Abstract
Fetal exposure to selective serotonin reuptake inhibitors (SSRI) has been associated with increased risk of adverse neurodevelopmental outcomes. In the adult brain, SSRI therapy regulates p11 (s100a10) expression and alters neurogenesis. The protein p11 indirectly regulates 5-HT signaling through 5-HT1B/D receptors. In the fetal brain, signaling through these receptors modulates axonal circuit formation. We determined whether p11 is expressed in the fetal mouse brain, and whether maternal SSRI exposure affects fetal p11 expression and neurogenesis. The SSRI ± citalopram was administered to pregnant mice from gestational day 8 to 17. Results show that p11 is expressed in fetal thalamic neurons and thalamocortical axons. Furthermore, p11 protein expression is significantly decreased in the fetal thalamus after in utero ±citalopram exposure compared to untreated controls, and neurogenesis is significantly decreased in specific fetal brain regions. These findings reveal differential regulation of p11 expression and altered neurogenesis in the fetal brain as a result of maternal SSRI exposure.
Keywords: P11, serotonin, SSRI, pregnancy, neurogenesis, fetal brain, citalopram
Graphical abstract
The prevalence of major depressive disorders (MDD) in pregnancy is approximately 13%.1 Selective serotonin reuptake inhibitor (SSRI) antidepressants remain the mainstay of treatment, despite an unclear safety profile in pregnancy.2 Although SSRI use may relieve symptoms of depression in pregnancy and ultimately lead to improved pregnancy outcomes, potential risks to the fetus has made both providers reluctant to prescribe them and pregnant women reluctant to accept them. Consequently, the use of SSRIs for depression during pregnancy remains a therapeutic challenge. Epidemiologic studies suggest that the use of SSRIs during pregnancy may be associated with increased risk of adverse neurodevelopmental outcomes in the offspring,3 with long-term consequences such as increased risk of autism spectrum disorders and postnatal language learning deficits.4–6 Timing of exposure appears critical, with the highest risk after exposure to SSRIs during the first trimester of pregnancy.4,5 A better understanding of the potential impact of these drugs on fetal brain development is needed.
Serotonin (5-HT) signaling provides important modulation of histogenic processes in the fetal brain, such as cell proliferation, migration, and axonal circuit formation.7 Since SSRIs specifically inhibit the 5-HT transporter function and can cross the human8,9 and mouse10 placenta, they could alter 5-HT availability in the fetal brain. Therefore, fetal brain exposure to SSRIs during pregnancy could have consequences on 5-HT-dependent neurogenic processes. Specifically, fetal brain exposure to SSRIs may affect thalamocortical axonal (TCA) circuit formation, a process exquisitely sensitive to disruptions of 5-HT signaling through 5-HT1B/1D receptors.11,12 In addition, the SSRI ± citalopram (CIT) was shown to directly affect the response of thalamic axons to guidance cues in vitro, suggesting potential direct alterations in TCA pathway formation in vivo.13 Any effect of SSRIs on the development of this particular pathway is critically important because thalamocortical circuits underlie social, emotional, and cognitive higher functions and disruption of their normal function can lead to life-long brain dysfunction.11,14–17
In the adult brain, the SSRI CIT increases neuronal expression of p11 (s100a10), a member of the S100 family of proteins with a well-established role in trafficking of transmembrane proteins to the cell surface.18,19 It has been shown that p11 regulates the cell surface translocation of the Gi-coupled 5-HT1B/1D receptors by forming molecular complexes.20 P11 has been implicated as a potential mediator in the pathophysiology of MDD in humans and in depression-like states in rodent models.21 This association is demonstrated by evidence of decreased p11 mRNA expression measured postmortem in brains of MDD patients who committed suicide and genetic rodent models of depression.22,23 Additional support has been generated by studies showing p11 knockout mice exhibit depression-like behaviors whereas p11 overexpressing mice display antidepressant-like responses in behavioral paradigms measuring depression-like states.22,24 Furthermore, Svenningson and Chergui showed that p11 increases the concentration of 5-HT1B receptors at the synapse, thereby increasing the overall efficiency of 5-HT signaling.22 This interaction appears to have a key role in regulating an individual’s susceptibility to depression and the response to SSRI therapy. In the adult brain, chronic SSRI therapy also increases hippocampal neurogenesis via largely unknown mechanisms.25 P11 knockout mice have demonstrated a reduced response to fluoxetine-induced cell proliferation, survival, and neurogenesis compared to wild-type controls.25 This suggests involvement of p11 in SSRI-induced adult neurogenesis. Despite the increasing understanding of the role of p11 in the adult brain, nothing is known about its role in fetal brain development or the molecular mechanisms by which treatment with SSRIs during pregnancy affects fetal neurodevelopment.
In the fetal brain, a precise level of 5-HT signaling through the 5-HT1B/1D receptors is critical for normal TCA pathway formation. If maternally administered SSRIs reach the fetal brain, they could functionally impact the p11/5-HT1B/1D molecular pathway, altering downstream 5-HT signaling and 5-HT-dependent TCA pathway formation. Our objectives were to determine (1) whether p11 is expressed in the fetal mouse brain, (2) whether maternal SSRI administration affects its expression, and (3) whether chronic maternal SSRI exposure affects neurogenesis in the fetal mouse brain.
RESULTS AND DISCUSSION
P11 Expression in the Fetal Brain
In the adult brain, p11 recently emerged as an important component of 5-HT signaling. It was shown to form molecular complexes with 5-HT1B/1D receptors and regulate their translocation to the cell surface, providing indirect modulation of signaling through these receptors.22,26 The role of p11 in fetal brain development is unknown. Since 5-HT modulates important neurogenic processes via signaling through 5-HT1B/1D receptors,11,12 we investigated p11 expression in the fetal brain in relation to 5-HT1B/1D receptors expression pattern. In situ hybridization data obtained from the Allen Developing Mouse Brain Atlas27 (see Allen Institute for Brain Science; available from http://developingmouse.brain-map.org) shows that p11 mRNA is expressed in the fetal cortex, thalamus, and hindbrain (Figure 1A). In particular, p11 mRNA is present in the developing thalamus at gestation day (GD)l8 in patterns overlapping with 5-HT1B receptor (htr1b), serotonin transporter (Slc6a4), and the TCA marker netrin-G1a (netG1a; Figure 1B–D). The presence of p11 transcripts in the fetal forebrain led us to investigate p11 protein expression pattern. Immunohistochemical staining shows that p11 protein is detected in the fetal cortex, thalamus, hippocampus (Figure 1F–H), and hypothalamus (Figure 1L) at GDl7. Most interestingly, p11 protein is detected in neuronal cell bodies located in the presumptive ventral posteriolateral thalamic nucleus (Figure 1K). Based on anatomical location, these p11+ cells correspond to neurons that give rise to the TCA pathway. As previously described, thalamic neurons and the corresponding TCAs can be clearly identified by netrin-G1 immunostaining (Figure 1E, I). Consistent with these observations, p11 immunoreactivity is detected in thalamic neurons (Figure 1K) and corresponding TCAs coursing through the internal capsule (Figure 1J). P11 protein is also detected in very specific patterns in the developing ventral hypothalamus (Figure 1L). Therefore, p11 mRNA and protein are detected in several regions where 5-HT1B/1D receptors are prenatally expressed, including the cortex, hindbrain and thalamus.28 5-HT1B/1D receptors have critical developmental roles in TCA pathway formation.11,12 These Gi-protein coupled 5-HT receptors are transiently expressed by thalamic neurons, from E12 to early postnatal stages (~P10).28,29 Prenatally, 5-HT1B/1D modulation of intracellular cAMP concentration in thalamic neurons changes the chemotactic response of their growing axons to the guidance cue netrin-1 and the direct in vivo manipulation of 5-HT1B/1D receptors gene expression in fetal thalamic neurons, by in utero electroporation, leads to abnormal TCA pathfinding.12 Thus, 5-HT signaling through 5-HT1B/1D receptors exerts a critical influence on TCA pathway formation. The colocalization of 5-HT1B/1D receptors and p11 in fetal thalamic neurons and axons suggests that p11 function might indirectly influence TCA pathway formation.
Figure 1.
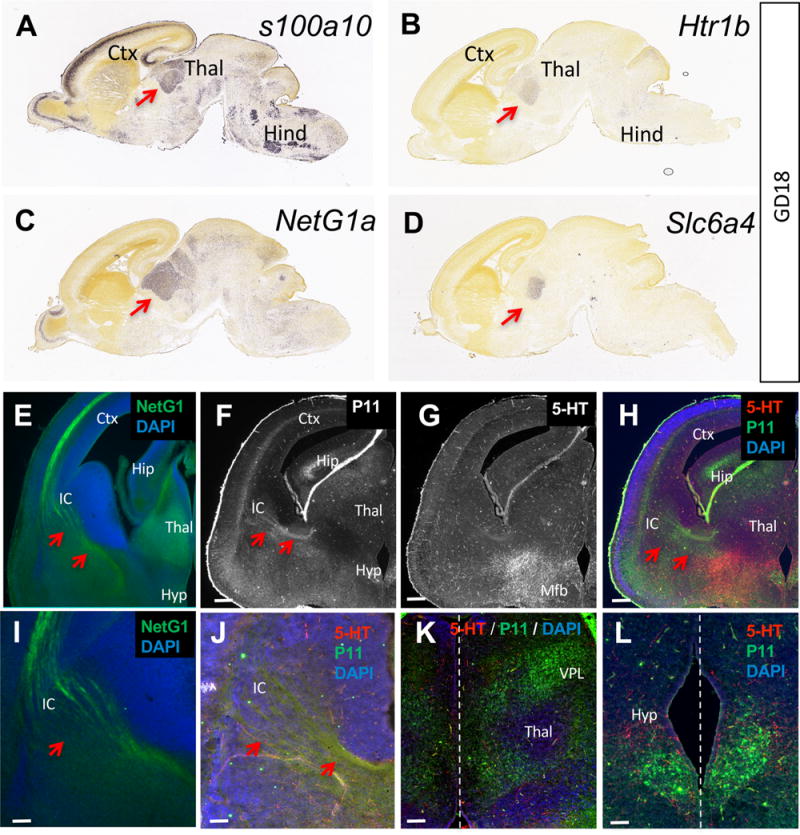
Expression of p11 in the fetal brain. (A) Allen Developing Mouse Brain Atlas in situ hybridization data depicting p11 (s100a10) mRNA expression in the fetal thalamus (Thal; red arrow), cortex (Ctx), and hindbrain (Hind) at GD18. (B, C, D) 5-HT1B (Htr1b) receptor, netrin-G1 (NetG1) and SERT (Slc6a4) mRNA are detected in overlapping patterns with p11 in the thalamus (red arrows). Image credit: Allen Institute. (E–L) Co-labeling with netrin-G1, p11, and 5-HT antibodies on coronal sections show p11+ thalamocortical axons (red arrows), some of which also faintly 5-HT+ (G), growing toward the internal capsule (IC; E, F), dorsal to strongly 5-HT+ axons running through the medial forebrain bundle (Mfb, G). (H) Overlay of p11 and 5-HT immunostainings. Red arrows point to the p11+ thalamic axons. Scale bars represent 200 μm. (I, J) Close-up view of NetG1+, p11+ and 5-HT+ thalamocortical axons (red arrows) coursing through the internal capsule. Scale bar =20 μm. (K, L) p11+ cell bodies are present in ventral posteriolateral nucleus (VPL) of the thalamus (K) and the ventral hypothalamus (Hyp, L). Dotted line represents the midline. Scale bars represent 20 μm. Ctx = cortex, Thal = thalamus, Hip = hippocampus, Mfb = medial forebrain bundle.
Effect of CIT on Fetal p11 Expression
In the adult brain, the SSRI CIT increases neuronal expression of p11,18 which affects the cell surface translocation of 5-HT1B/1D receptors.20 Our observations that p11 is expressed in the fetal brain in overlapping patterns with 5-HT1B/1D receptors raise the possibility that maternal CIT administration might affect p11 expression in the fetal brain as it does in the adult brain. In utero, CIT exposure might directly affect p11 expression if the drug reaches the fetal brain tissue. We first tested this possibility by measuring CIT tissue concentration in the brain of fetuses harvested from mothers chronically exposed to the drug. CIT was administered orally (20 mg/kg/day), through the drinking water to pregnant dams from GD8 to GD17. Our recent extensive study of the pharmacokinetics of fetal CIT exposure in pregnant mice showed that maternally administered CIT very quickly reaches the fetal blood and brain.10 Tissue concentration of CIT and its primary metabolite ± desmethylcitalopram (D-CIT) measured by HPLC reveal that this dosing of maternally administered oral CIT leads to accumulation of the drug and its metabolite in the fetal brain at GD17 (Figure 2A). To test if CIT exposure in utero affects the expression of p11 in the fetal brain, we used Western blotting to estimate p11 protein tissue concentration in forebrain regions where p11 immunoreactivity was detected (Figure 1). The frontal cortex, thalamus and hindbrain were harvested at GD17 from embryos whose mothers were exposed to regular drinking water, or water containing CIT (20 mg/kg/day) from GD8 to GD17 (Figure 2A). Western blot analyses show differential effects of in utero CIT exposure on p11 protein expression in the various fetal forebrain regions (Figure 2B). P11 protein expression in the fetal thalamus is significantly decreased by 45.1 ± 5.5% after in utero CIT exposure compared to untreated controls (95% confidence interval −58.6 to −31.6, p = 0.0002, t = 8.16, df = 6). In contrast, maternal exposure to CIT has no significant effect on p11 protein expression in the fetal cortex or hindbrain. In the fetal cortex, p11 protein expression decreased by 10.0 ± 40.3% (95% confidence interval −90.0 to 89.8, p = 0.9981, t = 0.002, df = 6), while in the hindbrain p11 protein expression increased by 45.1 ± 47.0% (95% confidence interval −59.6 to 149.8, p = 0.3600, t = 1.242, df = 6) (Figure 2B). Similarly, maternal exposure to CIT has no significant effect on 5-HT1B (95% confidence interval −3.4 to 3.8, p = 0.8840, t = 0.2166, df = 6) or 5-HT1D (95% confidence interval −16.0 to 27.6, p = 0.5385, t = 1.091, df = 6) receptor expression in the fetal thalamus (Figure 2C).
Figure 2.
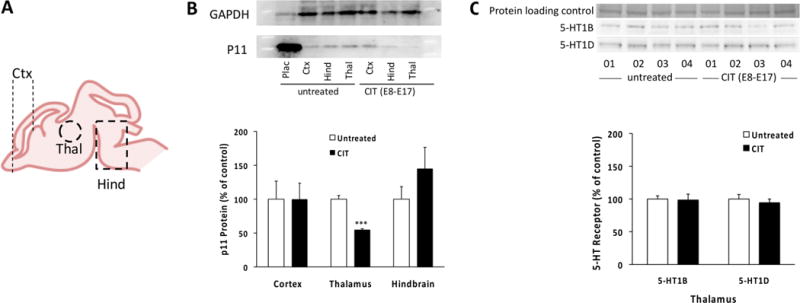
Effect of in utero exposure to CIT (20 mg/kg/day from GD8 to GD17) on p11 protein and 5-HT1B/1D receptor expression in the fetal brain. (A) Schematic representation of the fetal brain dissected out at GD17 for subsequent p11 expression analysis: Ctx = cortex (frontal half), Thal = thalamus, Hind = hindbrain region. (B) p11 protein concentration was estimated by Western blot in regions described in (A) in maternally untreated and maternally CIT-exposed fetal mouse brains. GAPDH was used as a loading control. The placenta (Plac) strongly expresses p11 and was used as a positive control. Western blot densitometric analyses show that chronic exposure of the fetus to CIT leads to a significant decrease in p11 protein concentration in the fetal thalamus specifically (***p = 0.0002 versus untreated, Student’s t test). Each column represents mean ± SEM (n = 4 dams per condition, with 2 pooled embryos per dam). (C) 5-HT1B and 5-HT1D receptor protein tissue concentration in fetal thalamus was estimated by Western blot in maternally untreated and maternally CIT-exposed. Each lane represents 2 pooled embryos obtained from an individual dam (dam number indicated below). Densitometric analyses show that chronic exposure of the fetus to CIT does not affect total 5-HT1B/1D receptor protein levels in the fetal thalamus. Each column represents mean ± SEM. For each dam, dissected pieces of tissue harvested from 2 randomly chosen embryos were pooled prior to processing–a pooled sample was subsequently considered n = 1 (n = 4 dams per condition).
The results show that, during pregnancy, maternally administered CIT reaches the fetal brain and produces specific patterns of change in p11 expression. In particular, it induces a significant decrease of p11 expression in the fetal thalamus. Importantly, we observe that 5-HT1B and 5-HT1D receptor tissue expression levels in the fetal thalamus did not change with in utero CIT exposure. The very limited amount of fetal thalamic tissue collectable per embryo from each litter did not allow us to measure the specific effect of CIT exposure on 5-HT1B/1D receptor membrane translocation in vivo. However, we speculate that if p11 endogenously regulates 5-HT1B/1D receptors plasma membrane translocation in the fetal brain as it does in the adult brain,22 decreasing p11 expression in fetal thalamic neurons is expected to alter 5-HT signaling and impact TCA circuit formation in a manner similar to the direct manipulation of 5-HT1B/1D receptors expression.12 Although the direct interaction between p11 and 5-HT1B/1D receptors in fetal thalamic neurons and axons remains to be demonstrated, the results suggest that p11 function might contribute to the development of this axonal pathway. In addition, understanding why in utero CIT exposure affects p11 expression specifically in the thalamus, but not cortex or hindbrain, needs further investigation.
Effect of CIT on Fetal Neurogenesis
P11 has also been implicated to play a role in SSRI-induced cell proliferation and adult hippocampal neurogenesis.25 To investigate the effects of chronic maternal CIT exposure on fetal neurogenesis, we performed immunohistochemistry staining for PH3 on control and treatment groups and quantified the number of proliferating cells in specific regions of the fetal brain at GD17. Brain regions with an abundance of PH3+ cells included the cortical subventricular zone (SVZ), ganglionic eminences (GE), and hippocampal neuroepithelium (Hip NE). These regions were examined in rostral, medial, and caudal brain sections at the levels depicted in Figure 3. Chronic maternal CIT exposure is associated with altered neurogenesis in different regions of the fetal mouse brain. In the SVZ, there was a significant interaction between the number of PH3+ cells and the rostro-caudal position in the brain (F(2, 15) = 7.427; p = 0.0057). In the caudal part of the cortical SVZ, the mean number of PH3+ cells was significantly decreased with in utero CIT exposure (95% confidence interval 1.4–59.7, F(1, 15) = 14.58, p = 0.0382). However, in the rostral and medial parts of the cortical SVZ, there was no significant difference in mean number of PH3+ cells between control and treatment groups (95% confidence interval −5.2 to 53.1, p = 0.1277, 95% confidence interval −12.1 to 46.1, p = 0.4107) (Figure 3A). In the fetal hippocampal neuroepithelium, there was a significant interaction between the number of PH3+ cells and the rostro-caudal position in the brain (F(1, 10) = 33.39, p = 0.0002). However, only the medial region showed a significant decrease in mean number of PH3+ cells with maternal CIT exposure (95% confidence interval 9.8 to 32.5, F(1, 10) = 27.68, p = 0.0012). While the caudal region of the hippocampal neuroepithelium did show a trend in reduced neurogenesis, this finding was not statistically significant (95% confidence interval −0.4 to 22.3, p = 0.0596) (Figure 3B). In the fetal GE, there was a significant interaction between the number of PH3+ cells and the rostro-caudal position in the brain (F(1, 10) = 116.8, p < 0.0001). A significant decrease in mean number of PH3+ cells was observed with in utero CIT exposure in both the lateral and medial GE (95% confidence interval 11.5 to 39.7, p = 0.0015, 95% confidence interval 5.6 to 33.8, p = 0.0086, F(1, 10) = 35.65) (Figure 3C).
Figure 3.
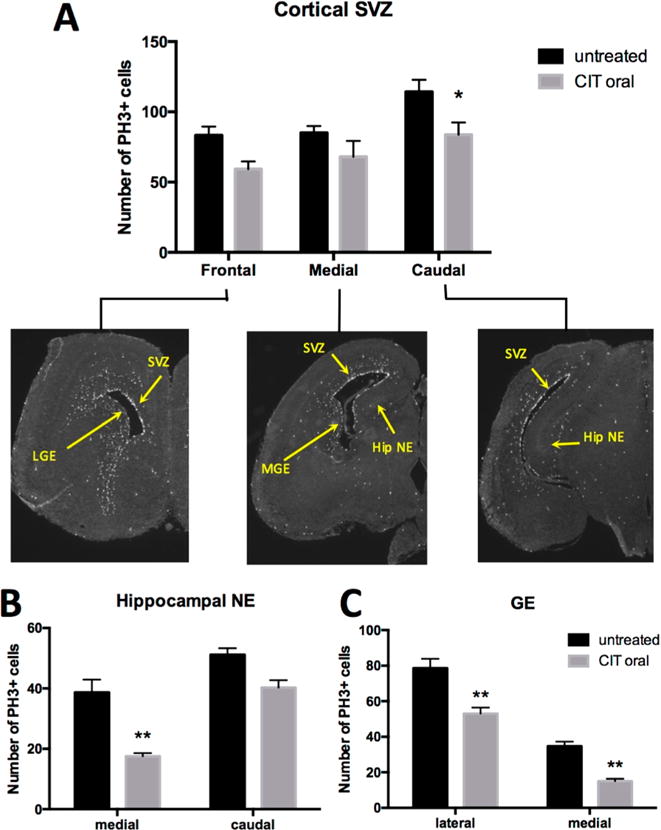
Effect of in utero exposure to CIT on fetal brain neurogenesis at GD17. (A) Immunohistochemistry shows a significant decrease in the mean number of cells staining positive for PH3 in the caudal sections of the fetal cortical SVZ after chronic exposure of timed-pregnant mice to CIT in drinking water: SVZ = cortical subventricular zone (*p = 0.0382 versus untreated). (B) The medial part of the fetal hippocampal neuroepithelium (Hip NE) showed a significant decrease in mean number of PH3+ cells in maternal CIT-exposed embryos (**p = 0.0012 vs untreated). (C) Chronic maternal CIT exposure results in a significant decrease in the mean number of PH3+ cells in the lateral and medial fetal ganglionic eminence (GE) in comparison to untreated (**p = 0.0015 and p = 0.0086, respectively). All comparisons were made by two-way ANOVA with Bonferroni correction. Each column represents mean ± SEM. Results were obtained from 1 embryo per dam with n = 4 dams for control and n = 3 dams for treatment.
The results show that specific fetal brain regions have evidence of decreased cell proliferation with in utero CIT exposure. Although the molecular mechanism linking p11 expression and function to neurogenesis is unknown, it is interesting to note that these events appear correlated both in the adult and fetal brain, albeit in opposing directions (i.e., SSRI exposure induces an increase in p11 expression and neurogenesis in the adult brain, but a decrease in both parameters in the fetal brain). Of particular interest is the decrease in PH3+ cells in the medial and caudal ganglionic eminence, a transitory brain structure that not only generates different cortical interneurons subtypes30,31 but also contributes to guiding TCAs toward the cortex. The ganglionic eminence provides an intermediate target for growing thalamic axons as they travel to the cerebral cortex and cortical axons traveling to the thalamus.32,33 Alterations in this structure may have significant long-term effects on neurodevelopment. Developmental neuropathy studies indicate the migration of ganglionic eminence neurons are critical and damage to the establishment of these connections have been implicated in cognitive deficits.32 These deficits have been demonstrated in premature infants suffering from hemorrhage or periventricular leukomalacia within the margin of the ganglionic eminence.32 The long-term consequences of altered cell proliferation triggered by SSRI treatment in the ganglionic eminence, cortical SVZ and hippocampal neuroepithelium remain to be investigated. In addition, future investigations are needed to elucidate the downstream effects of the CIT-mediated decrease of p11 protein expression on 5-HT1B/1D receptor translocation and signaling and the precise impact on TCA pathway formation.
METHODS
Animals and Chronic Maternal CIT Exposure
Experiments were conducted using timed-pregnant CD-1 mice obtained from Charles-River laboratories (San Diego, CA). Mice were housed 3–5 per cage with ad libitum access to food and water. Mice received 20 mg/kg/day ± citalopram hydrobromide (TCI America, C2370) from gestational day (GD) 8 to GD17 (treatment group). CIT was administered orally in drinking water containing 1% sucrose to mask the taste of the drug. The drug concentration in the drinking water (0.13g/l CIT) was determined from the average daily water consumption and the average body weight per mouse to achieve the target dose. The control group consisted of mice exposed to regular drinking water with 1% sucrose. CIT and control water solutions were monitored throughout the study and were replaced 5 days after the start of the treatment. Mice were randomly assigned to the treatment or control group (n = 4 dams/group). At GD17, mice were anaesthetized through inhalation of isoflurane gas (Western Medical Supply) and sacrificed by cervical dislocation. The uterus was immediately dissected and embryos from both treatment and control groups were harvested and placed on ice in 1× phosphate buffered saline (PBS). Maternal and fetal brains were dissected into frontal cortex, thalamus, and hindbrain regions (Figure 3B), weighed, snapfrozen in liquid nitrogen, and stored at −80 °C until use. Regions from 2 embryos per dam were pooled prior to storage Procedures were approved by the USC Institutional Animal Care and Use Committee.
Immunohistochemistry in the Fetal Brain
A subset of GD17 fetal brains from the control and treatment group were dissected in ice cold PBS and immediately transferred to 4% paraformaldehyde for incubation at 4 °C for 24 h. The fetal brains were then incubated at 4 °C in increasing amounts of sucrose (10%, 20%, 30% dissolved in PBS) for 24 h each. After the last incubation, the fetal brains were embedded in cryomolds on dry ice using tissue tek (VWR, 25608-930) and stored at −80 °C until sectioned. On the evening prior to sectioning, the embedded fetal brains were removed from −80 °C and allowed to warm to −20 °C. The entire rostro-caudal extent of each fetal brain was sectioned into 20 μm-thick coronal sections. Sections were stored at −80 °C until immunohistochemical analysis. Images for all sections were acquired with a Zeiss AxioCam MRm camera (Carl Zeiss) using Zeiss AxioVision 4.8.2 software (Zeiss).
P11, Netrin-G1, 5-HT, and Phospho-Histone H3
Sections from the control group were permeabilized with PBS containing 0.1% Triton X-100 (PBST) and 2% fetal bovine serum and incubated overnight at room temperature with primary antibodies to netrin-G1 (1:500 goat anti-Netrin-G1a, R&D Systems) or p11 (1:100 goat anti-S100A10, R&D Systems) and 5-HT (1:1000 rabbit anti-5HT, Sigma) or p11 and anti-phospho-histone H3 (PH3) (1:200 rabbit anti-phospho S10, Abcam). Slides were then washed in PBST before applying secondary antibodies. Incubation with secondary antibodies (1:800 anti-goat HRP and 1:800 anti-rabbit Rhodamine Red, Jackson ImmunoResearch Laboratories) occurred at room temperature for 2 h in the dark. Slides were again washed in PBST prior to amplification. For amplification, slides were incubated in Tyramide Signal Amplification-Fluorescein solution (1:50; PerkinElmer, NEL701A001 KT) for 10 min at room temperature in the dark. A final series of washes with PBST was performed and the slides were mounted using Prolong Gold with DAPI (to visualize nuclei; Life Technologies, P36931).
Measure of p11 Protein Expression in the Fetal Brain
Unless otherwise noted, all reagents were purchased from Sigma. Predissected and pooled (2 embryos per region) GD17 fetal mice brain samples were removed from the −80 °C freezer for both treatment and control groups. Total proteins were extracted by sonication in lysis buffer containing: 50 mM hepes pH7.4, 2 mM EGTA, 10 mM sodium orthovanadate, 30 mM sodium fluoride, 40 mM beta-glycerol phosphate, 1% Triton X-100, and complete mini protease inhibitor cocktail EDTA-free (1 tablet/10 mL; Roche). Protein concentrations were measured by Bradford protein assay per the manufacturer’s instruction (Biorad). All samples were separated on NuPAGE Novex 4–12% Bis-Tris protein gels (Life Technologies), transferred to polyvinylidine fluoride membrane, and blocked in 0.5% bovine serum albumin in Tris-buffered saline + 0.1% Tween 20. Western blots were incubated at 4 °C overnight in primary antibody to p11 (anti-S100A10 1:2000, R&D Systems) and GAPDH (anti-GAPDH, 1:1000, Abcam). Membranes were washed in Tris-buffered saline + 0.1% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature (rabbit anti-goat 1:1000; R&D Systems). Blots were incubated in enhanced chemiluminescent reagent (ECL; GE Healthcare Life Sciences); blots chemiluminescence was measured using Image Station 4000MM Pro (Carestream) using Carestream Molecular Imaging version 5.07.22 software. P11 protein tissue concentration was estimated by densitometric analysis. Values were normalized to GAPDH protein level measured in the same samples to correct for total protein loading differences.
Measure of 5-HT1B/D Protein Expression in the Fetal Brain
The procedures were similar as described above except that membranes were blocked in 1× iBind solution (Thermofisher). Western blots were incubated at room temperature for 2.5 h in the iBind Western System (Thermofisher) with primary antibody to 5-HT1B (anti-5HT1B 1:100, Abcam) or 5-HT1D (anti-5HT1D 1:50, Abcam) and secondary antibody (goat anti-rabbit, 1:1000, Jackson ImmunoResearch Laboratories). Membranes were washed in deionized water before incubation with enhanced chemiluminescent reagent (ECL; GE Healthcare Life Sciences); blots chemiluminescence was measured as described above for p11. 5-HT1B/D protein tissue concentration was estimated by densitometric analysis. Values were normalized to protein level detected using coomassie blue staining measured in the same samples to correct for total protein loading differences.
Statistical Analysis
P11 and 5-HT1B/D Protein Expression
Throughout the study, we used individual litter as the sampling unit in order to avoid potential litter effects. For each dam, dissected pieces of tissue harvested from two randomly chosen embryos were pooled (in order to obtain enough tissue for protein extractions/Western blotting) prior to processing–a pooled sample was subsequently considered N =1; this was repeated for each dam and the final number of samples used for statistical analysis was therefore the number of dams. Mean normalized chemiluminescence and standard error of the mean were calculated for each brain region (cortex, thalamus, hindbrain) in control and treatment groups. In all cases, the data passed the Shapiro-Wilk normality test (α = 0.05); in addition, F tests indicated that there were no significant differences in variances between the groups (p values: p > 0.999 for the cortex, p = 0.08 for thalamus, and p = 0.17 for hindbrain). For each individual region, comparisons between treatment and control means were made by unpaired Student t test. Results were considered statistically significant if the two-sided p-value was <0.05.
Anti-Phospho-Histone H3 (PH3) immunohistochemistry
Mean PH3 cell count for control and treatment groups were calculated for rostral, medial, and caudal sections of each brain region (cortical subventricular zone, ganglionic eminence, hippocampal neuroepithelium). Within each region, mean differences between treatment and control groups were made using two-way ANOVA with Bonferroni correction for multiple comparisons. Results were considered statistically significant if two-sided p-value was <0.05.
Acknowledgments
Funding
This work was supported by Grant #1R01MH106806 to A.B., and a Scott-Gentle Foundation Grant and Avery Translational Research Career Development Program Award (Children’s National Medical Center) to M.T. J.R.K. was supported by the LA+USC Maternal-fetal fellowship program.
Footnotes
ORCID
Alexandre Bonnin: 0000-0001-7549-6598
Author Contributions
J.R.K., J.V., and M.T. conducted the experiments, analyzed the results, and wrote the manuscript. A.B. designed the study, analyzed the results, and wrote the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med. 2011;365:1605–1611. doi: 10.1056/NEJMcp1102730. [DOI] [PubMed] [Google Scholar]
- 2.Bakker MK, Kölling P, van den Berg PB, de Walle HEK, de Jong-van den Berg LTW. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65:600–606. doi: 10.1111/j.1365-2125.2007.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 5.Harrington RA, Lee LC, Crum RM, Zimmerman AW, Hertz-Picciotto I. Prenatal SSRI Use and Offspring With Autism Spectrum Disorder or Developmental Delay. Pediatrics. 2014;133:e1241. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. Bmj. 2013;346:f2059–f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 8.Hendrick V, Stowe ZZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 9.Nagai M, Ohtani H, Satoh H, Matsuoka S, Hori S, Fujii T, Taketani Y, Sawada Y. Characterization of Transplacental Transfer of Paroxetine in Perfused Human Placenta: Development of a Pharmacokinetic Model to Evaluate Tapered Dosing. Drug Metab Dispos. 2013;41:2124–2132. doi: 10.1124/dmd.113.052332. [DOI] [PubMed] [Google Scholar]
- 10.Velasquez JC, Goeden N, Herod SM, Bonnin A. Maternal Pharmacokinetics and Fetal Disposition of (±)-Citalopram during Mouse Pregnancy. ACS Chem Neurosci. 2016;7:327–338. doi: 10.1021/acschemneuro.5b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Kleef ESB, Gaspar P, Bonnin A. Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. European journal of neuroscience. 2012;35:1563–1572. doi: 10.1111/j.1460-9568.2012.8096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonnin A, Torii M, Wang L, Rakic P, Levitt P. Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nat Neurosci. 2007;10:588–597. doi: 10.1038/nn1896. [DOI] [PubMed] [Google Scholar]
- 13.Bonnin A, Zhang L, Blakely RD, Levitt P. The SSRI citalopram affects fetal thalamic axon responsiveness to netrin-1 in vitro independently of SERT antagonism. Neuropsychopharmacology. 2012;37:1879–1884. doi: 10.1038/npp.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brummelte S, Mc Glanaghy E, Bonnin A, Oberlander TFF. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience. 2017;342:212. doi: 10.1016/j.neuroscience.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. New lessons from knockout mice: The role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. CNS spectrums. 2003;8:572–577. doi: 10.1017/s1092852900018848. [DOI] [PubMed] [Google Scholar]
- 16.Lin RCS, Rodriguez-Porcel F, Merzenich M, de Villers-Sidani E, Cai Z, Weaver KJ, Lu JYF, Pang Y, Simpson KL, Paul IA. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci U S A. 2011:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci U S A. 2005;102:5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 19.Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pfluegers Arch. 2008;455:575–582. doi: 10.1007/s00424-007-0313-4. [DOI] [PubMed] [Google Scholar]
- 20.Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, Greengard P. Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation. J Neurosci. 2009;29:1937–1946. doi: 10.1523/JNEUROSCI.5343-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svenningsson P, Kim Y, Warner-Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat Rev Neurosci. 2013;14:673–680. doi: 10.1038/nrn3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svenningsson P, Chergui K. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- 23.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- 24.Warner-Schmidt JL, Chen EY, Zhang X, Marshall JJ, Morozov A, Svenningsson P, Greengard P. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry. 2010;68:528–535. doi: 10.1016/j.biopsych.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Neurogenic effects of fluoxetine are attenuated in p11 (S100A10) knockout mice. Biol Psychiatry. 2010;67:1048–1056. doi: 10.1016/j.biopsych.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 26.Snyder SH. Serotonin, cytokines, p11, and depression. Proc Natl Acad Sci U S A. 2011;108:8923–8924. doi: 10.1073/pnas.1106103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson CL, Ng L, Lau C, Puelles L, Hohmann J, Jones A. The Allen Developing Mouse Brain Atlas: A resource for spatiotemporal gene expression. Int J Dev Neurosci. 2010;28:681. [Google Scholar]
- 28.Bonnin A, Peng W, Hewlett W, Levitt P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience. 2006;141:781–794. doi: 10.1016/j.neuroscience.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 29.Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N. Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci. 2002;22:886–900. doi: 10.1523/JNEUROSCI.22-03-00886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Q, Cobos I, La Cruz DE, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 32.Ulfig N. Ganglionic eminence of the human fetal brain–new vistas. Anat Rec. 2002;267:191–195. doi: 10.1002/ar.10104. [DOI] [PubMed] [Google Scholar]
- 33.López-Bendito G, Cautinat A, Sanchez JA, Bielle F, Flames N, Garratt AN, Talmage DA, Role LW, Charnay P, Marin O, Garel S. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


