Abstract
Background:
Overall prognosis of children with steroid-sensitive nephrotic syndrome (SSNS) is regarded as generally favorable. However, only a few recent studies have evaluated changes in kidney function and blood pressure over time in children with SSNS.
Objectives:
We describe clinical features of SSNS patients and characterize changes in calculated estimated glomerular filtration rate (eGFR) and use of antihypertensive medications during follow-up.
Design:
This is a retrospective cohort study.
Setting:
This study was conducted in a Canadian pediatric nephrology center.
Patients:
This study included patients aged 1 to 18 years with SSNS.
Measurements:
eGFR was calculated from recorded serum creatinine and height measurements using the modified Schwartz equation.
Methods:
eGFR was calculated at yearly intervals, and the trend of eGFR was assessed using linear mixed effects model. Patients were also evaluated for use of antihypertensive medications during follow-up.
Results:
Seventy-eight patients—median age, 3.2 years (interquartile range [IQR], 2.65) and median follow-up of 4.37 (IQR, 5.6)—were evaluated. Sixty-three (80.8%) had at least 1 relapse. Twenty-two (28.2%) and 20 (25.6%) were steroid dependent and frequently relapsing, respectively. Forty-three patients (55.1%) received at least 1 steroid-sparing agent, and of these, 18 (41.8%) received a calcineurin inhibitor. One patient had eGFR ≤90 mL/min/1.73 m2 during observation. eGFR remained unchanged over the follow-up period in this cohort of patients. Four patients (5.1%) were on antihypertensive medications at the end of follow-up.
Limitations:
Patients who had frequent relapses had more measurements available for serum creatinine and height, creating a sampling bias. The number of eGFR measurements was overall small, making it difficult to ascertain eGFR trend.
Conclusion:
eGFR remained unchanged over time in this cohort, and a small proportion of patients required antihypertensive therapy at the end of follow-up. Our study highlights the needs for carefully constructed long-term observational studies of children with nephrotic syndrome.
Keywords: steroid-sensitive nephrotic syndrome, glomerular filtration rate, hypertension, minimal change disease, calcineurin inhibitors
Abrégé
Mise en contexte:
De manière générale, on considère le pronostic du syndrome néphrotique stéroïdosensible chez l’enfant comme favorable. Toutefois, seules quelques études récentes ont mesuré les changements dans la fonction rénale et la pression sanguine au fil du temps chez les enfants atteints du syndrome néphrotique stéroïdosensible.
Objectifs de l’étude:
Décrire les manifestations cliniques du syndrome néphrotique stéroïdosensible, de même que caractériser les changements dans le débit de filtration glomérulaire estimé (DFGe) et la prise de médicaments contre l’hypertension au cours de la période de suivi.
Cadre et type d’étude:
Il s’agit d’une étude de cohorte prospective qui s’est tenue dans un centre de néphrologie pédiatrique canadien.
Patients:
Des enfants âgés de 1 à 18 ans atteints du syndrome néphrotique stéroïdosensible.
Mesures:
On a utilisé l’équation de Schwartz modifiée pour calculer le DFGe des participants à partir de leur taille et de mesures de créatinine sérique déjà enregistrées.
Méthodologie:
On a procédé au calcul du DFGe chaque année et on en a évalué la tendance à l’aide d’un modèle linéaire à effets mixtes. La prise de médicaments contre l’hypertension a également été évaluée au cours de la période du suivi.
Résultats:
Un total de 78 patients, dont l’âge médian se situait à 3,2 ans (écart interquartile = 2,65 ans), ont été évalués sur une période de 4,37 ans (EI = 5,6 ans) en moyenne, desquels 80,8% (63 patients) ont fait au moins une rechute. Des patients évalués, 28,2% (n = 22) étaient dépendants des stéroïdes et 25,6% (n = 20) faisaient des rechutes fréquentes. Quarante-trois patients (55,1%) ont reçu au moins un agent de préservation de stéroïdes, dont dix-huit (41,8%) ont reçu un inhibiteur de la calcineurine. Un seul patient a présenté une valeur de DFGe de plus de 90 ml/min/1,73 m2 au cours de la période d’observation. Le DFGe est demeuré inchangé tout au long de la période de suivi pour cette cohorte. À la fin de la période de suivi, quatre patients (5,1%) prenaient des médicaments contre l’hypertension.
Limites de l’étude:
Un biais d’échantillonnage est introduit par le fait qu’un nombre plus élevé de mesures de taille et de DFGe étaient disponibles pour les patients ayant fait plusieurs rechutes. Par ailleurs, l’établissement de tendances en regard des valeurs de DFGe s’avère difficile étant donné le nombre relativement faible de mesures disponibles.
Conclusions:
Les valeurs de DFGe sont demeurées inchangées tout au long de la période de suivi pour cette cohorte de patients, et seulement quatre d’entre eux ont dû être traités pour l’hypertension à la fin du suivi. Cette étude met en lumière le besoin pour la tenue d’études observationnelles à long terme et judicieusement élaborées chez les enfants atteints du syndrome néphrotique stéroïdosensible.
What was known before
Children with steroid-sensitive nephrotic syndrome (SSNS) have overall good prognosis regarding kidney function and blood pressure on long-term follow-up. Many of prior studies in SSNS included children with minimal change disease. Recent studies showed increasing prevalence of other histopathology diagnoses like focal segmental glomerulosclerosis. Also, there has been an increase in the use of calcineurin inhibitors over years to control relapses.
What this adds
This study evaluates the renal outcomes in a more recent cohort with changing histopathology and increasing use of calcineurin inhibitors.
Introduction
Despite frequent relapses and prolonged course of disease, the overall prognosis of childhood nephrotic syndrome is regarded as generally favorable in the majority of patients. In the literature to date, a number of studies have evaluated the long-term outcomes and kidney function in children with steroid-sensitive nephrotic syndrome (SSNS).1-11 The largest study documenting long-term outcomes was the report of the International Study of Kidney Disease in Children (ISKDC) conducted during the 1960s and 1970s. The results of this study showed that that there is a negligible risk for developing chronic kidney disease or other adverse outcomes in children who are steroid sensitive after a mean follow-up period of 9.4 years.1 In a cohort of children with minimal change disease on biopsy, Lewis et al reported in 1989 that no patients developed blood pressure greater than the 97th percentile for age or serum creatinine greater than 100 µmol/L over a period of follow-up of 10 to 20 years.2 Koskimies et al in 1982 reported in a prospective cohort of children with SSNS (77% had minimal change disease; others had mesangial proliferation or focal segmental glomerulosclerosis [FSGS]) that all patients had good kidney function after a follow-up of 5 to 14 years.3 In 1985, children with SSNS (n = 152) were followed for 14 to 32 years and showed no patients developed chronic kidney disease or hypertension.4
Many of these prior studies included children with minimal change disease on kidney biopsy; however, in recent years, it has been noted that a significant proportion of children with SSNS have histopathological findings other than minimal change disease. It is not known whether renal outcomes are different in more recent patient cohorts with changing histopathology.12 Furthermore, increasing use of calcineurin inhibitors for relapse control, which have inherent nephrotoxic properties, may be contributing to declining renal function over time in some patients.13,14 In 1985, Tejani15 reported in a group of children with minimal change disease that up to 62.5% of children who had frequent relapses but who remained steroid responsive developed chronic kidney disease over a period of 5 to 18 years. This finding has not been replicated in other studies. In a study of 11 children with steroid-dependent or frequently relapsing nephrotic syndrome who received tacrolimus, there was a significant increase in interstitial fibrosis in repeat kidney biopsy (on average 34 months after first kidney biopsy and an average duration of 19 months of exposure to tacrolimus).14 These studies cast doubt whether patients with SSNS in the recent era indeed have favorable prognosis.
Therefore, we sought to describe changes in estimated glomerular filtration rate (eGFR) and development of hypertension in a recent heterogeneous single-center cohort of patients with SSNS.
Methods
Design, Setting, and Patients
We reviewed the medical records of all children with nephrotic syndrome who were seen in the pediatric nephrology department at Alberta Children’s Hospital between January 1992 and September 2013. We included all patients who were steroid sensitive (as noted in the medical records) and between 1 and 18 years of age at first presentation. We excluded patients who developed steroid resistance at any point of time during the period of follow-up, those who had documented urinary tract abnormalities, recurrent urinary tract infection, renal impairment or hypertension prior to the onset of nephrotic syndrome, and those with unavailable medical records. Our hospital is a regional referral center for Southern Alberta, and we see all patients with childhood nephrotic syndrome in the region reducing potential referral bias in our cohort.
Patient’s paper-based charts and electronic medical record systems were used to retrieve relevant clinical information. Baseline data collected included age of the disease onset, gender, and ethnicity. We collected information regarding number of relapses with start and end dates of treatment for relapses, second-line medications, and antihypertensive medications used during follow-up. We also collected biopsy reports if available.
The patients were classified according to the clinical phenotype of nephrotic syndrome (steroid resistant, infrequently relapsing, frequently relapsing, and steroid dependent) based on the label given to each patient in the patient chart. We did not infer the status of the patient from observed relapse frequency, as it was very difficult to attribute these definitions retrospectively. It would also have caused a bias toward labeling patients as infrequently relapsing. We believe that this bias is minimized but not totally removed by using the label given for the type of nephrotic syndrome in the patient’s chart. The definitions of the types of nephrotic syndrome at our center were according to the ISKDC definitions. All patients received our standard protocol of prednisone at 60 mg/m2 daily for 6 weeks (maximum 80 mg) followed by 40 mg/m2 on alternative days for 6 weeks, 30 mg/m2 on alternative days for 14 days and then tapered by 10 mg/m2 every 8 days and then stopped. Relapses were treated with the 60 mg/m2 of prednisone until 3 consecutive days of urine protein remission and then changed to 60 mg/m2 on alternative days for another 8 days and then tapered by 10 mg/m2 every 8 days and then stopped. Prednisone treatment protocols are standardized at our center, and there is almost no variation between physicians in terms of prednisone prescribing practices.
Definitions of Outcomes
Our primary outcome was eGFR during yearly follow-up. eGFR was calculated using available data for height and serum creatinine measurements closest to or within 3 months before or after yearly intervals from the onset of disease, using the modified Schwartz formula (Constant k × height in centimeters/serum creatinine in micromoles per liter).16 Height was measured supine by clinic staff using a length board (for infants <1 year of age) and stadiometer (children >1 year). Same instruments were in use in our clinic for more than 10 years. The constant (k) for the modified Schwartz formula used in our institution is variable with age and sex being small at 33 in neonate and increases with the increase of age reaching 44 in girls more than 12 years of age and 52 in boys more than 13 years of age. Serum creatinine values are measured in our laboratory using the Roche enzymatic creatinine assay method since 2004, which is Isotope Dilution Mass Spectrometry (IDMS) traceable. No further details were available regarding the method used prior to 2004 or the total imprecision of the creatinine method at various time points.
A decreased eGFR was defined as less than 90 mL/min/1.73 m2. Our secondary outcome was hypertension defined as needing at least 1 antihypertensive medication at last observation.
Statistical Analysis
Baseline demographics of the patient cohort were described using medians with interquartile ranges (IQRs), 25th and 75th percentiles. We summarized the clinical characteristics of the cohort (relapse rate, clinical phenotype of nephrotic syndrome, second-line agents used in the course of treatment, kidney biopsy findings, and development of hypertension) using medians with IQRs, 25th and 75th percentiles. For the primary outcome eGFR, we used a linear mixed effects model adjusting for the repeated measures within patients and for the time observed to test for a trend seen in the plot of the means. This model was fitted to see whether there was a slope at all. An indicator variable indicating being followed past 4 years was also introduced, as graphical evaluation of the data seemed to indicate eGFR stabilization after 4th year of follow-up.
Results
We followed 78 patients for a median of 4.37 years (IQR, 5.6; 25th and 75th percentiles were 1.57 and 7.13, respectively), with median age at first presentation of 3.2 years [IQR, 2.65, 25th and 75th percentiles were 2.7 and 5.4, respectively). Most of the patients were males (73%). Sixty-three (80.8%) had at least 1 relapse during observation with a median relapse rate for all patients of 1.37/patient/year (IQR, 2.14; 25th and 75th percentiles were 0.37 and 2.51, respectively). Thirty-four patients (43.5%) were described as infrequently relapsing, 22 (28.2%) as steroid dependent, and 20 (25.6%) as frequently relapsing.
Forty-three patients (55.1%) received at least 1 steroid-sparing agent. Eighteen patients (23.1%) received a calcineurin inhibitor (either cyclosporine or tacrolimus). Cyclophosphamide was given to 36 (46.2%), levamisole to 14 (17.9%), and mycophenolate mofetil to 2 patients (2.6%). Ten patients (12.8%) received rituximab (Table 1). Rituximab at our center is given as a single dose if other second-line agents do not reduce the frequency of relapses or improve the steroid dependency.
Table 1.
Prevalence of the Use of Second-Line Agents (N = 78).
| Second-line agent used | No. of patients received,a n (%) |
|---|---|
| Cyclophosphamide | 36 (46.2) |
| Levamisole | 14 (17.9) |
| Cyclosporine | 13 (16.7) |
| Rituximab | 10 (12.8) |
| Pulse methylprednisolone | 5 (6.4) |
| Tacrolimus | 5 (6.4) |
| Mycophenolate mofetil | 2 (2.6) |
| Total exposed | 43 (55.1) |
Data for 3 patients were not available.
Kidney biopsy is not usually performed in SSNS patients at first presentation, as is common practice. In our cohort, 34 patients (43.6%) had kidney biopsy at some point during observation. The most common histopathological diagnosis was minimal change disease in 23 (67.6%) of those who has a kidney biopsy followed by FSGS in 3 (8.8%) patients. Seven patients had other histopathological diagnosis (eg, C1q nephropathy, IgM nephropathy, diffuse proliferative nephropathy, and mesangial proliferative nephropathy). Five of 18 patients who received calcineurin inhibitors had features of calcineurin toxicity on biopsy.
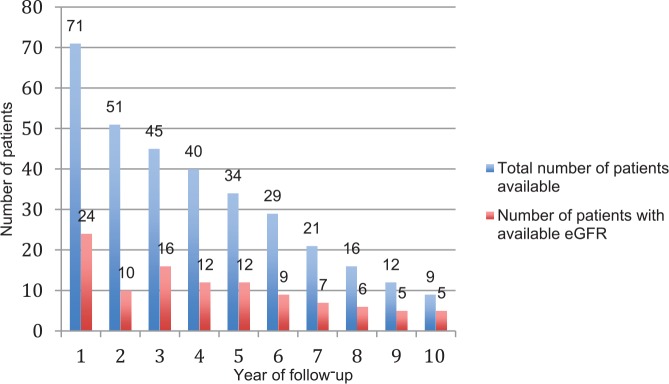
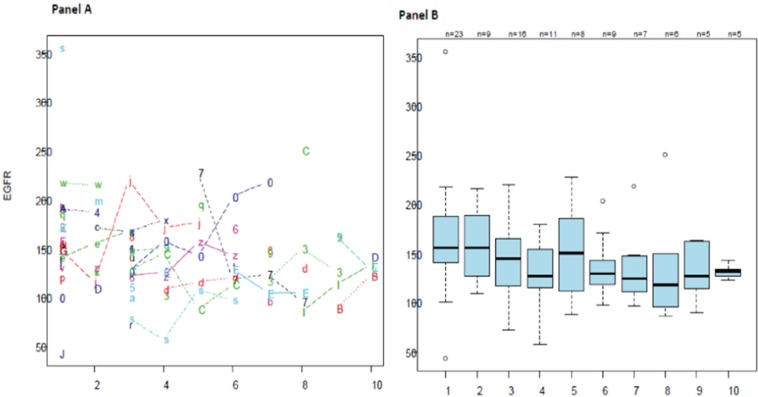
The median eGFR at first presentation was 165.1 mL/min/1.73 m2 (IQR, 54.4; 25th and 75th percentiles were 133.4 and 187.7, respectively). One patient had eGFR persistently ≤90 mL/min/1.73 m2 during observation. Only 24 patients had eGFR available at 1 year after first presentation, and this number decreased with increasing years of follow-up with only 5 patients had eGFR available at 10 years (Figure 1). The linear mixed effects model showed no significant trend in change of eGFR over time for each person (Figure 2, panel A). In Figure 2, panel B, the mean eGFR point estimates seem to indicate a potential downward slope, but this trend is not statistically significant.
Figure 1.
Number of patients and eGFR measurements available at yearly intervals.
Note. eGFR = estimated glomerular filtration rate.
Figure 2.
eGFR measurements over yearly follow-up intervals.
Note. Panel A shows changes in eGFR per person over yearly intervals. Panel B shows mean eGFR for all available measurements each year. eGFR = estimated glomerular filtration rate.
Thirty-nine patients (50%) received at least 1 antihypertensive medication during the period of observation. The most common antihypertensive medications used were calcium channel blockers in 18 patients (23.1%; Table 2). However, only 4 patients (5.1%) were on antihypertensive medications at the end of follow-up.
Table 2.
Use of Antihypertensive Medications (N = 78).
| Exposure to antihypertensive medications | n (%) |
|---|---|
| Yes | 39 (50.0) |
| No | 35 (44.9) |
| Not available in chart | 4 (5.1) |
| Frequency of medications used | n (%) |
| Amlodipine | 18 (23.1) |
| Nifedipine | 6 (7.7) |
| Enalapril | 13 (16.7) |
| Fosinopril | 3 (3.8) |
| Perindopril | 2 (2.6) |
| Hydrochlorothiazide | 6 (7.7) |
| Othersa | 4 (5.1) |
Note. aOne child each was given felodipine, losartan, ramipril, and spironolactone.
Discussion
We studied a population-based cohort of children with SSNS and found that there were very few patients who had serum creatinine measurements after 5 years of follow-up. Among those with measurements, eGFR remained relatively stable with no significant trend over time. No patients developed chronic kidney disease during follow-up, and only 1 patient had eGFR <90 mL/min/1.73 m2. A small number of patients were on antihypertensive medications at the end of follow-up. More than half of the cohort received at least 1 second-line agent to reduce relapse rate. The most common histopathological diagnosis was minimal change disease followed by FSGS.
It has been suggested that increasing use of nephrotoxic medications to control relapses of nephrotic syndrome and changing frequencies of histopathological diagnoses over time may have potential effects on kidney function long-term.17-19 Although 23% of patients were exposed to calcineurin inhibitors as second-line agents, we did not observe a significant change in kidney function during follow-up. Five patients in our cohort who received Calcineurin inhibitors (CNI) had features of CNI toxicity in their kidney biopsies (range 3-11 years after first presentation).
This finding has been shown in several previous studies that reported favorable outcome regarding kidney function in long-term follow-up of children with SSNS.1-11 As in previous studies, the most common histopathological diagnosis was minimal change disease among those patients who had a kidney biopsy. Focal Segmental Glomerulosclerosis (FSGS) and Membranoproliferative Glomerulonephritis (MPGN) were observed less frequently in our cohort, accounting for only 9% and 3%, respectively. Interestingly, histopathological entities like C1q nephropathy and IgM nephropathy were also observed in several patients. These histopathological features were not yet described when many of the previous studies were conducted and it is unknown if they carry a worse renal prognosis.20,21
The eGFR at initial presentation was unusually high with a median of 165.1 mL/min/1.73 m2. The use of serum creatinine to estimate GFR is affected by multiple factors including tubular secretion of creatinine, which accounts for 10% to 15% of the renally excreted creatinine.22,23 This will overestimate the GFR if the creatinine secreted by renal tubules is not accounted for. Overestimation is even higher in children with lower eGFR, as the tubular secretion of creatinine is higher in these patients. It has also been shown that serum albumin affects tubular excretion of creatinine in a way that lower serum albumin influences tubular secretion.24 During the initial presentation, children with nephrotic syndrome have very low serum albumin, which overestimates their GFR. This could explain the high eGFR at the initial presentation in our cohort. In subsequent eGFR measurement, many of these patients were in remission; therefore, the creatinine tubular secretion is not affected.
About half of the patients in our cohort received at least 1 antihypertensive medication at some point during their follow-up, but only very few remained on medications at the end of follow-up. The high proportion of children in this cohort who received antihypertensive medications is explained by the fact that many of these medications were used while patients were on corticosteroids for their relapse. While in relapse, some patients may require antihypertensive medications because of salt and fluid overload and if dietary measures were not sufficient to control blood pressure. Also, the use of calcineurin inhibitors may contribute to poor blood pressure control in these patients. Most patients in our cohort only received antihypertensive medications temporarily. Some patients who received angiotensin-converting enzyme inhibitors possibly received it for their antiproteinuric rather than antihypertensive effect. Long-term studies have reported a favorable outcome regarding blood pressure in children with SSNS. However, Kyrieleis et al reported that hypertension developed in 7 out of 15 of patients with Frequently relapsing nephrotic syndrome (FRNS) (biopsy-proven Mimimal Change Nephrotic Syndrome [MCNS]) after a median follow-up of 24 years (10-39).6 This implies that these patients had higher risk of developing hypertension with increasing age. Our study had a relatively short median length of follow-up and, therefore, did not have sufficient patients with longer observation periods.
Our study highlights an important issue in the routine follow-up and monitoring of patients with SSNS. A large proportion of patients did not have yearly serum creatinine measurements available, and many did not have yearly clinic visits recorded in the medical charts; the follow-ups may have stopped due to cessation of relapses. Lack of routine measurements of kidney function in nephrotic syndrome cohorts makes it difficult to assess eGFR trends over time, and only further prospective studies using a larger cohort with longer follow-up would answer this question. Lack of routine surveillance is likely the result of not knowing what is the ideal screening strategy for health maintenance in this group of patients, therefore introducing significant variation in care. Many clinicians are reassured by previous published reports with follow-up ranging from 11 months to 44 years that most steroid-sensitive patients remit their disease over time and do not experience significant morbidity. However, the long-term health of patients with SSNS is largely unknown.
Furthermore, we know that there is prevalent practice variation between physicians and pediatric nephrology centers in terms of choice of steroid protocols and steroid-sparing agents.25 It is not known whether this variation affects patient outcomes. In light of prevalent variation in care in both the choice of drugs and routine health surveillance, we believe that carefully constructed multicenter observational cohort and registry-based studies are necessary to obtain valuable generalizable data that will provide long-term observational patient outcome data. Observational cohort data with routinely collected assessments during relapse and remission will complement interpretation of published short-term clinical trial results and assist in strategic prioritization of future clinical trial questions regarding the benefit of new and old therapeutics. Such a pragmatic approach will enable clinicians to be more informed regarding benefits and harms of treatment and about the ideal health surveillance strategy for patients with nephrotic syndrome.
Our study has several limitations. First, calculating eGFR using serum creatinine measurements and anthropometrics collected in a retrospective fashion is problematic. There may be sampling bias in which patients who have more complicated course of nephrotic syndrome have regular blood work. It is also difficult to determine with certainty whether serum creatinine measurements were collected in either health or during periods of acute illness or during relapses. Many patients did not have serum creatinine checked on a regular basis as they were in remission and healthy for long periods. The sampling bias could have affected the observed eGFR trend. Second, there were considerable missing data in regard to measurement of serum creatinine and patients’ height. Also the number of patients was relatively small with an overall short follow-up. The number of eGFR measurements was also small, and it was decreasing with increasing years of follow-up. Due to the small sample size, we were unable to adjust for factors that could have potentially influenced eGFR over time. Many patients used antihypertensive medications temporarily for reasons like being on corticosteroids treatment or calcineurin inhibitors or during relapse and having salt and fluid overload. This does not indicate the development of persistent or true high blood pressure in these patients.
Our study was also significantly limited by retrospective ascertainment of calcineurin toxicity. We relied on data that were available within the charts for patients who underwent kidney biopsy for various reasons including confirmation of diagnosis prior to starting second-line agents and also to look for calcineurin toxicity after treatment for several months.
Conclusion
In conclusion, the present study shows overall stable GFR during the early follow-up period among SSNS patients. Our assessments of overall kidney function were limited due to lack of serial measurements of eGFR, and therefore, future work should involve determining the optimal frequency of monitoring for eGFR and routine health assessments in patients with SSNS using a larger cohort with longer follow-up. Potential effects of changing histopathology over recent years and the use of nephrotoxic calcineurin inhibitors for relapse control should be addressed.
Footnotes
Ethics Approval and Consent to Participate: This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary (REB13-0329).
Consent for Publication: Consent for publication was obtained from all authors.
Availability of Data and Materials: There is no data to share.
Authors’ Note: Sulaiman Alsaidi is now at Royal Hospital, Muscat, Oman.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Tarshish P, Tobin JN, Bernstein J, Edelmann CM., Jr. Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8(5):769-776. [DOI] [PubMed] [Google Scholar]
- 2. Lewis MA, Baildom EM, Davis N, Houston IB, Postlethwaite RJ. Nephrotic syndrome: from toddlers to twenties. Lancet. 1989;1(8632):255-259. [DOI] [PubMed] [Google Scholar]
- 3. Koskimies O, Vilska J, Rapola J, Hallman N. Long-term outcome of primary nephrotic syndrome. Arch Dis Child. 1982;57(7):544-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trompeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal-change nephrotic syndrome. Lancet. 1985;1(8425):368-370. [DOI] [PubMed] [Google Scholar]
- 5. Lahdenkari AT, Suvanto M, Kajantie E, Koskimies O, Kestila M, Jalanko H. Clinical features and outcome of childhood minimal change nephrotic syndrome: is genetics involved? Pediatr Nephrol. 2005;20(8):1073-1080. [DOI] [PubMed] [Google Scholar]
- 6. Kyrieleis HA, Lowik MM, Pronk I, et al. Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol. 2009;4(10):1593-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohlin AB. Clinical course and renal function in minimal change nephrotic syndrome. Acta Paediatr Scand. 1984;73(5):631-636. [DOI] [PubMed] [Google Scholar]
- 8. Ruth EM, Kemper MJ, Leumann EP, Laube GF, Neuhaus TJ. Children with steroid-sensitive nephrotic syndrome come of age: long-term outcome. J Pediatr. 2005;147(2):202-207. [DOI] [PubMed] [Google Scholar]
- 9. Al Salloum AA, Muthanna A, Bassrawi R, et al. Long-term outcome of the difficult nephrotic syndrome in children. Saudi J Kidney Dis Transpl. 2012;23(5):965-972. [DOI] [PubMed] [Google Scholar]
- 10. Hibino S, Uemura O, Nagai T, et al. Three year outcome of childhood idiopathic nephrotic syndrome under a unified immunosuppressive protocol. Pediatr Int. 2015;57(1):85-91. [DOI] [PubMed] [Google Scholar]
- 11. Ishikura K, Yoshikawa N, Nakazato H, et al. Morbidity in children with frequently relapsing nephrosis: 10-year follow-up of a randomized controlled trial. Pediatr Nephrol. 2015;30(3):459-468. [DOI] [PubMed] [Google Scholar]
- 12. Borges FF, Shiraichi L, da Silva MP, Nishimoto EI, Nogueira PC. Is focal segmental glomerulosclerosis increasing in patients with nephrotic syndrome? Pediatr Nephrol. 2007;22(9):1309-1313. [DOI] [PubMed] [Google Scholar]
- 13. Hino S, Takemura T, Okada M, et al. Follow-up study of children with nephrotic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis. 1998;31(6):932-939. [DOI] [PubMed] [Google Scholar]
- 14. Morgan C, Sis B, Pinsk M, Yiu V. Renal interstitial fibrosis in children treated with FK506 for nephrotic syndrome. Nephrol Dial Transplant. 2011;26(9):2860-2865. [DOI] [PubMed] [Google Scholar]
- 15. Tejani A. Morphological transition in minimal change nephrotic syndrome. Nephron. 1985;39(3):157-159. [DOI] [PubMed] [Google Scholar]
- 16. Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987;34:571-590. [DOI] [PubMed] [Google Scholar]
- 17. Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13(1):13-18. [DOI] [PubMed] [Google Scholar]
- 18. Bonilla-Felix M, Parra C, Dajani T, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55(5):1885-1890. [DOI] [PubMed] [Google Scholar]
- 19. Kitiyakara C, Kopp JB, Eggers P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23(2):172-182. [DOI] [PubMed] [Google Scholar]
- 20. Swartz SJ, Eldin KW, Hicks MJ, Feig DI. Minimal change disease with IgM+ immunofluorescence: a subtype of nephrotic syndrome. Pediatr Nephrol. 2009;24(6):1187-1192. [DOI] [PubMed] [Google Scholar]
- 21. Vizjak A, Ferluga D, Rozic M, et al. Pathology, clinical presentations, and outcomes of C1q nephropathy. J Am Soc Nephrol. 2008;19(11):2237-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832-1843. [DOI] [PubMed] [Google Scholar]
- 23. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Branten AJ, Vervoort G, Wetzels JF. Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant. 2005;20(4):707-711. [DOI] [PubMed] [Google Scholar]
- 25. Samuel S, Morgan CJ, Bitzan M, et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol. 2013;28(12):2289-2298. [DOI] [PubMed] [Google Scholar]