Abstract
Background
Little is known about risk attitudes and risk perceptions in multiple sclerosis (MS).
Objectives
The objectives of this paper are to investigate the range of risk attitudes and risk perceptions and examine associations between risk attitudes and risk perceptions and demographic and clinical features of the disease.
Methods
A total of 223 individuals completed a risk questionnaire. Risk attitude was measured using two rating scales and a standard gamble scenario. Risk perception was measured by asking participants to estimate the likelihood of disease progression and the likelihood of minor and serious side effects associated with common MS therapies.
Results
Participants were risk neutral overall and risk averse on issues related to health and safety. There was a significant association between disease duration and risk attitude, with patients with longer disease duration showing greater tolerance for risk. On the standard gamble scenario, males were significantly more likely to take treatments with a likelihood of death of 1:10,000 or 1:100,000 than females. Individuals with higher disability or a progressive disease course were significantly more likely to expect progression at two, five and 10 years.
Conclusion
Individuals with MS demonstrate low tolerance for risk. Risk attitudes and perceptions are influenced by some demographic and clinical features of the disease.
Keywords: Multiple sclerosis, risk attitude, risk perception, disease-modifying therapies
Introduction
Since 1993, 13 drugs have been approved by the Food and Drug Administration (FDA) for the treatment of relapsing forms of multiple sclerosis (MS). As new disease-modifying therapies (DMTs) become available, decisions regarding the selection of a particular DMT are becoming more complicated both for patients and physicians. The weighing of alternatives is particularly challenging for treatments that carry the potential for significant side effects and/or adverse events. One important factor that may influence treatment choice is an individual’s attitude toward risk.
Risk attitude is defined as an individual’s orientation toward taking or avoiding risk when deciding how to proceed in situations with uncertain outcomes.1,2 There are two competing views of risk attitude in the literature. The first view considers risk attitude a stable personality trait.1 The second sees risk attitude varying across domains such as financial, recreational and health.3 Risk-taking behavior, however, may be influenced by more than an individual’s risk attitude. It may also be a function of an individual’s risk perception, or the way that he or she perceives the likelihood of experiencing a negative event.4 In this view, treatment decision making in MS may reflect both an individual’s perception of risk and his or her attitude toward risk.
In the current study, we investigated the range of risk attitudes and risk perceptions in individuals with MS using risk attitude and risk perception rating scales and a standard gamble scenario. In addition, we examined the impact of demographic and clinical features of MS on risk attitudes and risk perceptions.
Participants and methods
Individuals were recruited from the Partners MS Center at the Brigham and Women’s Hospital in Boston, MA. All patients with a clinically isolated syndrome (CIS) or MS according to the revised McDonald criteria5 seen for a clinical visit between November 18, 2014 and December 16, 2014 were asked to complete a brief risk questionnaire (see Appendix A). The demographic and clinical characteristics of participants are shown in Table 1.
Table 1.
Demographic and clinical characteristics.
| Characteristics | |
|---|---|
| Participants | 223 |
| Females, n (%) | 173 (77.6) |
| Age, mean (SD) | 49.3 (11.7) |
| Race (White/Black or African American/Asian/more than one race) | (199/10/2/4) |
| Diagnosis (CIS/RRMS/SPMS/PPMS/PRMS) | 13/158/44/5/3 |
| Disease duration from onset, mean (SD) | 15.5 (10.3) |
| EDSS, median, range | 1.5 (0, 8.5) |
| Disease-modifying therapy, n (%) | 165 (74.0) |
Eight participants had unknown or not reported race and 24 participants did not have a recorded EDSS score.
CIS: clinically isolated syndrome; RRMS: relapsing–remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PPMS: primary progressive multiple sclerosis; PRMS: progressive relapsing multiple sclerosis; EDSS: Expanded Disability Status Scale.
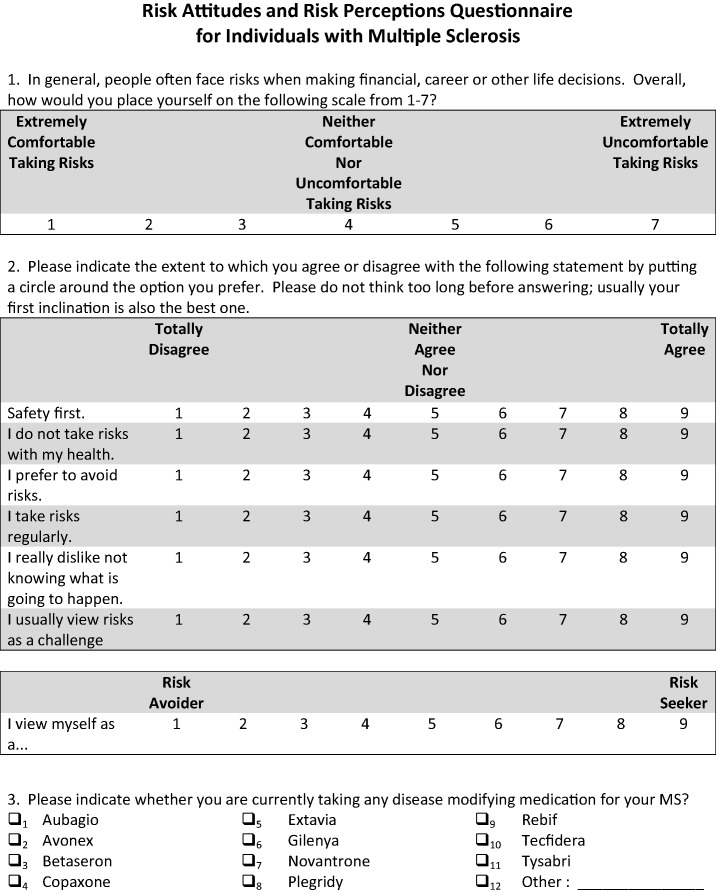
Risk attitude was measured using two rating scales and a standard gamble scenario. First, we used a single-item measure of risk orientation that asks participants to rate their overall comfort with taking risks from extremely comfortable to extremely uncomfortable.6 Second, we used the Risk Propensity Scale (RPS), a seven-item self-report measure of an individual’s general propensity to take risks.7 For the RPS, the summary score was calculated by rescaling each of the items so that higher scores were associated with preference for risk seeking. The theoretical range for the RPS is 7–63. The formula for the RPS is shown in Equation 1 where items with the suffix “rs” were rescaled.
| (1) |
Participants with missing information for any question were considered missing for the RPS. The reliability of the RPS score based on our sample was acceptable (Cronbach’s alpha = 0.721). Finally, we used a standard gamble scenario that asked individuals to consider a new MS drug that promised no new relapses or worsening of MS symptoms, but could cause death. Participants were asked to indicate their likelihood of taking the new drug if the risk of death was 1:2, 1:10, 1:100, 1:1000, 1:10,000 and 1:100,000.
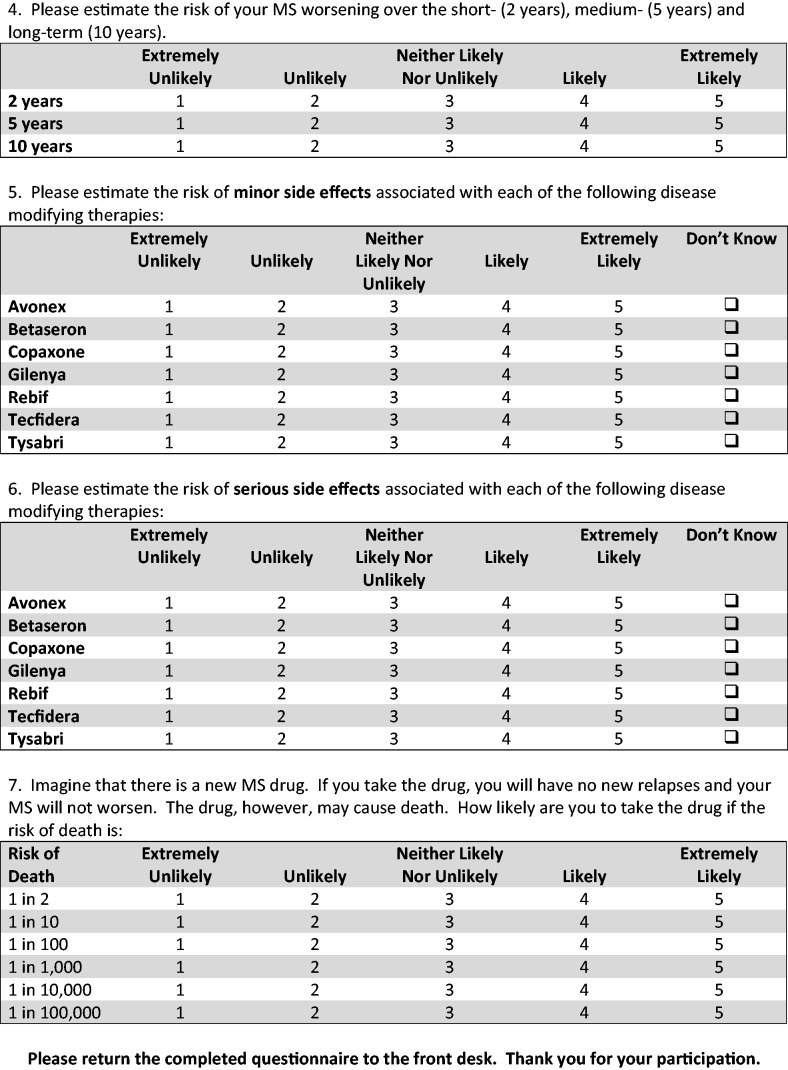
We measured risk perceptions in two ways using a five-point scale that ranged from extremely unlikely to extremely likely. First, individuals were asked to estimate the likelihood of becoming wheelchair-bound over the short- (two years), medium- (five years) and long-term (10 years). Second, participants were asked to estimate the likelihood of minor and serious side effects associated with interferon beta-1a intramuscular (IFNβ 1a IM), interferon beta-1b (IFNβ 1b), glatiramer acetate (GA), interferon beta-1a subcutaneous (IFNβ 1a SC), natalizumab (NTZ), fingolimod (FTY) and dimethyl fumarate (BG-12). These medications were chosen because they were the most commonly prescribed MS drugs at the Partners MS Center at the time that the survey was administered.
A total of 276 questionnaires were distributed and 224 were completed and returned for a response rate of 81.2%. One person completed the questionnaire twice within the interval, and only the first administration was used in our analysis for a final sample size of 223.
Demographic and clinical data including gender, age, marital status, disease duration, relapse rate and disability were obtained from the medical record. Current levels of disability were based on the physician-documented Expanded Disability Status Scale (EDSS).8 This study was approved by the Partners Human Research Committee at the Brigham and Women’s Hospital.
Statistical analysis
For each of the items related to risk attitudes, the mean and standard deviation (SD) was calculated. In addition, answers for each of the questions were classified as risk seeking, risk neutral or risk averse. For the risk-orientation item, risk seeking was a score of 1 or 2; risk neutral was a score of 3, 4 or 5; and risk averse was a score of 6 or 7. For the RPS when the items were scaled so that higher scores indicated risk seeking, risk seeking was a score of 7, 8, or 9; risk neutral was a score of 4, 5, or 6; and risk averse was a score of 1, 2 or 3. For each of the items related to risk perceptions, the proportion of individuals in each of the categories was calculated. For the risk of side-effects items, the proportions were calculated across all individuals and within participants treated with the specific DMT.
To assess the impact of demographic, clinical and treatment predictors on each risk measure, linear regression was used with continuous, dichotomous or categorical predictors as appropriate. To assess the impact of present DMT on each risk measure, treatment group was included in the model as a categorical variable, and the global test comparing all treatment groups was used to assess if there was a difference among the treatment groups. If a significant difference among the treatment groups was observed, pairwise comparisons were investigated. To compare the perceived likelihood of side effects in participants taking a specific DMT and those not taking the DMT, linear regression was used with the treatment group as the predictor variable. Since each of the scales could also be considered ordinal rather than continuous, an ordinal logistic regression model was fit, and the results were generally the same as for the linear regression. Therefore, the linear regression results are reported. Given that this was an exploratory analysis, no correction for multiple comparisons was applied.
Results
Risk attitudes
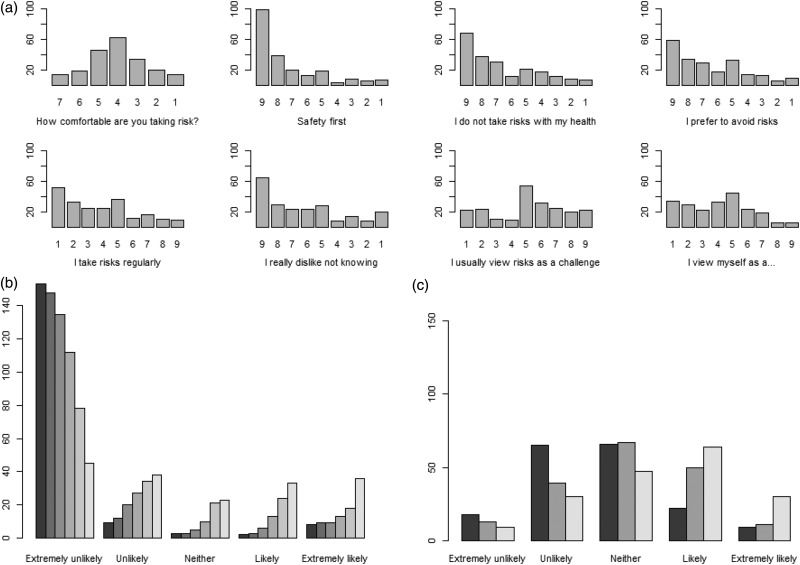
On a single-item measure of risk orientation, the majority of participants (66.8%) were risk neutral, and the mean score was almost exactly equal to the middle of the scale (Figure 1(a), Supplementary Table 1). Using the RPS, the mean (SD) risk propensity summary score was 26.3 (10.1), indicating an overall aversion to taking risks. When the individual items of the RPS were investigated, the majority of participants were classified as risk averse on the first five items of the scale (Figure 1(a), Supplementary Table 1).
Figure 1.
Descriptive graphics for risk attitude and risk perceptions.
(a) Bar graph showing the frequency of each response level for risk-attitude questions. Each graph is oriented so that higher risk seeking is shown on the right of the graph, but the actual scores are listed on the x-axis. (b) Bar graph for likelihood of taking a medication with a fatal side effect. The six bars from left to right show a probability of a fatal side effect of 1 in 2, 1 in 10, 1 in 100, 1 in 1000, 1 in 10,000, and 1 in 100,000, respectively. Only participants who answered all six questions were included in the graph (n = 175) to allow direct comparison. (c) Bar graph for the likelihood of disease worsening. The three bars from left to right show a likelihood of disease worsening in two years, five years and 10 years, respectively. Only participants who answered all three questions were included in the graph (n = 180) to allow direct comparison.
Using a standard gamble scenario, individuals reported the likelihood of taking a new MS drug given the probability of a fatal side effect (Figure 1(b), Supplementary Table 2). Six percent of participants indicated they were likely/extremely likely to take the drug if the risk of death was 1:2. The proportion increased to 16% if the risk of death was reduced to 1:1000, and to 41% if the risk of death was 1:100,000. Approximately 45% of participants indicated that they were unlikely/extremely unlikely to take the drug even if the probability of a fatal side effect was 1:100,000.
The associations between risk attitudes and demographic and clinical features of MS are presented in Table 2. There were no significant associations between risk-orientation scores or scores on the RPS and gender, age, race, marital status, number of children, EDSS, disease course or relapse rate. There was a significant association between disease duration and risk orientation, but not between disease duration and RPS. There was no significant association between current DMT and risk attitude using the global test (p = 0.052). The associations between responses to the standard gamble scenario and demographic and clinical features of MS are presented in Table 3. Males were significantly more likely to take the treatment with risk of fatal side effect equal to 1:10,000 or 1:100,000; and married individuals were significantly less likely to take a treatment with risk of fatal side effect equal to 1:10,000. No other features including current DMT were significantly associated with the likelihood of taking the treatment.
Table 2.
Associations between demographic and clinical characteristics and risk attitudes.
| Characteristic | Risk orientation | Risk propensity score |
|---|---|---|
| Male vs. female | −0.07 (−0.56, 0.43) | 0.68 (−2.63, 3.98) |
| Age | −0.02 (−0.03, 0.001) | 0.07 (−0.05, 0.19) |
| White race vs. other | −0.31 (−1.13, 0.50) | −0.91 (−6.19, 4.37) |
| Marital status | 0.07 (−0.39, 0.53) | −0.25 (−3.34, 2.84) |
| Number of children | 0.04 (−0.14, 0.22) | −0.65 (−1.85, 0.56) |
| EDSS | −0.03 (−0.13, 0.07) | −0.04 (−0.69, 0.61) |
| Disease duration | −0.03 (−0.05, −0.01)a | 0.11 (−0.03, 0.25) |
| Disease course (Progressive vs. relapsing) | 0.17 (−0.33, 0.67) | −0.30 (−3.65, 3.06) |
| Relapse rate in previous year | 0.02 (−0.53, 0.58) | 1.21 (−2.47, 4.89) |
| Current treatment | ||
| Untreated | Reference | Reference |
| IFN | 0.39 (−0.39, 1.17) | −5.54 (−10.84, −0.25) |
| GA | 0.26 (−0.38, 0.90) | −6.54 (−10.80, −2.28) |
| NTZ | −0.15 (−0.96, 0.66) | −1.44 (−6.73, 3.86) |
| FTY | −0.30 (−1.05, 0.46) | −1.17 (−6.20, 3.85) |
| BG-12 | 0.20 (−0.44, 0.84) | −1.66 (−5.85, 2.54) |
| TFL | −0.28 (−1.50, 0.95) | 3.08 (−4.84, 11.01) |
| Other | −0.44 (−1.53, 0.66) | −0.77 (−7.30, 5.78) |
Values reported are the estimated linear regression coefficient and associated 95% confidence interval from a model with the characteristic as the predictor and risk orientation or risk propensity score as the outcome. These values represent the change in the mean of the outcome for a one unit increase in the predictor. Participants with missing data on a specific covariate were not included in the analysis of that covariate (race: 8; marital status: 7; number of children: 16; EDSS: 24).
Association between predictor and covariate had a p value of less than 0.05. Since the global test comparing all treatments failed to be statistically significant (p = 0.68 for risk orientation, p = 0.052 for risk propensity score), no pairwise treatment comparison was considered statistically significant.
EDSS: Expanded Disability Status Scale; IFN: interferon; GA: glatiramer acetate; NTZ: natalizumab; FTY: fingolimod; BG-12: dimethyl fumarate; TFL: Teriflunomide.
Table 3.
Associations between demographic and clinical characteristics and risk attitudes.
| Characteristic | Likelihood of taking treatment with 1:100 | Likelihood of taking treatment with 1:10,000 | Likelihood of taking treatment with 1:100,000 |
|---|---|---|---|
| Male vs. female | 0.30 (−0.08, 0.68) | 0.58 (0.08, 1.07)* | 0.60 (0.08, 1.12)* |
| Age | 0.01 (−0.01, 0.02) | 0.01 (−0.01, 0.03) | −0.001 (−0.02, 0.02) |
| White race vs. other | 0.01 (−0.69, 0.71) | −0.21 (−1.12, 0.71) | −0.14 (−1.12, 0.83) |
| Marital status | −0.20 (−0.55, 0.15) | −0.63 (−1.08, −0.18)a | −0.33 (−0.81, 0.15) |
| Number of children | −0.02 (−0.16, 0.12) | −0.13 (−0.31, 0.06) | 0.02 (−0.18, 0.21) |
| EDSS | 0.02 (−0.05, 0.09) | 0.06 (−0.04, 0.16) | 0.05 (−0.06, 0.16) |
| Disease duration | −0.001 (−0.02, 0.02) | −0.001 (−0.02, 0.02) | −0.01 (−0.03, 0.01) |
| Disease course (progressive vs. relapsing) | 0.18 (−0.22, 0.58) | 0.40 (−0.12, 0.92) | 0.34 (−0.21, 0.89) |
| Relapse rate in previous year | −0.19 (−0.62, 0.25) | 0 (−0.58, 0.58) | 0.23 (−0.39, 0.84) |
| Current treatment | |||
| Untreated | Reference | Reference | Reference |
| IFN | −0.21 (−0.81, 0.39) | −0.18 (−0.95, 0.59) | −0.39 (−1.20, 0.43) |
| GA | −0.13 (−0.64, 0.39) | −0.01 (−0.68, 0.66) | −0.36 (−1.06, 0.35) |
| NTZ | −0.34 (−0.97, 0.30) | 0.76 (−0.03, 1.55) | 0.74 (−0.04, 1.53) |
| FTY | 0.09 (−0.48, 0.67) | 0.50 (−0.27, 1.28) | 0.48 (−0.30, 1.27) |
| BG-12 | 0.13 (−0.36, 0.63) | 0.19 (−0.46, 0.84) | 0.45 (−0.22, 1.12) |
| TFL | 0.14 (−0.80, 1.08) | 0.93 (−0.29, 2.14) | 0.74 (−0.54, 2.02) |
| Other | 0.02 (−0.71, 0.75) | 0.43 (−0.52, 1.37) | 0.56 (−0.43, 1.56) |
Values reported are the estimated linear regression coefficient and associated 95% confidence interval from a model with the characteristic as the predictor and likelihood of taking the treatment as the outcome. These values represent the change in the mean of the outcome for a one unit increase in the predictor. Participants with missing data on a specific covariate were not included in the analysis of that covariate (race: 8; marital status: 7; number of children: 16; EDSS: 24).
Association between predictor and covariate had a p value of less than 0.05.
EDSS: Expanded Disability Status Scale; IFN: interferon; GA: glatiramer acetate; NTZ: natalizumab; FTY: fingolimod; BG-12: dimethyl fumarate; TFL: Teriflunomide.
Risk perceptions
In Figure 1(c) and Supplementary Table 3, we show the distribution of responses regarding the perceived likelihood of disease progression. The percentages of patients reporting that they were likely or extremely likely to progress over the short-, medium- and long-term were 18.8%, 34.8% and 52.2%, respectively. Twenty-two percent of participants believed that it was unlikely/extremely unlikely that their MS would worsen over the next 10 years.
The associations between risk perceptions and demographic and clinical features of MS are presented in Table 4. Participants with higher EDSS or a progressive disease course had significantly increased scores for risk of progression items regardless of the time interval. In addition, a significant difference among the treatment groups was observed for perceived risk of progression over two years, and patients treated with GA, NTZ or FTY had lower perceived risk of progression over two years compared to untreated individuals. Finally, participants with higher relapse rate or lower disease duration had higher perceived risk at 10 years.
Table 4.
Associations between demographic and clinical characteristics and risk perceptions.
| Characteristic | Likelihood of progression in two years | Likelihood of progression in five years | Likelihood of progression in 10 years |
|---|---|---|---|
| Male vs. female | 0.10 (−0.24, 0.45) | 0.06 (−0.30, 0.41) | 0.06 (−0.33, 0.45) |
| Age | 0.01 (−0.01, 0.02) | 0.001 (−0.01, 0.01) | −0.01 (−0.03, 0) |
| White race vs. othera | 0.37 (−0.23, 0.97) | 0.05 (−0.55, 0.65) | −0.31 (−1.02, 0.40) |
| Marital status | 0.07 (−0.25, 0.39) | −0.05 (−0.38, 0.27) | −0.10 (−0.46, 0.25) |
| Number of children | 0.07 (−0.06, 0.19) | 0 (−0.13, 0.13) | −0.04 (−0.18, 0.11) |
| EDSS | 0.14 (0.08, 0.21)a | 0.12 (0.06, 0.19)a | 0.13 (0.06, 0.21)a |
| Disease duration | −0.002 (−0.02, 0.01) | −0.01 (−0.02, 0.01) | −0.02 (−0.03, −0.002)a |
| Disease course (progressive vs. relapsing) | 0.71 (0.36, 1.05)a | 0.51 (0.15, 0.87)a | 0.44 (0.04, 0.83)a |
| Relapse rate in previous year | 0.29 (−0.12, 0.69) | 0.32 (−0.09, 0.73) | 0.53 (0.09, 0.98)a |
| Current treatment | |||
| Untreated | Reference | Reference | Reference |
| IFN | −0.41 (−0.93, 0.12) | −0.06 (−0.61, 0.49) | 0.31 (−0.29, 0.91) |
| GA | −0.57 (−1.03, −0.11)a | −0.37 (−0.85, 0.12) | −0.11 (−0.64, 0.41) |
| NTZ | −0.55 (−1.07, −0.02)a | 0.09 (−0.46, 0.64) | 0.61 (0.01, 1.21) |
| FTY | −0.80 (−1.32, −0.28)a | −0.38 (−0.92, 0.15) | −0.01 (−0.59, 0.58) |
| BG-12 | −0.08 (−0.52, 0.37) | 0.18 (−0.28, 0.64) | 0.24 (−0.26, 0.74) |
| TFL | −0.02 (−0.88, 0.83) | −0.11 (−1.05, 0.84) | −0.04 (−1.07, 0.98) |
| Other | −0.39 (−1.05, 0.28) | 0.26 (−0.43, 0.94) | 0.76 (0.02, 1.50) |
Values reported are the estimated linear regression coefficient and associated 95% confidence interval from a model with the characteristic as the predictor and likelihood of progression as the outcome. These values represent the change in the mean of the outcome for a one unit increase in the predictor. Participants with missing data on a specific covariate were not included in the analysis of that covariate (race: 8; marital status: 7; number of children: 16; EDSS: 24).
Association between predictor and covariate had a p value of less than 0.05. Since global test comparing all treatments was statistically significant for progression at two years (p = 0.036) and failed to be statistically significant for progression at five years (p = 0.26) or at 10 years (p = 0.17), pairwise treatment comparisons are starred only for progression at two years.
EDSS: Expanded Disability Status Scale; IFN: interferon; GA: glatiramer acetate; NTZ: natalizumab; FTY: fingolimod; BG-12: dimethyl fumarate; TFL: Teriflunomide.
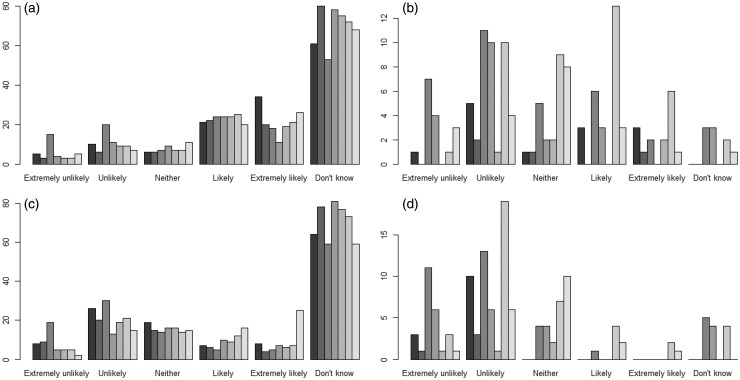
The perceived likelihood of minor and serious side effects associated with DMTs is presented in Figure 2 and Supplementary Tables 4 and 5. The most common response when all participants were surveyed was “Don’t know.” More than one-third of patients indicated that minor side effects were likely or extremely likely with IFNβ 1a IM (39.9%), BG-12 (34.4%), NTZ (33.3%) and IFNβ 1a SC (33.1%). Estimates of the likelihood of minor side effects were lower for GA (32.1%), IFNβ 1b (30.8%) and FTY (25.5%). The proportions of individuals who reported that serious side effects associated with injectable DMT use were likely/extremely likely ranged from 7.2% (IFNβ 1b) to 13.6% (IFNβ 1a IM). For the oral DMTs, the proportions of participants who indicated that serious side effects were likely or extremely likely were 18.1% for BG-12 and 11.9% for FTY. More than 30% of patients indicated that serious side effects were likely/extremely likely with NTZ.
Figure 2.
Likelihood of minor side effects (top row) and serious side effects (bottom row). For all panels, the bars from left to right show the likelihood of side effects on interferon-beta 1a intramuscular, interferon-beta 1b, glatiramer acetate, fingolimod, interferon-beta 1a subcutaneous, dimethyl fumarate, and natalizumab, respectively. Panel A shows the likelihood of minor side effects including all participants. Panel B shows the likelihood of minor side effects including only patients receiving the treatment of interest. Panel C shows the likelihood of serious side effects including all individuals. Panel D shows the likelihood of serious side effects including only participants receiving the treatment of interest.
When only patients who were taking a DMT were assessed in terms of the likelihood of side effects, a much lower proportion of individuals answered “Don’t know” (Figure 2 and Supplementary Tables 4 and 5). Further, the perceived likelihood of minor or serious side effects for participants taking the DMT was significantly lower than for individuals not taking the DMT for patients treated with IFNβ 1a IM, GA, FTY, BG-12 and NTZ (p < 0.05 for each comparison).
Discussion
We examined risk attitudes and risk perceptions in individuals with MS. On a single-item measure of risk attitude, MS patients appeared to be risk neutral. Using a more detailed RPS that included both general and health-related risk items, MS patients appeared to be risk neutral overall, and risk averse on issues related to health and safety. In fact, close to 65% of participants reported that they were risk averse when it comes to taking risks with their health. The reluctance of MS patients to take health-related risks was confirmed using a standard gamble scenario. Almost 50% of individuals indicated that they were unlikely/extremely unlikely to take a drug that promised no new relapses or worsening of MS symptoms but could cause death even when the probability of a fatal side effect was 1:100,000.
A small number of studies have similarly identified risk aversion in individuals with MS. Fox et al.9 presented MS patients with two standard-gamble paradigms. In the first, the outcomes were a cure for MS vs an immediate painless, death. In the second, the outcomes were the benefits of NTZ (68% reduction in clinical relapse rate, 42% slowing of disability and 90% reduction in new brain lesions) vs progressive multifocal leukoencephalopathy (PML). The median risk tolerance was 1:10,000 for both scenarios. Fifteen percent to 23% of respondents were highly risk averse and would accept no risk. The authors concluded that many individuals with MS are risk averse and unwilling to take high risks for greater benefits. In a study comparing decision making in MS patients and healthy control individuals using two decision-making tasks that involved risk, Simioni et al.10 found that individuals with MS showed greater risk aversion than healthy controls. They concluded that reduced tolerance for risk could affect treatment decision making in MS.
Several studies, by contrast, have demonstrated that MS patients are willing to accept risk for significant gain. Johnson et al.11 asked MS patients to choose hypothetical treatments from pairs of treatment alternatives with varying levels of efficacy and risk. In return for a decrease in relapse rate (four relapses to one over five years) and a delay of progression (progression in three years to five years), participants were willing to accept a 0.38% annual risk of death from PML. The authors concluded that individuals with MS are willing to accept higher levels of risk for greater improvements in clinical efficacy. Wilson et al.12 administered computer-based utility measures to 291 MS patients and used conjoint analysis to calculate the relative patient preferences for the current risk and benefit attributes of hypothetical DMTs. They found that MS patients were willing to accept a 0.08% risk of death or severe side effects for a year of delayed relapse, 0.22% risk of severe side effects for two years of delayed progression (from two to four years), and a 0.069% risk of severe side effects for eight years of delayed progression (from two to 10 years). Wilson et al.12 concluded that patients are willing to accept a relatively large risk of death and/or disability as long as the benefit is substantial.
We found a significant association between disease duration and risk attitude, with patients with longer disease duration showing greater tolerance for risk. Similar findings have previously been reported in MS. Caon et al.13 administered a series of standardized questions regarding the risks and benefits of new therapies to individuals treated with IFNβ or GA. Participants were divided into two groups, those with disease duration and treatment of more than five years or less than five years. Patients with more than five years of disease who were using injectable therapies were more likely to consider new treatments with greater risks as well as new oral therapies with significant risk and vigilance requirements. Fox et al.9 reported that longer MS disease duration was associated with greater risk tolerance among patients in the North American Research Committee on Multiple Sclerosis Registry.
We did not observe any significant associations between risk orientation scores or scores on the RPS and gender, age, race, marital status, number of children, disease course, EDSS or relapse rate. This is in contrast to an earlier report demonstrating that patients with greater disability were willing to take greater risks for treatments that would cure their MS or slow progression of the disease.9 The lack of association between risk attitude and disability in the current study may be due in part to the fact that participants were not very disabled overall. The median EDSS was 1.5, despite patients having a mean disease duration of 15.5 years. Using the standard gamble scenario, we found that female gender and being married were associated with lower tolerance for risk. In general, women may be less willing to accept treatment-related risks that would interfere with their ability to meet the responsibilities of home and/or work. Fox et al.9 found that female gender and having to care for dependents at home were both associated with reduced tolerance for risk.
In addition to examining risk attitudes, we assessed risk perceptions. Almost 20% of individuals reported that the risk of their MS worsening over the next two years was likely/extremely likely. Patients with greater disability or a progressive disease course were more likely to expect progression. Individuals with a higher relapse rate over the last year were more likely to expect disease progression at 10 years, but not at two and five years. These findings are in line with previous studies assessing disease expectations in MS. Janssens et al.14 asked MS patients to assess the perceived risk of becoming wheelchair dependent using 100 mm visual analogue scales. Participants tended to overestimate the two-year and 10-year risk of wheelchair dependence, but underestimated the lifetime risk. Patients with greater functional limitations had higher perceptions of risk. In a follow-up study,15 11% of participants did not discriminate between two-year, 10-year and lifetime risk. Heesen et al.16 examined risk perceptions in NTZ-treated patients by assessing individuals’ disease and therapeutic expectations. Participants were asked to estimate how many untreated patients and NTZ-treated patients would be wheelchair bound in 10 years and to estimate the number of patients who would be progression free through two years of treatment. Participants estimated that the 10-year risk of being wheelchair bound was 40% without therapy and 10% with NTZ. They also estimated that 50% of patients would have no progression after two years of NTZ therapy, suggesting that individuals treated with NTZ may be overestimating the benefits of treatment.
We surveyed individuals about the likelihood of minor and serious side effects occurring with the use of commonly prescribed DMTs. The most common response was “Don’t know.” Individuals were unwilling to estimate the risk of minor and serious side effects associated with DMTs they have not been prescribed. These results may indicate that MS patients do not have sufficient knowledge of DMT side effects to be able to make judgments about risk. When we compared responses in individuals taking the specific DMT with responses from the larger group, we found a much lower proportion of patients answered “Don’t know.” In addition, the perceived likelihood of minor or serious side effects among participants taking the specific DMT was significantly lower than the perceived likelihood of minor or serious side effects among individuals who were not taking the DMT. It may be that patients who choose a particular DMT choose it because they are comfortable with the side effect profile or it may be that once they start taking the DMT they become more comfortable with the risk.
There are several limitations associated with this study. First, patients were recruited from a tertiary referral center. Although we approached all patients seen at the MS Center during a five-week period, we cannot be sure that our study participants effectively represent the general population of MS patients. Second, our study was relatively short in duration, which resulted in a low sample size. The small sample size meant that we had limited power to detect associations between patient characteristics and risk outcomes in this study. Further studies with larger sample size will be required to better understand predictors of risk attitudes and perception in MS. Third, individuals who participated in this study had limited disability despite having had MS for an average of 15.5 years. It is important to note that this limited disability is unlikely to be due to sampling because all patients seen at the MS Center were eligible to participate, and the burden of study participation was small. It is possible that the 18.8% of patients who did not participate were more disabled than the 81.2% who completed the questionnaire. Fourth, given the wide range of available MS therapies and the small number of participants in each treatment group, we had limited power to detect differences in risk attitudes and risk perceptions across treatment groups. Fifth, we asked patients to respond to a hypothetical treatment scenario. Their answers to the standard gamble scenario might differ from actual decisions made by individuals making treatment decisions in the real world.
In summary, we found that individuals with MS are risk averse and that risk attitudes and risk perceptions are associated with some demographic and clinical features of the disease. Future studies with larger numbers of individuals should address whether risk attitudes and risk perceptions influence treatment decision making in MS.
Acknowledgments
The authors would like to thank the administrative staff at the Partners MS Center for their help with data collection.
Appendix A
Declaration of conflicting interests
Bonnie Glanz has received research support from Merck Serono and Verily Life Sciences. Emily Greeke has no disclosures to report. Allison LaRussa has no disclosures to report. Fiona Stuart has no disclosures to report. David Rintell was employed at the Partners MS Center, Brigham and Women’s Hospital, Harvard Medical School while the majority of this work was being performed. He continues to hold an appointment at Harvard Medical School and is currently a full-time employee at Sanofi Genzyme. Tanuja Chitnis has received research support from Biogen Idec, Novartis, Serono and Verily Life Sciences and fees for consulting from Biogen Idec, Novartis and Genentech-Roche. Brian Healy has received research support from Merck Serono, Verily Life Sciences, Genzyme and Novartis. He serves on the Worldwide Medical Biostatistics Multiple Sclerosis Advisory Board for Biogen Idec.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Rohrmann B. Risk attitude scales: Concepts, questionnaires, utilizations, Melbourne: University of Melbourne, Australia, 2005. [Google Scholar]
- 2.Rosen AB, Tsai JS, Downs SM. Variations in risk attitude across race, gender, and education. Med Decis Making 2003; 23: 511–517. [DOI] [PubMed] [Google Scholar]
- 3.Prosser LA, Wittenberg E. Do risk attitudes differ across domains and respondent types? Med Decis Making 2007; 27: 281–287. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JD, Young JM, Butow P, et al. Is it worth the risk? A systematic review of instruments that measure risk propensity for use in the health setting. Soc Sci Med 2005; 60: 1385–1396. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maestas CD and Pollock WM. Measuring generalized risk orientation with a single survey item, http://dx.doi.org/10.2139/ssrn.1599867 (3 May 2010, accessed 15 October 2014).
- 7.Meertens RM, Lion R. Measuring an individual’s tendency to take risks: The Risk Propensity Scale. J Appl Soc Psychol 2008; 38: 1506–1520. [Google Scholar]
- 8.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 9.Fox RJ, Salter A, Alster JM, et al. Risk tolerance to MS therapies: Survey results from the NARCOMS registry. Mult Scler Relat Disord 2015; 4: 241–249. [DOI] [PubMed] [Google Scholar]
- 10.Simioni S, Schluep M, Bault N, et al. Multiple sclerosis decreases explicit counterfactual processing and risk taking in decision making. PLOS One 2012; 7: e50718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson FR, Van Houtven G, Ozdemir S, et al. Multiple sclerosis patients’ benefit-risk preferences: Serious adverse event risks versus treatment efficacy. J Neurol 2009; 256: 554–562. [DOI] [PubMed] [Google Scholar]
- 12.Wilson L, Loucks A, Bui C, et al. Patient centered decision making: Use of conjoint analysis to determine risk-benefit trade-offs for preference sensitive treatment choices. J Neurol Sci 2014; 344: 80–87. [DOI] [PubMed] [Google Scholar]
- 13.Caon C, Memon A, Perumal J, et al. Patient response to new disease-modifying therapies: Results of a questionnaire study in RRMS patients receiving self-injected disease-modifying therapies. In: American Academy of Neurology (AAN) 63rd Annual Meeting, 9–16 April 2011, Honolulu, Hawaii. Abstract P01.208.
- 14.Janssens AC, de Boer JB, van Doorn PA, et al. Expectations of wheelchair-dependency in recently diagnosed patients with multiple sclerosis and their partners. Eur J Neurol 2003; 10: 287–293. [DOI] [PubMed] [Google Scholar]
- 15.Boeije HR, Janssens AC. ‘It might happen or it might not’: How patients with multiple sclerosis explain their perception of prognostic risk. Soc Sci Med 2004; 59: 861–868. [DOI] [PubMed] [Google Scholar]
- 16.Heesen C, Kleiter I, Nguyen F, et al. Risk perception in natalizumab-treated multiple sclerosis patients and their neurologists. Mult Scler 2010; 16: 1507–1512. [DOI] [PubMed] [Google Scholar]