Abstract
Cadmium is a naturally-occurring element, and humans are exposed from cigarettes, food, and industrial sources. Following exposure, cadmium accumulates in the kidney and is slowly released into the urine, usually proportionally to the levels found in the kidneys. Cadmium levels in a single spot urine sample have been considered indicative of long-term exposure to cadmium; however, such a potentially exceptional biomarker requires careful scrutiny. In this review, we report good to excellent temporal stability of urinary cadmium (intraclass correlation coefficient 0.66–0.81) regardless of spot urine or first morning void sampling. Factors such as changes in smoking habits and diseases characterized by increased excretion of proteins may produce short-term changes in urinary cadmium levels. We recommend that epidemiologists use this powerful biomarker in prospective studies stratified by smoking status, along with thoughtful consideration of additional factors that can influence renal physiology and cadmium excretion.
Keywords: Cadmium, Urinary-cadmium, Creatinine, Biomonitoring, Heavy metal, Biomarker
Introduction
Cadmium is a silver metal with a bluish tinge that occurs naturally in the earth’s crust. It has been heavily used in industrial activities and is also often found in phosphate-based fertilizers [1]. Human exposure to cadmium has increased over the past several hundred years; for example, levels in human bones from the twentieth century have been reported at about ten times above pre-industrial levels [2]. Following exposure, much of the cadmium accumulates in the kidney, and levels in urine have been shown to be proportional to levels in the kidney. Therefore, urinary cadmium (U-Cd) levels are often considered to be an indicator of long-term exposure [3]. In the realm of environmental exposure biomarkers, an easy-to-collect biomarker that is indicative of long-term exposure is exceedingly rare and merits careful scrutiny. Questions remain about this biomarker, and we seek to address some of them in this review paper to help assess the merits of using U-Cd as a biomarker of long-term exposure for the general population: (1) Are levels of U-Cd temporally stable across samples? and (2) What factors are predictive of U-Cd levels and do any of those factors produce variation in U-Cd over short periods of time?
In this review of the urinary cadmium biomarker, we open with a brief summary of the public health relevance and toxicokinetics of cadmium. Then, we discuss recent research on the temporal stability of U-Cd, followed by factors predictive of the marker, and then conclude with a brief discussion of broader implications for exposure assessment and environmental epidemiology.
Public Health Relevance of Cadmium
Primary sources of Cd exposure in the general population include food and tobacco, with key contributions from industrial emissions and Cd-containing fertilizer. Among nonsmokers, the primary source of Cd exposure is through the diet. For example, the application of phosphate fertilizer for a period of 36 years resulted in a 14-fold increase in Cd content of surface soils [4]. In general, Cd in soil accumulates in crops which are then consumed or smoked. Measured Cd levels in Fall wheat doubled from 1920 to 1979 [5], which has been attributed to the application of fertilizer and sewage sludge. Cd is present in virtually all foods, with more than 80 % of food-Cd coming from cereals, vegetables, and potatoes [6]. Average Cd intake in food varies from 8 to 25 μg/day, with another 1–3 μg per day among cigarette smokers [7]. The US Food and Drug Administration’s Total Diet Study update reported a 26 % increase in dietary Cd exposure from 1990 to 2003, from 8.8 to 11.1 μg/person/day [8]. Many European countries have national policies that limit Cd in phosphate fertilizers [9], but the USA has only recently implemented regulations and even then, only in a small number of states [1].
Other anthropogenic sources include human-made Cd emissions arising from the manufacture, use and disposal of products containing Cd such as batteries, or from the presence of Cd impurities in manufactured products [5, 10]. Cd emissions from over 12,500 facilities (e.g., solid waste incineration, iron and steel production, zinc mining, and metal finishing production) in the USA result in Cd deposition on agricultural soils and plant uptake [11], which then contributes to dietary exposure.
Toxicological and occupational studies confirm that Cd is a renal and bone toxicant and a lung carcinogen [1]. Renal effects have been observed in occupational studies from chronic inhalation of Cd in fumes and dust in excess of 10 μg/m3 [12–14] or from cumulative dietary exposure greater than 1600 mg [15]. Lung cancer has been observed from occupational exposures >8 years mg/m3, although concurrent arsenic exposure has been difficult to disentangle [16–19]; nonetheless, the International Agency for Research on Cancer has classified Cd as a human lung carcinogen (group 1) [20]. In the Jinzu river basin in Japan, Itai-Itai disease (literally translated as “ouch-ouch”), which is characterized by intense pain, fractures, and distortion of the long bones, was associated with cadmium in contaminated river water used to irrigate rice fields [21–24]. More recent studies of chronic human exposure to Cd at levels more common to the general population (<25 μg/day) suggest that Cd may be associated with renal effects, osteoporosis, cardiovascular outcomes, and several cancers, among other outcomes [25–35].
Toxicokinetics of Cadmium
Toxicological studies beginning in the 1950s demonstrated that following exposure via the digestive tract, Cd was initially highest in the liver and then highest in the kidneys [36, 37]. Conversely, studies in rodents showed that inhalation of cigarette smoke caused an increase of Cd in lung and kidney tissue, but not in the liver [38]. It was later shown that induction of metallothionein, a low molecular weight protein with a tertiary structure forming alpha and beta domains of metal clusters [3], explained the redistribution of Cd from the liver to the kidneys [39–41]. We now know that following exposure, Cd is absorbed more readily via inhalation than ingestion, and women tend to exhibit higher levels than men, most likely reflecting increased absorption due to lower iron levels [22, 42–45]. Once in systemic circulation, Cd is initially bound to albumin in blood plasma, then transferred to the liver where the Cd-albumin complex is taken up, degraded, and Cd is released [3] (Fig. 1, physiology of Cd excretion) (adapted from Nordberg [3] and Zalups [59], with permission from Elsevier.)
Fig. 1.
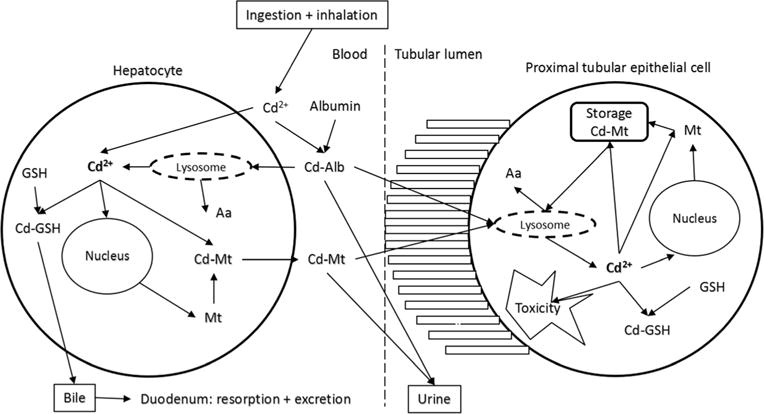
Physiology of Cd excretion (Adapted from Nordberg [3] and Zalups [59], with permission from Elsevier)
The released Cd induces synthesis of metallothionein [3]. Two major forms of metallothionein, MT-1 and MT-2, are inducible by Cd and can bind a range of metals, serving generally in the homeostasis of heavy metals (e.g., zinc) and thereby providing protection against many of their toxic effects [3, 46]. Both the constitutive cellular metallothioneins and those that are induced by chemicals are important in the detoxification of Cd [47]. After binding to metallothionein in liver cells, a small proportion of Cd-metallothionein is released into blood plasma and filtered through the glomerular membrane carrying Cd to the renal tubules [39, 48]. Like other small proteins, Cd-metallothionein is efficiently cleared from blood plasma by glomerular filtration and reabsorbed into the proximal tubules of the kidneys [3]. This pathway has been demonstrated using radiolabeling in animal experiments [49–51]. After uptake by the kidneys, Cd enters the lysosomes in the tubular cells where it is released from metallothionein [52] and may cause renal damage. Autopsy studies indicate that this process results in accumulation of Cd in the kidneys where it remains for many years, with an estimated half-life of 10–30 years [53–56]. A small portion of Cd is continuously but slowly excreted in urine [54]. Therefore, U-Cd is thought to reflect long-term exposure [57, 58], while blood Cd reflects a combination of both long-term and more recent exposures [3]. In addition, some Cd remains in, or is released back to, the gastrointestinal tract and excreted in the feces.
Introduction to Urine Cadmium
In the absence of occupational exposure to Cd, binding sites (e.g., metallothionein) are not saturated, and U-Cd generally increases in proportion to the amount of Cd stored in the kidney [1]. The degree to which U-Cd can be considered a reliable biomarker of long-term exposure in the general population will be discussed in the following sections on temporal stability and predictors of U-Cd.
An important consideration for any urinary biomarker is urine density. Urinary concentrations of contaminants are highly influenced by the degree of dilution of the urine and adjusting values for dilution is critical [60]. Normalizing urine biomarker levels using either urinary creatinine levels, specific gravity, osmolality, or urinary flow rate are most commonly used and correlate reasonably strongly with one another, yet each approach offers distinct benefits and drawbacks [61–65]. Urinary creatinine concentrations are most typically reported and therefore will be the focus of much of the discussion here. Creatinine normalized cadmium values will be abbreviated as U-Cdcr in this review. One important consideration of using U-CdCr, however, is that individuals with renal disease may excrete cadmium, creatinine, or other markers differently from healthy individuals [66, 67]; therefore, U-Cd should be used as a biomarker of exposure with caution in patients with kidney disease or diabetes.
Temporal Stability of Urine Cadmium
We identified seven studies [68–70, 71•, 72–74] that examined the temporal stability of U-Cdcr and reported intra class correlation (ICC) coefficients; ICCs ranged from 0.42 to 0.89. According to the criteria illustrated by Rosner [75], reproducibility is considered good when 0.40 ≤ ICC < 0.75 and excellent when ICC ≥ 0.75. In those studies, ICCs were highest for first morning void samples or 24 h samples measured within a few days of each other (ICC = 0.89) [72]; for samples collected anywhere from 1 to 12 months apart the ICCs ranged from 0.42 to 0.81 regardless of the type of sample [68, 69, 76]. It is unclear why these studies generated different ICC values. One potential difference between studies was whether or not they corrected for prevalent interferences by molybdenum oxide or tin [77]. Analysis of U-Cd is generally performed by inductively-coupled plasma mass spectrometry (ICP-MS), and these analyses can often suffer from both isobaric and polyatomic interferences that must be accounted for in the experimental design and data interpretation. There is currently some discussion within the analytical community regarding the appropriate approach of interference correction in U-Cd analysis. When we only included those studies which specified an attempted correction of polyatomic interferences when it may have been a concern (Table 1), the range of ICC values narrows to 0.66–0.81, with no clear difference whether the samples were spot urine or first morning void or whether the time interval between samples was months or a few years [71•, 72–74]. Therefore, studies of temporal stability generally support the interpretation that the U-Cd biomarker reflects long-term Cd body burden. However, it is important to note that these studies did not include individuals who experienced substantial variations in recent exposures (e.g., a new occupational Cd exposure or changes in smoking habits). Therefore, we do not know whether changes in recent exposures might impact the degree of temporal stability in the biomarker.
Table 1.
List of studies reporting ICC for repeated U-Cd measurements
| Author | Population (nind, nsamples) | Time frame between repeat samples | Type of urine sample | Range average U-Cdcr values (μg/g) | ICC U-Cdcr (type of samples, nsamples) | Analytical method | Polyatomic interferences on Cd (ICP-MS only) |
|---|---|---|---|---|---|---|---|
| Gunier et al. (2013) [68] | Healthy Californian women (141, 282) | 3–9 months | 24 h | 0.39–0.42 | 0.42 (24 h, 282)a 0.51 (3 months, NA) 0.59 (6 months, NA) 0.42 (9 months, NA) |
ICP-MS | Unclear correction approach; Possible interferences |
| Smolders et al. (2014) [70] | Healthy men and women (8, 352) | Every sample over a 6 days period | Spot | 0.08–0.66 | 0.75 (Spot, 352) | ICP-MS | Unclear correction approach; Possible interferences |
| Wang et al.(2015) [69] | Healthy men (11, 529) | 3 months | 24 h FMU Spot | 0.42–0.49 | 0.70 (24 h, 88)4 0.68 (FMU, 88) 0.53 (Spot, 529) |
ICP-MS | Unclear correction approach; Possible interferences |
| Akerstrom et al. (2014) [72] | Non-smoking healthy men and women (24, 288) | 7 days | 24 h FMU Spot | 0.08–0.17 | 0.89 (24 h, 48)a 0.89 (FMU, 48) 0.70 (spot, 288) |
ICP-MS | Addressed by analytical approach1 |
| Sanchez-Rodriguez et al. (2015) [71•] | Healthy men and women (83, 166) | 1 year | FMU | 0.10–0.25 | 0.72 (FMU, 166) | (DRC)-ICP-MS | Addressed by analytical approach2 |
| Vacchi-Suzzi et al. (2016) [74] | Healthy men and women (100, 244) | 1 week, 1 month, 6 months | FMU Spot | 0.19–0.21 | 0.76 (1 week FMU, 88) 0.66 (1 mo. FMU, 110) 0.78 (6 mo. spot, 156) |
ICP-MS | Addressed by analytical approach3 |
| Arisawa et al. (1997) [73] | Healthy Japanese men and womenina cadmium polluted area (48, 96) | 3 years | FMU | 6.70–8.40 | 0.81 (FMU, 96) | Zeeman effect electro thermal atomic absorption spectrometry | No concerns |
ICP-MS inductively coupled plasma mass spectrometry, DRC-ICP-MS dynamic reaction cell-inductively coupled plasma mass spectrometry, FMU first morning urine, NA not available, nind number of individual participants in a study, nsamples number of samples provided in a study (some studies had only two samples per participant; other studies had at least a dozen samples)
Not creatinine normalized
Potentially addressed by mathematical correction equations
Addressed by reactive gas oxygen to remove polyatomic interferences
Addressed by use of collision cell for polyatomic interferences and off-mass reporting for Sn isobaric interference
The 24-h samples are not creatinine normalized because they combine multiple urine samples over the course of a day
Predictors of Urine Cadmium
Another way to investigate the extent to which U-Cd is an indicator of long-term exposure is to assess which factors predict U-Cd and their short-term variation. The ideal study design to answer this question would be a longitudinal study which investigates the variation of U-Cd levels following changes in exposure. Unfortunately, in our review of the literature, we did not find any such longitudinal studies, with the exception of a small investigation on the decrease of secondhand smoke exposure in non-active smokers [71•]. However, a number of studies have measured U-Cd worldwide and investigated the correlation between exposure sources and those levels [78–84]. Significant correlations between estimated Cd exposure and U-Cd levels have been found in populations exposed to environmental contamination [23, 78, 79]. In the absence of unusually high environmental or occupational sources, the U-Cdcr concentration is usually <2 μg Cd/g creatinine in Western populations and is most strongly correlated with smoking, age, and female sex [78–84].
Cigarette smoking contributes to most of the variability in U-Cd due to the lungs’ high rate of absorption of Cd in tobacco. Current smokers have higher urinary concentrations than former and never smokers [54], and it has been widely reported that former smokers have significantly higher U-Cd than never smokers, which one would expect if U-Cd is a biomarker of long-term exposure [78–84]. Recently, however, at least two reports suggested there might be no such difference between former and never smokers [80, 85]. In order to further investigate the matter, we contacted study authors and were able to pool data from five study populations that reported U-Cdcr at levels generally <2 μg Cd/g creatinine, and also had information on smoking status and age [68, 80, 86, 87, 88•, 89] (Fig. 2). In pooling the data, studies that used the same study population, e.g., [88•, 89, 90], were not double-counted. In a pooled analysis, the mean ± SD U-Cdcr was 0.47 ± 0.50, 0.69 ± 0.88, and 0.91 ± 0.81 μg Cd/g creatinine, with n = 12630, 6309, and 7698 for never, former, and current smokers, respectively. The difference between these populations was highly significant, as evidenced by Student T test values for both never vs. former and never vs. current smokers of p < 0.01 (Fig. 2a). This overall difference between never and former smokers was driven by differences in U-Cdcr levels among the older population (Fig. 2b). While U-Cdcr levels appear to vary between never and former smokers, these data are unable to address whether levels of U-Cdcr decrease after smoking cessation. Sanchez-Rodriguez et al. (2015) report that a year after a public smoking ban went into effect, non-smokers showed a median drop in U-Cdcr of 0.07 μg/g, with 76 % of participants showing a drop in U-Cdcr [71•]. Adams and Newcomb (2014) modeled cross-sectional NHANES data and reported that U-Cd drops 23 % in the first year after quitting smoking among 55-year-old males with 20 pack years of smoking history [88•]. In their model, U-Cd levels in former smokers remain elevated compared with never smokers even 30 years following smoking cessation, suggesting that U-Cd reflects both recent smoking exposure and long-term smoking history.
Fig. 2.
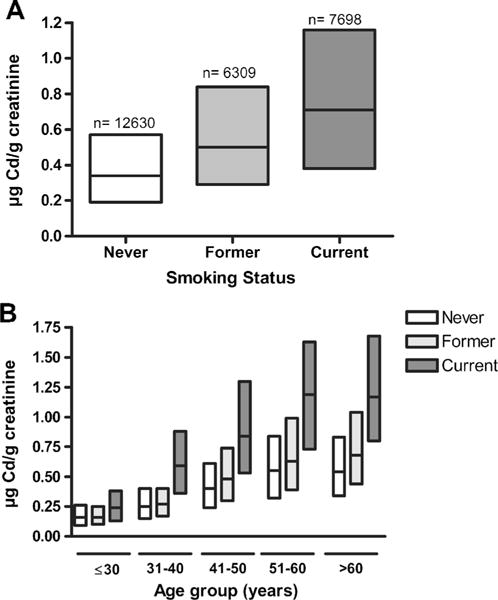
Creatinine-adjusted U-Cd median and IQR from six studies in never, former, and current smokers, overall (a) and stratified by age (b) [67, 79, 85–87, 88•]. The NHANES 1999–2010 data were included only once
Age is also consistently associated with U-Cdcr [78–84] (Fig. 2b), although the extent to which creatinine adjustment may inflate this association has not yet been established [80]. One possible explanation for this inflation is that urine creatinine levels tend to decrease with age as muscle mass diminishes [91]. In our analyses of the NHANES 1999–2012 data with at least 1500 people in each age group, the association of U-Cd with age is maintained regardless of creatinine adjustment (U-Cd: age 21–30; 0.24 μg/L, age 71–80; 0.55 μg/L and U-Cdcr: age 21–30; 0.17 μg/g, age 71–80; 0.56 μg/g).
U-Cd is also higher among women, with iron status and number of pregnancies (during which body iron stores are often depleted) being important factors because low iron increases Cd absorption [42, 68, 81–83, 92, 93]. Cd uses the same intestinal absorption transport system as zinc, calcium, and iron [94], three essential divalent cations. Iron (Fe) body stores were shown to especially influence the absorption rate of Cd: the lower the Fe body stores, the more Cd is absorbed from food in the intestinal tract [95]. Cd can be transported across the intestinal epithelium by the concerted action of the apical divalent metal transporter (DMT1) as well as the metal transported protein (MTP1). The expression of these proteins changes in response to the status of the body’s Fe stores, and Cd competes with Fe for absorption. This is particularly significant when cereals and green leafy vegetables, which can be relatively rich in Cd and poor in Fe, are consumed. This phenomenon is more prevalent in women, who tend to have lower body Fe stores, in particular during pregnancy [42, 43, 83, 93].
Linear models accounting for age, sex, number of pregnancies, and smoking habits typically contribute to less than 30 % of U-Cd variability, which drops to ∼10 % when the population is stratified by smoking status [82]. Even acknowledging the possibility of measurement error or nonlinearity, this suggests that other factors contribute to U-Cd variability, in particular an individual’s diet which can be a meaningful source of Cd. When dietary Cd intake is estimated using a food frequency questionnaire (FFQ), only a small portion of the observed variability in U-Cd is explained [81–84, 92, 96]. Other than organ meats like kidney and liver, which have very high levels of Cd but are seldom eaten regularly, low levels of Cd are found throughout the diet in diverse foods including meats, shellfish, vegetables, grains, soybeans/tofu, and dairy. This variety of dietary sources limits the likelihood of confounding in epidemiologic studies, but dietary sources have not been consistently associated with U-Cdcr in FFQ studies [81–84, 92, 96]. It is possible that the FFQ does not reflect historic exposure and that is the reason it so poorly estimates U-Cd. However, duplicate diet studies in which urine is collected within 24 h of dietary samples, tends to show better correlation. In two of three studies in which Cd was measured in a duplicate diet sample, U-Cdcr was positively associated with dietary Cd (ρ = 0.4 in both [79, 81], ρ = −0.1 in [97]). Julin et al. [81] argue that if the sampled dietary intake reflects historic patterns of dietary intake, then it may be more likely to be associated with U-Cdcr, although we cannot verify this assertion. In all three studies, the correlation between dietary intake and both blood Cd and U-Cd was similar, which might suggest that U-Cdcr is influenced to some degree by recent dietary exposures; however, the high degree of temporal stability of the marker (Table 1) suggests that the degree to which U-Cdcr reflects current dietary exposure to Cd is likely small. Therefore, it is suggested, but not confirmed, that the correlation between Cd dietary intake and U-Cd would be greater if duplicate diet samples were obtained many years before urine samples and not at the same time as in these studies.
A recent commentary [98•] also highlights two reports of co-excretion of Cd with plasma proteins in urine [80, 99]; factors responsible for excretion of these proteins might therefore also be predictive of U-Cdcr. A possible mechanistic explanation for this observation involves the capacity of the kidneys to filter and reabsorb low molecular weight proteins [80]. As described in the section on toxicokinetics above, following exposure, Cd-metallothionein is generally reabsorbed by the proximal tubules. The amount of the protein complex not reabsorbed is excreted in the urine, and as the body’s ability to reabsorb these proteins changes (e.g., due to disease), this can result in increased excretion of Cd along with other proteins. Therefore, because of co-excretion mechanisms, an increase in U-Cd might be observed in people with chronic diseases involving the kidneys and in those who experience an increase of plasma proteins in the urine (proteinuria or albuminuria), which may be a result of bone loss, cardiovascular diseases, or diabetic nephropathy [98•]. The greater risk predicted for certain diseases in cross-sectional epidemiology studies in the presence of elevated U-Cd may in fact be the result of reverse causality, in which higher U-Cd levels were caused by the disease and not vice versa. Therefore, prospective longitudinal studies are required to clarify the risks from relatively low levels of U-Cd that have been suggested through cross-sectional investigation.
Conclusions
In the universe of biomonitoring markers, U-Cd remains one of the best tools to assess long-term exposure to cadmium. The high degree of temporal stability in the biomarker, as evidenced by ICC values ranging from 0.66 to 0.81 regardless of spot samples or first morning voids, suggests that short-term variability in dietary exposures is likely only a small contributor to the U-Cd measure. Changes in U-Cd following smoking cessation, however, suggest that investigators should be careful to only investigate epidemiologic associations in separate strata of current, former, and never smokers. Researchers should also consider the physiology of cadmium exposure and excretion when designing epidemiologic studies to avoid being confounded by reverse causality and recent exposures. Prospective, longitudinal studies are recommended.
Acknowledgments
We wish to thank Scott Adams, Alfred Bernard, Esther Garcia-Esquinas, Meian He, Ana Navas-Acien, Peggy Reynolds, and Maria Tellez-Plaza who kindly provided additional data from their studies in order for us to prepare Fig. 2 of this manuscript.
Footnotes
This article is part of the Topical Collection on Metals and Health
Conflict of Interest Caterina Vacchi-Suzzi, Danielle Kruse, James Harrington, Keith Levine, and Jaymie R. Meliker declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.ATSDR (Agency for Toxic Substances & Disease registry) Toxicological profile for Cadmium. Atlanta, GA: 2012. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15. [Google Scholar]
- 2.Jaworowski Z, Barbalat F, Blain C, et al. Heavy metals in human and animal bones from ancient and contemporary France. Sci Total Environ. 1985;43(1–2):103–26. doi: 10.1016/0048-9697(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 3.Nordberg GF. Historical perspectives on cadmium toxicology. Toxicol Appl Pharmacol. 2009;238(3):192–200. doi: 10.1016/j.taap.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Singh BR. Trace element availability to plants in agricultural soils, with special emphasis on fertilizer inputs. Environ Rev. 1994;2(2):133–46. doi: 10.1139/a94-009. [DOI] [Google Scholar]
- 5.Kjellstrom T. Exposure and accumulation of cadmium in populations from Japan, the United States, and Sweden. Environ Health Perspect. 1979;28:169–97. doi: 10.1289/ehp.28-1637502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson IM, Eriksson J, Oborn I, et al. Cadmium in food production systems: a health risk for sensitive population groups. Ambio. 2005;34(4–5):344–51. doi: 10.1639/0044-7447(2005)034[0344:cifpsa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–8. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Egan SK, Bolger PM, Carrington CD. Update of US FDA’s total diet study food list and diets. J Expo Sci Environ Epidemiol. 2007;17(6):573–82. doi: 10.1038/sj.jes.7500554. [DOI] [PubMed] [Google Scholar]
- 9.van Balken J. IFA 2004 technical conference beijing. Beijing: 2004. Prospective EU Cd regulation for fertilizers. 04/20/2004 2004. [Google Scholar]
- 10.Plachy J. Cadmium recycling in the United States in 2000 vol US GEOLOGICAL SURVEY CIRCULAR 1196-O. Reston: U.S. Geological Survey; 2000. [Google Scholar]
- 11.Mobley JD, Rackley K, Pope A, et al. Persistent bioaccumulative toxic emissions in the US. Paper presented at the long range transport workshop; Ann Arbor, MI. 2003. [Google Scholar]
- 12.Falck FY, Jr, Fine LJ, Smith RG, et al. Occupational cadmium exposure and renal status. Am J Ind Med. 1983;4(4):541–9. doi: 10.1002/ajim.4700040408. [DOI] [PubMed] [Google Scholar]
- 13.Jarup L, Elinder CG, Spang G. Cumulative blood-cadmium and tubular proteinuria: a dose–response relationship. Int Arch Occup Environ Health. 1988;60(3):223–9. doi: 10.1007/BF00378700. [DOI] [PubMed] [Google Scholar]
- 14.Thun MJ, Osorio AM, Schober S, et al. Nephropathy in cadmium workers: assessment of risk from airborne occupational exposure to cadmium. Br J Ind Med. 1989;46(10):689–97. doi: 10.1136/oem.46.10.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogawa K, Kobayashi E, Honda R, et al. Renal dysfunctions of inhabitants in a cadmium-polluted area. Environ Res. 1980;23(1):13–23. doi: 10.1016/0013-9351(80)90088-2. [DOI] [PubMed] [Google Scholar]
- 16.Stayner L, Smith R, Thun M, et al. A dose–response analysis and quantitative assessment of lung cancer risk and occupational cadmium exposure. Ann Epidemiol. 1992;2(3):177–94. doi: 10.1016/1047-2797(92)90052-r. [DOI] [PubMed] [Google Scholar]
- 17.Sorahan T, Lancashire R. Lung cancer findings from the NIOSH study of United States cadmium recovery workers: a cautionary note. Occup Environ Med. 1994;51(2):139–40. doi: 10.1136/oem.51.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorahan T, Lancashire RJ. Lung cancer mortality in a cohort of workers employed at a cadmium recovery plant in the United States: an analysis with detailed job histories. Occup Environ Med. 1997;54(3):194–201. doi: 10.1136/oem.54.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thun MJ, Schnorr TM, Smith AB, et al. Mortality among a cohort of U.S. cadmium production workers—an update. J Natl Cancer Inst. 1985;74(2):325–33. [PubMed] [Google Scholar]
- 20.International Agency for Research on Cancer (IARC) Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Monogr Eval Carcinog Risk Hum. 1993;58 [PMC free article] [PubMed] [Google Scholar]
- 21.Shigematsu I. The epidemiological approach to cadmium pollution in Japan. Ann Acad Med Singapore. 1984;13(2):231–6. [PubMed] [Google Scholar]
- 22.Jarup L, Berglund M, Elinder CG, et al. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 23.Kido T, Nogawa K, Yamada Y, et al. Osteopenia in inhabitants with renal dysfunction induced by exposure to environmental cadmium. Int Arch Occup Environ Health. 1989;61(4):271–6. doi: 10.1007/BF00381425. [DOI] [PubMed] [Google Scholar]
- 24.Kagamimori S, Watanabe M, Nakagawa H, et al. Case–control study on cardiovascular function in females with a history of heavy exposure to cadmium. Bull Environ Contam Toxicol. 1986;36(4):484–90. doi: 10.1007/BF01623539. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Sun H, Wu Y, et al. Tubular and glomerular kidney effects in the Chinese general population with low environmental cadmium exposure. Chemosphere. 2016;147:3–8. doi: 10.1016/j.chemosphere.2015.11.069. [DOI] [PubMed] [Google Scholar]
- 26.Noonan CW, Sarasua SM, Campagna D, et al. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ Health Perspect. 2002;110(2):151–5. doi: 10.1289/ehp.02110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akesson A, Lundh T, Vahter M, et al. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113(11):1627–31. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akesson A, Bjellerup P, Lundh T, et al. Cadmium-induced effects on bone in a population-based study of women. Environ Health Perspect. 2006;114(6):830–4. doi: 10.1289/ehp.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallagher CM, Kovach JS, Meliker JR. Urinary cadmium and osteoporosis in U.S. Women >or= 50 years of age: NHANES 1988– 1994 and 1999–2004. Environ Health Perspect. 2008;116(10):1338–43. doi: 10.1289/ehp.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfven T, Elinder CG, Carlsson MD, et al. Low-level cadmium exposure and osteoporosis. J Bone Miner Res. 2000;15(8):1579–86. doi: 10.1359/jbmr.2000.15.8.1579. [DOI] [PubMed] [Google Scholar]
- 31.Staessen JA, Roels HA, Emelianov D, et al. Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet. 1999;353(9159):1140–4. doi: 10.1016/s0140-6736(98)09356-8. [DOI] [PubMed] [Google Scholar]
- 32.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, et al. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep. 2013;15(10):356. doi: 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res. 2008;68(15):6435–41. doi: 10.1158/0008-5472.CAN-08-0329. [DOI] [PubMed] [Google Scholar]
- 34.McElroy JA, Shafer MM, Trentham-Dietz A, et al. Cadmium exposure and breast cancer risk. J Natl Cancer Inst. 2006;98(12):869–73. doi: 10.1093/jnci/djj233. [DOI] [PubMed] [Google Scholar]
- 35.Arora M, Weuve J, Schwartz J, et al. Association of environmental cadmium exposure with periodontal disease in U.S. adults. Environ Health Perspect. 2009;117(5):739–44. doi: 10.1289/ehp.0800312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunn SA, Gould TC. Selective accumulation of Cd115 by cortex of rat kidney. Proc Soc Exp Biol Med. 1957;96(3):820–3. doi: 10.3181/00379727-96-23619. [DOI] [PubMed] [Google Scholar]
- 37.Nordberg GF, Nishiyama K. Whole-body and hair retention of cadmium in mice including an autoradiographic study on organ distribution. Arch Environ Health. 1972;24(3):209–14. doi: 10.1080/00039896.1972.10666071. [DOI] [PubMed] [Google Scholar]
- 38.Gairola CG, Wagner GJ. Cadmium accumulation in the lung, liver and kidney of mice and rats chronically exposed to cigarette smoke. J Appl Toxicol. 1991;11(5):355–8. doi: 10.1002/jat.2550110510. [DOI] [PubMed] [Google Scholar]
- 39.Nordberg GF, Piscator M, Nordberg M. On the distribution of cadmium in blood. Acta Pharmacol Toxicol (Copenh) 1971;30(3):289–95. doi: 10.1111/j.1600-0773.1971.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 40.Nordberg M. Studies on metallothionein and cadmium. Environ Res. 1978;15(3):381–404. doi: 10.1016/0013-9351(78)90120-2. [DOI] [PubMed] [Google Scholar]
- 41.Garty M, Wong KL, Klaassen CD. Redistribution of cadmium to blood of rats. Toxicol Appl Pharmacol. 1981;59(3):548–54. doi: 10.1016/0041-008x(81)90309-4. [DOI] [PubMed] [Google Scholar]
- 42.Akesson A, Berglund M, Schutz A, et al. Cadmium exposure in pregnancy and lactation in relation to iron status. Am J Public Health. 2002;92(2):284–7. doi: 10.2105/ajph.92.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berglund M, Akesson A, Nermell B, et al. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Perspect. 1994;102(12):1058–66. doi: 10.1289/ehp.941021058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kippler M, Ekstrom EC, Lonnerdal B, et al. Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol Appl Pharmacol. 2007;222(2):221–6. doi: 10.1016/j.taap.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Vahter M, Akesson A, Liden C, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104(1):85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Klaassen CD, Liu J, Diwan BA. Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol. 2009;238(3):215–20. doi: 10.1016/j.taap.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klaassen CD, Liu J, Choudhuri S. Metallothionein: an intracellular protein to protect against cadmium toxicity. Annu Rev Pharmacol Toxicol. 1999;39:267–94. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- 48.Nordberg GF, Nordberg M, Piscator M, et al. Separation of two forms of rabbit metallothionein by isoelectric focusing. Biochem J. 1972;126(3):491–8. doi: 10.1042/bj1260491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherian MG, Shaikh ZA. Metabolism of intravenously injected cadmium-binding protein. Biochem Biophys Res Commun. 1975;65(3):863–9. doi: 10.1016/s0006-291x(75)80465-7. [DOI] [PubMed] [Google Scholar]
- 50.Foulkes EC. Renal tubular transport of cadmium-metallothionein. Toxicol Appl Pharmacol. 1978;45(2):505–12. doi: 10.1016/0041-008x(78)90112-6. [DOI] [PubMed] [Google Scholar]
- 51.Nordberg M, Nordberg GF. Distribution of metallothionein-bound cadmium and cadmium chloride in mice: preliminary studies. Environ Health Perspect. 1975;12:103–8. doi: 10.1289/ehp.7512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler BA, Nordberg GF. The renal toxicity of cadmium metallothionein: morphometric and X-ray microanalytical studies. Toxicol Appl Pharmacol. 1978;46(3):609–23. doi: 10.1016/0041-008x(78)90307-1. [DOI] [PubMed] [Google Scholar]
- 53.Amzal B, Julin B, Vahter M, et al. Population toxicokinetic modeling of cadmium for health risk assessment. Environ Health Perspect. 2009;117(8):1293–301. doi: 10.1289/ehp.0800317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nordberg GF, Nogawa K, Nordberg M, et al. Handbook on the toxicology of metals: cadmium. Elsevier; Amsterdam: 2007. pp. 445–486. [Google Scholar]
- 55.Friberg L, Piscator M, Nordberg GF, et al. Cadmium in the environment. 2nd. Boca Raton: CRC Press; 1974. [Google Scholar]
- 56.Kjellstrom T, Nordberg GF. A kinetic model of cadmium metabolism in the human being. Environ Res. 1978;16(1–3):248–69. doi: 10.1016/0013-9351(78)90160-3. [DOI] [PubMed] [Google Scholar]
- 57.Roels H, Lauwerys R, Dardenne AN. The critical level of cadmium in human renal cortex: a reevaluation. Toxicol Lett. 1983;15(4):357–60. doi: 10.1016/0378-4274(83)90156-x. [DOI] [PubMed] [Google Scholar]
- 58.Roels HA, Lauwerys RR, Buchet JP, et al. In vivo measurement of liver and kidney cadmium in workers exposed to this metal: its significance with respect to cadmium in blood and urine. Environ Res. 1981;26(1):217–40. doi: 10.1016/0013-9351(81)90199-7. [DOI] [PubMed] [Google Scholar]
- 59.Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol. 2003;186(3):163–88. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 60.Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54(10):615–27. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 61.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suwazono Y, Akesson A, Alfven T, et al. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10(2–3):117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- 63.Imran S, Eva G, Christopher S, et al. Is specific gravity a good estimate of urine osmolality? J Clin Lab Anal. 2010;24(6):426–30. doi: 10.1002/jcla.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hays SM, Aylward LL, Blount BC. Variation in urinary flow rates according to demographic characteristics and body mass index in NHANES: potential confounding of associations between health outcomes and urinary biomarker concentrations. Environ Health Perspect. 2015;123(4):293–300. doi: 10.1289/ehp.1408944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Voinescu GC, Shoemaker M, Moore H, et al. The relationship between urine osmolality and specific gravity. Am J Med Sci. 2002;323(1):39–42. doi: 10.1097/00000441-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 66.Weaver VM, Kim NS, Lee BK, et al. Differences in urine cadmium associations with kidney outcomes based on serum creatinine and cystatin C. Environ Res. 2011;111(8):1236–42. doi: 10.1016/j.envres.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weaver VM, Vargas GG, Silbergeld EK, et al. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ Res. 2014;132:226–32. doi: 10.1016/j.envres.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunier RB, Horn-Ross PL, Canchola AJ, et al. Determinants and within-person variability of urinary cadmium concentrations among women in northern California. Environ Health Perspect. 2013;121(6):643–9. doi: 10.1289/ehp.1205524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YX, Feng W, Zeng Q, et al. Variability of metal levels in spot, first morning, and 24-hour urine samples over a 3-month period in healthy adult Chinese men. Environ Health Perspect. 2015 doi: 10.1289/ehp.1409551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smolders R, Koch HM, Moos RK, et al. Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 1: metals. Toxicol Lett. 2014;231(2):249–60. doi: 10.1016/j.toxlet.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 71•.Sanchez-Rodriguez JE, Bartolome M, Canas AI, et al. Anti-smoking legislation and its effects on urinary cotinine and cadmium levels. Environ Res. 2015;136:227–33. doi: 10.1016/j.envres.2014.09.033. Reports on the correlation between urine samples measured a year apart, and the impact of anti-smoking legislation on urinary cadmium levels in non-smokers. [DOI] [PubMed] [Google Scholar]
- 72.Akerstrom M, Barregard L, Lundh T, et al. Variability of urinary cadmium excretion in spot urine samples, first morning voids, and 24 h urine in a healthy non-smoking population: implications for study design. J Expo Sci Environ Epidemiol. 2014;24(2):171–9. doi: 10.1038/jes.2013.58. [DOI] [PubMed] [Google Scholar]
- 73.Arisawa K, Nakano A, Honda S, et al. Reproducibility of urinary beta 2-microglobulin and cadmium excretion among residents in a cadmium-polluted area during a 3-year period. Toxicol Lett. 1997;91(2):147–52. doi: 10.1016/s0378-4274(97)03884-8. [DOI] [PubMed] [Google Scholar]
- 74.Vacchi-Suzzi C, Porucznik CA, Cox KJ, et al. Temporal variability of urinary cadmium in spot urine samples and first morning voids. J Expos Sci Environ Epidemiol. 2016 doi: 10.1038/jes.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosner B. Multisample inference. Pacific Grove: Fundamentals of Biostatistics. (5th) 2002 [Google Scholar]
- 76.Ikeda M, Ezaki T, Tsukahara T, et al. Reproducibility of urinary cadmium, alpha1-microglobulin, and beta2-microglobulin levels in health screening of the general population. Arch Environ Contam Toxicol. 2005;48(1):135–40. doi: 10.1007/s00244-004-3034-0. [DOI] [PubMed] [Google Scholar]
- 77.Jarrett JM, Xiao G, Caldwell KL, et al. Eliminating molybdenum oxide interference in urine cadmium biomonitoring using ICP-DRC-MS. J Anal At Spectrom. 2008;23(7):962–7. doi: 10.1039/B801927D. [DOI] [Google Scholar]
- 78.Kobayashi E, Suwazono Y, Uetani M, et al. Association between lifetime cadmium intake and cadmium concentration in individual urine. Bull Environ Contam Toxicol. 2005;74(5):817–21. doi: 10.1007/s00128-005-0654-7. [DOI] [PubMed] [Google Scholar]
- 79.Shimbo S, Zhang ZW, Moon CS, et al. Correlation between urine and blood concentrations, and dietary intake of cadmium and lead among women in the general population of Japan. Int Arch Occup Environ Health. 2000;73(3):163–70. doi: 10.1007/s004200050023. [DOI] [PubMed] [Google Scholar]
- 80.Chaumont A, Voisin C, Deumer G, et al. Associations of urinary cadmium with age and urinary proteins: further evidence of physiological variations unrelated to metal accumulation and toxicity. Environ Health Perspect. 2013;121(9):1047–53. doi: 10.1289/ehp.1306607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Julin B, Vahter M, Amzal B, et al. Relation between dietary cadmium intake and biomarkers of cadmium exposure in premenopausal women accounting for body iron stores. Environ Health. 2011;10:105. doi: 10.1186/1476-069X-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vacchi-Suzzi C, Eriksen KT, Levine K, et al. Dietary intake estimates and urinary cadmium levels in Danish postmenopausal women. PLoS One. 2015;10(9):e0138784. doi: 10.1371/journal.pone.0138784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McElroy JA, Shafer MM, Hampton JM, et al. Predictors of urinary cadmium levels in adult females. Sci Total Environ. 2007;382(2–3):214–23. doi: 10.1016/j.scitotenv.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Adams SV, Newcomb PA, Shafer MM, et al. Sources of cadmium exposure among healthy premenopausal women. Sci Total Environ. 2011;409(9):1632–7. doi: 10.1016/j.scitotenv.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeda M, Moriguchi J, Ezaki T, et al. Smoking-induced increase in urinary cadmium levels among Japanese women. Int Arch Occup Environ Health. 2005;78(7):533–40. doi: 10.1007/s00420-005-0612-z. [DOI] [PubMed] [Google Scholar]
- 86.Liu B, Feng W, Wang J, et al. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut. 2016;210:1–8. doi: 10.1016/j.envpol.2015.11.046. [DOI] [PubMed] [Google Scholar]
- 87.Garcia-Esquinas E, Pollan M, Tellez-Plaza M, et al. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1306587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88•.Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Expo Sci Environ Epidemiol. 2014;24(2):163–70. doi: 10.1038/jes.2013.55. Models the impact of smoking cessation on urinary and blood cadmium levels and the degree to which each reflects short-term and long-term exposures. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tellez-Plaza M, Navas-Acien A, Caldwell KL, et al. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Perspect. 2012;120(2):204–9. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mortensen ME, Wong L-Y, Osterloh JD. Smoking status and urine cadmium above levels associated with subclinical renal effects in U.S. adults without chronic kidney disease. Int J Hyg Environ Health. 2011;214(4):305–10. doi: 10.1016/j.ijheh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113(2):192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olsson IM, Bensryd I, Lundh T, et al. Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects. Environ Health Perspect. 2002;110(12):1185–90. doi: 10.1289/ehp.021101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallagher CM, Chen JJ, Kovach JS. The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20–49 years. Environ Res. 2011;111(5):702–7. doi: 10.1016/j.envres.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 94.Vesey DA. Transport pathways for cadmium in the intestine and kidney proximal tubule: focus on the interaction with essential metals. Toxicol Lett. 2010;198(1):13–9. doi: 10.1016/j.toxlet.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Ryu DY, Lee SJ, Park DW, et al. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol Lett. 2004;152(1):19–25. doi: 10.1016/j.toxlet.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 96.Quraishi SM, Adams SV, Shafer M, et al. Urinary cadmium and estimated dietary cadmium in the Women’s Health Initiative. J Expo Sci Environ Epidemiol. 2016;26(3):303–8. doi: 10.1038/jes.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moon CS, Zhang ZW, Shimbo S, et al. Evaluation of urinary cadmium and lead as markers of background exposure of middle-aged women in Korea: dietary intake as an influential factor. Toxicol Lett. 1999;108(2–3):173–8. doi: 10.1016/s0378-4274(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 98•.Bernard A. Confusion about cadmium risks: the unrecognized limitations of an extrapolated paradigm. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509691. A thoughtful commentary bringing to light some of the challenges with the urinary cadmium biomarker, especially important for assessment of risk in cross-sectional studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Akerstrom M, Sallsten G, Lundh T, et al. Associations between urinary excretion of cadmium and proteins in a nonsmoking population: renal toxicity or normal physiology? Environ Health Perspect. 2013;121(2):187–91. doi: 10.1289/ehp.1205418. [DOI] [PMC free article] [PubMed] [Google Scholar]


