Abstract
Background
Neuroendocrine tumours (NETs) are heterogeneous, with varying presentations and treatment options. To our knowledge, there are no randomized and few long-term studies of patient outcomes. The role of surgical and medical therapy for local, regional and metastatic disease continues to be evaluated in the literature.
Methods
We conducted a population-based search of the provincial cancer registry to identify patients with gastrointestinal NETs from the stomach, small intestine, colon and rectum diagnosed between 1990 and 2005 and assessed their outcomes.
Results
We examined clinicopathological information on the outcomes of 530 patients with gastrointestinal NETs. The overall incidence of NETs increased from 11 per million to 19 per million during the study period. Advancing stage and patient age were associated with poor overall or disease-specific outcomes. Surgery, both curative and palliative, was associated with decreased risk of overall (hazard ratio [HR] 0.5, p < 0.001) and disease-specific (HR 0.5, p < 0.001) death. The biggest benefit was observed in patients with distant disease, in whom 5-year disease-specific survival for R0 resections was nearly double that for patients with macroscopic residual disease (92% v. 48%, p = 0.009). Older age was associated with poor 5-year overall and disease-specific survival (p < 0.001).
Conclusion
There has been a significant increase in incidence of gastrointestinal NETs, and advancing patient age, but not sex, is linked to poor outcomes in terms of overall and disease-specific survival. Surgery, both curative and palliative, was associated with decreased risk of overall and disease-specific death. Compared with patients with residual macroscopic disease, patients with distant disease were nearly twice as likely to survive 5 years if they had R0 resections. The use of radioisotope therapy and long-acting octreotide therapy was also associated with improved outcomes overall.
Abstract
Contexte
Les tumeurs neuroendocrines (TNE) sont hétérogènes, et les tableaux et options thérapeutiques sont variables. À notre connaissance, il n’existe pas d’études randomisées et il y a peu d’études à long terme sur les résultats chez les patients. Le rôle du traitement chirurgical et médicamenteux de la maladie locale, régionale et métastatique continue d’être évalué dans la littérature.
Méthodes
Nous avons procédé à une interrogation démographique du Registre provincial du cancer pour recenser les patients atteints de TNE gastro-intestinales provenant de l’estomac, de l’intestin grêle, du côlon et du rectum, diagnostiqués entre 1990 et 2005 et nous avons évalué les résultats.
Résultats
Nous avons examiné les données clinico-pathologiques des résultats enregistrés chez 530 patients atteints de TNE gastro-intestinales. L’incidence globale des TNE a augmenté de 11 par million à 19 par million pendant la période de l’étude. Le stade de la maladie et l’âge avancés ont été associés à des résultats globaux ou spécifiques à la maladie moins favorables. La chirurgie, curative et palliative, a été associée à un risque moindre de décès global (risque relatif [RR] 0.5, p < 0,001) et spécifique à la maladie (RR 0,5, p < 0,001). L’avantage le plus marqué a été observé chez les patients présentant une maladie distale, chez qui la survie à 5 ans spécifique à la maladie pour les résections R0 était près de 2 fois celle des patients présentant une maladie macroscopique résiduelle (92 % c. 48 %, p = 0,009). L’âge avancé a été associé à une survie à 5 ans globale et spécifique à la maladie défavorable (p < 0,001).
Conclusion
L’incidence des TNE gastro-intestinales a significativement augmenté, et l’âge avancé des patients, mais non le sexe, est lié à des résultats défavorables aux plans de la survie globale et spécifique à la maladie. La chirurgie, curative et palliative, a été associée à un risque moindre de décès global et spécifique à la maladie. Comparativement aux patients ayant une maladie macroscopique résiduelle, ceux qui avaient une maladie distale étaient près de 2 fois plus susceptibles de survivre 5 ans s’ils avaient des résections R0. Les traitements par radio-isotopes et octréotide à longue durée d’action ont aussi été associés à une amélioration globale des résultats.
Neuroendocrine tumours (NETs), known historically as carcinoid tumours, were first described by Lubarsch in 1888. These tumours are distributed throughout the body but are mainly found within the gastrointestinal (GI) tract.1–3 Much of what is known about the incidence, distribution and outcomes of carcinoid or GI NETs has been derived from large population-based studies.4,5 One of the largest and most recent population-based studies of GI tumours was completed in the United States using the Surveillance, Epidemiology, and End Results (SEER) database, which documented a nearly 5-fold increase from 1979 to 2004 in the incidence of GI NETs.6 These data have been mirrored in a recent Canadian study.7 However, patient outcomes in large database studies are difficult to compare given the lack of uniform descriptions of staging as well as varying treatment paradigms for this disease. The general trends indicate that more than 75% of patients survive 5 years and that the probability of survival is well reflected in stage classification of local, regional and distant disease.1,4,5,8–11
We undertook a study of the incidence, presentation, stage, anatomic distribution and survival characteristics of GI NET tumours in a Canadian population served by 2 academic centres with a common cancer registry. We endeavoured to show that the incidence of these tumours has changed over time and to understand how patient outcomes vary with disease presentation and different treatment modalities.
Methods
Alberta is the fourth most populous province in Canada, with a population of 3.8 million people. Between Jan. 1, 1990, and Dec. 31, 2005, all cases of gastric, small intestine, colonic and rectal NETs diagnosed in the province of Alberta were recorded in the Alberta Cancer Registry, as mandated by the Alberta Health Act. By probing this provincial registry, trained coders were able to identify all NETs diagnosed during the study period. Cases were excluded if the primary tumour location was not in the GI tract or if the pathology report was not consistent with a NET. Demographic characteristics extracted for each patient included age, sex and year of diagnosis. We extracted tumour-specific data, including clinical presentation, anatomic site of the tumour, stage at diagnosis (local, regional or metastatic) and evidence of carcinoid syndrome at diagnosis. The diagnosis of carcinoid syndrome was based on evidence of flushing, severe diarrhea, wheezing or cardiac symptoms consistent with elevated systemic 5-hydroxyindoleacetic acid (5-HIAA) or chromogranin A. We cross-referenced the registry data to 2 separate hospital data sets to confirm the completeness of the registry data. We were also able to obtain medical records from the Netcare electronic health repository or the Medical Records Department of the Cross Cancer Institute to validate the registry records. We assessed and validated the quality of the administrative data using the techniques outlined by Hinds and colleagues.12 In the present study we used the SEER staging system to compare outcomes. Stage of disease at presentation was defined as localized disease if there was no evidence of nodal involvement or distant metastatic disease, regional if there was evidence of nodal involvement, or metastatic. Anatomic location data were grouped as follows: stomach, small bowel, appendix, colon, rectum and other. We recorded whether the patient had surgery for either the primary tumour site or metastatic disease. The Alberta Cancer Board Research Ethics Board approved our study protocol.
Statistical analysis
We calculated the annual incidence of new cases using the number of new cases diagnosed each year and the total population of the province for the corresponding year. Descriptive statistics were obtained for the study variables. We report means and standard deviations for the continuous variables and frequencies and proportions for categorical variables. For the purpose of analysis comparing survival data across different anatomic sites, we used the primary tumour site within small bowel as the reference group. We used Kaplan–Meier estimates and the respective 95% confidence intervals (CIs) for median overall survival (OS) and disease-specific survival (DSS). We used log rank tests to compare the 2 survival curves, and we calculated age-adjusted 5-year OS and DSS rates for each stage, anatomic location and geographic location. To determine the predictors of OS and DSS, we used the Cox proportional hazard model. The final multivariate model was chosen with all the significant variables. All statistical analyses were conducted using SAS Software (SAS Institute Inc.), and we considered results to be significant at p < 0.05.
Results
A total of 530 cases of gastrointestinal NETs met our eligibility criteria. The overall incidence of NETs increased from 11 to 19 per million over the study period. The sex distribution did not vary significantly as a function of year in our study. Outcomes analyses historically used different staging criteria derived from different sources, including the American Joint Committee on Cancer (AJCC), European Neuroendocrine Tumor Society (ENETS) or the SEER databases, each with varying abilities to predict tumour behaviour.6–11 To facilitate comparison in the present study we use a single, simplified staging system comparable to that used by SEER, documenting local, regional and distant disease among the different tumour sites.
Presentation
The mean patient age was 59 (range 12–92) years, and half the patients were men (Table 1). Nearly half the patients (48%) presented with localized disease. There was evidence of carcinoid syndrome in 12% of cases. The most common tumour location was the small bowel (48%), followed by the colon or rectum, appendix and stomach. Of all the patients in the study 74% had surgery (Table 1).
Table 1.
Demographic and clinical characteristics
| Characteristic | No. (%)* |
|---|---|
| Male sex | 267 (50) |
| Age, mean (range), yr | 59 (12–92) |
| Stage at diagnosis | |
| Localized | 257 (48) |
| Regional | 104 (20) |
| Metastatic | 163 (32) |
| Missing/unknown | 6 (1) |
| Carcinoid syndrome at presentation | |
| Yes | 63 (12) |
| No | 426 (80) |
| Missing | 41 (7) |
| Anatomic distribution | |
| Stomach | 45 (9) |
| Small bowel | 293 (55) |
| Duodenum | 15 (3) |
| Jejunum | 13 (2) |
| Ileum | 156 (29) |
| Small bowel NOS | 109 (20) |
| Appendix | 95 (17) |
| Colorectal | 97 (19) |
| Right colon | 25 (5) |
| Left colon | 2 (0.5) |
| Sigmoid colon | 4 (1) |
| Colon NOS | 6 (1) |
| Rectal | 60 (11) |
| Surgery | |
| Yes | 401 (74) |
| No | 128 (25) |
| No data | 1 (< 1) |
| Radioisotope therapy | |
| Yes | 86 (17) |
| No | 434 (83) |
| No data | 10 (2) |
NOS = not otherwise specified.
Unless indicated otherwise.
The presentation of disease varied depending on the stage of disease. For patients with localized disease, 31% were identified from endoscopy, imaging, or incidental finding at the time of laparotomy for some other indication. The next most common presentations were suspected appendicitis (21%), abdominal pain not yet diagnosed (17%) and GI bleeding or anemia (10%). In the remaining 17% of cases we could not determine the inciting clinical factor that led to the diagnosis. For patients with regional disease, the most common presentation was abdominal pain (47%) followed by GI obstruction (36%), incidental finding (12%) and GI bleed or anemia (10%). Patients with metastatic disease most commonly presented with abdominal pain (55%), carcinoid syndrome (33%), GI obstruction (28%), incidental finding (13%) and GI bleed or anemia (6%).
Treatment
Most patients had surgery as part of their treatment, whereas 25% (n = 128) of the patients did not. Treatment by surgery varied according to the extent of disease at presentation; 70% of patients with localized disease, 99% of patients with regional disease and 65% of patients with distant metastases had surgery. Most patients treated with surgery had only 1 surgery (n = 387), whereas 40 patients had 2 surgeries and 3 patients had 3 surgeries. The rate of R0 resections among first surgical procedures was 62% compared with 45% for second surgical procedures and 33% for third surgical procedures. Of the patients who did not have surgery, 58% (75 of 128) had endoscopic removal of their tumour, representing type 1 and 2 gastric NETs and rectal NETs. In the group of patients who did not have either surgery or endoscopic removal of their primary tumour (n = 64), 92% presented with distant disease. Radionuclide therapy was given to a subset (n = 90) of patients who presented with metastatic disease or progressed to distant disease.
Survival
Patients were followed for on average 76 months. Table 2 shows the aggregate 5-year OS and DSS. Survival rates were calculated for the different groups according to stage, age, sex, anatomic distribution and whether or not surgery was performed. It is clear that for our cohort, increasing age but not sex was linked to poor outcomes, regardless of grade. On univariate analysis patients with metastatic disease had poorer outcomes for both OS and DSS (Table 3). For OS, there were significant differences on both univariate and multivariate analysis (Table 4). Patients older than 50 years were nearly 6 times as likely to die from any cause as patients younger than 50 years (Table 4). Sex, however, was not a factor in OS. Using NETs of the small bowel as a reference point, we found that patients with appendiceal and rectal tumours had significantly improved OS on univariate analysis, but the survival difference did not persist on multivariate analysis. Younger age was associated with improved disease-specific outcomes (Table 5). In addition, on multivariate analysis, men were less likely to die from their disease than women, and this was a significant deviation from the results seen for OS. For completeness, we examined patient outcomes comparing type 1 and 2 gastric NETs (n = 36) with type 3 (n = 9) to reveal that poor outcomes are almost solely attributable to patients with type 3 tumours.
Table 2.
Five-year overall and disease-specific survival
| Variable | 5-year OS, % | 5-year DSS, % |
|---|---|---|
| Stage | ||
| Localized | 81 | 97 |
| Regional | 78 | 90 |
| Metastatic | 51 | 58 |
| Age, yr | ||
| > 50 | 64 | 79 |
| < 50 | 86 | 91 |
| Sex | ||
| Male | 69 | 85 |
| Female | 70 | 80 |
| Site | ||
| Stomach | 70 | 89 |
| Small bowel | 70 | 83 |
| Appendix | 84 | 94 |
| Colon | 73 | 80 |
| Rectum | 100 | 100 |
DSS = disease-specific survival; OS = overall survival.
Table 3.
Five-year univariate survival analysis comparing stage
| Survival; stage | HR (95% CI) | p value |
|---|---|---|
| Overall survival | ||
| Localized | 1 | |
| Regional | 1.2 (0.8–2.0) | 0.38 |
| Metastatic | 2.9 (2.0–4.2) | < 0.001 |
| Disease-specific survival | ||
| Localized | 1 | |
| Regional | 2.7 (0.9–8.4) | 0.08 |
| Metastatic | 18.8 (7.6–46.8) | < 0.001 |
CI = confidence interval; HR = hazard ratio.
Table 4.
Univariate and multivariate analyses of 5-year overall survival
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age > 50 yr | 5.7 (3.2–10.0) | < 0.001 | 5.5 (2.9–10.4) | < 0.001 |
|
| ||||
| Sex, male v. female | 1.0 (0.7–1.4) | 0.94 | 0.9 (0.65–1.23) | 0.49 |
|
| ||||
| Site | ||||
|
| ||||
| Stomach v. small bowel | 0.6 (0.2–2.6) | 0.54 | 1.1 (0.28–4.7) | 0.85 |
|
| ||||
| Appendix v. small bowel | 0.3 (0.2–0.6) | < 0.001 | 1.0 (0.5–1.7) | 0.99 |
|
| ||||
| Colon v. small bowel | 0.9 (0.5–1.6) | 0.70 | 0.9 (0.5–1.7) | 0.89 |
|
| ||||
| Rectum v. small bowel | 0.5 (0.3–0.9) | 0.048 | 0.8 (0.4–1.6) | 0.51 |
|
| ||||
| Surgery, yes v. no | 0.5 (0.3–0.7) | < 0.001 | 0.6 (0.4–0.7) | 0.004 |
CI = confidence interval; HR = hazard ratio.
Table 5.
Univariate and multivariate analyses of 5-year disease-specific survival
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age > 50 yr | 5.2 (2.4–11.3) | < 0.001 | 4.1 (1.8–9.6) | 0.001 |
|
| ||||
| Sex, male v. female | 0.7 (0.4–1.0) | 0.06 | 0.6 (0.4–0.9) | 0.012 |
|
| ||||
| Site | ||||
|
| ||||
| Stomach v. small bowel | 0.5 (0.2–1.2) | 0.12 | 0.4 (0.2–1.3) | 0.14 |
|
| ||||
| Appendix v. small bowel | 0.2 (0.05–0.5) | 0.003 | 0.4 (0.1–1.5) | 0.17 |
|
| ||||
| Colon v. small bowel | 1.2 (0.6–2.4) | 0.54 | 1.6 (0.7–3.4) | 0.23 |
|
| ||||
| Rectum v. small bowel | 0.6 (0.2–1.3) | 0.17 | 2.3 (0.9–6.0) | 0.08 |
|
| ||||
| Surgery, yes v. no | 0.3 (0.2–0.5) | < 0.001 | 0.5 (0.3–0.7) | < 0.001 |
CI = confidence interval; HR = hazard ratio.
Response to treatment
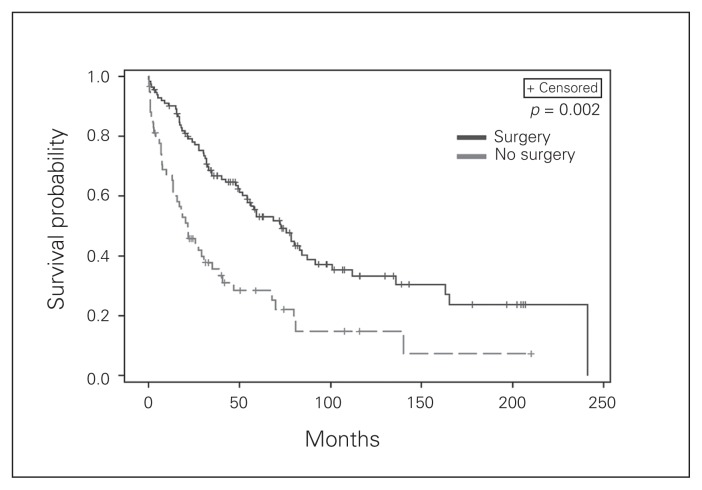
In our cohort surgical intervention was associated with improved OS and DSS by both univariate and multivariate analysis (Table 4, Table 5). When we looked at individual groups, surgery for localized tumours did not confer a DSS (p = 0.35) or OS benefit (p = 0.62) on multivariate analysis. The resection margins, whether R0, R1 or R2, did not appear to impact DSS for patients undergoing surgery for localized (p = 0.20) disease. All patients with regional disease had surgery, thus there was no comparator for analysis. Patients with distant disease who underwent surgery demonstrated significantly improved outcomes (Fig. 1). If the surgeon was able to obtain an R0 resection, 92% of patients survived 5 years; for R1 resections the DSS was 75% and for R2 resections it was 48%. This survival difference based on margin status was significant (p = 0.009).
Fig. 1.
Kaplan–Meier plots of disease recurrence as a function of time based on the whether or not the patients with distant disease underwent surgery with curative or palliative intent.
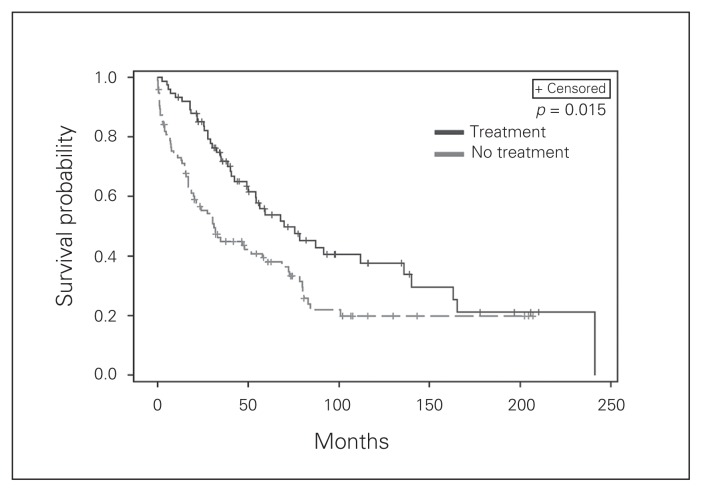
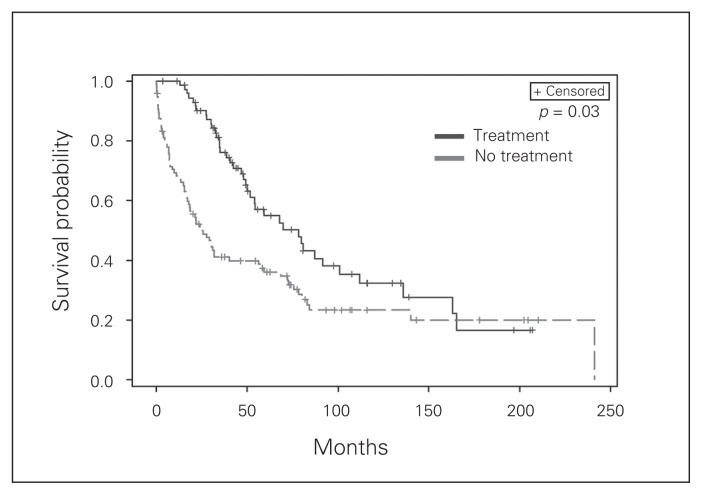
We also examined survival for patients receiving radioisotope therapy. Patient selection for radioisotope therapy included patients with symptomatic disease, those unable to undergo surgery owing to comorbidities and those with disease that was not amenable to resection. Radioisotope therapy appears to impact OS on multivariate analysis (Fig. 2). Moreover, as the number of radioisotope therapies for a given patient increased, we observed improved OS with a hazard ratio of 0.84 (p < 0.001) and improved DSS with a hazard ratio of 0.83 (p = 0.001). Finally, we examined systemic octreotide therapy, which was given to nearly 1 in 5 patients (n = 99) primarily to treat symptoms of serotonin excess. We observed a small but significant improvement in survival (Fig. 3).
Fig. 2.
Kaplan–Meier plots of disease recurrence as a function of time based on the whether or not the patients with distant disease underwent radioisotope therapy in the form of
Fig. 3.
Kaplan–Meier plots of disease recurrence as a function of time based on the whether or not the patients with distant disease were treated with long-acting octreotide.
Discussion
The overall incidence of NETs in our centre has increased significantly from 11 per million to 19 per million over a 15-year period. Our results are concordant with those of other North American centres as well as with those of studies completed in Europe, where the incidence of GI NETs is rising.3,4,6–10 At least part of this increase is likely due to the increased access and use of cross-sectional imaging for investigation of abdominal presentations, but the true prevalence remains unknown.11,13 The anatomic distribution of NETs in our study reflects that seen in other studies, with small bowel and appendiceal tumours accounting for more than two-thirds of all GI NETs.3,4,6,7,11 Our average age and equal incidence between men and women are generally concordant with studies performed worldwide. With respect to advancing stage and patient age, our results were similar to those published previously in terms of decreased survival.3,6,11 One of the novel findings of our study was that we did not see a sex bias in survival favouring women, as has been outlined by previous reports.6 In fact, male sex was associated with improved DSS (HR = 0.6, p = 0.014) on multivariate analysis.
We demonstrate potential value to surgical intervention in this challenging and complex group of patients. Surgery, both curative and palliative, is associated with a decreased risk of overall and disease-specific death by multivariate analysis. Surgical treatments of distant disease were associated with improved survival, where 5-year DSS for R0 resections was nearly double that for patients with macroscopic disease. As with many surgical studies, there is likely a selection bias to explain the association between surgery and improved survival, as patients with advanced metastatic disease and/or multiple comorbidities are less often candidates for surgery.14–18 However, we note that the most significant survival benefit associated with surgery was observed in patients with advanced disease. In addition, there was an association between R0 status and improved disease-specific outcomes (p = 0.009). Our evidence is concordant with that of other authors who documented improved outcomes with surgery for metastatic disease. 15,17,18 The survival rate in our study for R0 resections is higher than that reported in other studies (55%–80%).5,9,16,18 We attribute this improvement, in part, to the use of postoperative radioisotope therapy to minimize the risk of recurrence and control tumour growth. More than one-third of patients with distant disease received radioisotope therapy. Thus, our work further confirms that multimodality efforts anchored with surgery may go beyond symptom relief and may in fact improve OS and DSS.
Radioisotope therapy may be used to control symptoms, but it may also translate into improved outcomes for patients with distant disease. The most significant impact of radiopharmaceutical therapy was identified in those patients given multiple doses, likely reflecting avidity and improved efficacy. To our knowledge there is no level 1 evidence to suggest that radiotherapy has a survival benefit, although a small, nonrandomized study by Nguyen and colleagues19 documented improved survival for patients with metastatic NETs. In addition, a retrospective review by Sywak and colleagues20 showed improved 5-year OS when radioisotope therapy was used to treat metastatic midgut NETs.20 The survival benefit was most pronounced at 3 and 5 years. These studies suggest a combined role for surgery and radioisotope therapy for patients with metastatic NETs. The complexity of NETs in terms of origin, metastatic potential and variations in disease avidity for radioisotope therapy make performing large, prospective randomized trials difficult. Although the use of long-acting octreotide in this cohort was intended to treat carcinoid symptoms, we also saw a small improvement in survival overall, and there is recent evidence supporting its use to improve survival.21 Clearly, there is much more work needed to demonstrate the relative contributions of multimodal therapy to outcomes of patients with GI NETs.
Conclusion
In this population study of NETs, we report a significant increase in the incidence of disease. Surgery, both curative and palliative, was associated with decreased risk of overall (HR = 0.6, p = 0.004) and disease-specific death (HR = 0.5, p < 0.001) on univariate and multivariate analysis. Patients with distant disease were nearly twice as likely as patients with residual macroscopic disease to survive 5 years if they had R0 resections. The use of radioisotope therapy was also associated with improved outcomes overall. Advancing disease stage and patient age, but not male sex, were associated with poor outcomes in terms of OS and DSS.
Footnotes
Competing interests: T. McMullen is a paid consultant with Galapagos LLC and has received speaker fees from Novartis. No other competing interests declared.
Contributors: T. McMullen, C. de Gara and D. Schiller designed the study. T. McMullen and A. Al-Jahdali acquired and analyzed the data, which S. Ghosh, A. McEwan and D. Schiller also analyzed. T. McMullen wrote the article, which all authors reviewed and approved for publication.
References
- 1.Crocetti E, Paci E. Malignant carcinoids in the USA, SEER 1992–1999. An epidemiological study with 6830 cases. Eur J Cancer Prev. 2003;12:191–4. doi: 10.1097/00008469-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–22. doi: 10.1097/01.sla.0000129342.67174.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–59. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 4.Stamatakos M, Kontzoglou K, Sargedi C, et al. Gastrointestinal carcinoid tumors: diagnosis and treatment. Chirurgia (Bucur) 2010;105:759–66. [PubMed] [Google Scholar]
- 5.Valentino J, Evers BM. Recent advances in the diagnosis and treatment of gastrointestinal carcinoids. Adv Surg. 2011;45:285–300. doi: 10.1016/j.yasu.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 7.Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–97. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 8.Zar N, Garmo H, Holmberg L, et al. Long-term survival of patients with small intestinal carcinoid tumors. World J Surg. 2004;28:1163–8. doi: 10.1007/s00268-004-7610-2. [DOI] [PubMed] [Google Scholar]
- 9.Lepage C, Rachet B, Coleman MP. Survival from malignant digestive endocrine tumors in England and Wales: a population-based study. Gastroenterology. 2007;132:899–904. doi: 10.1053/j.gastro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563–9. doi: 10.1038/ajg.2010.341. [DOI] [PubMed] [Google Scholar]
- 11.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a SEER analysis. J Cancer. 2012;3:292–302. doi: 10.7150/jca.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinds A, Lix LM, Smith M, et al. Quality of administrative health databases in Canada: a scoping review. Can J Public Health. 2016;107:e56–61. doi: 10.17269/cjph.107.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouffon M, Iff S, Ziegler K, et al. Diagnosis and workup of 522 consecutive patients with neuroendocrine neoplasms in Switzerland. Swiss Med Wkly. 2014;144:w13924. doi: 10.4414/smw.2014.13924. [DOI] [PubMed] [Google Scholar]
- 14.Strosberg J, Nasir A, Coppola D, et al. Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2009;40:1262–8. doi: 10.1016/j.humpath.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–41. doi: 10.1002/cncr.25855. [DOI] [PubMed] [Google Scholar]
- 16.Habbe N, Fendrich V, Heverhagen A, et al. Outcome of surgery for ileojejunal neuroendocrine tumors. Surg Today. 2013;43:1168–74. doi: 10.1007/s00595-012-0408-1. [DOI] [PubMed] [Google Scholar]
- 17.Chambers AJ, Pasieka JL, Dixon E, et al. The palliative benefit of aggressive surgical intervention for both hepatic and mesenteric metastases from neuroendocrine tumors. Surgery. 2008;144:645–51. doi: 10.1016/j.surg.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Harring TR, Nguyen NT, Goss JA, et al. Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol. 2011;154:541–9. doi: 10.4061/2011/154541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen C, Faraggi M, Giraudet AL, et al. Long-term efficacy of radionuclide therapy in patients with disseminated neuroendocrine tumors uncontrolled by conventional therapy. J Nucl Med. 2004;45:1660–8. [PubMed] [Google Scholar]
- 20.Sywak MS, Pasieka JL, McEwan A, et al. 131I-metaiodobenzylguanidine in the management of metastatic midgut carcinoid tumors. World J Surg. 2004;28:1157–62. doi: 10.1007/s00268-004-7603-1. [DOI] [PubMed] [Google Scholar]
- 21.Shen C, Shih YC, Xu Y, et al. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: a population-based analysis. Cancer. 2014;120:2039–49. doi: 10.1002/cncr.28653. [DOI] [PubMed] [Google Scholar]