Abstract
Nickel-containing urease from Klebsiella aerogenes requires four accessory proteins for proper active site metalation. The metallochaperone UreE delivers nickel to UreG, a GTPase that forms a UreD:UreF:UreG complex which binds to urease apoprotein via UreD. Prior in silico analysis of the homologous structurally-characterized UreH:UreF:UreG complex from Helicobacter pylori identified a water tunnel originating at a likely nickel-binding motif in UreG, passing through UreF, and exiting UreH, suggestive of a role for the channel in providing the metal to urease apoprotein for its activation; however, no experimental support was reported for the significance of this tunnel. Here, specific variants were designed to disrupt a comparable 34.6 Å predicted internal tunnel, alternative channels, and surface sites for UreD. Cells producing a set of tunnel-blocking variants of UreD exhibited greatly reduced urease specific activities, whereas other mutants had no appreciable effect on activity. Affinity pull-down studies of cell-free extracts from tunnel-disrupting mutant cultures showed no loss of UreD interactions with urease or UreF:UreG. The nickel contents of urease samples enriched from activity-deficient cultures were decreased, while zinc and iron incorporation increased. Molecular dynamics simulations revealed size restrictions in the internal channels of the UreD variants. These findings support the role of a molecular tunnel in UreD as a direct facilitator of nickel transfer into urease, illustrating a new paradigm in active site metallocenter assembly.
TOC image

Urease catalyzes the hydrolysis of urea into ammonia and carbamate, with the latter compound spontaneously decomposing into a second molecule of ammonia and bicarbonate.1, 2 The enzyme is found in plants3 as well as some bacteria, archaea, algae, and fungi.4 Most of our knowledge about the formation of the nickel active site5 comes from studies of the urease activation machinery of Klebsiella aerogenes and Helicobacter pylori, as detailed below.
The urease gene cluster from K. aerogenes (ureDABCEFG)6 encodes three enzyme subunits (KaUreA, KaUreB, and KaUreC) and four accessory proteins (KaUreD, KaUreE, KaUreF, and KaUreG). The subunits assemble into (KaUreABC)3,7 with the dinuclear active site located in UreC. KaUreD, when fused to the maltose-binding protein (MBP-UreD), is soluble and binds over two equivalents of nickel.8 KaUreF alone is insoluble.9 KaUreG, which exists as a soluble monomer that binds one equivalent of nickel or zinc with similar affinities,10, 11 acts as a GTPase during urease maturation, although the precise role of GTP hydrolysis in this process remains unclear. KaUreG residues associated with a highly conserved Cys-X-His motif are hypothesized to function as the metal binding site, but substitution of one or both of these residues does not abolish metal binding.11 The crystal structure of KaUreE is known12 and the dimeric nickel-binding protein is proposed to deliver nickel for urease activation.13 Attempts to activate K. aerogenes urease in vivo in the absence of any accessory protein result in the production of inactive enzyme lacking nickel.6, 14 The purified apoprotein can be partially activated in vitro,15, 16 and the activation competence is enhanced for urease apoprotein in complex with UreD,16–19 UreD:UreF,18–20 and UreD:UreF:UreG.21 UreD:UreF:UreG is formed in vivo, though direct studies of this complex were limited due to its low solubility.10 In contrast, a soluble complex is formed with MBP-UreD, producing (MBP-UreD:UreF:UreG)2 that dissociates to the monomer of heterotrimers when bound to urease.22
The H. pylori urease gene cluster (ureABIEFGH)23 is similar to that from K. aerogenes, but the encoded proteins exhibit a few important distinctions. The enzyme contains two subunits (HpUreA is homologous to a KaUreA-UreB fusion, while HpUreB is homologous to KaUreC) that assemble into ((HpUreAB)3)4.24 HpUreF was structurally characterized25 and isothermal titration calorimetry demonstrates it binds nickel,26 although the functional relevance of metal binding was not tested. HpUreG is a monomer in the absence of metal, binds zinc with high affinity leading to dimerization, and binds nickel with low affinity without facilitating dimerization of the protein.27 The structure of dimeric HpUreE28 closely resembles that of KaUreE and it forms a complex with HpUreG.29 Finally, H. pylori encodes a proton-gated urea permease (UreI).30 Of great significance to urease activation, the crystal structure of (HpUreH:UreF:UreG)2 is known31 (HpUreH is homologous to KaUreD), although the interaction surface of this complex with urease has not been determined.
While the importance of the accessory proteins to urease maturation is clear, the exact mechanism of this process is still unknown with two main hypotheses posited. One proposal invokes a “hand-off” mechanism with cytosolic nickel binding to the UreE metallochaperone which then passes it to surface-exposed residues of UreG,11 possibly UreF, then UreD, and finally into the nascent active site of K. aerogenes urease apoprotein, all within the urease:UreD:UreF:UreG complex.8, 11 The second hypothesis involves the initial delivery of nickel from UreE to UreG, followed by the use of a buried channel connecting the proposed nickel-binding site of UreG, through UreF and UreH, directly into the nascent active site of H. pylori urease.26 Thus far, no experimental data have been reported to support the function of this tunnel in nickel delivery for urease maturation.
Here, the function of KaUreD in urease activation was examined. Targeted KaUreD sidechain substitutions were created, and the biological effects of these changes were characterized. The results support the existence of a tunnel in KaUreD that is used for nickel delivery to urease, with additional evidence derived from comparisons of the molecular dynamics (MD) of the internal channels in wild-type (WT) and variant proteins. This work provides insights into how UreD functions in urease activation and illustrates how a metal transfer tunnel can be utilized for metallocenter assembly.
MATERIALS AND METHODS
Plasmid Construction
To characterize the effects of point substitutions on the function of KaUreD in vivo, three types of plasmids were constructed; i.e., pMF001L*, pKK17D* and pKKD*G, where * indicates the mutant versions.
An EcoRI/HindIII fragment of pEC0028 containing ureD was inserted into similarly digested pUC8 to yield plasmid pMF001.This plasmid cannot be used to directly overproduce UreD because it was found to lack a critical upstream region needed for overexpression, so an EcoRI/AgeI fragment of pKK1713 (containing ureD and a 197-bp 5′ untranslated region) was substituted into similarly digested pMF001 to yield pMF001L. The accessory gene within pMF001L was mutated by polymerase chain reaction with overlapping oligonucleotides (Integrated DNA Technologies, Coralville, IA) containing the proper base-pair substitution(s) and amplified with PfuTurbo® polymerase (Agilent Technologies). The resulting pMF001L* products were digested with DpnI and transformed into E. coli MAX Efficiency® DH5α cells (Life Technologies). Mutagenesis was confirmed by sequencing (Davis Sequencing, Davis, CA, or Michigan State University Genomics Core, East Lansing, MI).
To study the effects of ureD mutations within the intact urease gene cluster, the pMF001L* versions were digested and the desired ureD-containing fragments were isolated as described above. These fragments were ligated into similarly treated pKK17 to yield the analogous versions of plasmid pKK17D*. These plasmids were also sequenced to ensure the proper insertions were present.
To examine the effects of substitutions in KaUreD on protein:protein interactions, WT and mutant EcoRI/AgeI ureD fragments from pMF001L* were ligated into the similarly digested and isolated backbone of pKKG.11 The resulting pKKD*G plasmids contain the ureD versions within the context of ureDABCEFGStr, where KaUreG has been modified with a C-terminal Strep-II tag (KaUreGStr). The resulting plasmid validities were confirmed by sequencing. A summary of all plasmids used in these studies can be viewed in Table 1.
Table 1.
Plasmids used in this study
| Plasmid | Description | Source |
|---|---|---|
| pUC8 | High-copy number plasmid containing the pBR322 origin of replication, the multiple cloning site of M13mp7, and conferring AmpR. Gene expression is driven by an upstream lac promoter. | 65 |
| pKK17 | EcoRI_HindIII fragment of K. aerogenes ureDABCEFG ligated into similarly digested pKK223-3 containing the pBR322 origin of replication, an upstream tac promoter, downstream rrnB ribosomal terminator sequence, and conferring AmpR | 13, 66 |
| pKK17D*, -V37L, -Y42D, -E46A, -E46Q, -C48A, -H49A, -H54A, -I59Y, -D63A, -D63Q, -L65I, -L65W, -S85K, -K86A, -Y88V, -Y88F, -R89A, -W111Y, -T128E, -D142A, -R148M, -E153A, -E153Q, -R163A, -E165A, -D169A, -E176A, -E176Q, -T196K, -R211A | EcoRI-AgeI ureD mutant fragments from pMF001L* ligated into similarly digested pKK17 | This study |
| pKKG | PstI-KpnI ureGStr fragment ligated into similarly digested pKK17 resulting in replacement of UreG with one containing a C-terminal Strep-II tag (ureDABCEFGStr) | 11 |
| pKKD*G, -D63A, -D63Q. –S85K, -D142A, -E176A, -E176Q, R211A | EcoRI-AgeI ureD mutant fragments from pMF001L* ligated into similarly digested pKKG | This study |
| pEC002 | pMal-c2X derived vector using the pBR322 origin of replication and conferring AmpR for the cytosolic overproduction of maltose binding protein fused at the N-terminus of UreD. Gene expression is governed by an upstream tac promoter and a downstream rrnB ribosomal terminator sequence. | 8 |
| pMF001 | EcoRI-HindIII ureD fragment from pEC002 ligated into similarly digested pUC8 | This study |
| pMF001L | EcoRI-AgeI 5’UTR-ureD fragment from pKK17 ligated into similarly digested pMF001 | This study |
UreD Homology Model Generation and Refinement, Conservation Mapping, Water Tunnel Prediction, and Molecular Dynamics
A homology model was prepared by using the Protein Homology/analogY Recognition Engine (Phyre2, version 2.0) server32 and the crystal structure of HpUreH from the HpUreH:UreF:UreG complex (PDB code 4HI0)31 as a template. A PSI-BLAST analysis33 was performed for HpUreH, sequences with more than 15% and less than 90% identity were identified, the top 30 were selected along with KaUreD for multiple sequence alignment,34 and the residue conservation scores were mapped onto the KaUreD homology model by using the ConSurf server.35 Initial homology models were refined with an MD-based protocol developed by us and validated in recent rounds of CASP (Critical Assessment of protein Structure Prediction).36 Briefly, ten 40-ns simulations with weak restraints on all Cα atoms were performed starting with the homology model and using a force constant of 0.5 kcal/mol/Å2. A subset of snapshots was selected from the generated sampling based on scoring. Subsequent averaging led to the refined structure that was used in subsequent simulations.
To determine the effects of point substitutions on the hypothetical water tunnels within KaUreD, a series of MD simulations were carried out for WT KaUreD and ten KaUreD variants (D176A, D176Q, D63A, D63Q, D142A, S85K, L65I, L65W, T128E and T196K) as well as the HpUreH:UreF:UreG complex. All of the variant structures were generated in VMD.37 The molecules were solvated with cubic boxes that were large enough to keep at least a 9 Å margin from any protein atom to the edge of the box. Cl− and Na+ were added by replacing water molecules randomly, yielding neutralized systems and a 20 mM NaCl solution, consistent with the concentration of the cytosol.38 The final systems contained about 27,000 atoms (KaUreD alone) or 152,000 atoms (HpUreH:UreF:UreG complex).
All of the simulations were performed with NAMD 2.939 using the CHARMM36 force field.40, 41. The particle-mesh Ewald method42 and the SETTLE algorithm43 were used to calculate the electrostatic interactions and to constrain heavy atom hydrogen bonds, respectively. The non-bonded interactions were truncated at 10 Å with a switching function at 8.5 Å. The systems were maintained at constant temperature and pressure of 298 K and 1 bar, respectively, using a Langevin-type thermostat and barostat.44, 45 The complete systems were first minimized over 5000 steps and equilibrated over 200 ps with the protein fixed. The temperature was gradually increased from 5 K to 300 K with a step of 50 K over 400 ps without any restraints. Simulations were run with a weak harmonic restraint on Cα atoms using a force constant of 10 kcal/mol/Å2, and reduced to zero in steps of 2 kcal/mol/Å2 over 500 ps. Subsequent 3 × 50 ns of MD simulations were accumulated without any restraints for each systems.
MOLE46 is a powerful tool to explore molecular channels, tunnels, and pores based on Voroni diagrams where the optimal path is searched for by the Dijkstra algorithm from a given starting point to the molecular surface. MOLE 2.0 was used to predict water tunnels within the refined KaUreD homology model and the HpUreH:UreF:UreG structure with a minimum radius of 1.2 Å. The electrostatics calculations were performed in APBS47 at pH 7.0 with default parameters. RMSDs were calculated for all Cα atoms except the N- and C-terminal loop (residues 1–10 and 255–270) and the fixed radius clustering analysis was carried out in MMTSB48 with an RMSD radius cutoff of 2.5 Å. Clusters were determined based on mutual, pairwise RMSD that results in overall similar structures to be grouped together. The conformation in a given cluster that was closest to the cluster center was used to represent the conformation of all the structures in this cluster. All the figures were generated in PyMOL.49
Urease Activity Assays
Enzyme activity was measured by quantifying ammonia release from urea using methods described by Weatherburn.50 The release of ammonia over time was monitored by the formation of indophenol on the basis of its absorption of light at 625 nm. One unit of urease activity is defined as the amount of enzyme required to hydrolyze 1 μmol of urea/min at 37 °C. The standard assay buffer was 50 mM of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.8, with 50 mM urea.
In vivo Activation of Urease by Variant KaUreDs
E. coli BL21(DE3) competent cells were transformed with pKK17D* or pKKD*G and plated on lysogeny broth (LB) agar plates supplemented with 300 μg/mL ampicillin. A single transformant colony was used to inoculate 2 mL of LB medium that was supplemented with 100 μg/mL ampicillin and cultured overnight. Aliquots (150 μl) of these overnight cultures were used to inoculate 15 mL of LB supplemented with 1 mM NiCl2 and 100 μg/mL ampicillin in 50 mL flasks, which were shaken at 200 RPM at 37 °C until reaching an optical density at 600 nm (OD600) of 0.5, induced with 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and grown overnight at 37 °C. Urease apoprotein was prepared in a similar manner, but with the culture medium lacking supplemental nickel. Cells were harvested by centrifugation and resuspended in 2.5 mL of 100 mM HEPES, pH 7.8, per g of wet cell paste. Cells were lysed by sonication while placed in an ethanol ice bath by using a Branson 450 sonifier with three 2-min cycles at 4 W output power and 50% duty cycle and 1 min of temperature recovery between cycles. Cell lysates were clarified by centrifugation at 100,000 g at 4 °C for one h. The soluble cell-free extracts were diluted 100-fold into 100 mM HEPES, pH 7.8, buffer for urease activity assays. Overproduction of urease subunits was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Protein Purification
To determine the metal contents of urease produced by cultures with a functionally-deficient KaUreD, E. coli BL21(DE3) cells were transformed with pKKD*G constructs of interest. A single transformant colony was inoculated into 5 mL of LB supplemented with 100 μg/mL ampicillin and cultured overnight. For each UreD variant used, 1 L of LB supplemented with 1 mM NiCl2 and 100 μg/mL of ampicillin was inoculated with 1 ml of overnight culture and grown until reaching an OD600 of ~0.6. These cultures were induced with 0.1 mM IPTG and grown overnight with shaking at 37 °C. Cells were harvested by centrifugation and resuspended in 2.5 volumes of buffer containing 50 mM Tris-base, pH 7.4, containing 1 mM EDTA and 1 mM β-mercaptoethanol (TEB) that was supplemented with 0.1 mM PMSF. Cells were lysed by sonication while immersed in an ethanol ice bath using a Branson 450 sonifier with three 2 min cycles at 4 W output power and 50% duty cycle including 1 min of temperature recovery between cycles. Soluble cell-free extracts were separated from unbroken cells and debris by centrifugation at 100,000 g for one h at 4 °C. Supernatants were diluted 1:1 in TEB buffer before being applied to a 90 mL Macro-Prep® DEAE Support (Bio-Rad) column pre-equilibrated in TEB buffer. The samples were washed with one volume of TEB buffer and eluted by using a 0 M to 1 M NaCl gradient in TEB buffer. Fractions containing urease were pooled, dialyzed into TEB buffer containing 25 mM NaCl, and concentrated to 1 mL by using an Amicon® Ultra-15 10K centrifugal filter (Millipore). Each concentrate was injected directly onto 100 mL columns containing either Superdex 200 (General Electric) or Sephacryl 300 HR (Sigma) resin pre-equilibrated in TEB buffer containing 25 mM NaCl. Proteins were eluted by using the same buffer and the fractions containing urease were pooled and concentrated to 2.5 mL. The pooled samples were analyzed for their purity by SDS-PAGE and assayed for urease specific activity.
Urease apoprotein was purified from cells containing the pKK17 plasmid as described previously.8
RESULTS
Creation of a KaUreD Homology Model and Targeting Residues to Substitute
To investigate the function of KaUreD in the maturation of K. aerogenes urease, we examined a series of ureD mutants for their effects on urease activity, metal content, and UreD:urease and UreD:UreF protein:protein interactions. Residues targeted for substitution were based, in part, on a multiple sequence alignment of 32 homologous sequences that identified the conserved residues in UreD/UreH proteins (Figure S1). Note that HpUreH is the only one of these proteins for which a crystal structure is known and that KaUreD is the only other representative that has been biochemically characterized. No metal-binding motifs were apparent within the alignment, nor were any metal-binding motifs identified for HpUreH within the HpUreH:UreF:UreG structure.31
To guide substitution of surface residues that may facilitate protein:protein interactions or participate in a hand-off mechanism of nickel transfer, we prepared a KaUreD homology model, with the crystal structure of HpUreH in the HpUreH:UreF:UreG complex31 serving as the template (Figure 1A), and refined the model by MD simulations. Sequence conservation mapped onto the homology model revealed a region of highly conserved, surface-exposed residues on the face opposite to that of the UreF binding site (Figure 1C). This region could function as the UreD:urease binding interface. Conserved surface residues in this region of the protein (Y42, E46, C48, H49, H54, D63, K86, Y88, R89, R148, and E153) were substituted. In addition, a few surface residues on the reverse face or near the KaUreD:UreF interface (R163, E165, D169, E176, T196, and R211) were chosen for substitution.
Figure 1.

Homology-model guided mutagenesis of KaUreD. (A) KaUreD model (green) aligned to HpUreH (yellow) from the HpUreH:UreF:UreG crystal structure. HpUreF (magenta) is shown to define the HpUreH:UreF interaction site. (B) Tunnels predicted for the KaUreD model. The color of residues corresponds to the associated exit (tunnel 1 = magenta, 2 = yellow, 3 = orange, and 4 = teal). Blue residues are positioned at the branch point shared by all tunnels, while red residues are located at the entrance point of the tunnel. (C) Two views (180° y-axis rotation) of the KaUreD model colored by conservation score and depicted in surface representation. Dark blue, white, and dark magenta denote low, average, and high conservation. Surface and buried residues not associated with predicted water tunnels are labeled. Residues are noted with K. aerogenes/H. pylori numbering.
Additional residues selected for substitution were predicted to be at least partially buried in the protein and may be important for urease activation if the tunnel hypothesis is correct. Channels within HpUreH:UreF:UreG26 had been predicted by using CAVER;51 we identified similar channels in the same complex (Figure S2) based on the average structure from 150 ns MD using MOLE.46 When applied to the refined KaUreD model, a set of tunnels was predicted to branch from a common entry site near E176, at the likely KaUreD:UreF interface, and exit through any of four pathways (Figure 1B). The origin of these tunnels is unchanged from the internal channel predicted for HpUreH, and tunnel 1 of the KaUreD homology model is similar to the tunnel predicted in HpUreH:UreF:UreG. However, the three novel exits in the KaUreD model (tunnels 2, 3 and 4 in Figure 1B) were not predicted by either CAVER or MOLE analysis of the HpUreH:UreF:UreG structure. To test the importance of the four channels in urease activation, non-surface (internal) residues were changed. For example, E176 in KaUreD, analogous to HpUreH D174 located at the HpUreH:UreF interface, was substituted with similarly sized or smaller residues, in both cases lacking a negative charge. Several additional substitutions were designed to place bulky (F, Y, or W) or lengthy (K or E) residues at positions within or at the termini of the tunnels (e.g., V37, I59, D63, L65, S85, W111, T128, and T196). For each alteration, the substitute residue was modeled into KaUreD using PyMOL and analyzed to ensure that most rotamers did not have steric clashes. Models containing potential tunnel-blocking changes were analyzed by MOLE to assess whether the substitutions exhibited the desired effect of eliminating the tunnel. Variants which blocked the water tunnels in silico were selected for experimental study. The selected residues and the corresponding H. pylori residue numbers are illustrated in Figure 1B and C. The set of substitutions, primers utilized, rationale for the mutations, and conservation scores of the residues are listed in Table S1.
Effects of KaUreD Variants on the in vivo Activity of Urease
To determine whether the mutations summarized above affected in vivo urease maturation, we cultured cells containing pKK17D* (the asterisk indicates ureD mutations) in LB in the presence of 1 mM Ni2+. SDS-PAGE was used to confirm equivalent levels of urease protein were produced in all cultures. Substitutions of surface-exposed residues in the KaUreD model did not appreciably alter the urease activities when assayed using soluble cell-free extracts (Figure 2). In contrast, significantly reduced levels (< 30%) of in vivo urease activity relative to that of cells containing WT KaUreD were noted for cells producing six other variants with substitutions surrounding a predicted 34.6 Å tunnel (Figure 1B). Cellular activation of urease using E176A or E176Q variants of KaUreD resulted in activities that were 24% and 16% of those with WT KaUreD. E176 of KaUreD corresponds to D174 in the H. pylori protein, located at the HpUreH:HpUreF interfacial site but not directly involved in bonding. The D142A variant of KaUreD led to 21% of WT urease activity. This residue (corresponding to E140 in HpUreH) is predicted to be buried, forming backbone-mediated contacts with the sidechain of T196 and a hydrogen bond between its carboxylate and the backbone carbonyl of L143. Since β-sheet formation and stability is dominated by backbone hydrogen bonding, loss of the polar interaction is unlikely to affect the overall structural stability of KaUreD. S85 is positioned with its sidechain facing the shared branch-point for the tunnels. The S85K variant yielded cell-free extract urease activities that were 19% relative to those for WT KaUreD. D63 resides on the face of KaUreD lying opposite the likely UreF interface and is positioned at the exit point of the 34.6 Å tunnel. Changing this residue (to A or Q) resulted in cell-free extracts with 12% and 15% of WT urease activities. Surprisingly, T196K KaUreD, containing a substitution designed to block the entrance point of the tunnel at the KaUreD:UreF interface, had only a mild effect on urease maturation (i.e. 71% of WT activity); however, the predicted effects were based on a static homology model so the outcome in a dynamic structure cannot be assured. In sum, these results support the existence of a channel within KaUreD that is important for urease activation, but they do not establish the function of the tunnel.
Figure 2.

Urease activities of cell-free extracts from cells containing KaUreD variants. E. coli BL21(DE3) cells were transformed with plasmid pKK17D* (encoding ureD*ABCEFG), grown in LB containing 1 mM NiCl2 (except for the sample producing urease apoprotein which was not supplemented with the metal), and soluble cell-free extracts were assayed for urease activity. Error bars represent triplicate analyses of biological replicates (n = 3 in all cases except R163A and T196K samples, where n = 2).
Pull-Down Assays
To identify whether urease:UreD or UreD:UreF protein:protein interactions were disrupted for the six KaUreD variants deficient in urease activation, we substituted the corresponding ureD sequences into pKKG (the urease gene cluster encoding KaUreG with a C-terminal Strep-II tag),11 cultured the E. coli pKKGD* cells in LB that lacked Ni2+, and examined the resulting urease:UreD:UreF:UreGStr complexes after Strep-Tactin enrichment. Constructs encoding WT and R211A KaUreD (providing 77% activity relative to WT) were used as controls (with replicate analysis of the WT sample yielding more intense bands for the accessory proteins). In all cases, the cell-free extracts contained similar levels of urease, and the UreGStr-fractions eluted from the Strep-Tactin resin contained the urease subunits, UreD, UreF, and UreG accessory proteins (Figure 3). These results demonstrated that urease:KaUreD and KaUreD:UreF interactions were maintained when using the KaUreD variants and it suggests that variant KaUreD proteins were properly folded.
Figure 3.
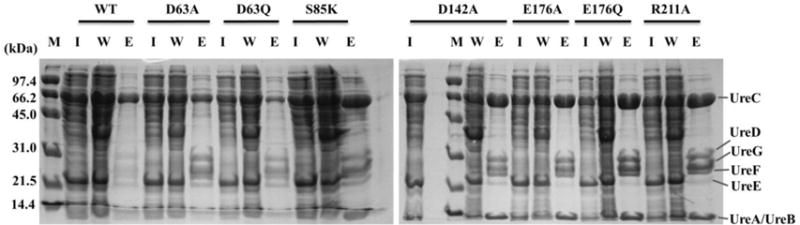
Interactions of KaUreGStr with other urease proteins. E. coli BL21(DE3) cells were transformed with pKKD*G producing the KaUreD variants noted above the lanes. Soluble cell-free extracts (I, for input) were chromatographed on Strep-Tactin resin with the unbound wash (W) and the desthiobiotin-eluted (E) fractions analyzed by SDS-PAGE.
Analysis of the Activities and Metal Contents of Urease Samples Activated by Variant KaUreDs
To examine whether ureD mutant cultures with reduced urease activities produced enzyme that was altered in metal content, we enriched urease samples from selected E. coli pKKD*G cells cultured in the presence of 1 mM Ni2+ (LB binds nickel with great affinity, thus limiting urease activation efficiency) and analyzed the enzyme specific activities and metal contents. The urease samples were enriched to > 90% purity (Figure S3), and their specific activities (Table 2) generally paralleled those measured in cell-free extracts where urease comprised approximately 15% of the cellular protein (Figure 2). While the KaUreD variants giving rise to reduced activity in the cell-free extracts also exhibited reduced specific activities for the enriched proteins, detailed comparisons of the results underscore differences. For example, urease activity in cell-free extracts produced by D63A KaUreD exhibited only 12% of the WT activity, whereas the purified urease from this sample exhibited 26% of the WT enzyme activity. The samples associated with E176A KaUreD also yielded greater activity for the purified enzyme than expected from the cell-free extracts. In contrast, the purified urease specific activities were lower than expected on the basis of cell-free urease activities for the samples associated with D63Q and D142A KaUreD. Inspection of the metal contents for the enriched ureases with low urease specific activity revealed less nickel than those activated with WT KaUreD and identified varying levels of zinc and iron (Table 2), with the greatest levels of zinc corresponding to the most inactive urease, generated with D142A KaUreD. These findings confirm that tunnel-blocking variants of KaUreD lead to deficiencies in urease nickel content, consistent with a nickel-transfer role for the channel.
Table 2.
Specific activities and metal contents of urease samples enriched from cells containing selected KaUreD variantsa
| KaUreD variant | Specific activity U/mg protein (% WT) | Ni per UreABC (% WT) | Zn per UreABC | Fe per UreABC |
|---|---|---|---|---|
| WT(-Ni) pKK17D | 0 | 0.004 (4 × 10−5) | 0.13 | 0.27 |
| WT pKKDG | 1690 (100) | 1.1 (100) | 0 | 0.02 |
| D63A pKKDG | 434 (25.7) | 0.54 (49.1) | 0.16 | 0.21 |
| D63Q pKKDG | 55.7 (3.29) | 0.22 (20.0) | 0.34 | 0.07 |
| S85K pKKDG | 487 (28.8) | 0.39 (35.5) | 0.06 | 0.30 |
| D142A pKKDG | 14.1 (0.84) | 0.07 (6.4) | 0.52 | 0.14 |
| E176A pKKDG | 1080 (63.7) | 0.51 (46.4) | 0.09 | 0.11 |
| E176Q pKKDG | 375 (22.2) | 0.43 (39.1) | 0.03 | 0.13 |
| R211A pKKDG | 1230 (72.4) | 0.76 (69.1) | 0.21 | 0.29 |
The urease samples were enriched from cell-free extracts of E. coli pKKD*G grown in LB containing 1 mM NiCl2, except for a sample of urease apoprotein that was obtained from E. coli pKK17D grown in LB lacking nickel (-Ni). Units of urease activity are μmole min−1 (mg protein)−1. Metal contents were determined by inductively coupled plasma-atomic emission spectroscopy.
MD of Activation-Deficient KaUreD Constructs
To analyze the effects of the KaUreD variants on the overall stabilities of the predicted tunnels, MD simulations were carried out on the refined KaUreD model and ten variants. These included the six substituted proteins that resulted in a loss of function (D63A/Q, S85K, E142A, and E176A/Q) along with four variants (L65I/W, T128E, and T196K) that were initially predicted to block the tunnel, but did not result in a loss of function. Root mean square deviations (RMSDs) of all heavy atoms for the variant systems were similar to WT, suggesting that the substitutions did not induce large conformational changes (Figure S4). KaUreD alone exhibited only slightly more flexibility (by about 0.5 Å RMSD) than either HpUreH protomer in (HpUreH:UreF:UreG)2, suggesting that separating KaUreD from the complex does not have a large effect on its conformation.
To analyze the effects of substituting side chains along the proposed channels and other possible channels, clustering analysis was applied to the combined conformations of the last 30 ns trajectories of all 11 KaUreD systems. All the sampled structures were grouped into eight clusters (C1–C8). The conformations with the smallest RMSD to the cluster centers were used as representative structures to probe the channels originating at E176 by MOLE (Figure 4 and Table S2). The largest cluster (C1), dominant in the WT and T196K models of KaUreD (green in Figure 4), possessed a channel capable of accommodating 6-coordinate Ni2+ with a radius of 0.69–0.715 Å.52 This channel is almost the same as the proposed channel. Other channels wide enough to allow Ni2+ to pass were also found in the smaller C6 and C7 clusters in the D63A/D142A and L65W/T128E KaUreD variants. All of these available channels have an ending/entrance located near D63. In other structures, viable channels leading from E176 were not identified because there was either a narrow bottleneck along the path (red channel in Figure 4 for C1 and channels in C4, C5, and C8), or the E176 (C2) or D63 (C3) ends were blocked (blue outlines in Figure 4) so that Ni2+ passage would be considered unlikely. The blocked channels in C2 and C3 resulted from sidechain dynamics, but this freedom of rotation might be occluded by KaUreD:UreF or KaUreD:urease interactions, as evidenced by the lower RMSD observed for the HpUreD:UreF protein:protein complex (Figure S4). Therefore, the cavity in C2 based on the KaUreD L65I/L65W/T196K models may not be representative of what would be observed in the KaUreD:UreF:UreG complex. All the eight representative structures were submitted to electrostatics calculations (Figure S5). The exit located near D63 with a negative electrostatic potential in cluster C1 turned into a positive electrostatic potential in other clusters. To conclude, the clustering analysis results revealed that all the systems have multiple dynamic channels initiating at the interface with UreF and ending near D63, providing a possible explanation for the remaining low urease activities levels in the variants.
Figure 4.

Channels associated with the eight main clustered conformations derived from MD simulations, with the constitutions of the corresponding clusters shown below in pie charts. All channels leading from the position around E176 (traversing KaUreD from left to right) are shown with different colors. E176 and D63 are shown as yellow sticks. Clusters C2 and C3 possess blocked channels at one or the other end, with the cavities depicted in blue outline.
DISCUSSION
It has been estimated that about 1/3 of all proteins contain metals, with ~40% of metalloproteins having metal centers essential for catalysis.53, 54 These proteins must overcome several challenges during their synthesis in order to incorporate the proper metal(s) to become functional. One approach to circumvent mismetalation is to use accessory proteins for binding the metal, synthesizing any metallocofactor shown to be present, and transferring it into the nascent metalloenzyme active sites.54–56 Examples of this strategy are seen in the Nif proteins involved in biosynthesis of the FeMoco cofactor of nitrogenase,57 the Hyp proteins used to generate the [NiFe]-hydrogenase cofactor,58 the copper chaperone for superoxide dismutase required for Cu,Zn-superoxide dismutase,59 and the focus here – the maturation of nickel-containing urease.5
Role of KaUreD in Urease Activation
KaUreD is required for in vivo maturation of K. aerogenes urease, it forms a complex with the apoenzyme, and it interacts with KaUreF and KaUreG, the latter of which accepts nickel from KaUreE.5 Two potential roles in transferring nickel have been posited for UreD/UreH homologues, beyond the structural role of connecting UreE and the urease apoprotein. One hypothesis suggests that KaUreD surface residues function in a “bucket brigade” approach to shuttle the nickel from its delivery site on KaUreG to the nascent active site.8, 11 Precedence for such a hand-off mechanism includes Cox17 and ScoI which pass Cu+1 into cytochrome c oxidase.60 An alternative hypothesis with no direct precedent is derived from the HpUreH:UreF:UreG crystal structure31 for which a buried water tunnel was predicted to span from the likely nickel-binding site in HpUreG through HpUreF and HpUreH, potentially functioning to deliver nickel for urease maturation.26 Significantly, no direct experimental support for a nickel transfer tunnel was reported. The studies described here provide compelling evidence that a buried channel in KaUreD is used to deliver nickel to urease apoprotein rather than transferring the metal ion via surface residues.
We characterized the effects on urease activity for 30 variant forms of KaUreD that were designed to disrupt potential surface metal-transfer sites and internal channels. None of the KaUreD surface substitutions led to significant losses of urease activity in cell-free extracts, implying that the surface residues examined are not of great importance to the function of KaUreD. In sharp contrast, KaUreD variants with alterations at the ends (E176A/Q and D63A/Q) and the initial branch point (D142A) of predicted tunnel 1 exhibit significant losses in urease activity. The S85K KaUreD variant, designed to block the shared branch point, also led to decreased urease activity. MD simulations reveal these substitutions either narrow the channel or block an exit. One KaUreD variant designed to block the exit of tunnel 1 (I59Y) had no appreciable effect on urease activity; however, this residue is located on a disordered loop in the model and likely adopts multiple conformations that would not be accurately depicted using analysis of a static structure. T196K KaUreD had no appreciable effect on urease activation in vivo, even though in silico analysis of the available rotamers predicted blockage of the tunnel entrance and the introduction of a positive charge (as in the S85K variant) could reasonably increase the energy barrier of Ni2+ transport. MD simulations suggest the tunnel of this variant exists largely in WT conformation, explaining this lack of effect. Unfortunately, no modifications to residues facing tunnel 2 could be modeled to block the tunnel without encountering severe steric clashes, but the lack of this tunnel in HpUreH and the clear tunnel 1-disrupting effects reduce the probability that this channel has a role. Overall, MD simulations support the existence of multiple dynamic channels (Figure 4), so that blocking one channel may be insufficient to abolish UreD function as new channels could form due to sidechain dynamics. In sum, the MD and cell-free extract activity results are consistent with a tunnel within KaUreD functioning in urease activation, but loss of urease activity also could arise from disruption of protein:protein interactions.
KaUreD variants of interest were shown to be capable of interacting with their known binding partners according to pull-down studies carried out using nickel-free cells containing pKKD*G. The WT construct activates urease comparable to non-tagged KaUreG,11 and Strep-Tactin enrichments contain KaUreGStr along with the urease subunits, KaUreD, and KaUreF. This result shows that protein:protein binding interactions are not disrupted when using the selected KaUreD variants and provides evidence for their proper folding. This finding is especially important in the case of the S85K variant, where the introduction of a positive charge buried within the protein was a point of concern.
The decreased urease activities in cells producing KaUreD variants correlate with lower nickel contents of the corresponding ureases. Of interest, urease samples that were deficient in activity and nickel content, including the urease apoprotein control, possess contaminating levels of zinc and iron. Most notably, ureases activated with the D63Q and D142A KaUreD variants exhibit the lowest urease activities and nickel contents as well as the highest zinc occupancies. These results are compatible with the selected variants disrupting a nickel transfer tunnel, leading to spurious metal incorporation (zinc and iron) into urease. The mechanism of mismetalation may occur via metal binding to the free urease apoprotein, which seems unlikely given the stability of KaUreABC:UreD:UreF:UreG during purification, or by binding to apoprotein within the complex, requiring a secondary metal access route that may inefficiently and opportunistically incorporate divalent metal ions when the nickel transfer tunnel is blocked. Upon apoprotein metalation, the complex dissociates to release the metal-substituted enzyme. The function of the other accessory proteins are presumably unaltered in cells containing the variant KaUreD proteins, so acquisition of nickel by KaUreE, transfer to KaUreG, and passage through KaUreF should remain unaffected. For WT cells with Ni2+ present, the mismetalation pathway is outcompeted by the GTP-dependent process that loads the correct metal into the nickel-transfer tunnel starting in UreF. The GTP dependence of nickel loading may provide the driving force so that nickel incorporation into urease apoprotein is not simply diffusion controlled.
Comparison of the KaUreD Internal Channel to Other Protein Tunnels
We have shown that KaUreD possesses a tunnel required for nickel delivery to the cognate urease. The residues lining this tunnel are shown in Figure 5, and lack positive charges which could impose a high thermodynamic penalty for cation movement. Negatively charged residues (D63, D142, and E176) are localized near the tunnel ends. The diameter of the tunnel, ranging from 1.2 Å to 2.5 Å, can accommodate Ni2+ with a radius of 0.69–0.715 Å,52 especially when considering the protein dynamics. The conclusion that KaUreD has a nickel-transfer tunnel fits well with results from nickel-binding studies of UreABC:KaUreD and MBP-KaUreD, as well as the computation-based proposal of a functional tunnel in HpUreH:UreF:UreG.8, 16, 26 Our data do not address the additional possibility that the tunnel also may be utilized for delivery of CO2 for carbamylating the lysine to form a bridging ligand of the dinuclear metallocenter.
Figure 5.

Characteristics of the main KaUreD internal channel. The main channel probed in WT KaUreD is shown in the upper panel in white with the KaUreF binding site to the left and the exit to the right. Residues lining the channel are shown in stick mode. The radius of the channel as a function of distance from the tunnel entrance is shown in the bottom panel.
Internal channels are known to serve important roles in proteins, other including the molecular tunnels used to prevent diffusion of toxic or short-lived intermediates in a variety of enzymes.61 Few precedents exist, however, for metal transfer tunnels. The cation transport proteins are designed for energy-driven, unidirectional transport across cell membranes,62 but the tunnels are not continuously open to prevent energy dissipation. A tunnel strategy is used for iron transfer into the ferritin core,63 but this process involves metal storage rather than metalloenzyme activation. The Fe-Fe hydrogenase HydA includes a cationic channel that allows for insertion of a 2Fe subcluster during metallocenter biosynthesis,64 but this partial tunnel is located within the enzyme itself rather than in an accessory protein. KaUreD thus serves as a new paradigm for metallocenter assembly using an internal metal transfer process.
Supplementary Material
Acknowledgments
We thank Eric Carter for initiating residue substitution studies of MBP-UreD.
Funding Sources
This work was supported in part by the National Institutes of Health (DK04586 to R.P.H.) and Michigan State University Dissertation Continuation and Completion Fellowships (to M.A.F.).
ABBREVIATIONS
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- LB
lysogeny broth
- MBP
maltose binding protein
- MD
molecular dynamics
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- WT
wild type
Footnotes
Supporting Information. Two additional tables and four supplementary figures are supplied as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zambelli B, Musiani F, Benini S, Ciurli S. Chemistry of Ni2+ in urease: Sensing, trafficking, and catalysis. Acc Chem Res. 2011;44:520–530. doi: 10.1021/ar200041k. [DOI] [PubMed] [Google Scholar]
- 2.Krajewska B. Ureases I. Functional, catalytic and kinetic properties: A review. J Molec Catalysis B: Enzymatic. 2009;59:9–21. [Google Scholar]
- 3.Witte CP. Urea metabolism in plants. Plant Sci. 2011;180:431–438. doi: 10.1016/j.plantsci.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Mobley HLT, Hausinger RP. Microbial ureases: Significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrugia MA, Macomber L, Hausinger RP. Biosynthesis of the urease metallocenter. J Biol Chem. 2013;288:13178–13185. doi: 10.1074/jbc.R112.446526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MH, Mulrooney SB, Renner MJ, Markowicz Y, Hausinger RP. Klebsiella aerogenes urease gene cluster: Sequence of ureD and demonstration that four accessory genes (ureD, ureE, ureF, and ureG) are involved in nickel metallocenter biosynthesis. J Bacteriol. 1992;174:4324–4330. doi: 10.1128/jb.174.13.4324-4330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabri E, Carr MB, Hausinger RP, Karplus PA. The crystal structure of urease from Klebsiella aerogenes. Science. 1995;268:998–1004. [PubMed] [Google Scholar]
- 8.Carter EL, Hausinger RP. Characterization of Klebsiella aerogenes urease accessory protein UreD in fusion with the maltose binding protein. J Bacteriol. 2010;192:2294–2304. doi: 10.1128/JB.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boer JL, Hausinger RP. Klebsiella aerogenes UreF: Identification of the UreG binding site and role in enhancing the fidelity of urease activation. Biochemistry. 2012;51:2298–2308. doi: 10.1021/bi3000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moncrief MBC, Hausinger RP. Characterization of UreG, identification of a UreD-UreF-UreG complex, and evidence suggesting that a nucleotide-binding site in UreG is required for in vivo metallocenter assembly of Klebsiella aerogenes urease. J Bacteriol. 1997;179:4081–4086. doi: 10.1128/jb.179.13.4081-4086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boer JL, Quiroz-Valenzuela S, Anderson KL, Hausinger RP. Mutagenesis of Klebsiella aerogenes UreG to probe nickel binding and interactions with other urease-related proteins. Biochemistry. 2010;49:5859–5869. doi: 10.1021/bi1004987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song HK, Mulrooney SB, Huber R, Hausinger RP. Crystal structure of Klebsiella aerogenes UreE, a nickel-binding metallochaperone for urease activation. J Biol Chem. 2001;276:49359–49364. doi: 10.1074/jbc.M108619200. [DOI] [PubMed] [Google Scholar]
- 13.Colpas GJ, Brayman TG, Ming LJ, Hausinger RP. Identification of metal-binding residues in the Klebsiella aerogenes urease nickel metallochaperone, UreE. Biochemistry. 1999;38:4078–4088. doi: 10.1021/bi982435t. [DOI] [PubMed] [Google Scholar]
- 14.Mulrooney SB, Hausinger RP. Sequence of the Klebsiella aerogenes urease genes and evidence for accessory proteins facilitating nickel incorporation. J Bacteriol. 1990;172:5837–5843. doi: 10.1128/jb.172.10.5837-5843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park IS, Hausinger RP. Requirement of carbon dioxide for in vitro assembly of the urease nickel metallocenter. Science. 1995;267:1156–1158. doi: 10.1126/science.7855593. [DOI] [PubMed] [Google Scholar]
- 16.Park IS, Hausinger RP. Metal ion interactions with urease and UreD-urease apoproteins. Biochemistry. 1996;35:5345–5352. doi: 10.1021/bi952894j. [DOI] [PubMed] [Google Scholar]
- 17.Park IS, Carr MB, Hausinger RP. In vitro activation of urease apoprotein and role of UreD as a chaperone required for nickel metallocenter assembly. Proc Natl Acad Sci USA. 1994;91:3233–3237. doi: 10.1073/pnas.91.8.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quiroz-Valenzuela S, Sukuru SCK, Hausinger RP, Kuhn LA, Heller WT. The structure of urease activation complexes examined by flexibility analysis, mutagenesis, and small-angle X-ray scattering. Arch Biochem Biophys. 2008;480:51–57. doi: 10.1016/j.abb.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang Z, Kuchar J, Hausinger RP. Chemical crosslinking and mass spectrometric identification of sites of interaction for UreD, UreF, and urease. J Biol Chem. 2004;279:15305–15313. doi: 10.1074/jbc.M312979200. [DOI] [PubMed] [Google Scholar]
- 20.Moncrief MBC, Hausinger RP. Purification and activation properties of UreD-UreF-urease apoprotein complexes. J Bacteriol. 1996;178:5417–5421. doi: 10.1128/jb.178.18.5417-5421.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano A, Hausinger RP. GTP-dependent activation of urease apoprotein in complex with the UreD, UreF, and UreG accessory proteins. Proc Natl Acad Sci USA. 1999;96:11140–11144. doi: 10.1073/pnas.96.20.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farrugia MA, Han L, Zhong Y, Boer JL, Ruotolo BT, Hausinger RP. Analysis of a soluble (UreD:UreF:UreG)2 accessory protein complex and its interactions with Klebsiella aerogenes urease by mass spectroscopy. J Am Soc Mass Spectrom. 2013;24:1328–1337. doi: 10.1007/s13361-013-0677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cussac V, Ferrero RL, Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992;174:2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ha NC, Oh ST, Sung JY, Cha KA, Lee MH, Oh BH. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nature Struct Biol. 2001;8:505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 25.Lam R, Romanov V, Johns K, Battaile K, Wu-Brown J, Guthrie JL, Hausinger RP, Pai E, Chirgadze NY. Crystal structure of a truncated urease accessory protein UreF from Helicobacter pylori. Proteins. 2010;78:2839–2848. doi: 10.1002/prot.22802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zambelli B, Berardi A, Martin-Diaconescu V, Mazzei L, Musiani F, Maroney MJ, Ciurli S. Nickel binding properties of Helicobacter pylori UreF, an accessory protein in the nickel-based activation of urease. J Biol Inorg Chem. 2014;19:319–334. doi: 10.1007/s00775-013-1068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellucci M, Zambelli B, Musiani F, Turano P, Ciurli S. Helicobacter pylori UreE, a urease accessory protein: Specific Ni2+- and Zn2+-binding properties and interaction with its cognate UreG. Biochem J. 2009;422:91–100. doi: 10.1042/BJ20090434. [DOI] [PubMed] [Google Scholar]
- 28.Shi R, Munger C, Asinas A, Benoit SL, Miller E, Matte A, Maier RJ, Cygler M. Crystal structures of apo and metal-bound forms of the UreE protein from Helicobacter pylori: Role of multiple metal binding sites. Biochemistry. 2010;49:7080–7088. doi: 10.1021/bi100372h. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Li H, Lai TP, Sun H. UreE-UreG complex facilitates nickel transfer and preactivates GTPase of UreG in Helicobacter pylori. J Biol Chem. 2015;290:12474–12485. doi: 10.1074/jbc.M114.632364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strugatsky D, McNulty R, Munson K, Chen CK, Soltis SM, Sachs G, Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2012;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fong YH, Wong HC, Yuen MH, Lau PH, Chen YW, Wong KB. Structure of UreG/UreF/UreH complex reveals how urease accessory proteins facilitate maturation of Helicobacter pylori urease. PLoS Biol. 2013;11:e1001678. doi: 10.1371/journal.pbio.1001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley LA, Sternberg MJ. Protein structure prediction on the web: A case study using Phyre server. Nature Protocols. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 33.Johnson MK, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. NCBI BLAST: A better web interface. Nucl Acids Res. 2008;36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McWilliams H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis tool web services from the EMBL-EBI. Nucl Acids Res. 2013;41:W597–W600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Celniker G, Nimrod G, Ashkenazy H, Glaser F, Martz E, Mayrose I, Pupko T, Ben-Tal N. ConSurf: Using evolutionary data to raise testable hypotheses about protein function. Isr J Chem. 2013;53:199–206. [Google Scholar]
- 36.Mirjalili V, Feig M. Protein structure refinement through structure selection and averaging from molecular dynamics ensembles. J Chem Theory Comp. 2013;9:1294–1303. doi: 10.1021/ct300962x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 38.van Eunen K, Bouwman J, Westerhoff HV, Bakker BM. Measuring enzyme activities under standardized in vivo-like conditions for systems biology. FEBS J. 2010;277:749–760. doi: 10.1111/j.1742-4658.2009.07524.x. [DOI] [PubMed] [Google Scholar]
- 39.Phillips JC, Braun R, Wang WJ, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD. Scalable molecular dynamics with NAMD. J Comp Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacKerell AD, Feig M, Brooks CL. Improved treatment of the protein backbone in empirical force fields. J Am Chem Soc. 2004;126:689–699. doi: 10.1021/ja036959e. [DOI] [PubMed] [Google Scholar]
- 41.Best RB, Zhu X, Shim J, Lopes PEM, Mittal J, Feig M, MacKerell AD. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi, and side-chain chi(1) and chi(2) dihedral angles. J Chem Theory Comp. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darden T, York D, Pedersen L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 43.Miyamoto S, Kollman PA. Settle: An analytical version of the shake and rattle algorithm for rigid water model. J Comp Chem. 1992;13:952–962. [Google Scholar]
- 44.Martyna GJ, Tobias DJ, Klein ML. Constant-pressure molecular dynamics algorithms. J Chem Phys. 1994;101:4177–4189. [Google Scholar]
- 45.Feller SE, Zhang YH, Pastor RW, Brooks CL. Constant pressure molecular dynamics simulation: The Langevin piston method. J Chem Phys. 1995;103:4613–4621. [Google Scholar]
- 46.Sehnal D, Varekovaa RS, Berka K, Pravda L, Navratilova V, Banas P, Ionescu CM, Otyepka M, Koca J. MOLE 2.0: Advanced approach for analysis of biomacromolecular channels. J Cheminform. 2013;5:39. doi: 10.1186/1758-2946-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feig M, Karanicolas J, Brooks CL., III MMTSB tool set: Enhanced sampling and multiscale modeling methods for applications in structural biology. J Mol Graph Modell. 2004;22:377–395. doi: 10.1016/j.jmgm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 49.The PyMOL Molecular Graphics System. Schrödinger, LCC: Version 1.7.4 ed. [Google Scholar]
- 50.Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]
- 51.Chovancova E, Pavelka A, Benes P, Strnad O, Brezovsky J, Koszlikova B, Gora A, Sustr V, Klvana M, Medek P, Biedermannova L, Sochor J, Damborsky J. CAVER 3.0: A tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput Biol. 2012;8:e1002708. doi: 10.1371/journal.pcbi.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Persson I. Hydrated metal ions in aqueous solution: How regular are their structures. Pure and Applied Chemistry. 2010;82:1901–1917. [Google Scholar]
- 53.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: From enzyme databases to general principles. J Biol Inorg Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 54.Waldron KJ, Robinson NJ. How do bacterial cells ensure that metalloproteins get the correct metal? Nature Rev Microbiol. 2009;6:25–35. doi: 10.1038/nrmicro2057. [DOI] [PubMed] [Google Scholar]
- 55.Kuchar J, Hausinger RP. Biosynthesis of metal sites. Chem Rev. 2004;104:509–526. doi: 10.1021/cr020613p. [DOI] [PubMed] [Google Scholar]
- 56.Cotruvo JA, Jr, Stubbe J. Metallation and mismetallation of iron and manganese proteins in vitro and in vivo: the class I ribonucleotide reductases as a case study. Metallomics. 2012;4:1020–1036. doi: 10.1039/c2mt20142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y, Ribbe MW. Biosynthesis of nitrogenase FeMoco. Coord Chem Rev. 2011;255:1218–1224. doi: 10.1016/j.ccr.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leach MR, Zamble DB. Metallocenter assembly of the hydrogenase enzymes. Curr Opin Chem Biol. 2007;11:159–165. doi: 10.1016/j.cbpa.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Schmidt PJ, Ramos-Gomez M, Culotta VC. A gain of superoxide dismutase (SOD) activity obtained with CSS, the copper metallochaperone for SOD1. J Biol Chem. 1999;274:36952–36956. doi: 10.1074/jbc.274.52.36952. [DOI] [PubMed] [Google Scholar]
- 60.Robinson NJ, Winge DR. Copper metallochaperones. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raushel FM, Thoden JB, Holden HM. Enzymes with molecular tunnels. Acc Chem Res. 2003;36:539–548. doi: 10.1021/ar020047k. [DOI] [PubMed] [Google Scholar]
- 62.Maret W, Wedd A, editors. Binding, Transport and Storage of Metal Ions in Biological Cells. Royal Society of Chemistry; Cambridge, U.K.: 2014. [Google Scholar]
- 63.Carrondo MA. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. EMBO J. 2003;22:1959–1968. doi: 10.1093/emboj/cdg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulder DW, Boyd ES, Sarma R, Lange RK, Endrizzi JA, Broderick JB, Peters JW. Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAΔEFG. Nature. 2010;465:248–251. doi: 10.1038/nature08993. [DOI] [PubMed] [Google Scholar]
- 65.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 66.Mulrooney SB, Pankratz HS, Hausinger RP. Regulation of gene expression and cellular localization of cloned Klebsiella aerogenes (K. pneumoniae) urease. J Gen Microbiol. 1989;135:1769–1776. doi: 10.1099/00221287-135-6-1769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


