It has been known for several years that there are 20% more males than females in institutions for the mentally retarded. The first report of sex-linked or X-linked inheritance of severe mental retardation in males was reported by Martin and Bell in 1943.1 Since that time, approximately 100 X-linked mental retardation syndromes have been recognized, or about 30% of all known X-linked conditions that affect males.2 Examples of X-linked mental retardation conditions include Allan-Herndon-Dudley, Renpenning, Wieacker-Wolff, Atkin-Flaitz, Waisman-Laxova, and fragile X syndromes. It has been estimated that X-linked genes may account for about 25% of mental retardation in males and 10% of learning problems in females.3 X-linked mental retardation accounts for about one ninth of all mental retardation between 14 and 64 years of age. Although several conditions contribute to X-linked mental retardation, the fragile X syndrome accounts for 30% to 50% of families with males affected with X-linked mental retardation (Figure 1).4 Therefore, the fragile X syndrome is a significant cause of mental deficiency in our society, particularly X-linked mental retardation.
Figure 1.
An example of a fragile X syndrome pedigree showing affected males (shaded), percentage fragile X chromosome expression, and carrier females (represented by a dot in the open circles).
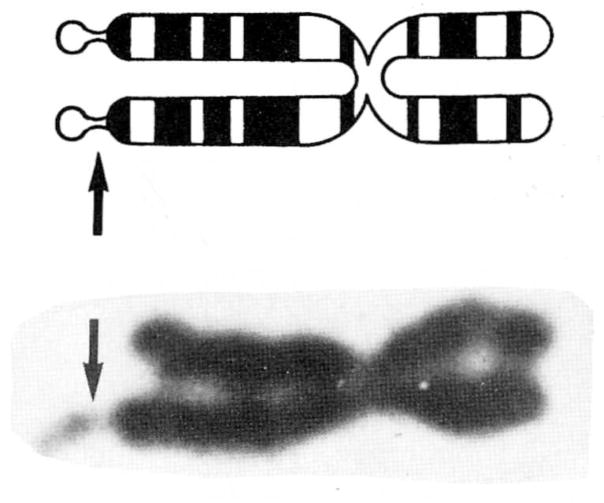
The fragile X syndrome is the second most common chromosome abnormality, after trisomy 21 or Down’s syndrome, among the mentally retarded. While trisomy 21 is usually sporadic, the fragile X syndrome is transmissible and is the most common genetic cause of mental retardation. The first report of a “marker X” chromosome containing a constriction near the terminus of the long arm of the X chromosome was in 1969 in a kindred with four mentally retarded males over three generations.5 With the use of folate-deficient cell culture media, the marker X chromosome was rediscovered in 1976 and subsequently identified with a fragile site at band q27 of the long arm of the X chromosome (Figure 2). Eighteen folate-sensitive fragile sites are now recognized on the human chromosomes, but the Xq27 site is the only one associated with a clinical syndrome.6 The original kindred of X-linked mental retardation reported by Martin and Bell 1 was also found to have the Xq27 site. Although one can rarely observe over 50% expression of the fragile X chromosome, the cytogenetic finding in at least 4% of male and 2% of female cells is considered diagnostic. Therefore, the Xq27 fragile site is a cytogenetic marker for the gene responsible for features recognized in the fragile X syndrome.
Figure 2.
Schematic drawing and photograph of a fragile X chromosome. The arrows indicate the location of the fragile site.
The first physical feature to be associated with the fragile X syndrome was macroorchidism. Testicular enlargement in fragile X syndrome individuals (87% of postpubertal males and 39% of prepubertal males) may result in a volume two to four times that of a normal adult (upper limit of normal is 25 mL), with an average adult testicular volume of 70 mL. Testicular size is measured by the use of an orchidometer (series of ellipsoid beads of various sizes used for determination of testicular volume) or calculated by the formula π/6 × (length) (width)2.
Other clinical features associated with the fragile X syndrome include large or prominent ears (80% of patients), relatively large head circumference, prominent forehead and supraorbital ridges, broad nose, long, narrow face (25% of adult patients), prominent chin, joint hyperflexibility (80% of prepubertal males), plantar dermatoglyphic crease between first and second toes (90% of patients), seizures (20% of patients), perseverative speech, and mental retardation (80% of males and 30% of females).7 The combination of mental retardation with significant speech delay, large prominent ears, and macroorchidism are characteristic of most fragile X syndrome patients (Figure 3).
Figure 3.
Frontal and profile views of a 28-year-old male with the fragile X syndrome, showing large prominent ears, prominent forehead and supraorbital ridges, and long, narrow face.
Mental retardation is the major or primary feature of individuals with fragile X syndrome. About 75% of fragile X syndrome patients are mildly to moderately retarded, with IQ scores between 50 and 75.7 Only 25% of fragile X syndrome patients have IQ scores lower than 50. Three percent to six percent of institutionalized males with mental retardation have the fragile X syndrome, thus the majority of patients with this condition are not institutionalized.
The behavior patterns of fragile X syndrome patients range from hyperactivity to severe autism.8 Three to ten percent of autistic individuals have the fragile X syndrome. Some patients with the fragile X syndrome are pleasantly social but avoid eye contact. Additional behavior traits observed in many patients include avoidance of physical contact; echolalia; decreased or absent nonverbal communication; catastrophic reactions to minor changes; rituals; hand posturing; mood changes; self-mutilations, especially of the hands; rocking; and hair pulling. These patients have a fascination with lyrics, schedules, and calendars, and may become upset when routines are changed. They have major deficits in math and may also have significant speech abnormalities, but they may perform better academically in reading, spelling, music, and art than their IQ scores would indicate. They have good imitative skills and will mimic sounds and behavior of other individuals. Therefore, behavior problems often improve in normal classroom settings with well-behaved children and worsen in classes with emotionally disturbed children. School activities should include a balance between structured programs in familiar surroundings and challenging programs that are not overwhelming for the child. The management of fragile X syndrome individuals will require a team approach, with the support of parents and educators, occupational, speech, and physical therapists, behavior and mental health specialists, geneticists, and physicians.
Population surveys indicate that one in every 1000 live-born males may be affected with the fragile X syndrome.4 More than one in every 2500 females are also affected, while the frequency of unaffected female carriers has been estimated at one in every 500. This syndrome has been seen in most ethnic groups including whites, blacks, Indians, Hispanics, Orientals, and Australian aborigines.7
The fragile X syndrome is an unusual type of X-linked inherited mental retardation condition. The classical X-linked pattern of inheritance involves an abnormal gene on the X chromosome. Females have two X chromosomes, while males have only one X chromosome and one Y chromosome. Therefore, females with one abnormal X chromosome have another that is normal. In most X-linked mental retardation syndromes, the normal X will mask the effects of the abnormal X so that females will appear normal but are carriers of the defective X chromosome, which can be passed on to their children. However, in the fragile X syndrome, 30% of these female carriers are found to be clinically affected with mental retardation or learning problems. This may be due to a mechanism known as lyonization, which leads to random inactivation or turning off of one of the two X chromosomes in a given female cell early in embryogenesis. The inactive X chromosome remains inactive in all descendent cells. While random inactivation of the X chromosome occurs in females with two normal X chromosomes, a non-random pattern is observed in females with a structurally abnormal X chromosome. Most chromosome studies of affected fragile X syndrome females indicate that the fragile X chromosome remains active in the majority of cells. This phenomenon may explain why some females are affected by the syndrome. If a male has an abnormal X chromosome he has no normal counterpart to mask the effects and will usually be affected; however, about 20% of males with the fragile X chromosome may not be affected or may be so mildly affected that they are considered normal.
Genetic counseling for the fragile X syndrome is more complicated than for the usual X-linked genetic disorders such as hemophilia, Duchenne muscular dystrophy, or color blindness, and detailed counseling should be provided by geneticists. In general, if a mother has the fragile X chromosome, 28% of her children (40% of her sons and 16% of her daughters) will have mental retardation and the fragile X syndrome.6 About 10% of the fragile X syndrome families appear to have the fragile X chromosome transmitted through a non-penetrant or unaffected male. These males will transmit their fragile X chromosome to all their daughters, who become carriers, but to none of their sons, although their grandsons are at risk.
Because of the expression of the fragile X chromosome in folate-deficient culture media, Lejeune and others 9 in the early 1980s treated fragile X syndrome patients with folic acid and reported behavior improvement and increased cognitive skills. Additional reports using placebo and cross-over design studies with folic acid (10 to 1000 mg per day) have demonstrated behavior improvement with decreased hyperactivity and increased attention span, particularly in prepubertal males. The mechanism of action of folate with fragile X syndrome patients is not well understood, considering that no abnormalities in serum or red blood cell folate levels or metabolism have been reported. Research with additional fragile X syndrome individuals, particularly at younger ages, is needed with folic acid and with central nervous system stimulants to determine their usefulness in the medical treatment of individuals with this syndrome.
Recent evidence indicates that certain antibiotics and seizure medications (e.g., phenytoin, sulfamethoxazole, and trimethoprim) will lower folic acid levels. Therefore, these medications are not advised in the treatment of fragile X syndrome patients.
The factor IX probe for the hemophilia B gene seemed to hold promise for uncovering a DNA marker linked to the gene responsible for the fragile X syndrome. However, more recent DNA studies indicate that the gene for the fragile X syndrome is neither as close nor as tightly linked to the factor IX gene as previously thought. Additional DNA probes (e.g., cX55.7, St14) are under investigation with fragile X syndrome families in the hope of determining the carrier status of an individual and, along with chromosome studies, improving the accuracy of prenatal diagnosis of fetuses at risk for this syndrome. At present, no specific DNA probe reliable in all families has been identified, but research continues.
Prenatal diagnosis of the fragile X syndrome is now available in a few centers around the country. The standard method involves amniocentesis at 15 to 17 weeks gestation to obtain fetal cells for chromosome and/or DNA studies. Prenatal diagnosis of well over 100 fetuses at risk for the fragile X syndrome has been accomplished, and at least 31 fetuses have had positive results.10 Prenatal diagnosis is 92% reliable for detecting a male fetus with the fragile X chromosome but is less accurate in female fetuses because they are less likely to demonstrate the fragile X chromosome.10 Because only 30% of females who carry the fragile X chromosome are mentally impaired, it is not possible to predict the degree of involvement in the female fetus if the fragile X chromosome study is positive.
Recently, fetal blood sampling and chorionic villus biopsy have been used for obtaining fetal cells for chromosome analysis and prenatal diagnosis of the fragile X syndrome. Since 1982, laboratories around the country have been organizing and testing several prenatal diagnostic methods and tissue culture procedures for enhancement of the fragile X chromosome expression in fetal cells to improve the speed and accuracy of prenatal diagnosis of fetuses at risk for the fragile X syndrome.
References
- 1.Martin JP, Bell J. A pedigree of mental defect showing sex linkage. J Neurol Psychiatry. 1943;6:154–157. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opitz JM. On the gates of hell and a most unusual gene. Am J Med Genet. 1986;23:1–10. doi: 10.1002/ajmg.1320230102. [DOI] [PubMed] [Google Scholar]
- 3.Turner G, Brookwell R, Daniel A, et al. Heterozygous expression of X-linked mental retardation and the X chromosome marker (fraX) (q27) N Engl J Med. 1980;303:662–664. doi: 10.1056/NEJM198009183031202. [DOI] [PubMed] [Google Scholar]
- 4.Herbst D, Miller J. Non specific X-linked mental retardation: II. The frequency in British Columbia. Am J Med Genet. 1980;7:461–469. doi: 10.1002/ajmg.1320070407. [DOI] [PubMed] [Google Scholar]
- 5.Lubs HA. A marker X chromosome. Am J Med Genet. 1969;21:231–244. [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland GR, Hecht F. Fragile Sites on Human Chromosomes. New York: Oxford University Press; 1985. [Google Scholar]
- 7.Hagerman RJ. Fragile X syndrome. Curr Probl Pediatr. 1987;17:623–674. doi: 10.1016/0045-9380(87)90011-9. [DOI] [PubMed] [Google Scholar]
- 8.Hagerman RJ, Smith ACM. The heterozygous female. In: Hagerman RJ, McBogg PM, editors. The Fragile X Syndrome: Diagnosis, Biochemistry and Intervention. Dillon, CO: Spectra Publishing Co, Inc; 1983. [Google Scholar]
- 9.Lejeune J, Rethore M-O, de Blois M-C, Ravel A. Assay of folic acid treatment in fragile-X syndrome. Ann Genet. 1984;27:230–232. [PubMed] [Google Scholar]
- 10.Jenkins EC, Brown TW. The prenatal diagnosis of the fragile X syndrome. In: Milunsky A, editor. Genetic Disorders of the Fetus. New York: Plenum Publishing Corp; 1986. [Google Scholar]