Abstract
Moyamoya disease is a rare steno-occlusive cerebrovascular disorder often resulting in hemorrhagic and ischemic strokes. Although sharing the same ischemic stimulus with atherosclerotic cerebrovascular disease, Moyamoya disease is characterized by a highly instable cerebrovascular system which is prone to rupture due to pathological neovascularization. To understand the molecular mechanisms underlying this instability, angiopoietin-2 gene expression was analyzed in middle cerebral artery lesions obtained from Moyamoya disease and atherosclerotic cerebrovascular disease patients. Angiopoietin-2 was significantly up-regulated in Moyamoya vessels, while serum concentrations of soluble angiopoietins were not changed. For further evaluations, cerebral endothelial cells incubated with serum from these patients in vitro were applied. In contrast to atherosclerotic cerebrovascular disease serum, Moyamoya disease serum induced an angiopoietin-2 overexpression and secretion, accompanied by loss of endothelial integrity. These effects were absent or inverse in endothelial cells of non-brain origin suggesting brain endothelium specificity. The destabilizing effects on brain endothelial cells to Moyamoya disease serum were partially suppressed by the inhibition of angiopoietin-2. Our findings define brain endothelial cells as the potential source of vessel-destabilizing factors inducing the high plasticity state and disintegration in Moyamoya disease in an autocrine manner. We also provide new insights into Moyamoya disease pathophysiology that may be helpful for preventive treatment strategies in future.
Keywords: Moyamoya, angiogenesis, cell culture, cerebrovascular disease, stroke
Introduction
Moyamoya disease (MMD) is a rare cerebrovascular disorder of unknown etiology.1 MMD patients develop steno-occlusive lesions located predominantly at the distal internal carotid artery and its main branches. Without surgical therapy, MMD patients face a grim prognosis due to a high incidence of cerebral strokes (up to 50–70% in five years), either of hemorrhagic nature due to vessel rupture or ischemic nature due to hemodynamic compromise.2,3
In comparison to patients suffering from hemodynamic insufficiency by atherosclerotic cerebrovascular disease (ACVD), MMD patients are characterized by an increased level of vascular plasticity and vascular instability underlying this high incidence of cerebrovascular events. This pathological cerebrovascular situation is reflected by an instable network of collateral peripheral blood vessels that coined the name of the disease (Moyamoya = “puff of smoke” seen in angiographic imaging) (Supplemental Figure 1).2 Importantly, these vascular hallmarks of MMD are specific to the brain vascular system and cannot be observed in any other organ. Understanding the molecular mechanisms underlying the increased instability of cerebral blood vessels in MMD might open novel avenues for preventing strokes in MMD patients. However, so far these pathophysiological mechanisms remain insufficiently understood.
Angiopoietins are important factors implicated in the fetal and postnatal development of the vascular system.4,5 They control angiogenic and vasculogenic processes and therefore, the plasticity state of blood vessels. Angiopoietins-1 and -2 bind with an equal affinity to the endothelial cell (EC)-specific receptor tyrosine kinase (RTK) Tie-2 leading to vessel stabilization by the constitutive angipoietin-1/Tie2 signaling, while activation of the angiopoietin-2/Tie-2 signaling leads to vessel destabilization.6 Thus, angiopoietin-1 promotes vascular silencing and EC barrier maintenance, whereas angiopoietin-2 mediates opposite effects.7 The biological functions of angiopoietin-2 have been observed to be closely linked to the bioavailability of vascular endothelial growth factor (VEGF).8
Herein we tried to understand the molecular mechanisms underlying the vascular hallmarks in MMD, such as the increased brain-specific blood vessel plasticity and vascular instability. We found a significant overexpression of the pro-angiogenic cytokine angiopoietin-2 in samples from human middle cerebral artery (MCA) obtained from MMD patients during the revascularization surgery. Moreover, we show that serum from MMD patients induces a unique expression of angiopoietin-2 and a subsequent down-regulation of endothelial tight junction (TJ) and adherens junction (AJ) molecules leading to impaired EC integrity. By the inhibition of the angiopoietin-2/Tie-2 activation using recombinant angiopoietin-1, these effects could be abrogated suggesting this pathway to play a significant role in establishing the pathological vascular network observed in MMD.
Materials and methods
Study approval
This study was approved by the local research and ethics committee of the Charité – Universitätsmedizin (reference # EA2/086/09). Informed consent was obtained from the patient or legal representative. Hereby we followed and strictly adhered to the Ethical Guidelines of the Charité which are in accordance to the Helsinki Declaration of 1975 and its revision of 1983.
Patients
Between 2009 and 2012, 24 patients with MMD and 13 ACVD with diagnosis were included in this study. We analyzed MMD patients who received superficial temporal artery- to middle cerebral artery (STA-MCA) bypass grafting in our neurosurgical department and from the C. Besta Neurological Institute for treatment of cerebrovascular hemodynamic impairment. All patients received a complete diagnostic workup consisting of neurologic examination, digital subtraction angiography, magnetic resonance imaging, and functional regional cerebral blood flow studies prior to surgery. Clinical data for all patients were recorded. Details on the study population can be found in Table 1.
Table 1.
Clinical characteristics of ACVD and MMD patients.
| MMD | ACVD | |
|---|---|---|
| Number | 24 | 13 |
| Age | 36 ± 11.8 | 56 ± 9.1 |
| Sex | 18 f (75%) 6 m (25%) | 3 f (23%) 10 m (67%) |
| NIHSS | 1.1 ± 0.4 | 1.1 ± 1.1 |
| Statins (%) | 3 (13%) | 8 (62%) |
ACVD: atherosclerotic cerebrovascular disease; MMD: Moyamoya disease; NIHSS: national institute of health scale; f: female; m: male.
Blood sampling and vessel specimens from revascularization surgery
Blood was obtained from all patients by venipuncture using CPT Vacutainer containing Na-citrate as anticoagulant (BD Biosciences, Heidelberg, Germany) prior to revascularization surgery. Vacutainers were centrifuged for 20 min at 1650 × g. Serum with lymphocytes was transferred into a new Falcon tube, centrifuged again at 1650 × g for 10 min and serum supernatant was removed and stored in aliquots at −80℃ until use. Samples of the MCA (M3 segment) were obtained during the surgical procedure. As controls, samples of the MCA and the bypass graft were obtained from patients undergoing STA-MCA surgery due to ACVD. Vessel specimens were shock-frozen in liquid nitrogen and stored at −80℃ until RNA extraction.
Messenger RNA isolation and quantitative real-time PCR
Extraction of total RNA from vessel material was performed with Qiazol (Qiagen, Hilden, Germany) in accordance to manufacturers’ instructions. The Human Angiogenesis RT2 Profiler PCR Array (Qiagen) was used to profile the expression of key genes involved in angiogenesis. In accordance to the manufacturers’ protocol, 102 µL cDNA was mixed with 2× SABiosciences RT2qPCR Master Mix (Qiagen) and H2O to a total volume of 2700 µL. Subsequently, 25 µL of the mixture was placed into each well of the PCR array (a 96-well array was used). The three steps of the cycling program were 95℃ for 10 min for 1 cycle, then 95℃ for 15 s, 60℃ for 1 min. This process was repeated for 40 cycles using CFX-96 real-time system (BioRad).
Total RNA isolation (PureLink RNA Mini Kit, Life Technologies, Karlsbad, CA, USA), cDNA synthesis in cEND and HuVEC cells (Onestep RT-PCR Kit, Qiagen), and quantitative real-time PCR (qPCR) (Premix ex Taq Perfect Real-Time Kit, Takara, Bio, Saint-Germain-en-Laye, France) were performed as previously described.9 For qPCR amplification, we used mouse and human gene-specific primers (obtained from TIB Molbiol Syntheselabor GmbH, Berlin, Germany) designed using Primer Express Software. The ABI PRISM 7300 SDS software (Relative quantification study) was used to determine the cycle threshold (CT) for each reaction and gene expression determined for each gene was normalized to expression of the endogenous housekeeping gene, 18 s RNA. The relative expression intensity was estimated by calculating 2−ΔΔCt for each sample. Specificity of PCR products was checked by melting curve analysis.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) to test human and mouse angiopoietin-1 and -2 in serum samples, and in cEND and HuVEC cell culture supernatants were performed in accordance to manufacturers’ instructions (R&D Systems, Wiesbaden, Germany).
Cell culture and treatment
Cerebral EC line (cEND) was generated from mouse brains and cultivated, as described previously.10 HuVECs (Lonza, Walkersville, USA) were used between passage 2 and 4. Cells were grown until 80% confluence, then transferred into serum-reduced cell culture medium with 2% (v/v) of dextran-coated charcoal-treated fetal calf serum and stimulated with 2.5% heat-inactivated human serum as described elsewhere11 and as indicated in the figure legends. CENDs were pretreated with the recombinant angiopietin-1 protein (10 and 20 ng/mL in PBS; R&D Systems) in the presence of His1Tag cross-linking antibody (5 µg/mL in PBS; R&D Systems) used to enhance the protein stability of angiopietin-1 for 2 h prior to serum exposition.12 Under serum treatment conditions, HuVEC cells were exposed to Endothelial Cell Growth Medium-2 (EGM-2, Lonza) without growth factor supplement.
Transendothelial electrical resistance measurements
Cells were plated on the top of Transwell chambers (0.4 µm pore size; Greiner Bio-One, Frickenhausen, Germany). Transendothelial electrical resistance (TER) was measured using an assembly containing current-passing and voltage-measuring electrodes (World-Precision Instruments Inc., New Haven, CT, USA). The resistance of blank filters was subtracted from the one of filters with cells before calculating the final resistance.
Electrophoresis and immunoblotting
Western blot analysis was performed according to the standard procedures. Primary antibodies were: goat polyclonal antibody against VE-cadherin (R&D Systems), mouse monoclonal antibodies against claudin-5 and β-actin (Life Technologies), and rabbit polyclonal antibody against occludin (Life Technologies), rabbit polyclonal antibodies against angiopoietin-1 and -2 (Abcam, Cambridge, UK). Secondary antibodies were obtained from Jackson Immuno Res. Laboratories, Suffolk, UK. Densitometric quantification was performed employing ImageJ 1.46 e.
In vitro tube formation assay
Growth factor-reduced Matrigel Basement Membrane Matrix (BD Biosciences, Heidelberg, Germany) was thawed on ice at 4℃ overnight and pipettes as well as flat bottom plates (Ibidi, Martinsried, Germany) were pre-cooled before use. Cell culture plates were coated with 50 µL Matrigel per well for 30 min at 37℃, cEND (3 × 104 cells per well) were seeded and images were taken after 48 h of cultivation at 37℃. Image analysis for tube parameters was performed by Wimasis, München Germany.
Cell proliferation assay
Cell proliferation was evaluated by the mitochondrial tetrazolium (MTT) assay. ECs were plated at 3 × 104 cells per well in 96-well culture plates. On the next day, medium was replaced by medium supplemented with 2.5% patients’ serum. After 24 or 48 h, the percentage of viable cells was determined using MTT reagent (Thiazolyl Blue Tetrazolium Bromide, Sigma-Aldrich, Taufkirchen, Germany) diluted with medium to a final concentration of 0.5 mg/mL, added into the cell supernatants and incubated at 37℃ for 4 h. The supernatant was removed and a resolving solution containing 1:1 ethanol and dimethyl sulfoxide was added to solubilize the formazan crystals. Absorbance was measured at 560 nm (reference wave length was 650 nm) by utilizing the microplate reader Infinite® 200 PRO (Tecan, Männedorf, Switzerland).
Immunocytochemistry
CEND cells were stimulated as indicated in the figure legends. Subsequently, cells were fixed with ice cold methanol for 8 min at −20℃ and washed with PBS. Cells were blocked with 2.5% bovine serum albumin for 30 min and stained with the polyclonal goat anti VE-cadherin (R&D Systems) and polyclonal rabbit-anti angiopoietin-2 (R&D Systems), both diluted 1:100 in blocking solution overnight at 4℃. As secondary antibodies, Alexa488-conjugated rabbit anti goat and Alexa561-conjugated goat anti rabbit (Jackson Immuno Res. Lab.) both diluted 1:200 were used. Cell nuclei were counterstained with 4.6-diamidin-2-phenylindol (DAPI) for 10 min at room temperature. Thereafter, coverslips were mounted onto slides using Immu-Mount mounting medium (Dako, Hamburg, Germany). Image acquisition of confocal microscopy was obtained with a confocal microscope (TCS SP5, Leica, Wetzlar, Germany). Confocal imaging of cEND cells was done using a z step of 0.1 µm and 63 × 1.4 NA oil immersion objective. All images were acquired using LCF AF software (all from Leica).
Statistical analysis
Data were obtained from three independent experiments performed in triplicates, as indicated in the figure legends. Values for densitometry and gene expression were averaged to establish a single value for ACVD (control) or MMD serum-incubated cells and compound-treated cells, as indicated. Data were analyzed through GraphPad Prism 6.1 (GraphPad Software) by T-test or ANOVA using the Turkey post hoc test for multiple comparisons assuming significance for *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001, as indicated in the figure legends.
Results
Angiopoietin-2 overexpression in MMD vessel specimens but not changed MMD serum
Our first hypothesis was that the increased vascular instability in MMD might be determined by a deregulated expression of genes involved or influencing the angiogenesis in the brain vasculature involved by the disease. By testing these pro- and anti-angiogenic mediators using the Human Angiogenesis RT2 Profiler PCR Array, we found angiopoietin-2 to be significantly overexpressed in the M3 segment of MMD MCAs compared to the expression of this gene in ACVD specimens (Figure 1(a)). Since angiopoietin-1 and -2 can be released by ECs into the circulation, the concentration of soluble angiopoietins in serum obtained from MMD and ACVD patients was measured by ELISA. Against our expectations, the concentration of these molecules did not differ in both patient cohorts, as indicated by the Ang-2/Ang-1 ratio calculated for inter-individual comparison (Figure 1(b)). This result suggested that the up-regulation of angiopoietin-2 in MCA ECs does not influence the concentration of this molecule in the circulation of MMD patients. We considered therefore rather a local, autocrine effect limited to the involved brain vasculature, which was tested by further experimental approaches.
Figure 1.
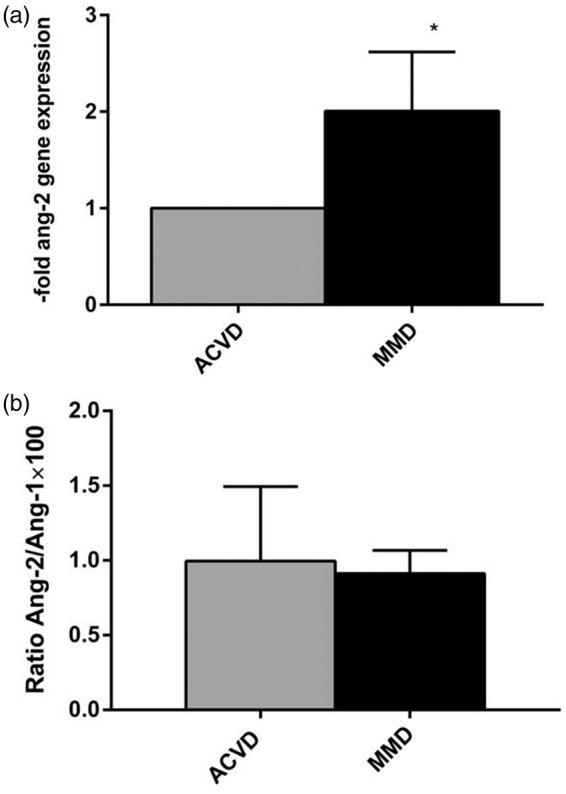
Increased angiopoietin-2 gene expression in MCA from MMD patients. (a) By screening factors involved in the biological process of angiogenesis by Angiogenesis RT2 Profiler (Qiagen), a significant angiopoietin-2 overexpression was found in samples of the M3 MCA segment of MMD patients compared to those obtained from ACVD patients (n = 3 for each group). Expression data were normalized to the expression of five housekeeping genes (ACTB, B2M, GAPDH, HPRT1, and RPLP0). Angiopoietin-2 expression in ACVD patients’ material was set as 1; mean ± SEM, *p < 0.05. (b) Qualitative measurements of Ang-1 and -2 were performed by ELISA. Protein concentrations of angiopoietin (Ang)-1 and Ang-2 were comparable in ACVD versus MMD serum as indicated by the Ang-2/Ang-1 ratio (%) being calculated for every patient.
Elevated angiopoietin-2 levels in MMD serum-exposed cEND cells
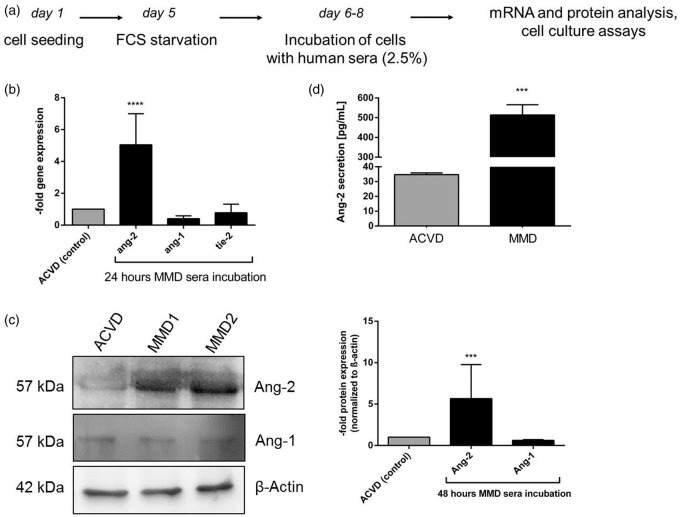
To follow our hypothesis, MMD and ACVD serum was analyzed for vasoactive effects on brain ECs by applying a standardized treatment protocol depicted in Figure 2(a). Compared to those stimulated with ACVD serum, cEND cells exposed to MMD serum showed approximately six-fold elevated transcript levels of angiopoietin-2, while the expression of angiopoietin-1 and tie-2 was not attenuated (Figure 2(b)). An increased protein expression of angiopoietin-2 was also detected by Western blot (Figure 2(c)). Protein expression of angiopoietin-1 was not changed in cells treated respectively with serum from both patient groups. Interestingly, we found a significantly increased extracellular release of angiopoietin-2 protein by MMD serum-treated cEND cells (Figure 2(d)). This prominent secretion of angiopoietin-2 was 14.8-fold when compared to the ACVD control.
Figure 2.
Impact of ACVD and MMD patients' serum on vessel-destabilizing factors in cENDs. (a) Scheme illustrating the protocol used for cEND cell treatment with patients’ serum followed by experimental analysis. (b) Relative gene expression of ang-2, ang-1, and tie-2 was assessed in the presence of ACVD versus MMD serum for 24 hours by qPCR. (c) Protein expression of Ang-2 and Ang-1 normalized to the expression of β-actin in response to MMD versus ACVD serum tested by Western blot. Images were chosen from a representative experiment. The respective densitometrical analysis was displayed in the graph. (d) Extracellular secretion of angiopoietin-2 in response to patients serum monitored in cENDs using ELISA. Values in the graphs are means ± SEM, (n = 3 independent experiments each including at least six different patients’ sera of each clinical type) ****p < 0.001, ***p < 0.001, **p < 0.01, *p < 0.05 versus ACVD-incubated cENDs.
Disturbed brain EC functionality by MMD serum treatment
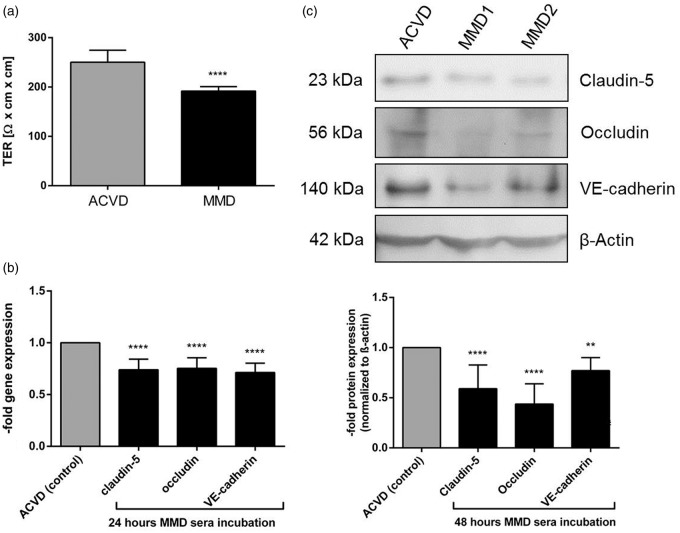
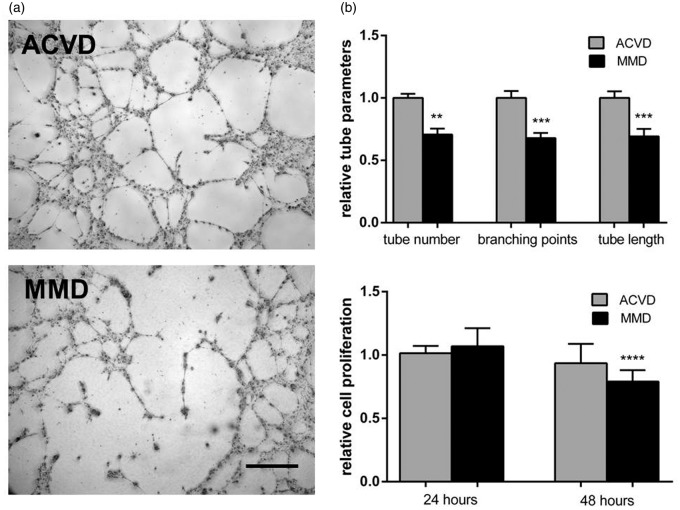
To test if the autocrine signaling of angiopoietin-2 might affect the EC barrier stability, first, the effect of MMD serum on the TER of cENDs was tested and revealed a significant reduction (by about 20% of the ACVD control) (Figure 3(a)) as a positive read out for MMD-induced EC monolayer disintegration. Secondly, a significantly decreased gene expression of the TJs claudin-5 and occludin, and the AJ VE-cadherin was verified in cEND cells kept in the presence of MMD and ACVD serum (Figure 3(b)). The responses mediated by MMD serum in cENDs were comparable for all genes tested encoding junctional proteins (mean down-regulation was about 20–30% of the ACVD serum-treated control cells) and resembled our findings gained in the TER experiment. A reduction of claudin-5, occludin and VE-cadherin could be further determined by Western blot (Figure 3(c)). Functional EC impairment resulting from MMD versus ACVD serum stimulations was further specified applying the Matrigel tube formation assay to test cerebral endothelial cell–cell interactions. The ability of ECs to form tubes in this assay depends on the potential of cells to migrate, establish cell–cell and cell-matrix contacts and to regulate cell survival. MMD sera application induced a clear cell detachment followed by a reduced number of tubes, branching points, and tube length in the network when compared to cells grown in ACVD serum (representative images and graph in Figure 4(a)). ECs exposed to ACVD serum retained the typical tube-like arrangement throughout the experiment. Moreover, we saw a significant reduction of cEND proliferation by up to 20% (Figure 4(b)).
Figure 3.
Effects of ACVD and MMD patients’ serum incubation on cEND barrier characteristics. (a) TER measured in MMD versus ACVD serum-stimulated cENDs over 48 h. Results were expressed as MMD-induced TER in relation to ACVD control. Gene expression (b) and protein expression (c) analysis of cell–ell contacts, claudin-5, occludin and VE-cadherin in response to MMD serum for 24 and 48 h, respectively, analyzed by qPCR and Western blot. Gene expression data were visualized as fold of the expression of respective molecules in ACVD-treated cENDs. Representative Western blot images and the respective densitometrical analysis presented in the graph. Values are means ± SEM, (n = 3 independent experiments each including five different patients’ sera per group performed in triplicates), ****p < 0.001, **p < 0.01, versus cENDs incubated in ACVD serum.
Figure 4.
Impaired cEND tube forming activity and cell proliferation in response to MMD serum. (a) CEND cell proliferation measured at 24 or 48 h of ACVD and MMD serum incubation determined employing MTT proliferation assay and expressed as cell proliferation rate relative to the ACVD control. (b) Phenotypic effect of ACVD and MMD serum incubation tested by tube formation assay. Representative low magnification images (10 × ) of tubes formed by serum-conditioned cENDs were chosen. Total tube number, branching points, and tube length were assessed by Wimasis Software. Values are means ± SEM, (n = 3 independent experiments each including five different patients’ sera per group performed in triplicates), ****p < 0.001, ***p < 0.001, **p < 0.01 versus cENDs incubated in ACVD serum.
Restoration of MMD-induced EC barrier destabilization by inhibition of angiopoietin-2
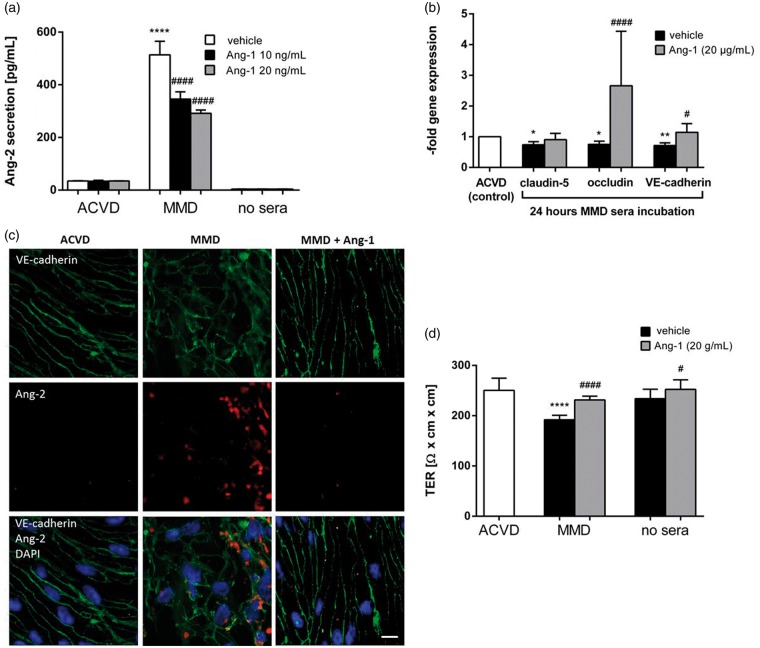
Inhibition of angiopoietin-2 is known to restore EC barrier properties. Therefore, the effects mediated by inhibition of these signaling pathways in brain ECs incubated with MMD serum were examined in our experimental setting. As demonstrated in Figure 5(a), exogenous angiopietin-1 protein was effective to decrease the autocrine release of angiopoietin-2 in response to MMD serum stimulation in a concentration dependent manner. The concentration of angiopoietin-2 protein in cEND cell supernatants remained, however, significantly increased in response to MMD serum as compared to the ACVD control. A minor restoring but not significant effect on claudin-5 gene expression was observed in cENDs incubated with angiopoietin-1 simultaneously to MMD serum treatment (Figure 5(b)). Also, a significant improvement of VE-cadherin expression was detected in cENDs co-treated with MMD serum and angiopoietin-1. The gene expression of occludin could be sufficiently reconstituted in the presence of exogenous angiopoietin-1 protein (Figure 5(b)). To explore whether incubations with serum obtained from ACVD and MMD patients had an effect on cEND cell morphology and cell–cell contact localization, immunocytochemical staining of the AJ protein VE-cadherin was performed and summarized in Figure 5(c). We observed changes in cellular morphology of MMD serum-incubated cells as compared to ACVD cENDs, which changed their spindle-like elongated shape into a more de-organized round phenotype with delocalized and discontinuous VE-cadherin protein stain. These morphological alterations were accompanied by a firm intercellular staining of angiopoietin-2 protein, which was to a much lesser extent detectable in ACVD patients’ serum-incubated cENDs. The MMD serum-mediated VE-cadherin de-organization and intracellular angiopietin-2 induction were attenuated by co-treating cENDs with exogenous angiopoietin-1. In addition, an elongated EC morphology with a membranous localization of VE-cadherin was detected by confocal fluorescence microscopy. Concomitantly, the MMD serum-mediated decrease of TER could be restored by applying recombinant angiopoietin-1 protein (Figure 5(d)).
Figure 5.
EC barrier stability upon blocking angiopoietin-2 in MMD serum-incubated cENDs. CEND cells were pre-stimulated with recombinant angiopoietin-1 (10 or 20 ng/mL) in the presence of His1 Tag cross-linking antibody (5 µg/mL) or left untreated for 2 h prior to exposition to 2.5% patients’ serum for 24 or 48 h, as indicated. (a) Effects of recombinant Ang-1 on secretion of Ang-2 analyzed by ELISA assay. (b) Gene expression of claudin-5, occludin and VE-cadherin in response to human serum and angiopoietin-2 blockade was analyzed by real-time qPCR. (c) Treated cEND cells were stained for VE-cadherin (green), Ang-2 (red), and DAPI (blue) in order to visualize cell nuclei. Angiopoietin-2 abrogated the MMD serum-induced morphology change and VE-cadherin localization (n = 3 independent experiments each including at least five different patients’ sera of each group). Bar: 10 µm. (d) The effect of ACVD and MMD serum on TER of cENDs in the presence or absence of angiopoietin-1 protein was determined, expressed in absolute TER values (Ω × cm2). Values from all graphs are means ± SEM, (n = 3 independent experiments performed in triplicates each including five different patients’ sera per group), ****/####p < 0.0001, ***/###p < 0.001, **/##p < 0.01, */#p < 0.05 versus cENDs incubated in ACVD or MMD serum, respectively.
MMD serum-induced pro-angiogenic factors and EC barrier weakening are brain EC-specific
Our results described for cerebrovascular cENDs were studied in relation to findings gained in experiments with non-brain ECs, i.e. HuVEC cells. Gene expression of angiogenic factors and some of the respective receptors in HuVECs were compared with data obtained for cENDs and summarized in Supplementary Table 1. A slight reduction of angiopoietin-2 and increased angiopoietin-1 transcript levels by 50% in MMD serum-treated HuVECs was detected when compared with HuVECs incubated with ACVD serum. The tie-2 receptor remained unaffected in both, MMD versus ACVD serum-stimulated cell cultures. Other growth factors encoding genes, including platelet-derived growth factor (PDGF), transforming growth factor β-1 (TGFβ-1), as well as the notch1 receptor, its’ ligands delta-like ligand 4 (dll4) and jag1 were tested in both cell types under the same conditions. There were both, down- and up-regulatory effects on the expression of these molecules in cENDs incubated with MMD serum. On the contrary, these effects were minor or not significant in MMD serum-stimulated HuVECs when related to cells exposed to ACVD serum, except for dll4 which was decreased in both EC cultures. Also, the expression of TJ and AJ molecules has been examined in HuVECs in the presence of both serum types (Supplementary Table 1). On the transcript level of these molecules, no significant changes could be identified in response to MMD serum when compared to cells kept in ACVD serum.
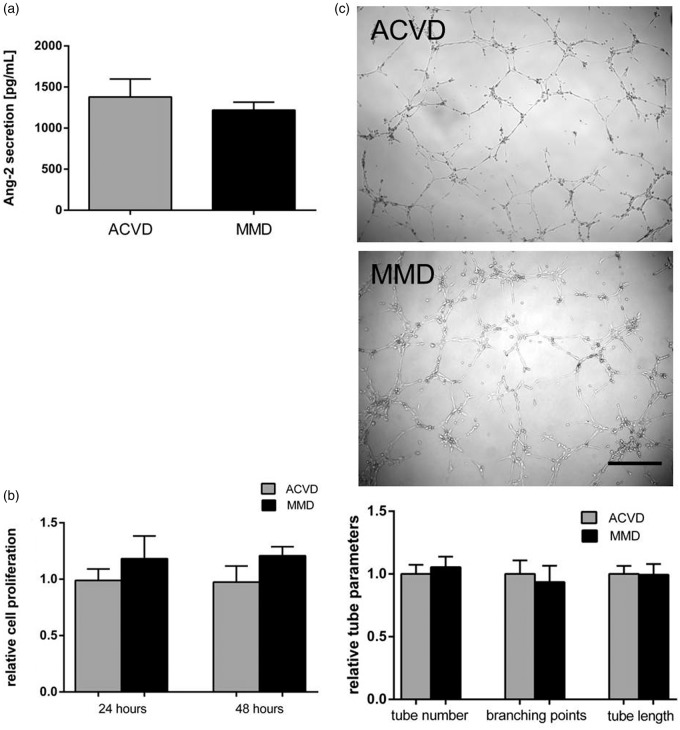
Testing functional changes of HuVECs in the presence of MMD against ACVD serum, no relevant differences in angiopoietin-2 secretion into the cell culture supernatant were observed (Figure 6(a)). In addition, no significant alterations in cell proliferation and tube forming ability of MMD versus ACVD serum-incubated HuVECs were detectable (Figure 6(b) and (c)).
Figure 6.
ACVD versus MMD serum responses observed in non-cerebral ECs, HuVEC cells. HuVEC cells were incubated in MMD or ACVD serum for 48 h applying the experimental protocol used for cENDs. (a) Secretion of human Ang-2 into the supernatant was analyzed by ELISA. (b) HuVEC cell proliferation was not significantly changed in response to ACVD and MMD serum incubations, as measured by MTT assay. (c) Phenotypic effects of ACVD and MMD patients' serum incubation were tested by Matrigel tube formation assay. Representative low magnification images (10 × magnification) of tubule tube formation in response to ACVD and MMD serum were chosen. Total number of tubes, branching points, and tube length was assessed by Wimasis Image Analysis and depicted by the graph. Values are means ± SEM, (n = 3 independent experiments each including five different sera per group).
Discussion
The principal novel finding of the herein presented study is that there is a significant overexpression of the pro-angiogenic and barrier-destabilizing factor angiopoietin-2 in the M3 segment of the MCA obtained from MMD patients, while the systemic concentration of this molecule remains unchanged compared to ACVD. This important result suggested a local effect of the angiopoietin-2 up-regulation in the vasculature involved by the disease, without influencing the systemic concentration of this molecule. Moreover, the cerebral EC culture cEND could be specified as an interesting in vitro model to study the effects mediated by the constituents of MMD serum on cerebral endothelium. With this respect, incubation of cerebral ECs with MMD serum provoked a disease-specific strong intra-cellular overexpression and secretion of angiopoietin-2 into the cell culture supernatant. In addition, MMD serum stimulation lowered the TER of brain ECs and induced a down-regulation of important cell–cell contacts. This in turn was followed by an impairment of cEND cell proliferation and reduced ability to form reliable EC–EC interactions. Interestingly, the MMD serum-mediated TJ and AJ barrier disruption accompanied by de-organized cell morphology was partially suppressed by antagonizing angiopoietin-2 by exogenous angiopoietin-1. As a control for these experiments, tissue and serum from ACVD patients were chosen since MMD and ACVD patients exhibit a similar occurrence of ischemic episodes and a comparable localization of cerebral infarctions.
Angiopoietin-1 and -2 facilitate vascular responses regulating angiogenesis and EC barrier integrity.13 Although both molecules seemed to be promising systemic markers with a potential impact on the development of Moyamoya collaterals, systemic concentrations of angiopoietins were not altered in our patient sera.
Overexpression of angiopoietin-2 has been implicated in the activation of signals leading to a disassembly of the junctional complex under physiological pro-angiogenic conditions and in a number of cerebrovascular disorders.8 We hypothesized, first, that circulating MMD serum constituents promote an elevation of angiopoietin-2 by brain ECs in an autocrine manner. Secondly, this local stimulus might be at least partially responsible for the development of Moyamoya vessels, irrespectively of the hemodynamic insufficiency that characterizes both diseases since these effects are absent in ACVD.
The composition of TJs and AJs corresponds with EC monolayer integrity in vitro and in vivo.14 Claudin-5 and occludin, both TJ molecules being prominently expressed in cerebral ECs, contribute to their characteristically high TER.15 A lowered expression of these molecules causes therefore EC monolayer leakage and was also described to participate in the regulation of angiogenic processes.16 An adequate expression of VE-cadherin is required for TJ sealing and functions as a control unit for the dynamics and the extent of angiogenic processes. While EC barrier strengthening is typical for quiescent and mature vasculature, its weakening promotes angiogenesis.17 Loss of these regulatory mechanisms of angiogenesis by, i.e., a decreased VE-cadherin expression or its delocalization results in lowered TER in in vitro and in uncontrolled neovascularization in vivo.17,18 In line with these facts, our study revealed a functional impairment of EC–EC interactions together with decreased EC proliferation and tube forming ability. The tube formation assay reflects the potential of cells to migrate and form cell–cell and cell-matrix contacts.19 Thus, our results show that weakening of cell–cell contacts and the inability to form a reliable tubular network might on one hand contribute to the hemorrhagic manifestations in MMD and on the other hand promote the development of aberrant collateral MMD vessel network.
A number of in vivo and in vitro studies demonstrated that exogenous angiopoietin-1 exerts anti-permeability effects on ECs by antagonizing angiopoietin-2.6,20–23 Our experiments demonstrate that the functionality of MMD serum-conditioned cENDs could be partially reconstituted by inhibiting the angiopietin-2/tie-2 signaling pathway. We show that simultaneous cENDs incubation with MMD serum and exogenous angiopoietin-1 partially re-established the expression and localization EC contact molecules. Occludin and VE-cadherin overcame completely the down-regulative effect of MMD serum on their expression. In contrast, antagonizing angiopoietin-2 failed to preserve claudin-5 in MMD serum-treated cEND cells. Moreover, by stimulating cEND cells with MMD serum and angiopietin-1, the secretion of angiopoietin-2 into the extracellular compartment could not be abrogated to the ACVD control level and remained relatively high. However, the re-establishment of cell–cell contacts mediated by angiopoietin-1 was sufficient enough to lead to a significant increase of TER values reaching the level of cell kept in ACVD serum. Therefore, in the context of MMD, our results provide new evidence supporting angiopoietin-2 to play an important role in promoting autocrine-mediated endothelial disturbances which strongly differ from those observed in vessel material from ACVD patients and brain ECs exposed to ACVD serum in vitro. We also suggest angiopoietin-2 to be a new promising target to prevent micro-bleeds or hemorrhagic stokes in MMD, since the inhibition of angiopoietin-2/tie-2 signaling in our in vitro model restored EC barrier properties. The signaling pathways of angiopoietins during vessel maturation are not completely understood until now. In contrast to angiopoietin-1, angiopoietin-2 is expressed almost exclusively in vascular endothelium.24 Angiopoietin-2 overexpressing mice show a similar phenotype as angiopoietin-1 and tie-2-deficient mice.25 Given the fact that the molecule was found to be prominently up-regulated in highly vascularized tumors (such as glioma, invasive ductal breast carcinoma and metastatic melanoma) and in pro-angiogenic diseases (such as macular degeneration, rheumatoid arthritis, osteoarthritis, psoriasis),26–29 its expression strongly correlates with tumor angiogenesis and poor prognosis.30–32 A number of in vivo studies implicated that blocking angiopoietin-2 efficiently decreases tumor angiogenesis and the metastatic potential.33,34 Interestingly, in the tumor context, it was demonstrated that healthy vessels were not affected by a selective anti-angiopoietin-2 treatment.35 This result is supported by the fact that angiopoietin-2 was found to be overexpressed under pathological conditions but without having any essential physiological role in healthy mature vessels.35 Selective angiopietin-2 therapy by specific antibodies or small molecules may therefore possess a beneficial safety profile accompanying their anti-tumor and anti-metastatic potential.36 These facts allow us to speculate that the inhibition of angiopoietin-2 in combination with other anti-angiogenic molecules might represent a promising strategy to prevent or reverse mechanisms leading to MMD pathology. Due to the numerous beneficial effects of anti-angiopoietin-2 therapy, an inta-arterial administration to the affected vessels might be therefore obvious. However, such approaches remain to be extensively tested in preclinical studies. Moreover, it is speculative if such targeting strategies may replace the perfusion restoring revascularization techniques (i.e. bypass surgery) which represent the actual treatment of choice despite its invasive nature.3
There are some shortcomings of our study that have to be mentioned. On one hand, no other specific angiopoietin-2 blocking substances, such as inhibitors or antibodies referred in the tumor context have been tested in our cell culture model. Moreover, owing to the complexity of the disease, it would be important to test on one hand downstream effectors of the angiopoietin-2 signaling pathway. Pathways that might be activated by the autocrine release of angiopoietin-2 are, i.e. activated integrins, the focal adhesion kinase (FAK), the extracellular regulated kinase 1/2 (ERK1/2), or phosphatidylinositide 3-kinase (PI3K)/Akt kinase signaling which are involved in cell proliferation, differentiation and motility.37 Also, other barrier-destabilizing or pro-angiogenic molecules playing a role in the MMD pathology should be tested in our experimental setting. They might have an own impact on the disease or even interplay with angiopietin-2.
On a technical note, as controls, we exclusively used in our study a part of the MCA and serum obtained from ACVD patients. Previous works, including our own, have pointed out that the chronic cerebral ischemia present in ACVD and MMD induces an elevation of pro-angiogenic and inflammatory serum constituents and a unique collateralization pattern.2,38–41 In our present investigation, we attended to emphasize disease-specific mechanisms driving the development of the characteristic MMD collaterals regardless of ischemic stimuli. Moreover, beside expression analysis performed on surgical specimen further analysis was conducted applying the cEND cell culture model. This fact was on one hand due to the rarity of MMD and to the access to the resection samples. Moreover, the isolation of ECs for functional analysis directly from vessel resections from both patients groups was proven to be difficult to realize. Given the small resection size, the extremely low EC yield hinders cultivation of these cells until forming a continuous monolayer. Therefore, and considering the lack of suitable animal models and the access to affected cells from patient lesions, we demonstrate that the cEND cell line represents a useful and standardized experimental approach for studies of the MMD pathophysiology.
Taken together, MMD represents a rare and clinically, as well as genetically heterogeneous disease. There is still a lack of animal models and the access to affected cells from patient lesions is limited. Here, we demonstrate for the first time that the pro-angiogenic and vessel-destabilizing mediator angiopoietin-2 is significantly overexpressed in MCA samples obtained from MMD patients. Importantly, the cEND cell line can be applied as an experimental approach to study the fragility of cerebral ECs under MMD conditions. Based on this in vitro model, we could confirm the up-regulation and secretion of angiopoietin-2 in cENDs stimulated with MMD serum indicating unknown MMD serum constituents that actively and in a local, autocrine manner. The release of angiopoietin-2 causes a disintegration of junctional components. These seemingly brain EC-specific effects are finally resulting in functional changes of cerebral EC properties. Moreover, we demonstrate that these EC barrier disintegrating effects mediated by MMD serum can be partially reconstituted by angiopoietin-1. Hence, our study could provide the basis for novel treatment strategies in order to prevent cerebrovascular events in this disease.
Significance
MMD is a rare steno-occlusive cerebrovascular disorder often resulting in hemorrhagic and ischemic strokes, while for ACVD, mature vessels and low neoangiogenic potential are indicated. MMD is characterized by a highly plastic and instable neovascularization, although the same ischemic stimulus was identified for both disorders. Here, we identify a mechanism that contributes to the high incidence of cerebrovascular hemorrhagic and ischemic events in MMD. We show that serum obtained from MMD patients induces impaired barrier properties of brain endothelial cells due to the autocrine secretion of the pro-angiogenic cytokine angiopoietin-2.
Supplementary Material
Acknowledgements
The authors are grateful to Irina Kremenetskaia for excellent technical assistance and to Dr. Francesco Acerbi and Paolo Ferroli of Neurosurgical Unit of IRCCS C.Besta Neurological Institute. VP is participant in the Charité Clinical Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KGB, DF, TS, VP, GB, SMK, MC, JW, MF, AB, and PV designed the experiments, collected, analyzed, and interpreted the data, and wrote the manuscript. PV interpreted the data and critically revised the manuscript.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data.
References
- 1.Suzuki J, Takaku A. Cerebrovascular “Moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20: 288–299. [DOI] [PubMed] [Google Scholar]
- 2.Czabanka M, Acker G, Jussen D, et al. Collateralization and ischemia in hemodynamic cerebrovascular insufficiency. Acta Neurochir (Wien) 2014; 156(11): 2051–2058. [DOI] [PubMed] [Google Scholar]
- 3.Vajkoczy P. Moyamoya disease: collateralization is everything. Cerebrovasc Dis 2009; 28: 258. [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000; 407: 242–248. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999; 5: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 6.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999; 286: 2511–2514. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Mori M, Sakamoto Y, et al. Biologic significance of angiopoietin-2 expression in human hepatocellular carcinoma. J Clin Invest 1999; 103: 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A 2002; 99: 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blecharz KG, Burek M, Bauersachs J, et al. Inhibition of proteasome-mediated glucocorticoid receptor degradation restores nitric oxide bioavailability in myocardial endothelial cells in vitro. Biol Cell 2014; 106(7): 219–235. [DOI] [PubMed] [Google Scholar]
- 10.Förster C, Silwedel C, Golenhofen N, et al. Occludin as direct target for glucocorticoid-induced improvement of blood-brain barrier properties in a murine in vitro system. J Physiol 2005; 565(Pt 2): 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blecharz KG, Haghikia A, Stasiolek M, et al. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cEND incubated with sera from patients with multiple sclerosis. Mult Scler 2010; 16: 293–302. [DOI] [PubMed] [Google Scholar]
- 12.Koh GY. Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med 2013; 19: 31–39. [DOI] [PubMed] [Google Scholar]
- 13.Peters S, Cree IA, Alexander R, et al. Angiopoietin modulation of vascular endothelial growth factor: Effects on retinal endothelial cell permeability. Cytokine 2007; 40: 144–150. [DOI] [PubMed] [Google Scholar]
- 14.Kleinschnitz C, Blecharz K, Kahles T, et al. Glucocorticoid insensitivity at the hypoxic blood-brain barrier can be reversed by inhibition of the proteasome. Stroke 2011; 42: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 15.Coisne C, Engelhardt B. Tight junctions in brain barriers during central nervous system inflammation. Antioxid Redox Signal 2011; 15: 1285–1303. [DOI] [PubMed] [Google Scholar]
- 16.Jain RK. Molecular regulation of vessel maturation. Nat Med 2003; 9: 685–693. [DOI] [PubMed] [Google Scholar]
- 17.Lampugnani MG, Dejana E. Adherens junctions in endothelial cells regulate vessel maintenance and angiogenesis. Thromb Res 2007120 Suppl 2): S1–S6. [DOI] [PubMed] [Google Scholar]
- 18.Blecharz KG, Drenckhahn D, Förster CY. Glucocorticoids increase VE-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cEND cells. J Cereb Blood Flow Metab 2008; 28: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 19.Birdsey GM, Dryden NH, Amsellem V, et al. Transcription factor Erg regulates angiogenesis and endothelial apoptosis through VE-cadherin. Blood 2008; 111: 3498–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 1996; 87: 1161–1169. [DOI] [PubMed] [Google Scholar]
- 21.Gamble JR, Drew J, Trezise L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res 2000; 87: 603–607. [DOI] [PubMed] [Google Scholar]
- 22.Kim I, Moon SO, Park SK, et al. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res 2001; 89: 477–479. [DOI] [PubMed] [Google Scholar]
- 23.Mammoto T, Parikh SM, Mammoto A, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 2007; 282: 23910–23918. [DOI] [PubMed] [Google Scholar]
- 24.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004; 103: 4150–4156. [DOI] [PubMed] [Google Scholar]
- 25.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997; 277: 55–60. [DOI] [PubMed] [Google Scholar]
- 26.Joussen AM. Vascular plasticity – the role of the angiopoietins in modulating ocular angiogenesis. Graefes Arch Clin Exp Ophthalmol 2001; 239: 972–975. [DOI] [PubMed] [Google Scholar]
- 27.Helfrich I, Edler L, Sucker A, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin Cancer Res 2009; 15: 1384–1392. [DOI] [PubMed] [Google Scholar]
- 28.Hu B, Guo P, Fang Q, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci U S A 2003; 100: 8904–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsui S, Inoue H, Yasuda K, et al. Angiopoietin 2 expression in invasive ductal carcinoma of the breast: its relationship to the VEGF expression and microvessel density. Breast Cancer Res Treat 2006; 98: 261–266. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad SA, Liu W, Jung YD, et al. Differential expression of angiopoietin-1 and angiopoietin-2 in colon carcinoma. A possible mechanism for the initiation of angiogenesis. Cancer 2001; 92: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 31.Wurmbach JH, Hammerer P, Sevinc S, et al. The expression of angiopoietins and their receptor Tie-2 in human prostate carcinoma. Anticancer Res 2000; 20: 5217–5220. [PubMed] [Google Scholar]
- 32.Zagzag D, Hooper A, Friedlander DR, et al. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol 1999; 159: 391–400. [DOI] [PubMed] [Google Scholar]
- 33.Huang H, Lai JY, Do J, et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin Cancer Res 2011; 17: 1001–1011. [DOI] [PubMed] [Google Scholar]
- 34.Mazzieri R, Pucci F, Moi D, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 2011; 19: 512–526. [DOI] [PubMed] [Google Scholar]
- 35.Thomas M, Kienast Y, Scheuer W, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared to Pan-Angiopoietin-1/-2 inhibitors. PLoS One 2013; 8: e54923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerald D, Chintharlapalli S, Augustin HG, et al. Angiopoietin-2: an attractive target for improved antiangiogenic tumor therapy. Cancer Res 2013; 73: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 37.Pafumi I, Favia A, Gambara G, et al. Regulation of angiogenic functions by angiopoietins through calcium-dependent signaling pathways. Biomed Res Int 2015; 2015: 965271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafat N, Beck GCh, Peña-Tapia PG, et al. Increased levels of circulating endothelial progenitor cells in patients with Moyamoya disease. Stroke 2009; 40: 432–438. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto M, Aoyagi M, Fukai N, et al. Differences in cellular responses to mitogens in arterial smooth muscle cells derived from patients with Moyamoya disease. Stroke 1998; 29: 1188–1193. [DOI] [PubMed] [Google Scholar]
- 40.Hojo M, Hoshimaru M, Miyamoto S, et al. A cerebrospinal fluid protein associated with Moyamoya disease: report of three cases. Neurosurgery 1999; 45: 170–173. discussion 173–174. [DOI] [PubMed] [Google Scholar]
- 41.Bedini G, Blecharz KG, Nava S, et al. vasculogenic and angiogenic pathways in Moyamoya disease. Curr Med Chem 2016; 23: 315–345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.