Abstract
Transarterial locoregional therapies (LRTs) are indispensable components of the modern interventional oncologic therapy of liver-dominant metastatic neuroendocrine tumors (NETs). The scope of available LRTs and their nuanced differences mandates a thorough understanding of their relative applicability and effectiveness in certain clinical circumstances to prescribe appropriate, patient-specific, image-guided therapy. This article aims to provide an overview of transarterial LRT options for liver-dominant metastatic NETs and therapy selection by reviewing procedure types, their advantages and disadvantages, and comparative efficacy in common case scenarios.
Keywords: neuroendocrine tumor, metastatic, locoregional therapy, Interventional radiology
Objectives : Upon completion of this article, the reader will be able to discuss the transarterial locoregional therapy options for liver-dominant metastatic neuroendocrine tumors, their advantages and disadvantages, and clinical outcomes.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that arise from the amine precursor uptake and decarboxylation (APUD) system found in nervous and endocrine tissue, including enterochromaffin (Kulchitsky) cells of the gastrointestinal (GI) tract, islet cells of the pancreas, and bronchial cells of the lung. These tumors occur at a yearly frequency of 5.25 cases per 100,000 people, with an annual age-adjusted incidence that has been rising over the past 30 years. 1 Carcinoid tumors—which represent the most common NET—typically arise in the GI tract (67%) or the pulmonary system (25%). 2 NETs of GI and pancreatic origin metastasize to the liver in 46 to 93% of cases. 3 Patients with NETs frequently experience hormone-induced flushing and diarrhea from “functional” tumors, and NET liver metastases result in clinical symptoms in the majority of cases given the bypass of normal hepatic hormonal deactivation. 4 Survival in cases of NET liver metastases ranges from 5 to 56 months depending on primary tumor site, 1 and 80 to 90% of patients with NET liver metastases are inoperable. 5 6 Because systemic therapies have modest benefit for most patients with metastatic NET, 7 8 9 interventional radiology (IR) administered transarterial locoregional therapies (LRTs) can play an important role in treatment. This article aims to provide practical guidance on transarterial LRT selection for NET hepatic metastases by offering a brief overview of currently available treatment options and then employing a case-based format to 1 : depict decision-making considerations in selecting an LRT in various clinical cases, 2 highlight the clinical and/or technical advantages or disadvantages of each modality, and 3 review their clinical outcomes.
Transarterial Locoregional Therapy Overview
A variety of transarterial LRTs are available to the practicing IR for treatment of liver dominant metastatic NET, including transarterial embolization (TAE), transarterial chemoembolization (TACE), and yttrium-90 radioembolization ( 90 Y RE). TAE is defined as blockade of hepatic arterial flow with a vascular occlusive agent, such as gelatin sponge, polyvinyl alcohol, or calibrated microspheres. 10 TAE results in tissue devascularization through distal arteriolar occlusion that spurs necrosis, inflammation, and fibrosis via the ischemic cascade. TACE can be broadly grouped into two distinct categories: (1) conventional TACE (c-TACE) is the infusion of a single or multiple chemotherapeutic agents emulsified with ethiodized oil, with or without additional embolization by particles such as gelatin sponge, polyvinyl alcohol, or calibrated microspheres 10 ; (2) drug-eluting embolic TACE (DEE-TACE) is the administration of calibrated microspheres onto which chemotherapeutic medication is loaded or adsorbed with the intention of sustained in vivo drug release. 10 Both forms of TACE result in tumor cellular necrosis via ischemic devascularization as well as chemotherapy-mediated cytotoxicity. 90 Y RE is defined as the transarterial infusion of radioactive microspheres containing 90 Y. 11 Tumor-embedded microspheres emit ionizing radiation that results in deoxyribonucleic acid (DNA) strand breaks and free radicals that undergo fixation upon reaction with oxygen in an aerobic environment, prompting cell death via apoptosis or mitotic catastrophe. Thus, 90 Y RE is more effective in an oxygenated environment, requiring preserved arterial flow via a minimally embolic infusion in the treated artery. This is in contrast to TAE and TACE, which rely on ischemia via a significant reduction in arterial flow secondary to embolization to achieve the desired tumoricidal effect. The technical details of each of these procedures are beyond the scope of this article, but are well described in the literature. 12
Procedure Indications and Preprocedure Considerations
Transarterial LRTs are indicated for inoperable and/or clinically symptomatic liver-dominant metastatic NETs. 13 14 Limited extrahepatic disease does not represent a procedure contraindication, particularly because LRTs can reduce or eliminate symptoms in cases of symptomatic functional tumors. 15 16 In the absence of clinical symptoms, transarterial LRTs may also be applied in cases with bulky metastatic liver disease. 13 In assessing patient eligibility for transarterial LRT, Eastern Cooperative Oncology Group (ECOG) score performance status should be determined, 17 with therapy reserved for those patients with maintained functional status (ECOG ≤ 2). Preprocedure laboratory evaluation should include a standard complete blood count, prothrombin time, and liver and kidney function; marked (e.g., bilirubin > 3.0 mg/dL) or uncorrectable abnormality of these parameters may preclude LRT. Measurement of relevant tumor markers such as serum chromogranin A and urine 5-hydroxyindoleacetic acid (5-HIAA) should be undertaken, and may be used as surrogate markers for postprocedure LRT response. As with any LRT, imaging studies should be obtained within 30 days of transcatheter therapy for baseline characterization of tumor size, number, burden, and location, as well as presence of extrahepatic disease. Special attention should be made to the history of bilioenteric anastomosis, as many patients with NET have undergone prior Whipple procedure, which markedly increases the risk of hepatic abscess, ranging from 33 to 86% even with aggressive antibiotic prophylaxis. 18 19 Periprocedural octreotide should be considered regardless of prior carcinoid symptoms, to control hormonal symptoms and/or hemodynamic status and avert carcinoid crisis, a syndrome characterized by profound blood pressure alterations and bronchospasm.
Case-Based Locoregional Therapy Selection
The following cases illustrate clinical application of various transarterial LRTs, with particular attention to the thought process and decision making underlying therapy selection. As both the experience and preference of individual IR practitioners as well as institutional inclinations impact therapy selection, definitive prescription of a particular transarterial LRT is unlikely, and treatments may be interchangeable, even among the particular example cases presented. This fact is supported by similar LRT clinical outcomes reported in the literature, 20 21 with survival more substantially influenced by NET characteristics, such as tumor grade, stage, and burden than by modality of LRT. 1 20
Case 1: TAE for Metastatic NET
A 49-year-old man presented to a community hospital with a chief complaint of hot flashes. Diagnostic workup included enteroscopy, which revealed a 9-cm small bowel mass. Pathologic evaluation from surgical resection confirmed carcinoid tumor, and five of ten regional lymph nodes exhibited metastatic disease. Liver masses were also evident at the time of surgery, and intraoperative biopsy corroborated hepatic metastatic disease. Medical oncology was consulted postoperatively, and the patient was started on monthly long-acting octreotide for symptomatic control.
The patient remained clinically asymptomatic for a year while on octreotide; however, his chromogranin A increased to more than 1,000 ng/mL from a baseline of 86 ng/mL. Magnetic resonance imaging (MRI) showed evidence of worsening multifocal metastatic NET involving the liver ( Fig. 1a ). The patient was started on everolimus 10 mg/day, and his chromogranin A level decreased to 226 ng/mL.
Fig. 1.

Metastatic neuroendocrine tumor treated with transarterial embolization (TAE). Coronal T2-weighted noncontrast MR image ( a ) demonstrates multinodular liver metastases (arrowheads) in right hepatic lobe. Digital subtraction right hepatic arteriogram ( b ) shows hypervascular right hepatic lobe liver metastases (arrowheads), which were embolized with 300–500 µm particles. Postembolization arteriogram ( c ) demonstrates no further tumor enhancement. Posttreatment coronal T2-weighted noncontrast MR image ( d ) performed 9 months after TAE reveals near-complete regression of right hepatic lobe liver metastases (arrowheads).
The patient remained symptom free for 2 years on octreotide and everolimus with stable hepatic tumor burden on cross-sectional imaging. However, his chromogranin A levels began to rise, and the patient was referred to IR to discuss LRT options for his liver-dominant metastatic NET.
At the time of consultation, the patient had maintained functional status (ECOG 0), normal liver function, and a chromogranin A level of 388 ng/mL. Various LRT options were discussed with the patient, and sequential bilobar TAE was planned. Visceral angiography was performed under IV moderate sedation, which demonstrated standard hepatic arterial anatomy and multifocal hypervascular masses in both hepatic lobes ( Fig. 1b ). The right hepatic artery was then embolized using 300 to 500 µm tris-acryl gelatin microspheres (Embosphere; Merit Medical, South Jordan, UT; Fig. 1c ). One month later, the left hepatic artery was embolized using the same technique. The patient reported mild transient abdominal pain that lasted approximately 1 week after each embolization.
The patient returned to IR for clinical and imaging follow-up 1 month after the left hepatic lobe embolization, and then at 3-month intervals. Sequential MRI studies revealed progressive size reduction of the treated tumors ( Fig. 1d ) associated with concurrent normalization of chromogranin A level. At present, the patient continues to do well 1.5 years after initial treatment, with continued objective radiologic response in the treated tumors.
Rationale for TAE and Clinical Outcomes
While there is no level 1 evidence for use of TAE for treatment of liver metastatic NET, this treatment is recognized by the National Comprehensive Cancer Network (NCCN) as a category 2B (uniform NCCN consensus that the intervention is appropriate based on lower level evidence) therapy for distant metastases spanning low tumor burden and asymptomatic to significant tumor burden and symptomatic. 13 Clinical outcomes of TAE for treatment of metastatic NET support the benefit of this procedure for this condition. Median survival times range from 3 to 6 years across studies, and hormonal symptoms resolve in up to 90% of cases. 16 22 23 24 25 26 27 Potential practical benefits of TAE compared with other transarterial LRTs include relatively reduced compound preparatory time (no need for pharmacy preparation of therapeutic agents and no need for mapping or planning arteriography), technical ease, and more forgiving impact of nontarget microsphere embolization. The lobar nature of this therapy also affords the need for only limited arteriography, thus making the TAE approach potentially useful in patients with renal insufficiency. This was the basis for selection of TAE in the described case. Downsides of TAE include occurrence of postembolization syndrome, as well as anecdotal basis for choice of embolic agent and particle size, which results in methodological variation that limits systematic comparability across published data.
Case 2: TACE for Metastatic NET
A 59-year-old woman presented for medical attention after experiencing fatigue and diarrhea for 5 months. Physical and laboratory examination was unremarkable. Initial workup with colonoscopy and esophagogastroduodenoscopy was normal. A computed tomographic (CT) scan revealed a subtle pancreatic head mass and synchronous liver masses, including an 8.0-cm enhancing segment 4 tumor and a 2.8-cm segment 8 tumor. Percutaneous ultrasound-guided biopsy of the liver masses diagnosed metastatic NET, which was staged with an octreotide scan ( Fig. 2a ). Endoscopic ultrasound (EUS)-guided biopsy of the pancreatic mass confirmed primary pancreatic NET. Chromogranin A level was normal. Given the size and location of the liver metastases, the patient was ineligible for surgical resection, and she was referred to IR for LRT.
Fig. 2.
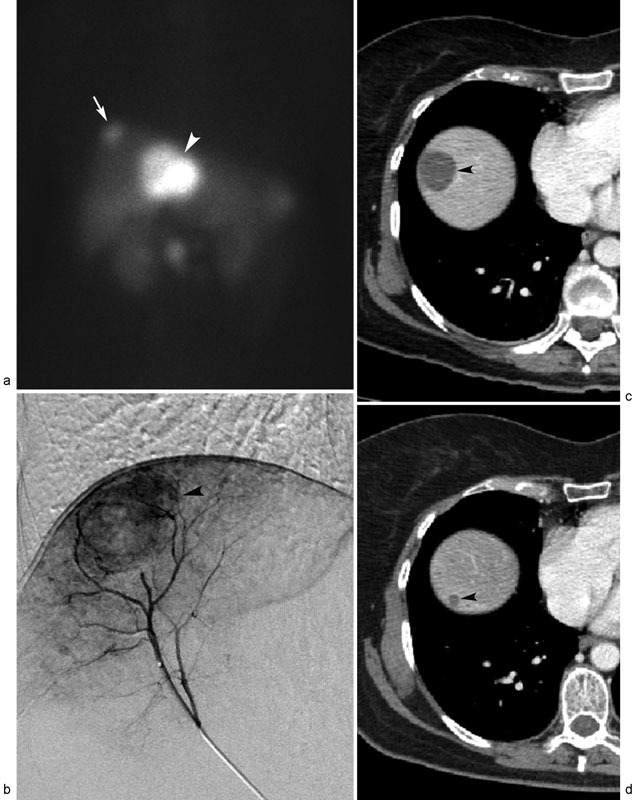
Metastatic neuroendocrine tumor treated with transarterial chemoembolization (TACE). Octreotide scan ( a ) displays oligonodular liver metastases spanning segment 4 (arrowhead) and segment 8 (arrow) tumors. ( b ) Digital subtraction arteriogram performed from segment 8 hepatic artery reveals hypervascular dome tumor (arrowhead), which was treated with c-TACE. Successive posttreatment contrast-enhanced CT scans performed 1 week ( c ) and 1 year ( d ) after TACE demonstrate gradual, near-complete regression of necrotic right hepatic lobe liver metastasis (arrowheads).
In clinic, the patient had normal functional status (ECOG 0) and preserved liver function. After discussion of the various LRT options with the patient, selective TACE to treat both the right hepatic lobe metastasis followed by the left hepatic lobe metastasis was planned.
Under IV moderate sedation, angiographic mapping demonstrated a replaced right hepatic artery, and confirmed a hypervascular tumor in segment 8 at the dome of the liver ( Fig. 2b ). The segment 8 hepatic artery was then embolized using a combination of three chemotherapy agents (cisplatin 100 mg, doxorubicin 50 mg, and mitomycin C 10 mg) emulsified with ethiodized oil (Lipiodol; Guerbet, Villepinte, France) in a 1:1 fashion. Following embolization, there was excellent uptake of chemotherapy emulsion in the tumor, and no further tumor angiographic enhancement. The patient tolerated the procedure well, and underwent treatment of the segment 4 tumor 1 month later.
After the procedures, the patient underwent serial CT scans at 3-month intervals for response assessment and tumor surveillance. Significant progressive size reduction in the treated tumors was evident ( Fig. 2c ). At her last clinical follow-up visit 8 years after the index TACE procedure, the patient remains alive and without diarrhea, but has developed regional lymph node metastases.
Rationale for TACE and Clinical Outcomes
Similar to TAE, there is no level 1 evidence for use of TACE in the treatment of liver metastatic NET, although this treatment is also recognized by the NCCN as a category 2B therapy for distant metastases spanning low tumor burden and asymptomatic to significant tumor burden and symptomatic. 13 TACE affords excellent clinical outcomes in metastatic NET: median survival times range from 3 to 6 years across studies, which is comparable to TAE. 15 16 22 23 24 25 26 27 28 29 30 31 Although early published reports of DEE-TACE for metastatic NET suggest efficacy, 32 safety results indicate the possibility of relatively higher rates of biliary complications that warrant caution in use of these agents until more robust safety data are available. 33 34 35 36 Advantages of TACE include a long track record of use for hepatic malignancies, utility in segmental or superselective therapy (although both TAE and 90 Y RE can be performed from a segmental approach as well), and straightforward capability for intra-procedure dose fractionation by manually splitting the prescribed chemotherapy emulsion. In the present case, TACE was prescribed due to intent for segmental treatment of a small right hepatic lobe tumor and larger left hepatic lobe tumor localized to hepatic segment 4. Disadvantages of TACE include occurrence of postembolization syndrome, as well as anecdotal basis for choice and dosing of specific chemotherapy agents and technical variability across IR operators and centers in TACE methodology, which limits reproducibility and comparability across other published data in a systematic fashion.
Case 3: 90 Y RE for Metastatic NET
A 61-year-old woman with metastatic large cell NET was referred to IR to discuss treatment options for enlarging hepatic masses. She had undergone prior resection of locally advanced disease including distal pancreatectomy, splenectomy, transverse colectomy, and partial gastrectomy, as well as resection of the left lateral lobe of the liver for a single hepatic metastasis. Notably, no biliary intervention or reconstruction was necessary. On review of her surgical pathology, she was considered an R0 resection, and the tumor demonstrated a K i -67 proliferation index of 10%. She was seen postoperatively by a medical oncologist, who offered adjuvant chemotherapy with cisplatin and etoposide; however, the patient deferred. Initial postresection follow-up imaging demonstrated new oligometastatic disease, and she was started on monthly long-acting octreotide. Repeat CT evaluation 3 months after the initiation of octreotide demonstrated progression of hepatic metastases, and she was referred to IR for consultation.
In IR clinic, she was healthy appearing with no diminishment in her level of activity (ECOG 0). She described two to three episodes of loose stools per week, but otherwise no episodes of flushing, hypertension, or shortness of breath attributable to carcinoid syndrome. Review of her imaging demonstrated multifocal bilobar hypervascular hepatic masses consistent with metastatic NET ( Fig. 3a ), a patent portal vein, and no biliary dilation. Liver function tests were within normal limits. Treatment options including TAE, TACE, and 90 Y RE were considered and discussed with the patient. 90 Y RE was selected, in part by patient preference to minimize postembolization syndrome.
Fig. 3.
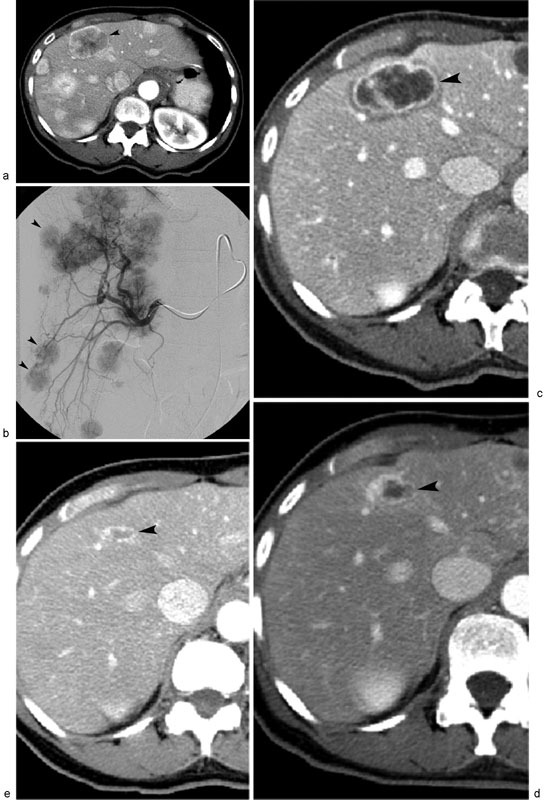
Metastatic neuroendocrine tumor treated with 90 Y RE. Contrast-enhanced CT scan ( a ) shows multifocal liver metastases, with index tumor (arrowhead) located in liver segment 4. Digital subtraction right hepatic arteriography ( b ) displays multifocal hypervascular right hepatic lobe liver metastases (arrowheads), which were treated with 90 Y-labeled resin microspheres. Sequential posttreatment contrast-enhanced CT scans performed 3 months ( c ), 6 months ( d ), and 1 year ( e ) after 90 Y RE demonstrate progressive, near-complete regression of necrotic segment 4 liver metastasis (arrowheads).
Planning mesenteric angiogram with technetium-99m macroaggregated albumin ( 99m Tc-MAA) was performed which confirmed multifocal bilobar hypervascular masses and conventional arterial anatomy ( Fig. 3b ). The gastroduodenal and right hepatic arteries were embolized with metallic coils (MicroNester; Cook Medical, Bloomington, IN) to minimize risk of non-target embolization, after which 5.3 mCi of 99m Tc-MAA was administered from the right hepatic artery and nuclear medicine scan lung shunt fraction was calculated at 13%. She subsequently underwent sequential bilobar treatment with 90 Y resin microspheres (SIR-Spheres; Sirtex Medical, Lane Cove, Australia) with 22.2 mCi (0.82 GBq) administered in the right hepatic artery followed 4 weeks later by 25.2 mCi (0.93 GBq) in the left hepatic artery. The patient tolerated each procedure well, and was discharged on lansoprazole, levofloxacin, and a methylprednisolone dose pack per institutional protocol.
Two months following her left lobe treatment, the patient returned to clinical and imaging evaluation. Her weight had stabilized, and she no longer had any episodes of diarrhea. Cross-sectional imaging demonstrated decreased size and enhancement of the liver metastases ( Fig. 3c ). She had progressive decrease in size of her liver metastases at 4 ( Fig. 3d ) and 9 months ( Fig. 3e ) posttreatment, and no return of her diarrhea. The patient was lost to further follow-up when she moved.
Rationale for 90 Y RE and Clinical Outcomes
The application of 90 Y RE for treatment of metastatic liver NETs is IR operator and institution specific. Again, similar to TAE and TACE, there is no level 1 evidence for use of 90 Y RE in the treatment of liver metastatic NET, although this treatment was recently recognized by the NCCN as a category 2B therapy for distant metastases spanning low tumor burden and asymptomatic to significant tumor burden and symptomatic. 13 90 Y RE for NET liver metastases has consistently demonstrated high tumor response rates associated with excellent survival outcomes. Studies including approximately 30 to 150 patients have shown objective tumor radiologic response rates ranging from 50 to 60% and median survival times spanning 2 to 6.5 years. 37 38 39 40 41 Purported benefits of 90 Y RE compared with other TAE and TACE include decreased symptoms of postembolization syndrome and excellent tolerance, low levels of toxicity, outpatient nature with same-day discharge, and relative objectivity in therapy prescription and dosimetry that affords more reliable systematic comparison across published data. Lobar therapy with 90 Y RE also has innate utility for multifocal hepatic metastases, which formed the basis for employment in the described case. Downsides of 90 Y RE include its relative technical challenge—requiring operator familiarity and expertise—as well as need for a second interventional procedure consisting of a mapping or planning arteriogram.
Conclusion
Despite the absence of level 1 data to determine the optimal LRT regimen, current data suggest that each transarterial treatment options described herein are effective options for the control of NET liver metastasis and palliation of related symptoms. While the paucity of data to suggest superiority of any single catheter-based treatment can make the ultimate therapeutic decision difficult, it does allow for flexibility in the management of this heterogeneous patient population, enabling the treating IR to tailor a treatment plan that accounts for the differences in patient presentation, anatomy, and local expertise, including use of different LRT therapies within the same patient. A thorough knowledge of the relative advantages and disadvantages of each LRT modality—including the unique risks of hepatic abscess and carcinoid crisis—thus allows the IR to play a key role in the multidisciplinary management of patients with NET liver metastases to optimize patient outcomes.
References
- 1.Yao J C, Hassan M, Phan A et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Modlin I M, Lye K D, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(04):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain R S, Canes D, Brown K T et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(04):432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 4.Touzios J G, Kiely J M, Pitt S Cet al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 200524105776–783., discussion 783–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proye C. Natural history of liver metastasis of gastroenteropancreatic neuroendocrine tumors: place for chemoembolization. World J Surg. 2001;25(06):685–688. doi: 10.1007/s00268-001-0013-8. [DOI] [PubMed] [Google Scholar]
- 6.Coppa J, Pulvirenti A, Schiavo Met al. Resection versus transplantation for liver metastases from neuroendocrine tumors Transplant Proc 200133(1-2):1537–1539. [DOI] [PubMed] [Google Scholar]
- 7.Delaunoit T, Van den Eynde M, Borbath I et al. Role of chemotherapy in gastro-entero-pancreatic neuroendocrine tumors: the end of a story? Acta Gastroenterol Belg. 2009;72(01):49–53. [PubMed] [Google Scholar]
- 8.Raymond E, Dahan L, Raoul J L et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(06):501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 9.Yao J C, Shah M H, Ito T et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(06):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaba R C, Lewandowski R J, Hickey R et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2016;27(04):457–473. doi: 10.1016/j.jvir.2015.12.752. [DOI] [PubMed] [Google Scholar]
- 11.Brown D B, Nikolic B, Covey A M et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23(03):287–294. doi: 10.1016/j.jvir.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 12.Habib A, Desai K, Hickey R, Thornburg B, Lewandowski R, Salem R. Transarterial approaches to primary and secondary hepatic malignancies. Nat Rev Clin Oncol. 2015;12(08):481–489. doi: 10.1038/nrclinonc.2015.78. [DOI] [PubMed] [Google Scholar]
- 13.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Neuroendocrine Tumors, Version 2.2016; Available at:https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. Accessed November 13, 2016
- 14.Kennedy A, Bester L, Salem R, Sharma R A, Parks R W, Ruszniewski P; NET-Liver-Metastases Consensus Conference.Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference HPB (Oxford) 2015170129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho A S, Picus J, Darcy M D et al. Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. AJR Am J Roentgenol. 2007;188(05):1201–1207. doi: 10.2214/AJR.06.0933. [DOI] [PubMed] [Google Scholar]
- 16.Ruutiainen A T, Soulen M C, Tuite C M et al. Chemoembolization and bland embolization of neuroendocrine tumor metastases to the liver. J Vasc Interv Radiol. 2007;18(07):847–855. doi: 10.1016/j.jvir.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Oken M M, Creech R H, Tormey D C et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(06):649–655. [PubMed] [Google Scholar]
- 18.Kim W, Clark T W, Baum R A, Soulen M C. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12(08):965–968. doi: 10.1016/s1051-0443(07)61577-2. [DOI] [PubMed] [Google Scholar]
- 19.Mezhir J J, Fong Y, Fleischer D et al. Pyogenic abscess after hepatic artery embolization: a rare but potentially lethal complication. J Vasc Interv Radiol. 2011;22(02):177–182. doi: 10.1016/j.jvir.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J X, Rose S, White S B et al. Embolotherapy for neuroendocrine tumor liver metastases: prognostic factors for hepatic progression-free survival and overall survival. Cardiovasc Intervent Radiol. 2017;40(01):69–80. doi: 10.1007/s00270-016-1478-z. [DOI] [PubMed] [Google Scholar]
- 21.Yang T X, Chua T C, Morris D L. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases - a systematic review. Surg Oncol. 2012;21(04):299–308. doi: 10.1016/j.suronc.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Pitt S C, Knuth J, Keily J M et al. Hepatic neuroendocrine metastases: chemo- or bland embolization? J Gastrointest Surg. 2008;12(11):1951–1960. doi: 10.1007/s11605-008-0640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maire F, Lombard-Bohas C, O'Toole D et al. Hepatic arterial embolization versus chemoembolization in the treatment of liver metastases from well-differentiated midgut endocrine tumors: a prospective randomized study. Neuroendocrinology. 2012;96(04):294–300. doi: 10.1159/000336941. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Yao J C, Ahrar K et al. Hepatic artery embolization and chemoembolization for treatment of patients with metastatic carcinoid tumors: the M.D. Anderson experience. Cancer J. 2003;9(04):261–267. doi: 10.1097/00130404-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Gupta S, Johnson M M, Murthy R et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(08):1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson B K, Larsson E G, Skogseid B M, Löfberg A M, Lörelius L E, Oberg K E. Liver embolizations of patients with malignant neuroendocrine gastrointestinal tumors. Cancer. 1998;83(11):2293–2301. [PubMed] [Google Scholar]
- 27.Loewe C, Schindl M, Cejna M, Niederle B, Lammer J, Thurnher S. Permanent transarterial embolization of neuroendocrine metastases of the liver using cyanoacrylate and lipiodol: assessment of mid- and long-term results. AJR Am J Roentgenol. 2003;180(05):1379–1384. doi: 10.2214/ajr.180.5.1801379. [DOI] [PubMed] [Google Scholar]
- 28.Vogl T J, Gruber T, Naguib N N, Hammerstingl R, Nour-Eldin N E. Liver metastases of neuroendocrine tumors: treatment with hepatic transarterial chemotherapy using two therapeutic protocols. AJR Am J Roentgenol. 2009;193(04):941–947. doi: 10.2214/AJR.08.1879. [DOI] [PubMed] [Google Scholar]
- 29.Ruszniewski P, Rougier P, Roche A et al. Hepatic arterial chemoembolization in patients with liver metastases of endocrine tumors. A prospective phase II study in 24 patients. Cancer. 1993;71(08):2624–2630. doi: 10.1002/1097-0142(19930415)71:8<2624::aid-cncr2820710830>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 30.Perry L J, Stuart K, Stokes K R, Clouse M E.Hepatic arterial chemoembolization for metastatic neuroendocrine tumors Surgery 1994116061111–1116., discussion 1116–1117 [PubMed] [Google Scholar]
- 31.Dong X D, Carr B I. Hepatic artery chemoembolization for the treatment of liver metastases from neuroendocrine tumors: a long-term follow-up in 123 patients. Med Oncol. 2011;28 01:S286–S290. doi: 10.1007/s12032-010-9750-6. [DOI] [PubMed] [Google Scholar]
- 32.Makary M S, Kapke J, Yildiz V, Pan X, Dowell J D. Conventional versus drug-eluting bead transarterial chemoembolization for neuroendocrine tumor liver metastases. J Vasc Interv Radiol. 2016;27(09):1298–1304. doi: 10.1016/j.jvir.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 33.de Baere T, Deschamps F, Teriitheau C et al. Transarterial chemoembolization of liver metastases from well differentiated gastroenteropancreatic endocrine tumors with doxorubicin-eluting beads: preliminary results. J Vasc Interv Radiol. 2008;19(06):855–861. doi: 10.1016/j.jvir.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Gaur S K, Friese J L, Sadow C A et al. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34(03):566–572. doi: 10.1007/s00270-011-0122-1. [DOI] [PubMed] [Google Scholar]
- 35.Bhagat N, Reyes D K, Lin M et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol. 2013;36(02):449–459. doi: 10.1007/s00270-012-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guiu B, Deschamps F, Aho S et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012;56(03):609–617. doi: 10.1016/j.jhep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 37.King J, Quinn R, Glenn D M et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer. 2008;113(05):921–929. doi: 10.1002/cncr.23685. [DOI] [PubMed] [Google Scholar]
- 38.Rhee T K, Lewandowski R J, Liu D M et al. 90Y Radioembolization for metastatic neuroendocrine liver tumors: preliminary results from a multi-institutional experience. Ann Surg. 2008;247(06):1029–1035. doi: 10.1097/SLA.0b013e3181728a45. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy A S, Dezarn W A, McNeillie P et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31(03):271–279. doi: 10.1097/COC.0b013e31815e4557. [DOI] [PubMed] [Google Scholar]
- 40.Saxena A, Chua T C, Bester L, Kokandi A, Morris D L. Factors predicting response and survival after yttrium-90 radioembolization of unresectable neuroendocrine tumor liver metastases: a critical appraisal of 48 cases. Ann Surg. 2010;251(05):910–916. doi: 10.1097/SLA.0b013e3181d3d24a. [DOI] [PubMed] [Google Scholar]
- 41.Memon K, Lewandowski R J, Mulcahy M F et al. Radioembolization for neuroendocrine liver metastases: safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83(03):887–894. doi: 10.1016/j.ijrobp.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]


