Abstract
Background
We previously demonstrated a gene-by-prenatal-environment interaction whereby the monoamine oxidase A gene (MAOA) modified the impact of prenatal tobacco exposure (PTE) on adolescent disruptive behavior (DB), with the MAOA risk genotype varying by sex. We extend this work by examining whether this mechanism is evident with another common adversity, prenatal stress exposure (PSE), and whether sex differences are present earlier in development in closer proximity to exposure.
Methods
Participants were 281 mothers and their 285 children derived from a prenatal cohort with in-depth prospective measures of PSE and PTE. We assessed DB at age 5 via dimensional developmentally-sensitive measurement. Analyses were stratified by sex based on prior evidence for sex differences.
Results
Concurrent stress exposure predicted DB in children (β=.310, p=.001), while main effects of prenatal exposures were seen only in boys. We found a three-way interaction of MAOAxPSExsex on DB (β=.813, p=.022). Boys with MAOA-H had more DB as a function of PSE, controlling for PTE (β=.774, p=.015), and as a function of PTE, controlling for PSE (β=.362, p=.037). Boys with MAOA-L did not show this susceptibility. MAOA did not interact with PSE (β=−.133, p=.561) nor PTE (β= −.144; p=.505) in predicting DB in girls. Examination of gene-environment correlation (rGE) showed a correlation between paternal MAOA-L and daughters’ concurrent stress exposure (r=−.240, p=.013).
Discussion
Findings underscore complex mechanisms linking genetic susceptibility and early adverse exposures. Replication in larger cohorts followed from the pregnancy through adolescence is suggested to elucidate mechanisms that appear to have varying developmental expression.
Keywords: monoamine oxidase A, pregnancy smoking, early adversity, disruptive behavior, gene x environment interaction, sex differences
1. Introduction
The monoamine oxidase A gene untranslated variable number of tandem repeats marker, referred to herein, as MAOA, influences the degradation of monoamines, thus may critically regulate risk for aggression and related phenotypes (Buckholtz & Meyer-Lindenberg, 2008, Sabol et al., 1998). In their seminal study nearly 15 years ago, Caspi and colleagues demonstrated how MAOA moderated the impact of childhood maltreatment on later aggressive antisocial behavior in adult males (Caspi et al., 2002). Since this time, at least 34 empirical papers and 3 reviews of the MAOA-adversity-antisocial behavior mechanism have followed (Buades-Rotger & Gallardo-Pujol, 2014, Byrd & Manuck, 2014, Goldman & Rosser, 2014, Kim-Cohen et al., 2006). Since the most recent meta-analysis published in 2014, an additional 8 papers have linked the MAOA x adversity interaction to a range of adult problem behaviors including criminal behavior (Lu & Menard, 2016), aggression (Hohmann et al., 2016, Rehan et al., 2015, Schlüter et al., 2016, Zhang et al., 2016), cigarette smoking (Huang et al., 2015), drug use (Harro & Oreland, 2016), and alcohol use (Cervera-Juanes et al., 2015). Yet very few studies to date have examined MAOA x adversity interactions in regards to the developmental expression of these patterns in young children (Enoch et al., 2010, Hill et al., 2013, Kim-Cohen et al., 2006).
Furthermore, despite accruing evidence of MAOA x adversity interactions, the direction of these patterns has been inconsistent. Results have been most robust regarding antisocial behavior in male offenders with the low-activity MAOA variant (MAOA-L) who were exposed to childhood maltreatment (Byrd & Manuck, 2014). However, a number of studies in offender and non-offender male samples have suggested instead, that the high activity variant (MAOA-H) confers greater antisocial risk (Gorodetsky et al., 2014, Lee, 2011, Prichard et al., 2008, Tikkanen et al., 2011, Tikkanen et al., 2010, Tikkanen et al., 2009, Van Der Vegt et al., 2009). Moreover, evidence from a growing number of studies that include female subjects suggests that MAOA interacts with environmental adversity in a sex-specific manner. To date there are 15 studies that have included females. Of these, 10 have suggested that females with the high-activity variant are at greater risk for antisocial behavior following exposure to childhood adversity or maltreatment (Åslund et al., 2011, Kim-Cohen et al., 2006, Kinnally et al., 2009, Mcgrath et al., 2012, Nikulina et al., 2012, Nilsson et al., 2011, Prom-Wormley et al., 2009, Sjöberg et al., 2007), while 5 studies suggest that the low-activity variant is associated with risk (Beach et al., 2010, Enoch et al., 2010, Hohmann et al., 2016, Kim-Cohen et al., 2006, Rehan et al., 2015). Thus, there is substantial evidence for sex differences in patterns, but the risk (or susceptibility) variant in each sex remains unclear.
Limitations of candidate gene-by-environment studies could contribute to observed discrepancies regarding MAOA. Behavioral phenotypes are associated with numerous genes, each of which accounts for a very small percentage of behavioral variability (Geschwind & Flint, 2015), while individual genes associated with specific behavioral phenotypes also affect multiple other traits (Plomin & Deary, 2015). In light of this concern, the field has largely shifted towards genome-wide approaches involving tens of thousands of individuals (Chabris et al., 2015, Dick et al., 2015, Gratten et al., 2014). Yet, GWAS approaches are not without limitations. Large epidemiologic samples offer significantly more power to detect small effect sizes, but are limited by the depth of measurement of environmental exposures. Poor measurement of environmental factors, then, could introduce error similar to measuring the wrong gene (Dick et al., 2015). In this way, candidate gene studies involving functional variants implicated in developmental pathways that utilize precise measures of environmental exposures can offer unique insights that much larger studies cannot. This may be especially true regarding environmental exposures that occur in utero, given the relative paucity of studies involving pregnant women (Wisner, 2012). While there is growing evidence to support the role of the intrauterine environment in shaping developmental trajectories (Babenko et al., 2015), how the prenatal environment may be modulated by MAOA has just begun to be explored (Hill et al., 2013, Hohmann et al., 2016, Wakschlag et al., 2010a).
Two environmental adversities commonly experienced concomitantly during the prenatal period are prenatal tobacco exposure (PTE) and prenatal stress exposure (PSE) (Flemming et al., 2013). PTE still affects some 1 in 10 births in the United States and has been linked to a wide range of adverse child outcomes including antisocial behaviors and their precursor phenotypes (U.S. Department of Health and Human Services, 2014). In a prior independent sample, we demonstrated moderation of vulnerability to PTE by MAOA in a sex-specific manner (Wakschlag et al., 2010a) with patterns similar to those previously observed for childhood maltreatment (Byrd & Manuck, 2014, Caspi et al., 2002). Specifically, adolescent boys with PTE and MAOA-L exhibited increased conduct disorder symptoms, compared to boys with MAOA-H. In adolescent girls, however, it was MAOA-H that interacted with PTE to predict conduct disorder symptoms, and also hostile attribution bias patterns on a face-processing task (Wakschlag et al., 2010a). The only other study to our knowledge that examined MAOA x PTE on antisocial behavior did not find sex-specific patterns (Hohmann et al., 2016), but assessed PTE by maternal report at 3 months postpartum, whereas we previously assessed PTE prospectively using a combination of interviews and biomarkers (Wakschlag et al., 2010a).
This discrepancy in results supports the notion that different ways of measuring environmental exposures could lead to different results (Dick et al., 2015). Indeed, as maternal cigarette smoking during pregnancy is an increasingly stigmatized behavior, under-reporting leading to misclassification of exposed versus non-exposed children is a well-established source of error (Estabrook et al., 2015, Pickett et al., 2005, Pickett et al., 2003). Moreover, as frequency, patterns, and topography of cigarette smoking are known to fluctuate significantly across gestation, prospective measurement of PTE that includes biomarker confirmation of reports is needed to most accurately capture this environmental exposure (Dukic et al., 2007, Estabrook et al., 2015, Pickett et al., 2005). Yet, even with ideal measurement of PTE, disentangling this particular exposure from the concomitant exposures is critical (Chiarella et al., 2015). As rates of cigarette smoking in the general population decline, PTE is increasingly intertwined with psychosocial stress during pregnancy (Flemming et al., 2015) but studies of PTE, including our previous study on MAOA x PTE (Wakschlag et al., 2010a), lack adequate control of PSE. More recently, we have shown that jointly accounting for PSE and PTE significantly enhances the prediction of behavioral disinhibition (Clark et al., 2015). In particular, PSE and PTE independently predicted higher levels of early childhood disruptive behavior, with the effect of PSE mediated by early difficult temperament and executive control.
Finally, the biological impact of environmental adversity could vary as a function of developmental timing (Dick et al., 2015). Advances in developmentally based measurement has increasingly enabled fine-grained characterization of disruptive behavior in very young children, (Wakschlag et al., 2014) in whom conduct disorder symptoms are impossible (i.e. truancy in preschool-aged children) or improbable (i.e. stealing while confronting a victim) (Wakschlag et al., 2010b). To our knowledge, the MAOA x prenatal adversity interaction has rarely been examined in close proximity to exposure in the first years of life (Byrd & Manuck, 2014, Enoch et al., 2010, Hill et al., 2013). In the current study, we extend our prior work by examining commonly co-occurring forms of prenatal adversity and their interaction with MAOA, independent of one another, utilizing in-depth prospective measurement of each of these exposures. Specifically, we tested the moderating effect of MAOA on PSE and PTE in predicting disruptive behavior in five-year-old children, probing for previously observed sex-effects in these gene x environment interactions, controlling for other prenatal exposures, postnatal exposures and parenting. We hypothesized that MAOA genotype would interact independently with both PSE and PTE to contribute to preschool disruptive behavior, with sex differences in the risk variant.
One of the primary challenges of causal modeling of prenatal exposures is the potential for genetic confounding (D'onofrio et al., 2010, D'onofrio et al., 2012, D'onofrio et al., 2008, Estabrook et al., 2015). In the present case, associations among PSE, PTE and disruptive behavior could result from underlying genetic factors that simultaneously influence parental traits, and by association, parental behaviors that influence the prenatal intrauterine environment, postnatal environment, and child traits (Gaysina et al., 2013, Harold et al., 2013, Jaffee & Price, 2007). Thus, using available data on parental MAOA genotype, we provided a partial test for genotype-environment correlation (rGE).
2. Material and Methods
2.1 Sample
Participants were 281 mothers and their 285 children (4 sets of twins; 141 boys, 144 girls) from the Midwest Infant Development Study - Preschool Phase (MIDS-P). In the initial phase of MIDS, mothers were recruited in early pregnancy (nearly three-quarters of women enrolled prior to 16 weeks gestation) using flyers distributed over a 4.5-year period to all obstetric clinics in two Midwestern cities. Smoking was oversampled (56% smokers at the start of the study), and women reporting binge drinking (> 2 drinks in any one sitting) or any illicit drug use were excluded. Non-smokers were matched broadly to smokers by demographic factors known to be associated with cigarette smoking (educational attainment, race, ethnicity, and Medicaid status). The sample was predominantly low-income women (56.8% non-Hispanic Caucasian; 43.2% other races and ethnicities) with a mean age of 25.7 years and a mean educational attainment of 13.1 years. Sixty percent of participants were unmarried, and 53% reported another smoker in the home during the pregnancy (Espy et al., 2011). In MIDS-P, children were assessed for disruptive behavior around age 5. (Descriptive statistics are shown in Table 2).
Table 2.
Sample characteristics for total sample (N = 285) and by sex
| Total | Boys n = 141 | Girls n = 144 | p | ||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Range | Mean (SD) | Mean (SD) | ||
| Predictors | |||||
| Prenatal stress exposure a | 50.1 (5.8) | 37.3–69.6 | 50.2 (6.1) | 50.0 (5.6) | .789 |
| Prenatal tobacco exposure b | 0.8 (2.2) | 0–16.7 | 0.7 (2.0) | 0.8 (2.1) | .730 |
| Outcome | |||||
| Disruptive behavior c | 61.0 (50.1) | 0 – 267 | 66.5 (53.3) | 55.7 (46.3) | .069 |
| Covariates | |||||
| Child age in years | 5.1 (0.3) | 4.6–6.0 | 5.1 (0.2) | 5.1 (0.2) | .382 |
| Percentage male | 49.5% | ||||
| Percentage MAOA-L | 54.7% | 44.0% | 65.3% | <.001 | |
| Prenatal alcohol exposure d | .03 (.05) | 0 –.33 | .04 (.06) | .03 (.05) | .298 |
| Parent antisocial behavior e | 5.2 (4.1) | 0–17 | 5.2 (4.3) | 5.1 (4.0) | .814 |
| Concurrent life stress f | 50.1 (6.2) | 37.3–68.4 | 50.0 (5.9) | 50.3 (6.4) | .456 |
| Concurrent tobacco exposure g | 4.2 (6.6) | 0–30.0 | 3.4 (5.9) | 4.8 (7.0) | .066 |
| Maternal responsiveness h | 3.61 (1.9) | 0–7.00 | 3.7 (1.7) | 3.6 (1.9) | .661 |
Life Stressors and Social Resources Scale, assessed at 28 weeks gestation
Cotinine-corrected mean cigarettes per day across pregnancy
Multidimensional Assessment Profile of Preschool Disruptive Behavior raw score
Average number of drinks per day reported across each trimester of pregnancy
Antisocial Behavior Questionnaire, sum of maternal and paternal scores
Life Stressors and Social Resources Scale, assessed at child age 5, raw score
Maternal smoking in cigarettes/day at child age 5
Early Childhood Home Observation for Measurement of the Environment, Responsivity subscale, raw score
2.2 Measures
2.2.1 Prenatal and concurrent stress exposure
In contrast to prior work examining maltreatment, in this study we examined intrauterine and preschool exposure to a range of normative psychosocial stressors. We assessed mothers using the Life Stressors and Social Resources Inventory (LISRES) (Moos et al., 1988) at 28 weeks of gestation (PSE), and again at the preschool follow-up when disruptive behavior was assessed, termed concurrent stress exposure (CSE). The LISRES is a 200-item structured interview that provides an integrated picture of an individual’s life context over the past 12 months. By assessing both life stressors (9 scales) and social resources (7 scales) available to manage these stressors, this unified framework recognizes the interdependence between the two (Moos & Moos, 1994).
The 9 Stressors Scales and sample questions are: physical health (Have you had asthma or allergies?); home/neighborhood (Is there enough heat in the winter? Has your home been burglarized?); financial (Do you have enough money to afford furniture or household equipment that needs to be replaced?); work (Did you find out that you were not going to get an expected promotion at work?); spouse/partner (Did your relationship change for the worse in the last year?); child (How often do any of your children get on your nerves?); extended family (When you spend time with your mother/stepmother, how often is she critical or disapproving of you?); friends & social activities (Have you had a serious conflict with a friend in the past year?); and negative life events (Did you lose your home through fire, flood, disaster, or major catastrophe?). The 7 Social Resources Scales and sample items are: financial (Has your financial situation improved?); work (Did you have a significant success at work?); spouse/partner (Did you start seeing someone exclusively?); children (Do you share mutual interests or activities with one or more of your children?); extended family (When you spend time with your mother can you count on her help when you need it?); friends (Do you confide in any of your friends?); and positive life events (Did you move to a better home?).
The LISRES scales have high internal reliability (α = .83 – .84) and test-retest reliability (r = .67 – .70). Raw scores on the 16 scales, which fell into the ‘average’ range, relative to normative samples (Moos & Moos, 1994), were converted into continuous factor scores using confirmatory factor analysis. These factor scores, representing PSE and CSE, were controlled in all regression models.
2.2.2 Prenatal and concurrent tobacco exposure
Smoking was assessed at each prenatal study visit (mean of 2.93 ± 0.70 visits; range = 1 − 4 visits) by self-report using timeline follow-back methodology (Sobell & Sobell, 1996), combined with repeated prospective blood and urine cotinine radioimmunoassays (Wang et al., 1997). Smoking patterns were established via a ‘best-estimate’ approach such that non-disclosure, under-reporting, and over-reporting were corrected based on serum cotinine values, employing statistical methods previously described (Dukic et al., 2007). Based on this calculation, 77.3% of women in this sample reported a lifetime smoking history and 69.4% of women smoked during pregnancy. Among pregnancy smokers, mean daily smoking after learning of the pregnancy was approximately one cigarette (M = 0.8; SD = 2.4; range = 0–16.7); 2.6% of women smoked an average of more than 10 cigarettes (half pack)/day. A continuous corrected mean serum cotinine measure of average cigarettes per day across pregnancy was used as the measure of PTE. Concurrent tobacco exposure (CTE) from mothers’ reported cigarettes/day smoked at the time of the preschool assessments was included as a covariate in all regression models.
2.2.3 Disruptive Behavior
Disruptive behavior was assessed with the Multidimensional Assessment Profile of Disruptive Behavior (MAP-DB), which utilizes a dimensional approach to differentiate normative misbehavior from facets of disruptive behavior (i.e., aggression, noncompliance, temper loss and low concern for others) within a developmental context (Wakschlag et al., 2014). Item Response Theory (IRT) modeling (Hambleton et al., 1991) was utilized to generate a continuous unidimensional total disruptive behavior score as the outcome measure (M = −.076; SD = 0.95; range = −2.60 2.510).
2.2.4 Covariates
Maternal parenting quality (responsiveness) was assessed by direct observation in the home at child age 5 using the responsivity subscale of the Early Childhood Home Observation for Measurement of the Environment (EC-HOME) (Totsika & Sylva, 2004). Additional covariates were child age, prenatal alcohol exposure, parent antisocial behavior (from mother and fathers’/partners’ reports) (Zoccolillo, 2000), concurrent stress exposure (CSE) (Moos & Moos, 1994), and concurrent tobacco exposure (CTE).
2.3 Genotyping
Participant saliva samples were collected with DNA Genotek Oragene Self-Collection Kits. DNA was extracted and quantified with Quanti-iT Pico Green dsDNA assay. Following Polymerase Chain Reaction, products were separated on a 3730 Genetic Analyzer (Wakschlag et al., 2010a). As MAOA is an X-linked gene, boys have one allele, and are classified as either MAOA-H or MAOA-L. With two alleles, girls are either homozygous or heterozygous. Previous investigators concur that variants with 4 repeats should be classified as MAOA-H and 3 repeats as MAOA-L. There is some discrepancy in the classification of the 5-repeat variant (Deckert et al., 1999, Sabol et al., 1998). Consistent with the approach of Sabol and Kim-Cohen (Kim-Cohen et al., 2006, Sabol et al., 1998), we classified variants with 5 repeats as MAOA-L. In girls, heterozygotes with 3.5/4 were classified as MAOA-H, along with 4/4 homozygotes. All other genotypes in girls were classified as low.
The distribution of MAOA genotypes for boys and girls by population are shown in Table 1. To test for Hardy-Weinberg Equilibrium, Likelihood Ratio tests were conducted with MAOA classified as multi-allelic with five possible alleles of 2, 3, 3.5, 4, and 5 repeats in unrelated females only. Allele frequencies met Hardy-Weinberg equilibrium (HWE) for each of the following populations: European American: χ2 = 0.427, df = 3, 2p = .934; Latino: χ2 = 2.085, df = 3, 2p = .555; African-American: χ2 = 5.183, df = 6, 2p = .521. HWE was not calculated for the remaining children due to small numbers (classified as “other” in Table 1). These populations were: Hispanic black (3 boys, 3 girls); Hispanic Native American (5 girls); Hispanic other (2 boys); non-Hispanic Asian (2 girls); and non-Hispanic other (1 boy, 3 girls). In the total sample of 285, there were 129 children (79 boys, 50 girls) with MAOA-H genotype and 156 children (62 boys, 94 girls) with MAOA-L genotype. For subsequent analyses, MAOA genotype was coded as 1 = low activity, 2 = high activity.
Table 1.
Distribution of MAOA genotypes for boys and girls by population (N = 285)
| Population | Boys | Girls | ||
|---|---|---|---|---|
|
| ||||
| MAOA Genotype | Freq (%) | MAOA Genotype | Freq (%) | |
| European American (Non-Hispanic whites) | 3/- | 36 (43.9%) | 3/3 | 12 (15.0%) |
| 0 | 3/3.5 | 1 (1.3%) | ||
| 0 | 3/4 | 36 (45.0%) | ||
| 0 | 3.5/4 | 1 (1.3%) | ||
| 4/- | 46 (56.1%) | 4/4 | 30 (37.5%) | |
|
| ||||
| MAOA-High | 46 (56.1%) | MAOA-High | 31 (39.7%) | |
| MAOA-Low | 36 (43.9%) | MAOA-Low | 49 (61.3%) | |
|
|
||||
| Total | 82 (100%) | Total | 80 (100%) | |
|
| ||||
| Latino (Hispanic whites) | 3/- | 1 (11.1%) | 3/3 | 4 (28.6%) |
| 0 | 3/4 | 5 (35.7%) | ||
| 4/- | 8 (88.9%) | 4/4 | 4 (28.6%) | |
| 0 | 4/5 | 1 (7.1%) | ||
|
| ||||
| MAOA-High | 8 (88.9%) | MAOA-High | 4 (28.6%) | |
| MAOA-Low | 1 (11.1%) | MAOA-Low | 10 (71.4%) | |
|
|
||||
| Total | 9 (100%) | Total | 14 (100%) | |
|
| ||||
| African American (Non-Hispanic blacks) | 2/- | 2 (4.7%) | 2/2 | 1 (2.9%) |
| 0 | 2/3 | 3 (8.6%) | ||
| 0 | 2/4 | 1 (2.9%) | ||
| 3/- | 17 (39.5%) | 3/3 | 8 (22.9%) | |
| 0 | 3/3.5 | 1 (2.9%) | ||
| 0 | 3/4 | 12 (34.3%) | ||
| 4/- | 23 (53.5%) | 4/4 | 9 (25.7%) | |
| 5/- | 1 (2.3%) | 5/5 | 0 | |
|
| ||||
| MAOA-High | 23 (53.5%) | MAOA-High | 9 (25.7%) | |
| MAOA-Low | 20 (46.5%) | MAOA-Low | 26 (74.3%) | |
|
|
||||
| Total | 43 (100%) | Total | 35 (100%) | |
|
| ||||
| Other (Includes Asian, Pacific Islander, Native American, and mixed race/ethnicity) | 3/- | 4 (57.1%) | 3/3 | 0 |
| 0 | 3/4 | 8 (53.3%) | ||
| 0 | 3/5 | 1 (6.7%) | ||
| 4/- | 3 (42.9%) | 4/4 | 5 (33.3%) | |
| 0 | 4/5 | 1 (6.7%) | ||
|
| ||||
| MAOA-High | 3 (42.9%) | MAOA-High | 5 (33.3%) | |
| MAOA-Low | 4 (57.1%) | MAOA-Low | 10 (66.7%) | |
|
|
||||
| Total | 7 (100%) | Total | 15 (100%) | |
|
| ||||
| Total all populations | 2/- | 2 (1.4%) | 2/2 | 1 (0.7%) |
| 0 | 2/3 | 3 (2.1%) | ||
| 0 | 2/4 | 1 (0.7%) | ||
| 3/- | 58 (41.1%) | 3/3 | 24 (16.7%) | |
| 0 | 3/3.5 | 2 (1.4%) | ||
| 0 | 3/4 | 61 (42.4%) | ||
| 0 | 3/5 | 1 (0.7%) | ||
| 0 | 3.5/4 | 1 (0.7%) | ||
| 4/- | 80 (56.7%) | 4/4 | 48 (33.3%) | |
| 0 | 4/5 | 2 (1.4%) | ||
| 5/- | 1 (0.7%) | 5/5 | 0 | |
|
| ||||
| MAOA-High | 80 (56.0%) | MAOA-High | 50 (34.7%) | |
| MAOA-Low | 61 (44.0%) | MAOA-Low | 94 (65.3%) | |
|
|
||||
| Total | 141 (100%) | Total | 144 (100%) | |
2.4 Inference of paternal genotype from maternal and female child genotype
Maternal, but not paternal genotypes were directly assessed in this cohort. Girls receive one MAOA allele from each parent. Mothers can transmit either of their two alleles, while fathers can only transmit their single allele. In this way, if mothers and daughters’ MAOA genotype is known, paternal genotype can be inferred in families in which daughters possess an allele that is not possessed by her mother. This allele, then, must have been transmitted from her father who is hemizygous. For example, in a daughter who is 3/4, if her mother is 4/4, her father must be 3/-. Paternal genotype can also be inferred in families in which daughters are homozygous. For example, if a daughter is 4/4 and her mother is 4/4, her father must be 4/-. If her mother is, instead, 3/4, her mother must have transmitted a 4 allele; the daughter’s other allele is also 4, which means her father must be 4/-. In families in which mothers and heterozygous daughters have the same MAOA genotype, paternal genotype cannot be inferred – here, one cannot discern which allele has been transmitted by the mother. Using this technique, we inferred paternal genotypes where possible (n = 107, or 74% of girls) for use in tests of gene-environment correlation.
2.5 Analysis
We evaluated variables for normality prior to use in regression models. PTE was left-skewed and thus log transformed after adding 1 to all values to obtain continuous values > 0. All interaction covariates were calculated by first mean-centering each covariate, then calculating the product terms.
2.5.1 Tests for G x E x sex
Linear regression analysis was used to test MAOA x PSE x sex on disruptive behavior, controlling for PTE and covariates, and MAOA x PTE x sex on disruptive behavior controlling for PSE and covariates. Based on previous literature showing differential effects of these interactions by sex, analyses were also conducted separately for boys and girls. Statistical significance of the interaction terms were tested using a Wald test.
2.5.2 Tests for rGE
To examine the possibility that findings regarding MAOA x PSE resulted from a relationship between parental genotype and environmental exposures, we used bivariate correlation analysis to examine relationships between parental genotypes and environmental exposures (PSE, PTE, CSE, CTE and maternal responsiveness).
3. Results
Descriptive characteristics for the total sample and for boys and girls separately are shown in Table 2. Due to hemizygosity in males, significantly more boys were classified as the MAOA-H genotype (56.0%) than girls (34.7%) (χ2 = 13.053, p < .001). Other variables did not significantly differ between boys and girls. Of the 94 girls (65.3%) classified as MAOA-L, 64 (68.1%) were heterozygotes with intermediate phenotypes (2/4, 3/4 or 4/5) (Table 1).
3.1 Main effects
CSE showed a main effect on disruptive behavior in the full sample (β = .310; p = .001) (Table 3). In models stratified by sex (Table 4), main effects were observed in boys with respect to PSE (β = −.676; p = .031), prenatal alcohol exposure (β = .185; p = .043), and CSE (β = .446; p < .001). A trend for PTE on was observed (β = .193; p = .057). In girls, we observed a main effect of MAOA (high) genotype on disruptive behavior (β = .215; p = .047).
Table 3.
Three-way interaction of MAOA genotype x prenatal stress exposure x sex on disruptive behavior in preschoolers a (N = 285)
| B | SE | β | 95% CI | t | Sig. | |
|---|---|---|---|---|---|---|
| Child age | −.296 | .239 | −.086 | −.768 − .176 | −1.237 | .218 |
| Sex b | .609 | .419 | .323 | −.220 − 1.437 | 1.451 | .149 |
| MAOA genotype c | .355 | .187 | .189 | −.013 − .724 | 1.902 | .059 |
| Prenatal stress exposure (PSE) d | .035 | .050 | .213 | −.065 − .135 | .694 | .489 |
| Prenatal tobacco exposure e | .041 | .032 | .094 | −.023 − .104 | 1.264 | .208 |
| Prenatal alcohol exposure f | 1.881 | 1.156 | .112 | −.402–4.163 | 1.627 | .106 |
| Parent antisocial behavior g | .005 | .018 | .024 | −.029 − .040 | .306 | .760 |
| Concurrent stress exposure h | .047 | .013 | .310 | .020 − .073 | 3.474 | .001 |
| Concurrent tobacco exposure i | .011 | .011 | .081 | −.010 − .033 | 1.045 | .297 |
| Maternal responsiveness | −.001 | .040 | −.002 | −.079 − .077 | −.032 | .975 |
| MAOA x sex | −.220 | .268 | −.202 | −.749 − .309 | −.822 | .412 |
| MAOA x PSE | −.212 | .216 | −.335 | −.638 − .215 | −.981 | .328 |
| PSE x sex | −.797 | .426 | −.587 | −.1.638 − .045 | −1.870 | .063 |
| MAOA x PSE x sex | .664 | .287 | .813 | .096–1.231 | 2.309 | .022 |
Multidimensional Assessment Profile of Preschool Disruptive Behavior, unidimensional IRT score
Boys coded as 1; girls coded as 0
Child MAOA genotype (MAOA-L = 1; MAOA-H = 2)
Life Stressors and Social Resources Scale (LISRES) assessed at 28 weeks gestation, factor score
Mean maternal cotinine during pregnancy, natural log-transformed
Mean reported drinks per day during pregnancy
Antisocial behavior questionnaire, sum of maternal and paternal scores
Life Stressors and Social Resources Scale (LISRES) assessed at child age 5, factor score
Maternal smoking in cigarettes/day at child age 5
Early Childhood Home Observation for Measurement of the Environment , responsivity subscale
Table 4.
Interaction of MAOA genotype x prenatal stress exposure (PSE) in predicting disruptive behavior a in boys (n = 141) versus girls b (n = 144)
| Predictors | Boys | Girls | ||
|---|---|---|---|---|
|
| ||||
| β [95% CI] | p | β [95% CI] | p | |
| Child age | −.059 [−.907 − .463] | .521 | −.099 [−.972 − .357] | .360 |
| Child MAOA c | .071 [−.245 − .523] | .472 | .215 [.005 − .787] | .047 |
| Prenatal stress exposure d | −.676[−.221 − .010] | .031 | .173 [−.080 − .135] | .610 |
| Prenatal tobacco exposure e | .193 [−.001 − .164] | .053 | −.012 [−.109 − .252] | .922 |
| Prenatal alcohol exposure f | .185 [.085–5.589] | .043 | −.045 [−.266 − .168] | .678 |
| Parent antisocial behavior g | −.048 [−.057 − .036] | .660 | .156 [−.151 − .299] | .196 |
| Concurrent stress exposure h | .446 [.034 − .110] | < .001 | .154 [−.045 − .425] | .270 |
| Concurrent tobacco exposure i | .180 [−.002 − .059] | .069 | .025 [−.212 − .221] | .838 |
| Maternal responsiveness j | .068 [−.074 − .154] | .483 | −.139 [−.180 − .040] | .207 |
| MAOA x PSE | .774 [.089 − .799] | .015 | −.133 [−.555 − .365] | .561 |
Multidimensional Assessment Profile of Preschool Disruptive Behavior, unidimensional IRT score
Boys coded as 1; girls coded as 0
Child MAOA genotype (MAOA-L = 1; MAOA-H = 2)
Life Stressors and Social Resources Scale (LISRES) assessed at 28 weeks gestation, factor score
Cotinine-corrected mean cigarettes per day across pregnancy, log-transformed
Mean reported drinks per day during pregnancy
Antisocial behavior questionnaire, sum of maternal and paternal scores
Life Stressors and Social Resources Scale (LISRES) assessed at child age 5, factor score
Maternal smoking in cigarettes/day at child age 5
Early Childhood Home Observation for Measurement of the Environment , responsivity subscale
3.2 MAOA x PSE x sex on disruptive behavior
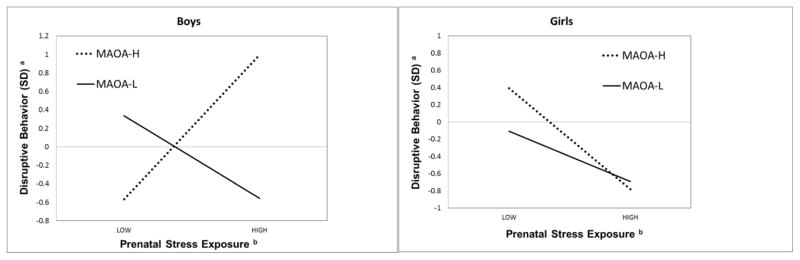
We found a significant 3-way interaction of MAOA x PSE x sex on disruptive behavior (β = .813; 95% CI: .096 to 1.231; p = .022) (Table 3). Figure 2 illustrates the interaction of MAOA x PSE on disruptive behavior in boys (left) versus girls (girls). In conditions of low PSE, boys with MAOA-H exhibited lower disruptive behavior symptoms compared with boys with MAOA-L. However, in conditions of high PSE, boys with MAOA-H had greater disruptive behavior, whereas those with MAOA-L appeared to be buffered. These patterns were not observed in girls.
Figure 2.
MAOA x prenatal stress exposure in boys versus girls.*
*Covariates: Child age, MAOA genotype, prenatal tobacco exposure, prenatal alcohol exposure, parent antisocial behavior, concurrent stress exposure, and concurrent tobacco exposure, maternal responsiveness
SD = standard deviations
aMultidimensional Assessment Profile of Preschool Disruptive Behavior, unidimensional IRT score
bLife Stressors and Social Resources Scale (LISRES) assessed at 28 weeks gestation, factor score
3.3 MAOA x PTE on disruptive behavior
For PTE, the three-way interaction of MAOA x PTE x sex was not significant (β = .135, p = .598). However, in the analyses stratified by sex, MAOA x PTE predicted disruptive behavior in boys (β = .362, p = .037), but not in girls (β = −.144, p = .505). Boys with MAOA-H exposed to more PTE exhibited more disruptive behaviors.
3.4 Passive rGE
Evidence of gene-prenatal environment correlation was not found. Correlations were as follows: maternal genotype and PSE (r = .056, p = .347), maternal genotype and PTE (r = .037, p = .538), paternal genotype (for girls only) and PSE (r = −.105, p = .284), paternal genotype and PTE (r = −.057, p = .567). We did observe a correlation between paternal MAOA genotype and CSE—girls whose fathers had the low activity MAOA genotype were exposed to higher concurrent stress (r = −.240, p = .013). No correlations were found between maternal genotype and CSE (r = .090, p = .130), maternal genotype and CTE (r = −.013; p = .827), paternal genotype and CTE (r = −.045, p = .643), maternal genotype and maternal responsiveness (r = −.081, p = .184), or paternal genotype and maternal responsiveness (r = .096, p = .329).
4. Discussion
There is increasing support for the role of early life adversity, in particular, prenatal adversity, in shaping disruptive behavior pathways (Aizer et al., 2015, Chiarella et al., 2015, Clark et al., 2015, Hanson et al., 2015, Ronald et al., 2010). How (and whether) these pathways are modulated by child MAOA genotype is just beginning to be examined (Hill et al., 2013, Hohmann et al., 2016, Wakschlag et al., 2010a). Adding to this small but growing subset of the MAOA literature (Byrd & Manuck, 2014), we found that the impact of two common prenatal adversities, PSE and PTE, like childhood maltreatment, may also be modulated by MAOA. We additionally present preliminary evidence for a gene-environment correlation between paternal MAOA-L and girls’ preschool stress exposure. We take a very cautious approach to making sense of these findings for several reasons. First, there are discrepancies in these results from our own earlier findings in an independent adolescent sample (Wakschlag et al., 2010a). Next, studies of preschool-aged children with measures of PSE and PTE with which these results could be compared are lacking. Finally, as alluded to in the introduction, MAOA has proved to be consistently inconsistent in its effects on behavior.
4.1 Association of MAOA-H with disruptive behaviors – susceptibility to prenatal adversity seen boys, but not in girls
Boys possessing the high-activity variant exhibited higher levels of disruptive behavior as a function of increasing prenatal adversity; PSE and PTE appeared to interact independently with MAOA. Girls with MAOA-H also showed more disruptive behaviors relative to MAOA-L girls, but this association was independent of the level of prenatal adversity. In fact, direct effects of prenatal exposures (tobacco, alcohol, stress) on boys’ disruptive behavior were not seen in girls. Taken together, girls appeared comparatively resilient to measured prenatal adversities. In our earlier study in an independent sample, we found MAOA x PTE interactions on conduct disorder symptoms in both sexes, but the low activity variant was associated with risk in adolescent boys, whereas the high-activity variant was associated with risk in adolescent girls (Wakschlag et al., 2010a). A potential explanation to consider in future work would be whether increasing testosterone levels associated with the pubertal transition in boys alters the function or influence of MAOA on behavior. Indeed, we have previously shown that testosterone levels in cerebrospinal fluid interact with MAOA to predict antisocial behavior in adult males, and have proposed a mediating effect of testosterone on gene transcription (Sjöberg et al., 2008). Ultimately, understanding the influence of MAOA across developmental periods could be enhanced by measuring hormones and their interactions.
4.2 MAOA x adversity interactions in young children
There is only one other study to our knowledge that examined the effect of MAOA x prenatal stress on disruptive patterns in pre-pubertal children. Hill and colleagues found that infants (of both sexes) with MAOA-L whose mothers reported more negative life events and more neighborhood deprivation during pregnancy exhibited greater negative emotionality at 5 weeks of age (Hill et al., 2013). We found that 5-year-old boys (but not girls) with the high- not low-activity variant, and greater PSE, exhibited more disruptive behavior. While different outcomes (negative emotionality versus disruptive behavior), different measures of prenatal stress (life history calendar versus LISRES interview), and different ages of children (5 weeks versus 5 years) could explain these discrepancies, both studies also show discrepancies with the predominant MAOA-L-maltreatment-antisocial behavior pattern observed in adolescent and adult males (Byrd & Manuck, 2014). Could the MAOA x adversity interaction vary as a function of developmental timing?
Indeed, the few studies that have examined G x E processes with other genes in preadolescent children are less consistent with the diathesis-stress model (Alexandra Burt et al., 2013, Burt & Klump, 2014b, Kim-Cohen et al., 2006). Rather, following a bioecological G x E model (Burt & Klump, 2014a), genetic influences may be most strongly expressed in average environments (Scarr, 1992), whereas deleterious environments could amplify environmental exposures (Pennington et al., 2009, Raine, 2002). Relatedly, we have recently shown that early life exposure to normative stressors is uniquely associated with higher regional homogeneity of resting state fMRI in prefrontal areas that underlie disruptive behavior pathways, after accounting for extreme violence exposure (Demir et al., under review). Clearly much more work is needed to confirm the modulation of the prenatal environment by MAOA. The present study provides clues that investigation of how adverse environments shape development and adaptation should to take genetic susceptibility and gene-environment correlations into account.
4.3 MAOA x PTE only in boys, and less robust than anticipated
While we had previously found a 3-way MAOA x PTE x sex interaction in the prediction of adolescent conduct disorder (Wakschlag et al., 2010a), here, we observed a MAOA x PTE interaction only in boys; the 3-way interaction of MAOA x PTE x sex in the full sample was not significant. This may be due to comparatively low levels of prenatal smoking in the current sample (0.8 cigarettes/day versus 12.8 cigarettes/day in our previous sample). Relatedly, PSE was not assessed in our previous study, but was, and was controlled for, in the current study. This difference could have further attenuated the independent effect of PTE. We also considered that detection of patterns in girls might have been hampered by lower rates of disruptive behavior at this young age (Schaeffer et al., 2006), but disruptive behavior scores did not differ significantly between boys and girls (Table 2). Finally, about two thirds of the girls characterized as MAOA-L in this sample possessed functionally intermediate phenotypes (2/4, 3/4 or 4/5). Hill and colleagues (2013) noted that their findings did not differ, however, whether they omitted or included heterozygote females, nor did outcomes differ among hetero- and homozygous females (Hill et al., 2013). Nonetheless, more information is needed on the molecular functionality of MAOA alleles of different repeat lengths in relevant cellular contexts.
4.4 Paternal MAOA-L – girls’ CSE correlation
Perhaps the most intriguing, albeit unexpected finding was that daughters whose fathers had the MAOA-L genotype had significantly higher concurrent stress exposure (CSE) as reported by their mothers, suggesting the possibility of a passive gene-environment correlation. The impact of this correlation in the current sample, however, is unclear. CSE showed a main effect on disruptive behavior in the full sample (Table 3; β = .310; p = .001), but seems to be driven by the effect of CSE in boys (Table 4; β = .446; p < .001) rather than girls (β = .154; p = .270). Moreover, while girls with MAOA-L fathers had more CSE, girls with MAOA-H actually exhibited higher disruptive behavior, regardless of prenatal stress or tobacco exposure (Table 4). It would be important to confirm this apparent paternal MAOA – preschool stress correlation using path analysis, and in a sample in which paternal genotypes were assessed directly. Comparison of genetically-related and genetically-unrelated parent-child dyads could further elucidate this correlation (Harold et al., 2013, Rice et al., 2013, Roos et al., 2016).
4.5 Limitations
There are additional limitations of this study not already mentioned that are worthy of consideration. First, the sample size may raise questions about adequate power to test for 3-way interactions. We conducted a post-hoc power analysis of the regression model used to test the MAOA x PSE x sex interaction and conclude that statistical power was in fact adequate (power = .999; R2 = .252, 14 predictors, probability level of .05, N = 285). Moreover, the depth of exposure and outcome measures in this study relative to large epidemiologic studies could have further increased our power to detect effects. Second, while we controlled for a number of prenatal and postnatal confounders including parenting quality (maternal responsiveness), there are undoubtedly still unmeasured factors, for example, the quality of the parent-child relationship, that could have influenced children’s disruptive behaviors (Kochanska & Kim, 2014). Third, as this cohort was oversampled for smokers to examine PTE, we cannot rule out the possibility that allele frequencies of MAOA are different from samples that are more normative – a tendency toward antisocial behavior could be over-represented (Wakschlag et al., 2003). Finally, the racial and ethnic diversity on the sample could have affected results—larger subpopulations met HWE, while very small subpopulations were not tested.
5. Conclusions
We provide preliminary evidence for the modulation of maternal psychosocial stress and maternal smoking during pregnancy by child MAOA genotype for preschool-aged boys in a racially and ethnically diverse population oversampled for smokers. It would be important to confirm these patterns in larger more representative samples. A longitudinal study that follows children across developmental periods and accounts for how the monoamine system may interact with the changing environmental and hormonal milieu would be ideal. We posit that transitions across the prenatal period to early childhood and across pubertal development could critically influence the function of apparently well-established G x E interactions.
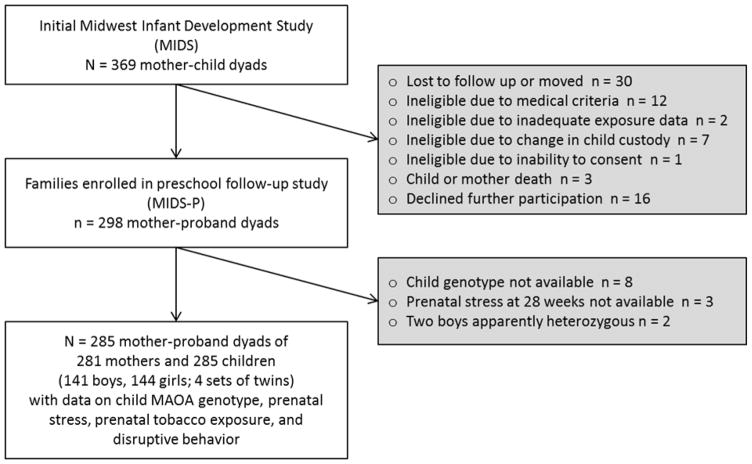
Figure 1.
Flow chart showing derivation of the analytic sample.
Highlights.
Whether and how MAOA moderates susceptibility to prenatal adversity is unclear.
Here MAOA moderated susceptibility to prenatal stress and tobacco exposure in boys.
Preliminary evidence for passive gene-environment correlation was found.
Girls whose fathers had MAOA-L genotype experienced higher stress at age 5.
Future research to elucidate developmental variation in mechanisms is recommended.
Footnotes
Role of funding sources
This research was supported by NIDA grants R01 DA014661 (Espy); R01 DA023653 (Espy, Wakschlag, Clark, Skol, Cook); and K23 DA037913 (PI Massey), and a 2015 American Academy of Child and Adolescent Psychiatry (AACAP) Summer Medical Student Fellowship (Hatcher). NIDA and AACAP had no role in the study design, data collection, analysis or interpretation of the data, or the decision to submit this paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizer A, Stroud L, Buka S. Maternal stress and child outcomes: Evidence from siblings. J Hum Resour. 2015 doi: 10.3386/w18422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandra Burt S, Klahr AM, Neale MC, Klump KL. Maternal warmth and directiveness jointly moderate the etiology of childhood conduct problems. J Child Psychol Psychiatry. 2013;54:1030–1037. doi: 10.1111/jcpp.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åslund C, Nordquist N, Comasco E, Leppert J, Oreland L, Nilsson KW. Maltreatment, MAOA, and delinquency: sex differences in gene – environment interaction in a large population-based cohort of adolescents. Behav Genet. 2011;41:262–272. doi: 10.1007/s10519-010-9356-y. [DOI] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Gunter TD, Packer H, Wernett P, Philibert RA. Child maltreatment moderates the association of MAOA with symptoms of depression and antisocial personality disorder. J Fam Psychol. 2010;24:12. doi: 10.1037/a0018074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buades-Rotger M, Gallardo-Pujol D. The role of the monoamine oxidase A gene in moderating the response to adversity and associated antisocial behavior: a review. Psychol Res Behav Manag. 2014;7:185. doi: 10.2147/PRBM.S40458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. Parent-child conflict as an etiological moderator of childhood conduct problems: an example of a 'bioecological' gene-environment interaction. Psychol Med. 2014a;44:1065–1076. doi: 10.1017/S0033291713001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL. Prosocial peer affiliation suppresses genetic influences on non-aggressive antisocial behaviors during childhood. Psychol Med. 2014b;44:821–830. doi: 10.1017/S0033291713000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB. MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol Psychiatry. 2014;75:9–17. doi: 10.1016/j.biopsych.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Cervera-Juanes R, Wilhem LJ, Park B, Lee R, Locke J, Helms C, Gonzales S, Wand G, Jones SR, Grant KA. MAOA expression predicts vulnerability for alcohol use. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabris CF, Lee JJ, Cesarini D, Benjamin DJ, Laibson DI. The fourth law of behavior genetics. Curr Dir Psychol Sci. 2015;24:304–312. doi: 10.1177/0963721415580430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarella J, Tremblay RE, Szyf M, Provençal N, Booij L. Impact of Early Environment on Children's Mental Health: Lessons From DNA Methylation Studies With Monozygotic Twins. Twin Research and Human Genetics. 2015;18:623–634. doi: 10.1017/thg.2015.84. [DOI] [PubMed] [Google Scholar]
- Clark CA, Espy KA, Wakschlag L. Developmental pathways from prenatal tobacco and stress exposure to behavioral disinhibition. Neurotoxicol Teratol. 2015;53:64–74. doi: 10.1016/j.ntt.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M, Neiderhiser JM, Langstrom N, Lichtenstein P. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry. 2010;67:529–538. doi: 10.1001/archgenpsychiatry.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Goodnight JA, Rathouz PJ, Lahey BB. Is maternal smoking during pregnancy a causal environmental risk factor for adolescent antisocial behavior? Testing etiological theories and assumptions. Psychol Med. 2012;42:1535–1545. doi: 10.1017/S0033291711002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Van Hulle CA, Waldman ID, Rodgers JL, Harden KP, Rathouz PJ, Lahey BB. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20:139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nothen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Demir OE, Voss J, O'Neill J, Briggs-Gowan MJ, Wakschlag L, Booth J. Early life stress exposure alters prefrontal resting-state fMRI local connectivity in young children. doi: 10.1016/j.dcn.2016.02.003. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, Hewitt JK, Kendler KS, Sher KJ. Candidate gene – environment interaction research reflections and recommendations. Perspect Psychol Sci. 2015;10:37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: a deterministic approach. Nicotine Tob Res. 2007;9:453–465. doi: 10.1080/14622200701239530. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Steer CD, Newman TK, Gibson N, Goldman D. Early life stress, MAOA, and gene-environment interactions predict behavioral disinhibition in children. Genes Brain Behav. 2010;9:65–74. doi: 10.1111/j.1601-183X.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA. Prenatal tobacco exposure: developmental outcomes in the neonatal period. Dev Psychol. 2011;47:153–156. doi: 10.1037/a0020724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrook R, Massey SH, Clark CA, Burns JL, Mustanski BS, Cook EH, O'Brien TC, Makowski B, Espy KA, Wakschlag LS. Separating Family-Level and Direct Exposure Effects of Smoking During Pregnancy on Offspring Externalizing Symptoms: Bridging the Behavior Genetic and Behavior Teratologic Divide. Behav Genet. 2015 doi: 10.1007/s10519-015-9762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming K, Graham H, Heirs M, Fox D, Sowden A. Smoking in pregnancy: a systematic review of qualitative research of women who commence pregnancy as smokers. J Adv Nurs. 2013;69:1023–1036. doi: 10.1111/jan.12066. [DOI] [PubMed] [Google Scholar]
- Flemming K, McCaughan D, Angus K, Graham H. Qualitative systematic review: barriers and facilitators to smoking cessation experienced by women in pregnancy and following childbirth. J Adv Nurs. 2015;71:1210–1226. doi: 10.1111/jan.12580. [DOI] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA psychiatry. 2013;70:956–963. doi: 10.1001/jamapsychiatry.2013.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–1494. doi: 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Rosser AA. MAOA-environment interactions: results may vary. Biol Psychiatry. 2014;75:2–3. doi: 10.1016/j.biopsych.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky E, Bevilacqua L, Carli V, Sarchiapone M, Roy A, Goldman D, Enoch MA. The interactive effect of MAOA-LPR genotype and childhood physical neglect on aggressive behaviors in Italian male prisoners. Genes, brain and behavior. 2014;13:543–549. doi: 10.1111/gbb.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Keller MC, Visscher PM. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci. 2014;17:782–790. doi: 10.1038/nn.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. Sage Publications; Newbury Park, Calif: 1991. [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Elam KK, Thapar A, Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D. The nature of nurture: Disentangling passive genotype – environment correlation from family relationship influences on children's externalizing problems. J Fam Psychol. 2013;27:12. doi: 10.1037/a0031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J, Oreland L. The role of MAO in personality and drug use. Prog Neuropsychopharmacol Biol Psychiatry. 2016;69:101–111. doi: 10.1016/j.pnpbp.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Hill J, Breen G, Quinn J, Tibu F, Sharp H, Pickles A. Evidence for interplay between genes and maternal stress in utero: monoamine oxidase A polymorphism moderates effects of life events during pregnancy on infant negative emotionality at 5 weeks. Genes, Brain and Behavior. 2013;12:388–396. doi: 10.1111/gbb.12033. [DOI] [PubMed] [Google Scholar]
- Hohmann S, Zohsel K, Buchmann AF, Blomeyer D, Holz N, Boecker-Schlier R, Jennen-Steinmetz C, Rietschel M, Witt SH, Schmidt MH. Interacting effect of MAOA genotype and maternal prenatal smoking on aggressive behavior in young adulthood. J Neural Transm. 2016:1–10. doi: 10.1007/s00702-016-1582-x. [DOI] [PubMed] [Google Scholar]
- Huang CL, Ou WC, Chen PL, Liu CN, Chen MC, Lu CC, Chen YC, Lin MH, Huang CS. Effects of Interaction Between Dopamine D2 Receptor and Monoamine Oxidase A Genes on Smoking Status in Young Men. Biol Res Nurs. 2015;17:422–428. doi: 10.1177/1099800415589366. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene–environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Huang Y-y, Haverly R, Burke AK, Galfalvy H, Brent DP, Oquendo MA, Mann JJ. Parental care moderates the influence of MAOA-uVNTR genotype and childhood stressors on trait impulsivity and aggression in adult women. Psychiatr Genet. 2009;19:126. doi: 10.1097/YPG.0b013e32832a50a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Kim S. A complex interplay among the parent–child relationship, effortful control, and internalized, rule-compatible conduct in young children: Evidence from two studies. Dev Psychol. 2014;50:8. doi: 10.1037/a0032330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS. Deviant peer affiliation and antisocial behavior: Interaction with monoamine oxidase A (MAOA) genotype. J Abnorm Child Psychol. 2011;39:321–332. doi: 10.1007/s10802-010-9474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y-F, Menard S. The interplay of MAOA and peer influences in predicting adult criminal behavior. Psychiatr Q. 2016:1–14. doi: 10.1007/s11126-016-9441-3. [DOI] [PubMed] [Google Scholar]
- McGrath L, Mustanski B, Metzger A, Pine D, Kistner-Griffin E, Cook E, Wakschlag L. A latent modeling approach to genotype–phenotype relationships: Maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Archives of women's mental health. 2012;15:269–282. doi: 10.1007/s00737-012-0286-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Fenn CB, Billings AG. Life stressors and social resources: an integrated assessment approach. Soc Sci Med. 1988;27:999–1002. doi: 10.1016/0277-9536(88)90291-2. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Life Stressors and Social Resources Inventory-Adult Form: Professional manual. PAR; Odessa, FL: 1994. [Google Scholar]
- Nikulina V, Widom CS, Brzustowicz LM. Child abuse and neglect, MAOA, and mental health outcomes: a prospective examination. Biol Psychiatry. 2012;71:350–357. doi: 10.1016/j.biopsych.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson KW, Comasco E, Åslund C, Nordquist N, Leppert J, Oreland L. MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict Biol. 2011;16:347–355. doi: 10.1111/j.1369-1600.2010.00238.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, McGrath LM, Rosenberg J, Barnard H, Smith SD, Willcutt EG, Friend A, Defries JC, Olson RK. Gene X environment interactions in reading disability and attention-deficit/hyperactivity disorder. Dev Psychol. 2009;45:77–89. doi: 10.1037/a0014549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstet Gynecol. 2003;101:140–147. doi: 10.1016/s0029-7844(02)02370-0. [DOI] [PubMed] [Google Scholar]
- Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry. 2015;20:98–108. doi: 10.1038/mp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard Z, Mackinnon A, Jorm AF, Easteal S. No evidence for interaction between MAOA and childhood adversity for antisocial behavior. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:228–232. doi: 10.1002/ajmg.b.30581. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley E, Eaves L, Foley D, Gardner C, Archer K, Wormley B, Maes H, Riley B, Silberg J. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol Med. 2009;39:579–590. doi: 10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J Abnorm Child Psychol. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Rehan W, Sandnabba NK, Johansson A, Westberg L, Santtila P. Effects of MAOA genotype and childhood experiences of physical and emotional abuse on aggressive behavior in adulthood. Nordic Psychology. 2015;67:301–312. [Google Scholar]
- Rice F, Lewis G, Harold GT, Thapar A. Examining the role of passive gene – environment correlation in childhood depression using a novel genetically sensitive design. Dev Psychopathol. 2013;25:37–50. doi: 10.1017/S0954579412000880. [DOI] [PubMed] [Google Scholar]
- Ronald A, Pennell CE, Whitehouse AJ. Prenatal Maternal Stress Associated with ADHD and Autistic Traits in early Childhood. Front Psychol. 2010;1:223. doi: 10.3389/fpsyg.2010.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos LE, Fisher PA, Shaw DS, Kim HK, Neiderhiser JM, Reiss D, Natsuaki MN, Leve LD. Inherited and environmental influences on a childhood co-occurring symptom phenotype: Evidence from an adoption study. Dev Psychopathol. 2016;28:111–125. doi: 10.1017/S0954579415000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Scarr S. Developmental theories for the 1990s: development and individual differences. Child Dev. 1992;63:1–19. [PubMed] [Google Scholar]
- Schaeffer CM, Petras H, Ialongo N, Masyn KE, Hubbard S, Poduska J, Kellam S. A comparison of girls' and boys' aggressive-disruptive behavior trajectories across elementary school: prediction to young adult antisocial outcomes. J Consult Clin Psychol. 2006;74:500. doi: 10.1037/0022-006X.74.3.500. [DOI] [PubMed] [Google Scholar]
- Schlüter T, Winz O, Henkel K, Eggermann T, Mohammadkhani-Shali S, Dietrich C, Heinzel A, Decker M, Cumming P, Zerres K. MAOA-VNTR polymorphism modulates context-dependent dopamine release and aggressive behavior in males. Neuroimage. 2016;125:378–385. doi: 10.1016/j.neuroimage.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell'Osso L, Virkkunen M, Goldman D. A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindström L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144:159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback user’s guide: A calendar method for assessing alcohol and drug use. Addiction Research Foundation; Toronto, Ontario, Canada: 1996. [Google Scholar]
- Tikkanen R, Auvinen-Lintunen L, Ducci F, Sjöberg RL, Goldman D, Tiihonen J, Ojansuu I, Virkkunen M. Psychopathy, PCL-R, and MAOA genotype as predictors of violent reconvictions. Psychiatry Res. 2011;185:382–386. doi: 10.1016/j.psychres.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen R, Ducci F, Goldman D, Holi M, Lindberg N, Tiihonen J, Virkkunen M. MAOA alters the effects of heavy drinking and childhood physical abuse on risk for severe impulsive acts of violence among alcoholic violent offenders. Alcoholism: Clinical and Experimental Research. 2010;34:853–860. doi: 10.1111/j.1530-0277.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen R, Sjöberg RL, Ducci F, Goldman D, Holi M, Tiihonen J, Virkkunen M. Effects of MAOA-Genotype, Alcohol Consumption, and Aging on Violent Behavior. Alcoholism: Clinical and Experimental Research. 2009;33:428–434. doi: 10.1111/j.1530-0277.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totsika V, Sylva K. The home observation for measurement of the environment revisited. Child and Adolescent Mental Health. 2004;9:25–35. doi: 10.1046/j.1475-357X.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking - 50 Years of Progress: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. [Google Scholar]
- van der Vegt EJ, Oostra BA, Arias-Vásquez A, van der Ende J, Verhulst FC, Tiemeier H. High activity of monoamine oxidase A is associated with externalizing behaviour in maltreated and nonmaltreated adoptees. Psychiatr Genet. 2009;19:209–211. doi: 10.1097/YPG.0b013e32832a5084. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi SW, Nichols SR, Kestler J, Burns JL, Carter AS, Henry D. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. J Am Acad Child Adolesc Psychiatry. 2014;53:82–96. e83. doi: 10.1016/j.jaac.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Kistner EO, Pine DS, Biesecker G, Pickett KE, Skol AD, Dukic V, Blair RJ, Leventhal BL, Cox NJ, Burns JL, Kasza KE, Wright RJ, Cook EH., Jr Interaction of prenatal exposure to cigarettes and MAOA genotype in pathways to youth antisocial behavior. Mol Psychiatry. 2010a;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Middlecamp MK, Walton LL, Tenzer P, Leventhal BL. Pregnant smokers who quit, pregnant smokers who don't: does history of problem behavior make a difference? Soc Sci Med. 2003;56:2449–2460. doi: 10.1016/s0277-9536(02)00248-4. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Tolan PH, Leventhal BL. Research Review:‘Ain’t misbehavin’:Towards a developmentally-specified nosology for preschool disruptive behavior. Journal of Child Psychology and Psychiatry. 2010b;51:3–22. doi: 10.1111/j.1469-7610.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Tager IB, Van Vunakis H, Speizer FE, Hanrahan JP. Maternal smoking during pregnancy, urine cotinine concentrations, and birth outcomes. A prospective cohort study. Int J Epidemiol. 1997;26:978–988. doi: 10.1093/ije/26.5.978. [DOI] [PubMed] [Google Scholar]
- Wisner KL. The last therapeutic orphan: the pregnant woman. Am J Psychiatry. 2012 doi: 10.1176/appi.ajp.2012.12030367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ming Q, Wang X, Yao S. The interactive effect of the MAOA-VNTR genotype and childhood abuse on aggressive behaviors in Chinese male adolescents. Psychiatr Genet. 2016;26:117–123. doi: 10.1097/YPG.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M. Longitudinal study of child development in Québec (ELDEQ 1998–2002) Institut de la statistique du Quebec; Quebec, Quebec, Canada: 2000. Parents’ health and social adjustment: part II, social adjustment; pp. 37–45. [Google Scholar]