Abstract
BACKGROUND
Developmental exposure to ethanol is recognized to produce long-term neurobehavioral impairment in multiple animal models. However, the molecular mechanisms underlying these deficits remain poorly understood. The present study was undertaken to ascertain whether two well-characterized targets of prenatal alcohol exposure, sonic hedgehog (Shh) and retinoic acid (RA), that induce the hallmark morphological phenotypes of fetal alcohol spectrum disorders (FASD), are involved in the generation of behavioral alterations as a result of alcohol exposure.
METHODS
Zebrafish embryos were exposed to ethanol (0%, 1%, 3%) at either 8–10 or 24–27 hours post-fertilization (hpf) and then evaluated during adolescence in the novel tank dive test to assess anxiety and risk-taking behavior. Overt signs of dysmorphogenesis were also scored and behavioral and morphological changes were compared for embryos treated with alcohol alone or in combination with subthreshold doses of shh or alhh1a3 morpholinos (MOs).
RESULTS
Ethanol treated fish displayed altered tank diving behavior that was not exacerbated by combined MO treatment. While treatment of embryos with either shha mRNA or RA prior to ethanol exposure only ameliorated the altered tank diving response in the case of shha mRNA overexpression, dysmorphogenesis was rescued by both treatments.
CONCLUSION
These results suggest that the effects of ethanol exposure on changes in anxiety and risk-taking behavior in adolescent zebrafish is manifested by a blunting of Shh, but not RA, signaling during early development.
Keywords: FASD, zebrafish, sonic hedgehog, retinoic acid, ethanol
INTRODUCTION
Prenatal exposure to ethanol results in a constellation of central nervous system (CNS) abnormalities that includes behavioral impairments, which are observed in a wide range of species (Gerlai et al., 2000; Cole et al, 2012; Ungerer et al., 2013). Studies in rodent have demonstrated that behavioral deficits similar to those observed in humans are produced following prenatal ethanol exposure. These behavioral defects include hyperactivity and deficiencies in response inhibition (Randall, 1987). Fetal alcohol exposure in mice assessed for open field exploration and fear-conditioned learning exhibit altered behavior as a consequence of prenatal ethanol exposure, with behaviors typical of reduced anxiety being exhibited, such as reduced contextual fear and increased exploration of novel objects (Allan et al, 2003). Rodent studies of behavior following prenatal ethanol exposure also demonstrate a constellation of behavioral defects that are remarkably similar to behaviors observed in FASD children, and include impaired attention, learning, executive function, and motor activity (Schneider et al, 2011). Thus, studies in rodents and humans indicate that prenatal ethanol exposure produces similar behavioral deficits in adults.
A wealth of evidence indicates that at least some of the effects of ethanol on CNS development may occur via disruption of extracellular matrix (ECM) signaling, in particular pathways involving sonic hedgehog (Shh) and retinoic acid (RA). Shh signaling is disrupted by ethanol exposure in multiple FASD animal models (Ahlgren et al., 2002; Arenzana et al., 2006; Li et al., 2007; Aoto et al., 2008; Loucks and Ahlgren, 2009; Zhang et al., 2013), and shh mRNA overexpression rescues many phenotypes induced by ethanol exposure in zebrafish (Loucks and Ahlgren 2009; Zhang et al, 2011, 2013). Recent studies using Shh +/− mice show that binge ethanol exposure at gastrulation results in more severe facial dysmorphologies, confirming Shh as a target of ethanol exposure in FASD (Kletzman et al, 2014).
A wealth of evidence supports RA as a target of prenatal ethanol exposure (Sulik et al, 1981; Duester, 1991; Pillarkat, 1991; Zachman et al, 1998; Leo and Lieber, 1999; Yelin et al, 2005; Yelin et al, 2007; Kot-Leibovich and Fainsod 2009; Kumar et al, 2010), and in zebrafish embryos RA can rescue FASD phenotypes that include microphthalmia (Marrs et al, 2010; Muralidharan et al, 2015). RA also exerts its function via the modulation of multiple other signaling systems, with several studies describing crosstalk between RA, Shh and Fgf signaling pathways. Especially relevant to the present study is the demonstration that RA, Shh and Fgfs regulate ventralization of spinal cord and telencephalon during CNS development (Appel and Eisen, 2003; Ribes et al, 2005). It is well established that RA signaling controls downstream expression and function of Fgfs and Shh in limb development (Diez del Corral and Storey 2004), with ethanol disrupting Fgf and Shh signaling during mouse limb development (Chrisman et al, 2004). Knockdown of retinaldehyde dehydrogenase 2 (Raldh2), a biosynthetic enzyme for RA, leads to ocular defects and diminished Shh and Fgf signaling (Ribes et al, 2009). Combined inhibition of Raldh2 and ethanol exposure in Xenopus recapitulates ethanol pathology and reduces Shh expression (Yelin et al, 2007; Kot-Leibovich and Fainsod, 2009). Recent studies from our laboratory show that RA interaction with Shh signaling is implicated in ethanol pathogenesis in midbrain-hindbrain boundary development in zebrafish, but not in ethanol-induced microphthalmia (Zhang et al, 2015). Thus, in multiple regions of the CNS ethanol appears to exert its teratogenic effects by disruption of a molecular signaling pathway that may involve RA and Shh.
As our previous studies have demonstrated a role for Shh and RA signaling pathways in the manifestation of morphological and cellular abnormalities in zebrafish FASD (Zhang et al, 2011; 2013; 2015), and we have shown that binge ethanol exposure during embryogenesis results in altered novel tank diving behavior in adolescent zebrafish (Bailey et al, 2015), we decided to assess whether these established molecular targets of ethanol exposure may also contribute to the altered zebrafish behaviors. Using the novel tank dive test we examined adolescent zebrafish, exposed as embryos to either alcohol alone or a combination of alcohol and MOs that perturb either Shh or RA function, for changes in anxiety and risk-taking behavior. Our studies surprisingly demonstrate a selective role for these signaling pathways in the alteration of anxiety behavior in adolescent zebrafish, with overexpression of shh mRNA preventing the effects of ethanol on altered anxiety-like behavior, but exposure of embryos to RA being unable to prevent the effects of ethanol on altered anxiety-like behavior in adolescent zebrafish. Our results are therefore the first demonstration for a role in Shh signaling in the generation of altered behavior in FASD.
Methods
Animals
Zebrafish (Danio Rerio) from the AB strain (obtained from the Zebrafish International Resource Center) were bred in-house and generated all embryos used in the present study. All fish were housed in automatic fish housing systems at 28.5°C. Fish were group housed with <20 fish per 3L tank on a 14 hr light,10 hr dark cycle 7 days per week. Approximately 20–30 male and female fish per ethanol exposure condition were reared for behavioral testing, fish with physical malformations were excluded from behavioral analyses. All behavioral testing was conducted between the hours of 12:00 and 5:00 pm, which was during the light phase. All fish were fed twice daily with brine shrimp (Brine Shrimp Direct, Ogden, Utah) in the morning and dry flake fish food (TetraMin®, Tropical Flakes, Melle, Germany) in the evening; evening feeding was always withheld until the completion of behavioral testing. All animal protocols used were approved by the Institutional Animal Care and Use Committee at North Carolina Central University in accordance with NIH and United States regulations.
Ethanol Treatment of Zebrafish Embryos
Zebrafish embryos in fish water (60 μg/ml Oceans natural sea salt mix dissolved in distilled water; Oceans Reefs & Aquariums) containing a 1:500 dilution of 0.1% methylene blue (to prevent fungal infection) were exposed to 1.0% and 3.0% ethanol from 8 to 10 hpf or 24 to 27 hpf. Ethanol was diluted with fish water to its final concentration, and at the selected developmental stage for ethanol treatment embryos were placed in fresh fish water containing ethanol. At the end of the exposure period, fish water containing ethanol was removed, embryos were washed once with fresh fish water and then transferred to fresh fish water and placed on an aquarium rack system for rearing. At approximately 2 months of age (which corresponds to the late fry juvenile stage of development) all fish were assessed for behavioral testing.
Antisense morpholino oligonucleotides injection
Antisense morpholino oligonucleotides (MOs) (Gene Tools, Philomath, OR) were designed against exon/intron splice sites for shha (5′-CAGCACTCTCGTCAAAAGCCGCATT-3′) (Nasevicius et al, 2000; Zhang et al, 2013). To target retinoic acid signaling, we used a MO to raldh3 (aldh1a3). This MO was designed to target the start codon (TATAGTCCCGTTCTGTGCCATAGC) ( Bill et al, 2009; Yahyavi et al, 2013). MOs were solubilized in water at a concentration of 0.03 mM (aldh1a3 MO) or 0.3 mM (shha MO). 1 nl MO was injected into one to two-cell stage embryos. Higher dosage (1.5 pm) of shh morphants display phenotypes characteristic of a shh mutation (Nasevicius et al, 2000). Control MO for the aldh1a3 does not produce any obvious defects and only wild-type human mRNA for ALDH1A3, but not mutant human mRNA for ALDH1A3/p.Lys190*, rescue the morphant phenotype (Yahyavi et al, 2013). These two MOs used in the current study have been well studied and validated in these previous publications with regard to specificity and absence of off-target effects.
To assess the role of specific cellular signaling pathways, Shh or RA, on altered novel tank diving behavior, zebrafish embryos were either injected with shha N183 mRNA to rescue the effects of Shh MO injection when combined with ethanol exposure, or treated with RA to rescue the effects of aldh1a3 MO injection when combined with ethanol exposure, as previously described (Zhang et al, 2015).
Facial and Brain Malformation Scoring
Eye size was measured at 2 days post-fertilization (dpf) as previously described (Zhang et al., 2011; 2014), and involved measuring the longest axis along the eye, and calculated against a standard 10 μm ruler under the same magnification. For 2 dpf eye measurements, we designated a diameter of less than 240 μm as the “small eye” phenotype, since all untreated eyes were at least 250 μm in diameter.
Midbrain-hindbrain boundary (MHB) perturbation was scored at 1 dpf as previously described (Zhang et a 2014). Malformation of the MHB was assessed visually based on absence of the defined border between the midbrain and hindbrain. The presence of the MHB was defined as the presence of 3 or 4 ridges (tectal and cerebellar boundaries) perpendicular to the anterior-posterior axis of the CNS at the midbrain–hindbrain junction. Absence of this defined border was scored as a disruption of MHB development.
Behavioral Testing
Novel tank dive task
The novel tank dive paradigm takes advantage of the zebrafish behavior of responding to a novel environment by initially dwelling on the tank floor before gradually exploring, swimming to the tank surface after several minutes in a novel environment (Bencan and Levin, 2008, Bencan et al., 2009, Levin, 2011 and Levin et al., 2007). This initial dive response is an effective strategy for predatory avoidance inasmuch as it removes the possibility of being consumed from below; the later exploration is presumably food-seeking/foraging behavior as zebrafish feed along the water column, including from the water’s surface. Therefore, any deviation from this normal dive/explore response pattern is considered maladaptive behavior.
Zebrafish were individually tested in 1.5-liter plastic tanks filled with 1350 ml of tank water, the surface of the water was approximately 10 cm from the tank floor. The trapezoid shaped tanks were 22.9 cm along the bottom and 27.9 cm along the top. The diagonal side of the tank was 15.9 cm and the opposite vertical side was 15.2 cm, the same design as used previously (see Levin et al., 2007). Behavior was tracked in real-time using EthoVision tracking software (Noldus Information and Technology, Wageningen, The Netherlands) that calculated distance from the tank floor and total distance traveled. Each trial was five-minutes in duration and commenced immediately after a fish was placed in the novel tank. The video signal was transmitted through a Everfocus camcorder (Duarte CA) that was positioned approximately 88 cm away from the testing tanks. A minimum of 12 fish were assayed for each treatment group.
Statistics
All ANOVA statistical analysis was performed using SPSS 22 (International Business Machines Corp, Armonk, New York). The Type1 error rate alpha (α) was set to 0.05. A repeated measures analysis of variance was conducted for each variable of interest. Morpholino, vehicle, and alcohol were used as the between factors with mean distance to floor, mean distance to wall, and total distance traveled per minute as the repeated measure. The Greenhouse-Geisser adjustment, which was used to control for sphericity, was applied to the novel tank dive data. To compare exposure groups, the mean was taken across all five (5) minutes for each exposure group. The Tukey post-hoc test was used to determine differences between exposure groups.
RESULTS
Effects of ethanol and perturbation of Shh or RA signaling on CNS development
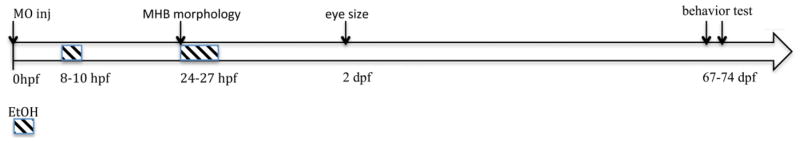
Zebrafish embryos were acutely exposed to ethanol in the presence or absence of MOs that target Shh or RA signaling, from either 8–10 hpf, representing late gastrulation to the initiation of neurulation, or 24–27 hpf, a period associated with formation of a defined five-vesicle brain (Figure 1). Following treatment embryos were assessed for morphological malformations at either 1 dpf (MHB formation) or 2 dpf (eye diameter to assess microphthalmia). While binge-like exposure to 1% ethanol did not produce dysmorphogenesis as assessed by MHB formation or eye diameter (Figures 2 and 3; 0/30 embryos), combined ethanol and shh or aldh1a3 MO treatment disrupted MHB formation at 1 dpf (Figure 2F,K; 28/60 embryos shh, 23/56 embryos aldh1a3) and induced microphthalmia at 2 dpf (Figure 3F, 22/52 embryos shh; Fig. 3J, 28/68 embryos shh; Fig. 3O, 19/51 embryos aldh1a3; Fig. 3Q, 13/34 embryos aldh1a3). To assess efficacy of treatments to prevent effects of ethanol on zebrafish development and behavior, we assessed the ability of either shha mRNA injection of one-cell embryos or RA treatment of embryos to rescue MHB and eye these malformation phenotypes. Either shha mRNA overexpression or treatment with exogenous RA during the time of ethanol exposure was used to confirm that the abnormal morphological phenotypes were reversible and indicated involvement of either Shh or RA signaling in this ethanol-induced dysmorphogenesis (Figures 2H,L and 3 H,L). Only 3/46 or 7/67 embryos displayed abnormal MHB for shha and aldh1a3 rescue, respectively. For rescue of microphthalmia, 0/32 embryos exposed to ethanol at 8–10 hpf, or 2/39 embryos exposed at 24–27 hpf, displayed abnormal eye size for shha rescue, and only 2/46 embryos with 8–10 hpf exposure or 2/40 embryos for 24–27 hpf exposure exhibited microphthalmia after aldh1a3 rescue. Thus, these treatments effectively rescued the dysmorphology induced by combined ethanol and MO treatment.
Figure 1. Time course of treatments and experimental analyses.
Timeline for MO injection and ethanol treatments are indicated, as well as times of morphological analysis of embryos (MHB and eye size) and behavioral testing.
Figure 2. Effect of ethanol and Shh or RA disrupting MOs on MHB formation.
Embryos were analyzed at 1 dpf for the presence or absence of the defined border between the midbrain and hindbrain (indicated by arrows). A, wild-type embryos; B, embryos injected with 0.3 pm shh MO only; C, embryos injected with 25 pg shha N183 mRNA only; D, embryos injected with both 0.3 pm shh MO and 25 pg shha mRNA; E, embryos exposed to 1% ethanol from 8–10 hpf; F, embryos exposed to 1% ethanol from 8–10 hpf and injected with 0.3 pm shh MO; G, embryos exposed to 1% ethanol from 8–10 hpf and injected with 25 pg shha mRNA; H, embryos exposed to 1% ethanol from 8–10 hpf and injected with both 0.3 pm shh MO and 25 pg shha mRNA; I, embryos injected with 0.03 aldh1a3; J, embryos treated with 1 nM RA and injected with 0.03 aldh1a3; K, embryos treated with 1% ethanol from 8–10 hpf and injected with 0.03 aldh1a3; L, embryos treated with 1% ethanol and RA from 8–10 hpf and injected with 0.03 aldh1a3. All embryos are lateral views. Noted that MHB formation was disrupted in embryos exposed to both ethanol and shh/aldh1a3 MO (F,K) and is rescued by shha mRNA overexpression or RA treatment respectively (H,L).
Figure 3. Ocular development in embryos exposed to ethanol or Shh and RA disrupting MOs.
Eye diameter was measured in 2 dpf embryos as described in Methods. A, wild-type embryos; B, embryos injected with 0.3 pm shh MO only; C, embryos injected with 25 pg shha N183 mRNA only; D, embryos injected with both 0.3 pm shh MO and 25 pg shha mRNA; E, embryos exposed to 1% ethanol from 8–10 hpf; F, embryos exposed to 1% ethanol from 8–10 hpf and injected with 0.3 pm shh MO; G, embryos exposed to 1% ethanol from 8–10 hpf and injected with 25 pg shha mRNA; H, embryos exposed to 1% ethanol from 8–10 hpf and injected with both 0.3 pm shh MO and 25 pg shha mRNA; I, embryos exposed to 1% ethanol from 24–27 hpf; J, embryos exposed to 1% ethanol from 24–27 hpf and shh MO ; K, embryos exposed to 1% ethanol from 24–27 hpf and injected with 25 pg shha mRNA; L, embryos exposed to 1% ethanol from 24–27 hpf and injected with both 0.3 pm shh MO and 25 pg shha mRNA; M, embryos injected with 0.03 aldh1a3; N, embryos treated with 1 nM RA and injected with 0.03 aldh1a3; O, embryos treated with 1% ethanol from 8–10 hpf and injected with 0.03 aldh1a3; P, embryos treated with 1% ethanol and RA from 8–10 hpf and injected with 0.03 aldh1a3. Q, embryos treated with 1% ethanol from 24–27 hpf and injected with 0.03 aldh1a3; R, embryos treated with 1% ethanol and RA from 24–27 hpf and injected with 0.03 aldh1a3 Note that microphthalmia is only observed in embryos exposed to both ethanol and shh/aldh1a3 MO (F, J, O, Q) and is rescued by shha mRNA overexpression or RA treatment respectively (H,L, P, R). The calibration bar is 50 μm.
Effect of ethanol exposure on novel tank diving test
Our previous studies analyzed novel tank diving behavior of adolescent zebrafish exposed to ethanol, demonstrating that binge-like ethanol exposure altered this behavior that is a correlate of anxiety and risk-taking behavior (Bailey et al, 2015). In the present study, following ethanol exposure either in the presence or absence of MO treatment, embryos were raised to juvenile/adolescence stage (approximately 2 months of age) and evaluated in the novel tank diving test. We did not observe a significant difference in survival between control embryos and the embryos exposed to the various treatments (data not shown).
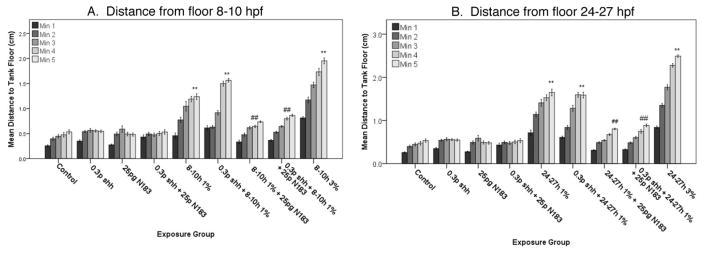
Ethanol exposed fish displayed a range of variability in total distance traveled when placed into a novel tank environment (Figures 4 and 6), with the differences in total distance traveled not being significant (all P values >0.05) except for the 8–10 hpf 1% ethanol, 25 pg shha N183 mRNA only, and control comparisons (P<0.05). However, for embryos treated at either 8–10 hpf and 24–27 hpf, both 1% and 3% ethanol treatment, as well as treatment with ethanol combined with subthreshold shh MO (F(5.7,236.8)=4.3, P<0.001) and aldh1a3 MO (F(5.8,479)=5.2, P<0.001), resulted in adolescent fish engaging in a reduced and more transient dive response than the control fish (see Figures 5 and 7). Tukey’s post-hoc testing revealed the 24–27 hpf exposure window, following the ethanol treatments, was the most sensitive exposure compared to control, in agreement with our previous studies (Bailey et al, 2015).
Figure 4. Total distance traveled in the novel tank diving test following 8–10 hpf (A) or 24–27 hpf (B) ethanol exposure and shh MO treatment.
Total distance traveled for each minute of a five-minute novel tank dive trial. Each bar represents the mean distance for one minute (min 1–5), data are organized by exposure group. Error bars represent SEM. ** indicates significant difference from control (post-hoc), P<0.05.
Figure 6. Total distance traveled in the novel tank diving test following 8–10 hpf (A) or 24–27 hpf (B) ethanol exposure and aldh1a3 MO treatment.
Total distance traveled for each minute of a five-minute novel tank dive trial. Each bar represents the mean distance for one minute (min 1–5), data are organized by exposure group. Error bars represent SEM.
Figure 5. Effect of 8–10 hpf (A) or 24–27 hpf (B) ethanol exposure and Shh signaling on risk-taking and anxiety behavior in juvenile zebrafish.
Distance from the tank floor for each minute of a five-minute novel tank dive trial. Each bar represents the mean distance from the tank floor for one minute (min 1–5), data are organized by exposure group. Error bars represent SEM. **significant difference from control (post-hoc), P<0.001. ##, significant difference from 1% ethanol and 1% ethanol + shh MO treatment groups, P<0.001.
Figure 7. Effect of 8–10 hpf (A) and 24–27 hpf (B) ethanol exposure and RA signaling on risk-taking and anxiety behavior in juvenile zebrafish.
Distance from the tank floor for each minute of a five-minute novel tank dive trial. Each bar represents the mean distance from the tank floor for one minute (min 1–5), data are organized by exposure group. Error bars represent SEM. **significant difference from control (post-hoc), P<0.001.
Effect of Shh mRNA rescue and RA treatment on novel tank diving test behavior
To assess the role of Shh or RA on altered novel tank diving behavior, zebrafish embryos were either injected with shha N183 mRNA to rescue the effects of Shh MO injection when combined with ethanol exposure, or treated with RA to rescue the effects of aldh1a3 MO injection when combined with ethanol exposure. With both ethanol exposure windows, Shha mRNA overexpression prevented the induction of altered novel tank diving behavior, with shha mRNA injected fish exhibiting diving behaviors that were significantly different when compared to ethanol treatment alone or ethanol combined with shha MO injection (Figure 5; F (9,420.5)=3.7, P<0.001). Conversely, RA treatment from either 8–10 or 24–27 hpf did not ameliorate the effects of ethanol, or combined ethanol and aldh1a3 MO treatment, on the novel tank diving behavior when compared to treated groups not exposed to RA (Figure 7). For both exposure times, ethanol-exposed fish treated with RA exhibited novel tank diving behavior that was significantly different from control groups (P<0.001).
DISCUSSION
The primary goal of the present study was to investigate whether the persisting neurobehavioral consequences of binge-like ethanol exposures in a zebrafish model are due to alteration of signaling pathways that are well established as target of prenatal ethanol exposure. This study therefore was a straightforward design to test the specific hypothesis that Shh and/or RA signaling pathways, while contributing to the hallmark dysmorphogenesis observed in FASD, also contribute to the altered behaviors observed during early and later life. To test this hypothesis we used the novel tank diving test, which assessed anxiety and risk-taking behavior in zebrafish, behavior shown to be affected by prenatal ethanol exposure in humans. Here we report that binge-like ethanol exposures during two defined stages of embryonic development in zebrafish alter novel tank diving behavior in a pathway-specific manner, with only Shh signaling being shown to play a key role in the alteration of zebrafish anxiety and risk-taking behavior. Importantly, the inability of RA to normalize the altered tank diving behavior highlights the specificity of our experimental design.
In the novel tank dive test, control fish will typically dive to the tank bottom for the five minutes of the testing period, and will not explore higher quadrants of the tank due to anxiety related behavior. Beyond this initial testing period control fish will begin to explore upper regions of the tank. In our previous studies using the novel tank diving test, we demonstrated that the zebrafish exposed to both 1% and 3% ethanol for both exposure windows spent significantly more time swimming near the top of the tank than did the controls, with the 24–27 hpf exposed fish being more sensitive to the ethanol exposures (Bailey et al, 2015). These data indicated ethanol-exposed fish are more risk-taking and are more likely to explore the novel regions of the tank, with these results being faithfully reproduced in the present study and extended to assess the role of Shh and/or RA signaling pathways in the manifestation of the altered novel tank diving behavior. One important conclusion that can be made from the present study, as well as our previous work, is that even exposure to lower doses of ethanol can have marked effects on zebrafish behavior, with the alteration in the novel tank diving response being similar between the 1% ethanol, 3% ethanol, and 1% ethanol plus MO treatment groups. Especially in the case of the 3% ethanol and 1% ethanol plus MO fish, these treatments produce dysmorphogenesis in 30–50% of the embryos that is both Shh- and RA-dependent, yet the behavioral change from fish treated with 1% ethanol with no embryo dysmorphogenesis is not significant. These data suggest that there is a limit to the altered novel tank diving response that can be elicited by these treatments, with 1% ethanol exposure alone being able to produce a behavioral change that is significantly different from controls and that is only slightly enhanced with treatments that also induce the hallmark phenotypes of FAS.
Our decision to focus on an involvement of Shh and/or RA signaling pathways as a possible molecular mechanism underlying alteration of the novel tank diving response was based on the wealth of evidence for disruption of Shh or RA signaling in the overt dysmorphogenesis observed in FAS. Shh signaling is disrupted by ethanol exposure in multiple FASD animal models (Ahlgren et al., 2002; Arenzana et al., 2006; Li et al., 2007; Aoto et al., 2008; Loucks and Ahlgren, 2009; Zhang et al., 2013), and shh mRNA overexpression rescues many phenotypes induced by ethanol exposure in zebrafish (Loucks and Ahlgren 2009; Zhang et al, 2011, 2013). Use of mice with mutations in the Shh pathway also provides support for the key role of Shh signaling in ethanol-induced facial malformations (Hong and Krauss, 2012; Kletzman et al, 2014). RA is also a key target of prenatal ethanol exposure (Sulik et al, 1981; Duester, 1991; Pillarkat, 1991; Zachman et al, 1998; Leo and Lieber, 1999; Yelin et al, 2005; Yelin et al, 2007; Kot-Leibovich and Fainsod 2009; Kumar et al, 2010), with RA rescuing FASD phenotypes in zebrafish (Marrs et al, 2010; Muralidharan et al, 2015). In addition, the aldh1a3 MO induced dysmorphology that is observed in FASD, and as we have shown here and previously (Zhang et al, 2015) RA can rescue the phenotypes induced by knockdown of raldh3 using this MO.
Our results presented here strongly suggest a role for Shh signaling in the control of development of the neural circuitry that regulates the novel tank diving behavior, since only shha mRNA expression could rescue the altered behavior following ethanol exposure alone or ethanol combined with either shh or aldh1a3 MO. The absence of normalization of the altered novel tank diving behavior with RA treatment is strongly suggestive of the specificity of the shh mRNA rescue and indicates that at least for the novel tank diving behavior, the development of the neural circuitry underlying this behavior shares a molecular mechanism with the pathways impacted by ethanol exposure during manifestation of the hallmark dysmorphogenesis associated with FASD. That both ethanol exposure at 8–10 and 24–27 hpf leads to altered novel tank diving behavior in adolescent fish, albeit the 24–27 hpf embryos being more sensitive to ethanol exposure, suggests that the development of the neural circuitry underlying this behavior occurs from gastrulation through neurulation in a Shh-dependent mechanism. As our previous studies also showed that sensorimotor behavior as assessed by the tap-startle response was affected by ethanol exposure at these developmental stages, it will be of interest in future studies to ascertain whether this behavior is also selectively Shh-dependent.
It is important to recognize that although our studies only analyzed the role of radlh3 in ethanol-mediated dysmorphology and behavior, it is possible that other raldh isoforms could be involved in ethanol-induced pathologies. In addition, RA expression in the presumptive zebrafish forebrain occurs prior to the 24–27 hpf ethanol exposures, and studies by Perz-Edwards et al (2001) have shown that robust raldh gene expression is not detected in neural tube until 18 hpf. Raldh2 is expressed in zebrafish, especially mesoderm (Begemann et al, 2001), raldh3 is expressed as early as 4 hpf in zebrafish and raldh4 is expressed in zebrafish beginning at 2 hpf (Liang et al, 2008). Thus, multiple isoforms of raldh are expressed during the periods embryos were exposed to ethanol. However, since RA was unable to rescue the observed behavioral alterations induced by ethanol exposure, while rescuing ocular and MHB dysmorphologies, these data strongly suggests that these other raldh isoforms are not involved in the altered novel tank diving behavior, at least as induced by the ethanol exposure windows employed in the present study.
Other molecular mechanisms are likely involved in the generation of behavioral abnormalities following developmental ethanol exposure. In zebrafish, binge-like exposure to 1% ethanol enhances tyrosine hydroxylase expression and dopamine levels in adult zebrafish brain, and indicates a role for the dopaminergic system in altered shoaling behavior as a consequence of ethanol exposure (Fernandes et al, 2015; Trans et al 2016a; 2016b). Relevant to the present study, exposure of adult zebrafish to 1% ethanol resulted in altered anxiety-like behavior that was shown to be mediated by the serotonergic system (Trans et al 2016c). While it is unclear if alterations in shoaling may also be mediated by signaling pathways such as Shh and/or RA, and if ethanol-mediated disruption of Shh signaling impairs normal development of these neurotransmitter systems, it would be of interest to investigate a possible role for Shh signaling in the development of the dopaminergic and serotonergic systems. Accordingly, Shh signaling has been shown to regulate the development of dopaminergic neurons in zebrafish forebrain (Holzschuh et al, 2003).
In conclusion, the data reported here extend our previous analysis of gastrulation/neurulation transition period (8–10 hpf) and early nervous system development (24–27 hpf) exposure to ethanol, and its effect on juvenile behavior, by assessing possible molecular pathways that may contribute to these behavioral alterations. Our data demonstrate that Shh signaling appears to play a role in the establishment of the novel tank diving behavior, since this behavior is altered following ethanol exposure, which can be rescued with shh mRNA overexpression. Interestingly, we also show that RA signaling does not contribute to this behavior since RA treatment is unable to rescue ethanol-induced changes in the novel tank diving behavior. These studies therefore begin to provide critical information on possible molecular mechanisms that may underlie ethanol’s effects on behavior, and importantly show that molecular pathways impacted by ethanol, that result in the hallmark facial abnormalities of FASD, may also modulate the behavior abnormalities that are observed in FASD.
Highlights.
Shh mRNA overexpression prevents ethanol-induced changes in zebrafish risk-taking behavior as measured in the novel tank diving test
Retinoic acid signaling does not appear to be involved in ethanol-induced alteration in zebrafish novel tank diving behavior since exogenous RA is unable to rescue ethanol-induced behavioral alterations.
This study provides novel insight into the role of ethanol sensitive genes, that are involved in ethanol-induced facial dysmorphologies associated with FASD, in zebrafish behavior.
Acknowledgments
This work was supported by NIH grant U54 AA019765.
This work was supported by NIH grant U54 AA019765. The authors thank Dr. ClarLynda Williams-Devane for help and guidance in the statistical analysis of the behavioral tests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlgren SC, Thakur V, Bronner-Fraser M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc Natl Acad Sci USA. 2002;99:10476–10481. doi: 10.1073/pnas.162356199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Higashiyama D, et al. Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Res (Part A) 2008;82:224–231. doi: 10.1002/bdra.20447. [DOI] [PubMed] [Google Scholar]
- Appel B, Eisen JS. Retinoids run rampant: multiple roles during spinal cord and motor neuron development. Neuron. 2003;40:461–464. doi: 10.1016/s0896-6273(03)00688-3. [DOI] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, 3rd, Aijon J, Sanchez-Gonzalez R, Arevalo R, Porteros A. Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol. 2006;28:342–348. doi: 10.1016/j.ntt.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Oliveri AN, Zhang C, Frazier JM, Mackinnon S, Cole GJ, Levin ED. Long-term behavioral impairment following acute embryonic ethanol exposure in zebrafish. Neurotoxicol Teratol. 2015;48:1–8. doi: 10.1016/j.ntt.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch G-J, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Bencan Z, Levin ED. The role of alpha7 and alpha4beta2 nicotinic receptors in the nicotine-induced anxiolytic effect in zebrafish. Physiology and Behavior. 2008;95:408–412. doi: 10.1016/j.physbeh.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill BR, Petzold AM, Clark KJ, et al. A primer for morpholino use in zebrafish. Zebrafish. 2009;6:69–77. doi: 10.1089/zeb.2008.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisman K, Kenney R, Comin J, Thai T, Suchocki L, Yueh YG, Gardner DP. Gestational ethanol exposure disrupts the expression of FGF8 and Sonic hedgehog signaling during limb patterning. Birth Defects Res (Part A) 2004;70:163–171. doi: 10.1002/bdra.20019. [DOI] [PubMed] [Google Scholar]
- Cole GJ, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay S. Effects of ethanol exposure on nervous system development in zebrafish. Int Rev Cell Mol Biol. 2012;299:255–315. doi: 10.1016/B978-0-12-394310-1.00007-2. [DOI] [PubMed] [Google Scholar]
- Dee CT, Szymoniuk CR, Mills PED, Takahashi T. Defective neural crest migration revealed by a Zebrafish model of ALx1-related frontonasal dysplasia. Hum Mol Genet. 2012;22:239–251. doi: 10.1093/hmg/dds423. [DOI] [PubMed] [Google Scholar]
- Diez del Corral R, Storey KG. Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays. 2004;26:857–869. doi: 10.1002/bies.20080. [DOI] [PubMed] [Google Scholar]
- Duester G. A hypothetical mechanism for fetal alcohol syndrome involving ethanol inhibition of retinoic acid synthesis at the alcohol dehydrogenase step. Alcohol Clin Exp Res. 1991;15:568–572. doi: 10.1111/j.1530-0277.1991.tb00562.x. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Rampersad M, Gerlai R. Embryonic alcohol exposure impairs the dopaminergic system and social behavioral responses in adult zebrafish. Int J Neuropsycopharmcol. 2015;18:1–8. doi: 10.1093/ijnp/pyu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–82. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Hauptmann G, Driever W. Genetic analysis of the roles of Hh, FGF8, and Nodal signaling during catecholaminergic system development in the zebrafish brain. J Neurosci. 2003;23:5507–5519. doi: 10.1523/JNEUROSCI.23-13-05507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Krauss RS. Cdon mutation and fetal ethanol exposure synergize to produce midline signaling defects and holoprosencephaly spectrum disroders in mice. PLOS Genetics. 2012:e1002999. doi: 10.1371/journal.pgen.1002999. [DOI] [PMC free article] [PubMed]
- Kletzman HW, Everson JL, Sulik KK, Lipinski RJ. The teratogenic effects of prenatal ethanol exposure are exacerbated by sonic hedgehog or Gli2 haploinsufficiency in the mouse. PLoS One. 2014;19:e89448. doi: 10.1371/journal.pone.0089448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis Model Mech. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Singh CK, DiPette DD, Singh US. Ethanol impairs activation of retinoic acid receptors in cerebellar granule cells in a rodent model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34:928–937. doi: 10.1111/j.1530-0277.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Reviews in the Neurosciences. 2011;22:75–84. doi: 10.1515/RNS.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Li YX, Yang HT, Zdanowicz M, et al. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest. 2007;87:231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- Liang D, Zhang M, Bao J, Zhang L, Xu X, Gao X, Zhao Q. Expressions of Raldh3 and Raldh4 during zebrafish early development. Gene Express Patterns. 2008;8:248–253. doi: 10.1016/j.gep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Loucks EJ, Ahlgren SC. Deciphering the role of Shh signaling in axial defects produced by ethanol exposure. Birth Defects Res (Part A) 2009;85:556–567. doi: 10.1002/bdra.20564. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Clendenon SG, Ratcliffe DR, et al. Zebrafish fetal alcohol syndrome model: effects of ethanol are rescued by retinoic acid supplement. Alcohol. 2010;44:707–715. doi: 10.1016/j.alcohol.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan P, Sarmah S, Marrs JA. Zebrafish retinal defects induced by ethanol exposure are rescued by retinoic acid and folic acid supplement. Alcohol. 2015;49:149–163. doi: 10.1016/j.alcohol.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic-acid-mediated gene expression in transgenic reporter zebrafish. Dev Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Pillarkat RK. Hypothesis: prenatal ethanol-induced birth defects and retinoic acid. Alcohol Clin Exp Res. 1991;15:565–567. doi: 10.1111/j.1530-0277.1991.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Randall CL. Alcohol as a teratogen: a decade of research in review. Alcohol Alcohol Suppl. 1987;1:125–132. [PubMed] [Google Scholar]
- Ribes V, Wang Z, Dolle P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2005;133:351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dolle P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signaling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 2011;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Tran S, Facciol A, Gerlai R. Alcohol-induced behavioral changes in zebrafish: the role of dopamine D2-like receptors. Psychopharmaoclogy (Berl) 2016a;233:2119–2128. doi: 10.1007/s00213-016-4264-3. [DOI] [PubMed] [Google Scholar]
- Tran S, Facciol A, Nowicki M, Chatterjee D, Gerlai R. Acute alcohol exposure increases tyrosine hydroxylase protein expression and dopamine synthesis in zebrafish. Behav Brain Res. 2016b;317:237–241. doi: 10.1016/j.bbr.2016.09.048. [DOI] [PubMed] [Google Scholar]
- Tran S, Nowicki M, Muraleetharan A, Chatterjee D, Gerlai R. Neurochemical factors underlying individual differences in locomotor activity and anxiety-like behavioral responses in zebrafish. 2016c doi: 10.1016/j.pnpbp.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Ungerer M, Knezovich J, Ramsay M. In utero alcohol exposure, epigenetic changes, and their consequences. Alcohol Res. 2013;35:37–46. doi: 10.35946/arcr.v35.1.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahyavi M, Abouzeid H, Gawdat G, et al. ALDH1A3 loss of function causes bilateral anophthalmia/microphthalmia and hypoplasia of the optic nerve and optic chiasm. Hum Mol Genet. 2013;22:3250–3258. doi: 10.1093/hmg/ddt179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelin R, Kot H, Yelin D, Fainsod A. Early molecular effects of ethanol during vertebrate embryogenesis. Differentiation. 2007;75:393–403. doi: 10.1111/j.1432-0436.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- Yelin R, Schyr RB, Kot H, et al. Ethanol exposure affects gene expression in the embryonic organizer and reduces retinoic acid levels. Dev Biol. 2005;279:193–204. doi: 10.1016/j.ydbio.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zachman RD, Grummer MA. The interaction of ethanol and vitamin A as a potential mechanism for the pathogenesis of fetal alcohol syndrome. Alcohol Clin Exp Res. 1998;22:1544–1556. [PubMed] [Google Scholar]
- Zhang C, Anderson A, Cole GJ. Analysis of crosstalk between retinoic acid and sonic hedgehog pathways following ethanol exposure in embryonic zebrafish. Birth Defects Res A Clin Mol Teratol. 2015;103:1046–1057. doi: 10.1002/bdra.23460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Frazier JM, Chen H, Liu Y, Lee JA, Cole GJ. Molecular and morphological changes in zebrafish following transient ethanol exposure during defined developmental stages. Neurotoxicol Teratol. 2014;44:70–80. doi: 10.1016/j.ntt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Ojiaku P, Cole GJ. Forebrain and hindbrain development in zebrafish is sensitive to ethanol exposure involving agrin, Fgf, and sonic hedgehog function. Birth Defects Res A Clin Mol Teratol. 2013;97:8–27. doi: 10.1002/bdra.23099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Turton QM, MacKinnon S, Sulik KK, Cole GJ. Agrin function associated with ocular development is a target of ethanol exposure in embryonic zebrafish. Birth Defects Res (Part A) 2011;91:129–141. doi: 10.1002/bdra.20766. [DOI] [PMC free article] [PubMed] [Google Scholar]