Abstract
Background
Adrenal tumors are commonly discovered on abdominal imaging. The majority of adrenal tumors are classified as “non-functional” and considered to pose no health risk, whereas a minority will be considered “functional” because they secrete hormones that increase risk for metabolic and cardiovascular diseases.
Objective
To evaluate the hypothesis that “non-functional” adrenal tumors (NFAT) increase risk for developing cardiometabolic outcomes when compared with having no adrenal tumor.
Design
Cohort study.
Setting
Integrated hospital system.
Participants
Exposed participants with benign NFAT (n=242) and unexposed participants with no adrenal tumor (n=1237).
Measurements
Medical records were reviewed from the time of abdominal imaging for development of incident outcomes (hypertension, composite diabetes [pre-diabetes or type 2 diabetes], hyperlipidemia, cardiovascular events, chronic kidney disease) (mean 7.7 years). Primary analyses evaluated independent associations between exposure status and incident outcomes using adjusted generalized linear models. Secondary analyses evaluated relationships between NFAT and cortisol physiology.
Results
NFAT were associated with significantly higher risk for incident composite diabetes when compared with no adrenal tumor (adjusted RR=1.87, 95% CI: 1.17, 2.98; absolute risk: 30/110 vs. 72/615, 15.6%). No significant associations between NFAT and other outcomes were observed. Higher “normal” post-dexamethasone cortisol levels (<1.8 mcg/dL) associated with larger NFAT size and a higher prevalence of type 2 diabetes.
Limitations
Potential bias in the selection of participants and ascertainment of outcomes.
Conclusions
Participants with NFAT had a significantly higher risk of developing diabetes when compared to participants without adrenal tumors. These results prompt a re-assessment of whether the classification of benign adrenal tumors as “non-functional” adequately reflects the continuum of hormone secretion and metabolic risk they may harbor.
INTRODUCTION
The use of cross-sectional abdominal imaging has increased the incidental detection of adrenal tumors (1, 2). Imaging and autopsy series estimate that the prevalence of adrenal tumors is 1–10% (3–5), with higher occurrences with older age (4, 5). Although nearly all of these adrenal findings will represent benign adrenocortical tumors that are “non-functional” in that they do not apparently secrete hormones (1, 2, 6), a substantial proportion may be “functional” in that they do secrete adrenocortical hormone(s). It is estimated that approximately 10% of adrenocortical tumors may secrete excess cortisol without the classical signs or symptoms of Cushing syndrome, known as subclinical hypercortisolism (1, 6–9). Subclinical hypercortisolism has been associated with hypertension, insulin resistance, type 2 diabetes, hyperlipidemia, osteoporosis, and obesity(10, 11), and recent studies suggest that subclinical hypercortisolism may increase the risk of developing incident cardiovascular events and mortality when compared to “non-functional” adrenal tumors (NFAT)(12–14). Therefore, it is recommended that all patients with adrenal tumors be screened for hypercortisolism (1, 2, 15, 16).
However, emerging evidence suggests that even apparent NFAT have a higher cross-sectional association with cardiometabolic derangements, such as insulin resistance, when compared with matched controls without adrenal tumors (17–19), possibly because NFAT secrete low-levels of glucocorticoids (17, 20). Although the size of these aforementioned studies was small and lacked longitudinal follow-up, collectively they suggest that NFAT, defined by our current clinical practice guidelines (1, 2, 15, 16, 21), may not be “non-functional” after all; they may impart cardiometabolic risk by secreting inappropriate amounts of adrenal hormones that evade our traditional clinical criteria and detection capabilities.
A better understanding of whether NFAT represent an independent risk factor for cardiometabolic disease is of public health importance. We hypothesized that NFAT are associated with an increased risk for developing incident diabetes and cardiovascular outcomes, when compared with similar patients without adrenal tumors.
METHODS
Study Population
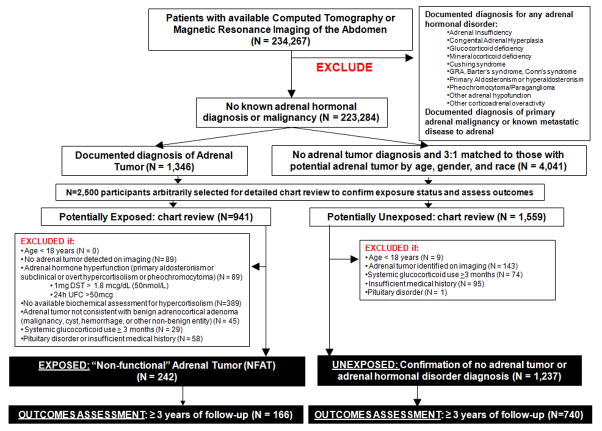
We retrospectively assembled a cohort to examine the prospective association between NFAT and cardiometabolic outcomes. The medical records of patients at Brigham and Women’s Hospital and Massachusetts General Hospital and their affiliated partner hospitals who had undergone either abdominal computed tomography or abdominal magnetic resonance imaging were evaluated (Figure 1). Detailed methodology for the selection of the study participants is presented in the Supplementary Appendix. After excluding participants who had a documented diagnosis of any adrenal hormone disorder or malignancy, we identified participants who potentially had NFAT (the exposure of interest) (n=941), and age-, sex-, and race-matched participants who potentially did not have an adrenal tumor (n=1,559) (Figure 1). All study procedures where approved by our Partners Inc. institutional research and ethics review board.
Figure 1. Selection of the study participants and identification of exposure status.
(NFAT vs. no adrenal tumors).
GRA=glucocorticoid-remediable aldosteronism; DST=dexamethasone suppression test; UFC=urinary free cortisol; NFAT= “non-functional” adrenal tumor.
Assessment of the Main Exposure: Non-functional Adrenal Tumor
Individual medical record review was used to confirm exposure status (Figure 1). Detailed methodology for confirming exposure status is presented in the Supplementary Appendix. We excluded participants with an adrenal tumor that did not appear benign on imaging or who lacked biochemical evaluation to assess for subclinical hypercortisolism. From the remaining participants with adrenal tumors who had assessments for hypercortisolism, we excluded those with evidence for potential subclinical or overt hypercortisolism defined as: a serum cortisol of >1.8 μg/dl (50 nmol/liter) following a 1 mg dexamethasone suppression testing (DST) (21) or a urinary free cortisol (UFC) ≥50 mcg/24h (21). We also excluded participants with potential subclinical or overt primary aldosteronism (22) (Supplementary Appendix). Following these exclusions, there were 242 participants with NFAT, of which subclinical hypercortisolism was excluded by DST in 164/242, by 24h UFC in 104/242, and by both DST and UFC in 28/242. Similarly, we used medical record review to confirm 1,237 unexposed participants who had no adrenal tumors on imaging and no known adrenal hormone dysfunction diagnoses (Figure 1).
Assessment of Other Relevant Exposures
Medical record review also collected details on pertinent demographic information (age, sex, race, and body mass index [BMI]), prevalent medical diagnoses (hypertension, hyperlipidemia, pre-diabetes, type 2 diabetes, heart failure, chronic kidney disease, myocardial infarction, ischemic stroke, atrial fibrillation, interventional coronary procedure [coronary catheterization or coronary artery bypass graft surgery]), smoking history, and use of relevant medication classes (anti-hypertensive, anti-diabetes [oral hypoglycemics or insulin], and for coronary artery disease and/or hyperlipidemia [aspirin, nitrates, statins, fibrates, niacin]).
Assessment of Main Outcome Measures: Incident Diabetes and Cardiovascular Disease
The main clinical outcomes of interest were: hypertension, composite diabetes, hyperlipidemia, cardiovascular events, and chronic kidney disease. These outcomes were assessed at baseline (the time of abdominal imaging to evaluate exposure status) and again in follow-up. Follow-up assessment of outcomes was conducted only after ≥ 3 years of longitudinal follow-up to ensure sufficient exposure time and opportunity for the reliable detection and documentation of clinical diagnoses by healthcare providers (n=166/242 with NFAT, n=740/1237 without any adrenal tumor) (Figure 1). Participants with < 3 years of available follow-up were not included in our dataset or analysis for incident outcomes. Prospective clinical outcomes were assessed at the most current annual or complete clinical evaluation. Examples of annual or complete evaluations include: comprehensive primary care or general internal medicine visit, cardiology or nephrology or endocrinology consultation, and pre-operative anesthesia consultation. Hypertension was defined by documented diagnosis. Pre-diabetes was defined by documented diagnosis and/or having a hemoglobin A1c value between 5.7–6.5% on ≥ 2 occasions among patients who were not on any hypoglycemic agent other than metformin. Type 2 diabetes was defined by documented diagnosis and/or having hemoglobin A1c values ≥ 6.5% on ≥ 2 occasions. Since pre-diabetes and type 2 diabetes are on a pathophysiologic continuum of insulin resistance, we defined “composite diabetes” as either pre-diabetes or type 2 diabetes. Hyperlipidemia was defined by documented diagnosis and/or low-density lipoprotein ≥ 150 mg/dL. Cardiovascular events were a composite of any documented diagnosis of history of myocardial infarction, ischemic stroke, heart failure, atrial fibrillation, or a coronary intervention procedure. Chronic kidney disease was defined by documented diagnosis.
Statistical analyses
We described demographic and clinical characteristics using the number of counts and proportions for categorical variables and mean with standard deviations (SD) for continuous variables. Categorical and continuous variables were compared using Fisher’s exact test or Chi-squared test, and Student’s t-test, respectively.
We fitted multivariable generalized linear regression models with binomial family and logarithmic link function to assess cross-sectional associations between NFAT and the prevalence of outcomes at baseline. Model 1 was adjusted for age, BMI, gender, race, and smoking status. Model 2 was adjusted for variables in model 1 as well as other clinically relevant cardiovascular and metabolic diagnoses (adjustments listed in table footnotes). For the above referred models we derived and reported unadjusted and multivariable adjusted prevalence ratios (PR) and 95% confidence intervals (CI).
Primary analyses evaluated the independent relation between exposure status and the development of each incident outcome using adjusted generalized linear models with Poisson family and link log to derive risk ratios (RR) (incidence) and rate ratios (cumulative incidence)(23). Risk and rate ratios were derived for individuals who had ≥ 3 years of follow-up and did not have the outcome in question at baseline. We used three multivariable models. Models 1 and 2 were as described above. Model 3 included all variables in models 1 and 2 as well as adjustments for the use of individual medication classes. In secondary analyses, we evaluated the association between NFAT size and cortisol levels using linear regression.
A two-tailed P value of ≤0.05 was deemed as statistically significant. All statistical analyses were performed using SAS v9.3 (Cary, North Carolina, USA).
Role of the Funding Source
The study investigators were funded by the National Institutes of Health and the Doris Duke Charitable Foundation. Neither funding source played a role in the study’s design, conduct, or reporting.
RESULTS
Study Population and Baseline Metabolic and Cardiovascular Outcomes
Baseline characteristics of the study population are described in Table 1. Participants with NFAT were slightly younger, had higher BMI and higher prevalence of pre-diabetes and diabetes when compared to those without adrenal tumors. In adjusted regression models, NFAT were independently associated with a higher prevalence of baseline composite diabetes (Appendix Table 1). This association between NFAT and baseline composite diabetes prevalence was present whether subclinical hypercortisolism was excluded using DST (adjusted PR=1.62 [1.16, 2.27]; n=164 with NFAT, n=1,237 without adrenal tumors) or using 24h UFC (adjusted PR=1.81 [1.13, 3.91]; n=104 with NFAT, n=1,237 without adrenal tumors).
Table 1.
Demographic and clinical characteristics of study participants at baseline by exposure status.
| Non-Functional Adrenal Tumor | No Adrenal Tumor | P | |
|---|---|---|---|
| N | 242 | 1237 | |
| Age (y) | 56.8 (11.4) | 59.6 (14.6) | 0.005 |
| BMI (kg/m2) | 30.9 (7.3) | 28.1 (6.8) | <0.001 |
| Female (%) | 77.7 | 70.7 | 0.03 |
| Race | 0.002 | ||
| White (%) | 60.7 | 62.3 | |
| Black (%) | 15.7 | 8.9 | |
| Hispanic (%) | 8.3 | 6.7 | |
| Other (%) | 15.3 | 22.1 | |
| Smoking | |||
| Non-smoker (%)+ | 68.6 | 72.4 | 0.24 |
| Current smoker (%) | 31.4 | 27.6 | |
| Hypertension (%) | 54.6 | 50.4 | 0.26 |
| Pre-Diabetes* (%) | 7.9 | 3.1 | <0.001 |
| Type 2 Diabetes**(%) | 20.7 | 14.2 | 0.014 |
| Composite diabetes † (%) | 28.5 | 17.3 | <0.001 |
| Hyperlipidemia (%) | 46.3 | 39.9 | 0.07 |
| Coronary Artery Disease (%) | 11.2 | 10.2 | 0.64 |
| Stroke (%) | 2.1 | 3.8 | 0.25 |
| Atrial Fibrillation (%) | 3.7 | 6.4 | 0.136 |
| Chronic Kidney Disease (%) | 6.2 | 5.7 | 0.76 |
| History of Cardiovascular Event (%)‡ | 16.9 | 18.1 | 0.71 |
| Medication Classes Used For: | |||
| Hypertension (%) | 48.8 | 48.3 | 0.94 |
| Diabetes# (%) | 15.3 | 10.3 | 0.03 |
| Hyperlipidemia or Coronary heart disease## (%) | 35.6 | 27.9 | 0.013 |
Data are mean (SD) or number of patients (%) where applicable. P values were calculated with t tests (continuous variables) or Chi χ2 or Fisher’s exact test (categorical variables).
Presumed never smokers or former smokers who quit > 6 months ago
Pre-Diabetes: Physician diagnosis and/or two or more separate HbA1c values between 5.7–6.5% without treatment with oral hypoglycemic agents (other than metformin) or injectable diabetes medications.
Diabetes: Physician diagnosis of “type 2 diabetes mellitus” or two or more separate HbA1c values ≥ 6.5%.
Composite diabetes: Combination of Pre-diabetes and Diabetes.
History of myocardial infarction, ischemic stroke, heart failure, atrial fibrillation, or interventional coronary procedure (such as coronary catheterization or coronary artery bypass graft surgery).
oral hypoglycemics or insulin
aspirin, nitrates, statins, fibrates, niacin.
“Non-Functional” Adrenal Tumors and Incident Outcomes
The mean duration of follow-up was 7.2 years (SD=3.5, range 3.0–23.1) for participants with NFAT and 7.8 years (SD=3.6, range 3.0–22.1) for participants without adrenal tumors. Incident composite diabetes was detected in 27.3% of participants with NFAT and 11.7% of participants without adrenal tumors (Figure 2A). Participants with NFAT had a significantly higher risk for developing composite diabetes when compared to participants without adrenal tumors (adjusted RR=1.87 [1.17, 2.98]; absolute risk 15.6% [6.9, 24.3]) (Table 2). There were no other significant associations between NFAT and other incident outcomes; however, wide confidence intervals preclude firm conclusions (Table 2). Importantly, among eligible participants in these analyses for incident composite diabetes, there were no major differences in demographic profiles, comorbidities, medication use, duration of follow-up or assessment of pertinent laboratories (Table 3).
Figure 2. Cases of incident composite diabetes during longitudinal follow-up.
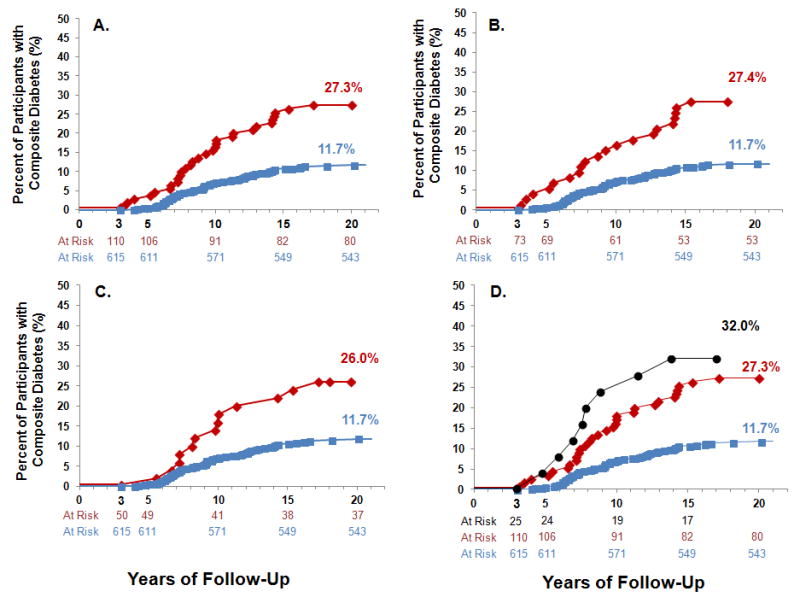
Shown are the proportion of participants who developed incident composite diabetes during follow-up. Eligible participants did not have any type of diabetes at baseline (the time of imaging), and had ≥3 years of follow-up. Panel (A) shows all 725 eligible participants (110 with NFAT and 625 without adrenal tumors), where participants with NFAT had subclinical hypercortisolism excluded using either a 1mg DST or a 24h UFC. Panel (B) shows only those participants with NFAT who had subclinical hypercortisolism excluded using a 1mg DST≤1.8 mcg/dL (73 eligible), and panel (C) shows only those participants with NFAT who had subclinical hypercortisolism excluded using a 24h UFC<50 mcg (50 eligible). Panel (D) depicts an exploratory analysis, whereby participants who were initially excluded from the main analysis due to potential subclinical hypercortisolism were included, and compared with all the eligible participants without adrenal tumors and with NFAT to assess incident composite diabetes.
Blue squares and lines indicate participants with no adrenal tumors.
Red diamonds and lines indicate participants with NFAT.
Black circles and lines indicate participants with adrenal tumors and subclinical hypercortisolism.
NFAT= “non-functional” adrenal tumor
Table 2. Non-functional adrenal tumors and the risk of developing incident outcomes.
The table shows the results of adjusted regression models to evaluate the risk for developing incident outcomes in participants with NFAT when compared to participants without any adrenal tumors.
| Incident Outcomes | Hypertension | Hyper-lipidemia | Composite Diabetes | Pre-Diabetes | Type 2 Diabetes | Chronic Kidney Disease | Cardio-Vascular Events |
|---|---|---|---|---|---|---|---|
| Number of Incident Events† Among Eligible Participants with NFAT+ | 19/74 (25.7%) | 15/87 (17.2%) | 30/110 (27.3%) | 23/110 (20.9%) | 14/126 (11.1%) | 15/152 (9.9%) | 13/138 (9.4%) |
| Person-years at risk | 519 | 617 | 807 | 807 | 902 | 1,104 | 1,019 |
|
| |||||||
| Number of Incident Events† Among Eligible Participants with no Adrenal Tumor | 125/400 (31.3%) | 66/461 (14.3%) | 72/615 (11.7%) | 36/615 (5.8) | 44/646 (6.8%) | 62/715 (8.7%) | 55/629 (8.7%) |
| Person-years at risk | 3,318 | 3,687 | 4,946 | 4,946 | 5,161 | 5,651 | 5,031 |
|
| |||||||
| Unadjusted Risk Ratios [95%CI] | 0.82 [0.51, 1.33] | 1.20 [0.69, 2.11] | 2.33 [1.52, 3.57] | 3.57 [2.12, 6.03] | 1.63 [0.89, 2.98] | 1.14 [0.65, 2.00] | 1.08 [0.59, 1.97] |
| Unadjusted Rate Ratios [95%CI] | 0.97 [0.56, 1.58] | 1.35 [0.72, 2.40] | 2.55 [1.61, 3.96] | 3.91 [2.21, 6.79] | 1.82 [0.92, 3.38] | 1.23 [0.65, 2.20] | 1.16 [0.59, 2.16] |
|
| |||||||
| Multivariable Model 1 Risk Ratios [95%CI]* | 0.83 [0.51, 1.35] | 1.06 [0.58, 1.92] | 2.09 [1.33, 3.27] | 3.34 [1.93, 5.80] | 1.54 [0.81, 2.92] | 1.10 [0.59, 2.05] | 0.94 [0.49, 1.78] |
| Multivariable Model 1 Rate Ratios [95%CI]* | 0.95 [0.58, 1.55] | 1.17 [0.64, 2.13] | 2.26 [1.44, 3.54] | 3.64 [2.10, 6.31] | 1.71 [0.90, 3.25] | 1.19 [0.64, 2.24] | 0.97 [0.50, 1.85] |
|
| |||||||
| Multivariable Model 2 Risk Ratios [95%CI]** | 0.76 [0.46, 1.26] | 1.03 [0.55, 1.91] | 2.03 [1.29, 3.19] | 3.26 [1.87, 5.69] | 1.50 [0.79, 2.87] | 0.99 [0.52, 1.86] | 0.78 [0.41, 1.50] |
| Multivariable Model 2 Rate Ratios [95%CI]** | 0.85 [0.51, 1.41] | 1.10 [0.59, 2.05] | 2.17 [1.37, 3.42] | 3.59 [2.05, .6.28] | 1.63 [0.85, 3.14] | 1.15 [0.60, 2.16] | 0.76 [0.39, 1.49] |
|
| |||||||
| Multivariable Model 3 Risk Ratios [95%CI]*** | 0.93 [0.55, 1.57] | 0.95 [0.51, 1.78] | 1.87 [1.17, 2.98] | 3.19 [1.83, 5.59] | 0.99 [0.49, 1.98] | 1.00 [0.53, 1.89] | 0.72 [0.37, 1.39] |
| Multivariable Model 3 Rate Ratios [95%CI]*** | 1.03 [0.61, 1.72] | 1.04 [0.55, 1.95] | 1.98 [1.23, 3.17] | 3.56 [2.02, 6.25] | 1.10 [0.54, 2.24] | 1.12 [0.59, 2.14] | 0.69 [0.34, 1.37] |
Incident event is defined as not having the specific outcome at baseline assessment and developing the outcome during the follow-up time.
Eligible participants include those who did not have the outcome of interest at baseline and who had ≥ 3 years of follow-up data to assess the incident development of this outcome over time.
Incident events of composite diabetes do not add up to the sum of incident type 2 diabetes events and pre-diabetes events because 15 individuals who developed incident type 2 diabetes had a diagnosis of pre-diabetes at baseline.
Multivariable Model 1 includes adjustment for: age, BMI, gender, race, smoking status
Multivariable Model 2 includes adjustment for: Model 1 + other clinically relevant baseline cardiovascular and metabolic diagnoses (hypertension, composite diabetes, hyperlipidemia, cardiovascular events, chronic kidney disease).
Multivariable Model 3 includes adjustment for: Model 2 + use of anti-hypertensive medications, use of anti-diabetes medications (oral hypoglycemics and insulin), and use of medications to treat coronary artery disease or hyperlipidemia (anti-platelets, nitrates, statins, fibrates, niacin).
NFAT= “non-functional” adrenal tumor
Table 3.
Demographic and clinical characteristics of the eligible participants for analyses on incident composite diabetes. This group of eligible participants had no baseline pre-diabetes or type 2 diabetes and ≥3 years of follow-up.
| Non-Functional Adrenal Tumor | No Adrenal Tumor | P | |
|---|---|---|---|
| N | 110 | 615 | |
| Age (y) | 56.1 (12.1) | 56.3 (14.5) | 0.92 |
| Female (%) | 84.6 | 80.5 | 0.37 |
| Race | |||
| White (%) | 65.5 | 67.3 | 0.97 |
| Black (%) | 9.1 | 9.3 | |
| Hispanic (%) | 7.3 | 6.2 | |
| Other (%) | 18.2 | 17.3 | |
| BMI (kg/m2) | 29.5 (6.9) | 27.7 (6.4) | 0.012 |
| Years of Follow-up (y) | 7.3 (3.3) | 8.0 (3.7) | 0.06 |
| Smoking | |||
| Non-smoker (%)+ | 66.4 | 75.0 | 0.08 |
| Current smoker (%) | 33.6 | 25.0 | |
| Hypertension (%) | 45.5 | 38.9 | 0.21 |
| Hyperlipidemia (%) | 40.0 | 31.7 | 0.099 |
| Coronary Artery Disease (%) | 8.2 | 5.5 | 0.27 |
| Stroke (%) | 0.9 | 3.4 | 0.23 |
| Atrial Fibrillation (%) | 1.8 | 4.2 | 0.29 |
| Chronic Kidney Disease (%) | 7.3 | 2.4 | 0.015 |
| History of Cardiovascular Event (%)‡ | 10.9 | 11.5 | 1.00 |
| Medication Classes Used For: | |||
| Hypertension (%) | 40.5 | 37.4 | 0.60 |
| Hyperlipidemia or coronary heart disease## (%) | 22.7 | 17.6 | 0.23 |
| Assessments within 1 year of final follow-up | |||
| Basic metabolic panel (%)^ | 100.0 | 100.0 | 1.00 |
| Lipid profile (%)^^ | 75.5 | 67.6 | 0.27 |
| Hemoglobin A1c (%)^^^ | 43.8 | 35.7 | 0.170 |
| Blood pressure (%)^^^^ | 100.0 | 100.0 | 1.00 |
Data are mean (SD) or number of patients (%) where applicable. P values were calculated with t tests (continuous variables) or Chi χ2 and Fischer’s exact tests (categorical variables).
Presumed never smokers or former smokers who quit > 6 months ago
Pre-Diabetes: Physician diagnosis and/or two or more separate hemoglobin A1c values between 5.7–6.5% without treatment with oral hypoglycemic agents (other than metformin) or injectable diabetes medications.
Type 2 Diabetes: Physician diagnosis of “type 2 diabetes mellitus” or two or more separate hemoglobin A1c values ≥ 6.5%.
Composite Diabetes: Combination of Pre-diabetes and Diabetes.
History of myocardial infarction, ischemic stroke, heart failure, atrial fibrillation, or interventional coronary procedure (such as coronary catheterization or coronary artery bypass graft surgery).
aspirin, nitrates, statins, fibrates, niacin.
Laboratory assessment of a basic metabolic panel (which includes electrolytes, creatinine, and glucose) within one year of the final assessment of outcomes.
Laboratory assessment of a lipid profile (which includes total cholesterol, triglycerides, and low and high density lipoprotein cholesterol) within one year of the final assessment of outcomes among participants not on lipid lowering medications.
Laboratory assessment of hemoglobin A1c within one year of the final assessment of outcomes among participants not on medications for diabetes.
Measurement of in-office blood pressure within one year of the final assessment of outcomes.
Sensitivity Analyses for Incident Composite Diabetes
The relationship between NFAT and incident composite diabetes remained stable regardless of whether subclinical hypercortisolism was excluded using the 1mg DST (unadjusted RR=2.34 [1.43, 3.84]; adjusted RR=1.78 [1.03, 3.08]) or the 24h UFC (unadjusted RR=2.22 [1.23, 4.01]; adjusted RR=2.10 [1.13, 3.91]) (Figure 2B,C). Since 24h UFC can be less sensitive at excluding subclinical hypercortisolism when compared to DST (21), we also used a stricter restriction criteria of 24h UFC <35 mcg/24h and observed no material difference in the result (adjusted RR=2.40 [1.26, 4.58]).
Higher adiposity may be a confounder of the association between NFAT and incident composite diabetes; however, higher adiposity may also represent a consequence of NFAT that secrete low-grade glucocorticoids and therefore may be in the causal pathway between NFAT and incident diabetes. Considering the potential for confounding by higher adiposity, we repeated our analysis for incident composite diabetes after adjusting for the change in BMI over time since weight gain may be an important risk factor for developing diabetes. We observed a stable adjusted RR of 2.05 (1.29, 3.27). Further, we repeated our analysis for incident composite diabetes after restricting the eligible participants to only those with BMI<30 kg/m2 such that the mean BMI for participants with NFAT was 25.0 (3.4) and the mean BMI for participants without adrenal tumors was 24.5 (3.1). There were no other notable differences in demographic or comorbid factors between exposure groups after restricting BMI to <30 kg/m2, and the adjusted RR for incident composite diabetes remained significant at 2.44 (1.17, 5.08).
Exploratory Analyses Including Subclinical Hypercortisolism
We explored whether there was a continuum of risk for incident composite diabetes when including participants with subclinical hypercortisolism. Of the 89 participants with adrenal tumors that we had excluded for adrenal hormone excess, 35 were excluded for subclinical hypercortisolism, and of these only 25 were eligible for analyses of incident composite diabetes (≥3 years of follow-up and no diabetes at baseline). Subclinical hypercortisolism was defined by DST in 21/25 with a mean post-dexamethasone cortisol of 2.8 (0.8) mcg/dL and by 24h UFC in 4/25 with a mean 24h UFC of 72.8 (19.3) mcg. During a mean follow-up of 7.9 (3.2) years, 32.0% of participants with subclinical hypercortisolism developed incident composite diabetes (Figure 2D).
Size of NFAT, Cortisol Levels, and Outcomes
In secondary analyses, larger NFAT size was associated with higher “normal” serum cortisol levels following dexamethasone (β=0.02, P<0.0001) and higher “normal” 24h urinary free cortisol levels (β=0.48, P<0.0001) (Figure 3A & 3B). Both relationships remained significant following adjustments for age, gender, race, BMI, smoking status, and prevalent hypertension, diabetes, hyperlipidemia, cardiovascular events, and chronic kidney disease. The prevalence of type 2 diabetes was higher with higher post-dexamethasone cortisol values (Figure 3C).
Figure 3.
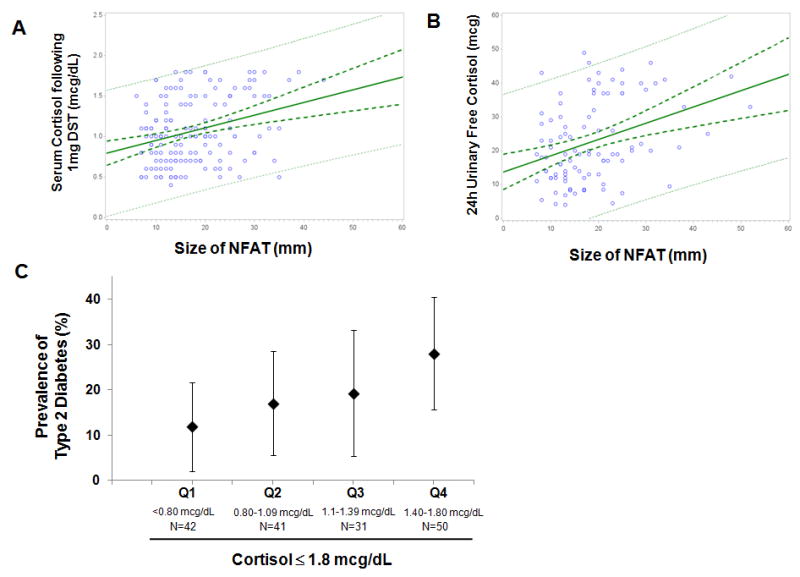
The relationship between the size of NFAT and (A) the degree of serum cortisol suppression following a 1 mg DST where all values are ≤1.8 mcg/dL, and (B) the 24 hour urinary free cortisol where all values are <50 mcg/24h. Shown are the mean regression line (bold solid green), the 95% C.I. for the mean regression (dashed bold green), and the 95% C.I. for the observed values (dashed green). (C) The prevalence of type 2 diabetes in participants with NFAT by quartile of “normal” serum cortisol levels following 1 mg DST, where all cortisol levels are ≤ 1.8 mcg/dL. Shown are the prevalence (%) and the 95% C.I..
NFAT= “non-functional” adrenal tumor
DST=dexamethasone suppression test
There was no significant association between the size of NFAT, or the degree of cortisol suppression following dexamethasone, with the incidence of composite diabetes or other outcomes; however, the number of incident cases in these analyses was small, thus limiting a robust analysis.
DISCUSSION
Adrenal tumors are discovered on 1–10% of abdominal imaging studies (1–5). There were >200 million computed tomography and magnetic resonance imaging studies worldwide in 2013, suggesting that the magnitude of the global prevalence of incidentally discovered adrenal tumors could be very high (24). Despite this high prevalence, adrenal tumors are often not given dedicated recognition or are lost to clinical follow-up (25), either because many health-care providers do not understand the importance of screening for adrenal hormone excess, and/or because they were considered unimportant at a time when another non-adrenal issue was the focus. Biochemical assessment of adrenal tumors is relevant because they may be “functional” in that they autonomously secrete hormones that cause overt or subclinical hormone excess and increase the risk for cardiovascular and/or metabolic outcomes. However, most adrenal tumors are ultimately determined to be “non-functional,” and therefore considered to pose no health risk (12, 14). The findings from our current investigation substantially extend and potentially redefine the current paradigm of a “non-functional” adrenal tumor: we observed that individuals with apparent NFAT, as defined by current clinical guidelines, had approximately 2-fold higher risk of developing incident diabetes when compared with participants without adrenal tumors. Further, our results indicate that a potential mechanism underlying this increased risk for diabetes may be glucocorticoid excess within a range that is currently considered “normal” by our accepted standards. In this regard, our findings suggest that “non-functional” adrenal tumors may not be non-functional after all; rather, they may secrete small and inappropriate amounts of glucocorticoids that increase the risk for metabolic disease over time.
Prior longitudinal studies by Di Dalmazi et al.(13), Debono et al.(12), and Morelli et al.(14) have described associations between adrenal tumors with subclinical hypercortisolism and incident cardiovascular events (14) and mortality(12, 13). These three studies used the NFAT group as the referent control, and did not include comparisons with individuals without adrenal tumors. Whether the higher risk of cardiovascular events and mortality associated with hypercortisolism in these studies was mediated by the development of incident insulin resistance or diabetes, a major risk factor for death (26), was not directly evaluated. In contrast, our study was designed to focus only on NFAT in comparison to no adrenal tumors; however, we were also able to conduct an exploratory analysis that included a small population of participants with subclinical hypercortisolism that suggested a continuum of risk across these classifications. Collectively, our studies (12–14) suggest a “continuum of metabolic and cardiovascular risk:” prevalent NFAT may impart an increased risk of developing insulin resistance and diabetes when compared to individuals without adrenal tumors, and the addition of subclinical hypercortisolism may further increase the risk of developing cardiovascular events and death, which are dramatically increased in the rare instance of overt Cushing syndrome (27, 28) (Appendix Figure 1). In contrast to the studies by Di Dalmazi and Morelli, we did not have measures of adrenocorticotropic hormone (ACTH) since our study was observational and ACTH measures in patients with NFAT and without adrenal tumors are rarely performed. These prior studies reported that ACTH levels were detectable and slightly higher in NFAT when compared to subclinical hypercortisolism (13, 14); therefore, the demonstration that ACTH levels are slightly higher in participants with no adrenal tumors when compared to NFAT could have further supported a continuum of glucocorticoid excess to parallel the risk for incident diabetes we observed.
Some small cross-sectional studies have suggested a potential link between NFAT and cardiometabolic diseases (10, 17–19). Androulakis et al. showed that individuals with NFAT had greater insulin resistance indices and endothelial dysfunction when compared with healthy controls without adrenal tumors (17). Further, they observed associations between higher carotid intima-media thickness and higher cortisol within the “non-functional” range, suggesting that even “normal” cortisol concentrations may confer a spectrum of risk. The findings of our secondary analyses demonstrated that higher cortisol secretion within the presumed “normal” range associated with greater NFAT size and a higher prevalence of type 2 diabetes. Although we did not observe a significant relation between NFAT size and incident outcomes, we presume that this effect was negated by the fact that larger NFAT (>4 cm) may have been preferentially surgically resected in accordance with current guidelines (1, 2, 4, 15, 16).
Cortisol is a potent glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) agonist. As elegantly demonstrated by Arlt et al. in their studies of adrenal steroid profiling using mass spectrometry (20), when compared with healthy controls without adrenal tumors, NFAT secrete higher levels of glucocorticoids that are not typically measured or captured by our standard clinical assays of cortisol. Thus, our reliance on assessing cortisol as a surrogate for the functionality of adrenal tumors may be inadequate in that the spectrum of GR and MR agonists that are secreted may be much greater. Together, our findings provide some general support for the speculation that adrenal tumors may continue to secrete low concentrations of GR and/or MR agonists that can contribute to diabetes and cardiovascular disease risk, and therefore, future studies of NFAT should incorporate methods such as broad adrenal steroid profiling to better evaluate the spectrum of GR and MR agonists that might account for our findings of incident diabetes (20).
Our findings must be interpreted in the context of the limitations of our study design. First, observational studies can suffer from confounding and selection bias. Second, since the root cause of adrenal neoplasia remains unresolved, an alternative interpretation of our findings could be that an unknown factor that induces adrenal neoplasia may also increase the risk of diabetes. Regardless of the interpretation, the ultimate clinical applicability of our findings remains that patients with adrenal tumors have a higher risk for developing diabetes and therefore risk stratification should be considered. Third, we recognize that selection bias may have played a role in how we classified NFAT and that bias in the ascertainment of outcomes could have influenced the result. We used documentation of diagnoses to screen for adrenal tumors and detected only 1,346 participants from the >220,000 eligible participants without an adrenal hormonal diagnosis who underwent abdominal imaging. This <1% prevalence of adrenal tumors on imaging is much lower than previous reports (3–5), and may represent the fact that most incidentally discovered adrenal tumors were not officially included as diagnoses in the patient record, or were lost to follow-up during the clinical care of the primary indication for imaging. Therefore, it is possible that our selection of NFAT may have represented participants who had more visits to their healthcare providers; and/or had more conscientious healthcare providers; and/or had more abdominal imaging due to a greater burden of medical problems. However, we observed no major demographic or comorbidity differences in the eligible participants included in our incident composite diabetes analyses, and both exposure groups were followed for comparable durations of time with comparable screening and assessments of outcomes at comprehensive health encounters. Fourth, our findings may not be generalizable to men since the majority of our study population was female. The female predominance of our study population was unexpected, and may be due to the fact that women are much more likely than men to visit with and maintain longitudinal follow-up with their physicians (29, 30) and more likely to undergo abdominal imaging than men (of the >234,000 of abdominal scans available, 65% were in women). Fifth, we cannot exclude meaningful associations between NFAT and other outcomes where confidence intervals suggest the potential for a relationship. It should be noted that our classification of diabetes outcomes was the most refined since it incorporated documented diagnosis and/or supportive HbA1c levels. In contrast, the classification of some other clinical outcomes (such as hypertension) relied on documentation of diagnoses alone and the baseline prevalence of some of these outcomes was already high. Sixth, we did not have repeated and longitudinal assessments of adrenal hormones or NFAT size to assess whether new or worsening adrenal hormone excess could account for our findings (12–14). It is possible that NFAT are related to risk for diabetes due to the development of incident subclinical hypercortisolism that we did not assess; however, the underlying message of our study, that NFAT should be recognized and monitored as potential risk-factors for diabetes, is unlikely to change even if we had repeated measures of hormones or NFAT size. Lastly, we did not have ACTH levels to analyze as discussed earlier.
In summary, our findings demonstrate a significantly higher risk of developing incident diabetes in individuals with NFAT when compared to those without adrenal tumors. Given the high prevalence of incidentally discovered adrenal tumors that predominantly represent benign NFAT, our findings have important implications for general clinical practice and future research investigations: 1) the classification of “non-functional” may be an inadequate and misleading term to ascribe to benign adrenal tumors since it minimizes the potential continuum of adrenal hormone secretion that can contribute to cardiometabolic risk; therefore, the current accepted criteria by which we classify adrenal tumors as “non-functional” may need re-evaluation; 2) these findings underscore the importance of recognizing incidentally discovered adrenal tumors as independent risk factors for developing diabetes that may warrant more frequent surveillance for glucose intolerance; and 3) future studies that include broad adrenal steroid metabolite profiling are needed to investigate whether NFAT secrete inappropriate amounts of glucocorticoid that evade our current clinical practice and contribute to adverse outcomes.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health and the Doris Duke Charitable Foundation.
We thank our funding sources. Anand Vaidya was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by Grant 2015085 from the Doris Duke Charitable Foundation, and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL111771. Dr. Adler was supported by National Institutes of Health award K24 HL103845 from the National Heart, Lung, And Blood Institute. Diana Lopez, Miguel Angel Luque-Fernandez, and Anand Vaidya had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Grant Support:
Anand Vaidya was supported by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health under Award Number R01 DK107407, by Grant 2015085 from the Doris Duke Charitable Foundation, and by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL111771.
Dr. Adler was supported by National Institutes of Health award K24 HL103845 from the National Heart, Lung, And Blood Institute.
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Nieman LK. Approach to the patient with an adrenal incidentaloma. J Clin Endocrinol Metab. 2010;95(9):4106–13. doi: 10.1210/jc.2010-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–10. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 3.Bovio S, Cataldi A, Reimondo G, Sperone P, Novello S, Berruti A, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29(4):298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 4.Terzolo M, Stigliano A, Chiodini I, Loli P, Furlani L, Arnaldi G, et al. AME position statement on adrenal incidentaloma. Eur J Endocrinol. 2011;164(6):851–70. doi: 10.1530/EJE-10-1147. [DOI] [PubMed] [Google Scholar]
- 5.Hedeland H, Ostberg G, Hokfelt B. On the prevalence of adrenocortical adenomas in an autopsy material in relation to hypertension and diabetes. Acta Med Scand. 1968;184(3):211–4. doi: 10.1111/j.0954-6820.1968.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 6.Patrova J, Jarocka I, Wahrenberg H, Falhammar H. Clinical Outcomes in Adrenal Incidentaloma: Experience from One Center. Endocr Pract. 2015;21(8):870–7. doi: 10.4158/EP15618.OR. [DOI] [PubMed] [Google Scholar]
- 7.Young WF., Jr Management approaches to adrenal incidentalomas. A view from Rochester, Minnesota. Endocrinol Metab Clin North Am. 2000;29(1):159–85. x. doi: 10.1016/s0889-8529(05)70122-5. [DOI] [PubMed] [Google Scholar]
- 8.Mantero F, Terzolo M, Arnaldi G, Osella G, Masini AM, Ali A, et al. A survey on adrenal incidentaloma in Italy. Study Group on Adrenal Tumors of the Italian Society of Endocrinology. J Clin Endocrinol Metab. 2000;85(2):637–44. doi: 10.1210/jcem.85.2.6372. [DOI] [PubMed] [Google Scholar]
- 9.Chiodini I. Clinical review: Diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96(5):1223–36. doi: 10.1210/jc.2010-2722. [DOI] [PubMed] [Google Scholar]
- 10.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscovo L, et al. Subclinical Cushing’s syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab. 2000;85(4):1440–8. doi: 10.1210/jcem.85.4.6515. [DOI] [PubMed] [Google Scholar]
- 11.Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012;166(4):669–77. doi: 10.1530/EJE-11-1039. [DOI] [PubMed] [Google Scholar]
- 12.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–70. doi: 10.1210/jc.2014-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. doi: 10.1016/S2213-8587(13)70211-0. [DOI] [PubMed] [Google Scholar]
- 14.Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, et al. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827–34. doi: 10.1210/jc.2013-3527. [DOI] [PubMed] [Google Scholar]
- 15.NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”) NIH Consens State Sci Statements. 2002;19(2):1–25. [PubMed] [Google Scholar]
- 16.Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, et al. The American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons medical guidelines for the management of adrenal incidentalomas. Endocr Pract. 2009;15(Suppl 1):1–20. doi: 10.4158/EP.15.S1.1. [DOI] [PubMed] [Google Scholar]
- 17.Androulakis II, Kaltsas G, Kollias GE, Markou A, Gouli A, Thomas D, et al. Patients with apparently non-functioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014:jc20134064. doi: 10.1210/jc.2013-4064. [DOI] [PubMed] [Google Scholar]
- 18.Peppa M, Boutati E, Koliaki C, Papaefstathiou N, Garoflos E, Economopoulos T, et al. Insulin resistance and metabolic syndrome in patients with nonfunctioning adrenal incidentalomas: a cause-effect relationship? Metabolism. 2010;59(10):1435–41. doi: 10.1016/j.metabol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Tuna MM, Imga NN, Dogan BA, Yilmaz FM, Topcuoglu C, Akbaba G, et al. Non-functioning adrenal incidentalomas are associated with higher hypertension prevalence and higher risk of atherosclerosis. J Endocrinol Invest. 2014;37(8):765–8. doi: 10.1007/s40618-014-0106-5. [DOI] [PubMed] [Google Scholar]
- 20.Arlt W, Biehl M, Taylor AE, Hahner S, Libe R, Hughes BA, et al. Urine steroid metabolomics as a biomarker tool for detecting malignancy in adrenal tumors. J Clin Endocrinol Metab. 2011;96(12):3775–84. doi: 10.1210/jc.2011-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–40. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266–81. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 24.(OECD) OfEC-oaD. Health at a Glance 2013: OECD Indicators. OECD Publishing; 2013. [Google Scholar]
- 25.Al-Thani H, El-Menyar A, Al-Sulaiti M, ElGohary H, Al-Malki A, Asim M, et al. Adrenal Mass in Patients who Underwent Abdominal Computed Tomography Examination. N Am J Med Sci. 2015;7(5):212–9. doi: 10.4103/1947-2714.157482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marczak L, O’Rourke K, Shepard D for the Institute for Health M. Evaluation. WHen and why people die in the united states, 1990–2013. JAMA. 2016;315(3):241. [Google Scholar]
- 27.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2015;100(8):2807–31. doi: 10.1210/jc.2015-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–27. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 29.Brett KM, Burt CW. Utilization of ambulatory medical care by women: United States, 1997–98. Vital Health Stat. 2001;13(149):1–46. doi: 10.1037/e309022005-001. [DOI] [PubMed] [Google Scholar]
- 30.Ashman JJ, HE, Talwalkar A. Variation in physician office visit rates by patient characteristics and state. National Center for Health Statistics Data Brief; 2015. p. 212. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.