Abstract
Background
We sought to determine the usefulness of electrospun dibutyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]), in wound treatment. We investigated the mechanisms of action of these polymers on wound healing.
Methods
We synthesized DBC, a newly identified ester derivative of chitin, using a patented method comprising the substitution of butyryl groups at positions C-3 and C-6 in chitin molecules. We confirmed the double substitution by the butyric groups using infrared spectrometry. The fibrous scaffolds were obtained using the electrospinning method. A polypropylene net was implanted subcutaneously in the rat and served as a wound model.
Results
Both DBC and PCL increased granulation tissue weight in the wound. In contrast to PCL, DBC did not abolish glycosaminoglycan changes in wounds. The tested samples did not impair total collagen synthesis or induce excessive fibrosis. In both PCL- and DBC-treated wounds, we observed a lower level of soluble collagen (compared with controls). The results show better hydration of the wounds in both the DBC and PCL groups. No induction of large edema formation by the tested materials was observed. These polymers induced almost identical macrophage-mediated reactions to foreign-body implantation. The implants increased the blood vessel number in a wound.
Conclusion
Both PCL and DBC could be used as scaffolds or dressings for wound treatment. The materials were safe and well tolerated by animals. As DBC did not disturb glycosaminoglycan accumulation in wounds and absorbed twice as much liquid as PCL, it can be considered superior.
Abstract
Contexte
Nous avons cherché à déterminer l’utilité du dibutyryl-chitine (DBC) ou du poly-(ɛ-caprolactone [PCL]) électrofilés dans le traitement des plaies. Nous avons étudié les mécanismes d’action de ces polymères sur la cicatrisation des plaies.
Méthodes
Nous avons synthétisé le DBC, un dérivé ester récemment identifié de la chitine, à l’aide d’une méthode brevetée incluant la substitution des groupes butyryl aux positions C-3 et C-6 des molécules de chitine. Nous avons confirmé la substitution double par les groupes butyriques à l’aide de la spectrométrie infrarouge. Les échafaudages fibreux ont été obtenus grâce à la méthode de filage électrostatique. Un filet en polypropylène a été implanté par voie sous-cutanée dans le rat et a servi de modèle de plaie.
Résultats
Le DBC et le PCL ont tous deux augmenté le poids du tissu de granulation dans la plaie. Contrairement au PCL, le DBC n’a pas supprimé les changements des glycosaminoglycanes des plaies. Les échantillons examinés n’ont pas perturbé la synthèse totale de collagène ni entraîné une fibrose excessive. Nous avons observé un niveau inférieur de collagène soluble (par rapport aux témoins) tant dans les plaies traitées par PCL que par DBC. Les résultats montrent une amélioration de l’hydratation des plaies tant pour les groupes DBC que PCL. Les matériaux à l’étude n’induisaient pas d’oedème étendu. Ces polymères ont induit des réactions macrophagiques presque identiques à l’implantation d’un corps étranger. Les implants ont accru le nombre de vaisseaux sanguins de la plaie.
Conclusion
Tant le PCL que le DBC pourraient être utilisés comme échafaudages ou pansements pour le traitement des plaies. Les matériaux étaient sécuritaires et ont été bien tolérés par les animaux. Comme le DBC n’a pas perturbé l’accumulation des glycosaminoglycanes des plaies et a absorbé 2 fois plus de liquide que le PCL, il peut être considéré comme étant supérieur.
A material used for the scaffold should not only be a good medium for cell growth, but also should not disturb healing or induce excessive fibrosis. High porosity of scaffolds ensures good cell migration inside implants and a suitable environment for their proliferation.1,2
Di-O-butyrylchitin (DBC) is an ester derivative of chitin. Synthesis of the polymer relies on the addition of butyric groups at positions C-3 and C-6 of chitin molecules. The method of DBC synthesis was described and patented. 3,4 Moreover, the polymer features good biocompatibility and a lack of cytotoxicity.5–7 Experiments using implantation of a nonwoven mat made of DBC fibres into a wound model8 revealed that the polymer has a beneficial effect on the repair process. The greatest biological effects were observed using dibutyrylchitin, with a lower molecular mass of 123 600 g/mol,3,9 with an intrinsic viscosity in dimethylacetamide (DMAc) of 2.05 g/dL.
Poly-(ɛ-caprolactone [PCL]) is applied as a drug delivery system for antibiotics or steroids.10,11 In addition, PCL is recommended for tissue engineering application as it exerts good compatibility with fibroblasts.11 The collagen-blended, porous nanofibrous membranes of PCL have been shown to be a good medium for the attachment and proliferation of fibroblasts, and could be applied as a scaffold for preparation of a skin substitute used to treat skin wounds.12–14
Limited data exist regarding DBC biological effects. Previous papers have shown the beneficial effects of a nonwoven mat, made from classical DBC fibres, on wound healing. 3,5,9 However, the influence of the thin fibrous structure of the material on wound repair has not yet been investigated. Therefore, the aim of the present study was to determine the influence of the electrospun thin DBC fibres on both the early and late stages of healing. In addition, we compared the results of the DBC experiments with the effects of PCL. We evaluated mechanisms of influence of the tested polymers on various phases of healing. Such a complex study addressing the physical and biological properties of PCL and DBC mats ensures better understanding of their influence on wound repair. Obtained findings are believed to define indications or contraindications of the material application in wound treatment.
Methods
Raw material
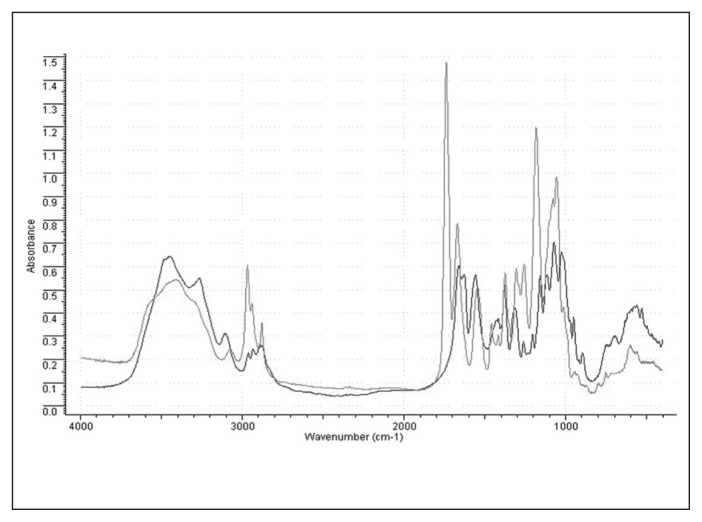
We obtained DBC by the esterification of shrimp chitin (Lodz University of Technology). The effects of double substitution by the butyric groups were confirmed by infrared spectrometry (Fig. 1).
Fig. 1.
Infrared spectra of di-O-butyrylchitin (grey line) and initial shrimp chitin (black line). The strong absorption visible on the spectra at 1738 cm−1 comes from the carboxyl group in an ester bond. The increased numbers of −CH2 and −CH3 groups are associated with greater absorption at 2950–2850 cm−1. Double substitution by ester groups is also confirmed by excellent solubility of the polymer in ethyl alcohol and dimethyl sulfoxide.
The molecular weight of the DBC was assessed using viscosimetry. Intrinsic viscosity was measured using a Ubelhode viscosimeter at 25°C with DMAc as the solvent (Sigma–Aldrich). We used DBC with an intrinsic viscosity of 1.93 g/dL in this study.
Technology
The electrospinning process was performed using a multinozzle laboratory stand designed and constructed at the Lodz University of Technology, Lodz, Poland.15 In this process, the polymer is delivered independently to each of the 32 spinning points of the spinning head of the stand. The spinning head is movable; the rate and the distance of movement are regulated. The electrostatic field is created by a high-voltage generator, and the applied voltage ranges from 0 kV to 50 kV. The distance between the nozzles and the collector was regulated across a range of 0.1–0.8 m. The electrospinning processes were optimized for both DBC and PCL.
Measurements
The thicknesses of the fibres and the web structure were analyzed using LUCIA G image analysis software (Laboratory Imaging). Porosity and pore size distribution were assessed using mercury porosimetry (AUTOPORE IV apparatus, Micromeritics) within a pressure range of 0.0036–412 MPa.16
As the bioresorbability of a polymer is influenced by its crystallinity, we assessed the supermolecular structure. The degree of crystallinity was measured using X–ray diffractometry (Bruker). Changes in supermolecular structure were analyzed using WAXSFIT software (University of Bielsko-Biala). We used the method of Hindeleh and Johnson17 to analyze WAXS patterns using WAXSFIT.
Measurement of wettability and wicking
We used a tensiometer Radian series 300 (Thermo Scientific) to measure contact angle and wicking. The contact angle was measured by immersing 25 mm samples. Depth of immersion was equal to 4 mm. We used both distilled water and cell culture medium (Gibco) to measure contact angle. Changes in the mass of the sample were noted. The contact angle was calculated on the basis of the following equation:
where cos θ is the contact angle, F is the force of interactions between samples and liquid at the point of first contact (in milligrams), g is the gravitational force (in square centimetres per second), γ is the surface tension of liquid (in dyne per centimetre), and PR is the surface of the contact (in square centimetres). We measured wicking of 10 mm samples. The depth of immersion was equal to 4 mm, and the duration of immersion was 5 minutes.
Biochemical evaluation of wound and local reaction after implantation
Animals
Eighty-four male Wistar rats weighing 240 g ± 30 g were housed with free access to commercial food and ad libitum access to tap water. The study was approved by the local Commission of Ethics in Lodz, Poland.
Study design
The animals were divided into 3 groups of 28 rats each: group 1 (control group) comprised rats implanted with a polypropylene net, group 2 comprised rats implanted with a polypropylene net covered by DBC, and group 3 comprised rats implanted with a polypropylene net covered by PCL. Each group comprised 4 subgroups of 7 rats. The implant was removed from the rat of the relevant subgroup after the second, fourth, eighth or twenty-fourth week of healing, respectively.
Wound model
A polypropylene net (3 cm × 2 cm) covered with 2 pieces of testing materials (DBC or PCL) was implanted subcutaneously in the left lumbar region. A polypropylene mesh was used as a control. An incision was made in the middle of the polypropylene implant. The incision was limited on each side by a 3 mm intact part of the net. After implantation, the skin wounds were closed with 5 sutures.
To measure the weight of the dry mass of the granulation tissue, the weight of the implanted polypropylene net with the nonwoven material was subtracted from that of the entire implant mass (polypropylene net with dry granulation tissue). Water content in the tissue was calculated by subtracting the dry tissue weight from the wet tissue weight and was expressed as a percentage of wet tissue weight.
Evaluation of the tensile strength of granulation tissue of the wound
The margins (3 mm each) connecting the 2 parts of the implants were cut off, so the 2 parts of the implant were linked only by granulation tissue. The breaking strength of the tissue was measured using a Instron Model 1112 Tensile Tester Machine. The maximal load of the measuring head was equal to 2 kg. The distance between clamps was 1 cm, and the test velocity was equal to 100 mm/min. On the basis of stress curves obtained during the stretching of samples, we determined the maximal force at breakage.
Determination of collagen
To assess soluble collagen, the macerated tissue was suspended in 10 times its weight of 0.45 M of sodium chloride (NaCl) solution with penicillin (10 000 units/sample) and kept for 24 hours at 2°C. The mixture was evaporated to dryness.
After hydrolysis with 6 N of hydrogen chloride (HCl), all the samples were evaporated to dryness, and the precipitates were dissolved in redistilled water and neutralized by 1 N of sodium hydroxide (NaOH). Hydroxyproline was oxidized to pyrrole by chloramine T in a citrate buffer. The perchloric acid was added to remove excess chloramine T. We then treated the samples with p-(dimethyl) aminobenzaldehyde and incubated them at 60°C for 20 minutes. The optical density was measured at 560 nm on a spectrophotometer.18
Determination of glycosaminoglycans
Samples were homogenized and dried to a constant weight at 90°C. A 50 mg portion of the dried sample was added to a mixture composed of 0.75 M of NaOH and 50 mM of natrium borate. After incubation, the pH was neutralized with 6 M of HCl, and then trichloroacetic acid (TCA) was added to each sample to precipitate proteins. After centrifugation, we added 6 mL of 100% ethanol to the supernatant. For glycosaminoglycan (GAG) precipitation, the samples were kept at −20°C overnight and then centrifuged. The precipitate containing GAG was resolved in distilled water.
A 1.2 mL aliquot of dimethylmethylene blue (DMMB) reagent composed of 51 mM of 1,9-DMMB, 45 mM of glycine and 41 mM of NaCl adjusted to a pH of 3.0 with 1 M of HCl was added to 50 mL of the sample. The absorbance was measured at 525 nm on a spectrophotometer.19
Alanine aminotransferase (ALT)20 and aspartate aminotransferase (AST)21 were assessed using kinetic methods. Jaffes’ method was applied for the evaluation of creatinine.22,23
Histological examination
Samples were frozen without initial fixation at −24°C and then sliced on a cryotome and stained with hematoxylin and eosin. The samples were washed in 96% ethanol containing carboxylene and xylene. The tissue was examined with a type 41 BX microscope (Olympus).
Statistical analysis
We used the Cochran Cox test for statistical analysis, and we considered results to be significant at p < 0.05. The null hypothesis was that differences between the average values of the determined variables of the studied samples and controls, as well as between samples, are statistically nonsignificant. The alternative hypothesis assumes that the differences between the studied samples and controls, as well as between samples, are statistically significant.
Results
Electrospinning of samples
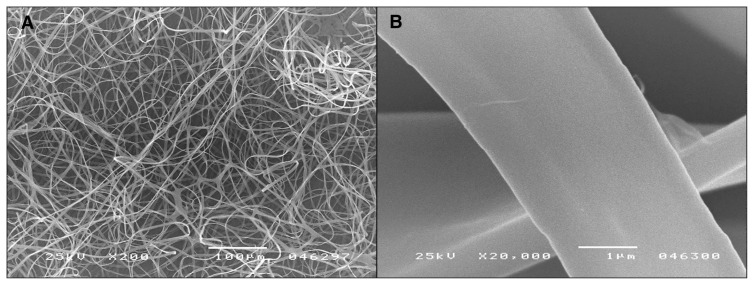
The DBC fibres were made from 6% solution in ethyl alcohol (for a polymer with an intrinsic viscosity in DMAc of 1.93 g/dL). This solution was drawn into an electrostatic field formed by 28 kV, with the distance between the nozzles and the collector being 15 cm at an air temperature of 23°C and 78% air humidity. Fibres with diameters of 2–10 mm (average 2.92 mm) were spun (Fig. 2).
Fig. 2.
Di-O-butyrylchitin (DBC) fibres formed from 6wt% solution in ethyl alcohol. Technological parameters: voltage 28 kV, distance between nozzles and collector 15 cm, air temperature 23°C, humidity 78%. Image A shows the web structure, and image B shows the fibre surface.
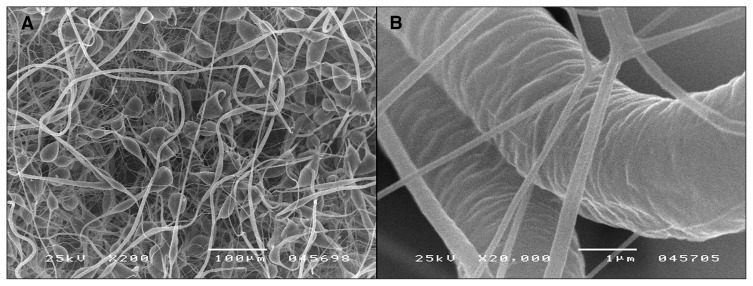
The following optimal parameters for PCL fibre preparation were applied: polymer solution concentration 7.5wt%, voltage 35 kV, distance between nozzles and collector 50 cm, temperature 13.6°C and air humidity 48%. Two types of PCL fibres are shown on the micrograph displayed in Figure 3: very fine fibres with a diameter of 280 nm and thick ones with a diameter of 7.08 mm.
Fig. 3.
Poly-(ɛ-caprolactone [PCL]) fibres formed from 7.5wt% solution in ethyl alcohol. Technological parameters: voltage 35 kV, distance between nozzles and collector 50 cm, air temperature 13.6°C, humidity 48%. Image A shows the web structure, and image B shows the fibre surface.
The DBC fibres were characterized by a 44% degree of crystallinity, whereas the PCL fibres were found to have a 45% degree of crystallinity (Table 1).
Table 1.
Structural characteristics of webs formed via electrospinning
| Treatment | Total pore area, m2/g | Average pore diameter, nm | Average fibre diameter, mm | Web thickness, mm | Surface mass, g/m2 |
|---|---|---|---|---|---|
| DBC | 5.52 | 2474.1 | 2.92 | 0.53 | 18.2 |
| PCL, fine fibres | 1.53 | 8016.1 | 0.28 | 0.13 | 28.5 |
| PCL, thick fibres | — | — | 7.08 | — | — |
DBC = di-O-butyrylchitin; PCL = poly-(ɛ-caprolactone).
Most pores detected in samples had an average diameter of about 10 000 nm, although the DBC samples had smaller pores (200 nm in diameter), which gives an average value of pore diameter at the level of 2500 nm. The smallest pore diameter for nonwoven mats made from PCL was 1000 nm, but average pore diameter was at the level of 8000 nm (data not shown). Values of water advancing contact angle were lower for DBC than PCL, although both results suggest that the samples have hydrophobic characteristics. Contact angle values for cell culture medium for 2 samples were similar. We observed that DBC adsorbed twice as much liquid as PCL (Table 2).
Table 2.
Surface properties of webs formed via electrospinning
| Treatment | Water | Cell culture medium | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Advancing contact angle, ° | Receding contact angle, ° | Wicking, mg/cm2 | Advancing contact angle, ° | Receding contact angle, ° | Wicking, mg/cm2 | |
| DBC | 86.86 | 30.9 | 18.3 | 81.12 | 0.00 | 25.32 |
|
| ||||||
| PCL | 105.96 | 0.00 | 6.3 | 78.05 | 0,00 | 9.24 |
DBC = di-O-butyrylchitin; PCL = poly-(ɛ-caprolactone).
The blood concentrations of creatinine, ALT and AST in rats treated with DBC or PCL were not significantly different from concentrations in controls (data not shown).
Wound biochemistry
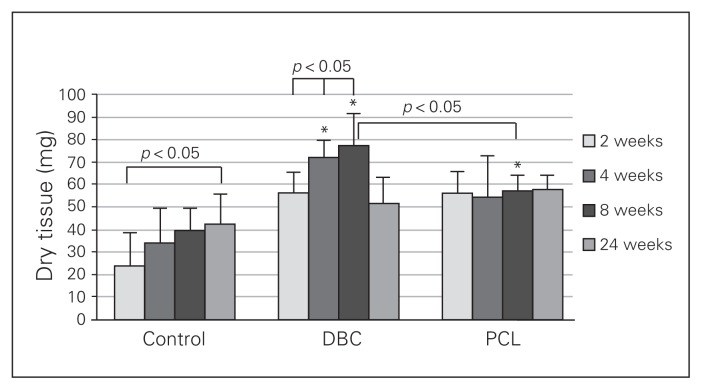
We observed a gradual elevation of the dry tissue collected from the control wounds (Fig. 4). The weight of the granulation tissue in the twenty-fourth week in controls was significantly greater than the weight of the wounded tissue from the second week of experimentation in the same group. The weights of the dry tissue in wounds of the DBC group obtained in both the fourth and eighth weeks of healing were significantly higher than those obtained in the second week. Granulation tissue weight in the fourth and eighth weeks of DBC-treated rats was significantly higher than the corresponding control data. Although the weight of dry tissue in the DBC group was greater than in the PCL group during the eighth week of healing, it had decreased by the twenty-fourth week (Fig. 4). The weight of the granulation tissue of the PCL group reached a maximal level in the second week of healing and remained unchanged during all periods of observation. During the eighth week of healing, the weight of the tissue in the PCL-treated wounds was significantly higher than in controls.
Fig. 4.
Weight of granulation tissue developed after 2, 4, 8 and 24 weeks of healing in control rats and rats treated with di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats. *Significant (p < 0.05) differences between the tested group and controls.
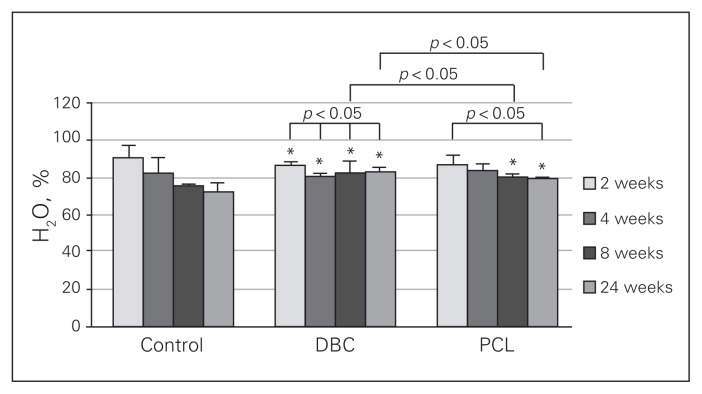
We observed a progressive decrease in water content of the wounds in the controls and PCL-treated rats (Fig. 5). The lower water content in the wound was present in the fourth, eighth and twenty-fourth weeks in DBC-treated rats compared with the results obtained in the second week in the same group. The water content of the DBC group was higher than in controls and PCL-treated rats at both the eighth and twenty-fourth weeks after injury, indicating that DBC application to the wound prevented reduction in water content. In the PCL-treated group, lower water content was observed in the twenty-fourth week than in the second week. Moreover, in the eighth and twenty-fourth weeks of healing, significantly higher water content was found in PCL-treated rats than in the control group.
Fig. 5.
Water content in granulation tissue after 2, 4, 8 and 24 weeks of healing in control rats and rats treated with di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats. *Significant (p < 0.05) differences between the tested group and controls.
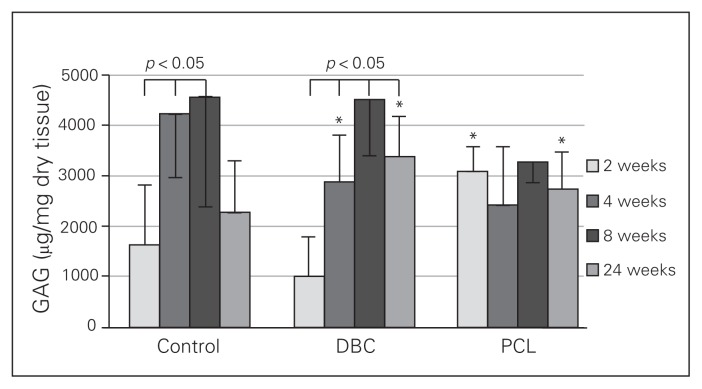
Glycosaminoglycan content in the granulation tissue of controls increased during repair, peaking from the fourth to the eighth week of the experiment and decreasing in the twenty-fourth week (Fig. 6). The GAG content of the wound was significantly higher in the fourth and eighth weeks than in the second week. This pattern of GAG changes was also observed in the DBC group and was abolished by PCL treatment. In the rats treated with DBC, GAG content in the wounds was higher in the fourth, eighth and twenty-fourth weeks than in the second week. The GAG content in the DBC-treated rats was markedly higher than in controls at the fourth and twenty-fourth weeks after implantation. In the PCL-treated rats, we observed a significantly higher level of GAG in both the second and twenty-fourth weeks of repair compared with controls.
Fig. 6.
Glycosaminoglycan (GAG) content in granulation tissue after 2, 4, 8 and 24 weeks in control rats and rats treated with di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats. *Significant (p < 0.05) differences between the tested group and controls.
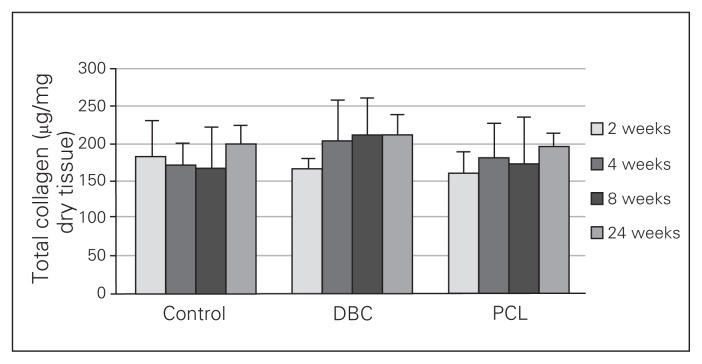
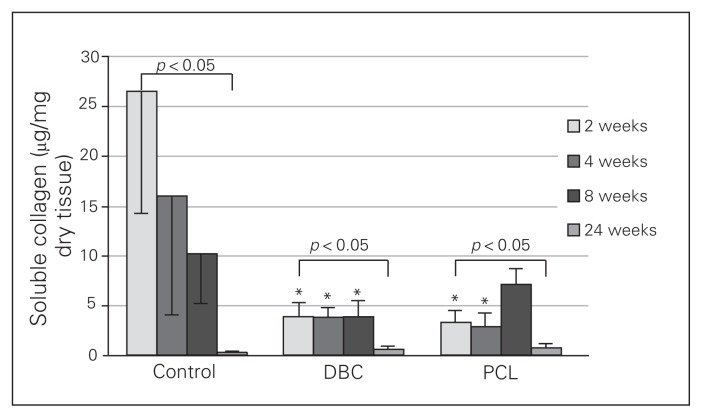
Neither DBC nor PCL significantly influenced total collagen levels in the wounds (Fig. 7). In the control wounds, the soluble collagen content gradually decreased from the second week to the twenty-fourth week (Fig. 8) and was significantly lower in the twenty-fourth week than the second week of healing. In both the DBC- and PCL-treated rats, the level of soluble collagen in the twenty-fourth week of healing was significantly lower than in the second week in either group. We found that DBC significantly decreased levels of soluble collagen from the second to the eighth week of wound healing compared with controls. Similarly, PCL reduced soluble collagen levels compared with controls from the second to the fourth week of wound healing.
Fig. 7.
Total collagen content in granulation tissue 2, 4, 8 and 24 weeks of healing in control rats and rats with insertions of dressings covered by di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats.
Fig. 8.
Soluble collagen content in granulation tissue 2, 4, 8 and 24 weeks of healing of control rats and rats with insertions of dressings covered by di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats. *Significant (p < 0.05) differences between the tested group and controls.
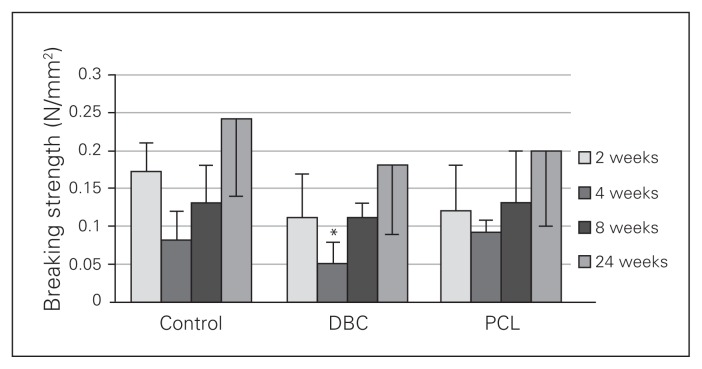
In the control group, breaking strength gradually increased and peaked during the twenty-fourth week of wound healing (Fig. 9), and this effect was also observed in the DBC- and PCL-treated groups. However, during the fourth week of healing, the breaking strength of the wound was significantly lower in the DBC group than the control and PCL-treated groups.
Fig. 9.
Breaking strength of granulation tissue after 2, 4, 8 and 24 weeks of healing in controls and rats with dressings covered by di-O-butyrylchitin (DBC) or poly-(ɛ-caprolactone [PCL]). Each bar represents a mean ± standard deviation of 7 rats. *Significant (p < 0.05) differences between the tested group and controls.
Wound histology
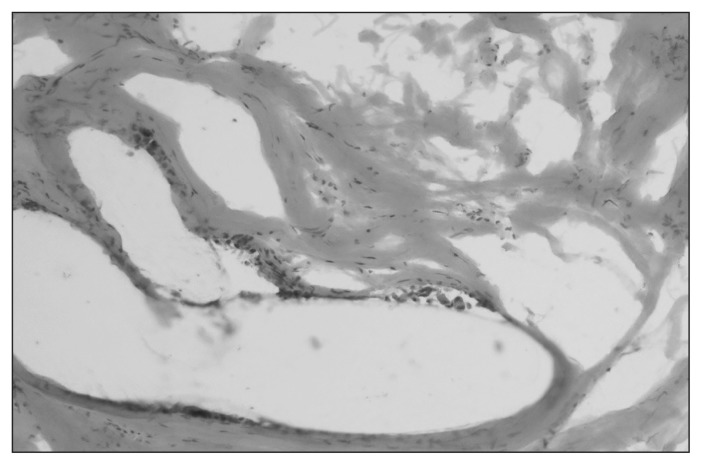
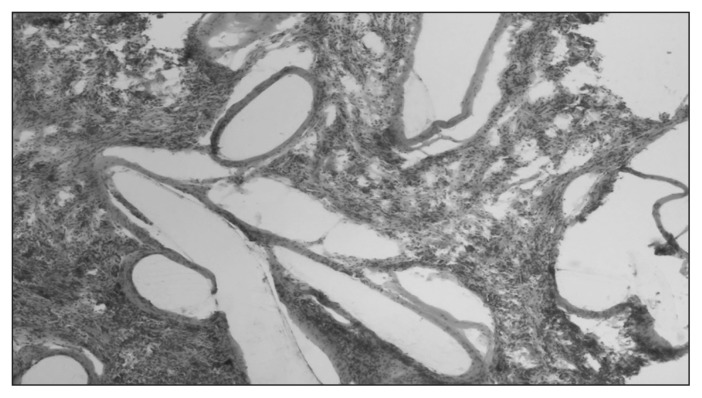
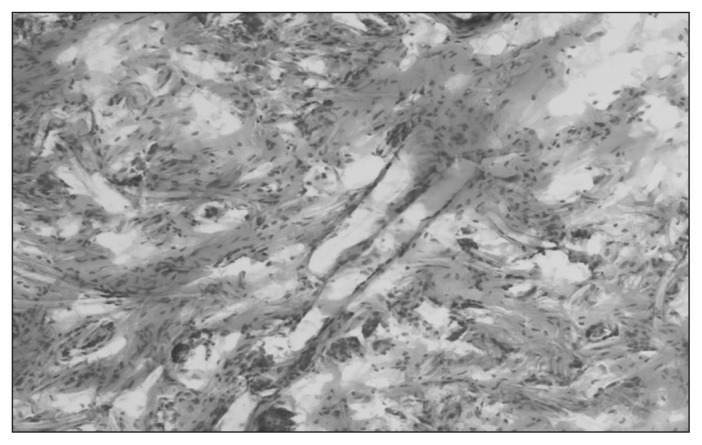
The inflammatory reaction was similar between the experimental and control groups. The intensity of inflammation was low in all groups. Inflammatory infiltration consisted of the same profile of cells. Differences were present in the size and location of “foreign body–type” macrophages. They were smaller, more numerous and more closely localized to fibres in the both the DBC and PCL groups compared with controls (Table 3 and Figs. 10–12).
Table 3.
Histological examination results*
| Group | Intensity of inflammation | Cellularity of connective tissue | “Foreign body type” macrophages | Number of blood vessels |
|---|---|---|---|---|
| Control | Low | Low | Low | Low |
| DBC | Low | Low | Intermediate | Intermediate |
| PCL | Low | Low | Intermediate | Intermediate |
DBC = di-O-butyrylchitin; PCL = poly-(ɛ-caprolactone).
The histological features were classified as low, intermediate, or high.
Fig. 10.
Granulation tissue in controls at 24 weeks. Staining with hematoxylin and eosin. Magnification ×200.
Fig. 12.
Granulation tissue in the poly-(ɛ-caprolactone [PCL]) group at 24 weeks. Staining with hematoxylin and eosin. Magnification ×80.
Discussion
The parameters of the electrospinning process allow the formation of a fibrous web. (Table 1). The literature gives similar characteristics for PCL electrospun fibres.25 The DBC webs (Table 1) have a smaller pore diameter than PCL webs: pore diameters of approximately 200 nm were seen in DBC webs, whereas the lowest observed pore diameter for PCL webs was 1000 nm. In addition, the total pore area was higher for DBC material than PCL material (Table 1). The DBC fibres were also found to have a more uniform diameter than the PCL webs, whose fibres were of nonuniform thickness, with nonfibrous elements also visible (Fig. 3). The DBC fibres demonstrated an oblate shape in cross-section. These features of DBC material allow better arrangement of the fibres in a web and result in better compaction.
Water-advancing contact angles were lower for DBC than PCL, which suggests that DBC is less hydrophobic than PCL, although the results showed that the 2 samples had hydrophobic characteristics (Table 2). Differences between values of advancing and receiving contact angles suggest that PCL samples have better wettability (Table 2). The DBC samples adsorbed twice the volume of liquid as PCL webs (Table 2), which corresponded with the total pore area values for both PCL and DBC webs. Thus, the total pore area of a DBC sample is 3 times that of a PCL sample (Table 1).
The dry tissue content was much higher in both the fourth and eighth weeks of repair in the wounds treated with DBC than control wounds (Fig. 4). Previous reports3 revealed that the use of DBC doubled the cell number in culture and lowered the necrotic cell number. In addition, DBC has been hypothesized to increase cell viability.3 Therefore, the DBC-induced augmentation of the granulation tissue weight, which we also observed in the present study, could be explained by an increased fibroblast number in the granulation tissue of the wound.3 Chitin derivatives have also been reported to stimulate cell proliferation in vivo.26 The pattern of the granulation tissue reaction to PCL treatment was different than that observed in both control and DBC-treated wounds. Therefore, the maximal level of granulation tissue in the PCL group was reached in the second week of healing and remained unchanged throughout the observation period. Good cell growth and fibroblast proliferation has previously been observed in a PCL/gelatin nanofibre scaffold.27 The reaction of the tissue was different for both PCL and DBC, but neither polymer diminished granulation tissue production nor caused continuous excessive accumulation (Fig. 4).
Water content in the wound was decreased in the control group (Fig. 5). We observed a similar process in both DBC- and PCL-treated wounds. The results showed better hydration of the wounds in both the DBC and PCL groups than the control group. No induction of large edema formation by the tested materials was observed (Fig. 5). The hydration of DBC samples was twice that of PCL obtained in vitro; this contrasts with only slightly better liquid retention observed in DBC samples implanted subcutaneously (Fig. 5). This discrepancy is supposed to be dependent on cell migration into the implanted samples and accumulation of extracellular matrix. The cells and elements of the extracellular matrix may modify water homeostasis within the tested sample.28,29 The poorer hydration of PCL implants than DBC implants may be linked with the impaired GAG accumulation pattern observed in this polymer (Fig. 6).
We observed a similar pattern of GAG level changes in the DBC and control groups. However, PCL implants altered the physiologic pattern of GAG changes in a wound (Fig. 6). Glycosaminoglycans are important regulators of the water–electrolyte balance in the tissue, modify growth factors and proteinase action, influence collagen fibre formation,30 and regulate gene expression.31
In the control group, the soluble collagen content decreased throughout the experiment (Fig. 8). The solubility of collagen in neutral salt solutions reflects its degree of maturation, cross-linking or catabolism.32 Collagen fibre maturation occurs when intermolecular covalent bond formation causes progressive loss of solubility of the molecule. In both the DBC and PCL groups, the soluble collagen level was lower in the second week of healing and persisted at the lower level for the duration of the experiment (Fig. 8). Thus, the 2 tested materials decreased the level of soluble collagen while the total collagen content remained unchanged (Fig. 7). This observation, suggests that these polymers may increase the deposition of insoluble collagen in wounds treated with DBC or PCL.32,33
Histological studies show similar responses of the tissue to implantation of both PCL and DBC. The implantation of biomaterials (Fig. 10–12) triggers an inflammatory response, with fibrosis following. The nonstained fibres and macrophage-mediated foreign body response are visible in the tissue. PCL is hydrolytically degradable.34 The enzymatic degradability of DBC has been studied by Muzzarelli and colleagues,34 who noted that DBC was modestly degraded by lipases. Collagenase was able to adhere to fibres and to exert limited activity on them, but little hydrolytic activity was exerted by cellulase and pectinase. DBC treatment with lysozyme reduced the tensile strength of fibres and released oligomers.34–36 Wounds treated with either DBC or PCL showed increased vascularization compared with control wounds (Table 3). Enhanced angiogenesis and accelerated wound healing has also been observed after implantation of chitosan-hyaluronan membranes with adipose-derived stem cells to the wound in the rat.37
Our main findings were focused on characterization of the processing techniques and structure and on describing the biological effects of biodegradable polymers. However, previous papers have examined only the classical forms, such as fibres, nonwovens, knitting products, foams or films. One of the main assumptions of this work was that the electrospinning process allows the production of webs with microfibres, which ensures better surface development and a more defined porous structure. The application of an electrostatic field for preparation of microfibrous webs enabled the production of a porous structure with a specified pore size distribution, suitable for cell migration.
Although methods of producing materials using electrospinning techniques are known, we used a unique, multinozzle laboratory stand in the present study. This stand enabled uniform material with a large surface to be produced in a single step, ensuring high repeatability of the samples. Also, polymer preparation techniques allowed for reproducible synthesis of the material featuring the same biological properties.
The electrospinning process was also found to produce fibres whose supermolecular structure was better than that of materials produced by classical methods from the same polymers. Tests of the surface properties of materials using water and cell culture medium indicate that interactions between tested materials and the media were significantly different. The higher values of wicking and the lower contact angle value suggest that the surface properties of the materials will not allow them to impede the passage of typical fluids existing in wounds. It should also be noted that better surface properties were observed for DBC samples.
The clinical effects of the polymers are dependent not only on the chemical composition, but also on the geometry and structure of the samples. The obtained pore sizes were sufficient for migration of the connective tissue cells, inflammatory cells and angiogenesis. The 2 scaffolds used induced adequate extracellular matrix synthesis in the wound. Thus, the DBC scaffold did not disturb the physiological course of repair of the wound and did not induce excessive fibrosis, edema or marked impairment of tensile strength. Ingrowth of the tissue into the sample after implantation modifies the hydrophilic properties of the polymers, but wounds treated with both PCL and DBC are significantly better hydrated. In both PCL and DBC samples, the process of degradation depends on the infiltration of macrophages. The resorption of the polymer is rather a long process that leaves degradation products that are neither nephrotoxic nor hepatotoxic.
Conclusion
The superiority of DBC was related to fact that, unlike PCL-treated wounds, it had not disturbed the pattern of GAG accumulation or had not reduced the weight of dry granulation tissue in the wound. Moreover, DBC webs absorbed twice as much liquid as PCL samples. Thus, dressings made of DBC could be recommended for the treatment of wounds with increased exudate. All other responses of the wounded tissue to DBC or PCL were similar, and both polymers could be used as scaffolds or dressings for wound treatment.
Fig. 11.
Granulation tissue in the di-O-butyrylchitin (DBC) group at 24 weeks. Staining with hematoxylin and eosin. Magnification ×200.
Acknowledgements
The research was partially funded by the Ministry of Science and Higher Education (PBZ-MNiSW-01/II/2007, Biodegradable Non-wovens for Medical, Agricultural and Technical applications). The authors are grateful to Teresa Staszewska for technical assistance.
Footnotes
Competing interests: None declared.
Contributors: J. Drobnik and I. Krucinska designed the study. J. Drobnik, A. Komisarczyk, S. Sporny and A. Szczepanowska acquired the data, which all authors analyzed. J. Drobnik, I. Krucinska, A. Komisarczyk and S. Sporny wrote the article, which all authors reviewed and approved for publication.
References
- 1.Venugopal RJ, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30:440–6. doi: 10.1111/j.1525-1594.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 2.Rentsch C, Schneiders W, Hess R, et al. Healing properties of surface-coated polycaprolactone-co-lactide scaffolds: a pilot study in sheep. J Biomater Appl. 2014;28:654–66. doi: 10.1177/0885328212471409. [DOI] [PubMed] [Google Scholar]
- 3.Blasinska A, Drobnik J. Effects of nonwoven mats of di-O-butyrylchitin and related polymers on the process of wound healing. Biomacromolecules. 2008;9:776–82. doi: 10.1021/bm7006373. [DOI] [PubMed] [Google Scholar]
- 4.Szosland LJ, Janowska G. Method for preparation of dibutyrylchitin. 169077B1. Polish Patent PL. 1996
- 5.Muzzarelli RAA, Guerrieri M, Goteri G, et al. The biocompatibility of dibutyryl chitin in the context of wound dressings. Biomaterials. 2005;26:5844–54. doi: 10.1016/j.biomaterials.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Szosland L, Krucinska I, Cislo R, et al. Synthesis dibutyrylchitin and preparation of new textiles made from dibutyrylchitin and chitin for medical applications. Fibres Text East Eur. 2001 Jul-Sep;:54–7. [Google Scholar]
- 7.Biological evaluation of medical devices, ISO 10993-5 (1992), ISO 10993–10; 1995.
- 8.Staniszewska-Kus J, Rutowski R, Paluch D. Our own experience in the evaluation of biocompatibility of surgical by microscopic multipoint estimation. Int J Art Organs. 1996;19:517. [Google Scholar]
- 9.Krucinska I, Komisarczyk A, Paluch D, et al. The impact of the dibutyrylchitin molar mass on the bioactive properties of dressings used to treat soft tissue wounds. J Biomed Mater Res Part B. 2012;100B:11–22. doi: 10.1002/jbm.b.31895. [DOI] [PubMed] [Google Scholar]
- 10.Yiling Teo E, Ong SH, Seow Khoon Chong M, et al. Polycaprolactone-based fused deposition modeled mesh for delivery of antibacterial agents to infected wounds. Biomaterials. 2011;32:279–87. doi: 10.1016/j.biomaterials.2010.08.089. [DOI] [PubMed] [Google Scholar]
- 11.Jaiswal M, Dinda AK, Gupta A, et al. Polycaprolactone diacrylate crosslinked biodegradable semi-interpenetrating networks of polyacrylamide and gelatin for controlled drug delivery. Biomed Mater. 2010;5:065014. doi: 10.1088/1748-6041/5/6/065014. [DOI] [PubMed] [Google Scholar]
- 12.Venugopal RJ, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblast on electrospun polycaprolactone collagen nanofibrous membrane. Artificial Organs. 2006;30:440–6. doi: 10.1111/j.1525-1594.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 13.Lou T, Leung M, Wang X, et al. Bi-layer scaffold of chitosan/PCL-nanofibrous mat and PLLA-microporous disc for skin tissue engineering. J Biomed Nanotechnol. 2014;10:1105–13. doi: 10.1166/jbn.2014.1793. [DOI] [PubMed] [Google Scholar]
- 14.Gholipour-Kanani A, Bahrami SH, Joghataie MT, et al. Tissue engineered poly(caprolactone)-chitosan-poly(vinyl alcohol) nanofibrous scaffolds for burn and cutting wound healing. IET Nanobiotechnol. 2014;8:123–31. doi: 10.1049/iet-nbt.2012.0050. [DOI] [PubMed] [Google Scholar]
- 15.Krucinska I, Komisarczyk A, Chrzanowski M, et al. Electrostatic field in electrospinning with a multicapillary head — modelling and experiment. Fibres Text East Eur. 2009;17:38–44. [Google Scholar]
- 16.Micromeritics. AutoPore IV Manual. Norcross (GA): Micromeritics; 2010. [Google Scholar]
- 17.Hindeleh AM, Johnson DJ. The resolution of multipeak data in fiber science. J Physics D: Applied Physic. 1971;4:259–63. [Google Scholar]
- 18.Woessner IF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1967;93:440–5. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 19.Farndale RW, Buttle DJ, Barrot AJ. Improved quantitation and determination of sulfated glycosaminoglycans by use dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin Chem Lab Med. 2002;40:718–24. doi: 10.1515/CCLM.2002.124. [DOI] [PubMed] [Google Scholar]
- 21.Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002;40:725–33. doi: 10.1515/CCLM.2002.125. [DOI] [PubMed] [Google Scholar]
- 22.Cook JG. Creatinine assay in the presence of protein. Clin Chim Acta. 1971;32:485–6. doi: 10.1016/0009-8981(71)90452-9. [DOI] [PubMed] [Google Scholar]
- 23.Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clin Chim Acta. 1972;38:475–6. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- 24.Krucinska I, Komisarczyk A, Paluch D, et al. The impact of the dibutyrylchitin molar mass on the bioactive properties of dressings used to treat soft tissue wounds. J Biomed Mater Res Part B. 2012;100B:11–22. doi: 10.1002/jbm.b.31895. [DOI] [PubMed] [Google Scholar]
- 25.Hsu ChM, Shivkumar S. Nano-sized beads and porous fiber constructs of Poly(ɛ-caprolactone) produced by electrospinning. J Mater Sci. 2004;39:3003–13. [Google Scholar]
- 26.Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–15. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 27.Chong EJ, Phan TT, Lim IJ, et al. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321–30. doi: 10.1016/j.actbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Asthana S, Goyal P, Dhar RKU, et al. Evaluation extracellular matrix-chitosan composite films for wound healing application. J Mater Sci Mater Med. 2015;26:220. doi: 10.1007/s10856-015-5551-y. [DOI] [PubMed] [Google Scholar]
- 29.Anilkumar TV, Muhamed J, Jose A, et al. Advantages of hyaluronic acid as a component of fibrin sheet for care of acute wound. Biologicals. 2011;39:81–8. doi: 10.1016/j.biologicals.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Kucharz E. The collagens: Biochemistry and pathophysiology. Berlin (Germany): Springer-Verlag; 1992. Biosynthesis of collagen; pp. 87–107. [Google Scholar]
- 31.Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 32.Gerstenfeld LC, Chipman SD, Kelly CM, et al. Collagen expression ultrastructural assembly and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol. 1988;106:979–89. doi: 10.1083/jcb.106.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblasts differentiation in MC3T3-E1. J Bone Miner Res. 1994;9:843–54. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 34.Muzzarelli C, Francescangelli O, Tosi G, et al. Susceptibility of dibutyryl chitin and regenerated chitin fibers to deacylation and depolymerization by lipases. Carbohydr Polym. 2004;56:137–46. [Google Scholar]
- 35.Szosland L, Szumilewicz J, Struszczyk H. Enzymatic degradation of dibutyrylchitin. In: Muzarrelli RAA, editor. Chitin Enzymology. Vol. 2. Atecm Edizioni; 1996. pp. 491–6. [Google Scholar]
- 36.Szosland L, Steplewski W. Rheological characteristic of dibutyrylchitin semi-concentrated solutions and wet spinning of dibutyrylchitin fibre. In: Domard A, Roberts GAF, Varum KM, editors. Advances in Chitin Science. II. 1998. pp. 531–536. [Google Scholar]
- 37.Hsu SH, Hsieh PS. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair Regen. 2014;23:57–64. doi: 10.1111/wrr.12239. [DOI] [PubMed] [Google Scholar]