Abstract
The Wingless/integrase-1 (Wnt) family of protein ligands and their functional antagonists, secreted frizzled-related proteins (sFRPs), regulate various biological processes ranging from embryonic development to immunity and inflammation. Wnt5a and sFRP5 comprise a typical ligand/antagonist pair, and the former molecule was recently detected at the messenger RNA (mRNA) level in human periodontitis. The main objective of this study was to investigate the interrelationship of expression of Wnt5a and sFRP5 in human periodontitis (as compared to health) and to determine their roles in inflammation and bone loss in an animal model. We detected both Wnt5a and sFRP5 mRNA in human gingiva, with Wnt5a dominating in diseased and sFRP5 in healthy tissue. Wnt5a and sFRP5 protein colocalized in the gingival epithelium, suggesting epithelial cell expression, which was confirmed in cultured human gingival epithelial cells (HGECs). The HGEC expression of Wnt5a and sFRP5 was differentially regulated by a proinflammatory stimulus (lipopolysaccharide [LPS] from Porphyromonas gingivalis) in a manner consistent with the clinical observations (i.e., LPS upregulated Wnt5a and downregulated sFRP5). In HGECs, exogenously added Wnt5a enhanced whereas sFRP5 inhibited LPS-induced inflammation, as monitored by interleukin 8 production. Consistent with this, local treatment with sFRP5 in mice subjected to ligature-induced periodontitis inhibited inflammation and bone loss, correlating with decreased numbers of osteoclasts in bone tissue sections. As in humans, mouse periodontitis was associated with high expression of Wnt5a and low expression of sFRP5, although this profile was reversed after treatment with sFRP5. In conclusion, we demonstrated a novel reciprocal relationship between sFRP5 and Wnt5a expression in periodontal health and disease, paving the way to clinical investigation of the possibility of using the Wnt5a/sFRP5 ratio as a periodontitis biomarker. Moreover, we showed that sFRP5 blocks experimental periodontal inflammation and bone loss, suggesting a promising platform for the development of a new host modulation therapy in periodontitis.
Keywords: inflammation, LPS, gingival epithelial cells, biomarkers, bone loss, Wnt proteins
Introduction
Periodontitis is a dysbiotic inflammatory disease that leads to the destruction of the periodontium and represents a major cause of tooth loss in adults (Lamont and Hajishengallis 2015). Current therapies are not always effective, and this oral disease continues to be a significant health and economic burden (Beikler and Flemmig 2011; Chapple 2014). Therefore, there is a need for better understanding of the underlying immunopathology and for rational therapeutic approaches.
The evolutionarily conserved Wingless/integrase-1 (Wnt) family of proteins is involved in various essential functions, including embryonic development, cell differentiation, and proliferation, and abnormal Wnt signaling is implicated in neoplastic and degenerative diseases (He et al. 1997; Kikuchi et al. 2009; Libro et al. 2016). At least 19 Wnt protein ligands interact with members of the Frizzled (Fz) family of serpentine receptors and induce signaling that is broadly classified into 2 main arms: β-catenin-dependent (canonical) and β-catenin-independent (noncanonical) Wnt signaling (Kikuchi et al. 2009). Accumulating evidence indicates that Wnt signaling pathways also play significant roles in immunity and inflammation and have been implicated in certain inflammatory disorders, such as psoriasis, sepsis, and obesity-associated metabolic dysfunction (George 2008; Staal et al. 2008; Ouchi et al. 2010; Maiti et al. 2012; Kumawat and Gosens 2016). The first direct evidence linking Wnt signaling to immunity was described in Drosophila, where the WntD homolog was shown to be upregulated by Toll/nuclear factor (NF)–κB signaling and to mediate protection against infection with Listeria monocytogenes (Gordon et al. 2005). A similar mechanism was shown to operate also in human mononuclear cells. Specifically, the upregulation of the noncanonical Wnt5a protein in response to microbial challenge also required Toll-like receptor-mediated NF-κB activation (Blumenthal et al. 2006).
The interaction of Wnt proteins with Fz receptors is antagonized by secreted frizzled-related proteins (sFRPs), which are structurally related to the Fz proteins and can thereby act as decoy receptors (Bovolenta et al. 2008). For instance, sFRP5 has been shown to antagonize Wnt5a-induced activation of c-Jun N-terminal kinase-mediated inflammation in the adipose (Ouchi et al. 2010) or cardiac (Nakamura et al. 2016) tissue. Recently, it was demonstrated that the expression of Wnt5a mRNA is upregulated in the gingiva of chronic periodontitis patients compared to healthy gingiva (Nanbara et al. 2012). Soon after, a genome-wide association study suggested an association between the WNT5A gene and severe chronic periodontitis (Divaris et al. 2013). However, no periodontal study to date has investigated how the expression of Wnt5a is correlated with that of sFRP5, which in fact dictates the activity of Wnt5a (Ouchi et al. 2010). Moreover, whether there is a causal relationship between Wnt5a and induction of periodontal inflammation and bone loss has yet to be addressed.
The main objectives of this study, therefore, were to investigate the relative abundance and interrelationship of Wnt5a and sFRP5 at both the messenger RNA (mRNA) and protein expression level in human periodontitis and to determine their roles in a mouse model of the disease. Our data presented here demonstrate a reciprocal relationship between the gingival expression of Wnt5a and sFRP5, which, respectively, mediate destructive and protective effects in experimental periodontitis. Our study thus provides a promising platform for the development of new host modulation therapies in periodontitis.
Materials and Methods
Human Gingival Tissue Specimens
Human gingival tissues were obtained during routine periodontal surgeries and crown-lengthening surgeries conducted on 69 systemically healthy adults at the University of Pennsylvania, School of Dental Medicine Graduate Periodontics Clinic. Prior to surgery, all of the periodontitis patients underwent scaling and root planing. Samples of gingival tissues were obtained from 29 individuals without periodontal disease (control group) during crown-lengthening surgery and 40 patients with generalized or localized moderate to severe chronic periodontitis. Appendix Table 1 presents the mean demographic and clinical parameters of the 2 groups. There were no significant differences in sex or age between periodontitis and healthy control individuals. All 69 samples were used for histochemistry, whereas 17 samples were used for real-time polymerase chain reaction (PCR) analysis. The probing depth and clinical attachment level of the sampling sites were evaluated using a periodontal probe (Hu-Friedy, Leimen, Germany). Research was performed under an institutional review board–approved protocol, and all donors provided informed consent.
Immunofluorescence Histochemistry
Gingival biopsy specimens were fixed in 4% paraformaldehyde and embedded in OCT compound. Cryostat sections were cut at 8 µm and mounted on glass slides. The sections were fixed in paraformaldehyde for 10 min and washed with mixed solution of phosphate-buffered saline (PBS) and 0.1% Tween 20 followed by PBS containing 0.1% Triton X-100, as well as PBS alone. Sections were stained using mouse monoclonal antibody against human Wnt5a (clone 6F2, IgG1; LSBio) and rabbit polyclonal antibody against human sFRP (Novus), followed by secondary reagents (Alexa Fluor 488–conjugated goat anti–rabbit IgG or Alexa Fluor 594–conjugated goat anti–mouse IgG; Life Technologies). The specificity of staining was confirmed by using appropriate isotype control (IgG1; eBioscience) or nonimmune rabbit IgG (Santa Cruz Biotechnology) followed by Alexa Fluor 488– or Alexa Fluor 594–conjugated anti-IgG. Images were captured using a Nikon Eclipse NiE automated upright fluorescent microscope.
Quantitative Real-time PCR
Total RNA was extracted from excised human and mouse gingival tissue or cultured cells using the PerfectPure RNA cell kit (5 Prime; Fisher) and quantified by spectrometry at 260 and 280 nm. The RNA was reverse-transcribed using the High Capacity RNA-to-cDNA Kit (Life Technologies), and quantitative PCR (qPCR) with complementary DNA (cDNA) was performed using the Applied Biosystems 7500 Fast Real-Time PCR System according to the manufacturer’s protocol (Life Technologies). Data were analyzed using the comparative (ΔΔCt) method. TaqMan probes, sense primers, and antisense primers for detection and quantification of genes investigated in this article were purchased from Life Technologies.
Human Gingival Epithelial Cells
Human gingival epithelial cells (HGECs) were prepared from clinically normal gingival tissue. The tissues were treated overnight with Dulbecco’s modified minimal essential medium containing 0.025% trypsin and 0.01% EDTA. After washing with PBS and subsequent chopping into small pieces, the tissues were suspended in EpiLife medium, containing supplement S7 and penicillin–streptomycin–amphotericin B solution (Thermo-Fisher). The tissues were removed when the cells started to grow and were maintained in culture until the cells reached confluence. For the stimulation experiments, HGECs were seeded into a 12-well culture plate. After 24 h of incubation, the attached cells were washed extensively with EpiLife medium and incubated in the same medium containing supplement S7 with Porphyromonas gingivalis lipopolysaccharide (LPS) (Invivogen), Wnt5a (R&D Systems), or sFRP5 (R&D Systems) for 12 h (to assess gene expression) or 24 h (to assess chemokine release). The various compounds were used at concentrations indicated in the figure legends and determined in preliminary experiments.
Chemokine Release Assay
The level of interleukin (IL)–8 in the supernatants of HGECs culture was determined using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience) according to the manufacturer’s instructions.
Mice and Periodontitis Model
All experiments were performed in accordance with the Regulations and Guidelines on Scientific and Ethical Care and Use of Laboratory Animals of the Science Council of Japan and approved by the Institutional Animal Care and Use Committee at Niigata University (permit number 26, 136-3). Six-wk-old male and female C57BL/6 mice were obtained from Japan SLC, Inc. The mice were maintained under specific pathogen-free conditions and fed regular chow and sterile water until the commencement of the experiment at 6 wk of age. To induce ligature-induced periodontitis, a 5-0 silk ligature was tied around the maxillary left second molar, whereas the contralateral molar tooth in each mouse was left unligated to serve as baseline control in the bone loss measurements. The mice were euthanized 5 d after placement of the ligatures. Gingiva were dissected and processed for qPCR determination of proinflammatory cytokine mRNA expression levels. Periodontal bone loss was assessed morphometrically in defleshed maxillae using a dissecting microscope (×40) fitted with a video image marker measurement system (Nikon Instruments). Specifically, the distance from the cement-enamel junction (CEJ) to the alveolar bone crest (ABC) was measured on 6 predetermined points on the ligated second molar and the affected adjacent regions (Abe and Hajishengallis 2013). To calculate bone loss, the 6-site total CEJ-ABC distance for the ligated side of each mouse was subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side. Negative values (in mm) indicated bone loss relative to the baseline (unligated control). In intervention experiments, PBS (control), Wnt5a (0.2 µg), sFRP5 (0.2 µg), or their combination was microinjected into the palatal gingiva of the ligated second maxillary molar. The microinjections were performed 1 d before placing the ligature and every day thereafter until the day before sacrifice (day 5).
Statistical Analysis
Data were evaluated by analysis of variance (ANOVA) and the Dunnett multiple-comparison test using the GraphPad Prism program (GraphPad Software). When 2 groups were compared, the 2-tailed unpaired t test was used. A P value <0.05 was taken as the level of significance.
Results
Reciprocal Expression of Wnt5a and sFRP5 in Human Gingival Tissue
To investigate the expression pattern of Wnt5a and sFRP5 and determine their possible association with periodontitis, we obtained gingival biopsies from periodontally involved and healthy control individuals. Wnt5a and sFRP5 mRNA was detected in all gingival samples examined (Fig. 1). However, the expression of Wnt5a was significantly higher (P < 0.01) in diseased than in healthy tissue (Fig. 1A), whereas the reverse was observed for sFRP5 (Fig. 1B). Immunofluorescence histochemistry analysis revealed colocalization of Wnt5a and sFRP5, which were predominantly expressed in the epithelial layer rather than in the subjacent connective tissue (Fig. 2). Consistent with the mRNA expression analysis, the expression of sFRP5 protein was stronger in healthy tissue compared to periodontitis tissue, whereas the expression of Wnt5a protein in health and disease did not reflect the obvious differences seen at the mRNA level (Fig. 2). Nevertheless, it was evident from the merged Wnt5a and sFRP5 images that Wnt5a predominated over sFRP5 expression in diseased but not in healthy tissue (Fig. 2).
Figure 1.
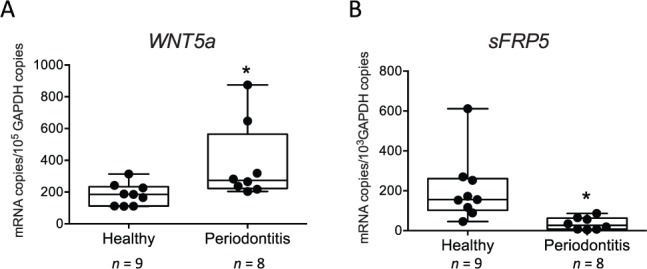
Upregulation of Wnt5a messenger RNA (mRNA) and downregulation of secreted frizzled-related protein 5 (sFRP5) mRNA expression in gingival biopsy specimens from periodontitis patients relative to healthy controls. Gingival mRNA expression of (A) Wnt5a and (B) sFRP5 in healthy and diseased sites was determined by quantitative polymerase chain reaction (qPCR). Results were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Dots represent individual values (n = 9 healthy and 8 periodontitis subjects), and each box in the box-and-whisker plots extends from the 25th to the 75th percentiles. *P < 0.01 versus healthy control.
Figure 2.
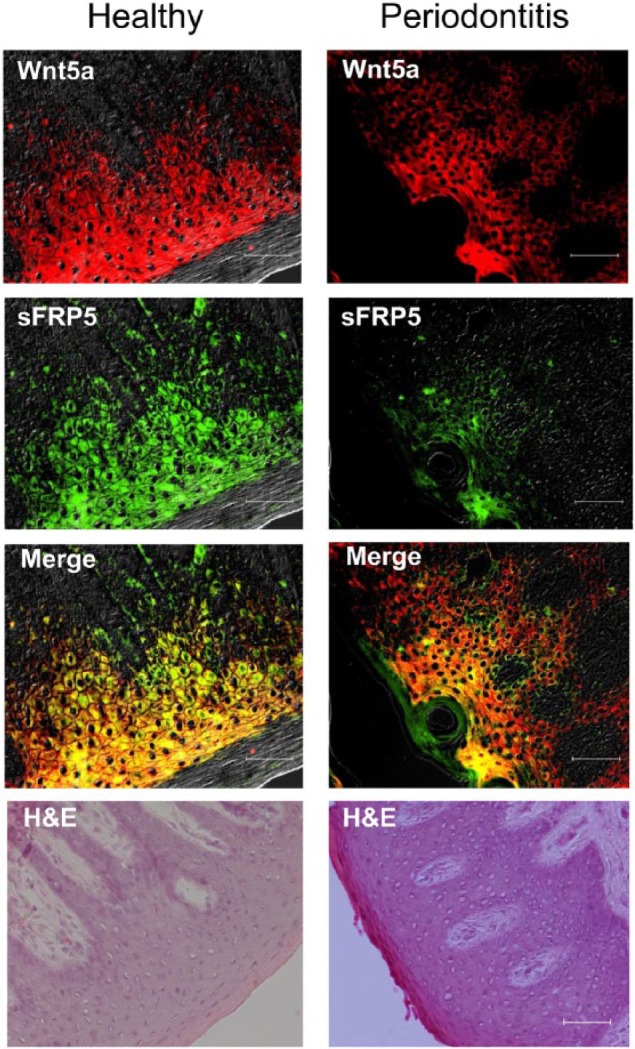
Protein expression of Wnt5a and secreted frizzled-related protein 5 (sFRP5) in healthy and diseased human gingival tissue. Periodontal biopsy specimens from healthy controls (left) and periodontitis patients (right) were processed for immunofluorescence and hematoxylin and eosin (H&E) staining. Shown are overlays of differential interference contrast and fluorescent images stained for Wnt5a (red) and sFRP5 (green), as well as merged images. Scale bar, 50 µm.
Differential Effects of Pg-LPS on Wnt5a and sFRP5 Expression in HGECs
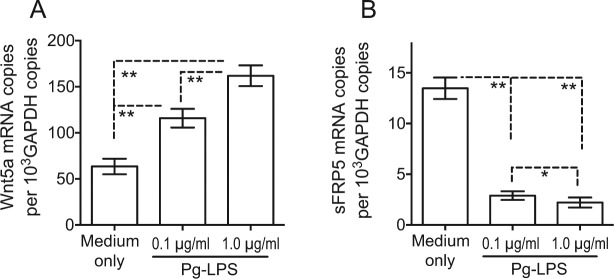
The observations from the clinical samples suggested that Wnt5a and sFRP5 are probably expressed in the gingival epithelium and that inflammatory stimuli in periodontitis shift the balance of expression between Wnt5a and sFRP5 in favor of the former. To investigate these notions, we used freshly isolated HGECs and exposed them to a bacterial proinflammatory stimulus, specifically LPS from P. gingivalis (Pg-LPS). Untreated HGECs were shown to express both Wnt5a and sFRP5 (Fig. 3), confirming the notion that the gingival epithelium can produce these molecules. Consistent with the dominant expression of Wnt5a in periodontitis, Pg-LPS upregulated Wnt5a mRNA expression (P < 0.01; Fig. 3A) and downregulated sFRP5 expression (P < 0.01; Fig. 3B).
Figure 3.
Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) upregulates Wnt5a expression and downregulates secreted frizzled-related protein 5 (sFRP5) expression in human gingival epithelial cells (HGECs). HGECs were stimulated for 12 h with the indicated concentrations of Pg-LPS and analyzed for messenger RNA (mRNA) expression of (A) Wnt5a and (B) sFRP5 by quantitative polymerase chain reaction. Results were normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Data are means ± SD (n = 5 sets of cultures). *P < 0.05 and **P < 0.01 between indicated groups.
Reversal of Pg-LPS– and Wnt5a-Induced IL-8 Expression by sFRP5 in HGECs
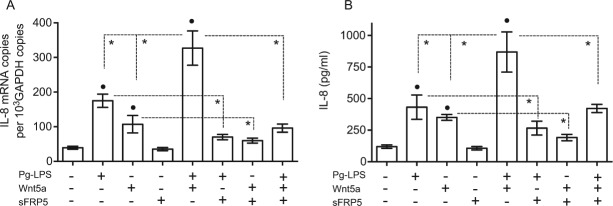
According to our findings, there is higher expression of sFRP5 in HGECs under basal than under inflammatory conditions (Fig. 3) and in healthy than in diseased gingival tissue (Figs. 1 and 2). These observations prompted us to determine whether sFRP5 can mediate anti-inflammatory effects. We first examined this possibility in vitro and specifically investigated whether sFRP5 can inhibit the expression of IL-8, a proinflammatory chemokine that can be produced by HGECs in response to a variety of proinflammatory stimuli (Eskan et al. 2007). Both Pg-LPS and Wnt5a, and particularly their combination, induced substantial levels of IL-8 mRNA and protein (Fig. 4A and B, respectively). However, the ability of Pg-LPS, Wnt5a, or their combination to induce IL-8 was significantly inhibited in the presence of sFRP5 (Fig. 4).
Figure 4.
Secreted frizzled-related protein 5 (sFRP5) inhibits interleukin (IL)–8 induction by Wnt5a and Porphyromonas gingivalis lipopolysaccharide (Pg-LPS) in human gingival epithelial cells (HGECs). HGECs were treated with medium only or with the indicated molecules or combinations thereof (Pg-LPS, 0.1 µg/mL; Wnt5a, 0.3 µg/mL /mL; sFRP5, 0.5 µg/mL) for 12 h (to assess IL-8 gene expression by quantitative polymerase chain reaction) or 24 h (to assess IL-8 protein release in culture supernatants by enzyme-linked immunosorbent assay). Data are means ± SD (n = 5 sets of cultures). *P < 0.01 between indicated groups; •P < 0.01 compared to untreated (medium only).
sFRP5 Inhibits Ligature-Induced Periodontitis
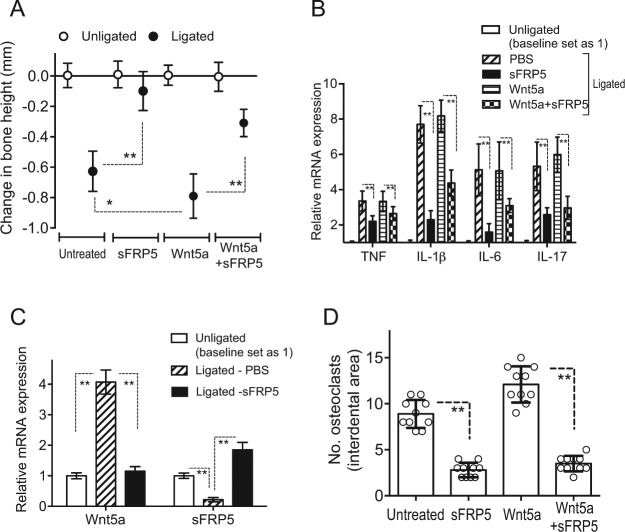
The above described data are consistent with the hypothesis that sFRP5 can mediate a protective role in periodontitis. We therefore next examined whether sFRP5 can inhibit disease activity in a validated murine model of ligature-induced periodontitis (Abe and Hajishengallis 2013). To this end, we microinjected sFRP5 in the gingiva of mice subjected to ligature-induced periodontitis, either alone or together with Wnt5a. Mice of either sex subjected to ligature-induced periodontitis for 5 d readily developed bone loss (to a comparable degree, thus data were pooled) (Fig. 5A). Importantly, the induction of bone loss was significantly inhibited by sFRP5 treatment (P < 0.01; Fig. 5A). The ligature-induced bone loss was significantly increased when the mice were also administered exogenous Wnt5a (P < 0.05; Fig. 5A); however, the bone loss induced by ligature and Wnt5a microinjection was also significantly inhibited by sFRP5 (P < 0.01; Fig. 5A). sFRP5-mediated inhibition of bone loss was associated with significantly reduced levels of gingival mRNA expression of proinflammatory cytokines (tumor necrosis factor [TNF], IL-1β, IL-6, and IL-17) (P < 0.01; Fig. 5B). In line with the reduced expression of proinflammatory cytokines due to sFRP5 treatment, sFRP5-treated mice also displayed a decreased inflammatory cell infiltrate relative to controls, as revealed by hematoxylin and eosin (H&E) staining of gingival tissue (Appendix Figure 1). Consistent with the clinical data (Fig. 1), ligature-induced periodontitis resulted in increased gingival mRNA expression of Wnt5a and decreased expression of sFRP5 compared to their expression in unligated control sites (P < 0.01; Fig. 5C). This profile of Wnt5a versus sFRP5 expression was reversed after microinjection of sFRP5 in ligated sites since this treatment inhibited the expression of Wnt5a and promoted the expression of sFRP5 (P < 0.01; Fig. 5C). Consistent with the bone loss measurements, local treatments with sFRP5 in a replica experiment caused a significant reduction of osteoclast numbers in bone tissue sections (P < 0.01; Fig. 5D).
Figure 5.
Secreted frizzled-related protein 5 (sFRP5) inhibits ligature-induced periodontitis. (A) Periodontal bone loss was induced for 5 d in mice by ligating a maxillary second molar and leaving the contralateral tooth unligated (baseline control). Groups of mice were locally microinjected into the palatal gingiva with phosphate-buffered saline (PBS; control), Wnt5a (0.2 µg), sFRP5 (0.2 µg), or their combination 1 d before placing the ligature and every day thereafter until the day before sacrifice (day 5). (B) In the same mice, ligature-induced periodontal inflammation was monitored in dissected gingiva processed for quantitative polymerase chain reaction (qPCR) to determine messenger RNA (mRNA) expression of the indicated cytokines. (C) In a similar ligature-induced periodontitis study where the mice were microinjected with PBS (control) or with sFRP5 (0.2 µg), dissected gingiva were processed and subjected to qPCR to determine the effect of sFRP5 on the mRNA expression of Wnt5a and sFRP5. In B and C, the data were normalized against glyceraldehyde 3-phosphate dehydrogenase mRNA and expressed as fold change in the transcript levels in the ligated side relative to those of the unligated side, assigned an average value of 1. Data are means ± SD (A and B, n = 10 including 5 female and 5 male mice; C, n = 6 including 3 female and 3 male mice). (D) In a replica experiment as in A, tartrate-resistant acid phosphatase (TRAP)-positive multinucleated cells (osteoclasts) were enumerated from 2 random coronal sections of the ligated molar from each of 5 male mice and averaged with the SD from the total 10 sections/group. *P < 0.05 and **P < 0.01 between indicated groups.
Discussion
In this article, we have demonstrated for the first time an inverse relationship in the expression of Wnt5a and sFRP5 in human periodontitis, which was reproduced in in vitro cultured HGECs exposed to a bacterial proinflammatory stimulus. The increased expression of sFRP5 in periodontal health compared to periodontitis prompted us to hypothesize that sFRP5 may play a protective role in the periodontium. This hypothesis was verified in the mouse model of ligature-induced periodontitis, where sFRP5 blocked inflammation and bone loss. These findings establish a cause-and-effect relationship between sFRP5 and periodontal disease activity that is therapeutically important.
P. gingivalis is a keystone pathogen that promotes the pathogenicity of the periodontal microbial community by manipulating the host response in ways that impair protective immunity while promoting inflammation (Hajishengallis et al. 2011; Maekawa et al. 2014). In this study, we showed that P. gingivalis can potentially enhance inflammation through the capacity of its LPS to upregulate the expression of Wnt5a in HGECs, whereas an earlier study found that Pg-LPS can induce Wnt5a in human monocytic THP-1 cells (Nanbara et al. 2012). Importantly, however, our study has also shown that Pg-LPS can simultaneously downregulate the expression of sFRP5, which is a functional antagonist of Wnt5a (Ouchi et al. 2010). Therefore, Pg-LPS regulates the Wnt5a/sFRP5 ratio toward a proinflammatory direction. We also found that sFRP5 can inhibit IL-8 induction in HGECs that were stimulated with Pg-LPS even in the absence of exogenous Wnt5a. In this context, it was previously shown that sFRP5 inhibits IL-6 induction in TNF-stimulated adipocytes, also in the absence of exogenous Wnt5a (Carstensen et al. 2014). One possible explanation for these independent findings is that sFRP5 mediated these anti-inflammatory effects by antagonizing endogenously produced Wnt5a in Pg-LPS–stimulated HGECs or TNF-stimulated adipocytes. However, at present, we cannot rule out the possibility that sFRP5 may act on additional targets (ligands or receptors) unrelated to Wnt signaling. In this regard, another member of the sFRP family, sFRP1, was shown to bind receptor activator for NF-κB ligand (RANKL), thereby preventing its interaction with RANK that would promote osteoclastogenesis (Häusler et al. 2004).
The diminished mRNA expression of sFRP5 we have detected in periodontitis is in line with findings from a recent study that showed significantly decreased sFRP5 transcript levels in ischemia/reperfusion injury-induced inflammation (Nakamura et al. 2016). The low mRNA expression of sFRP5 in periodontitis compared to health was consistent with its protein expression profile in periodontitis versus health as revealed by immunofluorescence histochemistry. On the other hand, the increased mRNA expression of Wnt5a in periodontitis relative to health was not reflected at the protein level. In this regard, the regulation of certain molecules at the mRNA level may not faithfully reflect protein expression levels due to the impact of posttranscriptional mechanisms regulating mRNA translation or stability or even posttranslational modifications affecting protein stability. Although Wnt5a expression was predominantly localized in the gingival epithelium rather than the subjacent connective tissue, it should be noted that our human gingival biopsies involved soft tissue without bone. However, Wnt5a can also be expressed by bone cells, specifically by osteoblasts although not by osteoclasts (Maeda et al. 2012). Interestingly, the binding of osteoblast-derived Wnt5a to a co-receptor complex involving receptor tyrosine kinase-like orphan receptor 2 (Ror2) and Frizzled (Ror2/Fz complex) on osteoclast precursors induces noncanonical Wnt signaling that upregulates RANK expression. This, in turn, renders the precursors more sensitive to the action of RANKL and consequently promotes osteoclast differentiation (Maeda et al. 2012). Moreover, β-catenin–dependent canonical Wnt signaling in osteoblasts (such as induced by the Wnt3a isoform) promotes bone formation and, moreover, suppresses bone resorption by inducing the secretion of osteoprotegerin, a natural inhibitor of RANKL (Baron et al. 2012). Interestingly, Wnt5a-induced noncanonical signaling can antagonize the β-catenin–dependent canonical pathway (Angers and Moon 2009), suggesting additional mechanisms by which Wnt5a could promote net loss of bone. Although it is conceivable that the microinjected sFRP5 into the mouse gingiva may have reached the bone, it is uncertain whether sFRP5 has acted to antagonize the ability of Wnt5a to promote osteoclastogenesis by upregulating RANK expression on osteoclast precursors (Maeda et al. 2012). Given the tight connection between inflammation and osteoclastogenesis (Miossec and Kolls 2012), our findings of significantly reduced numbers of osteoclasts in bone tissue sections from mice treated with sFRP5 may be explained by the anti-inflammatory action of sFRP5, as revealed by its capacity to inhibit the expression of proinflammatory cytokines as well as to reduce the inflammatory cell infiltrate in the murine gingival tissue.
An additional, not mutually exclusive, mechanism for the observed protective effect of sFRP5 in ligature-induced periodontitis is that sFRP5 inhibited inflammatory responses in the gingival epithelium (as suggested by our findings with HGECs), which is known to orchestrate downstream innate immune and inflammatory responses through the secretion of chemokines and other mediators (Kinane et al. 2008). In this regard, the expression of the IL-8 chemokine has been associated with human periodontal disease activity. Indeed, the IL-8 levels in the gingival crevicular fluid are upregulated in periodontitis (compared to health) but are downregulated after successful periodontal therapy (Gamonal et al. 2000). The in vivo anti-inflammatory potential of sFRP5 in our study was firmly established by the findings of reduced expression of several proinflammatory cytokines (TNF, IL-1β, IL-6, and IL-17) that have been implicated in periodontitis in humans and animal models (Garlet 2010; Zenobia and Hajishengallis 2015). The sFRP5-mediated inhibition of periodontal inflammation was associated with decreased expression of Wnt5a and increased expression of sFRP5 (compared to untreated ligature-induced periodontitis), consistent with our clinical observations that Wnt5a dominates in periodontitis, whereas sFRP5 is associated with periodontal health. Whether the Wnt5a/sFRP5 ratio can serve as a useful indicator of periodontal disease activity is a plausible hypothesis that requires clinical investigation. Interestingly, in this regard, the Wnt signaling pathway was identified by gene ontology analysis among the top 25 differentially expressed pathways in diseased and healthy gingival tissues (Demmer et al. 2008).
In summary, we have shown a novel inverse relationship between Wnt5a and sFRP5 expression in periodontal disease compared to health. Both molecules were shown to be expressed by cultured HGECs and to be differentially regulated by proinflammatory bacterial stimuli in a manner consistent with the clinical observations. In the same system, sFRP5 prevented LPS-induced inflammation, whereas Wnt5a was proinflammatory, in line with several other reports regarding proinflammatory Wnt5a effects on additional cell types (Sen et al. 2000; Blumenthal et al. 2006; Pereira et al. 2008; Nanbara et al. 2012). Importantly, local treatment with sFRP5 in mice subjected to ligature-induced periodontitis inhibited inflammation and bone loss, correlating with decreased numbers of osteoclasts in bone tissue sections. Therefore, Wnt5a appears to be an important target for intervention in periodontitis, and sFRP5 is likely a promising therapeutic compound. Moreover, our study provides a rationale for future studies investigating the Wnt5a/sFRP5 ratio as a potential biomarker in periodontitis.
Author Contributions
T. Maekawa, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; P. Kulwattanaporn, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; K. Hosur, H. Domon, M. Oda, Y. Terao, T. Maeda, contributed to data acquisition and analysis, drafted and critically revised the manuscript; G. Hajishengallis, contributed to conception, design, and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This work was supported by grants from the National Institutes of Health (DE015254, DE024153, and DE026152) to G.H. and from JSPS KAKENHI (16H06272E) and the Takeda Science Foundation to T.M.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abe T, Hajishengallis G. 2013. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 394(1–2): 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angers S, Moon RT. 2009. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 10(7):468–477. [DOI] [PubMed] [Google Scholar]
- Baron R, Saito H, Gori F. 2012. Bone cells crosstalk: noncanonical Roring in the Wnt. Cell Metab. 15(4):415–417. [DOI] [PubMed] [Google Scholar]
- Beikler T, Flemmig TF. 2011. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontology 2000. 55(1):87–103. [DOI] [PubMed] [Google Scholar]
- Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. 2006. The wingless homolog WNT5a and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 108(3):965–973. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. 2008. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 121(Pt 6):737–746. [DOI] [PubMed] [Google Scholar]
- Carstensen M, Wiza C, Rohrig K, Fahlbusch P, Roden M, Herder C, Ouwens DM. 2014. Effect of Sfrp5 on cytokine release and insulin action in primary human adipocytes and skeletal muscle cells. PLoS One. 9(1):e85906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple IL. 2014. Time to take periodontitis seriously. BMJ. 348:g2645. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Behle JH, Wolf DL, Handfield M, Kebschull M, Celenti R, Pavlidis P, Papapanou PN. 2008. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 79(11):2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, Lange EM, Moss K, Barros SP, Weyant RJ, et al. 2013. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 22(11):2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskan MA, Hajishengallis G, Kinane DF. 2007. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infect Immun. 75(2):892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. 2000. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 71(10):1535–1545. [DOI] [PubMed] [Google Scholar]
- Garlet GP. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 89(12):1349–1363. [DOI] [PubMed] [Google Scholar]
- George SJ. 2008. Wnt pathway: a new role in regulation of inflammation. Arterioscler Thromb Vasc Biol. 28(3):400–402. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Dionne MS, Schneider DS, Nusse R. 2005. Wntd is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature. 437(7059):746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, et al. 2011. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 10(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler KD, Horwood NJ, Chuman Y, Fisher JL, Ellis J, Martin TJ, Rubin JS, Gillespie MT. 2004. Secreted frizzled-related protein-1 inhibits rankl-dependent osteoclast formation. J Bone Miner Res. 19(11):1873–1881. [DOI] [PubMed] [Google Scholar]
- He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. 1997. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 275(5306):1652–1654. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A. 2009. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol. 19(3):119–129. [DOI] [PubMed] [Google Scholar]
- Kinane DF, Galicia JC, Gorr SU, Stathopoulou PG, Benakanakere M. 2008. P. gingivalis interactions with epithelial cells. Front Biosci. 13:966–984. [DOI] [PubMed] [Google Scholar]
- Kumawat K, Gosens R. 2016. Wnt-5a: signaling and functions in health and disease. Cell Mol Life Sci. 73(3):567–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Hajishengallis G. 2015. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 21(3):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libro R, Bramanti P, Mazzon E. 2016. The role of the Wnt canonical signaling in neurodegenerative diseases. Life Sci. 158:78–88. [DOI] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et al. 2012. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. 18(3):405–412. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, et al. 2014. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 15(6):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti G, Naskar D, Sen M. 2012. The wingless homolog wnt5a stimulates phagocytosis but not bacterial killing. Proc Natl Acad Sci U S A. 109(41):16600–16605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Kolls JK. 2012. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 11(10):763–776. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Sano S, Fuster JJ, Kikuchi R, Shimizu I, Ohshima K, Katanasaka Y, Ouchi N, Walsh K. 2016. Secreted frizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia/reperfusion injury. J Biol Chem. 291(6):2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanbara H, Wara-aswapati N, Nagasawa T, Yoshida Y, Yashiro R, Bando Y, Kobayashi H, Khongcharoensuk J, Hormdee D, Pitiphat W, et al. 2012. Modulation of Wnt5a expression by periodontopathic bacteria. PLoS One. 7(4):e34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. 2010. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 329(5990):454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. 2008. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 28(3):504–510. [DOI] [PubMed] [Google Scholar]
- Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. 2000. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 97(6):2791–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. 2008. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 8(8):581–593. [DOI] [PubMed] [Google Scholar]
- Zenobia C, Hajishengallis G. 2015. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000. 69(1):142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.