Abstract
Aim: The influence of serum urate on kidney disease is attracting attention, but the effects of uric acid (UA) on nephrosclerosis have not been elucidated.
Methods: We reviewed data from 45 patients diagnosed with arterial/arteriolar nephrosclerosis. The renal outcomes of the arterial/arteriolar nephrosclerosis patients were assessed by performing logistic and Cox regression analyses. A Kaplan-Meier analysis was used to evaluate the impact of hyperuricemia (HU) on kidney survival. The renal outcomes of patients with and without HU were compared by using a propensity score-matched cohort.
Results: The logistic regression models showed no significant differences in renal outcomes, according to baseline parameters or follow-up parameters, except the serum UA value and body mass index (BMI). Baseline serum UA level had the highest odds ratio (OR) for estimated glomerular filtration rate (eGFR) decline (OR, 1.86; 95% confidence interval (CI), 1.12 to 3.45), among the parameters assessed. In the multivariate Cox regression analysis, HU (UA ≥ 8.0 mg/dL) (P = 0.01) and BMI (P = 0.03) were significantly associated with a ≥ 50% eGFR decline or ESRD. The Kaplan-Meier analysis in the propensity score-matched cohort indicated that the renal survival rate of the group of arterial/arteriolar nephrosclerosis patients with HU was significantly lower than that of the group without HU (log rank, P = 0.03).
Conclusion: The results of this study suggest that the baseline serum UA value can serve as a renal outcome predictor in arterial/arteriolar nephrosclerosis patients.
Keywords: Arterial/arteriolar sclerosis, Biopsy, Hyperuricemia, nephrosclerosis, Prognosis
Introduction
Chronic kidney disease (CKD) is affected by multiple risk factors for disease progression1, 2), and it is extremely important to identify these factors. Various clinical factors have been identified as independent predictors of CKD progression3, 4), including proteinuria5, 6), elevated serum creatinine level5), hypertension7), smoking8, 9), anemia4, 5), sex10), race4), genetic disorders11), diabetes12), metabolic syndrome13), overweight14, 15), and obesity15). The impact of serum uric acid (UA) on renal prognosis in CKD patients has attracted recent attention16).
Nephrosclerosis is a major cause of CKD and subsequent end-stage renal disease (ESRD)17–19). A previous study found that 32% of patients with biopsy-proven nephrosclerosis developed ESRD during a 13-year follow-up period17). However, not all of the risk factors for progression of nephrosclerosis to ESRD have been identified due to lack of biopsy evidence. Regardless of the underlying etiology of CKD, the clinical risk factors of CKD progression described above may have significant predictive power for the long-term outcome of nephrosclerosis. Nevertheless, it remains difficult to predict the renal outcome of individual nephrosclerosis patients. Clinically, understanding each renal prognostic factor of the different primary diseases associated with CKD is important to treat the individual patient meticulously. The aim of the present study was to identify clinical prognostic factors for kidney disease progression and to elucidate the predictive value of hyperuricemia (HU) in patients with biopsy-proven nephrosclerosis.
Materials and Methods
Patient Selection
We examined the cases of 90 patients diagnosed with nephrosclerosis by kidney biopsy at Tokyo Woman's Medical University Hospital between February 1995 and November 2011. All kidney tissue specimens were obtained by percutaneous needle biopsy. Nephrosclerosis was diagnosed on the basis of renal pathology showing sclerosis of renal arterioles and small arteries20). The inclusion criteria in the present study were: (1) duration of follow-up ≥ 1 year (which excluded 27 patients), (2) absence of any other renal disease, including malignant nephrosclerosis (which excluded 16 patients), and (3) estimated glomerular filtration rate (eGFR) ≥ 15 mL/min/1.73 m2 (which excluded 2 patients). The remaining 45 patients who met these criteria were ultimately enrolled in the present study (Fig. 1). eGFR for patients was calculated as previously described21).
Fig. 1.
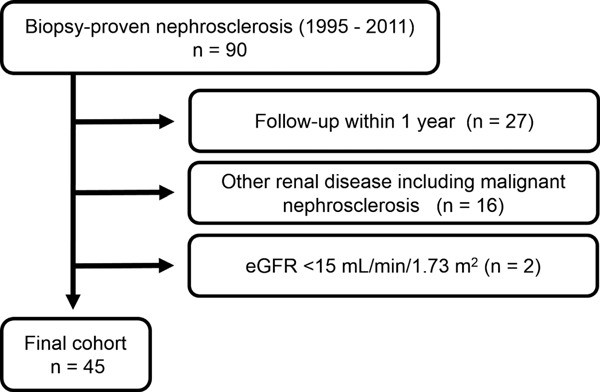
Flow chart of patient selection.
The 45 patients who did not meet the entry criteria were excluded from the 90 patients screened, and the other 45 patients were deemed eligible to enter this study.
The subjects' human rights and methods of protecting personal information were well considered. All the relevant and responsible staff adhered to the Helsinki Declaration (amended October 2013) and the Ethical Guidelines for Clinical Studies (revised July 31, 2008, referred to hereafter as the Clinical Studies Ethical Guidelines) in the execution of this study. This cohort study was approved by the Medical Ethics Committee of Tokyo Women's Medical University (#3667). Written informed consent for renal biopsy and use of clinical data at the time of the kidney biopsy, as well as subsequent histological data, was obtained from all patients.
Measurements of Covariates
The clinical parameters assessed at the time of the kidney biopsy (baseline) were as follows: age, gender, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), eGFR, serum creatinine, urea nitrogen, albumin, UA, total cholesterol (TC), LDL cholesterol (LDL-C), HDL cholesterol (HDL-C), triglyceride (TG) levels, and proteinuria (g/day). We also investigated concomitant drug use and comorbidities at the time of the kidney biopsy22, 23). The concomitant drugs were antihypertensive drugs, diuretics, and drugs for the treatment of hyperuricemia, dyslipidemia, and diabetes mellitus. Comorbidities are defined in the next section.
The clinical parameters assessed at the time of the 6-month follow-up examinations were as follows: SBP, DBP, BMI, eGFR, eGFR slope per year, and serum creatinine, albumin, UA, TC, LDL-C, HDL-C, and TG levels.
Definition of Comorbidities
Hypertension: Being treated with an oral antihypertensive agent, SBP ≥ 140 mmHg, DBP ≥ 90 mmHg
Hyperuricemia (HU): Being treated with an oral antihyperuricemic agent, serum UA level ≥ 6.0 mg/dL, serum UA level ≥ 7.0 mg/dL, or serum UA level ≥ 8.0 mg/dL
Hypercholesterolemia: Being treated with an oral antidyslipidemic agent, serum TC level ≥ 220 mg/dL, or serum LDL level ≥ 140 mg/dL
Hypertriglyceridemia: Being treated with an oral antidyslipidemic agent or a serum TG level ≥ 150 mg/dL
Diabetes mellitus: Being treated with an antidiabetic agent or a history of diagnosis with diabetes mellitus
Outcome Evaluation (Endpoint)
The outcome variable of interest was kidney disease progression, defined as a ≥ 50% decline in eGFR from baseline (≥ 50% eGFR decline) or ESRD requiring dialysis.
Statistical Analysis
Continuous variables are reported as the mean ± SD, and categorical variables are reported as percentages, unless otherwise stated. We compared participant outcomes by performing an unpaired t-test, chi-square test, or Fisher's exact test. The patients whose renal outcome was a ≥ 50% eGFR decline or ESRD were assigned to the poor outcome group. The patients whose renal outcome was not a ≥ 50% eGFR decline or ESRD were assigned to the benign outcome group. Data are expressed as the mean ± standard deviation (SD). Logistic-regression models were prepared to estimate the risk of ≥ 50% eGFR decline or ESRD associated with baseline and follow-up parameters, including clinical and laboratory variables.
Our principal goal was to determine whether the baseline serum UA value is a prognostic indicator in nephrosclerosis patients. The optimal cut-off serum UA value for discriminating ≥ 50% eGFR decline or ESRD during follow-up examination was determined by performing receiver operating characteristic (ROC) analyses. Patients were divided into an HU group (i.e., a group of patients being treated with an oral antihyperuricemic agent or whose UA level was ≥ 8.0 mg/dL) and a non-HU group (i.e., a group of patients being not treated with an oral administration antihyperuricemic agent or whose UA value was < 8.0 mg/dL). We compared participant characteristics of the two groups using the unpaired t -test, chi-square test, or Fisher's exact test. Prognostic variables for renal outcome were assessed by the univariate and multivariate Cox proportional hazards method. We included covariates for age, sex, BMI, eGFR, urine protein, and comorbidities, including HU, at baseline in Cox proportional hazards models. Variables with P-values of less than 0.1 in the univariate model were included in the multivariate model. The renal outcome, which was a ≥ 50% eGFR decline or ESRD and interval estimates between the HU group and the non-HU group, was calculated by the Kaplan–Meier method and evaluated by the log-rank test.
To further assess whether the associations were consistent across clinically matched subgroups, we fit propensity score-matched models that included several potential modifying variables (age, sex, eGFR, SBP, and BMI) and performed subgroup analyses of the groups. The caliper-matching method was used with a maximum tolerance level of 0.2. The standardized differences were calculated to assess the appropriateness of matching. The 95% confidence intervals (CIs) were calculated. P values < 0.05 were considered statistically significant. All statistical analyses were performed by using the JMP Pro ver.12.1.0 software program (SAS Institute, Cary, NC, USA).
Results
Patients
The 45 subjects consisted of 29 males and 16 females, and their mean age at the time of the kidney biopsy was 49.4 ± 12.5 years (range 16–67 years). The mean SBP was 136.1 ± 17.3 mmHg, DBP 83.4 ± 14.6 mmHg, BMI 25.7 ± 4.3 kg/m2, proteinuria 0.8 ± 0.8 g/day, and eGFR 54.6 ± 21.0 mL/min/1.73 m2 (Supplemental Table 1). The concomitant drug data showed that 38 were being treated with an antihypertensive agent, 14 with an antihyperuricemic agent, 16 with an antidyslipidemic agent, 22 with an antiplatelet agent, 2 with an antidiabetic agent, and 3 with a diuretic. The comorbidity data showed that 41 patients had hypertension; 18 had severe HU (UA ≥ 8 mg/dL or treatment with an antihyperuricemic agent); 25 had hypercholesterolemia; 24 had hypertriglyceridemia; and 10 had diabetes mellitus. The overall follow-up period was 6.8 ± 4.5 years. The rate of progression as measured by eGFR slope was −2.6 ± 3.1 mL/min/1.73 m2/year, and 11 patients had reached the endpoint (≥ 50% eGFR decline or ESRD) during the follow-up period.
Supplemental Table 1. Baseline and follow-up patient characteristics according to renal outcome.
| Variables | Total n = 45 |
Poor outcome n = 11 |
Benign outcome n = 34 |
P-value |
|---|---|---|---|---|
| Clinical Findings | ||||
| Age (years) | −49.4 ± 12.5 | −53.8 ± 9.0 | −48.0 ± 13.3 | −0.2 |
| Gender (Male; %) | −64.4 | −63.6 | −64.7 | −0.9 |
| SBP (mmHg) | −136.1 ± 17.3 | −137.1 ± 15.4 | −135.8 ± 18.0 | −0.8 |
| DBP (mmHg) | −83.4 ± 14.6 | −80.5 ± 12.2 | −84.3 ± 15.4 | −0.5 |
| BMI (kg/m2) | −25.7 ± 4.3 | −28.8 ± 4.3 | −24.7 ± 3.9 | −0.005 |
| Laboratory Findings | ||||
| Serum Albumin (g/dL) | −4.1 ± 0.4 | −4.1 ± 0.4 | −4.1 ± 0.4 | −0.9 |
| Blood Urea Nitrogen (mg/dL) | −19.3 ± 6.9 | −21.5 ± 7.8 | −18.6 ± 6.6 | −0.2 |
| Serum Creatinine (mg/dL) | −1.20 ± 0.40 | −1.23 ± 0.33 | −1.19 ± 0.43 | −0.8 |
| eGFR (mL/min/1.73 m2) | −54.6 ± 21.0 | −48.2 ± 15.0 | −56.6 ± 22.4 | −0.2 |
| Uric Acid (mg/dL) | −6.8 ± 1.4 | −7.7 ± 1.4 | −6.5 ± 1.3 | −0.02 |
| Total Cholesterol (mg/dL) | −218.4 ± 42.4 | −228.9 ± 50.9 | −214.9 ± 39.5 | −0.3 |
| LDL Cholesterol (mg/dL) | −124.5 ± 31.2 | −120.7 ± 29.6 | −125.8 ± 32.0 | −0.6 |
| HDL Cholesterol (mg/dL) | −51.1 ± 18.9 | −53.0 ± 27.7 | −50.5 ± 15.5 | −0.7 |
| Triglyceride (mg/dL) | −199.0 ± 116.7 | −249.7 ± 177.0 | −182.6 ± 116.7 | −0.1 |
| Proteinuria (g/day) | −0.84 ± 0.80 | −1.29 ± 0.97 | −0.69 ± 0.70 | −0.03 |
| Concomitant drugs | ||||
| Antihypertensive agents (%) | −84.4 | −90.9 | −82.4 | −0.5 |
| Antihyperuricemic agents (%) | −31.1 | −36.4 | −29.4 | −0.7 |
| Antidyslipidemic agents (%) | −35.6 | −54.5 | −29.4 | −0.1 |
| Antiplatelet agents (%) | −48.9 | −72.7 | −41.2 | −0.1 |
| Antidiabetic agents (%) | −4.4 | −9.1 | −2.9 | −0.4 |
| Diuretics (%) | −6.7 | −9.1 | −5.9 | −0.7 |
| Comorbidities | ||||
| Hypertension (%) | −91.1 | −90.9 | −91.2 | −1.0 |
| Hyperuricemia (UA ≧ 8 mg/dL) (%) | −40.0 | −63.6 | −32.4 | −0.1 |
| Hypercholesterolemia (%) | 55.6 | 72.7 | 50.0 | 0.2 |
| Hypertriglyceridemia (%) | 53.3 | 63.6 | 50.0 | 0.4 |
| Diabetes mellitus (%) | 22.2 | 36.4 | 17.6 | 0.2 |
| Clinical Findings (Follow-up Data) | ||||
| f/u SBP (mmHg) | 127.3 ± 13.0 | 133.0 ± 9.5 | 125.5 ± 13.5 | 0.1 |
| f/u DBP (mmHg) | 77.5 ± 8.3 | 80.4 ± 6.8 | 76.5 ± 8.6 | 0.2 |
| f/u BMI (kg/m2) | 25.6 ± 3.9 | 27.6 ± 3.6 | 25.0 ± 3.8 | 0.048 |
| Laboratory Findings (Follow-up Data) | ||||
| f/u Serum Albumin (g/dL) | 4.2 ± 0.3 | 4.1 ± 0.3 | 4.2 ± 0.3 | 0.5 |
| f/u Uric Acid (mg/dL) | 6.3 ± 1.0 | 6.7 ± 0.8 | 6.2 ± 1.0 | 0.2 |
| f/u Total Cholesterol (mg/dL) | 198.6 ± 31.4 | 207.0 ± 28.8 | 195.6 ± 32.2 | 0.3 |
| f/u LDL Cholesterol (mg/dL) | 108.9 ± 25.1 | 112.5 ± 20.2 | 108.0 ± 26.4 | 0.7 |
| f/u HDL Cholesterol (mg/dL) | 59.2 ± 20.5 | 60.2 ± 21.2 | 58.9 ± 20.7 | 0.9 |
| f/u Triglyceride (mg/dL) | 170.0 ± 68.2 | 197.6 ± 89.8 | 161.6 ± 59.4 | 0.1 |
| eGFR slope (mL/min/1.73 m2/year) | −2.6 ± 3.1 | −5.4 ± 2.6 | −1.8 ± 2.8 | 0.0004 |
Continuous values are expressed as means ± standard deviation. Count data are expressed as percentages. Abbreviation: n, number; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; HU (UA ≧ 8 mg/dL), Hyperuricemia (Uric Acid ≧ 8 mg/dL or with treatments); f/u, follow-up
Comparison of the Clinical and Pathological Findings between Groups According to Renal Outcome
The results of the comparison of clinical and laboratory findings at the time of the kidney biopsy in the two groups according to renal outcome are summarized in Supplemental Table 1. The following values were significantly higher in the poor outcome group than in the benign outcome group: BMI (28.8 ± 4.3 vs. 24.7 ± 3.9 kg/m2, P = 0.005), UA (7.7 ± 1.4 vs. 6.5 ± 1.3 mg/dL, P = 0.02), proteinuria (1.29 ± 0.97 vs. 0.69 ± 0.70 g/day, P = 0.03), f/u BMI (27.6 ± 3.6 vs. 25.0 ± 3.8 kg/m2, P < 0.05), and eGFR slope (−5.4 ± 2.6 vs. −1.8 ± 2.8 mL/min/1.73 m2/year, P = 0.0004). There were no significant differences between the groups in any of the other parameters.
Baseline Parameters and Serum Uric Acid Cut-Off Value as an Indicator of Kidney Disease Progression
We assessed the baseline and follow-up parameters of the nephrosclerosis patients. The logistic regression models showed no significant differences between the groups in any of the baseline parameters and follow-up parameters except BMI and serum UA level (Fig. 2). The baseline serum UA value yielded the highest odds for a ≥ 50% eGFR decline or ESRD (OR, 1.86; 95% CI, 1.12 to 3.45). Based on these results, we decided to use baseline parameters to predict kidney disease progression. We performed ROC analyses to identify the optimal UA cut-off value for discriminating a ≥ 50% eGFR decline or ESRD during the follow-up examination. The results of the ROC analyses showed that the optimal UA cut-off value was 8.0 mg/dL (AUC = 0.74, sensitivity = 54.6%, specificity = 85.3%, Fig. 3).
Fig. 2.
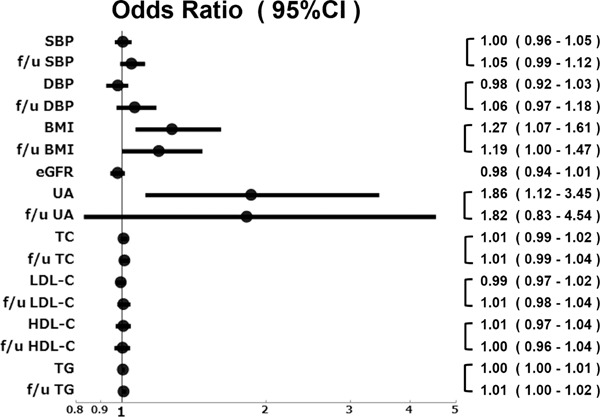
Odds ratio for a decline in eGFR by ≥ 50% from baseline or end-stage renal disease during the follow-up examination period.
Fig. 3.
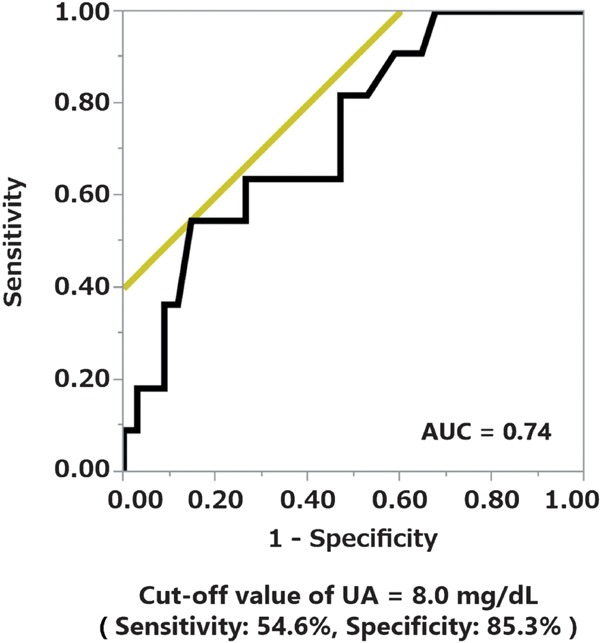
Receiver operating characteristic analysis to identify the optimal serum uric acid cut-off value for predicting an eGFR decline by ≥ 50% from baseline or end-stage renal disease during the follow-up examination period.
Hyperuricemia as a Prognostic Indicator in Nephrosclerosis Patients
To determine whether severe HU (UA ≥ 8.0 mg/dL) at the time of the renal biopsy was an independent predictor of a decline in renal function, we performed univariate and multivariate regression analyses based on the Cox hazard model for associations between the clinical findings and a ≥ 50% eGFR decline or ESRD during the follow-up (Table 1). The results showed that HU [Hazard Ratio (HR) = 18.2, P = 0.01] and BMI (HR = 1.30, P = 0.03) were significantly associated with a ≥ 50% eGFR decline or ESRD.
Table 1. Univariate and multivariate analysis of risk factors associated with ≥ 50% eGFR decline or ESRD (Total cohort n = 45).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Age (1 year increase) | 1.04 (0.99–1.11) | 0.2 | – | – |
| Male (vs. female) | 0.74 (0.21–2.93) | 0.7 | – | – |
| BMI (1 kg/m2 increase) | 1.22 (1.07–1.41) | 0.003 | 1.30 (1.01–1.68) | 0.03 |
| eGFR (1 mL/min/1.73 m2 increase) | 0.98 (0.95–1.02) | 0.3 | – | – |
| U-Prot (1 g/day increase) | 2.24 (1.19–4.10) | 0.009 | 2.07 (0.85–5.75) | 0.1 |
| Hypertension (vs. no) | 1.83 (0.32–35.5) | 0.6 | – | – |
| Hypercholesterolemia (vs. no) | 6.68 (1.59–45.8) | 0.02 | 2.42 (0.52–18.9) | 0.3 |
| Hypertriglyceridemia (vs. no) | 2.50 (0.68–11.8) | 0.2 | – | – |
| Diabetes mellitus (vs. no) | 1.04 (0.27–3.50) | 1.0 | – | – |
| Hyperuricemia (UA ≧ 8 mg/dL) (vs. no) | 5.81 (1.57–27.6) | 0.01 | 18.2 (2.68–278.8) | 0.01 |
Abbreviation: CI = confidence interval; BMI, body mass index; eGFR, estimated glomerular filtration rate; U-Prot, urinary protein excretion; vs, versus
Comparison between the Clinical Findings in Groups According to Serum Uric Acid Levels in the Total Cohort
We compared the clinical characteristics of two groups according to the UA value at the time of the kidney biopsy (Table 2). The serum UA levels (7.8 ± 1.3 vs. 6.5 ± 1.1 mg/dL, P = < 0.0001), blood urea nitrogen levels (22.5 ± 7.4 vs. 17.2 ± 5.8 mg/dL, P = 0.01), and serum creatinine levels (1.35 ± 0.40 vs. 1.10 ± 0.37 mg/dL, P = 0.04) were significantly higher in the HU group than in the non-HU group, and eGFR (46.4 ± 17.9 vs. 60.0 ± 21.5 mL/min/1.73 m2, P = 0.03) was significantly lower in the HU group than in the non-HU group. The percentages of patients being treated with an antihyperuricemic agent (77.8% vs. 0.0%, P < 0.0001) and a diuretic (16.7% vs. 0.0%, P = 0.03) were significantly higher in the HU group than non-HU group.
Table 2. Patient characteristics divided by uric acid status at the kidney biopsy (Total cohort n = 45).
| Variables | Total cohort |
||||
|---|---|---|---|---|---|
| Total n = 45 |
HU (UA ≧ 8 mg/dL) n = 18 |
Non HU (UA < 8 mg/dL) n = 27 |
P-value | Standardized Differences | |
| Clinical Findings | |||||
| Age (years) | 49.4 ± 12.5 | 50.4 ± 13.2 | 48.8 ± 12.2 | 0.7 | 0.126 |
| Gender (Male; %) | 64.4 | 61.1 | 66.7 | 0.7 | 0.117 |
| SBP (mmHg) | 136.1 ± 17.3 | 133.8 ± 17.5 | 137.7 ± 17.2 | 0.5 | 0.225 |
| DBP (mmHg) | 83.4 ± 14.6 | 80.5 ± 10.9 | 85.3 ± 16.6 | 0.4 | 0.342 |
| BMI (kg/m2) | 25.7 ± 4.3 | 26.4 ± 4.0 | 25.3 ± 4.6 | 0.4 | 0.255 |
| Laboratory Findings | |||||
| Serum Albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.4 | 1.0 | 0.000 |
| Blood Urea Nitrogen (mg/dL) | 19.3 ± 6.9 | 22.5 ± 7.4 | 17.2 ± 5.8 | 0.01 | 0.797 |
| Serum Creatinine (mg/dL) | 1.20 ± 0.40 | 1.35 ± 0.40 | 1.10 ± 0.37 | 0.04 | 0.649 |
| eGFR (mL/min/1.73 m2) | 54.6 ± 21.0 | 46.4 ± 17.9 | 60.0 ± 21.5 | 0.03 | 0.687 |
| Uric Acid (mg/dL) | 6.8 ± 1.4 | 7.8 ± 1.3 | 6.1 ± 1.1 | < 0.0001 | 1.412 |
| Total Cholesterol (mg/dL) | 218.4 ± 42.4 | 223.2 ± 36.1 | 215.1 ± 46.5 | 0.5 | 0.195 |
| LDL Cholesterol (mg/dL) | 124.5 ± 31.2 | 126.7 ± 25.8 | 123.0 ± 34.7 | 0.2 | 0.121 |
| HDL Cholesterol (mg/dL) | 51.1 ± 18.9 | 54.1 ± 24.6 | 49.1 ± 14.1 | 0.4 | 0.249 |
| Triglyceride (mg/dL) | 199.0 ± 116.7 | 218.5 ± 94.1 | 186.0 ± 129.7 | 0.4 | 0.287 |
| Proteinuria (g/day) | 0.84 ± 0.80 | 0.94 ± 0.69 | 0.77 ± 0.88 | 0.5 | 0.215 |
| Concomitant drugs | |||||
| Antihypertensive agent (%) | 84.4 | 94.4 | 77.8 | 0.1 | 0.494 |
| Antihyperuricemic agents (%) | 31.1 | 77.8 | 0.0 | < 0.0001 | 2.647 |
| Antidyslipidemic agents (%) | 35.6 | 50.0 | 25.9 | 0.1 | 0.513 |
| Antiplatelet agent (%) | 48.9 | 61.1 | 40.7 | 0.2 | 0.417 |
| Antidiabetic agents (%) | 4.4 | 5.6 | 3.7 | 0.8 | 0.090 |
| Diuretics (%) | 6.7 | 16.7 | 0.0 | 0.03 | 0.633 |
| Comorbidities | |||||
| Hypertension (%) | 91.1 | 94.4 | 88.9 | 0.5 | 0.200 |
| HU (UA ≧ 8 mg/dL) (%) | 40.0 | 100.0 | 0.0 | < 0.0001 | – |
| Hypercholesterolemia (%) | 55.6 | 66.7 | 48.1 | 0.2 | 0.383 |
| Hypertriglyceridemia (%) | 53.3 | 66.7 | 44.4 | 0.1 | 0.461 |
| Diabetes mellitus (%) | 22.2 | 22.2 | 22.2 | 1.0 | 0.000 |
| Clinical Findings (Follow-up Data) | |||||
| f/u SBP (mmHg) | 127.3 ± 13.0 | 127.8 ± 10.9 | 126.9 ± 14.4 | 0.8 | 0.070 |
| f/u DBP (mmHg) | 77.5 ± 8.3 | 77.7 ± 6.5 | 77.3 ± 9.4 | 0.9 | 0.049 |
| f/u BMI (kg/m2) | 25.6 ± 3.9 | 25.9 ± 4.3 | 25.5 ± 3.6 | 0.4 | 0.101 |
| Laborator Findings (Follow-up Data) | |||||
| f/u Serum Albumin (g/dL) | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.3 | 0.8 | 0.000 |
| f/u Uric Acid (mg/dL) | 6.3 ± 1.0 | 6.6 ± 1.1 | 6.2 ± 0.9 | 0.1 | 0.398 |
| f/u Total Cholesterol (mg/dL) | 198.6 ± 31.4 | 205.0 ± 35.9 | 194.1 ± 27.8 | 0.3 | 0.339 |
| f/u LDL Cholesterol (mg/dL) | 108.9 ± 25.1 | 111.0 ± 23.7 | 107.5 ± 26.5 | 0.7 | 0.139 |
| f/u HDL Cholesterol (mg/dL) | 59.2 ± 20.5 | 59.8 ± 20.5 | 58.7 ± 21.1 | 0.9 | 0.053 |
| f/u Triglyceride (mg/dL) | 170.0 ± 68.2 | 180.5 ± 72.2 | 162.4 ± 65.6 | 0.4 | 0.262 |
| Clinical Findings | |||||
| Age (years) | 49.9 ± 12.1 | 48.3 ± 13.3 | 51.5 ± 11.0 | 0.5 | 0.262 |
| Gender (Male; %) | 66.7 | 66.7 | 66.7 | 1.0 | 0.000 |
| SBP (mmHg) | 135.7 ± 18.4 | 135.2 ± 18.0 | 136.3 ± 19.5 | 0.9 | 0.059 |
| DBP (mmHg) | 82.5 ± 14.9 | 81.1 ± 11.1 | 83.9 ± 18.3 | 0.6 | 0.185 |
| BMI (kg/m2) | 26.4 ± 4.8 | 26.6 ± 4.3 | 26.2 ± 5.5 | 0.8 | 0.081 |
| Laboratory Findings | |||||
| Serum Albumin (g/dL) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.4 | 0.8 | 0.000 |
| Blood Urea Nitrogen (mg/dL) | 19.9 ± 7.0 | 21.1 ± 7.1 | 18.7 ± 6.9 | 0.3 | 0.343 |
| Serum Creatinine (mg/dL) | 1.27 ± 0.38 | 1.27 ± 0.39 | 1.27 ± 0.38 | 1.0 | 0.000 |
| eGFR (mL/min/1.73 m2) | 49.9 ± 18.1 | 50.0 ± 17.1 | 49.9 ± 19.7 | 1.0 | 0.005 |
| Uric Acid (mg/dL) | 7.1 ± 1.3 | 7.8 ± 1.2 | 6.5 ± 1.1 | 0.004 | 1.129 |
| Total Cholesterol (mg/dL) | 219.0 ± 42.3 | 222.7 ± 31.8 | 215.2 ± 51.6 | 0.6 | 0.175 |
| LDL Cholesterol (mg/dL) | 123.7 ± 29.3 | 125.0 ± 25.9 | 122.3 ± 33.3 | 0.8 | 0.091 |
| HDL Cholesterol (mg/dL) | 50.7 ± 20.0 | 56.0 ± 25.8 | 45.3 ± 10.1 | 0.1 | 0.546 |
| Triglyceride (mg/dL) | 213.4 ± 129.9 | 216.3 ± 93.0 | 210.5 ± 162.1 | 0.9 | 0.044 |
| Proteinuria (g/day) | 0.88 ± 0.86 | 0.88 ± 0.69 | 0.89 ± 1.02 | 1.0 | 0.011 |
| Concomitant drugs | |||||
| Antihypertensive agent (%) | 86.7 | 100.0 | 73.3 | 0.03 | 0.854 |
| Antihyperuricemic agents (%) | 36.7 | 73.3 | 0.0 | < 0.0001 | 2.343 |
| Antidyslipidemic agents (%) | 36.7 | 46.7 | 26.7 | 0.3 | 0.424 |
| Antiplatelet agent (%) | 46.7 | 53.3 | 40.0 | 0.5 | 0.269 |
| Antidiabetic agents (%) | 6.7 | 6.7 | 6.7 | 1.0 | 0.000 |
| Diuretics (%) | 3.3 | 6.7 | 0.0 | 0.3 | 0.379 |
| Comorbidities | |||||
| Hypertension (%) | 93.3 | 100.0 | 86.7 | 0.1 | 0.554 |
| HU (UA ≧ 8 mg/dL) (%) | 50.0 | 100.0 | 0.0 | < 0.0001 | – |
| Hypercholesterolemia (%) | 53.3 | 66.7 | 40.0 | 0.1 | 0.555 |
| Hypertriglyceridemia (%) | 58.6 | 73.3 | 42.9 | 0.1 | 0.648 |
| Diabetes mellitus (%) | 26.7 | 20.0 | 33.3 | 0.4 | 0.304 |
| Clinical Findings (Follow-up Data) | |||||
| f/u SBP (mmHg) | 128.5 ± 13.3 | 127.1 ± 11.4 | 130.2 ± 15.5 | 0.6 | 0.228 |
| f/u DBP (mmHg) | 78.1 ± 7.8 | 77.9 ± 6.6 | 78.3 ± 9.2 | 0.9 | 0.050 |
| f/u BMI (kg/m2) | 26.0 ± 4.4 | 26.1 ± 4.7 | 26.0 ± 4.3 | 0.9 | 0.022 |
| Laborator Findings (Follow-up Data) | |||||
| f/u Serum Albumin (g/dL) | 4.3 ± 0.3 | 4.2 ± 0.4 | 4.3 ± 0.2 | 0.6 | 0.316 |
| f/u Uric Acid (mg/dL) | 6.5 ± 0.8 | 6.7 ± 0.8 | 6.3 ± 0.8 | 0.1 | 0.500 |
| f/u Total Cholesterol (mg/dL) | 197.1 ± 34.3 | 202.0 ± 36.6 | 191.3 ± 32.1 | 0.5 | 0.311 |
| f/u LDL Cholesterol (mg/dL) | 108.2 ± 24.9 | 108.3 ± 22.2 | 108.2 ± 28.6 | 1.0 | 0.004 |
| f/u HDL Cholesterol (mg/dL) | 58.2 ± 17.7 | 60.6 ± 21.7 | 55.3 ± 11.9 | 0.5 | 0.303 |
| f/u Triglyceride (mg/dL) | 177.9 ± 73.8 | 177.5 ± 73.8 | 178.3 ± 76.8 | 1.0 | 0.011 |
Continuous values are expressed as means ± standard deviation. Count data are expressed as percentages. Abbreviation: HU (UA ≧ 8 mg/dL), Hyperuricemia (Uric Acid ≧ 8 mg/dL or with treatments); Non HU (UA < 8 mg/dL), Non Hyperuricemia (Uric Acid < 8 mg/dL or without treatments); n, number; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; f/u, follow-up
We performed a Kaplan-Meier analysis to assess kidney survival with a ≥ 50% eGFR decline or ESRD as the end-point. According to the kidney survival curves, the kidney survival rate of the nephrosclerosis patients in the HU group was significantly lower than in the non-HU group (Fig. 4A). At the 10-year follow-up examination, at least a 50% decrease in eGFR value was observed in 80% of the HU group (log rank, P = 0.005).
Fig. 4A.
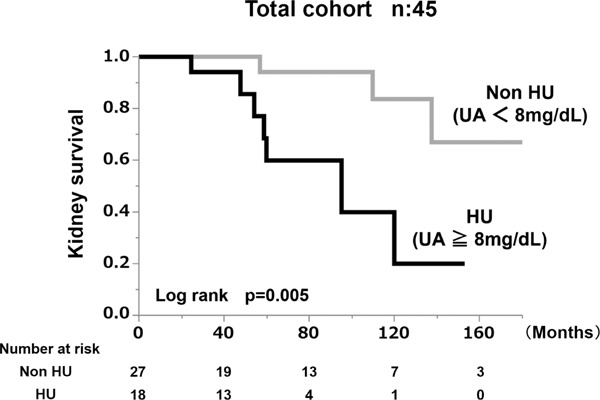
Kidney survival rate of the group with serum uric acid levels > 8 mg/dL and the group with serum uric acid levels < 8 mg/dL in the total cohort.
Comparison between the Clinical and Laboratory Findings in Groups According to Serum Uric Acid Levels in the Propensity Score-Matched Cohort
Since the serum UA levels may have been affected by age, sex, BMI, SBP, and kidney function, we created a propensity score-matched cohort of HU patients and non-HU patients. Comparisons between the clinical and laboratory findings at the time of kidney biopsy in the two groups are summarized in Table 2. There were no significant differences between the propensity score-matched groups in any of the parameters except the parameters associated with UA level and BMI value. The serum UA level in the HU group (7.8 ± 1.2 mg/dL) was significantly higher than in the non-HU group (6.5 ± 1.1 mg/dL, P = 0.004). The percentage of patients being treated with an antihyperuricemic agent (73.3% vs. 0.0%, P < 0.0001) was higher in the HU group than in the non-HU group. In this propensity score-matched cohort, we also performed univariate and multivariate regression analyses based on the Cox hazard model for associations between the clinical findings and a ≥ 50% eGFR decline or ESRD during the follow-up (Table 3). As is the case with the total cohort, HU (HR = 17.7, P = 0.02) was significantly associated with a ≥ 50% eGFR decline or ESRD.
Table 3. Univariate and multivariate analysis of risk factors associated with ≥ 50% eGFR decline or ESRD (Propensity score matched cohort n = 30).
| Variables | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Hazard Ratio (95% CI) |
P-value | Hazard Ratio (95% CI) |
P-value | |
| Age (1 year increase) | 1.03 (0.98–1.10) | 0.2 | – | – |
| Male (vs. female) | 0.60 (0.17–2.26) | 0.4 | – | – |
| BMI (1 kg/m2 increase) | 1.19 (1.04–1.36) | 0.01 | 1.25 (0.96–1.62) | 0.1 |
| eGFR (1 mL/min/1.73 m2 increase) | 0.99 (0.96–1.03) | 0.7 | – | – |
| U-Prot (1 g/day increase) | 1.91 (1.00–3.52) | 0.04 | 2.20 (0.85–6.70) | 0.1 |
| Hypertension (vs. no) | 1.53 (0.25–30.4) | 0.7 | – | – |
| Hypercholesterolemia (vs. no) | 6.49 (1.57–44.0) | 0.02 | 3.08 (0.61–27.7) | 0.2 |
| Hypertriglyceridemia (vs. no) | 3.39 (0.90–16.3) | 0.09 | 0.22 (0.03–1.75) | 0.1 |
| Diabetes mellitus (vs. no) | 1.05 (0.27–3.50) | 0.9 | – | – |
| Hyperuricemia (UA ≧ 8 mg/dL) (vs. no) | 4.14 (1.10–19.9) | 0.04 | 17.7 (2.26–307.4) | 0.02 |
Abbreviation: CI = confidence interval; BMI, body mass index; eGFR, estimated glomerular filtration rate; U-Prot, urinary protein excretion; vs, versus
Lastly, we performed a Kaplan–Meier analysis to assess kidney survival with a ≥ 50% eGFR decline or ESRD as the end-point. According to the kidney survival curves, the kidney survival rate of the HU group of nephrosclerosis patients was significantly lower than in the non-HU group (Fig. 4B). At the 10-year follow-up examination, there was at least a 50% decrease in eGFR value or ESRD in 81.4% of the HU patients (log rank, P = 0.03).
Fig. 4B.
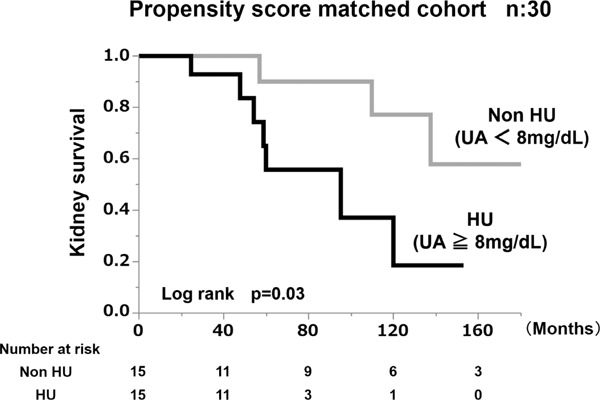
Kidney survival rate of the group with serum uric acid value > 8 mg/dL and the group with serum uric acid < 8 mg/dL in the propensity score-matched cohort.
Discussion
Several problems need to be solved in the research on nephrosclerosis. The first problem is that the diagnosis of nephrosclerosis is generally made on the basis of the characteristic clinical features, and confirmation by renal biopsy is rarely indicated. As a result, the pathophysiology of nephrosclerosis has not been fully elucidated.
The second problem is that there are several different opinions regarding the pathological diagnosis of nephrosclerosis20, 24–27). Because arterial and arteriolar sclerosis is generally accompanied by global glomerulosclerosis and interstitial fibrosis, glomerular and interstitial lesions tend to be included among the diagnostic criteria for nephrosclerosis, but their inclusion may cause confusion influenced by aging, primary glomerular disease, or primary interstitial disease. We chose simple diagnostic criteria for nephrosclerosis focusing on initiators of kidney injury. In the present study, nephrosclerosis was defined as the renal pathology associated with sclerosis of renal arterioles and small arteries from the pathophysiological point20).
The third problem, which is the main topic of this study, is that arterial/arteriolar nephrosclerosis can be influenced by various causes and multiple risk factors. Arterial/arteriolar nephrosclerosis is usually associated with hypertension20, 28). Hypertension is thought to be both a cause of arterial/arteriolar nephrosclerosis and a renal prognostic factor29). On the other hand, Hsu doubted the conventional theory that non-malignant hypertension is a common cause of CKD and ESRD, because there is little evidence30), and Tracy et al. reported finding that arterial/arteriolar nephrosclerosis precedes the development of hypertension31). Furthermore, renal vascular lesions are sometimes observed in the absence of hypertension in animal models32).
Based on the definition of nephrosclerosis as renal pathology associated with sclerosis of renal arterioles and small arteries20), we postulate that the etiology of arterial/arteriolar nephrosclerosis is multifactorial. In addition to hypertension33), other clinical factors, including aging26, 34), systemic atherosclerosis35), systemic vasculitis36, 37), obesity38), and diabetes mellitus39), may contribute to development of the pathological features of arterial/arteriolar nephrosclerosis. In the same manner, we also consider that arterial/arteriolar nephrosclerosis can have multiple renal risk factors. Since there is little evidence regarding prognostic risk factors of biopsy-proven nephrosclerosis, we attempted to identify clinical prognostic risk factors for biopsy-proven arterial/arteriolar nephrosclerosis, focusing especially on the UA level.
The results of our multivariate analysis of the Cox proportional hazards model showed that HU (P = 0.01) and BMI (P = 0.03) were significantly associated with a ≥ 50% eGFR decline or ESRD in the patients with biopsy-proven arterial/arteriolar nephrosclerosis (Table 1). Since the blood pressure of our cohort was relatively well controlled (mean SBP/DBP = 136/83 mmHg) with antihypertensive agents (84.4%), hypertension was not a significant prognostic risk factor. Rather, HU was proved to be the most significant prognostic risk factor of arterial/arteriolar nephrosclerosis in the blood pressure-controlled cohort. Furthermore, the Kaplan–Meier analysis showed that the kidney survival rate of the biopsy-proven arterial/ arteriolar nephrosclerosis patients with HU was significantly lower than that of arterial/arteriolar nephrosclerosis patients without HU in a propensity-matched cohort (Fig. 4B; P = 0.03).
HU has been found to be a risk factor for development of hypertension40–43), and the results of several epidemiologic studies have indicated the existence of an association between the development of CKD and HU44, 45). HU has been found to independently predict the progression of kidney disease in diabetic nephropathy46, 47), IgA nephropathy48–51), chronic allograft nephropathy52), and CKD45, 53, 54). However, although HU has been associated with the presence of kidney arteriolar sclerosis33, 50, 55, 56) in CKD patients, there is little evidence of an association between UA and disease progression of biopsy-proven nephrosclerosis. In animal models, HU has been found to induce systemic hypertension and afferent arteriolar sclerosis57, 58). Although the precise mechanism of the nephrotoxicity of HU has not been fully elucidated, recent data showed a direct harmful effect of UA on endothelial cells59) and smooth muscle cells60). In humans, it has been reported that serum uric acid level is independently associated with an elevated carotid intimamedia thickness61). Therefore, UA injures renal vessels, which are the major lesion of arterial/arteriolar nephrosclerosis.
Conclusion
In conclusion, the results obtained by using a propensity score-matched cohort in the present study showed that HU is a clinical predictive marker for progression of biopsy-proven arterial/arteriolar nephrosclerosis. Since treatment of hypertension has recently become widespread, treatment for HU will become more important in patients with arterial/arteriolar nephrosclerosis.
Competing Interests
The authors have declared that no competing interests exist.
References
- 1). Nenov VD, Taal MW, Sakharova OV, Brenner BM: Multi-hit nature of chronic renal disease. Current Opinion in Nephrology and Hypertension 2000; 9: 85-97 [DOI] [PubMed] [Google Scholar]
- 2). Taal MW, Brenner BM: Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney International 2006; 70: 1694-1705 [DOI] [PubMed] [Google Scholar]
- 3). Yu HT: Progression of chronic renal failure. Arch Intern Med 2003; 163: 1417-1429 [DOI] [PubMed] [Google Scholar]
- 4). Hunsicker LG, Adler S, Caggiula A, England BK, Greene T, Kusek JW, Rogers NL, Teschan PE: Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int 1997; 51: 1908-1919 [DOI] [PubMed] [Google Scholar]
- 5). Norris KC, Greene T, Kopple J, Lea J, Lewis J, Lipkowitz M, Miller P, Richardson A, Rostand S, Wang X, Appel LJ: Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J Am Soc Nephrol 2006; 17: 2928-2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Lea J, Greene T, Hebert L, Lipkowitz M, Massry S, Middleton J, Rostand SG, Miller E, Smith W, Bakris GL: The relationship between magnitude of proteinuria reduction and risk of end-stage renal disease: results of the African American study of kidney disease and hypertension. Arch Intern Med 2005; 165: 947-953 [DOI] [PubMed] [Google Scholar]
- 7). Reynolds K, Gu D, Muntner P, Kusek JW, Chen J, Wu X, Duan X, Chen CS, Klag MJ, Whelton PK, He J: A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol 2007; 18: 1928-1935 [DOI] [PubMed] [Google Scholar]
- 8). Regalado M, Yang S, Wesson DE: Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. Am J Kidney Dis 2000; 35: 687-694 [DOI] [PubMed] [Google Scholar]
- 9). Orth SR, Stockmann A, Conradt C, Ritz E, Ferro M, Kreusser W, Piccoli G, Rambausek M, Roccatello D, Schafer K, Sieberth HG, Wanner C, Watschinger B, Zucchelli P: Smoking as a risk factor for end-stage renal failure in men with primary renal disease. Kidney Int 1998; 54: 926-931 [DOI] [PubMed] [Google Scholar]
- 10). Coggins CH, Breyer Lewis J, Caggiula AW, Castaldo LS, Klahr S, Wang SR: Differences between women and men with chronic renal disease. Nephrol Dial Transplant 1998; 13: 1430-1437 [DOI] [PubMed] [Google Scholar]
- 11). Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr, Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ: APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 2013; 369: 2183-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853 [PubMed] [Google Scholar]
- 13). Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD: Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2011; 6: 2364-2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Kataoka H, Ohara M, Shibui K, Sato M, Suzuki T, Amemiya N, Watanabe Y, Honda K, Mochizuki T, Nitta K: Overweight and obesity accelerate the progression of IgA nephropathy: prognostic utility of a combination of BMI and histopathological parameters. Clinical and Experimental Nephrology 2012; 16: 706-712 [DOI] [PubMed] [Google Scholar]
- 15). Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Annals of Internal Medicine 2006; 144: 21-28 [DOI] [PubMed] [Google Scholar]
- 16). Goicoechea M, Garcia de Vinuesa S, Verdalles U, Verde E, Macias N, Santos A, Perez de Jose A, Cedeno S, Linares T, Luno J: Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 2015; 65: 543-549 [DOI] [PubMed] [Google Scholar]
- 17). Vikse BE, Aasarod K, Bostad L, Iversen BM: Clinical prognostic factors in biopsy-proven benign nephrosclerosis. Nephrology Dialysis Transplantation 2003; 18: 517-523 [DOI] [PubMed] [Google Scholar]
- 18). Dasgupta I, Porter C, Innes A, Burden R: ‘Benign’ hypertensive nephrosclerosis. Qjm-an International Journal of Medicine 2007; 100: 113-119 [DOI] [PubMed] [Google Scholar]
- 19). Sumida K, Hoshino J, Ueno T, Mise K, Hayami N, Suwabe T, Kawada M, Imafuku A, Hiramatsu R, Hasegawa E, Yamanouchi M, Sawa N, Fujii T, Ohashi K, Takaichi K, Ubara Y: Effect of Proteinuria and Glomerular Filtration Rate on Renal Outcome in Patients with Biopsy-Proven Benign Nephrosclerosis. PLoS One 2016; 11: e0147690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kumar V, Abbas AK, Fausto N: Robbins and Cotran pathologic basis of disease. 9th ed, ed by Kumar V, Abbas AK, Fausto N, pp938-939, Saunders, Philadelphia, PA: 2015 [Google Scholar]
- 21). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A: Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 22). Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA: Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci 2007; 62: 281-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Ording AG, Sorensen HT: Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol 2013; 5: 199-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Tracy RE, Ishii T: What is ‘nephrosclerosis’? lessons from the US, Japan, and Mexico. Nephrol Dial Transplant 2000; 15: 1357-1366 [DOI] [PubMed] [Google Scholar]
- 25). Meyrier A: Nephrosclerosis: update on a centenarian. Nephrol Dial Transplant 2015; 30: 1833-1841 [DOI] [PubMed] [Google Scholar]
- 26). Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD: The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 2010; 152: 561-567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Kopp JB: Rethinking hypertensive kidney disease: arterionephrosclerosis as a genetic, metabolic, and inflammatory disorder. Curr Opin Nephrol Hypertens 2013; 22: 266-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Moritz AR, Oldt MR: Arteriolar Sclerosis in Hypertensive and Non-Hypertensive Individuals. Am J Pathol 1937; 13: 679-728 [PMC free article] [PubMed] [Google Scholar]
- 29). Luke RG: Hypertensive nephrosclerosis: pathogenesis and prevalence. Essential hypertension is an important cause of end-stage renal disease. Nephrol Dial Transplant 1999; 14: 2271-2278 [DOI] [PubMed] [Google Scholar]
- 30). Hsu CY: Does non-malignant hypertension cause renal insufficiency? Evidence-based perspective. Curr Opin Nephrol Hypertens 2002; 11: 267-272 [DOI] [PubMed] [Google Scholar]
- 31). Tracy RE: Renovasculopathies of hypertension and the rise of blood pressure with age in blacks and whites. Semin Nephrol 1996; 16: 126-133 [PubMed] [Google Scholar]
- 32). Meyrier A, Simon P: Nephroangiosclerosis and hypertension: things are not as simple as you might think. Nephrol Dial Transplant 1996; 11: 2116-2120 [DOI] [PubMed] [Google Scholar]
- 33). Kubo M, Kiyohara Y, Kato I, Tanizaki Y, Katafuchi R, Hirakata H, Okuda S, Tsuneyoshi M, Sueishi K, Fujishima M, Iida M: Risk factors for renal glomerular and vascular changes in an autopsy-based population survey: the Hisayama study. Kidney Int 2003; 63: 1508-1515 [DOI] [PubMed] [Google Scholar]
- 34). Erten S, Gungor O, Sen S, Ozbek SS, Kircelli F, Hoscoskun C, Toz H, Asci G, Basci A, Ok E: Nephrosclerosis and carotid atherosclerosis: lessons from kidney donor histology. Nephrology (Carlton) 2011; 16: 720-724 [DOI] [PubMed] [Google Scholar]
- 35). Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 1987; 31: 1153-1159 [DOI] [PubMed] [Google Scholar]
- 36). Boers M, Croonen AM, Dijkmans BA, Breedveld FC, Eulderink F, Cats A, Weening JJ: Renal findings in rheumatoid arthritis: clinical aspects of 132 necropsies. Ann Rheum Dis 1987; 46: 658-663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Katz SM, Korn S, Umlas SL, DeHoratius RJ: Renal vascular lesions in systemic lupus erythematosus. Ann Clin Lab Sci 1990; 20: 147-153 [PubMed] [Google Scholar]
- 38). Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD: Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 2001; 59: 1498-1509 [DOI] [PubMed] [Google Scholar]
- 39). Shimizu M, Furuichi K, Yokoyama H, Toyama T, Iwata Y, Sakai N, Kaneko S, Wada T: Kidney lesions in diabetic patients with normoalbuminuric renal insufficiency. Clin Exp Nephrol 2014; 18: 305-312 [DOI] [PubMed] [Google Scholar]
- 40). Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH: Resurrection of uric acid as a causal risk factor in essential hypertension. In: Hypertension. United States; 2005: 18-20 [DOI] [PubMed] [Google Scholar]
- 41). Sundstrom J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS: Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45: 28-33 [DOI] [PubMed] [Google Scholar]
- 42). Forman JP, Choi H, Curhan GC: Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol 2007; 18: 287-292 [DOI] [PubMed] [Google Scholar]
- 43). Nagahama K, Inoue T, Iseki K, Touma T, Kinjo K, Ohya Y, Takishita S: Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27: 835-841 [DOI] [PubMed] [Google Scholar]
- 44). Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS: Uric acid and incident kidney disease in the community. J Am Soc Nephrol 2008; 19: 1204-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45). Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M: Association of uric acid with change in kidney function in healthy normotensive individuals. Am J Kidney Dis 2010; 56: 264-272 [DOI] [PubMed] [Google Scholar]
- 46). Zoppini G, Targher G, Chonchol M, Ortalda V, Abaterusso C, Pichiri I, Negri C, Bonora E: Serum uric acid levels and incident chronic kidney disease in patients with type 2 diabetes and preserved kidney function. Diabetes Care 2012; 35: 99-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Ficociello LH, Rosolowsky ET, Niewczas MA, Maselli NJ, Weinberg JM, Aschengrau A, Eckfeldt JH, Stanton RC, Galecki AT, Doria A, Warram JH, Krolewski AS: High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 2010; 33: 1337-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M: Serum uric acid and renal prognosis in patients with IgA nephropathy. Nephron 2001; 87: 333-339 [DOI] [PubMed] [Google Scholar]
- 49). Syrjanen J, Mustonen J, Pasternack A: Hypertriglyceridaemia and hyperuricaemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 2000; 15: 34-42 [DOI] [PubMed] [Google Scholar]
- 50). Wu J, Chen X, Xie Y, Yamanaka N, Shi S, Wu D, Liu S, Cai G: Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant 2005; 20: 719-727 [DOI] [PubMed] [Google Scholar]
- 51). Moriyama T, Itabashi M, Takei T, Kataoka H, Sato M, Shimizu A, Iwabuchi Y, Nishida M, Uchida K, Nitta K: High uric acid level is a risk factor for progression of IgA nephropathy with chronic kidney disease stage G3a. J Nephrol 2015; 28: 451-456 [DOI] [PubMed] [Google Scholar]
- 52). Akalin E, Ganeshan SV, Winston J, Muntner P: Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation 2008; 86: 652-658 [DOI] [PubMed] [Google Scholar]
- 53). Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S: Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004; 44: 642-650 [PubMed] [Google Scholar]
- 54). Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS: Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 2009; 169: 342-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Kohagura K, Kochi M, Miyagi T, Kinjyo T, Maehara Y, Nagahama K, Sakima A, Iseki K, Ohya Y: An association between uric acid levels and renal arteriolopathy in chronic kidney disease: a biopsy-based study. Hypertens Res 2013; 36: 43-49 [DOI] [PubMed] [Google Scholar]
- 56). Kojima C, Takei T, Ogawa T, Nitta K: Serum complement C3 predicts renal arteriolosclerosis in non-diabetic chronic kidney disease. J Atheroscler Thromb 2012; 19: 854-861 [DOI] [PubMed] [Google Scholar]
- 57). Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38: 1101-1106 [DOI] [PubMed] [Google Scholar]
- 58). Nakagawa T, Mazzali M, Kang DH, Kanellis J, Watanabe S, Sanchez-Lozada LG, Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ: Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol 2003; 23: 2-7 [DOI] [PubMed] [Google Scholar]
- 59). Sanchez-Lozada LG, Lanaspa MA, Cristobal-Garcia M, Garcia-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ: Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp Nephrol 2012; 121: e71-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML: Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 2008; 26: 269-275 [DOI] [PubMed] [Google Scholar]
- 61). Chen Y, Xu B, Sun W, Sun J, Wang T, Xu Y, Xu M, Lu J, Li X, Bi Y, Wang W, Ning G. Impact of the Serum Uric Acid Level on Subclinical Atherosclerosis in Middle-aged and Elderly Chinese. J Atheroscler Thromb 2015; 22: 823-832 [DOI] [PubMed] [Google Scholar]


