Abstract
The attribution of incentive-motivational value to reward-related cues contributes to cue-induced craving and relapse in addicted patients. Recently, it was demonstrated that subanesthetic ketamine increases motivation to quit and decreases cue-induced craving in cocaine-dependent individuals. Although the underlying mechanism of this effect is currently unknown, one possibility is that subanesthetic ketamine decreases the incentive-motivational value of reward-related cues. In the present study, we used a Pavlovian conditioned approach procedure to identify sign-trackers, rats that attribute incentive-motivational value to reward-related cues, and goal-trackers, rats that assign only predictive value to reward cues. This model is of interest because sign-trackers are more vulnerable to cue-induced reinstatement of drug-seeking behavior and will persist in this drug-seeking behavior despite adverse consequences. We tested the effect of subanesthetic ketamine on the expression of Pavlovian conditioned approach behavior and the conditioned reinforcing properties of a reward-related cue in sign-trackers and goal-trackers. We found that subanesthetic ketamine decreased sign-tracking and increased goal-tracking behavior in sign-trackers, though it had no effect on conditioned reinforcement. These results suggest that subanesthetic ketamine may be a promising pharmacotherapy for addiction that acts by decreasing the incentive-motivational value of reward-related cues.
Keywords: Pavlovian conditioned approach, conditioned reinforcement, sign-tracking, salience, addiction
1. Introduction
The attribution of incentive-motivational value to reward-related cues is believed to contribute to relapse and cue-induced craving in addiction (Robinson and Berridge, 1993; Sinha and Li, 2007). In support of this, drug-related cues can acquire incentive-motivational value (Wolfling et al., 2008), bias attention (Waters et al., 2003; Attwood et al., 2008), and rapidly induce craving (Michalowski and Erblich, 2014; Charboneau et al., 2013) in addicted patients. In addition, relapse is associated with increased cue-induced neural activity within the mesocorticolimbic reward system of addicted patients (Li et al., 2015). Even during prolonged periods of drug abstinence, drug-related cues can maintain sustained incentive-motivational value in both humans (Preller et al., 2013) and rodents (Weiss et al., 2001; Ciccocioppo et al., 2001).
N-methyl-D-aspartate (NMDA) receptor signaling is critical for reward-cue associations (Vengeliene et al., 2015), and glutamatergic synaptic plasticity in the mesocorticolimbic system is believed to underlie addiction pathophysiology (van Huijstee and Mansvelder, 2014; Kalivas et al., 2009; Kalivas and Volkow, 2011). Targeting NMDA receptor signaling using subanesthetic doses of ketamine, a noncompetitive NMDA receptor antagonist, has been investigated previously for the treatment of major depressive disorder, where it has been found to produce a rapid reduction in symptomology that endures long after drug clearance (aan het Rot et al., 2010; Price et al., 2009). Based upon the results of these and other studies, subanesthetic ketamine has been investigated for the treatment of addiction, and Dakwar et al. showed that subanesthetic ketamine administration increases motivation to quit and reduces cue-induced craving in cocaine-dependent subjects twenty-four hours after infusion (2014). In an earlier study, Krupitsky et al. also demonstrated that subanesthetic ketamine reduces cravings and increases abstinence for up to two years in heroin-dependent individuals (2002). Currently, it is unknown how subanesthetic ketamine affects reward processing to increase motivation to quit and reduce cue-induced craving; however, one possibility is that it decreases the incentive-motivational value of reward-related cues.
In order to investigate this possibility, we used a Pavlovian conditioned approach (PCA) procedure in rats. During PCA training, rats are presented with a conditioned stimulus (CS; e.g., a lever) that response-independently predicts the delivery of an unconditioned stimulus (US; e.g., a food pellet). Over the course of training, three patterns of conditioned responses (CRs) typically develop: sign-tracking (CS-directed CRs), goal-tracking (US-directed CRs), and an intermediate response (both CRs). Previously, it has been demonstrated that sign-trackers (STs), compared to goal-trackers (GTs) and intermediate-responders (IRs), attribute incentive-motivational value to reward-related cues, which become attractive, powerful motivators of behavior in and of themselves (Robinson and Flagel, 2009). It has also been shown that STs have increased cue-induced reinstatement of drug-seeking and continue to seek drugs despite adverse consequences, two hallmarks of addiction (Saunders and Robinson, 2010; Saunders et al., 2013). PCA procedures are useful in determining how pharmacological manipulations can alter the incentive-motivational value of reward-related cues without the confounds inherently associated with long-term exposure to drugs of abuse. In the current study, we investigated how subanesthetic ketamine influences the incentive-motivational value and conditioned reinforcing properties of reward-related cues in rats. In Experiment 1, rats underwent PCA training to phenotype rats as STs and GTs, and then subanesthetic ketamine was administered systemically to determine its effect on PCA behavior. In Experiment 2, rats underwent PCA training sessions to phenotype rats as STs and GTs, and then subanesthetic ketamine was administered systemically before a conditioned reinforcement test.
2. Methods
2.1. Animals
Fifty-three, adult male Sprague Dawley rats (275–300g) were purchased from Harlan Laboratories and Charles River Laboratories in order to obtain a relatively equal distribution of sign- and goal-trackers. Although it is not always necessary to purchase rats from different barriers, it oftentimes provides additional behavioral heterogeneity and phenotypic diversity by capitalizing on slight differences in genetic composition and possibly rearing practices among barriers (Fitzpatrick et al., 2013). In Experiment 1, 28 rats were used (Harlan = 16; Charles River = 12), in Experiment 2, 25 rats were used (Charles River, n = 25). Subjects were counterbalanced for vendor origin as part of the experimental design. Rats were maintained on a 12:12-hr light/dark cycle, and food and water were available ad libitum for the duration of the study. Rats were acclimatized to the housing colony for two days prior to handling. All procedures were approved by the University Committee on the Use and Care of Animals (University of Michigan; Ann Arbor, MI).
2.2. Drugs
Ketamine hydrochloride was used (racemic mixture; Hospira, Inc.; Lake Forest, IL). Ketamine (100 mg/kg) was diluted in sterile saline to make a subanesthetic dose of ketamine (32 mg/kg; 1 mL/kg; pH = 7.34–7.36). This dose was selected based upon previous studies showing that subanesthetic ketamine (30–35 mg/kg) increases brain metabolism and glutamatergic transmission in rats (Duncan et al., 1998b; Kim et al., 2011). Sterile saline was used as the vehicle control.
2.3. Pavlovian Conditioned Approach: Apparatus
Modular conditioning chambers (24.1 cm width × 20.5 cm depth × 29.2 cm height; MED Associates, Inc.; St. Albans, VT) were used for Pavlovian conditioning. Each chamber was located in a sound-attenuating cabinet equipped with a ventilation fan to provide ambient white noise. Each chamber was equipped with a pellet magazine, an illuminated, retractable lever (counterbalanced on the left or right of the pellet magazine), and a red house light on the wall opposite of the pellet magazine. When inserted into the chamber, the retractable lever was illuminated by an LED light within the lever housing. A pellet dispenser delivered banana-flavored food pellets into the pellet magazine. An infrared sensor measured head entries into the pellet magazine.
2.4. Pavlovian Conditioned Approach: Procedure
For two days prior to pretraining, rats were familiarized with banana-flavored food pellets (45 mg; Bioserv; Frenchtown, NJ) in their home cages. Twenty-four hours later, rats were placed into the operant chambers and underwent one pretraining session during which the red house-light remained on but the lever was retracted. Fifty food pellets were delivered on a variable time (VT) 30-s schedule (i.e., one food pellet was delivered on average every 30 s, but actual delivery varied between 0–60 s). All rats consumed all the food pellets by the end of the pretraining session. Each trial during a test session consisted of extension of the illuminated lever (conditioned stimulus; CS) into the chamber for 8 s on a VT 90-s schedule (i.e., one food pellet was delivered on average every 90 s, but actual delivery varied between 60–120 s). Retraction of the lever was immediately followed by the response-independent delivery of one food pellet (unconditioned stimulus; US) into the pellet magazine. Each test session consisted of 25 trials of CS-US pairings, resulting in a total session length of approximately 40 min. Each rat consumed all the food pellets that were delivered.
2.5. Conditioned Reinforcement: Procedure
For the conditioned reinforcement test, which lasted 40 min, each chamber was equipped with two nose-poke ports adjacent to a lever located in the center of the front wall of the chamber. Nose-poke responses in the active nose-poke port resulted in presentation of the lever-CS for 2 s on a fixed ratio 1 (FR1) schedule, whereas nose pokes of the inactive nose-poke port did not result in presentation of the lever-CS.
2.6. Experimental Procedure
In Experiment 1, rats underwent a total of ten daily PCA training sessions. The eighth PCA training session served as a baseline to ensure that rats within each phenotype, which would be divided into drug conditions, did not differ in their conditioned responding. During the ninth PCA training session, rats were administered subanesthetic ketamine (32 mg/kg) or vehicle 30 min before testing. During the tenth PCA training session, rats were tested without any drug or vehicle injection to determine whether acute, subanesthetic ketamine administration had enduring effects. In Experiment 2, rats underwent seven daily PCA training sessions followed twenty-four hours later by a test of conditioned reinforcement. Similar to Experiment 1, rats were administered subanesthetic ketamine (32 mg/kg) or vehicle 30 min before testing.
2.7. Statistical Analysis
PCA behavior was scored using an index that incorporates the number, latency, and probability of lever presses (sign-tracking CR) and magazine entries (goal-tracking CR) during CS presentations within a session. Briefly, we averaged the response bias (i.e., number of lever presses and magazine entries for a session; [lever presses – magazine entries] / [lever presses + magazine entries]), latency score (i.e., average latency to perform a lever press or magazine entry during a session; [magazine entry latency – lever press latency]/8), and probability difference (i.e., proportion of lever presses or magazine entries; lever press probability – magazine entry probability). The PCA index score ranges from +1.0 (absolute sign-tracking) to −1.0 (absolute goal-tracking), with 0 representing no bias. PCA index scores were used to classify rats as STs (score ≥ 0.5), GTs (score ≤ −0.5), and IRs (−0.5 < score < 0.5). For conditioned reinforcement, inactive and active nose-poke port responses were quantified and compared between groups.
SPSS (Version 22; IBM, Inc.) was used for all statistical analysis. For all linear mixed models, the covariance structure was selected based upon Akaike’s information criterion (i.e., the lowest number criterion represents the highest quality statistical model using a given covariance structure). PCA behavior across training sessions were analyzed using a linear mixed model with an autoregressive (AR1) covariance structure with Phenotype (GT and ST) and Drug (Ketamine and Vehicle) as between-subject factors when appropriate. In Experiment 1, latency of pellet retrieval and non-CS magazine entries during PCA training were analyzed using a two-way analysis of variance (ANOVA) with Phenotype (GT and ST) and Drug (Ketamine and Vehicle) as between-subject factors. In Experiment 2, conditioned reinforcement was analyzed using a three-way ANOVA with Phenotype (GT and ST), Drug (Ketamine and Vehicle), and Port (Active and Inactive) as between-subject factors. When significant effects or interactions were revealed, multiple comparisons were performed using Fisher’s Least Significant Difference (LSD) post hoc test.
3. Results
3.1. Experiment 1: Subanesthetic ketamine administration decreases sign-tracking behavior and does not affect goal-tracking behavior
Rats underwent PCA training and were classified as STs, GT, and IRs; however, only STs (n = 12) and GTs (n = 16) were used for further experimental testing. Figure 1 shows that during eight daily PCA training sessions STs and GTs differed in their lever press number (F(1,28.83) = 41.88, p = 4.53 × 10−7), latency (F(1,30.53) = 46.17, p = 1.41 × 10−7), and probability (F(1,32.44) = 61.78, p = 5.21 × 10−9) as well as their magazine entry number (F(1,31.69) = 25.63, p = 1.7 × 10−5), latency (F(1,37.04) = 38.65, p = 3.18 × 10−7), and probability (F(1,34.7) = 33.48, p = 2.0 × 10−6). STs and GTs differed on their PCA index scores over the eight daily PCA training sessions, (F(1,32.44) = 61.78, p = 5.21 × 10−9), and the PCA index score of Session 8, which also served as the baseline session for subanesthetic ketamine administration, was used to determine PCA phenotypes.
Figure 1.
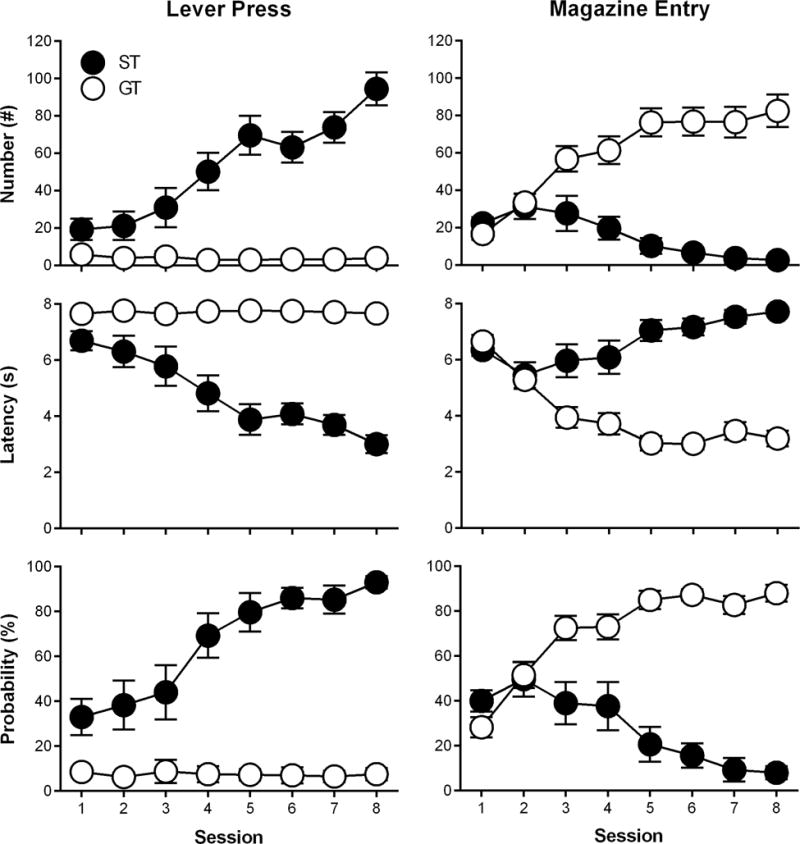
Rats underwent Pavlovian conditioned approach training over eight daily sessions and were classified as sign-trackers (STs) or goal-trackers (GTs) based on their lever press and magazine entry number, latency, and probability during Session 8. Data are presented as mean ± S.E.M.
Figure 2 shows the PCA behavior of rats during baseline, test (drug-on), and post-test (drug-off) sessions. During the baseline session, STs continued to lever-press more than GTs across CS trials (effect of Phenotype; F(1,123.8) = 367.99, p = 6.83 × 10−39), and there was no difference in the respective conditioned responding of GTs (effect of Drug; F(1,75.42) = 0.15, p = 0.7) or STs (effect of Drug; F(1,52.28) = 1.31, p = 0.26) that would later receive subanesthetic ketamine (ST, n = 5; GT, n = 8) or vehicle (ST, n = 7; GT, n = 8). Likewise, GTs continued to enter the magazine more than STs across CS trials (effect of Phenotype; F(1,131.53) = 237.04, p = 3.21 × 10−31). Subanesthetic ketamine decreased sign-tracking (effect of Drug; F(1,55.74) = 21.44, p = 2.12 × 10−5) and increased goal-tracking (effect of Drug; F(1,73.22) = 19.01, p = 4.19 × 10−5) in STs; however, subanesthetic ketamine did not affect sign-tracking (effect of Drug; F(1,63.81) = 1.68, p = 0.2) or goal-tracking (effect of Drug; F(1,67.91) = 3.19, p = 0.078) in GTs. During the post-test (drug-off) session, sign-tracking behavior in STs previously treated with subanesthetic ketamine was still decreased compared to saline-treated STs (effect of Drug; F(1,54.87) = 3.98, p = 0.05), but goal-tracking was no longer different between ketamine- and saline-treated STs (effect of Drug; F(1,89.63) = 1.24, p = 0.27). In addition, GTs that were previously administered saline or subanesthetic ketamine continued to show no within-session differences in sign-tracking (effect of Drug; F(1,67.91) = 3.19, p = 0.08) or goal-tracking (effect of Drug; F(1,57.91) = 0.48, p = 0.49) during the post-test (drug-off) session. During the test session, subanesthetic ketamine administration did not alter the latency to retrieve food pellets from the magazine following CS presentation (Figure 3A; effect of Drug; F(1,23) = 0.048, p = 0.83; interaction of Phenotype × Drug; F(1,23) = 0.03, p = 0.86), and as previously mentioned, all rats consumed all food pellets that were delivered. Subanesthetic ketamine did, however, increase non-CS magazine entries for both phenotypes (i.e., increased overall activity; Figure 3B; effect of Drug; F(1,24) = 7.44, p = 0.012).
Figure 2.
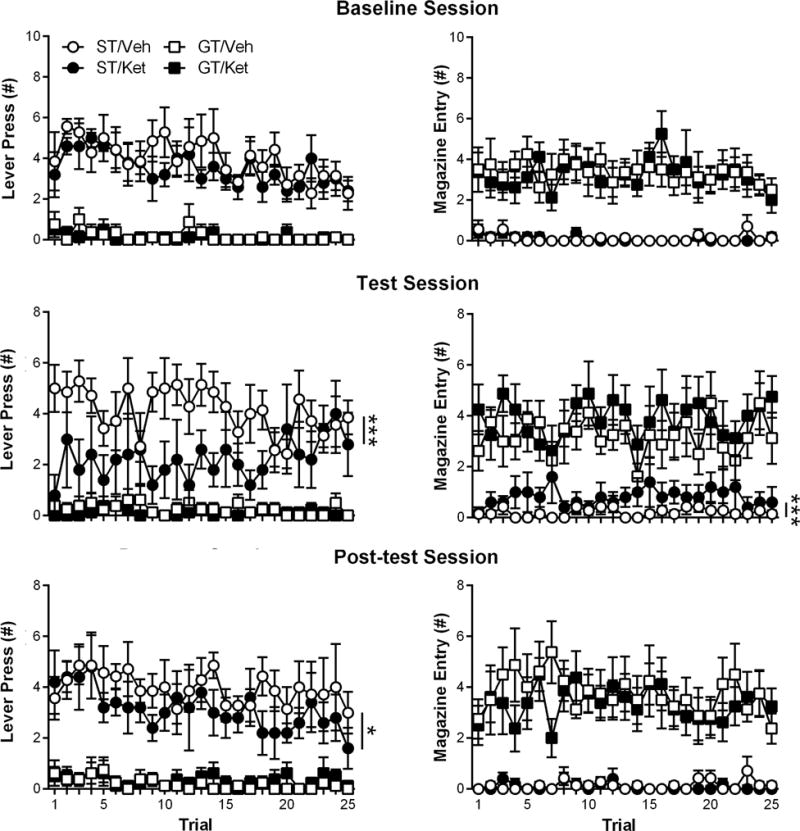
In Experiment 1, sign- and goal-tracking behavior was measured in sign-trackers (STs) and goal-trackers (GTs) during three additional Pavlovian conditioned approach sessions: baseline, test, and post-test. During the Pavlovian conditioned approach test session, subanesthetic ketamine (Ket; 32 mg/kg) or vehicle (Veh; saline) were administered 30 min prior to testing. Data are presented as mean ± S.E.M.
Figure 3.
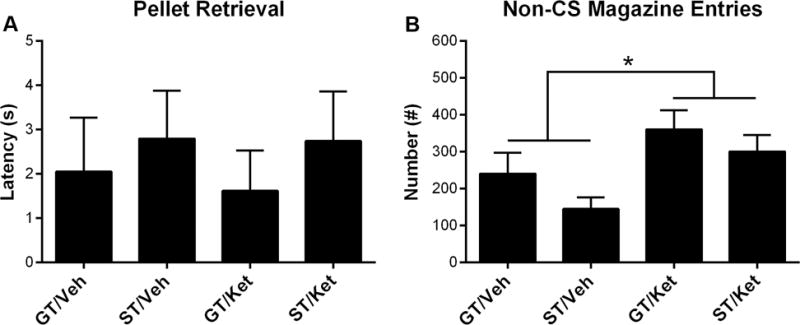
During the Pavlovian conditioned approach test session, the latency of pellet retrieval and number of non-CS magazine entries (i.e., the number of magazine entries performed outside presentation of the lever-CS) were measured between sign-trackers (STs) and goal-trackers (GTs) that were administered subanesthetic ketamine (Ket; 32 mg/kg) or vehicle (Veh; saline). Data are presented as mean + S.E.M. * − p < 0.05.
3.2. Experiment 2: Subanesthetic ketamine administration does not affect conditioned reinforcement
Rats underwent PCA training and were classified as STs, GT, and IRs; however, only STs (n = 14) and GTs (n = 11) were used for further experimental testing. During seven daily PCA training sessions, STs and GTs differed in their lever press number (F(1,23.57) = 32.61, p = 7.0 × 10−6), latency (F(1,24.78) = 50.38, p = 2.05 × 10−7), and probability (F(1,25.02) = 63.84, p = 2.39 × 10−8) as well as their magazine entry number (F(1,28.88) = 41.06, p = 5.34 × 10−7), latency (F(1,31.62) = 60.16, p = 8.26 × 10−9), and probability (F(1,28.11) = 51.06, p = 8.73 × 10−8). STs and GTs differed on their PCA index scores over the seven daily PCA training sessions, (F(1,25.41) = 97.5, p = 3.5 × 10−10), and the average PCA index score of Sessions 6 and 7 were used to determine PCA phenotypes.
Following PCA training, rats were administered ketamine (ST, n = 7; GT, n = 6) or vehicle (ST, n = 7; GT, n = 5) before undergoing a conditioned reinforcement test. Figure 4A shows that all rats performed more nose pokes into the active relative to the inactive port (effect of Port; F(1,42) = 15.65, p = 2.87 × 10−4). Consistent with previous findings, STs performed more active nose-poke responses than GTs (effect of Phenotype; F(1,21) = 16.97, p = 4.88 × 10−4). Subanesthetic ketamine did not affect conditioned reinforcement (Figure 4A; interaction of Drug × Port; F(1,42) = 0.34, p = 0.56; interaction of Phenotype × Drug × Port; F(1,42) = 0.13, p = 0.72) or discrimination (i.e., ratio of active/inactive nose-pokes) between ports (data not shown; interaction of Phenotype × Drug; F(1,21) = 1.28, p = 0.27); however, it did decrease the number of lever presses per CS presentation as a result of active nose-poke responding (data not shown; interaction of Phenotype × Drug; F(1,21) = 4.67, p = 0.042), ultimately decreasing total conditioned approach to the lever-CS (i.e., lever presses over all lever-CS presentations; Figure 4B; interaction of Phenotype × Drug; F(1,21) = 6.0, p = 0.023). Post-hoc comparisons revealed that vehicle-treated STs had higher lever presses than vehicle-treated GTs (p = 2.51 × 10−6) and that ketamine decreased the number of lever presses in STs (p = 0.0012) but not GTs (p = 0.97).
Figure 4.
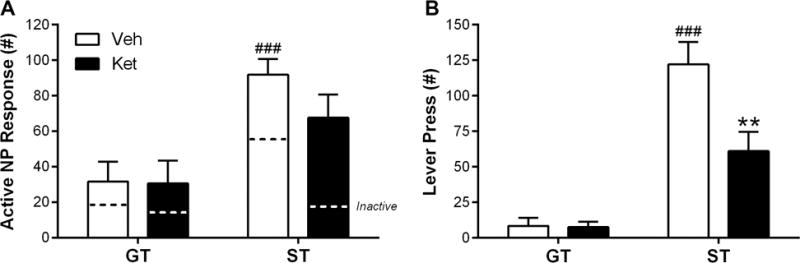
In Experiment 2, sign-trackers (STs) and goal-trackers (GTs) were administered subanesthetic ketamine (Ket; 32 mg/kg) or vehicle (Veh; saline) before undergoing a conditioned reinforcement test during which nose-poke responses and lever presses were measured. Data are presented as mean + S.E.M. ** − p < 0.01, Ket vs Veh; ### − p < 0.001, ST vs GT.
4. Discussion
In Experiment 1, we demonstrated that a subanesthetic dose of ketamine (32 mg/kg) decreases the expression of sign-tracking behavior in STs without affecting goal-tracking behavior in GTs. Interestingly, this effect was still detectable twenty-four hours after administration during a post-test (drug-off) PCA training session. In addition, subanesthetic ketamine increased goal-tracking behavior in STs, although the effect was not detectable during the post-test (drug-off) session. During the test session, subanesthetic ketamine did not influence food pellet consumption (i.e., all rats ate all food pellets during the test session) or the latency to retrieve food pellets following lever retraction. Subanesthetic ketamine did, however, increase non-CS magazine entries (a measure of general exploratory activity), which is in accordance with previous findings that subanesthetic ketamine increases locomotor activity (Littlewood et al., 2006b). We do not believe that this influenced the interpretation of our results, however, because locomotor hyperactivity, in the absence of effects on the incentive-motivational value of the lever-CS, would have increased the likelihood of STs approaching and interacting with the lever-CS, which it did not. Moreover, if subanesthetic ketamine-induced alterations in PCA behavior resulted from locomotor effects, both phenotypes would have presumably been affected equally, which they were not. In Experiment 2, subanesthetic ketamine did not affect conditioned reinforcement (i.e., the number of times a rat performed a nose-poke response for presentation of the lever-CS), however, it reduced conditioned approach (i.e., number of lever presses during lever-CS presentation) in STs, but not GTs during the conditioned reinforcement test.
In both rats and humans, subanesthetic doses of ketamine produce global increases in neural activity, as compared to anesthetic doses of ketamine, which produce global suppression of neural activity (Duncan et al., 1998b). In humans, subanesthetic ketamine increases cerebral glucose metabolism (Duncan et al., 1998a; Langsjo et al., 2004; Vollenweider et al., 1997; Breier et al., 1997), cerebral blood perfusion (Langsjo et al., 2003; Holcomb et al., 2001) and blood oxygen level-dependent contrast (De Simoni et al., 2013) in brain regions such as the frontal cortex, thalamus, hippocampus, and striatum; and, similar findings have been reported in rats using glucose metabolism (Duncan et al., 1998b) and blood oxygen level-dependent contrast (Littlewood et al., 2006b; Littlewood et al., 2006a). It has been suggested that this differential regulation of neural activity involves a dose-dependent bias between antagonizing NMDA receptors on inhibitory GABAergic interneurons (low-dose, subanesthetic ketamine) and excitatory pyramidal neurons (high-dose, anesthetic ketamine) (Miller et al., 2016). Therefore, subanesthetic doses of ketamine are believed to increase neural activity in brain regions by inhibiting GABAergic interneurons and disinhibiting glutamatergic neurons. In support of this, subanesthetic ketamine decreases extracellular GABA and increases extracellular Glu concentrations within the rat prefrontal cortex (PFC) (Perrine et al., 2014; Moghaddam et al., 1997). Because sign-tracking behavior has been suggested to result from low “top-down” modulation of subcortical structures (Haight and Flagel, 2014), it is possible that subanesthetic ketamine decreases the expression of sign-tracking behavior in STs by increasing glutamatergic activity in the PFC. Moreover, subanesthetic ketamine may decrease sign-tracking behavior by increasing connectivity between other brain regions and the PFC. For example, subanesthetic ketamine administration has been shown to increase thalamocortical connectivity in humans (Dawson et al., 2014; Rivolta et al., 2015), and it has been previously shown that GTs but not STs have increased functional connectivity between the thalamus and PFC in their neural responses to lever-CS presentations (Flagel et al., 2011a; Haight and Flagel, 2014).
Increased glutamatergic activity in the PFC may also explain the enduring effect of subanesthetic ketamine on the expression of sign-tracking behavior in STs twenty-four hours following administration. Ketamine has a half-life of 2.5 hours (Wieber et al., 1975) and subanesthetic ketamine alters glutamate release only up to two hours following administration (Moghaddam et al., 1997). The enduring behavioral effects of subanesthetic ketamine have been hypothesized to result from an increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-to-NMDA receptor ratio (Maeng et al., 2008; Du et al., 2006) with AMPA receptors in the PFC facilitating brain-derived neurotrophic factor (BDNF) release (Lepack et al., 2015; Zhou et al., 2014). Moreover, infusion of anti-BDNF antibodies into the PFC blocks the behavioral effects of subanesthetic ketamine (Lepack et al., 2015). Previously, it has been shown that STs have lowered levels of BDNF in the PFC compared to GTs (Morrow et al., 2015). Therefore, it is plausible that subanesthetic ketamine decreases sign-tracking behavior in STs by normalizing low levels of BDNF in the prefrontal cortex of STs.
In addition to its effects in the PFC, subanesthetic ketamine increases dopamine (DA) release in the nucleus accumbens (NAc) of rats (Littlewood et al., 2006b; Moghaddam et al., 1997). This action presumably arises from local NMDA receptor inhibition in the NAc, as subanesthetic ketamine does not alter metabolism or tyrosine hydroxylase levels in the rat ventral tegmental area, the primary source of DA afferents to the NAc (Baptista et al., 2015). It is known that lever-CS presentations result in discrete cue-associated increases in DA in the NAc core of STs but not GTs, which underlie the attribution of incentive salience to reward-related cues (Flagel et al., 2011b), and administration of flupenthixol, a nonselective D1/D2 receptor antagonist, into the NAc core impairs the expression of sign-tracking (Di Ciano et al., 2001; Saunders and Robinson, 2012; Flagel et al., 2011b). Acute amphetamine administration, however, also decreases sign-tracking behavior and increases goal-tracking behavior, similar to our results with subanesthetic ketamine (Holden and Peoples, 2010; Simon et al., 2009). These results suggest that indiscriminately increasing DA levels may interfere with the cue-evoked DA release that imbues reward-related cues with incentive-motivational value. One possibility is that ketamine-induced DA release shifts conditioned responding from the reward-distal lever-CS (i.e., sign-tracking) to the reward-proximal pellet magazine (i.e., goal-tracking) (Simon et al., 2009; Tindell et al., 2012). This could explain why sign-tracking behavior decreased and goal-tracking behavior increased, rather than sign-tracking behavior being exclusively affected.
The subanesthetic ketamine-induced shift from sign- to goal-tracking behavior could have important therapeutic implications because they are believed to represent model-free and model-based reinforcement learning, respectively (Huys et al., 2014). Clinically, a departure from model-free to model-based reinforcement learning would represent a transition from habitual, stimulus-driven responses to goal-directed cognitive control (Otto et al., 2015). One possibility is that subanesthetic ketamine could produce this shift through a combination of increased prefrontal cortical activation and altered striatal DA homeostasis (Deserno et al., 2015; Doll et al., 2016).
During the conditioned reinforcement test, subanesthetic ketamine did not influence conditioned reinforcement (i.e., the number of times a rat performed an active nose-poke response for presentation of the lever-CS); however, it decreased conditioned approach to the lever-CS in STs but not GTs. These results confirm that, while PCA and conditioned reinforcement measure closely related incentive-motivational processes, the two are dissociable and depend on neural substrates that do not completely overlap (Hitchcott and Phillips, 1998). Because NMDA receptor antagonism (i.e., AP-5) has previously been shown to decrease conditioned reinforcement, these results also suggest that subanesthetic ketamine has different pharmacological actions than other NMDA receptor antagonists (Wickham et al., 2015).
Although only two clinical studies have investigated the effects of subanesthetic ketamine in addicted patients, interest in the use of subanesthetic ketamine as a treatment for neuropsychiatric disorders has surged over the past decade, and many studies have already been performed to optimize its use as a pharmacotherapy. For example, a sublingual preparation of subanesthetic ketamine was recently reported to produce rapid and enduring anti-depressant effects in refractory depression with no euphoric or dissociative effects (Lara et al., 2013). In addition, ketamine stereoisomers have been investigated to maximize therapeutic potential while minimizing side effects. For example, R-ketamine is more potent, longer lasting, produces less psychotomimetic effects, and more robustly increases BDNF signaling in the PFC than S-ketamine (Yang et al., 2015; Zhang et al., 2014). Alongside these pharmacological advances, it is also important to understand how a potential pharmacotherapy affects the underlying behaviors of a particular neuropsychiatric disorder. Currently, it is unknown how subanesthetic ketamine decreases craving in addicted patients, and our results provide insight into a potential mechanism, suggesting that subanesthetic ketamine decreases the incentive-motivational properties of reward-related cues in subjects vulnerable to addiction-like behaviors.
Acknowledgments
This work was funded by the University of Michigan Department of Psychiatry (U032826 [JDM]), the Department of Defense (DoD) National Defense Science and Engineering Graduate (NDSEG) Fellowship (CJF), and the National Institute on Drug Abuse (NIDA; K08-DA037912-01 [JDM]).
References
- aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Attwood AS, O’Sullivan H, Leonards U, et al. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103(11):1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Baptista PP, Saur L, Bagatini PB, et al. Antidepressant Effects of Ketamine Are Not Related to (1)(8)F-FDG Metabolism or Tyrosine Hydroxylase Immunoreactivity in the Ventral Tegmental Area of Wistar Rats. Neurochem Res. 2015;40(6):1153–1164. doi: 10.1007/s11064-015-1576-3. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, et al. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154(6):805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Charboneau EJ, Dietrich MS, Park S, et al. Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: preliminary results. Psychiatry Res. 2013;214(2):122–131. doi: 10.1016/j.pscychresns.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Sanna PP, Weiss F. Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A. 2001;98(4):1976–1981. doi: 10.1073/pnas.98.4.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakwar E, Levin F, Foltin RW, et al. The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry. 2014;76(1):40–46. doi: 10.1016/j.biopsych.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, McDonald M, Higham DJ, et al. Subanesthetic ketamine treatment promotes abnormal interactions between neural subsystems and alters the properties of functional brain networks. Neuropsychopharmacology. 2014;39(7):1786–1798. doi: 10.1038/npp.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni S, Schwarz AJ, O’Daly OG, et al. Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage. 2013;64:75–90. doi: 10.1016/j.neuroimage.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Deserno L, Wilbertz T, Reiter A, et al. Lateral prefrontal model-based signatures are reduced in healthy individuals with high trait impulsivity. Transl Psychiatry. 2015;5:e659. doi: 10.1038/tp.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, et al. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21(23):9471–9477. doi: 10.1523/JNEUROSCI.21-23-09471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Bath KG, Daw ND, et al. Variability in Dopamine Genes Dissociates Model-Based and Model-Free Reinforcement Learning. J Neurosci. 2016;36(4):1211–1222. doi: 10.1523/JNEUROSCI.1901-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Machado-Vieira R, Maeng S, et al. Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov Today Ther Strateg. 2006;3(4):519–526. doi: 10.1016/j.ddstr.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Leipzig JN, Mailman RB, et al. Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res. 1998a;812(1–2):65–75. doi: 10.1016/s0006-8993(98)00926-3. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Knapp DJ, et al. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res. 1998b;787(2):181–190. doi: 10.1016/s0006-8993(97)01390-5. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, et al. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One. 2013;8(10):e75042. doi: 10.1371/journal.pone.0075042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Cameron CM, Pickup KN, et al. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight JL, Flagel SB. A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci. 2014;8:79. doi: 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcott PK, Phillips GD. Double dissociation of the behavioural effects of R(+) 7-OH-DPAT infusions in the central and basolateral amygdala nuclei upon Pavlovian and instrumental conditioned appetitive behaviours. Psychopharmacology (Berl) 1998;140(4):458–469. doi: 10.1007/s002130050790. [DOI] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, et al. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25(2):165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behav Brain Res. 2010;208(1):270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huys QJ, Tobler PN, Hasler G, et al. The role of learning-related dopamine signals in addiction vulnerability. Prog Brain Res. 2014;211:31–77. doi: 10.1016/B978-0-444-63425-2.00003-9. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, et al. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lee H, Kim HJ, et al. In vivo and ex vivo evidence for ketamine-induced hyperglutamatergic activity in the cerebral cortex of the rat: Potential relevance to schizophrenia. NMR Biomed. 2011;24(10):1235–1242. doi: 10.1002/nbm.1681. [DOI] [PubMed] [Google Scholar]
- Krupitsky E, Burakov A, Romanova T, et al. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. J Subst Abuse Treat. 2002;23(4):273–283. doi: 10.1016/s0740-5472(02)00275-1. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Kaisti KK, Aalto S, et al. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99(3):614–623. doi: 10.1097/00000542-200309000-00016. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Salmi E, Kaisti KK, et al. Effects of subanesthetic ketamine on regional cerebral glucose metabolism in humans. Anesthesiology. 2004;100(5):1065–1071. doi: 10.1097/00000542-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Lara DR, Bisol LW, Munari LR. Antidepressant, mood stabilizing and procognitive effects of very low dose sublingual ketamine in refractory unipolar and bipolar depression. Int J Neuropsychopharmacol. 2013;16(9):2111–2117. doi: 10.1017/S1461145713000485. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, et al. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18(1) doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li W, Wang H, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015;20(5):968–978. doi: 10.1111/adb.12182. [DOI] [PubMed] [Google Scholar]
- Littlewood CL, Cash D, Dixon AL, et al. Using the BOLD MR signal to differentiate the stereoisomers of ketamine in the rat. Neuroimage. 2006a;32(4):1733–1746. doi: 10.1016/j.neuroimage.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Littlewood CL, Jones N, O’Neill MJ, et al. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology (Berl) 2006b;186(1):64–81. doi: 10.1007/s00213-006-0344-0. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Michalowski A, Erblich J. Reward dependence moderates smoking-cue- and stress-induced cigarette cravings. Addict Behav. 2014;39(12):1879–1883. doi: 10.1016/j.addbeh.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OH, Moran JT, Hall BJ. Two cellular hypotheses explaining the initiation of ketamine’s antidepressant actions: Direct inhibition and disinhibition. Neuropharmacology. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, et al. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Saunders BT, Maren S, et al. Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats. Behav Brain Res. 2015;276:59–66. doi: 10.1016/j.bbr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Skatova A, Madlon-Kay S, et al. Cognitive control predicts use of model-based reinforcement learning. J Cogn Neurosci. 2015;27(2):319–333. doi: 10.1162/jocn_a_00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Ghoddoussi F, Michaels MS, et al. Ketamine reverses stress-induced depression-like behavior and increased GABA levels in the anterior cingulate: an 11.7 T 1H-MRS study in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:9–15. doi: 10.1016/j.pnpbp.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Preller KH, Wagner M, Sulzbach C, et al. Sustained incentive value of heroin-related cues in short- and long-term abstinent heroin users. Eur Neuropsychopharmacol. 2013;23(10):1270–1279. doi: 10.1016/j.euroneuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta D, Heidegger T, Scheller B, et al. Ketamine Dysregulates the Amplitude and Connectivity of High-Frequency Oscillations in Cortical-Subcortical Networks in Humans: Evidence From Resting-State Magnetoencephalography-Recordings. Schizophr Bull. 2015;41(5):1105–1114. doi: 10.1093/schbul/sbv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65(10):869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67(8):730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33(35):13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology (Berl) 2009;202(4):699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- van Huijstee AN, Mansvelder HD. Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction. Front Cell Neurosci. 2014;8:466. doi: 10.3389/fncel.2014.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Olevska A, Spanagel R. Long-lasting effect of NMDA receptor antagonist memantine on ethanol-cue association and relapse. J Neurochem. 2015 doi: 10.1111/jnc.13350. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, et al. Metabolic hyperfrontality and psychopathology in the ketamine model of psychosis using positron emission tomography (PET) and [18F]fluorodeoxyglucose (FDG) Eur Neuropsychopharmacol. 1997;7(1):9–24. doi: 10.1016/s0924-977x(96)00039-9. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, et al. Attentional shifts to smoking cues in smokers. Addiction. 2003;98(10):1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, et al. Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology. 2001;25(3):361–372. doi: 10.1016/S0893-133X(01)00238-X. [DOI] [PubMed] [Google Scholar]
- Wickham RJ, Solecki WB, Nunes EJ, et al. Distinct effects of ventral tegmental area NMDA and acetylcholine receptor blockade on conditioned reinforcement produced by food-associated cues. Neuroscience. 2015;301:384–394. doi: 10.1016/j.neuroscience.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieber J, Gugler R, Hengstmann JH, et al. Pharmacokinetics of ketamine in man. Anaesthesist. 1975;24(6):260–263. [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27(4):976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29(7):419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]


