Abstract
Very low-density lipoprotein (VLDL) receptor is a member of the low-density lipoprotein (LDL) receptor family. It binds triglyceride rich lipoprotein (TGRL) but not LDL, because it recognizes apolipoprotein (apo)E only but not apoB. The VLDL receptor functions as a peripheral lipoprotein receptor in concert with lipoprotein lipase (LPL) in heart, muscle, adipose tissue and macrophages. In contrast to the LDL receptor, VLDL receptor binds apo E2/2 VLDL and apoE3/3 VLDL particles, and its expression is not down-regulated by intracellular lipoproteins. It has been reported that both LDL-cholesterol (LDL-C) and postprandial triglyceride (chyromicron and VLDL remnants) are risk factors for human atherosclerotic cardiovascular disease (ASCVD). True ligands such as lipoprotein particles of the VLDL receptor are chyromicron remnant (CMR) and VLDL remnant (postprandial hyperlipidemia). Although the oxidized LDL (oxLDL)-scavenger receptors pathway is considered to be the main mechanism for macrophage foam cell formation, it seems that the TGRL-LPL-VLDL receptor pathway is also involved. Since Lp(a) is one of the ligands for the VLDL receptor, the Lp(a)- VLDL receptor pathway is another potential alternative. The expression of VLDL receptor protein in mouse macrophages is modest compared to that in rabbit and human macrophages, both in vitro and in vivo. Therefore, we need to elucidate the mechanism of human ASCVD not by using the mouse model and scavenger receptors pathway but instead using the rabbit model and VLDL receptor pathway, respectively.
Keywords: VLDL receptor, Lipoprotein lipase, Lp(a), Atherosclerosis, Macrophage foam cell formation
Introduction
Macrophage foam cell formation is a critical step in the initial stages of the atherosclerotic process. The “Response to injury” hypothesis indicates that injury of endothelial cells (ECs) results in a series of reactions that include overexpression of adhesion molecules and secretion of various chemokines, which in turn result in the recruitment of circulating monocytes and T lymphocytes1). The alternative “response to retention” hypothesis argues that LDL enters the intimal space, where it is retained by subendothelial extracellular matrix molecules, predominantly proteoglycans. Proteoglycan-bound LDL increases the susceptibility of LDL to oxidation. The latter (oxLDL) enhances the synthesis of monocyte chemoattractant protein (MCP-1) by ECs and smooth muscle cells (SMCs), and acts directly as a chemoattractant to monocytes2). The atherosclerotic process starts with the transmigration of monocytes into the arterial intima, where they differentiate into macrophages. Simultaneously, macrophages take up oxLDL through the scavenger receptors (SR-A, CD36, and Lox1)3). However, evidence suggests that the SR-A and CD36 are not involved in macrophage foam cell formation since targeted deletion of SR-A and CD36 does not abrogate macrophage foam cell formation or reduces the atherosclerotic area in apoE knockout (KO) mice4, 5). Although LDL-C remains the primary treatment target to reduce the risk of ASCVD, epidemiological studies have shown that high triglyceride levels (remnant lipoprotein or non-fasting triglycerides) are independently associated with increased incidence of cardiovascular events in Japanese patients6, 7). Both LDL-C and postprandial triglycerides (CMR and VLDL remnant) are risk factors for human ASCVD on a global basis8–10). But there remains some uncertainty regarding the direct causal role of TGRL in ASCVD. True ligands such as lipoprotein particles of the VLDL receptor are CMR, VLDL remnant, and Lp(a). In addition macrophages express both VLDL receptor and LPL. In this review, I want to advocate the importance of TGRL-LPL-VLDL receptor and Lp(a)-VLDL receptor pathways for macrophage foam cell formation.
Difference between VLDL and LDL Receptors
We first cloned and characterized the VLDL receptor and found structural domains that were similar to those of the LDL receptor, including: (i) an aminoterminal ligand binding domain composed of multiple cysteine-rich repeats, (ii) an epidermal growth factor (EGF) precursor homology domain, (iii) an O-linked sugar domain with clustered serine and threonine, (iv) a single transmembrane domain, and (v) a cytoplasmic domain with an FDNPVY sequence described in rabbit and human VLDL receptor cDNA11, 12) (Fig. 1). The exon-intron organization of the VLDL receptor gene is almost identical to that of the LDL receptor gene, except for an extra exon that encodes an additional repeat in the ligand binding domain (LDL receptor contains a 7-fold repeat while the VLDL receptor has an 8-fold repeat) (Fig. 1). The two genes are located on different chromosomes; the VLDL receptor gene on chromosome 9 (9p24) and the LDL receptor gene on chromosome 19 (19p13.2). Subsequent studies indicated that LDL receptor mutation causes familial hypercholesterolemia (FH)8). In 2005, a human VLDL receptor mutant was discovered and homozygous deletion of the VLDL receptor gene was found to be the cause of autosomal recessive cerebellar hypoplasia with cerebral gyral simplification13) (Table 1).
Fig. 1.
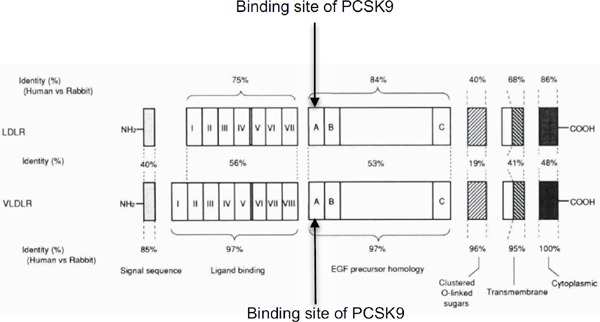
Structure of the VLDL receptor and the LDL receptory10).
PCSK9 binds the EGF-A site of both LDL receptor and VLDL receptor.
Table 1. Differences between VLDL receptor and LDL receptor.
| VLDL receptor | LDL receptor | |
|---|---|---|
| Gene location | 9p24 | 19p13.2 |
| Phenotype of human mutant | Cerebellar hypoplasia with cerebral gyral simplification | Familial hypercholesterolemia (FH) |
| Ligands | ApoE, LPL, Lp(a), RAP, PCSK9, Reelin, Thrombospondin-1, uPA/plasminogen activator inhibitor-1 complex, Serine protease-serpin complex, Tissue factor pathway inhibitor (TFPI), Fibrin, Hepatitis C virus | ApoE, ApoB, RAP, PCSK9, Hepatitis C virus |
| Main expression sites | Heart, Muscle, Adipose tissue, Macrophages, Endothelial cells, Brain | Liver, Adrenal gland |
| Binding capacity of apoE2/2 | Equal to apoE3/3 | Less than 1% |
| Sterol regulation | None | Negative feedback |
| Alternative splicing | + | − |
Apo: apolipoprotein, LPL: lipoprotein lipase, RAP: receptor-associated-protein, PCSK9: proprotein convertase subtilisin/kexin type 9, uPA: urokinase plasminogen activator
It is controversial that whether LDL receptor binds Lp(a). It seems that Lp(a) is recognized by LDL receptor in vitro, but most of the in vivo data including human have shown that LDL receptor does not involved in the catabolism of Lp(a).
VLDL receptor cDNA overexpressing ldlA-7 (LDL receptor-deficient CHO) cells bound apoE-containing lipoproteins, including VLDL, intermediate-density lipoprotein (IDL) from Watanabe heritable hyperlipidemic (WHHL) rabbits, and β-VLDL (β-migrating VLDL) from cholesterol-fed rabbits, but did not bind LDL from WHHL rabbits11). On the other hand, ldlA-7 cells transfected with the LDL receptor cDNA bound both apoB- and apoE-containing lipoproteins, including VLDL, IDL, LDL from WHHL rabbits, and β-VLDL from cholesterol-fed rabbits11). Notably, VLDL from fasted normal human subjects bound with lower affinity compared to VLDL prepared from WHHL rabbits11). The VLDL from WHHL rabbits was recognized by VLDL receptor cDNA overexpressing ldlA-7 cells because LDL receptor deficiency induced the accumulation of remnant particles in VLDL. More than 25 years ago, I thought the new cloned gene producing protein was not a lipoprotein receptor even though the new gene was structurally similar to LDL receptor gene. Over a period of six months, I examined the ligand binding properties using my own fasted-VLDL and LDL, but could not obtain clear binding data. My fasted-VLDL and LDL particles were never recognized by new gene overexpressing ldlA-7 cells (data not shown). Accidentally, I once obtained my own postprandial VLDL and LDL after eating curry rice in the Co-op café at Tohoku University. I found that postprandial VLDL, but not LDL, was bound and internalized by the new gene overexpressing ldlA-7 cells. At that stage, I was sure that the true ligands of VLDL receptor for lipoprotein particles were CMR and VLDL remnant (postprandial lipoproteins). To ensure the ligand specificity of VLDL receptor in vivo, we generated the VLDL receptor and LDL receptor double KO mice and compared them to LDL receptor KO mice. Non-fasting serum total cholesterol was not different between the two, but non-fasting serum triglyceride was elevated in the double KO mice. High-performance liquid chromatography (HPLC) analysis found high LDL-cholesterol fraction during all fasting times in LDL receptor KO mice. When VLDL receptor deletion was induced in LDL receptor KO mice, the VLDL-cholesterol fraction was higher than the LDL-cholesterol fraction at all fasting times (Fig. 2). These data indicate that the VLDL receptor acts on TGRL metabolism both quantitatively and qualitatively. On the other hand, the low affinity binding of fasting human VLDL to the VLDL receptor could be overcome by enriching VLDL with either apoE or LPL14). Niemeier et al.15) reported that the same mechanism was the case for chylomicron particles. The VLDL receptor mediated the uptake of CMR, and this uptake was further increased by the addition of apoE and inactivated LPL. Argraves et al. found that LPL itself bound with high affinity to purified VLDL receptor16). In vivo, VLDL receptor KO mice have reduced LPL activity17). Taking into account that the VLDL receptor and LPL are expressed in the same tissues (heart, muscle, adipose tissue, and macrophages), these findings suggest that CMR and VLDL remnant are true ligands for the VLDL receptor in concert with LPL. In contrast to the LDL receptor, VLDL receptor bound apoE2/2 VLDL and apoE3/3 VLDL identically in vitro18). Further adenovirus-mediated VLDL receptor expression in the liver of apoE2/2 and apoE3-Leiden transgenic mice resulted in lowering plasma cholesterol levels, indicating that the VLDL receptor recognized apoE2/2 and apoE3-Leiden. The reduction in plasma cholesterol was mainly due to a reduction in VLDL levels19). Ruiz et al. showed that the VLDL receptor recognizes all apoE isoforms (apoE2, apoE3, and apoE4) and avidly binds lipid-free apoE20).
Fig. 2.
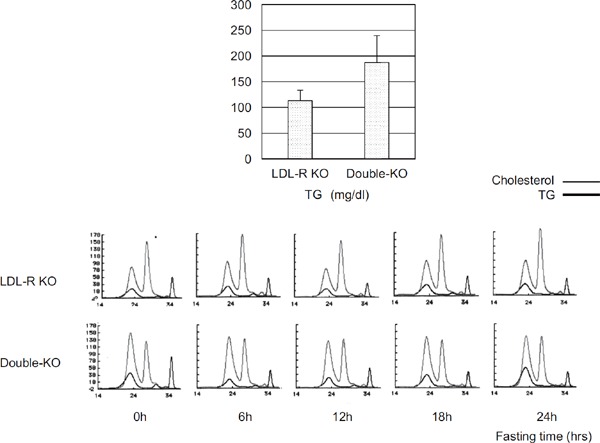
Top: Non-fasting serum triglyceride (TG) in LDL-R KO and double KO mice (n = 6).
Bottom: HPLC profile during 24 h fasting times in LDL-R KO and double KO mice.
The VLDL receptor expression is highly abundant in heart, muscle, adipose tissue, macrophages, ECs and brain, but barely detectable in the liver, in which the LDL receptor is expressed abundantly.
It is currently understood that the LDL receptor is down-regulated by intracellular lipoproteins. We were the first group to report that the VLDL receptor is not down-regulated by sterols in THP-1 and rabbit resident alveolar macrophages12, 21). Western blots also showed the disappearance of LDL receptor protein following the addition of 100 µg/ml of β-VLDL to the medium for 48 h, though the VLDL receptor protein level did not change in THP-1 cells22). The human LDL receptor gene contains a sterol regulatory element (SRE)-1 while the VLDL receptor gene includes two SRE-1-like sequences12). SRE-1 contains a direct repeat of the nucleotide sequence CAC on the same DNA strand separated by two Cs. The sequences of the two CAC are believed to be the target of the SRE-binding protein 1 that controls transcription of the LDL receptor gene23). The SRE-1-like sequences in the VLDL receptor contain single nucleotide substitutions that disrupt the direct CAC repeats. This could be the reason why the VLDL receptor expression is not regulated by intracellular lipoproteins.
The VLDL receptor mRNAs produce mainly two kinds of VLDL receptor proteins by alternative splicing; type 1 VLDL receptor and type 2 VLDL receptor that lacks O-linked sugar domain encoded by exon 1612).
Multiple Ligands for VLDL and LDL Receptors
In addition to apoE and LPL, the VLDL receptor binds Lp(a)24), receptor-associated protein (RAP)25), proprotein convertase subtilisin/kexin type 9 (PCSK9)26–28), reelin29), thrombospondin-130–32), urokinase plasminogen activator (uPA)/plasminogen activator inhibitor-1 complex16, 33–36), serine protease-serpin complex37), and tissue factor pathway inhibitor (TFPI)36). It seems that ECs are important sites for the action of the VLDL receptor because the movement of active LPL across ECs involves both heparan sulfate proteoglycan and the VLDL receptor38). In addition, it is intriguing that fibrin is one of the ligands for the VLDL receptor. Interaction of fibrin with the VLDL receptor promotes transendothelial migration of leukocyte as in the case for LPL and thereby enhances inflammation39). Anti-VLDL receptor antibodies inhibit fibrin-VLDL receptor interaction and significantly reduce myocardial injury induced by ischemia-perfusion40). The common ligands of the VLDL and LDL receptors are apoE, RAP, PCSK9 and hepatitis C virus41–43). Because anti- PCSK9 monoclonal antibody (evolocumab and alirocumab) increases the protein expression of both VLDL and LDL receptors44), patients with hepatitis C treated with anti-PCSK9 monoclonal antibody should be watched carefully (Table 1, Fig. 1).
TGRL-LPL-VLDL Receptor and Lp(a)-VLDL Receptor Pathways for Macrophage Foam Cell Formation
The expression of VLDL receptor primarily in macrophages has been confirmed in human and rabbit atherosclerotic lesions24, 45–47). In vitro, we reported that IFN-γ inhibited VLDL receptor expression and foam cell formation in three types of human macrophages (PMA-induced THP-1, PMA-induced HL-60, and human monocyte-derived macrophages) by β-VLDL, a representative lipoprotein in the metabolic syndrome and type III hyperlipoproteinemia48). These results suggest that the VLDL receptor could be a macrophage β-VLDL receptor, which is one of the receptors involved in macrophage foam cell formation. However, controversial in vivo findings using a mouse model were reported. Atherosclerotic lesions were not different between HuB (human apoB) transgenic mice and VLDL receptor-deficient HuB transgenic mice fed atherogenic diet for 4 months17). Tacken et al. also showed that both VLDL receptor deficiency and endothelial VLDL receptor overexpression did not affect the size of atherosclerotic lesions. Interestingly, they indicated that deficiency of the VLDL receptor profoundly increased intimal thickening after vascular injury49). We also compared the area of atherosclerotic lesions in double KO and LDL receptor KO mice, but found no difference in the area even though we showed clear difference in lipoprotein profile (Fig. 2). Fortunately, we were able to obtain rabbit polyclonal anti-VLDL receptor antibody that recognized human, rabbit, rat, and mouse VLDL receptors. A synthetic peptide, CASVGHTYPAISVVSTDDDL, which corresponds to the carboxy-terminus of the human, rabbit, rat, and mouse VLDL receptors, was synthesized and injected into Japanese White rabbits to obtain polyclonal antibody (named VR2). VR2 reacted only with human VLDL receptor, but not with human LDL receptor or human ApoER2 cDNA transfected ldlA-7 cells. Furthermore, VR2 specifically recognized the human and wild-type mouse heart VLDL receptor while it did not detect VLDL receptor bands in hearts of VLDL receptor KO mice. Western blots showed that although VLDL receptor protein was detected in PMA-treated THP-1 human macrophages and wildtype mouse heart, it was not detected in cell lines derived from mouse macrophages (Raw264.7 and J774.2) and also mouse peritoneal macrophages. The VR2 antibody detected rabbit VLDL receptor protein in heart but not in liver by immunohistochemistry. VLDL receptor proteins were clearly detected in some of the RAM11-positive macrophages in the thoracic aorta of WHHLMI rabbits, which are indicative of atherosclerotic lesion. In contrast to the atherosclerotic lesions in WHHLMI rabbit thoracic aorta, no VLDL receptor protein was observed in BM8-positive mouse macrophages in aortic atherosclerotic lesions in chowfed apoE KO mice and LDL receptor KO mice whose diet had been supplemented with 1.25% cholesterol for 12 weeks50). We detected abundant amounts of VLDL receptor protein in human atherosclerotic coronary arteries but not in non-atherosclerotic coronary arteries, using the same VR2 antibody (data not shown). Argraves et al. have already detected the VLDL receptor protein in human atherosclerotic plaque and the VLDL receptor protein was co-located with plaque KP-1-positive macrophages and foam cells24). TGRL has also been isolated from human artery segments51). Recently Matsuo et al. reported that serum remnant lipoprotein levels were positively correlated with the necrotic components of the coronary plaques and negatively correlated with the fibrotic components evaluated by intravascular ultrasound (IVUS) in patients with stable angina52) and it is known that both LDL-C and TGRL are independent risk factors for human ASCVD8–10). Therefore, I consider that the mechanisms of macrophage foam cell formation are somewhat different between mice and humans or rabbits. Finally, I want to call up the TGRL-LPL-VLDL receptor pathway for macrophage foam cell formation, especially in rabbit and human. Since Lp(a) is one of the ligands for the VLDL receptor24), the Lp(a)-VLDL receptor pathway may be considered as another alternative pathway (Fig. 3). Since both rabbit and human macrophages express VLDL receptor protein, studies on the importance of VLDL receptor signaling for TGRL should focus on these species rather than on the mouse systems (mouse peritoneal macrophages, apoE KO mice, and LDL receptor KO mice).
Fig. 3.
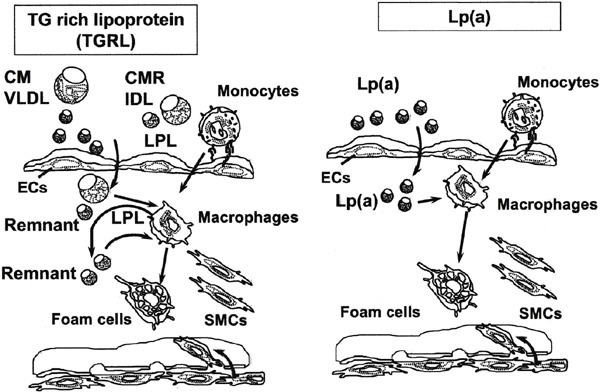
Schematic diagram of the putative TGRL-LPL-VLDL receptor and Lp(a)-VLDL receptor pathways for macrophage foam cell formation.
CM: chyromicron, CMR: chyromicron remnant, VLDL: very low-density lipoprotein, IDL: intermediate-density lipoprotein, LPL: lipoprotein lipase, ECs: endothelial cells, SMCs: smooth muscle cells
Conclusions
The VLDL receptor could be a so-called macrophage β-VLDL receptor which is involved in macrophage foam cell formation. Notably researchers in atherosclerosis should be known that macrophage VLDL receptor protein expression is different among species. I want to see more detailed researches about TGRL-LPL-VLDL receptor and Lp(a)-VLDL receptor pathways for macrophage foam cell formation in the future.
Aknowledgments
I thank Akihisa Kamataki (Hirosaki University Graduate School of Medicine), Tokuo Yamamoto (Tohoku University), and Takafumi Ishida (Fukushima Medical University) for collaboration. I also appreciate Jinya Suzuki (University of Fukui) for preparation of the manuscript.
Conflict of Interest
The author declares no conflict of interest.
References
- 1). Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature, 1993; 362: 801-809 [DOI] [PubMed] [Google Scholar]
- 2). Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol, 1995; 15: 551-561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Chistiakov DA, Bobryshev YV, Orekhov AN. Macrophage-mediated cholesterol handling in atherosclerosis. J Cell Mol Med, 2016; 20: 17-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest, 2005; 115: 2192-2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol, 2009; 29: 19-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Kugiyama K, Doi H, Takazoe K, Kawano H, Soejima H, Mizuno Y, Tsunoda R, Sakamoto T, Nakano T, Nakajima K, Ogawa H, Sugiyama S, Yoshimura M, Yasue H. Remnant lipoprotein levels in fasting serum predict coronary events in patients with coronary artery disease. Circulation, 1999; 99: 2858-2860 [DOI] [PubMed] [Google Scholar]
- 7). Iso H, Naito Y, Sato S, Kitamura A, Okamura T, Sankai T, Shimamoto T, Iida M, Komachi Y. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am J Epidemiol, 2001; 153: 490-499 [DOI] [PubMed] [Google Scholar]
- 8). Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet, 2014; 384: 607-617 [DOI] [PubMed] [Google Scholar]
- 9). Budoff M. Triglycerides and triglyceride-rich lipoproteins in the causal pathway of cardiovascular disease. Am J Cardiol, 2016; 118: 138-145 [DOI] [PubMed] [Google Scholar]
- 10). Toth PP. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc Health Risk Manag, 2016; 12: 171-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T. Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A, 1992; 89: 9252-9256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Sakai J, Hoshino A, Takahashi S, Miura Y, Ishii H, Suzuki H, Kawarabayasi Y, Yamamoto T. Structure, chromosome location, and expression of the human very low density lipoprotein receptor gene. J Biol Chem, 1994; 269: 2173-2182 [PubMed] [Google Scholar]
- 13). Boycott KM, Flavelle S, Bureau A, Glass HC, Fujiwara TM, Wirrell E, Davey K, Chudley AE, Scott JN, McLeod DR, Parboosingh JS. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am J Hum Genet, 2005; 77: 477-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Takahashi S, Suzuki J, Kohno M, Oida K, Tamai T, Miyabo S, Yamamoto T, Nakai T. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J Biol Chem, 1995; 270: 15747-15754 [DOI] [PubMed] [Google Scholar]
- 15). Niemeier A, Gafvels M, Heeren J, Meyer N, Angelin B, Beisiegel U. VLDL receptor mediates the uptake of human chylomicron remnants in vitro. J Lipid Res, 1996; 37: 1733-1742 [PubMed] [Google Scholar]
- 16). Argraves KM, Battey FD, MacCalman CD, McCrae KR, Gafvels M, Kozarsky KF, Chappell DA, Strauss JF, 3rd, Strickland DK. The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem, 1995; 270: 26550-26557 [DOI] [PubMed] [Google Scholar]
- 17). Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, Bensadoun A, Goldberg IJ. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J Biol Chem, 2002; 277: 10037-10043 [DOI] [PubMed] [Google Scholar]
- 18). Takahashi S, Oida K, Ookubo M, Suzuki J, Kohno M, Murase T, Yamamoto T, Nakai T. Very low density lipoprotein receptor binds apolipoprotein E2/2 as well as apolipoprotein E3/3. FEBS Lett, 1996; 386: 197-200 [DOI] [PubMed] [Google Scholar]
- 19). van Dijk KW, van Vlijmen BJ, van der Zee A, van't Hof B, van der Boom H, Kobayashi K, Chan L, Havekes LM, Hofker MH. Reversal of hypercholesterolemia in apolipoprotein E2 and apolipoprotein E3-Leiden transgenic mice by adenovirus-mediated gene transfer of the VLDL receptor. Arterioscler Thromb Vasc Biol, 1998; 18: 7-12 [DOI] [PubMed] [Google Scholar]
- 20). Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res, 2005; 46: 1721-1731 [DOI] [PubMed] [Google Scholar]
- 21). Suzuki J, Takahashi S, Oida K, Shimada A, Kohno M, Tamai T, Miyabo S, Yamamoto T, Nakai T. Lipid accumulation and foam cell formation in Chinese hamster ovary cells overexpressing very low density lipoprotein receptor. Biochem Biophys Res Commun, 1995; 206: 835-842 [DOI] [PubMed] [Google Scholar]
- 22). Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb, 2004; 11: 200-208 [DOI] [PubMed] [Google Scholar]
- 23). Smith JR, Osborne TF, Goldstein JL, Brown MS. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J Biol Chem, 1990; 265: 2306-2310 [PubMed] [Google Scholar]
- 24). Argraves KM, Kozarsky KF, Fallon JT, Harpel PC, Strickland DK. The atherogenic lipoprotein Lp(a) is internalized and degraded in a process mediated by the VLDL receptor. J Clin Invest, 1997; 100: 2170-2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Battey FD, Gafvels ME, FitzGerald DJ, Argraves WS, Chappell DA, Strauss JF, 3rd, Strickland DK. The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J Biol Chem, 1994; 269: 23268-23273 [PubMed] [Google Scholar]
- 26). Poirier S, Mayer G, Benjannet S, Bergeron E, Marcinkiewicz J, Nassoury N, Mayer H, Nimpf J, Prat A, Seidah NG. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem, 2008; 283: 2363-2372 [DOI] [PubMed] [Google Scholar]
- 27). Roubtsova A, Munkonda MN, Awan Z, Marcinkiewicz J, Chamberland A, Lazure C, Cianflone K, Seidah NG, Prat A. Circulating proprotein convertase subtilisin/kexin 9 (PCSK9) regulates VLDLR protein and triglyceride accumulation in visceral adipose tissue. Arterioscler Thromb Vasc Biol, 2011; 31: 785-791 [DOI] [PubMed] [Google Scholar]
- 28). Roubtsova A, Chamberland A, Marcinkiewicz J, Essalmani R, Fazel A, Bergeron JJ, Seidah NG, Prat A. PCSK9 deficiency unmasks a sex- and tissue-specific subcellular distribution of the LDL and VLDL receptors in mice. J Lipid Res, 2015; 56: 2133-2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell, 1999; 97: 689-701 [DOI] [PubMed] [Google Scholar]
- 30). Mikhailenko I, Krylov D, Argraves KM, Roberts DD, Liau G, Strickland DK. Cellular internalization and degradation of thrombospondin-1 is mediated by the aminoterminal heparin binding domain (HBD). High affinity interaction of dimeric HBD with the low density lipoprotein receptor-related protein. J Biol Chem, 1997; 272: 6784-6791 [DOI] [PubMed] [Google Scholar]
- 31). Oganesian A, Armstrong LC, Migliorini MM, Strickland DK, Bornstein P. Thrombospondins use the VLDL receptor and a nonapoptotic pathway to inhibit cell division in microvascular endothelial cells. Mol Biol Cell, 2008; 19: 563-571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Blake SM, Strasser V, Andrade N, Duit S, Hofbauer R, Schneider WJ, Nimpf J. Thrombospondin-1 binds to ApoER2 and VLDL receptor and functions in postnatal neuronal migration. EMBO J, 2008; 27: 3069-3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Webb DJ, Nguyen DH, Sankovic M, Gonias SL. The very low density lipoprotein receptor regulates urokinase receptor catabolism and breast cancer cell motility in vitro. J Biol Chem, 1999; 274: 7412-7420 [DOI] [PubMed] [Google Scholar]
- 34). Webb DJ, Thomas KS, Gonias SL. Plasminogen activator inhibitor 1 functions as a urokinase response modifier at the level of cell signaling and thereby promotes MCF-7 cell growth. J Cell Biol, 2001; 152: 741-752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Jensen JK, Malmendal A, Schiott B, Skeldal S, Pedersen KE, Celik L, Nielsen NC, Andreasen PA, Wind T. Inhibition of plasminogen activator inhibitor-1 binding to endocytosis receptors of the low-density-lipoprotein receptor family by a peptide isolated from a phage display library. Biochem J, 2006; 399: 387-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Di Y, Liu Z, Tian J, Zong Y, Yang P, Qu S. TFPI or uPAPAI-1 complex affect cell function through expression variation of type II very low density lipoprotein receptor. FEBS Lett, 2010; 584: 3469-3473 [DOI] [PubMed] [Google Scholar]
- 37). Kasza A, Petersen HH, Heegaard CW, Oka K, Christensen A, Dubin A, Chan L, Andreasen PA: Specificity of serine protease/serpin complex binding to very-lowdensity lipoprotein receptor and α2-macroglobulin receptor/low-density-lipoprotein-receptor-related protein. Eur J Biochem, 1997; 248: 270-281 [DOI] [PubMed] [Google Scholar]
- 38). Obunike JC, Lutz EP, Li Z, Paka L, Katopodis T, Strickland DK, Kozarsky KF, Pillarisetti S, Goldberg IJ. Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor. J Biol Chem, 2001; 276: 8934-8941 [DOI] [PubMed] [Google Scholar]
- 39). Yakovlev S, Mikhailenko I, Cao C, Zhang L, Strickland DK, Medved L. Identification of VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrindependent transendothelial migration of leukocytes. Blood, 2012; 119: 637-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Yakovlev S, Belkin AM, Chen L, Cao C, Zhang L, Strickland DK, Medved L. Anti-VLDL receptor monoclonal antibodies inhibit fibrin-VLDL receptor interaction and reduce fibrin-dependent leukocyte transmigration. Thromb Haemost, 2016; 116: 1122-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci U S A, 1999; 96: 12766-12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Ujino S, Nishitsuji H, Hishiki T, Sugiyama K, Takaku H, Shimotohno K. Hepatitis C virus utilizes VLDLR as a novel entry pathway. Proc Natl Acad Sci U S A, 2016; 113: 188-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Yamamoto S, Fukuhara T, Ono C, Uemura K, Kawachi Y, Shiokawa M, Mori H, Wada M, Shima R, Okamoto T, Hiraga N, Suzuki R, Chayama K, Wakita T, Matsuura Y. Lipoprotein Receptors Redundantly Participate in Entry of Hepatitis C Virus. PLoS Pathog, 2016; 12: e1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Seidah NG. New developments in proprotein convertase subtilisin-kexin 9's biology and clinical implications. Curr Opin Lipidol, 2016; 27: 274-281 [DOI] [PubMed] [Google Scholar]
- 45). Multhaupt HA, Gafvels ME, Kariko K, Jin H, Arenas-Elliot C, Goldman BI, Strauss JF, 3rd, Angelin B, Warhol MJ, McCrae KR. Expression of very low density lipoprotein receptor in the vascular wall. Analysis of human tissues by in situ hybridization and immunohistochemistry. Am J Pathol, 1996; 148: 1985-1997 [PMC free article] [PubMed] [Google Scholar]
- 46). Nakazato K, Ishibashi T, Shindo J, Shiomi M, Maruyama Y. Expression of very low density lipoprotein receptor mRNA in rabbit atherosclerotic lesions. Am J Pathol, 1996; 149: 1831-1838 [PMC free article] [PubMed] [Google Scholar]
- 47). Hiltunen TP, Luoma JS, Nikkari T, Yla-Herttuala S. Expression of LDL receptor, VLDL receptor, LDL receptor-related protein, and scavenger receptor in rabbit atherosclerotic lesions: marked induction of scavenger receptor and VLDL receptor expression during lesion development. Circulation, 1998; 97: 1079-1086 [DOI] [PubMed] [Google Scholar]
- 48). Kosaka S, Takahashi S, Masamura K, Kanehara H, Sakai J, Tohda G, Okada E, Oida K, Iwasaki T, Hattori H, Kodama T, Yamamoto T, Miyamori I. Evidence of macrophage foam cell formation by very low-density lipoprotein receptor: interferon-gamma inhibition of very low-density lipoprotein receptor expression and foam cell formation in macrophages. Circulation, 2001; 103: 1142-1147 [DOI] [PubMed] [Google Scholar]
- 49). Tacken PJ, Delsing DJ, Gijbels MJ, Quax PH, Havekes LM, Hofker MH, van Dijk KW. VLDL receptor deficiency enhances intimal thickening after vascular injury but does not affect atherosclerotic lesion area. Atherosclerosis, 2002; 162: 103-110 [DOI] [PubMed] [Google Scholar]
- 50). Takahashi S, Ito T, Zenimaru Y, Suzuki J, Miyamori I, Takahashi M, Takahashi M, Ishida T, Ishida T, Hirata K, Yamamoto TT, Iwasaki T, Hattori H, Shiomi M. Species differences of macrophage very low-density-lipoprotein (VLDL) receptor protein expression. Biochem Biophys Res Commun, 2011; 407: 656-662 [DOI] [PubMed] [Google Scholar]
- 51). Rapp JH, Lespine A, Hamilton RL, Colyvas N, Chaumeton AH, Tweedie-Hardman J, Kotite L, Kunitake ST, Havel RJ, Kane JP. Triglyceride-rich lipoproteins isolated by selected-affinity anti-apolipoprotein B immunosorption from human atherosclerotic plaque. Arterioscler Thromb, 1994; 14: 1767-1774 [DOI] [PubMed] [Google Scholar]
- 52). Matsuo N, Matsuoka T, Onishi S, Yamamoto H, Kato A, Makino Y, Kihara S. Impact of remnant lipoprotein on coronary plaque components. J Atheroscler Thromb, 2015; 22: 783-795 [DOI] [PubMed] [Google Scholar]


