Abstract
We report here a novel biomimetic approach to the regeneration of human enamel. The approach combines the use of inorganic pyrophosphate (PPi) to control the onset and rate of enamel regeneration and the use of leucine-rich amelogenin peptide (LRAP), a nonphosphorylated 56–amino acid alternative splice product of amelogenin, to regulate the shape and orientation of growing enamel crystals. This study builds on our previous findings that show LRAP can effectively guide the formation of ordered arrays of needle-like hydroxyapatite (HA) crystals in vitro and on the known role mineralization inhibitors, like PPi, play in the regulation of mineralized tissue formation. Acid-etched enamel surfaces of extracted human molars, cut perpendicular or parallel to the direction of the enamel rods, were exposed to a PPi-stabilized supersaturated calcium phosphate (CaP) solution containing 0 to 0.06 mg/mL LRAP for 20 h. In the absence of LRAP, PPi inhibition was reversed by the presence of etched enamel surfaces and led to the formation of large, randomly distributed plate-like HA crystals that were weakly attached, regardless of rod orientation. In the presence of 0.04 mg/mL LRAP, however, densely packed mineral layers, comprising bundles of small needle-like HA crystals, formed on etched surfaces that were cut perpendicular to the enamel rods. These crystals were strongly attached, and their arrangement reflected to a significant degree the underlying enamel prism pattern. In contrast, under the same conditions with LRAP, little to no crystal formation was found on enamel surfaces that were cut parallel to the direction of the enamel rods. These results suggest that LRAP preferentially interacts with ab surfaces of mature enamel crystals, inhibiting their directional growth, thus selectively promoting linear growth along the c-axis of enamel crystals. The present findings demonstrate a potential for the development of a new approach to regenerate enamel structure and properties.
Keywords: restorative materials, enamel biomineralization/formation, extracellular matrix, mineralized tissue/development, hydroxyapatite, scanning electron microscopy
Introduction
Dental enamel is the hardest and most mineralized tissue in the human body and comprises the outer layer of tooth crowns. Unlike other calcified tissues such as dentin and bone, the mature enamel tissue lacks an inherent means of self-repair. However, studies (Koulourides et al. 1965; Backer Dirks 1966) indicate that saliva, which continuously bathes teeth within the oral cavity, has the capacity to remineralize incipient enamel caries lesions. This finding is related to the fact that saliva is supersaturated with respect to tooth enamel mineral and other calcium phosphate (CaP) phases (Hay et al. 1982), thus providing the potential to support mineral growth within the incipient enamel defect. These observations point to a key functional role of salivary proteins: to maintain high levels of supersaturation by inhibiting precipitation of CaP phases in saliva and on sound tooth surfaces, a conclusion based on extensive in vitro studies (Moreno et al. 1979; Hay et al. 1984; Hay et al. 1987). Inhibitors of mineralization play key roles in the regulation of mineralized tissue formation, including that in bones and teeth, in part, by maintaining high levels of local supersaturation and by controlling the onset of mineral growth through the inactivation or removal (e.g., via proteolytic digestion) of the inhibitor (Margolis et al. 2014). In the present study, we have applied this concept to the biomimetic regeneration of human enamel by using inorganic pyrophosphate (PPi), which has been shown to play an essential role in the regulation of bone formation (e.g., Russell et al. 1971), based on its known ability to inhibit CaP crystal growth (Fleisch and Bisaz 1962; Fleisch et al. 1969; Meyer and Nancollas 1973; Moreno et al. 1987). The inhibition of CaP crystal growth has been attributed to the adsorption of PPi to hydroxyapatite (HA) crystal growth sites (Moreno et al. 1987). Here, upon adsorption, PPi undergoes surface-catalyzed hydrolysis, resulting in a reduction in the concentration of the PPi inhibitor.
Most carious lesions are found in fissures of occlusal surfaces (Ekstrand et al. 2001) that are prone to demineralization in vivo and can lead to extensive mineral loss and the complete destruction of enamel surfaces. Hypomineralized teeth, having low mineral content, a translucent appearance, and soft enamel, can also result from genetic diseases (e.g., amelogenesis imperfecta) and environmental factors (e.g., high fluoride levels causing fluorosis, acidic beverages). Accordingly, there is a need to develop more effective means to regenerate defective enamel, to ultimately replace the widespread use of filling materials with compositions and structures that differ from those of the enamel, which do not form good interfaces with the natural tissue surrounding the lesion. As a result of this mismatch, secondary caries frequently develop over time (Totiam et al. 2007; Spencer et al. 2010). As a promising goal, biomimetic synthesis of enamel-like materials with a dense interface can be an attractive alternative (Ruan and Moradian-Oldak 2014). Numerous prior studies have shown that thin layers of enamel-like structures can be regenerated on human enamel using fluorapatite-gelatin composites (Busch et al. 2001, Busch 2004), an acidic paste of fluoridated HA (Onuma et al. 2005; Yamagishi et al. 2005), or recombinant amelogenin and fluoride solutions (Fan et al. 2009). These findings demonstrate the feasibility of applying chemical approaches to enamel regeneration, although additional studies are warranted.
The mechanism of enamel formation is still not fully understood, although much has been learned about the capacity of amelogenin, the predominant enamel matrix protein, to guide the elongated growth of enamel crystals. (Wen et al. 2000; Iijima and Moradian-Oldak 2004; Beniash et al. 2005; Kwak et al. 2009; Kwak et al. 2011; Wiedemann-Bidlack et al. 2011). In particular, studies have shown that full-length recombinant amelogenins, which contain highly conserved N- and C-terminal domains of amelogenin that are essential for enamel formation (Simmer and Fincham 1995; Fincham et al. 1999; Margolis et al. 2006), have the capacity to regulate spontaneous CaP formation and guide the formation of ordered bundles of enamel-like HA crystals in vitro. The leucine-rich amelogenin peptide (LRAP), a nonphosphorylated 56-residue alternative splice variant of full-length porcine amelogenin that contains only the N- and the C-terminal domains of the parent amelogenin, was similarly found to regulate ordered mineralization in vitro, like the nonphosphorylated full-length amelogenins (Le Norcy et al. 2011).
Building on this background, we report here on the development of a novel biomimetic approach to the regeneration of mature enamel structure that, for the first time, simultaneously employs 2 key elements of the biomineralization process: 1) kinetic control of mineralization by using a known inhibitor of CaP formation, PPi, and 2) the regulation of crystal growth shape and orientation using a relevant biomolecule, nonphosphorylated LRAP.
Materials and Methods
Preparation of LRAP
Porcine LRAP was synthesized commercially (NEO Peptide and RS Synthesis) and repurified (Nagano et al. 2009). Lyophilized peptides were dissolved in filtered (0.22 µm) distilled deionized water (DDW) at room temperature to yield a 7-mg/mL stock solution. Complete dissolution of the peptide was confirmed using dynamic light scattering that exhibited mean hydrodynamic radii values (RH) that were ~1 nm (Le Norcy et al. 2011). Protein stock solutions were centrifuged (11,340 × g; Eppendorf Centrifuge 5403) at 4°C for 20 min, just prior to use.
Preparation of Acid-Etched Enamel
Extracted human teeth were collected according to guidelines approved by Forsyth’s Institutional Review Board. Sections of outer enamel were cut using an Isomet Low Speed (Buehler Ltd.) saw and wafering blade (series 15LC; Buehler Ltd.) in 1) a direction roughly perpendicular to the enamel rods (group A) and 2) a direction parallel to the enamel rods (group B). Cut enamel surfaces were then ground and sequentially polished with a 6-, 1-, and 0.25-µm MetaDi supreme diamond suspension on the polishing pad for 4 min each using a Buehler Minimet1000 polisher (Buehler Ltd.). A 12- to 16-mm2 window on each enamel specimen was prepared by coating with nail varnish. All sections were immersed into 30% (w/w) phosphoric acid for 10 s, followed by extensive rinsing with DDW. The acid-etched sections were placed in a 1.5-mM KH2PO4 solution (~pH 7.4) and stirred overnight to remove residual acidity.
Enamel Regeneration Using LRAP and PPi-Stabilized CaP Solution
Highly supersaturated CaP solutions (degree of saturation with respect to HA, DSHA = 24.1) (Moreno and Margolis, 1988) containing 1.5 mM KH2PO4 (99.0%; Sigma), 1.3 mM CaCl2 (99.0%; Sigma), and 50 mM NaCl were prepared in 50 mL, using degassed DDW (DGDDW). All stock solutions were filtered with a 0.22-µm Millipore filter and then analyzed for Ca using atomic absorption spectroscopy (Analyst200; Perkin Elmer) and phosphate using UV-VIS spectrophotometry at 390 nm (Genesis5; Milton Roy). PPi (Na4P2O7) stock solution was made daily and added to the supersaturated solution prior to the addition of calcium. Final PPi concentrations were 0.01 to 2 µM. The initial pH was adjusted to ~pH 7.4 with KOH solution in a 37°C water bath with pure nitrogen gas bubbling through the solution to prevent carbonate contamination from air. Three enamel sections were then placed in the CaP solution, with and without added LRAP (final concentration: 0.02–0.06 mg/mL). Solution pH was monitored continuously. At selected times, the enamel sections were removed, rinsed with DGDDW, and examined, as described below. Ten experiments were carried out using sections for group A and 7 experiments for group B (3 sections per experiment). Control experiments were carried out in the absence of enamel sections, without and with added LRAP.
Characterization of Regenerated Enamel Layers
Treated and untreated acid-etched enamel surfaces were characterized using scanning electron microscopy (SEM; Zeiss EVO-LS10 Instrument; Carl Zeiss Microscopy Ltd.), energy-dispersive spectroscopy (EDS; XMAX; Oxford Instruments), Fourier transform infrared spectroscopy (FT-IR), and grazing incidence angle X-ray diffraction (GIXRD) to assess mineral composition, phase, and morphology at the nano- and micro-scale. In EDS analyses, a standard curve (Ca/P atomic ratio vs. theoretical molar ratios of known standards) was obtained using HA (2910 standard reference material; NIST), octacalcium phosphate (OCP; Clarkson), and dicalcium phosphate (DCPD, synthesized at Forsyth). FT-IR spectra were obtained using Spectrum One with Multi-scope (PerkinElmer) in reflectance mode with 128 scans and 4-cm–1 resolution. GIXRD patterns were collected using a Bruker D8 Advance with Gadds Multipurpose Diffractometer (Bruker) with CuKα radiation (λ = 1.542 Å) and a 2-dimensional detector operating in a reflection geometry. Graphical representations were prepared using SigmaPlot v10.0 software (Systat Software). Cross-sectional images were obtained using an NVision 40 Dual-Beam focused ion-beam (FIB) and SEM after gold sputter coating (Denton Desk V; Denton Vacuum).
Results
Control Studies: Effects of PPi and LRAP on Spontaneous CaP Formation
As shown in Figure 1A(a), as indicated by a marked decrease in pH, spontaneous precipitation was observed in about 40 to 50 min using the described CaP mineralization solution. In contrast, the CaP solution containing just 0.01 µM PPi did not exhibit a significant change in pH until 10 h (Fig. 1A(b)), which corresponded to the onset of bulk solution precipitation observed at 20 h. At 1 µM PPi, however, complete inhibition of spontaneous precipitation was observed during the 20-h period (Fig. 1A(c)), in general agreement with earlier studies (Fleisch and Bisaz 1962). The stabilized supersaturated solution containing 0.01 µM PPi was chosen for the present study.
Figure 1.
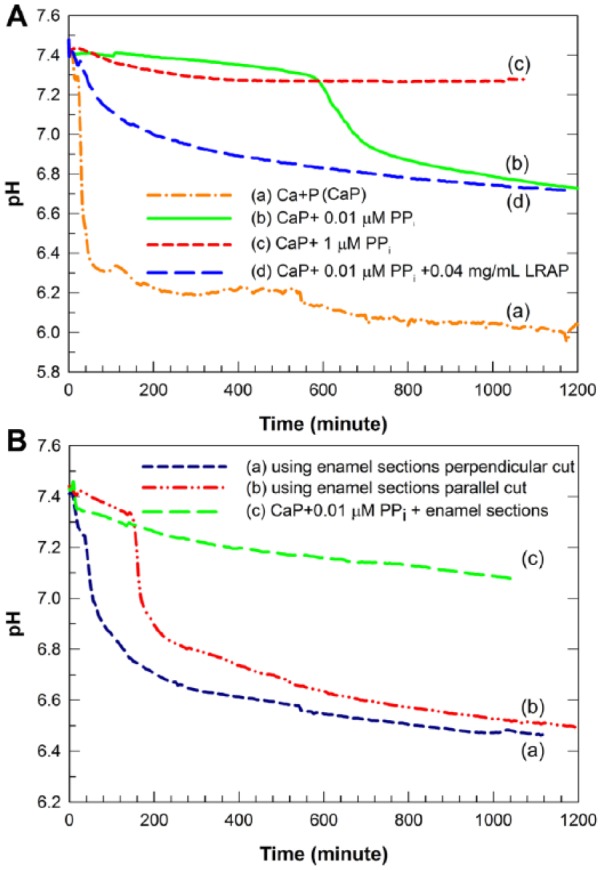
Changes in pH of supersaturated calcium phosphate (CaP) solutions containing 1.3 mM Ca, 1.5 mM P, 50 mM NaCl, pHi = 7.4 as a function of time under various conditions. (A) In the absence of acid-etched enamel: Control, without added inorganic pyrophosphate (PPi) and leucine-rich amelogenin peptide (LRAP), a marked decrease in solution pH was observed (a). The mineralization solution was stabilized by the addition of 0.01 µM PPi for up to 10 h (b) while the same solution was stabilized by the addition of 1 µM PPi for up to 20 h (c). In the presence of 0.04 mg/mL LRAP, the 0.01-µM PPi-stabilized mineral solution exhibited a slight drop in pH within the first 2 h that was followed by a more gradual decrease to pH 6.7 over the 20-h experimental time period (d). (B) In the presence of acid-etched enamel: With 0.04 mg/mL LRAP and 3 acid-etched enamel sections cut in a direction perpendicular to enamel rods (a) and 3 acid-etched enamel sections cut parallel to enamel rods (b) in the PPi-stabilized mineralizing solution, significant decreases in pH were observed within 40 to 50 and 180 min, respectively. As a control, the 0.01-µM PPi-stabilized mineral solution in the presence of enamel sections cut in a direction perpendicular to enamel rods but without LRAP showed a gradual and slight pH decrease to pH 7.1 over 20 h (c).
When 0.04 mg/mL LRAP was added to the supersaturated CaP solution containing 0.01 µM PPi, a slight pH drop from pH 7.4 to 7.0 was observed within 2 h, followed by a gradual decrease to pH 6.7 at 20 h (Fig. 1A(d)) that corresponded to the formation of a mixture of bundles of needle-like crystals and plate-like crystals (Appendix Fig. 1(a)). Hence, the addition of LRAP was found to promote spontaneous mineralization to some degree. When enamel sections cut perpendicular to the direction of the enamel rods were immersed in the mineralizing solution in the absence of LRAP, the pH value decreased gradually from pH 7.4 to 7.1 over the 20-h period (Fig. 1B(c)). Corresponding to this pH drop, randomly distributed plate-like crystals formed on the enamel surface, as seen at 10 h (Appendix Fig. 2B) and 20 h (Fig. 2C, D) by SEM analysis. At higher concentrations of PPi, such as 0.1, 1, and 2 µM, crystal growth was limited or prevented, respectively (Appendix Fig. 3B, C), and no bulk precipitation was observed.
Figure 2.
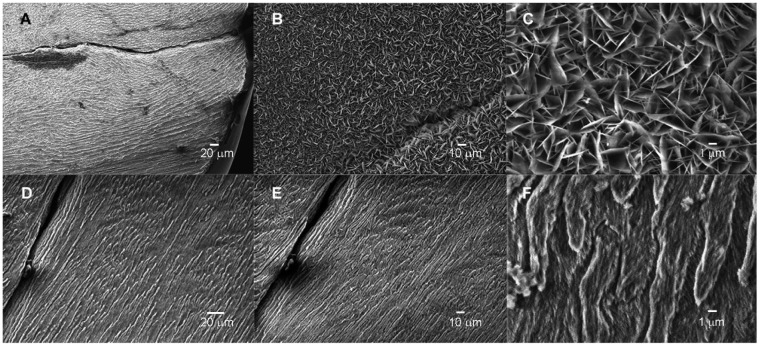
Scanning electron microscopy images of acid-etched enamel cut in a direction perpendicular to enamel rods at ×1K (A), ×20K (B), and the mineral layer deposited on acid-etched enamel cut in a direction perpendicular to the enamel rods following a 20-h exposure to the inorganic pyrophosphate (PPi)–stabilized supersaturated calcium phosphate (CaP) solution in the absence (C, at ×1K and D, at ×20K) and in the presence (E, at ×1K and F, at ×20K) of 0.04 mg/mL leucine-rich amelogenin peptide (LRAP). In the absence of LRAP (C and D), large randomly arranged plate-like crystals formed on the enamel surface, while in the presence of LRAP (E and F), the regenerated layer was composed of a dense packing of small needle-like crystals in somewhat parallel arrangement and reflected to a very significant degree the typical enamel prismatic pattern of the underlying original enamel surface.
Effect of the Combined Use of PPi and LRAP on the Regeneration of Acid-Etched Enamel
In the presence of enamel sections cut in a direction perpendicular to that of enamel rods and 0.04 mg/mL LRAP using the PPi-stabilized CaP solution, a relatively rapid pH drop from pH 7.4 to 6.9 was observed within 80 min (Fig. 1B(a)), in comparison to that observed when enamel sections were exposed to the same solution containing just PPi without LRAP (Fig. 1B(c)). The initial pH change was followed by a slower decrease to pH 6.5, along with bulk precipitation observed by transmission electron microscopy (TEM) at 20 h (Appendix Fig. 1(b)). Astonishingly, under these conditions in the presence of 0.04 mg/mL LRAP, a layer of mineral was found to form (Fig. 2E) that reflects to a very significant degree the typical enamel prismatic pattern of the underlying etched enamel surface, as shown at the same low magnification by SEM (Fig. 2A). As shown in Figure 2E, substantial crystal growth was observed after 20 h that covered the entire exposed enamel surface. The textured surface deposit that formed in the presence of LRAP appears to comprise a dense packing of small needle-like crystals in somewhat parallel arrangement (Fig. 2F). These findings were clearly not seen with the use of the mineralizing solution without added LRAP where, instead, formed rhombohedral plate-like crystals were randomly distributed over the enamel surface (Fig. 2C, D). All XRD patterns (Appendix Fig. 4) and FT-IR spectra (Appendix Fig. 5) measured confirmed the formation of HA. These characterizations, combined with EDS measurements of Ca/P ratios, revealed that the regenerated mineral layer formed in 0.04 mg/mL LRAP (Ca/P = 1.57) is similar to acid-etched enamel (Ca/P = 1.62), while the mineral layer formed in the absence of LRAP (Ca/P = 1.37) is consistent with a Ca-deficient HA phase (Elliott 1994; Sui et al. 2014). Under the same conditions, in the presence of 0.02 mg/mL LRAP, isolated globular assemblies of crystals with varying morphologies were observed (Appendix Fig. 6A, B). In the presence of 0.06 mg/mL LRAP, enamel crystal growth was not clearly observed (Appendix Fig. 6C, D).
Cross-sectional images of specimens generated in the absence and presence of LRAP (Fig. 3A, B, respectively) were also obtained. In the presence of 0.04 mg/mL LRAP in the PPi-stabilized supersaturated solution, the regenerated layer exhibited densely packed bundles of crystals and was about 2 µm in thickness (Fig. 3B). Importantly, although the outer new growth layer appeared to be somewhat more porous than the underlying enamel, the interface connecting the 2 layers appeared to be almost seamless. This fact was also observed in a fractured specimen using conventional SEM (Fig. 3C). The particles of the newly regenerated layer had very similar sizes and morphology with those of acid-etched enamel substrate and were found to grow in a well-aligned fashion in the direction of existing enamel crystals (Fig. 3C, arrows). In contrast, the mineral layer formed and the resulting interface generated in the absence of LRAP was readily distinguishable from the underlying natural enamel (Fig. 3A), using specimens in which the grown layer was partially removed by ultra-sonication treatment (see below). Large thin plate-like crystals with sharp edges grew perpendicular to the enamel surface and exhibited morphologies and sizes distinctively different from those seen in the underlying enamel crystals. The thickness of the resulting mineral layer was about 7 to 8 µm, although the layer appeared to be quite porous. Of further significance, the mineral layers formed in the presence of LRAP adhered strongly to the enamel surface and were unaffected by sonication (Fig. 4A). In contrast, 70% ± 0.5% of the mineral layer formed in the absence of LRAP was readily removed by sonication (area analysis by ImageJ 1.44o) (Fig. 4B).
Figure 3.
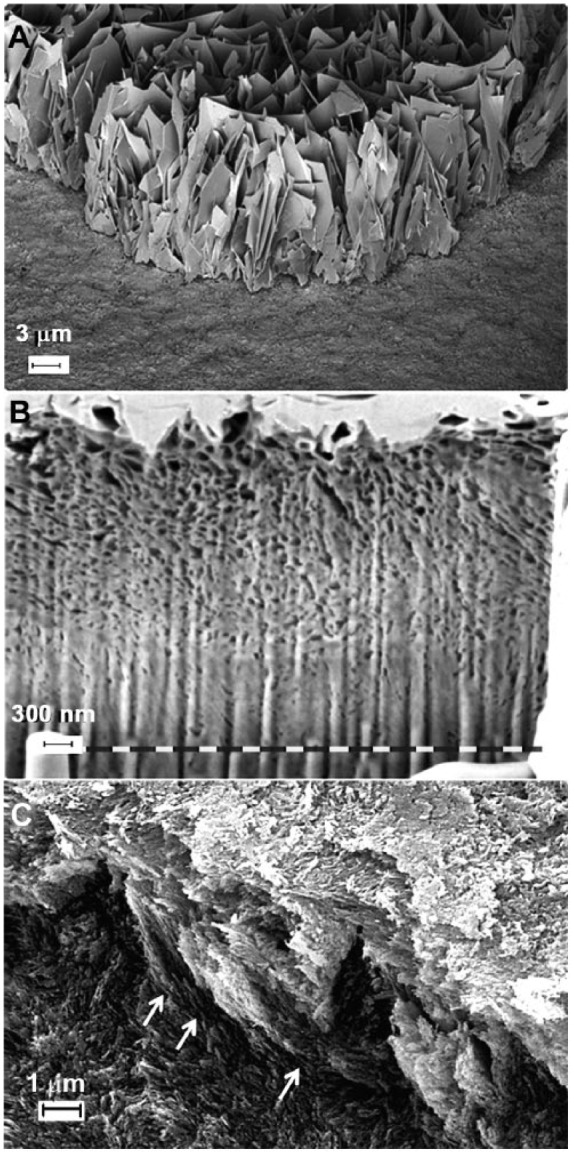
Scanning electron microscopy (SEM) or focused ion-beam/SEM cross-sectional images of regenerated mineral layers on acid-etched enamel cut in a direction perpendicular to enamel rods using inorganic pyrophosphate (PPi)–stabilized supersaturated calcium phosphate (CaP) solution (A). A mineral layer (7–8 µm in thickness) composed of large thin plate-like crystals with sharp edges was formed perpendicular to the enamel surface and exhibited morphologies and sizes distinctively different from those of human enamel crystals seen in the underlying enamel. By contrast, the regenerated layer on treated enamel sections in the presence of 0.04 mg/mL leucine-rich amelogenin peptide (LRAP) in PPi-stabilized supersaturated CaP solution exhibited densely packed bundles of crystals and was about 2 µm in thickness (B). The fractured surface (C) of the latter layer showed that the particles of newly regenerated layer had very similar sizes and morphology in comparison to those of the acid-etched enamel substrate and that the similarly shaped dense bundles of newly formed crystals were found to grow in a fashion well aligned to the direction of existing enamel crystals (arrows).
Figure 4.
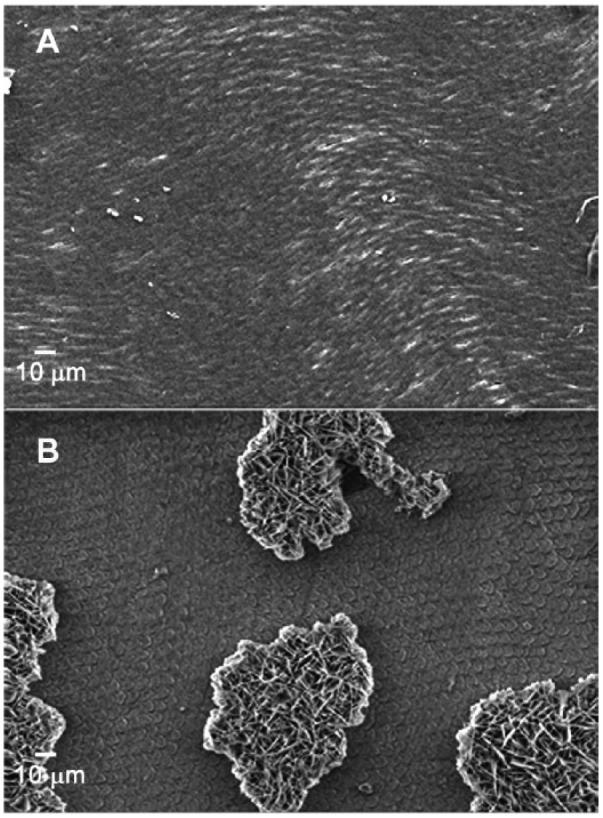
After a 5-s sonication treatment, scanning electron microscopy images of regenerated layers obtained from inorganic pyrophosphate (PPi)–stabilized supersaturated calcium phosphate (CaP) solution in the presence (A) and the absence of 0.04 mg/mL leucine-rich amelogenin peptide (LRAP) (B). The mineral layers formed in the presence of LRAP adhered strongly to the enamel surface and were unaffected by sonication (A). In contrast, the mineral layer formed in the absence of LRAP was readily removed by sonication (B).
The influence of the enamel prism orientation on the effect of LRAP on enamel regeneration was explored using acid-etched enamel sections cut in a direction parallel to the enamel rods (Fig. 5A, D). In the absence of LRAP under the same conditions used for enamel sections cut in a direction perpendicular to the enamel rods, plate-like crystals on the enamel surface were formed (Fig. 5B, C). The shape, size, and random orientation of the plate-like crystals shown are similar to those formed on the enamel sections that were cut perpendicular to the rod direction (Fig. 2C, D) and again quite distinct from the size and morphology of acid-etched enamel crystal seen (Fig. 5A). However, crystal growth did not take place on acid-etched enamel surfaces cut in a direction parallel to that of the enamel rods in the presence of LRAP (Fig. 5E, F) under the same conditions used to induce the needle-like growth of acid-etched enamel cut perpendicular to the enamel rods (Fig. 2E, F). As shown in Figure 1B(b), the solution pH dropped rapidly to pH 6.8 after the first 3 h of the reaction, followed by an additional gradual pH decrease to ~pH 6.6 at 20 h, similar to the final pH of the reaction using sections cut perpendicular to the direction of the enamel rods under the same conditions. Bulk precipitation was observed by TEM, similar to that seen in Appendix Figure 1(b).
Figure 5.
Scanning electron microscopy images of surfaces of acid-etched enamel cut in a direction parallel to the enamel rods before (A and D) and after exposure to the inorganic pyrophosphate (PPi)–stabilized supersaturated calcium phosphate (CaP) solution in the absence (B, at ×1K and C, ×20K) and in the presence (E, at ×1K and F, ×20K) of 0.04 mg/mL leucine-rich amelogenin peptide (LRAP). In the presence of 0.04 mg/mL LRAP (E, F), crystal growth was not observed, while in the absence of LRAP (B, C), plate-like crystals were found to randomly cover the entire exposed enamel surface.
Discussion
As noted in the Introduction, PPi was selected in the present study since its removal by surface-catalyzed hydrolysis by the enamel surface (Moreno et al. 1987) could promote the reversal of mineralization inhibition (Fig. 1B(c)) and the preferential formation of mineral directly on acid-etched enamel surfaces. Our present findings have confirmed this idea by showing that the presence of acid-etched human enamel, or HA itself (Appendix Fig. 7), induces a reversal of PPi inhibition and promotes enamel regrowth (Figs. 1 and 2).
To enhance our ability to regenerate the enamel surface structure, a nonphosphorylated LRAP was also used in our biomimetic system to induce the elongation of mature enamel crystals, based on its demonstrated ability to guide the formation of ordered arrays of enamel-like crystals in vitro under conditions that support spontaneous CaP formation in solution (Le Norcy et al. 2011). Results obtained clearly show that its effect on enamel regeneration under the chosen experimental conditions is quite sensitive to LRAP concentration (Fig. 2 and Appendix Fig. 6). Nevertheless, at an intermediate concentration in the presence of PPi, LRAP was able to guide the formation of a textured enamel surface deposit composed of a dense packing of partially aligned small needle-like crystals (Fig. 2F), unlike the large plate-like crystals that formed in the absence of LRAP (Fig. 2D). Notably, LRAP was found to initiate the formation of the regenerated enamel layer within 15 min (Appendix Fig. 2C, D), while the onset of crystal growth on the enamel substrate was not observed until 5 to 10 h in the absence of LRAP, due to the apparent inhibitory effect of PPi (Appendix Fig. 2A, B). Hence, under these experimental conditions (0.04 mg/mL), LRAP was found to promote the rate of mineral deposition on etched enamel surfaces, while PPi stabilized the mineral solution. In comparison to results of prior studies (Onuma et al. 2005; Yamagishi et al. 2005; Hossein et al. 2015; Shafiei et al. 2015), we were able to rapidly generate a well-oriented enamel-like mineral structure directly on human enamel that replicates enamel surface morphology (Fig. 2). Dense crystals were found to grow in a well-aligned and seamless fashion in the direction of existing enamel crystals (Fig. 3B, C). As a result of the seamless or epitaxial growth, the regenerated enamel layer was strongly adhered, in comparison to that formed in the absence of LRAP (Fig. 4A, B).
Importantly, we also found that crystal growth does not occur on acid-etched enamel surfaces that are cut in a parallel direction to that of enamel rods in the presence of LRAP (Fig. 5E, F) under the same conditions used to induce the oriented growth of acid-etched enamel specimens cut perpendicular to the direction of the enamel rods (Fig. 2E, F). In contrast, in the absence of LRAP, randomly oriented plate-like crystals similarly grew on acid-etched enamel surfaces, regardless of enamel prism orientation (Figs. 2C and 5B). Such a difference suggests that LRAP preferentially interacts with enamel crystallite faces that are parallel to the c-axis, preventing crystal growth in ab planes and promoting crystal elongation, as Fincham et al. (1999) speculated for amelogenin nanospheres and as demonstrated by our earlier studies using recombinant full-length amelogenins (Beniash et al. 2005; Kwak et al. 2009) and LRAP (Le Norcy et al. 2011).
In conclusion, LRAP has the capacity to promote the linear growth of mature enamel crystals along the c-axis and regulate the size, shape, and orientation of a strongly adherent mineral layer that reflects the arrangement of existing enamel crystals in the presence of PPi-stabilized supersaturated CaP solutions. Although additional studies are needed to exploit this approach to produce regenerated enamel layers of greater thickness, the present findings demonstrate a potential for the development of a new and effective approach to regenerate enamel structure and properties.
Author Contributions
S.Y. Kwak, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Litman, contributed to data acquisition and analysis, critically revised the manuscript; H.C. Margolis, contributed to conception, design, and data interpretation, critically revised the manuscript; Y. Yamakoshi, J.P. Simmer, contributed to data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is available online.
This work was supported by grant R03-DE023620 (S.Y.K.) from the National Institute of Dental and Craniofacial Research. Early aspects of this study were also supported by a grant from the Colgate-Palmolive Company. The FIB work was performed at the Center for Nanoscale Systems (CNS), located at Harvard University in Cambridge, Massachusetts. CNS is a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the National Science Foundation (NSF) under NSF award no. ECS-0335765. GIXRD analyses were carried out at the MIT Center for Materials Science and Engineering that is also sponsored by NSF.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Backer Dirks O. 1966. Post eruptive changes in dental enamel. J Dent Res. 45(3):503–511. [Google Scholar]
- Beniash E, Simmer JP, Margolis HC. 2005. Effects of recombinant mouse amelogenins on the formation and organization of hydroxyapatite crystals in vitro. J Struct Biol. 149(2):182–190. [DOI] [PubMed] [Google Scholar]
- Busch S. 2004. Regeneration of human tooth enamel. Angew Chem Int Ed. 43(11):1428–1431. [DOI] [PubMed] [Google Scholar]
- Busch S, Schwarz U, Kniep R. 2001. Morphogenesis and structure of human teeth in relation to biomimetically grown fluorapatite-gelatine composites. Chem Mater. 13(10):3260–3271. [Google Scholar]
- Ekstrand KR, Ricketts DN, Kidd EA. 2001. Occlusal caries: pathology, diagnosis and logical management. Dent Update. 28(8):380–387. [DOI] [PubMed] [Google Scholar]
- Elliott JC. 1994. Structure and chemistry of the apatites and other calcium orthophosphates. Amsterdam: Elsevier. [Google Scholar]
- Fan Y, Sun Z, Moradian-Oldak J. 2009. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials. 30(4):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham AG, Moradian-Oldak J, Simmer JP. 1999. The structural biology of the developing dental enamel matrix. J Struct Biol. 126(3):270–299. [DOI] [PubMed] [Google Scholar]
- Fleisch H, Bisaz S. 1962. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 195:911. [DOI] [PubMed] [Google Scholar]
- Fleisch H, Graham R, Russell G, Francis MD. 1969. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathological calcification in vivo. Science. 165(3899):1262–1264. [DOI] [PubMed] [Google Scholar]
- Hay DI, Carlson ER, Schluckebier SK, Moreno EC, Schlesinger DH. 1987. Inhibition of calcium phosphate precipitation by human salivary acidic proline-rich proteins: structure-activity relationships. Calcif Tissue Int. 40(3):126–132. [DOI] [PubMed] [Google Scholar]
- Hay DI, Schluckebier SK, Moreno EC. 1982. Equilibrium dialysis and ultrafiltration studies of calcium and phosphate binding by human salivary proteins: implications for salivary supersaturation with respect to calcium phosphate salts. Calcif Tissue Int. 34(6):531–538. [DOI] [PubMed] [Google Scholar]
- Hay DI, Smith DJ, Schluckebier SK, Moreno EC. 1984. Relationship between concentration of human salivary statherin and inhibition of calcium phosphate precipitation in stimulated human parotid saliva. J Dent Res. 63(6):857–863. [DOI] [PubMed] [Google Scholar]
- Hossein BG, Sadr A, Espigares J, Hariri I, Nakashima S, Hamba H, Shafiei F, Moztarzadeh F, Tagami J. 2015. Study on the influence of leucine-rich amelogenin peptide (LRAP) on the remineralization of enamel defects via micro-focus x-ray computed tomography and nanoindentation. Biomed Mater. 10(3):035007. [DOI] [PubMed] [Google Scholar]
- Iijima M, Moradian-Oldak J. 2004. Interactions of amelogenins with octacalcium phosphate crystal faces are dose dependent. Calcif. Tissue Int. 74(6):522–531. [DOI] [PubMed] [Google Scholar]
- Koulourides T, Feagin F, Pigman W. 1965. Remineralization of dental enamel by saliva in vitro. Ann N Y Acad Sci. 131(2):751–757. [DOI] [PubMed] [Google Scholar]
- Kwak SY, Green S, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Regulation of calcium phosphate formation by amelogenins under physiological conditions. Eur J Oral Sci. 119(Suppl. 1):103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SY, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2009. Role of 20-kDa amelogenin (P148) phosphorylation in calcium phosphate formation in vitro. J Biol Chem. 284(28):18972–18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Norcy E, Kwak S-Y, Wiedemann-Bidlack FB, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Leucine-rich amelogenin peptides regulate mineralization in vitro. J Dent Res. 90(9):1091–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HC, Beniash E, Fowler CE. 2006. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J Dent Res. 85(9):775–793. [DOI] [PubMed] [Google Scholar]
- Margolis HC, Kwak SY, Yamazaki H. 2014. Role of mineralization inhibitors in the regulation of hard tissue biomineralization: relevance to initial enamel formation and maturation. Front Physiol. 5:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JL, Nancollas GH. 1973. The influence of multidentate organic phosphonates on the crystal growth of hydroxyapatite. Calcif Tissue Res. 13(4):295–303. [DOI] [PubMed] [Google Scholar]
- Moreno EC, Aoba T, Margolis HC. 1987. Pyrophosphate adsorption onto hydroxyapatite and its inhibition of crystal growth. Compend Suppl. 1987(8):S256–S258, S260,, S262–S266. [PubMed] [Google Scholar]
- Moreno EC, Margolis HC. 1988. Composition of human plaque fluid. J Dent Res. 67(9):1181–1189. [DOI] [PubMed] [Google Scholar]
- Moreno EC, Varughese K, Hay DI. 1979. Effect of human salivary proteins on the precipitation kinetics of calcium phosphate. Calcif Tissue Int. 28(1):7–16. [DOI] [PubMed] [Google Scholar]
- Nagano T, Kakegawa A, Yamakoshi Y, Tsuchiya S, Hu JC-C, Gomi K, Arai T, Bartlett JD, Simmer JP. 2009. Mmp-20 and Klk4 cleavage site preferences for amelogenin sequences. J Dent Res. 88(9):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onuma K, Yamagishi K, Oyane A. 2005. Nucleation and growth of hydroxyapatite nanocrystals for nondestructive repair of early caries lesions. J Cryst Growth. 282(1–2): 199–207. [Google Scholar]
- Ruan Q, Moradian-Oldak J. 2014. Development of amelogenin-chitosan hydrogel for in vitro enamel regrowth with a dense interface. J Vis Exp. 89: doi: 10.3791/51606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RG, Bisaz S, Donath A, Morgan DB, Fleisch H. 1971. Inorganic pyrophosphate in plasma in normal persons and in patients with hypophosphatasia, osteogenesis imperfecta, and other disorders of bone. J Clin Invest. 50(5):961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiei F, Hossein BG, Farajollahi MM, Fathollah M, Marjan B, Tahereh JK. 2015. Leucine-rich amelogenin peptide (LRAP) as a surface primer for biomimetic remineralization of superficial enamel defects: an in-vitro study. Scanning. 37(3):179–185. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fincham AG. 1995. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 6(2):84–108. [DOI] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, et al. 2010. Adhesive/dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 38(6):1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui T, Lunt AJ, Baimpas N, Sandholzer MA, Hu J, Dolbnya IP, Landini G, Korsunsky AM. 2014. Hierarchical modelling of in situ elastic deformation of human enamel based on photoelastic and diffraction analysis of stresses and strains. Acta Biomater. 10(1):343–354. [DOI] [PubMed] [Google Scholar]
- Totiam P, González-Cabezas C, Fontana MR, Zero DT. 2007. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 41(6):467–473. [DOI] [PubMed] [Google Scholar]
- Wen HB, Moradian-Oldak J, Zhong JP, Greenspan DC, Fincham AG. 2000. Effects of amelogenin on the transforming surface microstructures of bioglass in a calcifying solution. J Biomed Mater Res. 52(4):762–773. [DOI] [PubMed] [Google Scholar]
- Wiedemann-Bidlack FB, Kwak SY, Beniash E, Yamakoshi Y, Simmer JP, Margolis HC. 2011. Effects of phosphorylation on the self-assembly of native full-length porcine amelogenin and its regulation of calcium phosphate formation in vitro. J Struct Biol. 173(2):250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, Senawangse P. 2005. A synthetic enamel for rapid tooth repair. Nature. 433(7028):819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.