levels were up regulated. This suggests that sestrin2 exerts its neuroprotective properties by inhibiting mTOR signaling via AMPK activation. On the other hand, rapamycin, a pharmacological inhibitor of mTOR, showed to decrease sestrin2 expression after HIE. It was worthy that rapamycin cannot completely suppress sestrin2 activation, which indicated that other unknown pathways may regulate sestrin2 expression in neonatal rats hypoxic-ischemic insult. In age-related disease studies, it has been shown that sestrin2 can act as a negative regulator of mTOR, resulting in improved autophagic clearance of impaired mitochondria.12
A recent study showed sestrin2 knockout mice that were subjected to ischemia and reperfusion injury in the heart did not display any significant differences between wild-type and sestrin2 knockout mice.34 In contrast, a study done on Drosophila (which only expresses one type of sestrin) indicated that sestrin deficient heart resulted in heart dysfunction.12 As previously mentioned, invertebrates only express one type of sestrin, while vertebrates like mammals, express three types (sestrin1, sestrin2 and sestrin3). It is not surprising that in our study we did not observe any substantial changes in brain infarct area or neurological function in comparison with sham when silencing sestrin2 without hypoxic-ischemic insult as the other two isoforms of sestrin may be able to compensate for the loss of sestrin2. Although there was no abnormal basal brain function performance, silencing sestrin2 in injured animals showed aggravated brain infarct area and neurological function compared to sham group.
In this study, we also elucidate that sestrin2 was an essential regulator of AMPK after hypoxic-ischemic injury. Previous studies have demonstrated that under detrimental insults, sestrin2-AMPK signaling pathway exerts protective effects on cellular processes and function, such as scavenging reactive oxygen species, inhibiting apoptosis, improving autophagy, and metabolism.34–38 Consistent with these results, our results indicated the mechanism of decreased sensitivity to hypoxic-ischemic injury in sestrin2-deficency neonatal brain, due to AMPK activator as evidenced by activating AMPK and follow-up suppressing apoptotic proteins. Interestingly, there was still moderate AMPK phosphorylation after silencing sestrin2, indicating that sestrin2 was not the only regulator of AMPK after HIE. As shown in other studies, sestrin1 and sestrin3 also modulate AMPK activation in various harmful insults.38–41 Moreover, liver kinase B1 is another essential upstream kinase of AMPK in ischemic heart,34 but not in HeLa cells, suggesting sestrin2-induced AMPK activation is much more complicated and still requires future exploration.10
In summary, we demonstrated that sestrin2 reduced brain infarct area, brain atrophy, and improved long-term neurological function after hypoxic-ischemic injury. Consistent with previous studies, we demonstrated that neonatal hypoxic-ischemic injury activated mTOR signaling which may lead to cell death and that AMPK activation can suppress mTOR signaling. However, in our study, we showed that this AMPK activation is induced by sestrin2, which resulted in inhibition of mTOR signaling and attenuation of apoptosis after neonatal HIE.
Overall, sestrin2 is an attractive candidate for therapeutic treatment as it does not have any known side effects while it has shown to have neuroprotective properties, specifically attenuating long-term brain damage and improving long-term neurological function. Future studies are needed in better understanding sestrin2 and its possible side effects as well as signaling mechanisms.
Supplementary Material
Funding
This study is partially support by NIH NS078755 to JHZ.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
For this paper, the conception and design were made by XS, LX, DMD, JHZ, JT, and MY. Drafting the article was done by XS. Critically revising the article was done by all the authors. Reviewing submitted version of manuscript was done by all the authors. Approval of the final version of the manuscript on behalf of all authors was done by XS and JHZ.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Mental Retard Develop Disabil Res Rev 2001; 7: 56–64. [DOI] [PubMed] [Google Scholar]
- 2.Chicha L, Smith T, Guzman R. Stem cells for brain repair in neonatal hypoxia-ischemia. Childs Nerv Syst 2014; 30: 37–46. [DOI] [PubMed] [Google Scholar]
- 3.Ranasinghe S, Or G, Wang EY, et al. Reduced cortical activity impairs development and plasticity after neonatal hypoxia ischemia. J Neurosci 2015; 35: 11946–11959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain JM, Moore L, Ren Z, et al. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res 2013; 4: 158–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracewell M, Marlow N. Patterns of motor disability in very preterm children. Mental Retard Develop Disabil Res Rev 2002; 8: 241–248. [DOI] [PubMed] [Google Scholar]
- 6.Ferriero DM. Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 7.Bartha AI, Shen J, Katz KH, et al. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol 2007; 37: 85–90. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013; 1: CD003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budanov AV, Lee JH, Karin M. Stressin' Sestrins take an aging fight. EMBO Mol Med 2010; 2(: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 2013; 18: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Budanov AV, Talukdar S, et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab 2012; 16: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Budanov AV, Park EJ, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010; 327: 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-Sahra I, Dirat B, Laurent K, et al. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell Death Differ 2013; 20: 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YS, Chen SD, Wu CL, et al. Induction of sestrin2 as an endogenous protective mechanism against amyloid beta-peptide neurotoxicity in primary cortical culture. Exp Neurol 2014; 253: 63–71. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Burris M, Fajilan A, et al. Prolonged exposure to isoflurane ameliorates infarction severity in the rat pup model of neonatal hypoxia-ischemia. Transl Stroke Res 2011; 2: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topkoru BC, Altay O, Duris K, et al. Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage. Stroke 2013; 44: 3189–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Zhang J, Guo J, et al. Chronic hydrocephalus and perihematomal tissue injury developed in a rat model of intracerebral hemorrhage with ventricular extension. Transl Stroke Res 2015; 6: 125–132. [DOI] [PubMed] [Google Scholar]
- 18.Lekic T, Klebe D, McBride DW, et al. Protease-activated receptor 1 and 4 signal inhibition reduces preterm neonatal hemorrhagic brain injury. Stroke 2015; 46: 1710–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Ma Q, Suzuki H, et al. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke 2011; 42(3): 764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmued LC, Stowers CC, Scallet AC, et al. Fluoro-jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 2005; 1035: 24–31. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Hu X, Yang W, et al. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke 2010; 41: 2341–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ten VS, Bradley-Moore M, Gingrich JA, et al. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav Brain Res 2003; 145: 209–219. [DOI] [PubMed] [Google Scholar]
- 23.Zhou DX, Zhan CY, Zhong Q, et al. Upregulation of Sestrin-2 expression via P53 protects against 1-Methyl-4-Phenylpyridinium (MPP plus) neurotoxicity. J Mol Neurosci 2013; 51: 967–975. [DOI] [PubMed] [Google Scholar]
- 24.Wu CL, Chen SD, Yin JH, et al. Nuclear factor-kappaB-dependent Sestrin2 induction mediates the antioxidant effects of BDNF against mitochondrial inhibition in rat cortical neurons. Mol Neurobiol. Epub ahead of print 26 July 2015. DOI: 10.1007/s12035-015-9357-1. [DOI] [PubMed] [Google Scholar]
- 25.Budanov AV, Shoshani T, Faerman A, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 2002; 21: 6017–6031. [DOI] [PubMed] [Google Scholar]
- 26.Chuang YC, Yang JL, Yang DI, et al. Roles of Sestrin2 and ribosomal protein S6 in transient global ischemia-induced hippocampal neuronal injury. Int J Mol Sci 2015; 16: 26406–26416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher L, Evans TM, Watts LT, et al. Rapamycin treatment improves neuron viability in an in vitro model of stroke. PLoS One 2013; 8: e68281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong ZZ, Yao Q, Li HH. The rationale of targeting mammalian target of rapamycin for ischemic stroke. Cell Signal 2013; 25: 1598–1607. [DOI] [PubMed] [Google Scholar]
- 29.Maiese K. Cutting through the complexities of mTOR for the treatment of stroke. Curr Neurovasc Res 2014; 11: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie L, Sun F, Wang J, et al. mTOR signaling inhibition modulates macrophage/microglia-mediated neuroinflammation and secondary injury via regulatory T cells after focal ischemia. J Immunol 2014; 192: 6009–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong X, Xie R, Zhang H, et al. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis 2014; 66: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim GT, Lee SH, Kim YM. Quercetin Regulates Sestrin 2-AMPK-mTOR signaling pathway and induces apoptosis via increased intracellular ROS in HCT116 colon cancer cells. J Cancer Prevent 2013; 18: 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budanov AV, Sablina AA, Feinstein E, et al. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004; 304: 596–600. [DOI] [PubMed] [Google Scholar]
- 34.Morrison A, Chen L, Wang J, et al. Sestrin2 promotes LKB1-mediated AMPK activation in the ischemic heart. FASEB J 2015; 29: 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seo K, Ki SH, Shin SM. Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity. Cell Signal 2015; 27: 1533–1543. [DOI] [PubMed] [Google Scholar]
- 36.Eid AA, Lee DY, Roman LJ, et al. Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 2013; 33: 3439–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parmigiani A, Nourbakhsh A, Ding B, et al. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep 2014; 9: 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Niu Y, Yuan H, et al. AMPK binds to Sestrins and mediates the effect of exercise to increase insulin-sensitivity through autophagy. Metabolism 2015; 64: 658–665. [DOI] [PubMed] [Google Scholar]
- 39.Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes 2015; 64: 1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang YL, Loh KS, Liou BY, et al. SESN-1 is a positive regulator of lifespan in Caenorhabditis elegans. Exp Gerontol 2013; 48: 371–379. [DOI] [PubMed] [Google Scholar]
- 41.Hong-Brown LQ, Brown CR, Navaratnarajah M, et al. Adamts1 mediates ethanol-induced alterations in collagen and elastin via a FoxO1-sestrin3-AMPK signaling cascade in myocytes. J Cell Biochem 2015; 116: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.