SUMMARY
Ubiquitin (Ub) signaling is a diverse group of processes controlled by covalent attachment of small protein Ub and the polyUb chains to a range of cellular protein targets. Best documented Ub signaling pathway is the one that delivers polyUb-proteins to the 26S proteasome for degradation. However, studies of molecular interactions involved in this process have been hampered by the transient and hydrophobic nature of these interactions and the lack of tools to study them. Here, we develop Ub-phototrap (UbPT), a synthetic Ub variant containing a photoactivatable crosslinking side chain. Enzymatic polymerization into chains of defined lengths and linkage types provided a set of reagents that led to identification of Rpn1 as a third proteasome ubiquitin-associating subunit that coordinates docking of substrate shuttles, unloading of substrates, and anchoring of polyUb-conjugates. Our work demonstrates the value of UbPT and we expect that its future uses will help define and investigate the ubiquitin interactome.
eTOC
Application of polyubiquitin-phototrap (polyUbPT), a novel set of chain-specific inducible photo-crosslinking probes, enables trapping of transient partners through the hydrophobic patch of ubiquitin. PolyUbPT captured Rpn1 from intact proteasome complexes. Rpn1 joins Rpn10 and Rpn13 as proteasome subunits with affinity for polyUb and Ub-like domains.
INTRODUCTION
Myriad intracellular processes in eukaryotes are directed through ubiquitin (Ub) signaling (Glickman and Ciechanover, 2002; Hershko and Ciechanover, 1998). The versatility of Ub signaling is largely due to the numerous ways in which individual Ub units can be assembled into polymers. Forming an isopeptide bond, the carboxyl-terminus of one Ub module links the ε-amine residue of a lysine side chain of another in poly-Ubiquitin (polyUb) chains. Eight different linkages in polyUb chains include conjugation at the seven-lysine side chains on each Ub molecule (K6, K11, K27, K29, K33, K48, and K63) and elongation through the N-terminus of Ub. In a similar manner, polyUb chains are attached either to a lysine residue or to the N-terminus of a target protein. The linkage type and length of the polyUb signal determines the fate of the conjugated target: for example, K48-linkages are the canonical signal for degradation by proteasomes, whereas K63-linked chains are involved in non-degradative pathways (e.g. intracellular sorting, membrane-associated trafficking, or DNA damage response). The outcome often requires ubiquitin-binding proteins that interpret each specific signal. Ubiquitin binding domains (UBDs) span several distinct protein families and are broadly distributed throughout the cell (Husnjak and Dikic, 2012; Rahighi and Dikic, 2012; Scott et al., 2015). Most UBDs show a marked preference for polyUb chains over monoUb, and in some cases recognition is linkage specific (Fushman and Wilkinson, 2011; Hofmann, 2009; Hurley et al., 2006; Raasi et al., 2005; Sims and Cohen, 2009). For instance, one important class of ubiquitin-binding proteins shuttles polyUb-conjugates, primarily K48-linked, from various cellular locations to proteasome complexes, where they are degraded. As molecular mediators, association of UBDs with polyUb tends to be transient in order to facilitate relay of cargo at its final destination. Capturing these relatively intermediate-strength interactions is an experimental challenge.
The 26S proteasome, a 2.5 MDa multisubunit complex composed of a proteolytic 20S core particle (CP) and a 19S regulatory particle (RP), is the final destination for many polyUb-tagged cellular proteins (Mayor et al., 2016). Two proteasome subunits, Rpn10 and Rpn13 (recently joined by Rpn1 (Shi et al., 2016)), are established polyUb receptors and are thought to serve as docking sites for polyUb conjugates. In addition, a number of transiently proteasome-associated shuttle proteins facilitate degradation by targeting ubiquitin-conjugates to proteasomes. The bivalent shuttles capture polyUb by means of a ubiquitin-associated (UBA) domain and simultaneously dock at the 19S RP via a ubiquitin-like (UBL) domain. Although UBL domains associate with Rpn1 at the proteasome, docking of these shuttles may partially overlap with the site of direct polyUb binding since Rpn10 and Rpn13 display affinity for UBLs as well as for polyUb (Elsasser et al., 2004; Elsasser et al., 2002; Fatimababy et al., 2010; Hamazaki et al., 2015; Husnjak et al., 2008; Kim et al., 2004; Matiuhin et al., 2008; Rosenzweig et al., 2012; Sakata et al., 2012; Schreiner et al., 2008; Zhang et al., 2009b). Once anchored, the hexameric ring of AAA-ATPases resident in the 19S RP (Rpt1–6) unfolds the substrate and promotes translocation into the proteolytic core of the 20S CP (Schweitzer et al., 2016). In parallel, proteasome-associated deubiquitinases (DUBs) remove the polyUb signal from the substrate (Finley, 2009; Glickman and Adir, 2004; Guterman and Glickman, 2004; Lee et al., 2011; Mansour et al., 2015). DUB and ATPase activities are carefully coordinated in the 19S RP to allow for proteolytic efficiency and recycling of ubiquitin (Aufderheide et al., 2015; Matyskiela et al., 2013; Peth et al., 2009; Peth et al., 2013a, b; Peth et al., 2013d; Singh et al., 2016; Verma et al., 2002; Verma et al., 2000). These activities are coordinated, to a large extent, by the two largest subunits in the 19S RP, Rpn1 and Rpn2 that function as flexible scaffolds. Both proteins contain a central domain of multiple alpha-turn-alpha proteasome/cyclosome (PC) repeats that fold into structurally similar highly curved toroids, extended by divergent flexible N- and C-terminal regions (Effantin et al., 2009; He et al., 2012; Kajava, 2002; Rosenzweig et al., 2012). Although Rpn1 and Rpn2 share much in common structurally (Effantin et al., 2009; He et al., 2012; Lander et al., 2012; Unverdorben et al., 2014), their different positions within the 19S RP and different binding partners make them fascinating candidates for functional analysis.
Rpn1 associates with UBL domains found in auxiliary proteins Rad23/hHR23, Dsk2/hPLIC/Ubiquilin, Ddi1, and Ubp6/USP14, all of which also contain a domain with high affinity for polyUb (Aufderheide et al., 2015; Elsasser et al., 2002; Kim et al., 2004; Nowicka et al., 2015; Peth et al., 2009; Peth et al., 2013a; Rosenzweig et al., 2012). The paralog subunit, Rpn2, has been shown to form tight interactions with the polyUb receptor Rpn13/ADRM1, and with the proteasome-associated DUB, UCH37/UCH-L5 (Aufderheide et al., 2015; Bashore et al., 2015; Hamazaki et al., 2006; He et al., 2012; Sakata et al., 2012). Determining how proteasome recognizes and processes substrates is the subject of intense research efforts. Beyond K48-linked polyUb modifications that have long been considered the primary proteasome targeting signal, a diverse range of polyUb signals can apparently be recognized by the proteasome (Lu et al., 2015; Mansour et al., 2015; Meyer and Rape, 2014; Nathan et al., 2013; Saeki et al., 2009). The limited binding capacity of Rpn10 and Rpn13, and the fact that they are not essential for viability of S. cerevisiae, indicate that additional proteasomal subunits interact either directly with polyUb, or with shuttle factors that aid targeting. In fact, a report suggests that Ubp6, as a rather slow acting DUB, is a principal proteasomal polyUb receptor (Peth et al., 2009). Thus, Ubp6, a transiently associating proteasomal subunit has been reported to double up as an anchor for polyUb-conjugates (Aufderheide et al., 2015; Peth et al., 2009). In contrast, Rpn11, a tightly incorporated proteasomal DUB, has a weak binding affinity for polyUb, raising the possibility that neighboring subunits bind and present polyUb to its catalytic site (Mansour et al., 2015; Pathare et al., 2014; Unverdorben et al., 2014; Worden et al., 2014; Yu et al., 2015). The relatively transient nature of polyUb binding coupled with many potential binding partners and ATP-dependent conformational changes upon substrate engagement (Beckwith et al., 2013; Matyskiela et al., 2013; Sledz et al., 2013b; Unverdorben et al., 2014) pose experimental challenges to track the trajectory of polyUb at proteasomes. The hydrophobic nature of most polyUb recognition events restricts application of many crosslinking approaches, typically modification of polar groups (i.e. crosslinking amine residues and thiols).
In this study, we introduce Ub-phototrap (UbPT), a variant of ubiquitin in which native leucine residues at a position of choice are replaced by a photoactivatable crosslinking leucine mimic, photoleucine (pLeu). By using linear total chemical synthesis of the 76 amino acid Ub polypeptide, pLeu was introduced at position 8 or 73 in the Ub sequence with high efficiency. The resulting UbPT is recognized and activated by ubiquitination enzymes, and is smoothly incorporated into homogenously linked polyUb chains (i.e. K48 and K63) of desired length. Next, these conjugates prove to be specifically recognized by UBDs, and to be disassembled by DUBs. We validated the use of polyUbPT on intact 26S proteasome complexes in trapping Rpn10 and Rpn13. We then identified Rpn1 as a third proteasome ubiquitin-associating subunit by applying polyUbPT. With isolated Rpn1, the binding region on Rpn1 was narrowed down to the first PC repeat cluster. Nuclear magnetic resonance (NMR) experiments demonstrated that monoUb and polyUb bind Rpn1 through the canonical hydrophobic patch (formed by L8, I44, V70). Competition experiments demonstrated that binding of UBL domains from shuttling factors partially overlaps with binding of polyUb to Rpn1. We conclude that pLeu is a modular and versatile reagent with a unique ability to trap, irreversibly, protein-protein interactions of hydrophobic nature. Due to these properties, polyUbPT is particularly useful for studying Ub-associating proteins in complex or in isolation.
RESULTS
Chemical synthesis of UbPT and hybrid approach for the generation of polyUbPT reagents
Ub-phototrap (UbPT) was prepared in a linear fashion by the solid phase peptide synthesis reported earlier (El Oualid et al., 2010) (see Supplementary Information and Supplementary Schemes 1–10 for general protocol and details of chemical synthesis). Here, the stepwise elongation of the Ub polypeptide is facilitated by the use of pseudoproline and dimethoxybenzyl dipeptides (by preventing the formation of folded and/or aggregated intermediates on-resin). The required Fmoc protected photoleucine building block (Janz et al., 2011) was prepared from commercially available L-photoleucine (Figure 1A) and incorporated into the Ub sequence (Zhou et al., 2016). After global deprotection of the synthetic Ub with 90% TFA followed reversed phase HPLC, pure UbPT was obtained in 20 – 25% overall yield.
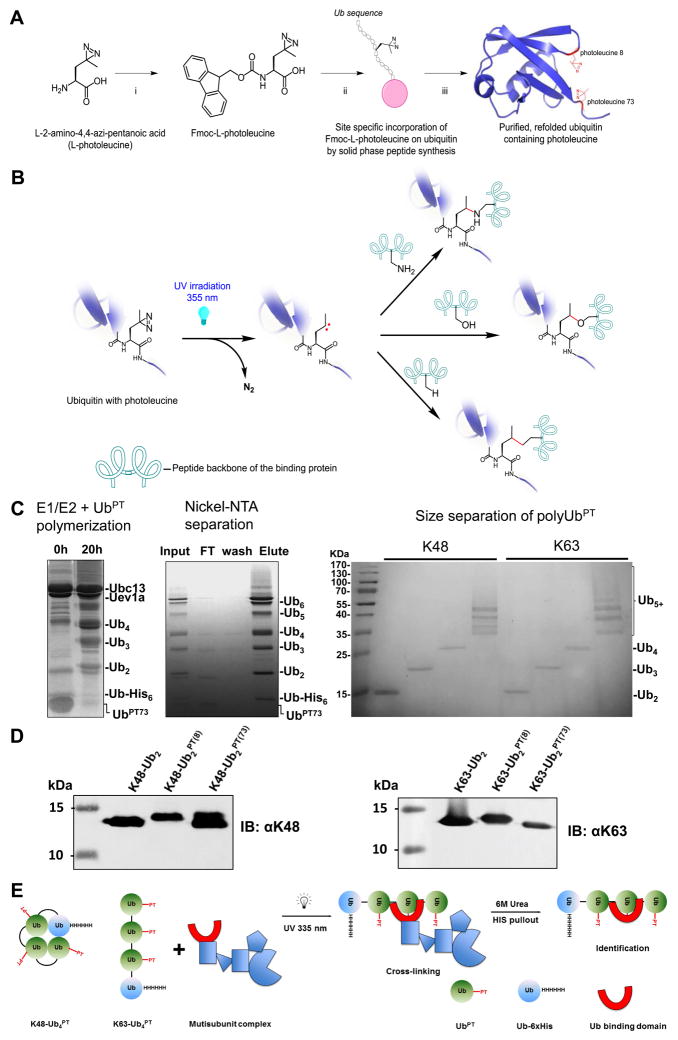
Figure 1.
Polyubiquitin-phototrap (polyUbPT), a ubiquitin variant containing photoactivatable crosslinking groups. (A) Synthesis of ubiquitin containing photoleucine. Intermediates shown in the diagram with reaction conditions for each step: i. Fmoc-OSu, 10% aq Na2CO3, THF, 96%; ii. SPPS; iii. 95% TFA, 2.5% triisopropylsilane, 2.5% H2O. (B) Mechanism of photo-crosslinking. After UV irradiation at 355 nm, the diazirine moiety (left) is released as N2 and forms a highly reactive singlet carbene on the alkyl side-chain of Ub (center). The carbene can then react with nearby protein residues or chemical functional groups forming a new covalent bond (right). (C) Enzymatic polymerization of polyUbPT. UbPT was polymerized into polyUb chains by incubating with E1, linkage-specific E2 (example show for K63 chains), and substoichiometric Ub-His6 (left). Ni2+-NTA separation removed unpolymerized Ub monomers (center). Chains of homogenous length separated by size exclusion (right). (D) Linkage-specific antibodies recognize diUbPT in which Leu8 or Leu73 were replaced with photoleucine, similar to cognate diUb. (E) Scheme to detect linkage-specific polyUb-binding subunits using 6xHis tagged polyUbPT initiated by photoactivation followed by denaturing isolation.
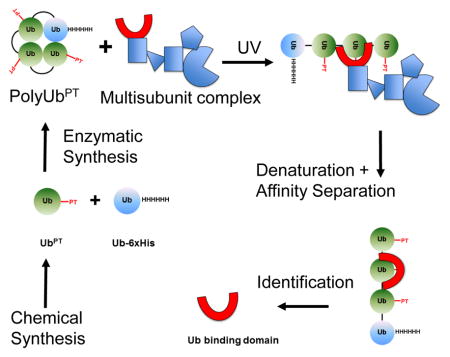
Provided that the photoleucine residue (pLeu) is in close proximity to another protein, there are multiple ways for crosslinking to occur allowing it to be a potent crosslinker (Figure 1B). Following photo-activation, the reactive singlet carbene on the alkyl side-chain of pLeu can bond covalently with a number of common functional groups in proteins, thereby increasing the likelihood of trapping binding partners. However, as hydroxyl groups are also prevalent in aqueous environments, the effective chemical half-life of the reactive singlet carbene on exposed or unattached pLeu is short; the trap is essentially self-limiting due to quenching by water. This property decreases crosslinking to spurious proteins thereby increasing specificity of pLeu embedded in a protein to trap specific binding partners. To expand the use of UbPT, monomeric Ub molecules in which Leucine either at position 8 or 73 was replaced by pLeu, UbPT(8) and UbPT(73) respectively, were ligated enzymatically into homogenous K48- or K63-linked polyUb chains of defined length with efficiencies comparable to unmodified ubiquitin (Figure 1C, 1D). Herein we refer to polyUbPT variants according to their linkage type, chain length, and position of photoleucine: K48-Ub2PT(8), K48-Ub2PT(73), K48-Ub4+PT(8), K48-Ub4+PT(73), K63-Ub2PT(8), K63-Ub2PT(73), K63-Ub4+PT(8), and K63-Ub4+PT(73). Mixing UbPT with natural, tagged, or mutated ubiquitin and careful choice of E2 ubiquitin conjugation enzymes allows polymerization of chains of modular compositions for use as highly adaptable tools (e.g., for monitoring association with distal vs. proximal Ub units in a chain). For instance, by enzymatically polymerizing UbPT onto a proximal Ub6xHis module we designed a scheme that allows the isolation of individual Ub-binding subunits from multi-domain complexes after crosslinking under denaturing conditions (Figure 1E).
PolyUbPT traps polyUb-binding proteins
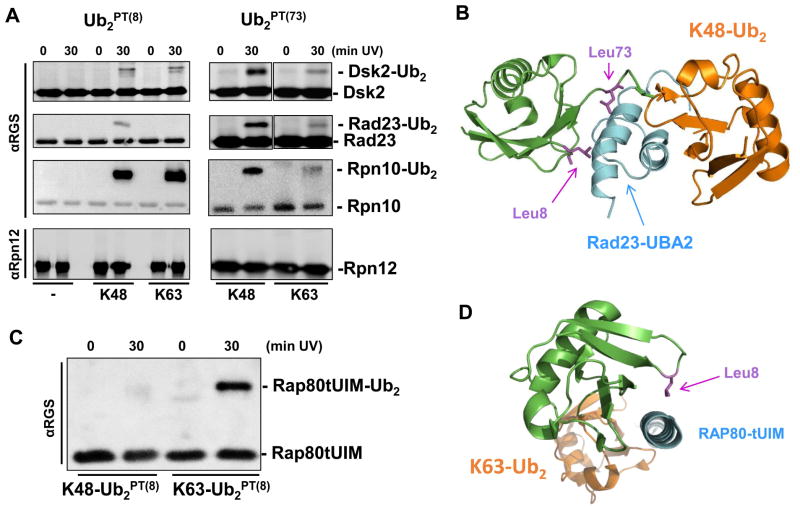
Linkage-selective antibodies recognized homogenous K48-linked or K63-linked diUb polymerized from UbPT(8) or UbPT(73) with similar efficiency to dimers from unmodified wild-type Ub (Figure 1D). This encouraging observation indicated that polyUbPT could be used to trap linkage-specific Ub-binding proteins. We confirmed the ability of polyUbPT to crosslink proteasome-associated polyUb-shuttles and polyUb receptors. For this purpose, we chose dual-function proteins known to function both independently and at the proteasome: Rad23, Dsk2, and Rpn10. The first two are representatives of the UBL-UBA family of shuttle proteins (Diaz-Martinez et al., 2006; Hofmann and Bucher, 1996; Lowe et al., 2006; Ohno et al., 2005; Raasi et al., 2004; Wilkinson et al., 2001; Zhang et al., 2008), whereas Rpn10 is a UIM-containing receptor (Matiuhin et al., 2008; Riedinger et al., 2010). Recombinant Rad23, Dsk2 and Rpn10 crosslinked to both Ub2PT(8) and Ub2PT(73) of either K48 or K63 linkage type with varying efficiencies, depending on the particular Ub-binding protein (Figure 2A). This is a notable observation given that all three aid proteasome function by shuttling ubiquitin-conjugates. Thus, Dsk2 was able to crosslink with both K48- and K-63 linked polyUb chains in agreement with our earlier results that the human ortholog, ubiquilin-1, binds both linkage types comparably (Raasi et al., 2004; Sims et al., 2009; Zhang et al., 2008), though in the current experiment it did display mildly higher efficiency when pLeu was located at position 73 of the Ub signal (Figure 2A). Likewise, Rad23 showed a marked preference for K48-Ub2PT(73) over K63-linked diUb, consistent with published reports of K48-linkage specificity of UBA1 and UBA2 polyUb-binding domains of the mammalian ortholog hHR23 (Raasi et al., 2004; Varadan et al., 2005). The molecular structure of UBA2 in complex with K48-Ub2 highlights the proximity of leucine residues 73 and 8 to the UBA binding surface (Figure 2B). In agreement with expectations based on earlier reports (Girod et al., 1999; Matiuhin et al., 2008; Miller et al., 2004; Zhang et al., 2009a; Zhang et al., 2009b), also Rpn10 crosslinked to either linkage type, albeit more efficiently to Ub2PT(8) than to Ub2PT(73), alluding to the residues they contact on the surface of Ub. This property probably reflects the orientation of Ub in association with UBA or UIM domains of binding proteins (Hurley et al., 2006). No crosslinked product was detected with Rpn12, a proteasome subunit that served as a negative control, supporting the specificity of polyUbPT for trapping Ub-associating proteins (Figure 2A).
Figure 2.
Ub2PT crosslink to polyUb-binding proteins. (A) K48-linked or K63-linked diUbPT was crosslinked to Dsk2, Rad23, Rpn10, or a control protein Rpn12 according to protocol described in Figure 1B. (B) Model of Rad23-UBA2 molecular structure (cyan) in complex with K48-Ub2, distal Ub (green) and proximal Ub (orange) based on PDB: 1ZO6. In this orientation, proximity of Leu73 and of Leu8 (magenta sticks) of the distal Ub to the receptor are apparent. (C) RAP80-tUIM selectively crosslinks to K63-Ub2PT(8). In this case, RAP80-tUIM efficiently crosslinked to K63-Ub2PT(8) but not to a dimer linked via Lys48, demonstrating that UbPT allows for trapping of K63-linkage selectivity. (D) Crystal structure of RAP80-tUIM (cyan) in complex with K63-Ub2 (distal Ub green and proximal Ub orange) from PDB:3A1Q, highlights that Leu8 (magenta stick) on the distal Ub is in close proximity to the ligand.
Through work with both ubiquitination enzymes and polyUb shuttles, we demonstrated that alteration of the environment or nature of the interactions by this replacement is minimal and readily recapitulates known behaviors of unmodified proteins. Use of pLeu has proven successful to map intra-complex interactions in cis, by demonstrating that pre-attached ubiquitin on histone H2B comes in contact with the N-terminus of histone H2A (Zhou et al., 2016). By replacing one of the leucine residues involved in binding of ubiquitin to many receptors or shuttles, we show herein that pLeu provides an unrivaled tool to study transient hydrophobic associations typical of ubiquitin and polyUb chains.
An extensively studied UIM-containing protein is RAP80, a polyUb-binding protein that participates in DNA repair presumably unrelated to proteasome function (Wang et al., 2007). Rap80 has been documented to associate selectively with K63-linked polyUb (binding affinity of RAP80 tandem ubiquitin interacting motif (tUIM) to K63-Ub2 or K48-Ub2 reported to be KD=21.6 ± 0.8 μM and KD=157 ± 8 μM, respectively (Sims and Cohen, 2009)). Indeed, RAP80 retained its K63-linkage specificity in crosslinking to Ub2PT(8) (Figure 2C). The orientation of Rap80-tUIM (cyan) in complex with K63-Ub2 positions Leu8 on the distal Ub unit in close proximity to the UIM of RAP80 (Figure 2D), explaining the efficiency of crosslinking with K63-Ub2PT(8). Crosslinking of RAP80tUIM with K48-Ub2PT(8) was negligible. These results highlight the importance of optimizing the position of the photoleucine residue in UbPT, depending on targeted receptors. With validation of UbPT on established Ub receptors, we set out to capture Ub-binding components of protein complexes.
PolyUbPT identifies Rpn1 as a polyUb-binding protein
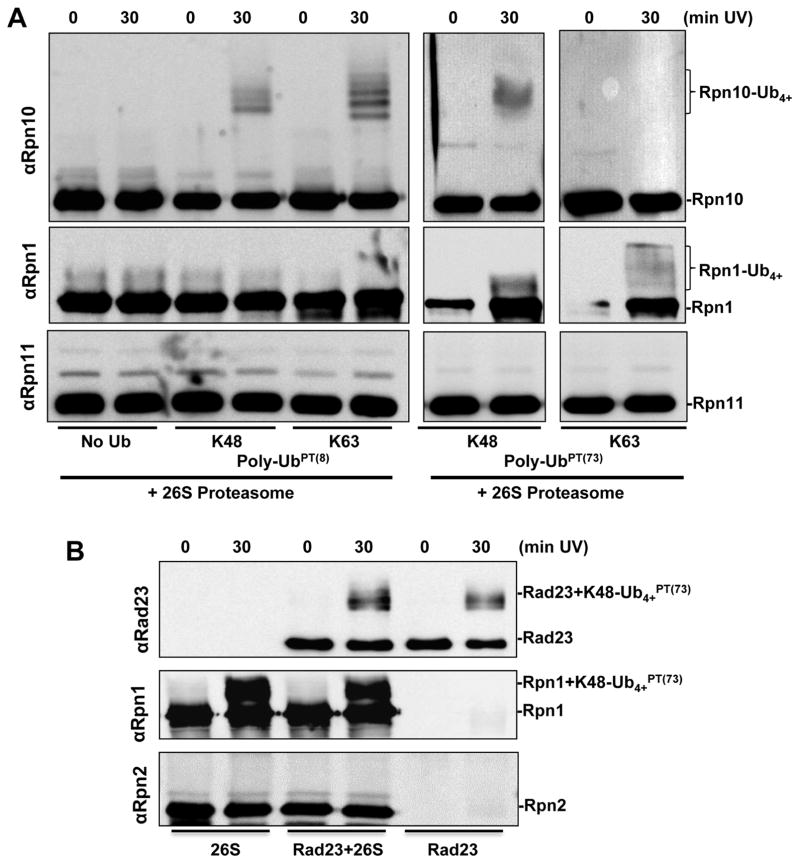
Having confirmed that pLeu does not interfere with the hydrophobic nature of recognition by typical Ub-binding proteins and that it can be useful to trap shuttles or standalone receptors, we set out to evaluate its specificity in pinpointing Ub-binding proteins within multi-subunit complexes. Proteasomes are made up of some 35 subunits, at least 6 of which associate with polyUb during the catalytic cycle. Applying our approach to isolate ubiquitin-binding components of a multi-subunit complex specifically (Figure 1E), we found that polyUbPT trapped Rpn10 and Rpn1 from intact and functional proteasome complexes, without spuriously crosslinking to neighboring subunits (Figure 3A). Proteasome-bound Rpn10 crosslinked more efficiently to polyUbPT(8), maintaining its properties as a stand-alone protein (Figure 2). In contrast, proteasome-incorporated Rpn1 was trapped more efficiently by K48-polyUbPT(73) suggesting that the orientation by which it binds Ub chains differs from Rpn10 (Figure 3A). It is important to point out that polyUbPT was specific for polyUb-binding subunits, and despite being in close proximity to other subunits in the same multi-subunit complex, other subunits did not crosslink to polyUbPT (Figure 3). Interestingly, a proteasome-associated DUB, Ubp6, was trapped by polyUbPT (Figure S1). Successful crosslinking of Ubp6C118A with polyUbPT(8) highlights the utility of UbPT to uncover a range of interaction affinities, even transient enzyme-substrate interactions. Together, these data establish that polyUbPT can pinpoint subunits that directly associate with Ub within a multi-subunit, multi-tasking complex.
Figure 3.
PolyUbPT is tested against purified yeast proteasome. (A) In the proteasome Rpn10 shows a preference to bind polyUbPT(8) (top panel), while Rpn11 shows no detectable interaction with polyUbPT. (B) K48-Ub4+PT(73) retains its ability to crosslink to Rad23 (top panel); Rpn1 retains its ability to recognize polyUbPT in the proteasome regardless of Rad23 (middle panel), no crosslinking is detected with Rpn2 (bottom panel).
PolyUb chains can anchor at proteasome complexes directly, or may be tethered by auxiliary factors. In order to test the effect of shuttle proteins on crosslinking of polyUbPT to ubiquitin receptors at proteasome, we incubated purified 26S proteasomes with K48-Ub4+PT(73), with or without excess Rad23 to emulate the role of a polyUb-substrate shuttle. We found that presence of Rad23 had no effect on K48-Ub4PT(73) crosslinking to Rpn1 in the proteasome (Figure 3B). Under these conditions, Rad23 did not alter the ability of Rpn1 to recognize polyUb while retaining its own ability to bind polyUb as evident by crosslinked product with polyUbPT (Figure 3B). Thus far, polyUbPT emerged as a potent tool to accurately and rapidly pinpoint and isolate polyUb-binding proteins in mixed or complex environments. With Rpn1 being the newest and least studied of the proteasome-associated polyUb-binding proteins, our focus turned to what additional information polyUbPT can provide on Rpn1 as a potential Ub receptor in the proteasome.
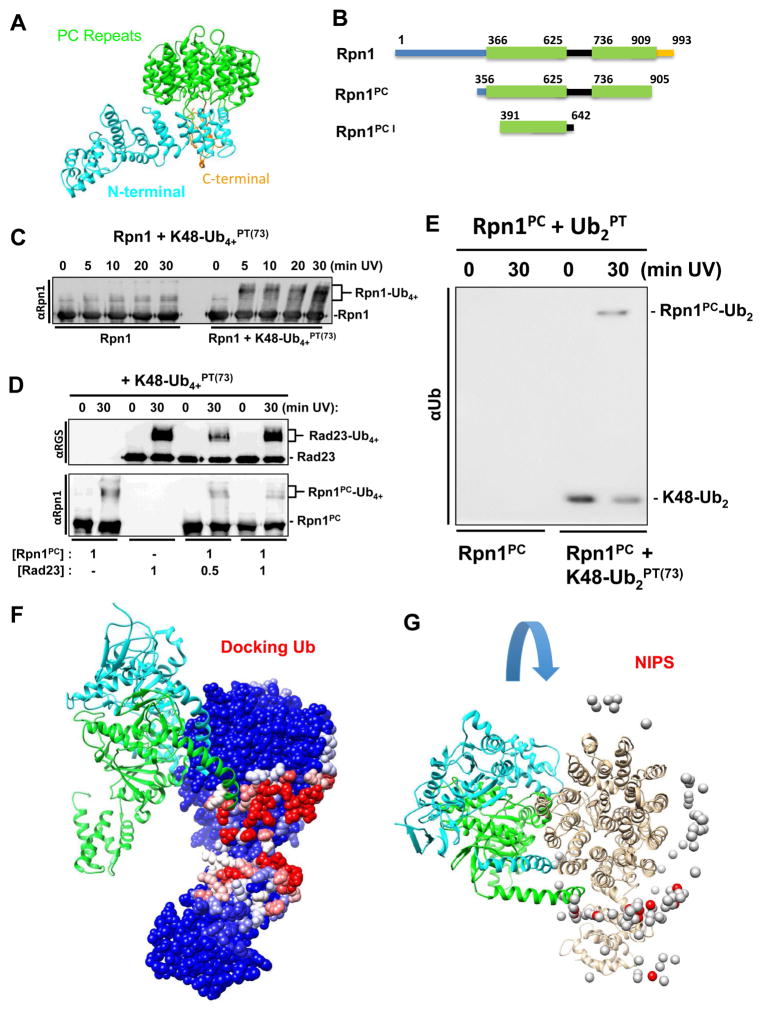
UBL-UBA proteins such as Rad23 constitute the main class of shuttles for polyUb-conjugates, dock at the proteasome through their UBL domain to PC repeats situated in the central region of the Rpn1 proteasome subunit. Structurally, Rpn1 can be divided into three segments: a central toroid made up of repetitive α-turn-α repeats termed PC repeat (Effantin et al., 2009; Kajava, 2002; Lupas et al., 1997), flanked by flexible N- and C- extensions (Figure 4A). In each PC repeat, the outer α-helix contains bulkier amino acid side chains causing the repetitive structure to curve inwards into a concave arc imaged as a closed donut shaped torroid in proteasomes (Aufderheide et al., 2015; Effantin et al., 2009; Schweitzer et al., 2016). The PC repeats cluster in two – which we term PC1 and PC2 (Figure 4B) – interspersed with a highly charged segment for which little structural information is available (Unverdorben et al., 2014). As it happens, UBL (and most likely Ub) binding maps to PC1 (Gomez et al., 2011; Shi et al., 2016). First, we confirmed that polyUbPT was competent to trap full-length recombinant Rpn1 as a stand-alone protein unassociated with 26S complexes (Figure 4C). The relatively fast appearance of crosslinked product, within five minutes, suggests that the interaction is significant. However, the size and conformational flexibility of Rpn1 posed hurdles for purification strategies and solubility. Therefore, we found that a truncated segment of Rpn1 covering the PC repeats (aa 356–905; Rpn1PC), or even just the first set of PC1 repeats (aa 391–642; Rpn1PC1) were easily purified as soluble monomeric proteins and retained competence to crosslink polyUbPT (Figure 4D, 4E, S2). Compared to the full-length Rpn1 protein, these smaller polypeptides were more amenable for subsequent biophysical assays such as NMR experiments. K48-linked polyUbPT efficiently trapped Rpn1PC. Once again, the presence of Rad23 had little effect on K48-Ub4PT(73) crosslinking to Rpn1PC, even though Rad23 itself was also competent to bind and crosslink to these K48-linked polyUb chains (Figure 4D). This result may indicate either that multiple sites on Rpn1 associate with Ub or UBLs, or that at stoichiometric ratios neither ligand binds Rpn1 tightly enough to exclude the other. Nevertheless, association of Rpn1PC with ubiquitin was significant enough that crosslinked products were detected even with unanchored dimeric ubiquitin (K48-Ub2PT(73); Figure 4E, S2), indicating that Rpn1 is an inherent Ub-binder.
Figure 4.
(A) Rpn1 structure from PDB 4CR2 (chain Z) with PC repeats (green), N-terminal region (cyan), and flexible linker (gold). (B) Schematic dissection of Rpn1 domains used in the study (PC repeats in green). C) Time course of crosslinking of K48-Ub4+PT(73) and full-length Rpn1. (E) K48-linked diUbPT(73) was crosslinked to Rpn1PC (Rpn1356–905). (D) K48-Ub4+PT(73) successfully crosslinks with Rad23 alone and in varying concentrations of Rpn1PC (top). Unexpected crosslinking products of K48-Ub4+PT(73) and Rpn1PC are uncovered with anti-Rpn1 (bottom). (F) Molecular docking analysis using PDB 4CR2 for the Rpn1 structure and the PDB 1UBQ for ubiquitin. The position of two nearest neighbors in the 19S, Rpt1 and Rpt2, are shown in light blue and green respectively according to the EM model. (G) Rpn1 colored by NIP values (see methods, higher values in red) in the context of the proteasome with its interacting subunits. 26S protease regulatory subunit 7 homologs (Rpt1, chain H, colored cyan) and 26S protease regulatory subunit 4 homolog (Rpt6, chain I, colored green). Ub docking does not interfere with binding of RPN1 to the proteasome.
PolyUb-binding region of Rpn1
As an integral subunit of the proteasome complex, Rpn1 associates with several neighboring subunits in the 19S. Does this leave sufficient surface area exposed to bind its ligands such as UBLs or polyUb? From current cryoEM-derived proteasome models (PDB 4cr2), Rpn1 is situated peripherally on the 19S RP touching two AAA ATPase subunits Rpt1 and Rpt2 (Figure S3). Not surprisingly, most evolutionary conserved residues on the surface of Rpn1 identified with the ConSurf server contacted neighboring subunits Rpt1 and Rpt2 when incorporated into the proteasome, although a few exposed residues that are not in direct contact with any other proteasome subunit are also highly conserved between Rpn1 sequences across eukaryotes (Figure S3). By focusing on Rpn1 (chain Z in PDB 4cr2) and its two nearest neighbors, it becomes apparent that half of the PC toroid (see previous paragraph) is solvent-exposed suitably positioned to serve as a docking station for substrates or substrate-shuttles possibly explaining the presence of highly conserved exposed residues (Figure 4F, S3).
An in silico docking experiment with PyDock WEB server revealed a surface on Rpn1 with high-energy docking poses for complexes with free Ub (Figure 4F, G, S3). The docking experiment was performed on Rpn1 structure and though interactions were not restricted (e.g. free docking), the most preferred Ub docking sites mapped to the exposed region of the PC domain and to the hinge between the PC domain and the N-terminus (Red; Figure 4F). When performing docking of Ub onto the PC domain structure (Figure 4G), the 100 most preferred docking results distribute along the exposed edge of the PC ring particularly along the segment aa ~350–640 that encompasses the highly conserved hydrophobic residues in the first PC repeat (PC1; Figure 4A). This is precisely the area that has been shown before to come in contact with UBL domains of substrate shuttles or with polyUb chains (Shi et al., 2016), encouraging us to utilize Rpn1PC1 for further studies.
Features of polyUb binding to the first PC region of Rpn1
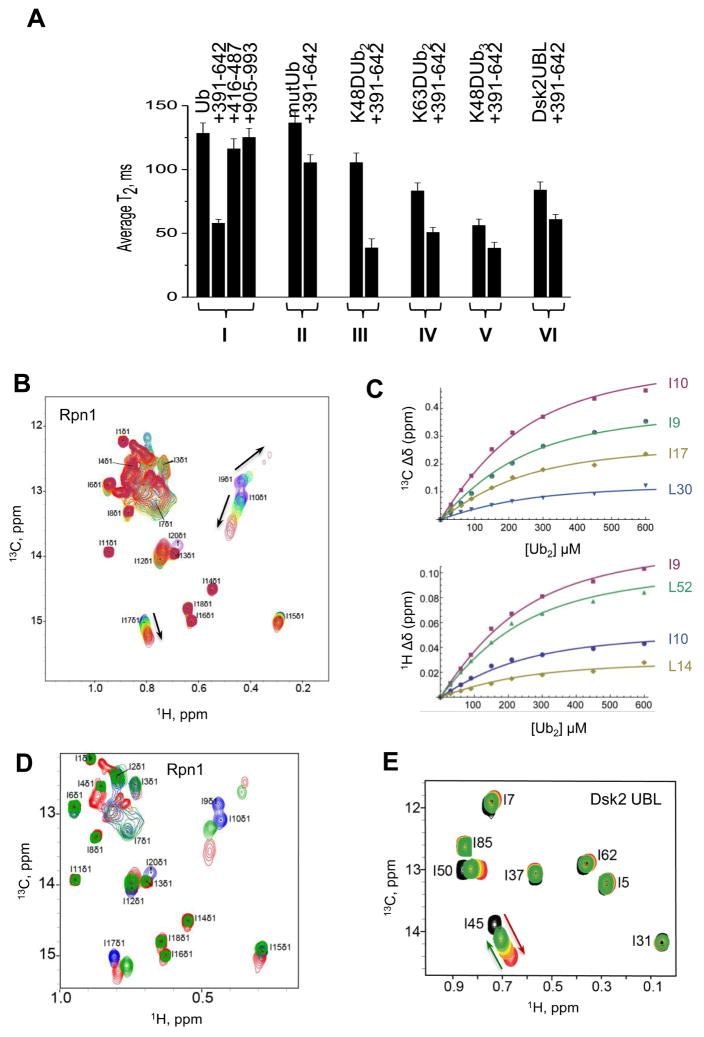
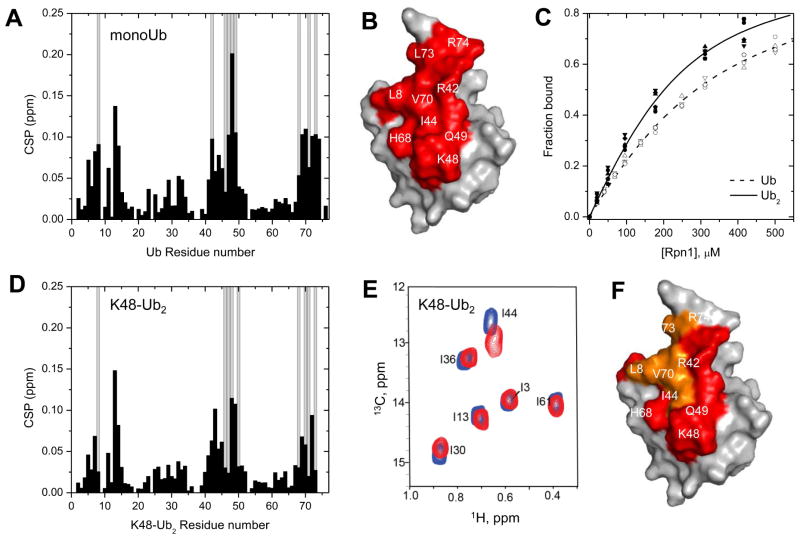
The PC1 fragment of Rpn1 is small enough to make it amenable for biophysical characterization by NMR assays. Measurements of the 15N transverse relaxation time (T2) of backbone amides in monoUb in complex with Rpn1 fragments narrowed the region of association to the PC region, and specifically to the first set of PC repeats, PC1 (Figure 5A, lane I). No binding of polyUb was detected to truncated fragments of Rpn1 that did not contain the first PC region of Rpn1, we deduced that the first PC region (~aa 356–625) is essential for association of Rpn1 with monoUb through hydrophobic interactions typical of Ub-recognition by most receptors (Figure 5A; lanes I, II). The combined results indicate that PC1 is sufficient to bind free Ub (Figure 5A), though we do not rule out that other segments of Rpn1 may participate in binding or in anchoring ligands, as alluded to by the docking experiments (Figure 4F, G, S3). However, merely substituting Leu8 or Ile44 in Ub with Alanine, diminished its affinity for Rpn1PC1 confirming that the hydrophobic patch on the surface of ubiquitin is central to association with Rpn1 (Figure 5A, S4). Interestingly, using a similar experimental approach, the same PC1 segment could associate with multiple ligands: K48-Ub2, K63-Ub2 or the UBL domain of Dsk2 (Figure 5A, lanes III–VI). In order to observe the corresponding changes in Rpn1 upon binding of Ub, we selectively labeled methyl residues of Ile, Leu, Val, and Met in Rpn1PC1 with 13CH3 and measured chemical shift perturbations (CSPs) upon titration with increasing concentrations of K48-Ub2 (Figure 5B). Titration of 13CH3-labeled Rpn1PC1 (Ile, Leu, Val and Met residues have a labeled methyl group; 13CH3-ILVM-Rpn1PC1) with diUb produced a KD of 112±29 μM (Figure 5C, Table 1), which places it comparable or slightly weaker than other polyUb-receptors such as Rpn10 (Rosenzweig et al., 2012). Select CSPs of Ile and Leu groups demonstrated that specific hydrophobic residues in the first PC repeat region of Rpn1 participate in binding to Ub and to diUb (Figure 5B, C). In addition, these same methyl residues displayed chemical shift changes upon Rpn1 association with Rad23UBL (Figure 5D). A structure of Rad23UBL bound to residues in the same region was independently observed (Chen et al., 2016), confirming that Rpn1PC1 likely contains overlapping (or partially overlapping) binding sites for polyUb and for Rad23UBL.
Figure 5.
Analysis of the competition between Ub or Ub2 and UBL domains for binding to Rpn1. (A) 15N T2 of backbone amides (averaged over secondary structure residues) for the following proteins (left to right): (I) monoUb alone and in the presence of Rpn1391–642 or Rpn1416–487 or Rpn1905–993, at 1:1 molar ratio; (II) Ub mutant (L8A, I44A) alone and in the presence of Rpn1391–642 at 1:1 molar ratio; (III) distal Ub of K48-Ub2 alone, and in the presence of Rpn1391–642 (at 1:1 molar ratio); (IV) distal Ub of K63-Ub2 alone and in the presence of Rpn1391–642 at 1:1 molar ratio; (V) distal Ub of K48-Ub3 alone and in the presence of Rpn1391–642 at 1:1 molar ratio; (VI) Dsk2UBL alone, in the presence of Rpn1391–642. (B) Overlay of 1H-13C HMQC spectra of 13CH3-ILVM-labeled perdeuterated Rpn1391–642 free (purple) and at various points in titration with K48-Ub2 (from dark blue to red, 3:1 molar ratio). Shown is the spectral region containing CH3-Ile δ signals; the Rpn1 signals are numbered arbitrarily; the arrows show the directions of signal shifts. (C) Representative titration curves for select CH3-Ile δ signals of Rpn1391–642 as a function of K48-Ub2 concentration, for 13C (top) and 1H (bottom) resonances. The solid lines represent the results of a global fit to a 1:1 binding model. The Rpn1 signals are numbered arbitrarily. The average KD values are summarized in Table 1. (D) Overlay of 1H-13C HMQC spectra of 13CH3-ILVM-labeled perdeuterated Rpn1391–642 in the absence (blue) and presence of K48-Ub2 (red) or Rad23UBL (green). Shown is the spectral region containing CH3-Ile δ signals; the Rpn1 signals are numbered arbitrarily. Rpn1 concentration was 250 μM, and Rad23 and K48-Ub2 were 500 μM each. (E) Overlay of 1H-13C HMQC spectra of 13CH3-ILVM-labeled perdeuterated Dsk2UBL alone (black), in the presence of 2H-Rpn1391–642 at 2:1 molar ratio (red) and upon subsequent additions of unlabeled K48-Ub2 up to 16-fold excess (green). At the end-point of titration, the concentrations are: [Rpn1] = 300 μM, [Dsk2] = 300 μM, [Ub2] = 4.8 mM. Shown is the spectral region containing CH3-Ile δ signals; the assignment of Dsk2UBL signals is from (Chen et al., 2008). The red and green arrows highlight the signal shifts upon addition of Rpn1391–642 and Ub2, respectively.
Table 1.
Summary of Rpn1 interactions
| Analyte* | Ub (NH) | K48-Ub2(Dist,NH) | Rpn1 (CH3) | K63-Ub2 (Dist,NH) | Dsk2 UBL (NH) | Ubp6 UBL (NH) | Rub1 (NH) |
|---|---|---|---|---|---|---|---|
| ligand | Rpn1PC1 | Rpn1PC1 | K48-Ub2 | Rpn1PC1 | Rpn1PC1 | Rpn1PC1 | Rpn1PC1 |
| KD (μM) | 214±68 | 116±30 | 112±29 | 103±59 | 22±12 | 40±31 | 280±20 |
Titration of Rpn1 to 15N-Rad23 caused severe signal broadening in amide signals that precluded accurate determination of signal shifts for KD determination. The observation of signal broadening indicates intermediate or slow exchange regime, likely due to slow off-rates.
Rpn1PC1 bound UBL domains of proteasome shuttles tighter than Ub: Dsk2UBL with KD=22±12 μM and Ubp6UBL with KD=40±31 μM (Table 1). Titration of Rpn1 with 15N-Rad23UBL caused severe signal broadening in amide signals that precluded accurate determination of signal shifts for KD determination. The observation of signal broadening indicates intermediate or slow exchange likely due to slow off-rates, compatible with reported tight affinity (Shi et al., 2016). Since signal broadening was not observed for Dsk2 nor for diUb at similar conditions, this signifies fundamentally tighter Rpn1 binding to Rad23UBL compared to the other two ligands. Rub1, the UBL protein most closely resembling Ub (Singh et al., 2012) also bound the first PC stretch of Rpn1 with a KD=280±20 μM, an affinity comparable to that of monoUb (Table 1). These results indicate that UBL domains of proteasome shuttles have a greater affinity for Rpn1PC1 compared to reversible protein modifiers such as polyUb or Rub1 (Table 1).
Having mapped binding of both diUb and UBLs to the first PC region of Rpn1, we wished to evaluate whether they compete for the same site. Although binding of Rad23UBL and Ub have been mapped to same site (Shi et al., 2016), competition assays have not tested their relative affinities, and binding of similar UBLs have not been mapped. We designed a competition experiment to test whether prebound Dsk2UBL is displaced from Rpn1PC1 by excess diUb (Figure 5E). Initially, chemical shifts of methyl groups in 13CH3-ILVM-labeled Dsk2UBL were recorded in the free state (i.e. the ligand unbound to a receptor) and in complex with Rpn1PC1 (Figure 5E, black-to-red signals). Titration of this pre-formed complex with increasing concentrations of K48-Ub2 resulted in partial displacement of Dsk2UBL from Rpn1 at high ratios of diUb to UBL. Comparing the magnitudes of Dsk2UBL signal shifts (average over several residues; Figure 5E) before and after adding K48-Ub2 we estimate that 33.5 ± 2.4% of Dsk2UBL molecules remain in complex with Rpn1PC1 in the presence of 16X concentration of K48-Ub2. Using a mathematical model for competitive binding of two different ligands to the same site on a protein (Wang, 1995) and taking into account the respective experimental KD values for Dsk2UBL and for diUb (22 ± 12 μM, 112 ± 29 μM; Table 1), we predicted that the fraction of Rpn1PC1-bound Dsk2UBL should drop from 76.3 ± 5.7% of Dsk2UBL before the addition of diUb to 20.6 ± 10.7% at the end-point of our titration. Indeed our experimental results demonstrate a similar behavior to this prediction, with a partial overlap of the respective statistical ranges. The somewhat higher percentage of the Rpn1-bound Dsk2UBL observed in this assay may be the result of errors in the protein concentration measurements, but could also point to another binding site on Rpn1 for Ub2 molecules. Additional studies will be required to verify this.
The uniqueness of pLeu as a crosslinking reagent is the ability to capture interactions of a hydrophobic nature. Most documented receptors such as Rpn10, Rad23, Dsk2 and Rap80 (Figure 2) recognize ubiquitin via the so-called hydrophobic patch on its surface (centered on Leu8, Ileu44, Val70; (Pickart and Fushman, 2004)), therefore embedding pLeu into a polyubiquitin chain to generate a ubiquitin PhotoTrap (UbPT) minimally perturbs hydrophobic residues on its surface offering the potential to study the “sphere-of-interactions” revolving around ubiquitin. Having used this approach to pin down association of ubiquitin to the PC stretch of Rpn1 on proteasome complexes (Figure 3, 4), we turned our focus to the complementary binding surfaces on ubiquitin. Upon titrating 15N-labeled monoUb with Rpn1PC1, the majority of significant CSPs pointed to hydrophobic residues on the surface of Ub centered on the canonical hydrophobic patch (Figure 6A, B). Binding affinities of Rpn1 for K48-linked diUb or for monoUb were derived from a global fit of multiple CSP values upon titrations with ligand and estimated to have a KD of 116±30 μM or 214±68 μM, respectively (Figure 6C, D, Table 1). Even K63-Ub2 bound Rpn1PC1 with a KD of 103±59 μM (Figure S5), suggesting that Rpn1 can interact with an array of polyUb signals without being particularly discriminatory of linkage type. It is important to clarify that these are apparent affinities that may reflect a variation in interactions between multiple residues on both receptor and ligand. A two-fold increase in the binding affinity of dimeric over monomeric Ub (KD dropping from ~215 to ~115 μM) does not imply cooperative binding. Indeed, upon binding of Rpn1, the CSPs from either amide (15N) or methyl (13CH3-ILVM) groups in either unit of K48-Ub2 pointed out that L8, I44, and V70 of both proximal and distal Ub units of K48-Ub2 were perturbed upon binding to Rpn1 (Figure 6E, F). These observations suggest that a change in the interface between the two units of ubiquitin occurs from the free to Rpn1-bound K48-Ub2 (Figure S5).
Figure 6.
NMR analysis of the binding interactions between Rpn1391–642 and mono and diUb. (A) Amide CSPs (black bars) in monoUb at the endpoint of titration with Rpn1PC1 (Rpn1391–642), as a function of residue number. Residues exhibiting strong signal attenuations (>75%) during the titration are marked with grey bars. (B) Map of the perturbed residues (red: CSP > 0.05 ppm and/or signal attenuations) on the surface of Ub. Some residues are indicated. (C) Titration curves for several residues in monoUb (open symbols) or K48-Ub2 (solid symbols) as a function of Rpn1 concentration. The lines (dashed or solid, respectively) represent the results of global fit of multiple CSP values upon titrations with ligand to a 1:1 binding model. The titrations started with 200 μM Ub or Ub2, and went up to 4.1-fold molar excess of Rpn1PC1 for monoUb and 3.1 for Ub2. Binding affinities of Rpn1 for monoUb or for K48-linked diUb were derived from a global fit (residues 7, 13, 14, 70, 72 in monoUb; 14, 44, 45, 49, 69 in diUb) and estimated to have a KD of 214±68 and 116±30 μM, respectively, which was identical within experimental error to the KD of 112±29 μM obtained from the reciprocal titration of 13CH3-ILVM-Rpn1PC1 with unlabeled K48-Ub2 (summarized in Table 1). (D) Amide CSPs (black bars) in the distal Ub of K48-Ub2 at the endpoint of titration with Rpn1PC1, as a function of residue number. Residues showing strong signal attenuations (>75%) during the titration are marked with grey bars. Note that the residues exhibiting perturbations in K48-Ub2 are essentially the same as in monoUb (panels A, B). Similar residues in K63-Ub2 showed perturbations upon titration with Rpn1PC1 (Figure S5). (E) Overlay of 1H-13C HMQC spectra of 13CH3-labeled ILVM-residues in perdeuterated K48-Ub2 in the absence (blue) or presence (red) of perdeuterated Rpn1PC1. Only methyl groups of Ile, Leu, Val, and Met were selectively 13CH3- labeled in an otherwise deuterated background (2H, 13CH3-ILVM). Strong CSPs were recorded primarily in L8, I44, and V70 of both proximal and distal Ub unit. Shown is the spectral region containing CH3-Ile δ signals. (F) Residues on Ub that exhibited spectral perturbations upon addition of Rpn1 to K48-Ub2: amide data are colored red and on top of them methyl data are colored orange.
To summarize, UbPT is a novel reagent to trap hydrophobic interactions of a variety of polyubiquitin modifications. The first experimental application of polyUbPT pinpointed PC repeats in Rpn1 (Rpn1PC1) as the primary docking site of polyUb on proteasomes Monitoring reciprocal changes determined that this association is coordinated by hydrophobic residues on the surface of Ub (Figure 6). Beyond ubiquitin recognition, incorporating pLeu into proteins of interest should extend similar possibilities to investigate hydrophobic associations of a plethora of signaling molecules.
DISCUSSION
In this study, we show how polyUbPT can be used effectively to selectively bind, trap and even isolate the preferred binding partner from a protein mixture, or to pinpoint a receptor on a protein complex containing multiple subunits with diverse properties. By fixing interactions, followed by isolation and identification of copurifying subunits, crosslinking is a particularly powerful tool to identify composition of complexes. Crosslinkers can even narrow down recognition elements in each participant. However, transient interactions pose an experimental hurdle for traditional crosslinking approaches. Increasing reactivity of the functional group in the hope of stabilizing fleeting associations would only amplify probability of trapping spurious or non-specific interactions during the “off time” between main signaling partners. In the current study, we have introduced ubiquitin-phototrap as a general tool for unbiased screening binding partners of ubiquitin without prior knowledge of binding partners in order to trap weak transient binders without decreasing specificity that would render results uninformative due to false positives.
Design of modifications on side chains amenable for crosslinking (or of fluorescent or paramagnetic tags for other biophysical techniques) to map protein-protein interactions often requires knowledge of protein sequence and structure to obtain successful results. Chemical crosslinkers traditionally link between neighboring amine or thiol groups, which can be either in cis on a single protein or in trans between binding partners. This property may pose a hurdle for traditional crosslinkers to capture Ub-binding proteins as recognition of ubiquitin often utilizes hydrophobic interactions. At the same time, the Ub molecule is naturally suited for integration of photoleucine given that hydrophobic residues partake in interactions with Ub-interacting proteins such as receptors, DUBs or conjugating enzymes. Specifically, two key leucine residues on ubiquitin – Leu8 and Leu73 – are solvent exposed and are known to participate in binding associations. Integration of photoleucine into Ub allowed for a highly reactive crosslinking agent that could react with protein backbones in close proximity, yet facilely quenched by solvent to limit spurious interactions (Figure 1). Following photoactivation, the reactive singlet carbene on the alkyl side-chain of pLeu can bond covalently with a number of common functional groups in proteins, guaranteeing that efficient crosslinking is not restricted to precise positioning of a limited set of residues (e.g. lysine, cysteine) thereby increasing likelihood of trapping binding partners. However, as hydroxyl groups are also prevalent in aqueous environments, the effective chemical half-life of the reactive singlet carbene on unattached “Trap” is short; the trap is essentially self-limiting due to quenching by water. This property decreases crosslinking to spurious “non-specific” proteins ensuring that pLeu is specific for meaningful nearest neighbors, even of transient associations. As we demonstrated, the reaction was rapid with detectable product within five minutes. The added benefit of a photoactivatable group gives UbPT users complete control over when to initiate the crosslinking reaction. Importantly, UbPT is a modular reagent that is easily incorporated into polyUbPT and can be used to differentiate between linkage-specific UBDs.
Crosslinking approaches have been successful for determining proteasome architecture (Bohn et al., 2010; Forster et al., 2009; Hartmann-Petersen et al., 2001; Lasker et al., 2012; Sharon et al., 2006), however they were not successful in detecting transient interactions of proteasome-interacting-proteins (PIPs). Our polyUb-based photo-crosslinking reagents, which we term polyUbPT, were successfully applied to 26S proteasome complexes, subunits, and associated receptors. Rpn1 emerged as the highest capacity Ub-binding subunit of the proteasome, able to form a complex with polyUb and UBL domains. By docking shuttles and associating with Ub, Rpn1 may aid unloading of ubiquitinated cargo onto the proteasome for further treatment (Figure S6). While this study was under preparation, the capacity of Rpn1 to associate with Ub was substantiated independently (Shi et al., 2016). Using a combination of techniques, the authors elegantly demonstrated that Rpn1 harbors two binding sites: T1 for UBL domains and for ubiquitin, and T2 for the UBL domain of Ubp6. The specific residues on the T1 site that bind ubiquitin were identified by solving an NMR structure of a segment of Rpn1 associated to ubiquitin or diUb. This site falls into the first PC repeat of Rpn1, the same region that was found sufficient to be captured by polyUbPT. Moreover, PolyUbPT was able to pinpoint and isolate Rpn1 out of the intact 26S proteasome complex, demonstrating that association with ubiquitin is retained in both free and proteasome-incorporated forms. The same residues also associate with Rad23, and can be competed out by excess Dsk2UBL, hinting at possible unloading of ubiquitinated cargo from shuttle proteins to proteasome.
Rpn1 is the first Ub-binding protein associated with the proteasome complex that is encoded by an essential gene in S cerevisiae. Typically, proteasome-associated polyUb-binding proteins have been classified into two categories: (i) delivery proteins or shuttles, whose association with the proteasome is transient in nature (e.g. Rad23/hHR23, Dsk2/hPLIC/Ubiquilin, Ddi1/DDI1) and (ii) bona fide receptors (Rpn10/S5a, Rpn13/ADRM-1). Yet, in S. cerevisiae none of the Ub-associating proteins are strictly essential (Finley et al., 2012). Two additional proteasome subunits interact with polyUb - the DUB Ubp6/USP14 (Aufderheide et al., 2015; Mansour et al., 2015; Peth et al., 2009) and an ancillary tethering component Sem1/Dss1 (Paraskevopoulos et al., 2014) – yet they are also non-essential. On a tangential note, the metalloprotease Rpn11/PSMD14 (Aufderheide et al., 2015; Luan et al., 2016; Mansour et al., 2015; Pathare et al., 2014) and the ATPase Rpt5 (Lam et al., 2002) have also been suggested to interact with polyUb in some capacity and are essential subunits, yet their contribution to recruitment or anchoring of polyUb at proteasome complexes has not been defined. Thus far, the prevailing view has been that these subunits work in parallel as redundant receptors and no single Ub-binding subunit serves as the primary docking site on the proteasome. This view is being revised, now that independent studies have demonstrated the propensity of Rpn1 to associate with polyUb at the proteasome.
Through its sheer size (being the largest subunit in the 26S proteasome complex) and its structural features, Rpn1 is naturally set up to scaffold several adjacent subunits and provide a docking site for proteasome-associating factors (Effantin et al., 2009; He et al., 2012; Rosenzweig et al., 2012). Light-induced crosslinking with engineered UbPT narrowed down polyUb binding to the first PC region of Rpn1, overlapping with binding sites reported for UBL domains such as those found in proteasome shuttles (Elsasser et al., 2002; Gomez et al., 2011; Rosenzweig et al., 2012; Yun et al., 2013). Yet functional relationship of Rpn1 to other polyUb-binding components on the proteasome is unclear, given the convoluted network of interactions of polyUb and UBLs (at comparable affinities) to multiple receptors at the proteasome (Kang et al., 2007; Kang et al., 2006; Matiuhin et al., 2008; Mueller and Feigon, 2003; Mueller et al., 2004; Zhang et al., 2009a; Zhang et al., 2008; Zhang et al., 2009b). While Rpn1 is capable of directly binding both K48-and K63-linked polyUb, its affinity for the UBL domain of the UBL-UBA family of shuttles is tighter than for unanchored chains. It is, therefore, likely that shuttles direct and aid targeting of polyUb conjugates to Rpn1. In this manner, a single proteasomal subunit – Rpn1 – coordinates docking of substrate shuttles, unloading of substrates, and anchoring of polyUb-conjugates, defining the first mechanistic step of proteasome action.
PolyUb is a complex signal made up of repeating units that are assembled in an almost endless number of possible configurations (Nakasone et al., 2013). In order to achieve the desired outcome, each configuration of polyUb should be recognized precisely, deciphered, and conveyed to the proper pathway. To this end, a multitude of proteins discriminate among the plethora of polyUb signals by means of embedded Ub-binding domains (UBDs). Consequently, most interactions with Ub are transient, with intermediate complexes serving to shuttle polyUb-conjugates as cargo while also protecting the signal from disassembly by DUBs (Hartmann-Petersen et al., 2003; Wilkinson et al., 2001). Moderate affinities (tens to hundreds of μM) for polyUb chains (Fushman and Wilkinson, 2011; Winget and Mayor, 2011) often reflect high off-rates from shuttles, and hence the transient nature of many polyUb signals. Even at a destination such as the proteasome, recruitment and anchoring of polyUb is just the beginning of a multi-step trajectory. As a substrate unfolds and is translocated into the 20S CP, the polyUb signal is relayed between receptors and finally handed over to proteasome-associated DUBs for release (Aufderheide et al., 2015; Bhattacharyya et al., 2014; Matyskiela et al., 2013; Peth et al., 2013c; Sledz et al., 2013a; Sledz et al., 2013b; Unverdorben et al., 2014). Although many proteins with affinity for Ub or polyUb have been uncovered through a variety of experimental approaches (Fushman and Wilkinson, 2011; Husnjak and Dikic, 2012; Scott et al., 2015; Winget and Mayor, 2011), the transient nature of association and fast exchange rates pose a hurdle to full mapping of the associated Ub-interactome. The novel set of phototrap reagents based on the ubiquitin polymer proved powerful in exposing new insight on Ub-binding entities.
PolyUbPT, as its name implies, was able to trap a specific transient interaction in a multi-subunit, multi-catalytic, molecular machine. This study lays the foundation for the future use of polyUbPT to discover interactions of Ub in new systems, and beyond to unrelated proteins. The lability of light-induced photoleucine as a crosslinking reagent, combined with flexibility of enzymatic polymerization of UbPT enables low-resolution surface mapping of receptor-ligand interfaces. PolyUbPT demonstrates that different Ub-binding domains such as the newly exposed PC repeat stretch in Rpn1, UIMs, or UBA domains, contact different elements in the Ub ligand. Specificity of Ub chain recognition is not limited to the linkage or to residues directly surrounding the isopeptide linkage, but involves additional residues. Hence, the UIM of RAP80 meets different surface areas on the Ub chain than does UIM of Rpn10. The properties of polyUbPT should allow characterization of interactions with intermediate binding affinities and even for unambiguous detection of elusive polyUb-binding proteins. With UbPT validated on a diverse set of established Ub receptors, UbPT emerges as a powerful tool to chart the plethora of Ub-associating proteins found in the Ub signaling system. From a qualitative point of view, the broad incorporation of UbPT into diverse polyUb chains highlights the non-invasive nature of the photoleucine probe on Ub chain synthesis and most importantly, without altering the hydrophobic nature on which many of its partners rely for proper recognition. We conclude that UbPT is a modular reagent that provides advantages over conventional crosslinking reagents for studying Ub-associating proteins in extract, in complex or in isolation.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents can be directed to the leading author: Michael H. Glickman (glickman@tx.technion.ac.il).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Proteasome was purified from widely used laboratory yeast strain, BY4741 obtained from EUROSCARF (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), considered in this study as “wildtype” yeast.
All recombinant proteins were cloned from cDNA isolated from BY4741 yeast and expressed in E. coli (either M15, BL21(DE3) or Rosetta (II)).
METHOD DETAILS
Plasmid construction and protein purification
Smt3 (SUMO) was ligated into the pET28b vector. Subsequently, the full-length Rpn1 DNA sequence was amplified from yeast genomic DNA and ligated in the smt3-pET28b vector, downstream of smt3. Shorter fragments, Rpn1416–487, Rpn1905–993, Rpn1391–642, and Rpn1356–905 were created by applying the appropriate primer pair to the full length Rpn1 for ligation into the smt3-pET28b vector. All ligations were performed using T4 DNA fast ligase (Promega) according to the manufacturer’s protocol. Ligation products were transformed into chemically competent E. coli DH5α (Life technologies) cells and selected against 50μg/mL kanamycin. Plasmids were extracted and sequenced from the forward and reverse directions to confirm their integrity. Full length Rpn12 was ligated into the same smt3-pET28b vector. Plasmids for other proteins used have been reported in previous studies; Rpn10 and DSK2 variants (Zhang et al., 2009), Rap80-tUIM (Nakasone et al., 2013), Rad23 constructs (Rosenzweig et al., 2012), Rub1 (Singh et al., 2012), and Ubp6C118A (Mansour et al., 2015).
Proteins in pQE30 vectors were expressed in E. coli M15 cells (Novagen), while those in pET28b were expressed in BL-21 (DE3) Rosetta II cells (Novagen). 2 L cultures of LB media supplemented with the respective antibiotic (pQE30 100 μg/mL ampicillin or pET28b 50 μg/mL kanamycin) were grown to OD600~0.6 at 37ºC, induced with 0.5 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG), and expression was carried out for 18 hrs at 16ºC. Cells were harvested and stored at −80°C until purification. Cells were resuspended in HisTrap buffer A (20 mM phosphate, 200 mM NaCl, 10 mM imidazole, pH 7.4) buffer and lysed using French Press. The lysate was cleared with centrifugation, syringe filtered and loaded on to a pre-equilibrated 10 mL His-Trap (GE Life Sciences) column in the same buffer. Proteins were eluted using steps of 5 column volumes 10% and 70% HisTrap buffer B (20 mM phosphate, 200 mM NaCl, 280 mM imidazole, pH 7.4). Following elution, fractions containing proteins of interest were pooled and dialyzed against PBS pH 7.4 buffer. To obtain the highest purity, gel filtration was performed using a Superdex 200 16/60 (GE Life Sciences) in PBS pH 7.4 buffer. Purity was confirmed with SDS-PAGE and proteins were aliquoted and stored at −80 °C.
Purification of Rpn1 and Rpn1 fragments
Cell pellets expressing His6-Smt3-Rpn1 and fragments were suspended in HisTrap buffer A (50 mM HEPES pH 7.5, 400 mM KCl, 2.5% glycerol, 10 mM imidazole and 5 mM β-ME) and lysed using a French press. The cellular debris were cleared by centrifugation and the supernatant was loaded onto a 10 mL HisTrap column in the same buffer. Elution was performed with HisTrap buffer B (50 mM HEPES pH 7.5, 400 mM KCl, 2.5% glycerol, 280 mM imidazole and 5 mM β-ME) in two 5 cv steps, 10% then 70%. 13CH3-ILVM labeled Rpn1 constructs were obtained following (ref Kay – see section bellow) and purified in the same as above, except that the HisTrap buffers were changed to HisTrap buffer A (50 mM Tris pH 8.0, 300 mM KCl, 10 mM imidazole) for loading, and HisTrap buffer B (50 mM Tris pH 8.0, 500 mM KCl, 250 mM imidazole) for elution. Fractions containing Rpn1 were detected using SDS-PAGE, pooled and dialyzed at 4°C against (40 mM HEPES pH8, 250 mM KCl and 5 mM β-ME) or 50 mM Hepes pH 7.6, 500 mM KCl, 5 mM DTT, 5% glycerol for 13CH3-ILVM-Rpn1. In the respective buffers, the His6-smt3 tag was removed from Rpn1 constructs using His6-ULP1 and the desired Rpn1 constructs were isolated using Ni-NTA resin. To obtain the appropriated oligomeric state and purity, Rpn1 constructs were then injected on a Superdex S75 16/60 column in 20 mM HEPES pH 8.0, 100 mM KCl and 2.5% glycerol or 50 mM Hepes pH 7.6, 500 mM KCl, 2 mM TCEP for 13CH3-ILVM-Rpn1.
Methyl labelling Rpn1PC1
Cells were grown at 37°C in M9 D2O media supplemented with 14NH4Cl and [2H,12C]-glucose as the sole nitrogen and carbon sources, respectively. Methyl labeling of the Ile-δ1-[13CH3] and Val/Leu-[13CH3, 12CD3] variety (referred to as ILV-protein in what follows, that is U-[15N,2H], Ileδ1-[13CH3], Leu,Val-[13CH3,12CD3]-labeled) followed a published procedure (Tugarinov et al., 2006).
Assembly of K48- and K63-linked polyUbPT(8) and polyUbPT738) chains
Monomeric Ub mutants, E2 conjugating enzymes, and human E1 were obtained recombinantly as described (Nakasone et al., 2013; Volk et al., 2005). Enzymatically synthesized K48-, and K63-linked Ub chains were assembled by combining a proximally blocked Ub mutant (Ub-His6) in combination with pLeu8 or pLeu73 modified Ub (Castaneda et al., 2013; Nakasone et al., 2013). K48-linked Ub chains were obtained from a reaction containing 1 mg of Ub-His6 and 10 mg of each UbPT(8) or UbPT(73), 80 nM E1 (UBA1), 40 μM E2-25K, 4 mM TCEP, and 15 mM ATP in a volume of 1 mL with a 50 mM Tris pH 8.0 buffer incubated at 37 ºC for 20 hours. In a similar fashion, reactions to generate K63-linked Ub chains contained 30 μM of each Ubc13 and Uev1a with same monomers in addition to 50 ng of UbK63R to influence chain length. Following the completion of each reaction, Ub-His6 chains were diluted into a volume of 40 mL HisTrap buffer A (20 mM phosphate, 200 mM NaCl, 10 mM imidazole, pH 7.4), and loaded onto a 5 mL HisTrap column. Side products of the reaction flowed through the columns and polyUbPT chains with Ub-His6 in the proximal position were eluted in HisTrap buffer B (20 mM phosphate, 200 mM NaCl, 280 mM imidazole, pH 7.4). PolyUbPT reactions without Ub-His6 were first passed through a 1 mL GST column in PBS pH 7.4 buffer to remove E1 and E2 enzymes. Defined polymers of polyUbPT were resolved on a Superdex 75 16/60 size exclusion column (GE Life Sciences) in PBS, pH 7.4. Fractions containing the desired chain lengths were confirmed with SDS-PAGE and stored at −20°C until needed. We note that each step of polyUbPT was carried out in the dark to preserve the crosslinking group.
Yeast proteasome purification
Highly pure yeast 26S proteasome obtained from yeast in stationary phase in a total of 6L YPD media, following established protocol (Glickman and Coux, 2001). The activity and structure (RP2CP) of proteasomes was confirmed using the Suc-LLVY-AMC peptidase activity assay. Proteasome concentration was determined by Bradford assay (Thermo Scientific). Proteasomes were flash frozen in liquid nitrogen and stored at −80°C until use.
Western blot analysis
Samples from UV crosslinking reactions were taken and the indicated time point and mixed with 5xPLD for SDS-PAGE. Gels were transferred to nitrocellulose membranes (GE Life Sciences), blocked in 5%(w/v) non-fat milk for 1 hour at room temperature, washed and incubated with the primary antibody for 1 hour at room temperature (see Table S2). Membranes were then washed and incubated with the respective secondary HRP conjugate antibody (Bio-Rad) for chemiluminescence analysis with an Image Quant LAS 4000 (GE Healthcare).
Assembly of Ub2 chains for NMR measurements
K48-linked and K63-linked diUb with 15N-enriched distal Ub were assembled using chain-termination mutations (K48R or K63R on the distal Ub and D77 on the proximal) as described (Varadan et al., 2004; Varadan et al., 2002). K48-linked diUb with heavy isotope labeling 13C-ILVM on both ubiquitin units were assembled using E1 and E2-25K enzymes and ILVM-labeled monoUb; the dimers were separated from the rest of the reaction products using cation exchange chromatography followed by size exclusion.
NMR measurements
NMR-based titration assays were performed by monitoring changes in NMR spectra of isotope-labeled component (protein) upon addition of unlabeled binding partner (ligand). The KD values were derived from a global fit model that provides errors based on the fit calculated by a nonlinear least square fit to a single-site binding model using the equation:
where [P]T and [L]T are the total protein and ligand concentrations at each titration point, Δδ is the change in peak position from the apo state and ΔδMAX is the chemical shift difference between apo and fully bound states of the protein (Varadan et al., 2004). For 13C measurements, binding isotherms were quantified separately for 1H or 13C chemical shifts with Δδ calculated from the following relation:
| [2] |
where ΔδH(C) is the shift change between methyl group 1H (13C) nuclei in apo and fully saturated forms of the protein, α (β) is one standard deviation of the methyl 1H (13C) chemical shifts (separate values of α (β) are used for different methyl groups), as tabulated in the Biological Magnetic Resonance Data Bank (www.bmrb.wisc.edu). For 15N measurements, combined chemical shift perturbations were used, calculated as follows:
where ΔδH and ΔδN are shifts in 1H and 15N resonances, respectively.
15N relaxation rates were measured using standard methods as described (Hall and Fushman, 2003).
UbPT crosslinking conditions
The crosslink reaction was performed in 96 well-plates, allowing for a 30 minute preincubation at 30°C. Samples were placed 10 cm from the light source and UV-irradiated for 30 min using 5X8W UV Bulbs 302/355 nm (Cleaver Scientific – UV Crosslinker). Rad23 competition reactions were conducted in PBS pH 7.4 buffer using 1 μM of Rpn1PC, 5 μM K48-Ub4+PT(73), and 0.5 μM or 1 μM of Rad23. Proteasome crosslinking was carried out in 25 mM Tris pH 7.4, 10 mM MgCl2, 10% glycerol, 2 mM ATP and 1 mM DTT buffer. 200 nM proteasome was first pre-incubated with 0.1 mM NEM for 30 minutes. UV Crosslinking occurred after addition of 400 nm Rad23 and 2 μM of the indicated polyUb4+PT.
Docking simulations and Bioinformatics analysis
pyDockWEB
Docking analysis were done with pyDockWeb (Jimenez-Garcia et al., 2013), a web tool for the structural prediction of protein-protein interactions. Given the 3D coordinates of two interacting proteins (which can be modeled or experimental PDB structures), pyDockWEB returns the best rigid-body docking orientations generated by FTDock (Gabb et al., 1997) and evaluated by pyDock scoring function (Cheng et al., 2007), which includes electrostatics, desolvation and limited van der Waals contribution energy terms.
NIP method
Normalized interface propensity (NIP) values derived from rigid body docking with electrostatics and desolvation scoring for the prediction of interaction hotspots (Grosdidier et al., 2007). The ensembles of the rigid-body docking solutions generated by the simulations were subsequently used to project the docking energy landscapes onto the protein surfaces. Highly populated low-energy regions consistently correspond to actual binding sites. Most of the predicted hot-spot residues are above NIP values of 0.3.
ConSurf server
The ConSurf server (Glaser et al., 2003) is a bioinformatics tool for estimating the evolutionary conservation of amino positions in a protein molecule based on the phylogenetic relationships between homologous sequences. The degree to which an amino (or nucleic) acid position is evolutionarily conserved is strongly dependent on its structural and functional importance; rapidly evolving positions are variable while slowly evolving positions are conserved. Thus, conservation analysis of positions among members from the same family can often reveal the importance of each position for the protein structure or function.
UCSF CHIMERA
Molecular graphics and analyses were performed with the UCSF Chimera package. Chimera is developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco ((http://www.cgl.ucsf.edu/chimera) supported by NIGMS P41-GM103311). (Pettersen et al., 2004)
Chemical Methods Synthesis of monomeric UbPT(8) and UbPT(73)
All commercial materials (Aldrich, Fluka, Novabiochem, Biosolve, Thermo Scientific) were used without further purification. L-2-amino-4,4-azi-pentanoic acid (L-photoleucine) was purchased from Thermo Scientific. Peptide synthesis reagents (standard amino acid building blocks and PyBop) were purchased from Novabiochem. All solvents were reagent grade or HPLC grade. Unless stated otherwise, reactions were performed under an inert atmosphere. NMR spectra (1H and 13C) were recorded on a Bruker Avance 300 spectrometer, referenced to TMS or residual solvent. LC-MS analysis was performed on a system equipped with a Waters 2795 separation Module (Alliance HT), Waters 2996 Photodiode Array Detector (190–750 nm), Phenomenex KinetexTM C18 (100A, 100 × 21 mm, 2.6 μm) reversed phase column or Phenomenex KinetexTM XB-C18 100A (50 × 2 mm, 2.6 μm) reversed phase column and a Micromass LCT-TOF mass spectrometer. Samples were run at 0.40 mL/min using a gradient of two mobile phases, A: 0.1% aq. formic acid and B: 0.1% formic acid in acetonitrile. Data processing was performed using Waters MassLynx 4.1 software. Preparative HPLC was performed on a Waters XBridge™ Prep C18 Column (30 × 150 mm, 5μm OBD™) at a flow rate of 37.5 ml/min. The solvents used were aq. 0.05% TFA (Solvent A) and acetonitrile containing 0.05% TFA (Solvent B) using gradient elution.
Compound synthesis and characterization
L-2-amino-4,4-azi-pentanoic acid (L-photoleucine, 100 mg, 0.7 mmol) was dissolved in 5 mL of 10% aq Na2CO3. To this, a solution of Fmoc N-hydroxysuccinimide ester (Fmoc-OSu, 1.2 eq, 0.84 mmol, 283 mg) in 5 mL THF was added (Scheme 1). The reaction mixture was stirred overnight at RT. A sample from the reaction mixture was analyzed by LC-MS (LCT, micromass) to determine the formation of Fmoc-photoleucine. LC-MS Rt 7.03 min; MS ES+ calculated: 366.39; found 365.93. Phenomenex KinetexTM C18 (100A, 100 × 21 mm, 2.6 μm); solvents − 0.1% aq. formic acid (Solvent A) and acetonitrile containing 0.1% formic acid (Solvent B), flow rate = 0.4 mL/min, runtime = 12 min, column T = 45°C. Gradient: 5% ⇨ 95% solvent B over 7.5 min.
The THF was removed by evaporation under reduced pressure and the remaining aqueous phase washed with ethyl acetate (20 mL). The organic layer was separated and washed with water (20 mL). Both aqueous layers were combined and acidified with 1M aq HCl until the pH dropped between 1 and 2. The product was extracted two times with ethyl acetate. The combined organic layers were dried over sodium sulphate, filtered and evaporated under reduced pressure. After purification by column chromatography (1% → 5% MeOH/DCM), the product was obtained as a colourless oil (yield: 245 mg, 0.67 mmol, 96%, purity: 90 %, according to NMR). This compound can be further purified to 99% by a preparative reversed phase HPLC. 1H NMR (300 MHz, CDCl3): δ = 11.23 (s, 1 H), 7.79 (d, J=7.6 Hz, 2 H), 7.67 (d, J=7.6 Hz, 2 H), 7.60 – 7.28 (m, 4 H), 5.66 (d, J=7.56 Hz, 1 H), 4.62 – 4.42 (m, 3 H), 4.29 (t, J=6.9 Hz, 1 H), 2.13 and 1.69 (m, 2H), 1.09 and 0.9 (2s, 3H). See Supplemental Data for supporting information.
Solid phase peptide synthesis of Ub containing photoleucine
The synthesis of ubiquitin by solid phase peptide synthesis was carried out according to the previously reported protocol (El Oualid et al., 2010). Ubiquitin with photoleucine incorporated at positions 8 or 73 and ubiquitin containing photoleucine at positions 8 and 73 were synthesized by solid phase peptide synthesis on TentaGel Trt R resin. After acid (TFA) cleavage, the ubiquitin was precipitated in ether, dried and lyophilized. See Supplemental Data for supporting information.
HPLC purification of Ub containing photoleucine
Ubiquitin containing the photoleucine was first dissolved in DMSO. This solution was slowly added to MQ water containing 0.05% TFA and filtered through a GfxO/0.45μm GHP membrane Acrodisc® Premium 25mm syringe filter. The sample was then injected onto a Waters XBridge™ Prep C18 Column (30 × 150 mm, 5μm OBD™) at a flow rate of 37.5 ml/min. The protein was purified with the gradient outlined Table 1 using aq. 0.05% TFA (Solvent A) and acetonitrile containing 0.05% TFA (Solvent B) as eluents.
The retention time for the ubiquitin mutants was approximately 10 minutes. All fractions containing the protein were confirmed by checking the mass using a LC-MS: Rt 2.8 min; Phenomenex KinetexTM XB-C18 100A (50 × 2 × 10 mm, 2.6 μm); solvents - MQ water with 0.1% formic acid (Solvent A) and acetonitrile containing 0.1% formic acid (Solvent B), flow rate = 0.5 mL/min, runtime = 6 min, column T = 45°C. Gradient: 5% ⇨ 95% B over 3.5 min. All samples containing pure protein were pooled and lyophilized. See Supplemental Data for supporting information. See Supplemental Data for detailed information on gradient used in the HPLC purification of the ubiquitin mutants.
Analysis of purified ubiquitin incorporated with photoleucine
The ubiquitin mutants were dissolved in DMSO to a concentration of 10 mg/mL. 0.2 μL of this sample was resuspended in 10 μL MQ water. To this solution, 5 μL 3x SDS buffer (containing 7.5% 2-mercaptoethanol) was added and the samples were heated at 70°C for 10 minutes. Samples were then loaded on a Nova 12 % Bis-Tris gel and run at 190 V for 47 mins using MES buffer. See Supplemental Data for supporting information.
LC-MS analysis of the purified ubiquitin containing photo-leucine
All purified proteins were confirmed by checking the mass using LC-MS. Rt 4.45 min; Phenomenex KinetexTM C18 (100A, 100 × 21 mm, 2.6 μm); solvents – aq. 0.1% formic acid (Solvent A) and acetonitrile containing 0.1% formic acid (Solvent B), flow rate = 0.4 mL/min, runtime = 12 min, column T = 45°C. Gradient: 5% ⇨ 95% B over 7.5 min. (purity > 98%). See Supplemental Data for supporting information.
Supplementary Material
SIGNIFICANCE.
How shuttles, receptors and multiple binding subunits on the proteasome relay the polyUb signal between them has not been deciphered. Through the application of novel UV light-inducible crosslinking agents, UbPT and UbPT-spiked polyUb chains, we were successful in capturing proteasome-associated Ub-binding subunits. The embedded photoleucine crosslinker minimally interfered with recognition of Ub moieties and thus enabled characterization of polyUb association with an essential proteasome subunit, Rpn1. A hydrophobic patch centered on L8, I44, and V70 on the surface of Ub tethers to hydrophobic residues on the exposed surface of proteasome-incorporated Rpn1. Rpn1 binds ubiquitin chains polymerized through either K48- or K63- linkages, and retains its ubiquitin-binding properties as a free stand-alone protein. The flexible alpha-helical PC repeat sequence on Rpn1 is sufficiently broad to anchor polyUb and UBL-containing proteins simultaneously. This provides insight as to how the polyUb signal is transferred from shuttles to receptors and expands our knowledge of Ub-binding subunits at the proteasome. Hybrid synthesis (i.e. chemical and enzymatic) of polyUb chains of well-defined linkage, combined with site of photoactivatable crosslinker, is highly adaptable for covalently trapping hydrophobic interactions in diverse systems beyond the ubiquitin system. To conclude, UbPT is a modular reagent that provides advantages over conventional crosslinking reagents for studying Ub-associating proteins in complex or in isolation.
Highlights.
Photoleucine was successfully incorporated into fully synthetic ubiquitin monomers.
Embedded Photoleucine permitted binding to the hydrophobic patch of ubiquitin.
Enzymatically polymerized ubiquitin phototrap (polyUbPT) captured Ub-binding receptors.
The first PC region of Rpn1, either isolated or proteasome-incorporated, bound polyUb.
Acknowledgments
We thank Dris El Atmioui for peptide synthesis. We thank Carlos A. Castañeda for aid in synthesis of isotopically labeled diUb. Noa Reis is acknowledged for help in cloning design and construction of plasmids and general advice. WM is supported in part by an ISF council for higher education outstanding minority (VATAT) fellowship, MC is supported through the EU Seventh Framework Programme (FP7A-PEOPLE-2011-ITN), MAN is supported by a Fulbright postdoctoral fellowship and the Aly Kaufman Fellowship Trust at the Technion. This work was supported in part by a grant from the Netherlands Foundation for Scientific Research (N.W.O.) to HO and a grant from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada (L.E.K) and by National Institutes of Health Grants GM065334 and R21NS093454 (DF) and GM095755 (DF and MHG), a USA-Israel Binational Science Foundation grant (to DF and MHG), and an Israel Science Foundation grant to MHG.
Footnotes
Competing financial interests
HO and FEO declare competing financial interests as co-founder and shareholder of UbiQ Bio BV. H.O. is part of the DUB Alliance that includes Cancer Research Technology and FORMA Therapeutics.
Supplemental Information including six supplemental figures can be found online linked to this article.
Author Contributions
FEO devised the UbPT design. DSH and FEO carried out the synthesis of UbPT monomers. WM and MC constructed plasmids for Rpn1 fragments and performed crosslinking experiments. WM, MC and MAN isolated recombinant proteins and carried out hybrid synthesis of polyUbPT. ZY purified 26S proteasome and aided in proteasome based crosslinking experiments. RR and LEK isolated methyl labeled Rpn1PC1 and carried out methyl-TROSY based experiments. 15N experiments were designed and carried out by RS and DF. Docking simulations and bioinformatics analysis was managed by FG. LEK, DF, HO and MHG funded the project and coordinated the cooperation and experimental design. All authors contributed to writing the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aufderheide A, Beck F, Stengel F, Hartwig M, Schweitzer A, Pfeifer G, Goldberg AL, Sakata E, Baumeister W, Forster F. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc Natl Acad Sci U S A. 2015;112:8626–8631. doi: 10.1073/pnas.1510449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashore C, Dambacher CM, Goodall EA, Matyskiela ME, Lander GC, Martin A. Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome. Nat Struct Mol Biol. 2015;22:712–719. doi: 10.1038/nsmb.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith R, Estrin E, Worden EJ, Martin A. Reconstitution of the 26S proteasome reveals functional asymmetries in its AAA+ unfoldase. Nat Struct Mol Biol. 2013;20:1164–1172. doi: 10.1038/nsmb.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Yu H, Mim C, Matouschek A. Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol. 2014;15:122–133. doi: 10.1038/nrm3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn S, Beck F, Sakata E, Walzthoeni T, Beck M, Aebersold R, Forster F, Baumeister W, Nickell S. Structure of the 26S proteasome from Schizosaccharomyces pombe at subnanometer resolution. Proc Natl Acad Sci U S A. 2010;107:20992–20997. doi: 10.1073/pnas.1015530107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaneda CA, Kashyap TR, Nakasone MA, Krueger S, Fushman D. Unique structural, dynamical, and functional properties of k11-linked polyubiquitin chains. Structure. 2013;21:1168–1181. doi: 10.1016/j.str.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhang D, Matiuhin Y, Glickman M, Fushman D. 1H, 13C, and 15N resonance assignment of the ubiquitin-like domain from Dsk2p. Biomol NMR Assign. 2008;2:147–149. doi: 10.1007/s12104-008-9107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Randles L, Shi K, Tarasov SG, Aihara H, Walters KJ. Structures of Rpn1 T1:Rad23 and hRpn13:hPLIC2 Reveal Distinct Binding Mechanisms between Substrate Receptors and Shuttle Factors of the Proteasome. Structure. 2016;24:1257–1270. doi: 10.1016/j.str.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TM, Blundell TL, Fernandez-Recio J. pyDock: electrostatics and desolvation for effective scoring of rigid-body protein-protein docking. Proteins. 2007;68:503–515. doi: 10.1002/prot.21419. [DOI] [PubMed] [Google Scholar]
- Diaz-Martinez LA, Kang Y, Walters KJ, Clarke DJ. Yeast UBL-UBA proteins have partially redundant functions in cell cycle control. Cell Div. 2006;1:28. doi: 10.1186/1747-1028-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effantin G, Rosenzweig R, Glickman MH, Steven AC. Electron microscopic evidence in support of alpha-solenoid models of proteasomal subunits Rpn1 and Rpn2. J Mol Biol. 2009;386:1204–1211. doi: 10.1016/j.jmb.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Oualid F, Merkx R, Ekkebus R, Hameed DS, Smit JJ, de Jong A, Hilkmann H, Sixma TK, Ovaa H. Chemical synthesis of ubiquitin, ubiquitin-based probes, and diubiquitin. Angew Chem Int Ed Engl. 2010;49:10149–10153. doi: 10.1002/anie.201005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser S, Chandler-Militello D, Muller B, Hanna J, Finley D. Rad23 and Rpn10 serve as alternative ubiquitin receptors for the proteasome. J Biol Chem. 2004;279:26817–26822. doi: 10.1074/jbc.M404020200. [DOI] [PubMed] [Google Scholar]
- Elsasser S, Gali RR, Schwickart M, Larsen CN, Leggett DS, Muller B, Feng MT, Tubing F, Dittmar GA, Finley D. Proteasome subunit Rpn1 binds ubiquitin-like protein domains. Nat Cell Biol. 2002;4:725–730. doi: 10.1038/ncb845. [DOI] [PubMed] [Google Scholar]
- Fatimababy AS, Lin YL, Usharani R, Radjacommare R, Wang HT, Tsai HL, Lee Y, Fu H. Cross-species divergence of the major recognition pathways of ubiquitylated substrates for ubiquitin/26S proteasome-mediated proteolysis. FEBS J. 2010;277:796–816. doi: 10.1111/j.1742-4658.2009.07531.x. [DOI] [PubMed] [Google Scholar]
- Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Ulrich HD, Sommer T, Kaiser P. The ubiquitin-proteasome system of Saccharomyces cerevisiae. Genetics. 2012;192:319–360. doi: 10.1534/genetics.112.140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster F, Lasker K, Beck F, Nickell S, Sali A, Baumeister W. An atomic model AAA-ATPase/20S core particle sub-complex of the 26S proteasome. Biochem Biophys Res Commun. 2009;388:228–233. doi: 10.1016/j.bbrc.2009.07.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushman D, Wilkinson KD. Structure and recognition of polyubiquitin chains of different lengths and linkage. F1000 biology reports. 2011;3:26. doi: 10.3410/B3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabb HA, Jackson RM, Sternberg MJ. Modelling protein docking using shape complementarity, electrostatics and biochemical information. J Mol Biol. 1997;272:106–120. doi: 10.1006/jmbi.1997.1203. [DOI] [PubMed] [Google Scholar]
- Girod PA, Fu HY, Zryd JP, Vierstra RD. Multiubiquitin chain binding subunit MCB1 (RPN10) of the 26S proteasome is essential for developmental progression in Physcomitrella patens. Plant Cell. 1999;11:1457–1471. doi: 10.1105/tpc.11.8.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, Ben-Tal N. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19:163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]
- Glickman MH, Adir N. The proteasome and the delicate balance between destruction and rescue. PLoS Biol. 2004;2:E13. doi: 10.1371/journal.pbio.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological reviews. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- Glickman M, Coux O. Purification and characterization of proteasomes from Saccharomyces cerevisiae. Curr Protoc Protein Sci. 2001;Chapter 21(Unit 21–25) doi: 10.1002/0471140864.ps2105s24. [DOI] [PubMed] [Google Scholar]
- Gomez TA, Kolawa N, Gee M, Sweredoski MJ, Deshaies RJ. Identification of a functional docking site in the Rpn1 LRR domain for the UBA-UBL domain protein Ddi1. BMC Biol. 2011;9:33. doi: 10.1186/1741-7007-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosdidier S, Pons C, Solernou A, Fernandez-Recio J. Prediction and scoring of docking poses with pyDock. Proteins. 2007;69:852–858. doi: 10.1002/prot.21796. [DOI] [PubMed] [Google Scholar]
- Guterman A, Glickman MH. Deubiquitinating enzymes are IN/(trinsic to proteasome function) Curr Protein Pept Sci. 2004;5:201–211. doi: 10.2174/1389203043379756. [DOI] [PubMed] [Google Scholar]
- Hall JB, Fushman D. Characterization of the overall and local dynamics of a protein with intermediate rotational anisotropy: Differentiating between conformational exchange and anisotropic diffusion in the B3 domain of protein G. J Biomol NMR. 2003;27:261–275. doi: 10.1023/a:1025467918856. [DOI] [PubMed] [Google Scholar]
- Hamazaki J, Hirayama S, Murata S. Redundant Roles of Rpn10 and Rpn13 in Recognition of Ubiquitinated Proteins and Cellular Homeostasis. PLoS Genet. 2015;11:e1005401. doi: 10.1371/journal.pgen.1005401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki J, Iemura S, Natsume T, Yashiroda H, Tanaka K, Murata S. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 2006;25:4524–4536. doi: 10.1038/sj.emboj.7601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann-Petersen R, Hendil KB, Gordon C. Ubiquitin binding proteins protect ubiquitin conjugates from disassembly. FEBS Lett. 2003;535:77–81. doi: 10.1016/s0014-5793(02)03874-7. [DOI] [PubMed] [Google Scholar]
- Hartmann-Petersen R, Tanaka K, Hendil KB. Quaternary structure of the ATPase complex of human 26S proteasomes determined by chemical cross-linking. Arch Biochem Biophys. 2001;386:89–94. doi: 10.1006/abbi.2000.2178. [DOI] [PubMed] [Google Scholar]
- He J, Kulkarni K, da Fonseca PC, Krutauz D, Glickman MH, Barford D, Morris EP. The structure of the 26S proteasome subunit Rpn2 reveals its PC repeat domain as a closed toroid of two concentric alpha-helical rings. Structure. 2012;20:513–521. doi: 10.1016/j.str.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hofmann K. Ubiquitin-binding domains and their role in the DNA damage response. DNA Repair (Amst) 2009;8:544–556. doi: 10.1016/j.dnarep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P. The UBA domain: a sequence motif present in multiple enzyme classes of the ubiquitination pathway. Trends Biochem Sci. 1996;21:172–173. [PubMed] [Google Scholar]
- Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem. 2012;81:291–322. doi: 10.1146/annurev-biochem-051810-094654. [DOI] [PubMed] [Google Scholar]
- Husnjak K, Elsasser S, Zhang N, Chen X, Randles L, Shi Y, Hofmann K, Walters KJ, Finley D, Dikic I. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–488. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz JM, Ren Y, Looby R, Kazmi MA, Sachdev P, Grunbeck A, Haggis L, Chinnapen D, Lin AY, Seibert C, et al. Direct interaction between an allosteric agonist pepducin and the chemokine receptor CXCR4. J Am Chem Soc. 2011;133:15878–15881. doi: 10.1021/ja206661w. [DOI] [PubMed] [Google Scholar]
- Jimenez-Garcia B, Pons C, Fernandez-Recio J. pyDockWEB: a web server for rigid-body protein-protein docking using electrostatics and desolvation scoring. Bioinformatics. 2013;29:1698–1699. doi: 10.1093/bioinformatics/btt262. [DOI] [PubMed] [Google Scholar]
- Kajava AV. What curves alpha-solenoids? Evidence for an alpha-helical toroid structure of Rpn1 and Rpn2 proteins of the 26 S proteasome. J Biol Chem. 2002;277:49791–49798. doi: 10.1074/jbc.M204982200. [DOI] [PubMed] [Google Scholar]
- Kang Y, Chen X, Lary JW, Cole JL, Walters KJ. Defining how ubiquitin receptors hHR23a and S5a bind polyubiquitin. J Mol Biol. 2007;369:168–176. doi: 10.1016/j.jmb.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Vossler RA, Diaz-Martinez LA, Winter NS, Clarke DJ, Walters KJ. UBL/UBA ubiquitin receptor proteins bind a common tetraubiquitin chain. J Mol Biol. 2006;356:1027–1035. doi: 10.1016/j.jmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kim I, Mi K, Rao H. Multiple interactions of rad23 suggest a mechanism for ubiquitylated substrate delivery important in proteolysis. Mol Biol Cell. 2004;15:3357–3365. doi: 10.1091/mbc.E03-11-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YA, Lawson TG, Velayutham M, Zweier JL, Pickart CM. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature. 2002;416:763–767. doi: 10.1038/416763a. [DOI] [PubMed] [Google Scholar]
- Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A. Complete subunit architecture of the proteasome regulatory particle. Nature. 2012;482:186–191. doi: 10.1038/nature10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker K, Forster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P, Beck F, Aebersold R, Sali A, Baumeister W. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci U S A. 2012;109:1380–1387. doi: 10.1073/pnas.1120559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Lee BH, Hanna J, King RW, Finley D. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol Cell Proteomics. 2011;10:R110. doi: 10.1074/mcp.R110.003871. 003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe ED, Hasan N, Trempe JF, Fonso L, Noble ME, Endicott JA, Johnson LN, Brown NR. Structures of the Dsk2 UBL and UBA domains and their complex. Acta Crystallogr D Biol Crystallogr. 2006;62:177–188. doi: 10.1107/S0907444905037777. [DOI] [PubMed] [Google Scholar]
- Lu Y, Lee BH, King RW, Finley D, Kirschner MW. Substrate degradation by the proteasome: a single-molecule kinetic analysis. Science. 2015;348:1250834. doi: 10.1126/science.1250834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan B, Huang X, Wu J, Mei Z, Wang Y, Xue X, Yan C, Wang J, Finley DJ, Shi Y, et al. Structure of an endogenous yeast 26S proteasome reveals two major conformational states. Proc Natl Acad Sci U S A. 2016;113:2642–2647. doi: 10.1073/pnas.1601561113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Baumeister W, Hofmann K. A repetitive sequence in subunits of the 26S proteasome and 20S cyclosome (APC) Trends Biochem Sci. 1997;22:195–196. doi: 10.1016/s0968-0004(97)01058-x. [DOI] [PubMed] [Google Scholar]
- Mansour W, Nakasone MA, von Delbruck M, Yu Z, Krutauz D, Reis N, Kleifeld O, Sommer T, Fushman D, Glickman MH. Disassembly of Lys11 and mixed linkage polyubiquitin conjugates provides insights into function of proteasomal deubiquitinases Rpn11 and Ubp6. J Biol Chem. 2015;290:4688–4704. doi: 10.1074/jbc.M114.568295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matiuhin Y, Kirkpatrick DS, Ziv I, Kim W, Dakshinamurthy A, Kleifeld O, Gygi SP, Reis N, Glickman MH. Extraproteasomal Rpn10 restricts access of the polyubiquitin-binding protein Dsk2 to proteasome. Mol Cell. 2008;32:415–425. doi: 10.1016/j.molcel.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyskiela ME, Lander GC, Martin A. Conformational switching of the 26S proteasome enables substrate degradation. Nat Struct Mol Biol. 2013;20:781–788. doi: 10.1038/nsmb.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor T, Sharon M, Glickman MH. Tuning the proteasome to brighten the end of the journey. American journal of physiology Cell physiology. 2016 doi: 10.1152/ajpcell.00198.2016. ajpcell 00198 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HJ, Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157:910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SLH, Malotky E, O’Bryan JP. Analysis of the role of UIMs in ubiquitin-binding and ubiquitylation. J Biol Chem. 2004;279:33528–33537. doi: 10.1074/jbc.M313097200. [DOI] [PubMed] [Google Scholar]
- Mueller T, Feigon J. Structural determinants for the binding of ubl domains to the proteasome. EMBO J. 2003;22:4634–4645. doi: 10.1093/emboj/cdg467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller TD, Kamionka M, Feigon J. Specificity of the interaction between ubiquitin-associated domains and ubiquitin. J Biol Chem. 2004;279:11926–11936. doi: 10.1074/jbc.M312865200. [DOI] [PubMed] [Google Scholar]
- Nakasone MA, Livnat-Levanon N, Glickman MH, Cohen RE, Fushman D. Mixed-linkage ubiquitin chains send mixed messages. Structure. 2013;21:727–740. doi: 10.1016/j.str.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan JA, Kim HT, Ting L, Gygi SP, Goldberg AL. Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? EMBO J. 2013;32:552–565. doi: 10.1038/emboj.2012.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka U, Zhang D, Walker O, Krutauz D, Castaneda CA, Chaturvedi A, Chen TY, Reis N, Glickman MH, Fushman D. DNA-damage-inducible 1 protein (Ddi1) contains an uncharacteristic ubiquitin-like domain that binds ubiquitin. Structure. 2015;23:542–557. doi: 10.1016/j.str.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno A, Jee J, Fujiwara K, Tenno T, Goda N, Tochio H, Kobayashi H, Hiroaki H, Shirakawa M. Structure of the UBA domain of Dsk2p in complex with ubiquitin molecular determinants for ubiquitin recognition. Structure. 2005;13:521–532. doi: 10.1016/j.str.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Paraskevopoulos K, Kriegenburg F, Tatham MH, Rosner HI, Medina B, Larsen IB, Brandstrup R, Hardwick KG, Hay RT, Kragelund BB, et al. Dss1 is a 26S proteasome ubiquitin receptor. Mol Cell. 2014;56:453–461. doi: 10.1016/j.molcel.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare GR, Nagy I, Sledz P, Anderson DJ, Zhou HJ, Pardon E, Steyaert J, Forster F, Bracher A, Baumeister W. Crystal structure of the proteasomal deubiquitylation module Rpn8-Rpn11. Proc Natl Acad Sci U S A. 2014;111:2984–2989. doi: 10.1073/pnas.1400546111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Besche HC, Goldberg AL. Ubiquitinated proteins activate the proteasome by binding to Usp14/Ubp6, which causes 20S gate opening. Mol Cell. 2009;36:794–804. doi: 10.1016/j.molcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Kukushkin N, Bosse M, Goldberg AL. Ubiquitinated proteins activate the proteasomal ATPases by binding to Usp14 or Uch37 homologs. J Biol Chem. 2013a;288:7781–7790. doi: 10.1074/jbc.M112.441907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Kukushkin N, Bosse M, Goldberg AL. Ubiquitinated Proteins Activate the Proteasomal ATPases by Binding to Usp14 or Uch37 Homologs. Journal of Biological Chemistry. 2013b;288:7781–7790. doi: 10.1074/jbc.M112.441907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Nathan JA, Goldberg AL. The ATP costs and time required to degrade ubiquitinated proteins by the 26 S proteasome. J Biol Chem. 2013c;288:29215–29222. doi: 10.1074/jbc.M113.482570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peth A, Nathan JA, Goldberg AL. The ATP Costs and Time Required to Degrade Ubiquitinated Proteins by the 26 S Proteasome. Journal of Biological Chemistry. 2013d;288:29215–29222. doi: 10.1074/jbc.M113.482570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Raasi S, Orlov I, Fleming KG, Pickart CM. Binding of polyubiquitin chains to ubiquitin-associated (UBA) domains of HHR23A. J Mol Biol. 2004;341:1367–1379. doi: 10.1016/j.jmb.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Raasi S, Varadan R, Fushman D, Pickart CM. Diverse polyubiquitin interaction properties of ubiquitin-associated domains. Nat Struct Mol Biol. 2005;12:708–714. doi: 10.1038/nsmb962. [DOI] [PubMed] [Google Scholar]
- Rahighi S, Dikic I. Selectivity of the ubiquitin-binding modules. FEBS Lett. 2012;586:2705–2710. doi: 10.1016/j.febslet.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Riedinger C, Boehringer J, Trempe JF, Lowe ED, Brown NR, Gehring K, Noble ME, Gordon C, Endicott JA. Structure of Rpn10 and its interactions with polyubiquitin chains and the proteasome subunit Rpn12. J Biol Chem. 2010;285:33992–34003. doi: 10.1074/jbc.M110.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig R, Bronner V, Zhang D, Fushman D, Glickman MH. Rpn1 and Rpn2 coordinate ubiquitin processing factors at proteasome. J Biol Chem. 2012;287:14659–14671. doi: 10.1074/jbc.M111.316323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y, Kudo T, Sone T, Kikuchi Y, Yokosawa H, Toh-e A, Tanaka K. Lysine 63-linked polyubiquitin chain may serve as a targeting signal for the 26S proteasome. EMBO J. 2009;28:359–371. doi: 10.1038/emboj.2008.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata E, Bohn S, Mihalache O, Kiss P, Beck F, Nagy I, Nickell S, Tanaka K, Saeki Y, Forster F, et al. Localization of the proteasomal ubiquitin receptors Rpn10 and Rpn13 by electron cryomicroscopy. Proc Natl Acad Sci U S A. 2012;109:1479–1484. doi: 10.1073/pnas.1119394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner P, Chen X, Husnjak K, Randles L, Zhang NX, Elsasser S, Finley D, Dikic I, Walters KJ, Groll M. Ubiquitin docking at the proteasome through a novel pleckstrin-homology domain interaction. Nature. 2008;453:548–552. doi: 10.1038/nature06924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer A, Aufderheide A, Rudack T, Beck F, Pfeifer G, Plitzko JM, Sakata E, Schulten K, Forster F, Baumeister W. Structure of the human 26S proteasome at a resolution of 3.9 A. Proc Natl Acad Sci U S A. 2016;113:7816–7821. doi: 10.1073/pnas.1608050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Oldham NJ, Strachan J, Searle MS, Layfield R. Ubiquitin-binding domains: mechanisms of ubiquitin recognition and use as tools to investigate ubiquitin-modified proteomes. Proteomics. 2015;15:844–861. doi: 10.1002/pmic.201400341. [DOI] [PubMed] [Google Scholar]
- Sharon M, Taverner T, Ambroggio XI, Deshaies RJ, Robinson CV. Structural organization of the 19S proteasome lid: insights from MS of intact complexes. PLoS Biol. 2006;4:e267. doi: 10.1371/journal.pbio.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen X, Elsasser S, Stocks BB, Tian G, Lee BH, Shi Y, Zhang N, de Poot SA, Tuebing F, et al. Rpn1 provides adjacent receptor sites for substrate binding and deubiquitination by the proteasome. Science. 2016:351. doi: 10.1126/science.aad9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JJ, Cohen RE. Linkage-specific avidity defines the lysine 63-linked polyubiquitin-binding preference of rap80. Mol Cell. 2009;33:775–783. doi: 10.1016/j.molcel.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims JJ, Haririnia A, Dickinson BC, Fushman D, Cohen RE. Avid interactions underlie the Lys63-linked polyubiquitin binding specificities observed for UBA domains. Nat Struct Mol Biol. 2009;16:883–889. doi: 10.1038/nsmb.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Zerath S, Kleifeld O, Scheffner M, Glickman MH, Fushman D. Recognition and cleavage of related to ubiquitin 1 (Rub1) and Rub1-ubiquitin chains by components of the ubiquitin-proteasome system. Mol Cell Proteomics. 2012;11:1595–1611. doi: 10.1074/mcp.M112.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Sahu I, Mali SM, Hemantha HP, Kleifeld O, Glickman MH, Brik A. Synthetic Uncleavable Ubiquitinated Proteins Dissect Proteasome Deubiquitination and Degradation, and Highlight Distinctive Fate of Tetraubiquitin. J Am Chem Soc. 2016;138:16004–16015. doi: 10.1021/jacs.6b09611. [DOI] [PubMed] [Google Scholar]
- Sledz P, Forster F, Baumeister W. Allosteric effects in the regulation of 26S proteasome activities. J Mol Biol. 2013a;425:1415–1423. doi: 10.1016/j.jmb.2013.01.036. [DOI] [PubMed] [Google Scholar]
- Sledz P, Unverdorben P, Beck F, Pfeifer G, Schweitzer A, Forster F, Baumeister W. Structure of the 26S proteasome with ATP-gammaS bound provides insights into the mechanism of nucleotide-dependent substrate translocation. Proc Natl Acad Sci U S A. 2013b;110:7264–7269. doi: 10.1073/pnas.1305782110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugarinov V, Kanelis V, Kay LE. Isotope labeling strategies for the study of high-molecular-weight proteins by solution NMR spectroscopy. Nat Protoc. 2006;1:749–754. doi: 10.1038/nprot.2006.101. [DOI] [PubMed] [Google Scholar]
- Unverdorben P, Beck F, Sledz P, Schweitzer A, Pfeifer G, Plitzko JM, Baumeister W, Forster F. Deep classification of a large cryo-EM dataset defines the conformational landscape of the 26S proteasome. Proc Natl Acad Sci U S A. 2014;111:5544–5549. doi: 10.1073/pnas.1403409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Raasi S, Pickart C, Fushman D. Structural determinants for selective recognition of a Lys48-linked polyubiquitin chain by a UBA domain. Mol Cell. 2005;18:687–698. doi: 10.1016/j.molcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D. Solution conformation of Lys63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem. 2004;279:7055–7063. doi: 10.1074/jbc.M309184200. [DOI] [PubMed] [Google Scholar]
- Varadan R, Walker O, Pickart C, Fushman D. Structural properties of polyubiquitin chains in solution. J Mol Biol. 2002;324:637–647. doi: 10.1016/s0022-2836(02)01198-1. [DOI] [PubMed] [Google Scholar]
- Verma R, Aravind L, Oania R, McDonald WH, Yates JR, 3rd, Koonin EV, Deshaies RJ. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- Verma R, Chen S, Feldman R, Schieltz D, Yates J, Dohmen J, Deshaies RJ. Proteasomal proteomics: identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol Biol Cell. 2000;11:3425–3439. doi: 10.1091/mbc.11.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk S, Wang M, Pickart CM. Chemical and genetic strategies for manipulating polyubiquitin chain structure. Methods Enzymol. 2005;399:3–20. doi: 10.1016/S0076-6879(05)99001-0. [DOI] [PubMed] [Google Scholar]
- Wang B, Matsuoka S, Ballif BA, Zhang D, Smogorzewska A, Gygi SP, Elledge SJ. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science. 2007;316:1194–1198. doi: 10.1126/science.1139476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX. An exact mathematical expression for describing competitive binding of two different ligands to a protein molecule. FEBS Lett. 1995;360:111–114. doi: 10.1016/0014-5793(95)00062-e. [DOI] [PubMed] [Google Scholar]
- Wilkinson CR, Seeger M, Hartmann-Petersen R, Stone M, Wallace M, Semple C, Gordon C. Proteins containing the UBA domain are able to bind to multi-ubiquitin chains. Nat Cell Biol. 2001;3:939–943. doi: 10.1038/ncb1001-939. [DOI] [PubMed] [Google Scholar]
- Winget JM, Mayor T. The diversity of ubiquitin recognition: hot spots and varied specificity. Mol Cell. 2011;38:627–635. doi: 10.1016/j.molcel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Worden EJ, Padovani C, Martin A. Structure of the Rpn11-Rpn8 dimer reveals mechanisms of substrate deubiquitination during proteasomal degradation. Nat Struct Mol Biol. 2014;21:220–227. doi: 10.1038/nsmb.2771. [DOI] [PubMed] [Google Scholar]
- Yu Z, Livnat-Levanon N, Kleifeld O, Mansour W, Nakasone MA, Castaneda CA, Dixon EK, Fushman D, Reis N, Pick E, et al. Base-CP proteasome can serve as a platform for stepwise lid formation. Biosci Rep. 2015:35. doi: 10.1042/BSR20140173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun JH, Ko S, Lee CK, Cheong HK, Cheong C, Yoon JB, Lee W. Solution structure and Rpn1 interaction of the UBL domain of human RNA polymerase II C-terminal domain phosphatase. PLoS One. 2013;8:e62981. doi: 10.1371/journal.pone.0062981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Chen T, Ziv I, Rosenzweig R, Matiuhin Y, Bronner V, Glickman MH, Fushman D. Together, Rpn10 and Dsk2 can serve as a polyubiquitin chain-length sensor. Mol Cell. 2009a;36:1018–1033. doi: 10.1016/j.molcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Raasi S, Fushman D. Affinity makes the difference: nonselective interaction of the UBA domain of Ubiquilin-1 with monomeric ubiquitin and polyubiquitin chains. J Mol Biol. 2008;377:162–180. doi: 10.1016/j.jmb.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang Q, Ehlinger A, Randles L, Lary JW, Kang Y, Haririnia A, Storaska AJ, Cole JL, Fushman D, et al. Structure of the s5a:k48-linked diubiquitin complex and its interactions with rpn13. Mol Cell. 2009b;35:280–290. doi: 10.1016/j.molcel.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Holt MT, Ohashi N, Zhao A, Muller MM, Wang B, Muir TW. Evidence that ubiquitylated H2B corrals hDot1L on the nucleosomal surface to induce H3K79 methylation. Nat Commun. 2016;7:10589. doi: 10.1038/ncomms10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.