Abstract
A possibly causal relationship between multiple sclerosis and chronic cerebrospinal venous insufficiency has recently been hypothesized. Studies investigating chronic cerebrospinal venous insufficiency have reported conflicting results and few have employed multiple diagnostic imaging modalities across a large patient and control population. In this study, three complementary imaging modalities were used to investigate the chronic cerebrospinal venous insufficiency hypothesis in patients with multiple sclerosis and two age- and sex-matched control groups: healthy volunteers and patients with other neurological diseases. Strictly blinded Doppler ultrasound according to the original chronic cerebrospinal venous insufficiency hypothesis; four-dimensional flow magnetic resonance imaging of venous flow in the head, neck, and chest; and contrast-enhanced magnetic resonance venography for neck and chest venous luminography were acquired. An internal jugular vein stenosis evaluation was also performed across modalities. Percentage of subjects meeting ultrasound-based chronic cerebrospinal venous insufficiency criteria was small and similar between groups. In group-wise and pairwise testing, no four-dimensional flow magnetic resonance imaging variables were statistically significantly different, for any measurement location. In contrast-enhanced magnetic resonance venography of the internal jugular and azygos veins, no statistically significant differences were observed in stenosis scores between groups. These results represent compelling evidence against the chronic cerebrospinal venous insufficiency hypothesis in multiple sclerosis.
Keywords: Doppler ultrasound, four-dimensional flow magnetic resonance imaging, multiple sclerosis, venous blood flow, venous luminography
Introduction
Multiple sclerosis (MS) is a disease of the central nervous system characterized by immune-mediated demyelination and neurodegeneration, causing neurocognitive dysfunction with highly variable severity and time course. The underlying etiology of MS has remained unknown since its first clinical description1 despite exhaustive study of many potential genetic, metabolic, and environmental risk factors. A vascular mechanism underlying MS was considered and studied several decades ago2 but fell out of favor as mounting evidence pointed to neuroimmunological mechanisms.
Recently, there has been renewed interest in a possible vascular component to MS since studies by Zamboni et al. suggested the presence of abnormal venous drainage of the brain and spinal cord (dubbed “chronic cerebrospinal venous insufficiency” or CCSVI) in a cohort of MS patients.3–5 The CCSVI hypothesis suggests that impaired venous drainage promotes tissue iron deposition which either triggers or potentiates a neuroinflammatory cascade of events that manifest as MS. Zamboni et al. reported 100% sensitivity and specificity in distinguishing MS from non-MS subjects with flow-specific B-mode and Doppler ultrasound (US) criteria for CCSVI.5 Additionally, they proposed a treatment for MS patients with CCSVI using angioplasty with or without stenting (so-called liberation procedure) of internal jugular veins (IJV) to open a presumably chronic stenosis.
Subsequent studies have produced mixed results: most other investigators could not replicate the early promise of Zamboni’s results,6–22 though a few follow-up studies seemed confirmatory23–26 (Table 1). Of two recent meta-analyses that were conducted, one found correlation between CCSVI and MS,27 while the other pointed to considerable heterogeneity across case–control studies.28 Notably, both of these meta-analyses concluded that there was no causative role of CCSVI in MS. Recent studies have been limited by small sample sizes6–11,13–16,18,22–24 or by the absence of a confirmatory comparative imaging modality.12,17,19,21,23,25,26 The single exception is the study by Traboulsee et al.20
Table 1.
Literature review of CCSVI imaging studies and numbers of participants from different imaging modalities.
| CCSVI Studies Numbers |
||||||
|---|---|---|---|---|---|---|
| First author | Journal | Year | Modality | MS | OND | HC |
| Zamboni | J Neurol Sci | 2009 | TCCS-ECD | 109 | – | 177 |
| Zamboni | J Neurol Neurosurg Ps | 2009 | TCCS-ECD | 65 | 45 | 190 |
| Al-Omari | Int Angiol | 2010 | TCCS-ECD | 25 | – | 25 |
| Doepp | Ann Neurol | 2010 | TCCS-ECD | 56 | – | 20 |
| Sundstrom | Ann Neurol | 2010 | PC MR | 21 | – | 20 |
| Baracchini | Ann Neurol | 2011 | TCCS-ECD | 60 | – | 60 |
| Marder | Arch Neurol | 2011 | TCCS-ECD | 18 | – | 11 |
| Centonze | Ann Neurol | 2011 | TCCS-ECD | 84 | – | 56 |
| Mayer | J Neurol Neurosurg Ps | 2011 | TCCS-ECD | 20 | – | 20 |
| Tsivgoulis | Neurology | 2011 | TCCS-ECD | 42 | – | 43 |
| Auriel | J Neurol Sci | 2011 | TCCS-ECD | 27 | – | 32 |
| Wattjes | J Neurol Neurosurg Ps | 2011 | 3D PC MRI/CE-MRV | 20 | – | 20 |
| Zivadinov | Neuroradiology | 2011 | CE-MRV | 57 | – | 21 |
| Blinkenberg | Acta Neurol Scand | 2012 | TCCS-ECD/PC MR | 24 | – | 15 |
| Kantarci | Eur Radiol | 2012 | TCCS-ECD | 62 | – | 54 |
| Amato | Mult Scler | 2012 | TCCS-ECD | 15 | – | 16 |
| McTaggart | AJNR | 2012 | CE-MRV | 19 | – | 20 |
| Barreto | Ann Neurol | 2013 | TCCS-ECD | 206 | 37 | 11 |
| Comi | J Neurol Sci | 2013 | TCCS-ECD | 1165 | 376 | 226 |
| Traboulsee | The Lancet | 2014 | TCCS-ECD/catheter venography | 79 | – | 98 |
| MacGowan | JMRI | 2014 | PC MR | 26 | – | 26 |
| Mancini | PLoS One | 2014 | TCCS-ECD | 58 | 7 | 13 |
| Tromba | Phlebology | 2015 | TCCS-ECD | 112 | – | 67 |
| This study | JCBFM | 2016 | TCCS-ECD/CE-MRV/4D flow MRI | 76 | 53 | 43 |
The aim of this study was to test the CCSVI hypothesis using Doppler US flow assessment based on Zamboni’s CCSVI criteria5 and 4D flow MRI (Phase contrast vastly undersampled isotropic projection reconstruction (PC-VIPR)29,30), augmented by contrast-enhanced MR venography (CE-MRV). These techniques were used to compare venous flow dynamics in the head, neck, and chest between patients with MS and two control groups: patients with other neurological disorders (OND) and healthy controls (HC).
Materials and methods
Demographics
This study was designed as a single-center, comparative, cross-sectional investigation in patients with MS,31 age- and sex-matched HCs, and controls with ONDs. The study population consisted of a total of 172 subjects: 76 MS patients (age: 46.1 ± 11.1 yrs, 27M/49F), 53 HCs (age: 45.8 ± 11.2 yrs, 22M/31F), and 43 patients with other neurological disease (age: 49.0 ± 12.7 yrs, 14M/29F. Numbers of OND subjects by disease were as follows: Parkinson’s disease—14; epilepsy—12; migraine headaches—8; essential tremor—2; oculopharyngeal muscular dystrophy—1; cervical trauma—1; vestibular hypersensitivity—1; myoclonus—1; cerebellar degeneration—1; cervical dystonia—1; and Charcot–Marie–Tooth disease—1). See Table 2.
Table 2.
Demographic data for subjects in this study.
| MS | HC | OND | |
|---|---|---|---|
| Number of subjects (female/male) | 76 (49/27) | 53 (31/22) | 43 (29/14) |
| Mean age ± SD (years) | 46.1 ± 11.1 | 45.8 ± 11.2 | 49.0 ± 12.7 |
| Mean disease duration ± SD (years) | 9.9 ± 7.8 | – | 15.4 ± 13.8 |
| Median expanded disability status scale (range) | 2.50 (0–7) | – | – |
| Number RRMS early/mid/late, SPMS, PPMS | 10/19/27, 12, 8 | – | – |
HC: healthy controls; MS: multiple sclerosis; OND: other neurological disease; PPMS: primary progressive MS; RRMS: relapsing–remitting MS; SPMS: secondary progressive MS.
Recruitment invitations were approved by the local IRB and included clinic posters, patient newsletters, and queries of a HC subject database. Medical records for prospective subjects were reviewed by the study neurologist, and in ambiguous cases the subjects were examined by the study neurologist. Virtually all MS and OND subjects were outpatients initially diagnosed by attending neurologists in neurology subspecialty clinics at the University of Wisconsin Hospital and Clinics. Recruitment was consecutive for MS subjects; for OND and HC subjects, an effort was made, insofar as was possible, to match demographic characteristics with the recruited MS group. The MS group consisted of subjects meeting the McDonald 2010 criteria31 after assessment by the study neurologist and having no confounding neurological, psychiatric, vascular, immune, or systemic inflammatory conditions. All MS subtypes were included; for the relapsing–remitting MS subjects, disease was classified as early (clinically isolated syndrome or relapses ≤ 2 years from diagnosis), middle (duration 2–5 years from diagnosis), or late (duration > 5 years from diagnosis). HC individuals were age- and sex-matched to MS group with no history of neurological, psychiatric, vascular, immune, or systemic inflammatory conditions.
The University of Wisconsin Institutional Review Board approved all study procedures and protocols following the policies and guidance established by the campus Human Research Protection Program. Each participant provided signed written informed consent before participation.
MRI acquisition
A clinical 3T scanner (Discovery MR750, GE Healthcare, WI, USA) was used to collect 4D flow MRI at three anatomical stations using PC-VIPR. The imaging protocol for the head included: FOV (covering all brain vasculature) = 22 cm × 22 cm × 22 cm, reconstructed resolution = (0.69 mm)3 isotropic, TE/TR/α = 3.5 ms/9.0 ms/15°, Velocity ENCoding “VENC” = 20 cm/s. The imaging protocol for the neck included: FOV (covering confluens sinuum to aortic arch) = 18 cm × 18 cm × 18 cm, reconstructed resolution = (0.70 mm)3 isotropic, TE/TR/α = 3.0 ms/7.9 ms/15°, VENC = 40–70 cm/s. The imaging protocol for the chest included: FOV (covering all heart vasculature and azygos vein (AV)) = 32 cm × 32 cm × 32 cm, resolution = (1.25 mm)3 isotropic, TE/TR/α = 2.7 ms/6.9 ms/15°, VENC = 40 cm/s). Cardiac triggers were recorded for retrospective cardiac gating with an offline reconstruction. All 4D flow scans were reconstructed to 20 cardiac timeframes irrespective of subject heart rate and anatomical station.
The PC-VIPR technique was used to assess for venous stenosis, collaterals, and flow velocity data. However, because the expected range of flow velocities (the “VENC” setting) must be specified a priori, unexpectedly low velocities can then result in poor signal-to-noise ratio. Therefore, conventional CE-MRV sequences were added to ensure high-resolution visualization of the AV and IJVs, where virtually all venous stenoses reported by Zamboni et al.3–5 were located. A single injection of intravascular contrast agent, gadofosveset trisodium (Ablavar, Lantheus Medical Imaging, MA, USA) at a dose of 0.03–0.05 mmol/kg, was used for both scans. Additional scan parameters for the neck were as follows: FOV (covering aortic root to confluens sinuum) = 28 cm × 28 cm × 26 cm, resolution = 0.55 mm × 0.55 mm × 0.80 mm, TE/TR/α = 1.3 ms/3.4 ms/28°. And those for the chest were as follows: FOV = 36 cm × 36 cm × 22.5 cm, resolution = 0.7 mm × 0.7 mm × 0.7 mm, TE/TR/α = 0.98 ms/2.9 ms/15°.
US acquisition
All US studies were conducted by a single board certified vascular sonographer. Before scanning any subjects, the study sonographer underwent two days of dedicated training in the evaluation of CCSVI “Zamboni Criteria” at University of British Columbia; this training was provided by a sonographer who in turn trained directly with Dr Zamboni at the University of Ferrara, Italy. State-of-the-art US imaging employed high-resolution B-mode imaging with color and spectral Doppler to investigate venous drainage (S2000, Siemens, Erlangen, Germany). The 9 MHz linear vascular probe was used to assess the IJV and vertebral veins, and the 4 MHz probe was used to assess the deep cerebral veins (DCVs) through the temporal window. The ultrasonographer was blinded to the subjects’ group status (conversation forbidden during sonography; subject greeted and prepared for sonography by study coordinator uninvolved in data acquisition or analysis; any assistive devices such as canes, walkers, etc. hidden from view).
The US study protocol replicated that of Zamboni et al.5 All measurements were initially taken with the patient in the supine position and then repeated in the upright position on the bed. The jugular vein diameter was measured at the smallest location using both the machine’s auto trace package as well as a manual tracing by the sonographer. Color Doppler and pulsed wave Doppler were utilized to verify IJV and vertebral vein patency, presence, and direction of blood flow. The valve between the IJV and subclavian vein was assessed for abnormalities using B-mode imaging. The DCVs were visualized through the temporal window, with the settings at 2 MHz to penetrate adequately. Blood flow was assessed for reversal with Color and pulsed wave Doppler.
MRI analysis
All MRI flow processing was completed by one examiner with five years flow processing experience. The examiner was uninvolved with image acquisition and blinded to group membership. Figure 1 displays anatomical locations of the analysis planes in a single subject in the head, neck, and chest, as well as corresponding CE-MRV limited MIPs in the neck and chest. In the head, attention was directed to the superior sagittal sinus, left/right transverse sinus, left/right internal cerebral vein, and left/right basal vein. In the neck, measurements were made of the left/right IJV at three stations (Upper, Mid, Lower). And in the chest, measurements were made of the AV 2 cm from its junction with the superior vena cava. Measured flow parameters were as follows: total flow (ml/cardiac cycle), peak flow over the cardiac cycle (ml/s), and percent retrograde flow (%RF). These MRI measurements correspond to Zamboni’s original criteria for detection of CCSVI using Doppler US5: our analysis allows for assessment of directional flow in the cervical and intracranial veins (criteria 1 and 2), anomalies/stenoses of IJVs (criteria 3), and blocked outflow in cervical veins (criteria 4).
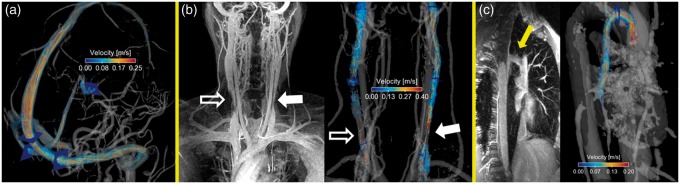
Figure 1.
All images shown in 36 y/o female with migraine. Planes mark PC-VIPR measurement locations. (a) Velocity streamlines in the head from PC-VIPR angiogram. (b) Limited maximum intensity projection (MIP) of CE-MRV of neck veins (left) and PC-VIPR streamlines (right). Areas of stenosis viewed on the CE-MRV MIP (IJV morphology scores < 3) are complemented by PC-VIPR information: slow flow (open arrow) and velocity jet (closed arrow). (c) Limited CE-MRV MIP (left) and corresponding PC-VIPR angiogram and streamlines (right) in the azygos vein. CE-MRV: contrast-enhanced MR venography; IJV: internal jugular vein; PC-VIPR: phase contrast vastly undersampled isotropic projection reconstruction.
For semiquantitative assessment of venous caliber, CE-MRVs were scored by two radiologists blinded to subject identity, date/sequence of scan, and each other’s scores. The scoring followed the scale introduced by Zivadinov et al.15: ability to assess the IJV and AV (1, poor; 2, acceptable; 3, good; 4, excellent), IJV morphology at its narrowest point (1, absent; 2, pinpoint; 3, flattened; 4, crescentic; 5, ellipsoidal/round), and AV morphology (1, diffusely irregular/narrowed; 2, focally narrowed at central aspect; 3, caliber increasing from peripheral to central).
Statistics
Group blood flow differences were assessed on a per vessel/location basis using two-sample unpaired Student’s t-tests. Similarly, age- and sex-matched pairs were assessed on a per vessel/location basis using paired Student’s t-tests. Group CE-MRV morphology scoring differences were assessed on a per-vessel basis using a Wilcoxon sum-rank test. False discovery rate control was used to correct for multiple comparisons.32 Cohen’s κ with linear weights was used to assess inter-rater reliability for CE-MRV morphology measurements (all IJV and AV scores and dichotomized IJV scoring, see below). These results were compared with US, in which a subject having ≥ 2 of the five CCSVI criteria was considered positive for CCSVI.4
Trimodality narrowing assessment
One of the main criteria proposed for CCSVI lies in determining IJV stenosis using B-mode US.4 A binary IJV narrowing evaluation was made for each method: CE-MRV (morphology vessel score < 3), PC-VIPR (any measurement plane with %RF > 5%), and B-mode US (“evidence of proximal IJV stenosis”).5 The percentage of total subjects who exhibited narrowing was compared between readers, groups, and modalities.
Results
Protocol
Table 3 provides an overview of data collection and analysis from MRI scans. PC-VIPR scans were successfully performed and analyzed for 163/172 (94.8%) of all subjects in the head, 155/172 (90.1%) in the neck, and 146/172 (88.9%) in the AV. The main reason for acquisition or analysis failure was due to data archiving problems (head—n = 5; neck—n = 7; AV—n = 9). CE-MRV of the neck was successfully scanned and scored in 97.3% of cases, while that of the chest was slightly lower at 96.1%. CCSVI US scans were successfully performed in all subjects the same day the MRI exam was performed.
Table 3.
Overview of successful data collection and analysis from MRI scans.
| Successfully completed and analyzed imaging—number of subjects (% of total) | ||||||
|---|---|---|---|---|---|---|
| PC-VIPR | MS | HC | OND | |||
| Head | 74 (97.4) | 47 (88.7) | 42 (97.7) | |||
| Neck | 71 (93.4) | 46 (86.8) | 38 (88.4) | |||
| Chest | 65 (85.5) | 46 (86.8) | 35 (81.4) | |||
| CE-MRV |
R1 |
R2 |
R1 |
R2 |
R1 |
R2 |
| Neck | 74 (97.4) | 75 (98.7) | 47 (88.7) | 46 (86.8) | 43 (100) | 37 (86.0) |
| Chest | 75 (98.7) | 74 (97.4) | 46 (86.8) | 46 (86.8) | 39 (90.7) | 34 (79.1) |
CE-MRV: contrast-enhanced MR venography; HC: healthy controls; MS: multiple sclerosis; OND: other neurological disease; PC-VIPR: phase contrast vastly undersampled isotropic projection reconstruction; %RF: percent retrograde flow; R1: reader 1; R2: reader 2.
Note that all ultrasound scans were successfully performed.
MRI flow analysis
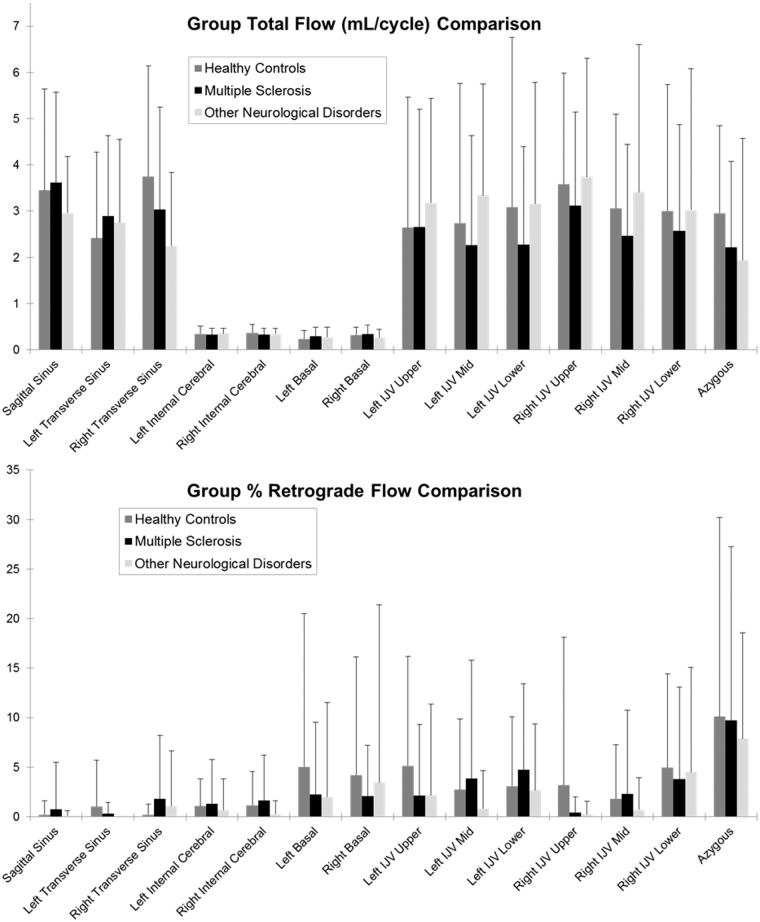
Figure 2 displays group-wise bar plot results of total flow and %RF across all vessels measured using PC-VIPR. No major differences are observed for any flow parameter or in any vessel between groups. No statistically significant differences were observed for any flow parameter (total flow, peak flow, or %RF) across all vessels and between all combinations of groups. Likewise, no statistically significant differences were observed between age- and sex-matched pairs.
Figure 2.
Group-wise comparisons of average ( ± standard deviation) total flow (in ml/cycle, top) and percent retrograde flow (%RF) over the cardiac cycle (bottom) for all measured locations. No statistically significant differences were found at any location between any of the three groups. %RF: percent retrograde flow.
CE-MRV analysis
Good IJV image quality scores were observed (all subjects averaged: reader 1 = 3.3 ± 0.7, reader 2 = 2.8 ± 0.5), with moderate inter-rater reliability in morphology scores (κ = 0.56). For dichotomized IJV scoring, inter-rater reliability was greater (κ = 0.60). For the AV in the chest, image quality scores were good (all subjects averaged: reader 1 = 3.2 ± 0.9, reader 2 = 2.7 ± 0.5), yet inter-rater reliability in morphology scores was poor (κ = 0.16).
Despite variable inter-rater reliability scores, no statistically significant differences between any group combinations were observed for CE-MRV morphology measurements, for either reader.
MRI flow and US assessment
Subjects with %RF > 5% in any part of the MRI regional flow assessment (head, IJVs, AV) were considered to exhibit “CCVSI-like” criteria. The percentage of total subjects showing this feature was compared to the percentage of subjects with ≥ 2 US CCSVI criteria and is shown in Table 4. The number of subjects that exhibited %RF in one or more regions and tested positively for CCSVI criteria from the US exam was nine (14.1%) in MS patients, five (11.4%) in HC subjects, and one (3.2%) in subjects with other neurological disease.
Table 4.
Group-wise comparison of total number of subjects exhibiting CCSVI criteria.
| Number of subjects (% of total with all data) |
|||
|---|---|---|---|
| MS | HC | OND | |
| PC-VIPR | |||
| Reflux flow any DCV (%RF > 5%) | 14 (18.9%) | 16 (34.0%) | 3 (7.1%) |
| Reflux flow any IJV (%RF > 5%) | 32 (45.1%) | 25 (54.3%) | 13 (34.2%) |
| Reflux flow AV (%RF > 5%) | 25 (38.5%) | 12 (26.1%) | 15 (42.9%) |
| Ultrasound | |||
| Positive US CCSVI (≥2 criteria met) | 12 (15.8%) | 9 (17.0%) | 4 (9.3%) |
AV: azygos vein; CCSVI: chronic cerebrospinal venous insufficiency; DCV: deep cerebral vein; HC: healthy controls; IJV: internal jugular vein; MS: multiple sclerosis; OND: other neurological disease; PC-VIPR: phase contrast vastly undersampled isotropic projection reconstruction; %RF: percent retrograde flow.
Note that percentage of total represents number of subjects that had all measurements.
Trimodality IJV narrowing assessment
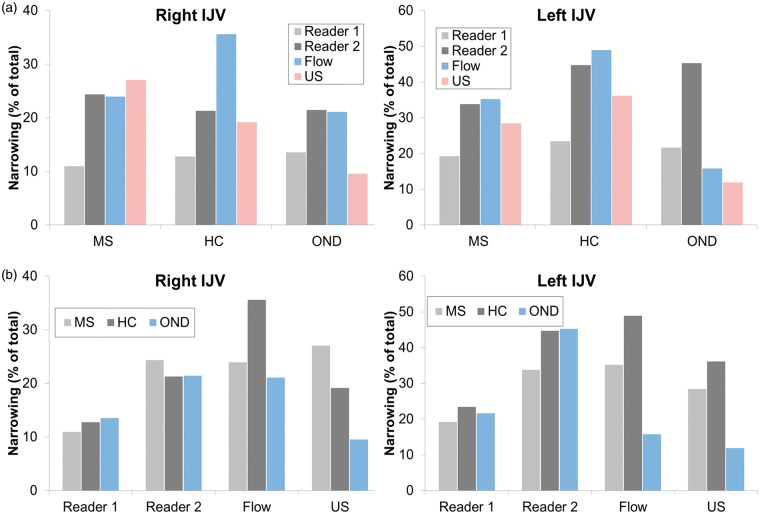
Figure 3 demonstrates percent of total subjects exhibiting IJV narrowing across groups and readers (as well as flow and US results). Higher occurrence of narrowing is evident in the left IJV, and greater variability is seen for both PC-VIPR and US compared with CE-MRV.
Figure 3.
(a) Group-wise comparisons of detected narrowing, shown as percentage of total subjects for measured cases in the right and left IJV. (b) Reader (and US) comparisons of detected IJV narrowing, shown as a percentage of total subjects. From the same CE-MRV data, reader 2 appears to detect narrowing more frequently. Prevalence is higher in the left IJV than right IJV. CE-MRV: contrast-enhanced MR venography; IJV: internal jugular vein.
Discussion
This study tested the CCSVI hypothesis with three complementary imaging techniques (US, 4D flow MRI, and CE-MRV) in a large cohort of MS patients and age- and sex-matched controls, including HCs and subjects with other neurological disease. Our study was designed to address limitations of small sample sizes and single modality assessment found in other CCSVI investigations.4–26 To our knowledge, this is the only study that provides a comprehensive assessment of blood flow and luminography using 4D flow MRI in three cerebrospinal venous regions (totaling 14 measurement locations of blood flow), CE-MRV for semiquantitative assessment of vessels in both the IJV and AV, and strictly blinded US exams performed according to Zamboni’s CCSVI criteria.5
Without question, the CCSVI hypothesis has led to renewed interest in both intra- and extracranial venous flow measurements, namely in the DCVs, the IJVs, and the AV. From an imaging perspective, venous flow assessment for CCSVI is problematic. First, unlike rigid and muscular arteries, veins have thin, nonmuscular walls that make for an easily compressible and distensible lumen. Hence, veins are highly variable in appearance on cross-sectional imaging, which substantially compromises the test–retest and inter-rater reliability of venous caliber measurements on CE-MRV exams.33 Such variability is especially evident in IJVs which can take a number of different cross-sectional configurations (pinpoint, crescentic, flattened, oblong, etc.). Additionally, unlike stenotic arterial flow jets, a collapsing vein may produce slow flow or even flow reversal. In B-mode US images of IJVs, which are inherently 2D projections, this may lead to over- or under-estimation of lumen size, depending on the vessel shape and transducer direction. Underestimation may also occur if the ultrasonographer uses too much transducer pressure, collapsing the vein. Second, venous flow velocity (within a volume) is sensitive to a number of variables including body position,34 head position or rotation,35 respiratory state,36,37 hydration level,38 diurnal changes, and caffeine intake. Finally, the DCVs of interest in CCSVI—which include the internal cerebral and basal veins—are small and located in areas that are often difficult to assess. In flow MRI, this translates to a need for higher resolution scans and careful correction of phase offsets and displacement artifacts that lead to flow measurement errors. The problem is even more vexing in transcranial Doppler US due to the small or nonexistent temporal acoustic window (roughly 10% of individuals have no acoustic window),39 the variability of operator skill, and the inherent methodological problem adequately blinding technicians to subject status. Many of these criticisms relative to Zamboni’s original diagnosis of CCSVI using US (and the subsequent endovascular treatment) have been examined in more detail previously.40
In general, MRI has several benefits compared with US: MRI is the standard-of-care modality for diagnosis of MS, it is less operator dependent and is conducted according to prospective protocols that can be faithfully repeated by fully blinded operators, and it can be used to noninvasively assess vessels inaccessible to US. Drawbacks of MRI include image acquisition is not real time, ECG-gated acquisitions last several cardiac cycles, and measurements can only be made in the supine position, precluding the assessment of positional flow changes.3
PC-VIPR29,30 is a particularly powerful technique for flow assessment that utilizes a 3D, time-resolved radial acquisition k-space trajectory. This technique provides several advantages for assessing CCSVI beyond other MRI methods that measure blood flow. First, PC-VIPR is a 3D technique that covers an extensive anatomical volume with high isotropic spatial resolution, allowing entire vascular territories to be rendered during a single scan. Second, compared to a standard 3D Cartesian sampling scheme, the radial acquisition, in which central k-space is oversampled and outer portions of k-space are undersampled, allows for faster acquisition and high temporal resolution. This in turn creates high image contrast and sparse signal representation with background suppression. Finally, the artifacts associated with PC-VIPR do not substantially compromise the images.
In summary, each imaging technique used in this study has its own strengths and weaknesses, and therefore provides complementary information on cerebrospinal venous vasculature. B-mode and Doppler US provides high temporal resolution and allows for strict adherence to Zamboni’s CCSVI criteria. The inherent 2D nature of US, however, may cause over- or underestimation of cross-sectional area. Further, it is impossible to interrogate the entire cerebrospinal venous system. 3D MRI techniques are more easily blinded and do not have these limitations. CE-MRV provides excellent high-resolution visualization of venous structures. This method acquires results at a single time point and thus may fail to capture potential respiratory or cardiac-induced change. Further, there are currently no established standards for normal venous lumen caliber.15,24 Without such standards, inter-rater agreement measures are unlikely to be high. PC-VIPR provides dynamic measurement of 3D velocities in a large volume with high spatial resolution; however, unexpectedly low velocities may result in poor signal-to-noise ratio and potentially compromise flow measurements.
With 4D flow MRI (PC-VIPR), there were no statistically significant differences observed between groups (or age- and sex-matched pairs) for any flow measurement, at any anatomical location. Concordant with other IJV flow studies,6,25,33 larger total flow values are seen for the right IJV compared with the left IJV across all groups. However, large standard deviations are also observed, which possibly stemmed from the grouping of varying age and gender for group comparisons. This grouping encompassed a wide range of participant ages (MS: 19–68 years, HCs: 23–74 years, other neurological disease: 22–68 years) and a higher number of women than men (MS: 49F/27M; HCs: 31F/22; other neurological disease: 29F/14M). At baseline, IJV flow measurements are extremely variable. Individual cerebrospinal venous flow measurements are known to be subject to several normal day-to-day variations, such as related to body position,34 head position,35 and hydration levels.38 Previous work has shown these day-to-day flow changes to be as high as 20%, even while demonstrating good technical reproducibility in cerebrospinal venous vasculature measurements using PC-VIPR.33 A previous study using US and CE-MRV in MS patients suggests CE-MRV provides a more robust assessment of IJV narrowing than B-mode US, which can be limited by operator skill and misleading 2D projections.41 Scoring of the CE-MRV analysis indicated good image quality for both the IJV and AV, with particularly high values for the IJV. Although CE-MRV in the chest was performed during a single breath hold, the images are not cardiac resolved. This cardiac motion likely caused the lower image quality seen for the AVs compared with the IJV. Despite good image quality, CE-MRV results from this study differ from previous work concerning extracranial venous scoring24 in which IJV caliber was assessed using a linearly increasing flattening scale. Our semiquantitative approach to venous lumen morphology has precedence15 but may have made consistent scoring problematic, and resulted in moderate (IJV) and poor (AV) interobserver agreement. That said, no significant differences in scoring were observed between subject groups for either scorer.
Our US studies found that 15.8% of MS patients met at least two CCSVI criteria, but so did a nearly equal percentage of HC subjects (17.0%). These findings are in concert with published CCSVI studies using Doppler and B-mode US which reported high heterogeneity in CCSVI diagnosis (≥2 criteria met). Across the studies listed in Table 1 (excluding Zamboni et al. studies3–5), the percentage of all patients with MS who met criteria range from 06,9,11,18 to 84%23 (across all studies average ± stdev = 20.9 ± 27.6%). The percentage of all HC subjects who met criteria range from 06,9,18,19,23,26 to 45.0%20 (average ± stdev = 9.9 ± 16.0%). Our findings further highlight the high variability in venous anatomy and physiology across subject groups.
There were relatively equal percentages of subjects who met CCSVI criteria by US and MR. However, US seems to be a more conservative measure of IJV stenosis; lower percentages of subjects were found to have IJV stenosis by US than by either MRI measurement. Results across all subjects suggest that some small amount of reflux in the cerebrospinal venous system exists in both a normal population and MS patients, and that there is no significant difference in the percentage of these groups exhibiting reflux.
In conclusion, our study demonstrates no significant relationship between MS and IJV stenosis and abnormal flow. We found no difference between subjects with MS and those with other neurological disease, or HC subjects, with regard to venous anatomy and physiology, irrespective of imaging modality. Our results indicate that the CCSVI hypothesis is not strongly explanatory as a cause of MS.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National MS Society Grant #RC1003-A-1, NIH Grant 2R01HL072260
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The University of Wisconsin-Madison receives research support from GE Healthcare.
Authors’ contributions
EMS—4D flow data archiving, reconstruction and postprocessing, statistical analysis, manuscript and figure preparation.
SK—neuro-ultrasonographer, ultrasound data collection and storage, editing of manuscript.
JM—data postprocessing analysis, editing of manuscript.
KMJ—optimization of 4D flow MRI sequence designs and image reconstruction.
MK—oversight of ultrasound imaging and analysis, editing of manuscript.
SF—clinical neuroradiologist, contrast-enhanced MRV scoring.
JOF—study design, clinical MS consultant, recruitment of subjects, editing of manuscript.
OW—study design, imaging protocol, 4D flow MRI sequence design, editing of manuscript.
AF—clinical neuroradiologist, study design, scoring of contrast-enhanced MRV, editing of manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Goldenberg MM. Multiple sclerosis review. P T 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- 2.Putnam TJ. Studies in multiple sclerosis IV. “Encephalitis” and sclerotic plaques produced by venular obstruction. Arch Neuro Psychiatr 1935; 33: 929–940. [Google Scholar]
- 3.Zamboni P, Galeotti R. The chronic cerebrospinal venous insufficiency syndrome. Phlebology 2010; 25: 269–279. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni P, Galeotti R, Menegatti E, et al. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamboni P, Menegatti E, Galeotti R, et al. The value of cerebral Doppler venous haemodynamics in the assessment of multiple sclerosis. J Neurol Sci 2009; 282: 21–27. [DOI] [PubMed] [Google Scholar]
- 6.Doepp F, Paul F, Valdueza JM, et al. No cerebrocervical venous congestion in patients with multiple sclerosis. Ann Neurol 2010; 68: 173–183. [DOI] [PubMed] [Google Scholar]
- 7.Sundström P, Wåhlin A, Ambarki K, et al. Venous and cerebrospinal fluid flow in multiple sclerosis: a case-control study. Ann Neurol 2010; 68: 255–259. [DOI] [PubMed] [Google Scholar]
- 8.Baracchini C, Gallo P. Prevalence, sensitivity, and specificity of chronic cerebrospinal venous insufficiency in MS. Neurology 2011; 77: e124–e125. author reply e125–e126. [PubMed] [Google Scholar]
- 9.Marder E, Gupta P, Greenberg BM, et al. No cerebral or cervical venous insufficiency in US veterans with multiple sclerosis. Arch Neurol 2011; 68: 1521–1525. [DOI] [PubMed] [Google Scholar]
- 10.Centonze D, Floris R, Stefanini M, et al. Proposed chronic cerebrospinal venous insufficiency criteria do not predict multiple sclerosis risk or severity. Ann Neurol 2011; 70: 51–58. [DOI] [PubMed] [Google Scholar]
- 11.Mayer CA, Pfeilschifter W, Lorenz MW, et al. The perfect crime? CCSVI not leaving a trace in MS. J Neurol Neurosurg Psychiatry 2011; 82: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsivgoulis G, Mantatzis M, Bogiatzi C, et al. Extracranial venous hemodynamics in multiple sclerosis: a case-control study. Neurology 2011; 77: 1241–1245. [DOI] [PubMed] [Google Scholar]
- 13.Wattjes MP, van Oosten BW, de Graaf WL, et al. No association of abnormal cranial venous drainage with multiple sclerosis: a magnetic resonance venography and flow-quantification study. J Neurol Neurosurg Psychiatry 2011; 82: 429–435. [DOI] [PubMed] [Google Scholar]
- 14.Auriel E, Karni A, Bornstein NM, et al. Extra-cranial venous flow in patients with multiple sclerosis. J Neurol Sci 2011; 309: 102–104. [DOI] [PubMed] [Google Scholar]
- 15.Zivadinov R, Lopez-Soriano A, Weinstock-Guttman B, et al. Use of MR venography for characterization of the extracranial venous system in patients with multiple sclerosis and healthy control subjects. Radiology 2011; 258: 562–570. [DOI] [PubMed] [Google Scholar]
- 16.Blinkenberg M, Akeson P, Sillesen H, et al. Chronic cerebrospinal venous insufficiency and venous stenoses in multiple sclerosis. Acta Neurol Scand 2012; 126: 421–427. [DOI] [PubMed] [Google Scholar]
- 17.Kantarci F, Albayram S, Demirci NO, et al. Chronic cerebrospinal venous insufficiency: does ultrasound really distinguish multiple sclerosis subjects from healthy controls? Eur Radiol 2012; 22: 970–979. [DOI] [PubMed] [Google Scholar]
- 18.Amato MP, Saia V, Hakiki B, et al. No association between chronic cerebrospinal venous insufficiency and pediatric-onset multiple sclerosis. Mult Scler 2012; 18: 1791–1796. [DOI] [PubMed] [Google Scholar]
- 19.Barreto AD, Brod SA, Bui TT, et al. Chronic cerebrospinal venous insufficiency: case-control neurosonography results. Ann Neurol 2013; 73: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Traboulsee AL, Knox KB, Machan L, et al. Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study. Lancet 2014; 383: 138–145. [DOI] [PubMed] [Google Scholar]
- 21.Comi G, Battaglia MA, Bertolotto A, et al. Observational case-control study of the prevalence of chronic cerebrospinal venous insufficiency in multiple sclerosis: results from the CoSMo study. Mult Scler 2013; 19: 1508–1517. [DOI] [PubMed] [Google Scholar]
- 22.Macgowan CK, Chan KY, Laughlin S, et al. Cerebral arterial and venous blood flow in adolescent multiple sclerosis patients and age-matched controls using phase contrast MRI. J Magn Reson Imaging 2014; 40: 341–347. [DOI] [PubMed] [Google Scholar]
- 23.Al-Omari MH, Rousan LA. Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis. Int Angiol 2010; 29: 115–120. [PubMed] [Google Scholar]
- 24.McTaggart RA, Fischbein NJ, Elkins CJ, et al. Extracranial venous drainage patterns in patients with multiple sclerosis and healthy controls. AJNR 2012; 33: 1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mancini M, Lanzillo R, Liuzzi R, et al. Internal jugular vein blood flow in multiple sclerosis patients and matched controls. PLoS One 2014; 9: e92730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tromba L, Blasi S, Vestri A, et al. Prevalence of chronic cerebrospinal venous insufficiency in multiple sclerosis: a blinded sonographic evaluation. Phlebology 2015; 30: 52–60. [DOI] [PubMed] [Google Scholar]
- 27.Zwischenberger BA, Beasley MM, Davenport DL, et al. Meta-analysis of the correlation between chronic cerebrospinal venous insufficiency and multiple sclerosis. Vasc Endovasc Surg 2013; 47: 620–624. [DOI] [PubMed] [Google Scholar]
- 28.Tsivgoulis G, Sergentanis TN, Chan A, et al. Chronic cerebrospinal venous insufficiency and multiple sclerosis: a comprehensive meta-analysis of case-control studies. Ther Adv Neurol Disord 2014; 7: 114–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu T, Korosec FR, Block WF, et al. PC VIPR: a high-speed 3D phase-contrast method for flow quantification and high-resolution angiography. AJNR 2005; 26: 743–749. [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson KM, Lum DP, Turski PA, et al. Improved 3D phase contrast MRI with off-resonance corrected dual echo VIPR. Magn Reson Med 2008; 60: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol 2014; 67: 850–857. [DOI] [PubMed] [Google Scholar]
- 33.Schrauben EM, Johnson KM, Huston J, et al. Reproducibility of cerebrospinal venous blood flow and vessel anatomy with the use of phase contrast-vastly undersampled isotropic projection reconstruction and contrast-enhanced MRA. AJNR 2014; 35: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klabunde RE. Cardiovascular physiology concepts, 2nd ed Philadelphia, PA: Lippincott Williams & Wilkins/Wolters Kluwer, 2012. [Google Scholar]
- 35.Khatri VP, Wagner-Sevy S, Espinosa MH, et al. The internal jugular vein maintains its regional anatomy and patency after carotid endarterectomy: a prospective study. Ann Surg 2001; 233: 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordenström B, Norhagen A. Effect of respiration on venous return to the heart. Am J Roentgenol Radium Ther Nucl Med 1965; 95: 655–661. [DOI] [PubMed] [Google Scholar]
- 37.Schrauben EM, Anderson AG, Johnson KM, et al. Respiratory-induced venous blood flow effects using flexible retrospective double-gating. J Magn Reson Imaging 2015; 42: 211–216. [DOI] [PubMed] [Google Scholar]
- 38.Lu M, Raber L, Baus L, et al. Ultrasound Evaluations of Chronic Cerebrospinal Venous Insufficiency (CCSVI): Important factors to consider. AAN; Honolulu, Hawaii, 2011.
- 39.Markus HS. Transcranial Doppler ultrasound. Br Med Bull 2000; 56: 378–388. [DOI] [PubMed] [Google Scholar]
- 40.Valdueza JM, Doepp F, Schreiber SJ, et al. What went wrong? The flawed concept of cerebrospinal venous insufficiency. J Cereb Blood Flow Metab 2013; 33: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doepp F, Wurfel JT, Pfueller CF, et al. Venous drainage in multiple sclerosis: a combined MRI and ultrasound study. Neurology 2011; 77: 1745–1751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.