Abstract
Background
Lubricin, or proteoglycan 4 (PRG4), is a glycoprotein responsible for joint boundary lubrication. PRG4 has been previously shown to be down-regulated following traumatic joint injury such as a meniscal tear. There is preliminary evidence suggesting that intra-articular injection of PRG4 post-injury will reduce cartilage damage in rat models of surgically-induced post-traumatic osteoarthritis.
Objective
To determine the efficacy of intra-articular injection of full length recombinant human lubricin (rhPRG4) for reducing cartilage damage after medial meniscus destabilization (DMM) in a pre-clinical large animal model.
Study Design
Controlled laboratory study
Methods
Unilateral DMM was performed in 29 Yucutan minipigs. One week post-DMM, animals received 3 weekly intra-articular injections (3cc/injection): 1) rhPRG4 [1.3mg/ml; n=10], 2) rhPRG4+hyaluronan [1.3mg/ml rhPRG4 and 3mg/ml hyaluronan (~950 kDA); n=10], and 3) phosphate buffered saline [PBS; n=9]. Hind limbs were harvested 26 weeks post-surgery. Cartilage integrity was evaluated using macroscopic (India Ink) and microscopic (Safranin O-fast green and hematoxylin & eosin) scoring systems. Secondary outcomes evaluated using ELISA included PRG4 levels in synovial fluid, CTX-II concentrations in urine and serum, and IL-1β levels in synovial fluid and serum.
Results
The rhPRG4 group had significantly less macroscopic cartilage damage in the medial tibial plateau compared to the PBS group (p=.002). No difference was found between the rhPRG4+hyaluronan and PBS groups (p=.23). However, no differences in microscopic damage scores were observed between the three groups (p=.70). PRG4 production was elevated in the rhPRG4 group synovial fluid compared to the PBS group (p=.033). The rhPRG4 group presented significantly lower urinary CTX-II levels, but not serum levels, when compared to the PBS (p=.013) and rhPRG4+hyaluronan (p=.011) groups. In serum and synovial fluid, both rhPRG4 (p=.006; p=.017) and rhPRG4+hyaluronan groups (p=.009; p=.03) presented decreased IL-1β levels.
Conclusion
All groups exhibited significant cartilage degeneration following DMM surgery. However, animals treated with rhPRG4 had the least amount of cartilage damage and less inflammation, providing evidence that intra-articular injections of rhPRG4 may slow the progression of post-traumatic osteoarthritis.
Clinical Relevance
Patients with meniscal trauma are at high risk for post-traumatic osteoarthritis. This study demonstrates that an intra-articular injection regimen of rhPRG4 may attenuate cartilage damage following meniscal injury.
Keywords: meniscus, osteoarthritis, lubricin, PRG4, hyaluronan, inflammation
INTRODUCTION
Acute meniscal injuries account for approximately 30% of meniscal tears and are known to predispose the patient to post-traumatic osteoarthritis.14 The menisci protect the articular cartilage by maximizing joint congruity and minimizing contact stress within the knee.16 While the link between acute meniscal injury and the increased risk for post-traumatic osteoarthritis is not fully understood, altered joint contact mechanics and inflammation likely play a role. The standard surgical treatments for a meniscal tear are partial meniscectomy or meniscal repair. While these procedures alleviate symptoms, they do not eliminate the risk for post-traumatic osteoarthritis.34,41,46 Currently, no disease modifying osteoarthritis drugs are clinically available to slow or prevent post-traumatic osteoarthritis.32
Lubricin, or proteoglycan 4 (PRG4), is a mucinous glycoprotein found in synovial fluid that is expressed by the tissues lining synovial joints, binds to the articular cartilage surfaces, and serves in part as a boundary lubricant.12 Following joint trauma (e.g., meniscal injury, ACL tear) or joint surgery (e.g., partial meniscectomy, meniscal repair), PRG4 expression is down-regulated and the concentration in synovial fluid is reduced.9,11,25,37 Therefore, traumatized joints due to injury or surgery are at risk for arthrosis, possibly due to increased friction, which in turn may promote cartilage breakdown.11,25,26,47 Given that PRG4 expression is down-regulated and that the PRG4 concentration in synovial fluid is reduced after joint injury,37 intra-articular injections of recombinant human lubricin (rhPRG4) (i.e. tribosupplementation) could potentially prevent disease progression. rhPRG4 has been shown to have similar properties as native PRG4 in that it binds to the articular cartilage surfaces and acts as a boundary lubricant.1
Currently, viscosupplementation with hyaluronan (HA), another component of synovial fluid, is a FDA approved therapy to treat symptoms associated with osteoarthritis. While there is clinical evidence to show that intra-articular HA injections provide short-term pain relief due to its anti-inflammatory properties, its disease modifying effects remain doubtful. Only one clinical study, out of several, reported an increase in cartilage thickness following high-molecular weight HA treatment.18,49 While it has been proposed that there is a synergistic interaction between PRG4 and HA ex vivo,1,7,21,30 the role of this interaction in vivo remains controversial.6,47
It has been demonstrated that intra-articular injections of PRG4 and rhPRG4 have a chrondroprotective effect in small animal models of post-traumatic osteoarthritis.15,24,26 The next step in translating rhPRG4 to clinical use for the treatment and prevention of post-traumatic osteoarthritis is to demonstrate its effectiveness in a large animal model. The first study objective was to determine if three weekly intra-articular injections of rhPRG4 following surgical destabilization of the medial meniscus (DMM) reduced cartilage damage after 26 weeks in the porcine model. The second objective was to determine if there was an added benefit by using rhPRG4+HA in comparison to rhPRG4 alone, in vivo. It was hypothesized that the groups treated with injections of rhPRG4 or rhPRG4+HA would have less cartilage damage than the group treated with placebo injection (PBS) after DMM, and that injection of rhPRG4+HA would result in improved outcomes when compared to the group treated with rhPRG4 alone. Joint integrity was evaluated using macroscopic (India Ink) and microscopic (Safranin O-fast green and hematoxylin & eosin) assessment. Secondary outcomes include the level of PRG4 in synovial fluid, the concentrations of a Type II collagen breakdown marker (CTX-II) in urine and serum, and the presence of an inflammatory cytokine (IL-1β) in synovial fluid and serum.
METHODS
Study Design
The study was approved by the Institutional Animal Care and Use Committees of Brown University and Rhode Island Hospital and was performed to meet the ARRIVE guidelines. Thirty Yucatan mini-pigs (14 castrated males, 16 females, age, 13.5±0.79 months, weight, 55.5±2.1kg) underwent unilateral destabilization of the medial meniscus and were randomized into one of three injection treatment groups: rhPRG4 (n=10), rhPRG4+HA (n=10), and PBS (sham control, n=10). The DMM model was selected as it is commonly performed to induce OA in small animal models48 and because meniscal injuries are extremely common in humans.14 The minipig model also exhibits features of OA that are common to those seen in people.29,36 All animals were housed individually in adjacent pens for a minimum of eight weeks at Brown University and then transported to an offsite animal housing facility (Accuro Farms, Southbridge MA). The minimum pen size was 22.5 sq ft.
Surgical Technique
After induction of general anesthesia (See supplemental Table), all animals underwent surgical DMM. The surgical knee was selected at random. An incision was made approximately 2cm in length over the medial joint line at the medial edge of the patellar tendon. The dissection was carried through the subcutaneous and bursal tissues. Once through the capsule, part of the fat pad was excised and the incision was opened to expose the anterior horn of the medial meniscus. A snap was passed underneath the anterior horn of the medial meniscus to isolate this structure and protect the tibial cartilage during cutting. A 3-5mm full thickness segment of the anterior horn was then removed using a scalpel blade. Complete transection was confirmed via direct observation of retraction of the anterior portion of the meniscus relative to the cartilage of the tibial plateau and the medial femoral condyle. The wound was then irrigated with sterile phosphate buffered saline. The knee was closed in layers with buried sutures: Arthrotomy: 0 vicryl, interrupted; Bursa: 2-0 vicryl, running; Subcutaneous tissue: 2-0 vicryl, interrupted; and Subcuticular layer: 3-0 vicryl, interrupted. Post-operative pain was managed using a protocol (Supplemental Table) that included buprenorphine, fentanyl, bupivacaine/lidocaine (incision site only after closure), and Tylenol elixir (as needed). Animals were treated with an antibiotic (Ceftiofur) and an anti-vomiting agent immediately post-operatively (Odansetron). Animals were kept in pens for the duration of the study. They were not forced to exercise, though they appeared active when checked daily.
Intra-articular Injections
Full-length rhPRG4 was manufactured and provided by Lubris BioPharma (Framingham, MA). It was derived from Chinese Hamster Ovary (CHO) cells as previously described.1,43 The apparent molecular weight of 460 kDa, as assessed by SDS-PAGE, is larger than its predicted molecular weight.1 It is rich in O-linked Core 1 glycosylations43 that are responsible for lubricating activity27 and recapitulate native PRG4 lubricating activity on cartilage bearings in vitro.1 The dosing strategy was scaled by weight based on previously published work on rodents,26 which also used the same full-length rhPRG4 from CHO cells. For the rhPRG4+HA preparation, a non-cross-linked lyophilized HA preparation (R&D Systems, Minneapolis, MN), with a predicted molecular weight of ~950 kDa, was mixed with the rhPRG4. The injection solutions (3cc volume per injection with 0.1% Tween) were as follows: PBS, rhPRG4 (1.3mg/ml), and rhPRG4+HA (1.3mg/ml rhPRG4 and 3mg/ml HA). Under general anesthesia, intra-articular injections were given 7, 14, and 21 days after surgery using ultrasound guidance (Logiq e; General Electric Health Care, United Kingdom). Animals were euthanized 26 weeks from surgery and both hind limbs were harvested. The surgical leg was immediately processed for testing while the control knee was stored at −20°C.
Macroscopic Cartilage Assessment
The articulating surfaces of the medial tibial plateau and medial femoral condyle of the surgical limbs were stained with India ink and photographed for independent scoring by two blinded reviewers (BCF, KAW). When a difference existed, the two scores were averaged. Scores ranged from 0 (smooth surface) to 4 (lesion with more than 10% exposed bone).36,44 In addition, the lesion areas were measured. The length and width of each lesion were measured using calipers and the lesion area was approximated as an ellipse.36
Histological Assessment
Osteochondral specimens consisting of the medial compartment of the tibial plateau were dissected from the knee. They were fixed in 10% formalin for 7 days and then washed in PBS for 24 hours. Specimens were decalcified in EDTA for seven months and embedded in paraffin. Slices 6μm thick from the central region of the tibial plateau were obtained in the coronal plane. Histology slides were prepared for light microscopy by staining with Safranin O-fast green to evaluate glycosaminoglycan content (scored 0-4) while an adjacent slice was stained with hematoxylin and eosin (H&E) to score cartilage structure (scored 0-10), chondrocyte density (scored 0-4), and cell cloning (scored 0-4). Note that a low score is indicative of minimal damage while a high score is associated with severe damage. Three independent experienced examiners (KAW, BCF, GDJ), who were blinded to the animal number and experimental condition, scored the slides by consensus using a system that was adapted from that recommended for the sheep and goat by the Osteoarthritis Research Society International (OARSI).31 The four sub-scores were reported independently and also added to produce a total sum score (maximum possible score=22).
Secondary Outcomes
Synovial fluid (without lavage), serum and urine samples were obtained at the time of harvest. All samples were stored at −80°C until testing. Synovial fluid was recovered from the surgical knees of 8, 10 and 6 animals of the rhPRG4, rhPRG4+HA, and PBS groups, respectively. All reagents, samples, and standards for the biochemical assays were prepared as instructed by the manufacturer and performed in triplicate.
PRG4 ELISA
Relative comparisons of the PRG4 concentrations in the synovial fluid between treatment groups were determined using mAb 5C11 (EMD Millipore; Darmstadt, Germany), a monoclonal antibody for PRG4, in an inhibition ELISA format.2 A standard concentration curve was constructed by diluting a concentrated rhPRG4 solution as we did not have purified porcine PRG4 available.
CTX-II
Concentrations of carboxy-terminal telepeptide of type II collagen (CTX-II), a type II collagen cleavage marker, were measured in urine and serum using the Cartilaps ELISA kit (Immunodiagnostic systems IDS Inc., Gaithersburg, MD, USA).47 Urinary CTX-II concentrations were normalized to urinary creatinine levels using the Creatinine Assay Kit (Abcam, Cambridge, MA, USA).12 Normalized urinary CTX-II was reported as ug CTX-II/nmol creatinine and serum CTX-II was reported as pg/ml.
Interleukin-1β (IL-1β)
Concentrations of IL-1β in synovial fluid and serum were evaluated using a commercially available ELISA kit designed for swine (Thermo Scientific, Frederick, MD, USA).47 With a dilution factor of 10, the synovial fluid and serum concentrations were evaluated.
Statistical Methods
Nonparametric Kruskal-Wallis one-way analyses of variance were used to compare treatment groups on macroscopic cartilage damage scores (medial tibial plateau and medial femoral condyle), histologic damage scores (medial tibial plateau), and concentrations of PRG4, CTX-II and IL-1β in the fluid samples. In order to control the type I error rate, Exact Wilcoxon Rank Sum tests were used to make pair-wise comparisons between groups when the Krustal-Wallis tests indicated significant differences. Additionally, one-way analysis of variance was used to compare mean lesion areas between treatment groups in the medial tibial plateau and lateral femoral condyle. All analyses were performed using SAS statistical software, Version 9.4 (SAS Institute, Cary, NC). Statistical significance was determined based on p<.05.
Power for the macroscopic cartilage damage score was estimated to be 80% for detecting group differences with the Wilcoxon Rank Sum test when the probability that a randomly selected observation in one group was less than a randomly selected observation in the other group was equal to 0.85. This corresponds to a relatively large effect size.
RESULTS
Animal Welfare
All animals were fully weight bearing within seven days of surgery and at the time of harvest. Twenty nine of the thirty animals survived the 26-week follow-up period. One animal from the PBS group was fully weight bearing within six days of surgery, however she refused food post-surgery. She was euthanized 31 days after surgery as recommended by the veterinarian. Upon necropsy, it was determined by a veterinarian that the cause of anorexia appeared unrelated to any specific pathology of the gastrointestinal tract or the surgery. This animal was excluded from the analysis.
Macroscopic Cartilage Assessment
All subjects showed macroscopic degenerative changes in the articular surfaces of the medial tibial plateau of the surgically treated knee (Fig.1a). Subjects receiving rhPRG4 treatment had significantly lower macroscopic scores in the medial tibial plateau compared to PBS control (p=.002) (Fig. 1b), while there were no significant differences between the PBS treated group and the rhPRG4+HA group (p=.23). Likewise, there was no significant difference between the rhPRG4 and the rhPRG4+HA groups (p=.070). All lesions were centrally located within the medial tibial plateau (Fig. 1a). The mean lesions in the medial femoral condyles were similar in grade (2.1±0.77, 2.5±1.32, and 2.5±1.09 for the rhPRG4, rhPRG4+HA, and PBS groups, respectively) to those in the medial tibial plateau, but did not significantly differ between treatment groups (p=.75). The medial femoral condyle lesions were located in the anterior portion of the condyle, with some extending into the posterior region. There was minimal damage in the lateral compartment of the joint for all treatment groups. As for the mean lesion areas, there were no treatment group differences in the medial tibial plateau (rhPRG4: 22±19.0 mm2; rhPRG4+HA: 27±19.0 mm2; PBS: 33±19.3 mm2; p=.51) or the medial femoral condyle (rhPRG4: 6±19.0 mm2; rhPRG4+HA: 15±19.0 mm2; PBS: 20±20.0 mm2; p=.27).
1.
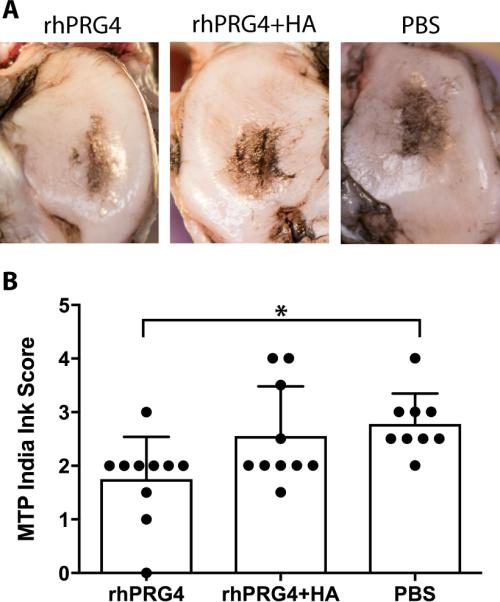
Macroscopic Cartilage Assessment for the medial tibial plateau (MTP). a) Images from the median lesion severity score for each treatment group: rhPRG4, rhPRG4+HA, and PBS. b) India ink scoring revealed significantly lower medial tibial plateau scores in the rhPRG4 group compared to the PBS group (p=.002). Error bars represent the standard deviations.
Histological Assessment
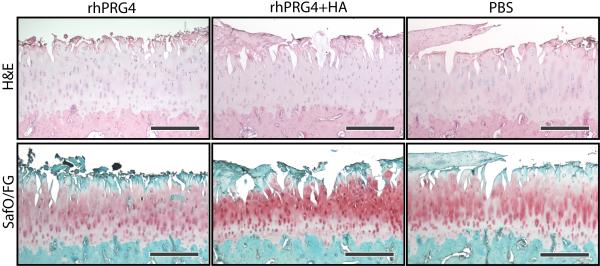
There were no statistically significant differences in the mean histological sum scores of the medial tibial plateaus between groups (p=.70) (Fig. 2). The mean±standard deviations of the sum scores were 15.5±3.95, 16.3±3.57, and 15.0±4.72 for rhPRG4, rhPRG4+HA and PBS groups, respectively. No differences were found between groups for any of the sub scores (p>.26) (Fig. 3).
2.
Histological images the median medial tibial plateau from the mid-coronal plane stained with H&E (top row) and Safranin O-fast green (bottom row). Scale bar is 500μm.
3.
Mean histological sub-scores for the three treatment groups. A) Structure (p=.78), B) Chondrocyte Density (p=.93), C) Cell Cloning (p=.45), and D) Glycosaminoglycan staining (p=.26). There were no significant differences between groups. Error bars represent the standard deviations.
Secondary Outcomes
Elevated levels of PRG4 were observed in the synovial fluid of the rhPRG4 surgical limbs (n= 8) compared to those of the PBS group (n=6, p=.033) (Fig. 4). No differences were observed between the rhPRG4 and the rhPRG4+HA (n=9) groups (p=.17) or in the rhPRG4+HA treated group compared to the PBS group (p=.09).
4.
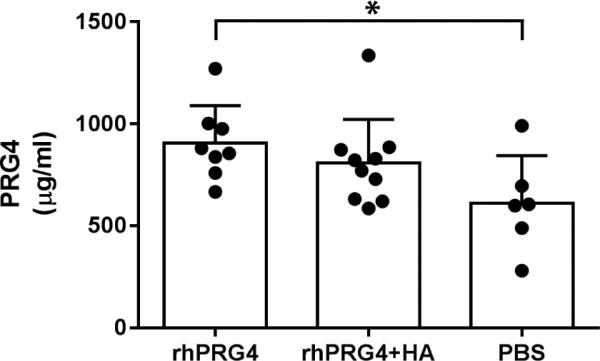
Elevated levels of PRG4 levels were present in the synovial fluid of the rhPRG4 treated limbs compared to the PBS treated limbs (p=.033). There was no significant difference between the rhPRG4 group and the rhPRG4+hyaluronan group (p=0.17) or between the rhPRG4+HA and PBS group (p=.09). Error bars represent the standard deviations.
Urinary CTX-II concentrations were higher in the PBS (n=9) and rhPRG4+HA (n=10) groups compared to the rhPRG4 group (n=10, p=.013 and .011, respectively) (Fig. 5a). However, in the serum, CTX-II levels were not significantly different between groups (p=.94; Fig. 5b).
5.
CTX-II concentrations. a) Urinary CTX-II levels normalized by creatinine. rhPRG4 treated joints exhibited reduced urinary CTX-II levels compared to PBS and rhPRG4+HA treated joints (p=.013 and .011, respectively). b) CTX-II levels in the serum were not significant between treatment groups (p=.94). Error bars represent the standard deviations.
There were increased serum levels of IL-1β in the PBS (n=9) compared to the rhPRG4 (n=10, p=.006) and the rhPRG4+HA groups (n=10, p=.009), indicating the presence of systemic inflammation in the PBS treated group (Fig. 6a). No difference was observed between the rhPRG4 and rhPRG4+HA groups (p=.791). Likewise, the surgical limb synovial fluids had elevated IL-1β concentrations in the PBS (n=6) group compared to the rhPRG4 (n=8, p=.017) and the rhPRG4+HA (n=9, p=.029) groups (Fig. 6b). No difference in IL-1β was observed between the rhPRG4 group and the rhPRG4+HA group (p=.81).
6.
IL-1β concentrations. a) Serum IL-1β concentration was also reduced in the rhPRG4 and rhPRG4+HA groups compared to the PBS group (p=.006; p=.009). b) The synovial fluid IL-1β concentration was diminished in the rhPRG4 and rhPRG4+HA groups compared to the PBS group (p=.017; p=.029). There was no significant difference between the rhPRG4 group and the rhPRG4+hyaluronan group (p=.81). Error bars represent the standard deviations.
DISCUSSION
The results of the study support the primary hypothesis that intra-articular injections of rhPRG4 would reduce arthrosis. Specifically, in the medial tibial plateau, the mean macroscopic score for the rhPRG4 group was significantly lower than that of the PBS group (Fig. 1). The reduction in macroscopic cartilage damage in the medial tibial plateau was likely due to the restoration of boundary lubrication and secondarily by anti-inflammatory activity.4 However, differences were not evident in the medial femoral condyles. The porcine results agree with those of previous small animal studies post-meniscal injury15 and after ACL transection,12,13,24,26,47 in which PRG4 supplementation reduced cartilage damage in treated knees in vivo. The CTX-II data, a marker of Type II collagen cleavage, also support these findings as the rhPRG4 group had lower mean CTX-II levels in urine as compared to the PBS group (Fig. 4a), indicative of less systemic cartilage degradation. This finding also recapitulates similar observations in a prior small animal study.26 The rhPRG4 group had reduced IL-1β levels in both the serum and synovial fluid supporting less catabolic enzymatic activity in the cartilage. This group also showed the highest levels of synovial PRG4 after 26 weeks. In antigen-induced arthritis in rats, Elsaid et al showed PRG4 levels were diminished in arthritic joints immediately following induction of arthrosis.10 Furthermore, the loss of PRG4, which likely resulted from high levels of IL-1β and cathepsin B (a protease that degrades PRG4), leads to increased joint coefficients of friction. In a recent rat ACL transection study, intra-articular injections of rhPRG4 were found to significantly reduce the number of caspase-3 positive chondrocytes, thus preserving chondrocytes and possibly preventing cartilage degeneration.12 Ruan et al also observed decreased levels of CTX-II and less cartilage damage in arthritic mice following intra-articular injections of an adenoviral vector over expressing PRG4.42 Most recently in transgenic mice, which conditionally express diphtheria toxin, it was shown that significantly diminishing the number of chondrocytes in a joint does not cause arthrosis;50 however, loss of chondrocytes occurs in PRG4 null mice.28 All of these observations and the present findings indicate that PRG4 is crucial for preventing chondrocyte death and cartilage degeneration.
Our results did not support the secondary hypothesis that the rhPRG4+HA group would be more effective than treatment with rhPRG4 alone. While both treatment groups had reduced IL-1β levels in the synovial fluid and serum, the rhPRG4 group had less macroscopic cartilage damage than the rhPRG4+HA group in the medial tibial plateau, and surprisingly, the rhPRG4+HA group failed to show significant improvements over the PBS injection group (Fig. 1). It has been previously shown that the cartilage damage in PRG4+HA treated animals were similar to PRG4 injected rats.47 It should be noted that the rat study utilized a HA formulation with a high molecular weight (5,000 kDa) compared to that of the present study (~950 kDa).47 For the present study, it was postulated that the lack of a difference may be attributed to the CD44-mediated anti-inflammatory effects of rhPRG4 and HA, in which both molecules compete for the cell-surface receptor CD44.3 Additionally, a recent study in canines reported that viscosupplementation with three different doses of HA [2 dosing strategies with a high molecular weight HA (Hylan G-F; molecular weight = 6,000KDa), and 1 dosing strategy with a low molecular weight HA (Hyalgan; 500-730 KDa)] provided symptomatic relief for post-traumatic osteoarthritis induced by radial transection of the medial meniscus; however, none of the doses reduced gross cartilage morphology or histological damage compared to the saline treated groups by 6 months.38 One reason for these findings may have been due to the late timing of supplementation, which was initiated four months after surgery and may have been too late to reverse damage. Smith et al also reported no significant differences in a canine ACL transection model in gross morphology, histology, or gait at 12 weeks and 32 weeks after treatment with a low molecular weight HA (5 weekly injections of Hyalgan starting one day after surgery).45 The lack of differences between treatment groups (HA vs. control) may be due to the low molecular weight formulation that was used.
There is current debate over whether rhPRG4 and HA function synergistically. Synergy has been reported in friction studies in which PRG4 or rhPRG4 are combined with HA to reduce joint friction in vitro.1,7,33 In contrast, rhPRG4 and HA do not appear to be synergistic in vivo based on our current large animal study or our previous work in the ACL transection rat model.47 The molecular weight of HA used in this study was ~950 kDa and in the previous rat study it was 5,000 kDa.47
Although there were varying degrees of macroscopic cartilage damage, microscopic histological scoring of medial tibial plateau lesions revealed no significant differences between groups. The lack of difference via histology is not surprising given that all three groups had significant chondral lesions in the medial compartment post-DMM surgery, and the most severe region of the lesion was evaluated for each sample. While the data indicate that the macroscopic damage was less in the rhPRG4 treated group, the mean lesion damage in the medial tibial plateau was 1.8, where 1 indicates surface scuffing and 2 represents a significant lesion but with no bone exposure.44 The histological assessment was performed through a central slice and encompassed the center of the lesion, which was present in the surgical limbs of all treatment groups. Furthermore, we created a mechanical defect in the meniscus that was left untreated. Thus, it is unlikely that full chondral protection would be possible. For example, partial meniscectomy has been shown to reduce meniscal hoop stresses, which are transferred to the bone through the meniscal roots in intact menisci. Thus removal of the root would compromise meniscal function, decrease joint contact area, and increase joint contact pressure.17 These biomechanical changes along with the associated increase of joint friction may ultimately lead to joint arthrosis. Nonetheless, it is noteworthy that the rhPRG4 injections were able to provide some long-term chondroprotection in light of this mechanical instability, and it seems plausible that rhPRG4 injections in combination with soft tissue repair (i.e., meniscal root or meniscal repair), would further prevent chondral damage. Clinically, meniscal repair has been shown to lower the risk of post-traumatic osteoarthritis compared to partial meniscectomy.34,46 Future studies are being designed to address this issue.
DMM and/or full transection of the meniscus in small animal models are commonly performed to study mechanisms of post-traumatic osteoarthritis and to evaluate potential disease modifying therapeutics.15,20,22,23,35 While these models provide significant insight into disease mechanisms and are relatively inexpensive compared to large animal models, it is important to validate the findings of potential therapeutics in a larger animal model with a knee similar in size to the human joint. The porcine model was selected for this study as it has been shown to be anatomically and functionally similar to the human knee when compared to other animal models,39 and has been accepted by the FDA as a pre-clinical model to demonstrate the safety and efficacy of intra-articular ligament repair.40
The DMM approach has been used with other large animal models such as sheep8 and canines.38 Cake et al compared three different surgically-induced meniscal injuries in sheep: 1) complete medial meniscectomy, 2) meniscal body transection, and 3) transection of the cranial meniscotibial ligament (cranial pole release). Gross morphology and histological assessment revealed that meniscal injury via the second and third surgical techniques produced significantly different scores compared to the sham group but no significant differences were found between the surgically-induced models. However, there were subtle differences where the lesions were located between the surgical methods.8 In a recent canine study, arthroscopic destabilization of the medial meniscus was performed by making a radial transection at the posterior horn junction with the caudal meniscotibial ligament.38 Four months later the animals exhibited clinical signs of lameness. At six months, gross cartilage assessment revealed articular cartilage damage mainly in the medial compartment and histology scores demonstrated osteoarthritis had progressed in all animals. Surgical advantages of DMM compared to complete meniscectomy are that a smaller incision can be made and a shorter procedure time.
ACL transection in large animals is also used to initiate joint arthrosis in studies of post-traumatic osteoarthritis.5,36 In comparison to ACL transection, the porcine DMM model produced more consistent lesions, both in size and magnitude, located in the central region of the medial tibial plateau. The resulting mean ±standard deviation of the macroscopic scores of the untreated DMM group (PBS injection) was 2.8±0.57 at 26 weeks post-surgery. The location of the lesions produced with our porcine DMM model was in agreement with those following medial meniscal body transection surgery in sheep at 12 weeks.8 In our study, the animals exhibited significant macroscopic and histological cartilage lesions by 26 weeks. In a clinical study, Roemer et al determined that patients who underwent partial meniscectomy showed radiographic signs of osteoarthritis within 1 year follow-up.41
There are several study limitations to consider. A limitation of the DMM model was that the anterior horn was not mechanically repaired after it was cut. Thus, the DMM procedure would continue to promote long-term arthrosis despite early treatment with any short-term therapeutic agent. It should be noted that the rhPRG4 injections were completed within four weeks of surgery (1 injection per week) while joint integrity was assessed after 26 weeks. Therefore, it is not surprising that chondral damage was present in all treatment groups. Despite this limitation, the short-term rhPRG4 injections in this study showed evidence of slowing disease progression.
For the present study, we elected to study a lower molecular weight HA preparation (~950 kDa) in comparison to commercially available viscosupplements (~6,000 kDa). Nonetheless, the HA preparation we used had a molecular weight reported to be useful in animal models19 and was not a cross-linked viscosupplement. Unfortunately, a cross-linked HA would not have readily mixed with the rhPRG4. However, it was not the goal of this study to evaluate the performance of rhPRG4 in comparison to current viscosupplements. We only designed this study to check if the addition of HA would further improve the performance of rhPRG4.33 Thus, an HA only group was not included. Nonetheless, the study results regarding HA could have been different if a higher MW HA preparation was used.
Another limitation is that we did not include a true control group. We elected not to use the contralateral knee since there is evidence that minor cartilage damage occurs in the contralateral knee following surgical introduction of post-traumatic osteoarthritis in the porcine model.36 Due to the prohibitive cost of large animal studies, we utilized the PBS treated group as a positive control.
The relatively small sample size is another limitation to consider. The power analysis determined that we had sufficient power to detect group differences corresponding to relatively large effect sizes. Nonetheless, we were able to detect differences between the rhPRG4 and the sham (PBS) group.
Additional limitations are that the secondary analyses of synovial fluids between groups were of a lower sample size as we were unable to collect samples from all animals. This smaller sample size could potentially bias the results and/or limit the power of the synovial fluid analyses (i.e., PRG4 and IL-1β). The values of the PRG4 concentration reported may not be fully accurate because purified rhPRG4 was used as a standard in ELISA due to a lack of purified porcine PRG4, which was not available. However, the relative comparison of PRG4 levels across the three treatment groups indicates that rhPRG4 may have restored native PRG4 expression. A measure of lameness was not included in this study, which could have provided insight into changes in joint function following treatment. Finally, another limitation in extrapolating our results to humans is that the pig is a quadruped. Nonetheless, there is evidence of anatomical and biomechanical similarities of the knee joint between pigs and humans.29,39
In conclusion, the study demonstrated that three weekly injections of rhPRG4 immediately following an acute joint injury attenuate cartilage damage in an in vivo pre-clinical model. Intra-articular injections of rhPRG4 decreased macroscopic cartilage damage in the medial tibial compartment, lowered a urinary biomarker of Type II collagen breakdown (but not serum), and reduced the concentration of IL-1β in the synovial fluid and serum. Although rhPRG4+HA treated groups, which utilized a HA formulation of ~950 kDa, exhibited low levels of IL-1β, this combination did not reduce cartilage damage compared to the positive control (PBS injection). Thus, rhPRG4 may provide a potential supplementation therapy to reduce post-traumatic osteoarthritis progression and warrants further study in an effort to translate the therapy to humans.
Supplementary Material
ACKNOWLEDGEMENTS
The project was funded by the National Institutes of Health [R42-AR057276 (STTR); R01-AR056834; P20-GM104937 (COBRE Bioengineering Core); T32-AR055885], the Department of Defense [PR110746] and the Lucy Lippitt Endowed Professorship. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or DOD.
The authors would like to thank Matthew Akelman and Katherine Larson for providing assisting with procedures and data collection, Naga Padmini Karamchedu for her help with histology, and Mark Hurtig DVM (University of Guelph) for teaching us the surgical approach.
Dr. Schmidt has received consulting fees from Lubris BioPharma, owns stock or stock options in Lubris Biopharma, and holds patents related to the use of recombinant human proteoglycan 4 (rhPRG4). Dr. Jay owns stock or stock options in Lubris BioPharma and holds patents related to the use of rhPRG4. Lubris BioPharma provided the rhPRG4 for use in the study.
REFERENCES
- 1.Abubacker S, Dorosz SG, Ponjevic D, Jay GD, Matyas JR, Schmidt TA. Full-Length Recombinant Human Proteoglycan 4 Interacts with Hyaluronan to Provide Cartilage Boundary Lubrication. Ann Biomed Eng. 2016;44:1128–1137. doi: 10.1007/s10439-015-1390-8. [DOI] [PubMed] [Google Scholar]
- 2.Ai M, Cui Y, Sy MS, et al. Anti-lubricin monoclonal antibodies created using lubricin-knockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PLoS One. 2015;10:e0116237. doi: 10.1371/journal.pone.0116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sharif A, Jamal M, Zhang LX, et al. Lubricin/Proteoglycan 4 Binding to CD44 Receptor: A Mechanism of the Suppression of Proinflammatory Cytokine-Induced Synoviocyte Proliferation by Lubricin. Arthritis Rheumatol. 2015;67:1503–1513. doi: 10.1002/art.39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alquraini A, Garguilo S, D'Souza G, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. doi: 10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atarod M, Ludwig TE, Frank CB, Schmidt TA, Shrive NG. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthritis Cartilage. 2015;23:640–647. doi: 10.1016/j.joca.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Barton KI, Ludwig TE, Achari Y, Shrive NG, Frank CB, Schmidt TA. Characterization of proteoglycan 4 and hyaluronan composition and lubrication function of ovine synovial fluid following knee surgery. J Orthop Res. 2013;31:1549–1554. doi: 10.1002/jor.22399. [DOI] [PubMed] [Google Scholar]
- 7.Bonnevie ED, Galesso D, Secchieri C, Cohen I, Bonassar LJ. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS One. 2015;10:e0143415. doi: 10.1371/journal.pone.0143415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cake MA, Read RA, Corfield G, et al. Comparison of gait and pathology outcomes of three meniscal procedures for induction of knee osteoarthritis in sheep. Osteoarthritis Cartilage. 2013;21:226–236. doi: 10.1016/j.joca.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58:1707–1715. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 11.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1632–1633. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 12.Elsaid KA, Zhang L, Shaman Z, Patel C, Schmidt TA, Jay GD. The impact of early intra articular administration of interleukin-1 receptor antagonist on lubricin metabolism and cartilage degeneration in an anterior cruciate ligament transection model. Osteoarthritis Cartilage. 2015;23:114–121. doi: 10.1016/j.joca.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsaid KA, Zhang L, Waller K, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin's effect in exercised joints following ACL transection. Osteoarthritis Cartilage. 2012;20:940–948. doi: 10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Englund M, Roos EM, Roos HP, Lohmander LS. Patient-relevant outcomes fourteen years after meniscectomy: Influence of type of meniscal tear and size of resection. Rheumatol. 2001;40:631–639. doi: 10.1093/rheumatology/40.6.631. [DOI] [PubMed] [Google Scholar]
- 15.Flannery CR, Zollner R, Corcoran C, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60:840–847. doi: 10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 16.Fox AJ, Wanivenhaus F, Burge AJ, Warren RF, Rodeo SA. The human meniscus: a review of anatomy, function, injury, and advances in treatment. Clin Anat. 2015;28:269–287. doi: 10.1002/ca.22456. [DOI] [PubMed] [Google Scholar]
- 17.Freutel M, Seitz AM, Ignatius A, Durselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23:74–82. doi: 10.1007/s00167-014-2951-3. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher B, Tjoumakaris FP, Harwood MI, Good RP, Ciccotti MG, Freedman KB. Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med. 2015;43:734–744. doi: 10.1177/0363546514533777. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. doi: 10.1053/sarh.2002.33720. [DOI] [PubMed] [Google Scholar]
- 20.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Greene GW, Banquy X, Lee DW, Lowrey DD, Yu J, Israelachvili JN. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc Natl Acad Sci U S A. 2011;108:5255–5259. doi: 10.1073/pnas.1101002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iijima H, Aoyama T, Ito A, et al. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthritis Cartilage. 2015;23:1563–1574. doi: 10.1016/j.joca.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10:785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 24.Jay GD, Elsaid KA, Kelly KA, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64:1162–1171. doi: 10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jay GD, Elsaid KA, Zack J, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–564. [PubMed] [Google Scholar]
- 26.Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62:2382–2391. doi: 10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jay GD, Harris DA, Cha CJ. Boundary lubrication by lubricin is mediated by O-linked beta(1-3)Gal-GalNAc oligosaccharides. Glycoconjugate J. 2001;18:807–815. doi: 10.1023/a:1021159619373. [DOI] [PubMed] [Google Scholar]
- 28.Karamchedu NP, Tofte JN, Waller KA, Zhang LX, Patel TK, Jay GD. Superficial zone cellularity is deficient in mice lacking lubricin: a stereoscopic analysis. Arthritis Res Ther. 2016;18:64. doi: 10.1186/s13075-016-0967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiapour AM, Shalvoy MR, Murray MM, Fleming BC. Validation of porcine knee as a sex-specific model to study human anterior cruciate ligament disorders. Clin Orthop Relat Res. 2015;473:639–699. doi: 10.1007/s11999-014-3974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwiecinski JJ, Dorosz SG, Ludwig TE, Abubacker S, Cowman MK, Schmidt TA. The effect of molecular weight on hyaluronan's cartilage boundary lubricating ability - alone and in combination with proteoglycan 4. Osteoarthritis Cartilage. 2011;19:1356–1362. doi: 10.1016/j.joca.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Little CB, Smith MM, Cake MA, Read RA, Murphy MJ, Barry FP. The OARSI histopathology initiative -recommendations for histological assessments of osteoarthritis in sheep and goats. Osteoarthritis Cartilage. 2010;18(Suppl 3):S80–92. doi: 10.1016/j.joca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Losina E, Daigle ME, Suter LG, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage. 2013;21:655–667. doi: 10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ludwig TE, Cowman MK, Jay GD, Schmidt TA. Effects of concentration and structure on proteoglycan 4 rheology and interaction with hyaluronan. Biorheology. 2014;51:409–422. doi: 10.3233/BIR-14037. [DOI] [PubMed] [Google Scholar]
- 34.Majewski M, Stoll R, Widmer H, Muller W, Friederich NF. Midterm and long-term results after arthroscopic suture repair of isolated, longitudinal, vertical meniscal tears in stable knees. Am J Sports Med. 2006;34:1072–1076. doi: 10.1177/0363546505284236. [DOI] [PubMed] [Google Scholar]
- 35.Moore EE, Bendele AM, Thompson DL, et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage. 2005;13:623–631. doi: 10.1016/j.joca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musumeci G, Loreto C, Carnazza ML, Cardile V, Leonardi R. Acute injury affects lubricin expression in knee menisci: an immunohistochemical study. Ann Anat. 2013;195:151–158. doi: 10.1016/j.aanat.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Pashuck TD, Kuroki K, Cook CR, Stoker AM, Cook JL. Hyaluronic acid versus Saline intra-articular injections for amelioration of chronic knee osteoarthritis: A canine model. J Orthop Res. 2016;34:1772–1779. doi: 10.1002/jor.23191. [DOI] [PubMed] [Google Scholar]
- 39.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:469–476. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proffen BL, Perrone G, Roberts G, Murray MM. Bridge-enhanced ACL repair: A review of the science and the pathway through FDA investigational device approval. Ann Biomed Engin. 2015;43:805–818. doi: 10.1007/s10439-015-1257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roemer FW, Kwoh CK, Hannon MJ, et al. Partial meniscectomy is associated with increased risk of incident radiographic osteoarthritis and worsening cartilage damage in the following year. Eur Radiol. (27) 2016 Aug; doi: 10.1007/s00330-016-4361-z. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan MZ, Erez A, Guse K, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5:176ra134. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samsom ML, Morrison S, Masala N, et al. Characterization of full-length recombinant human Proteoglycan 4 as an ocular surface boundary lubricant. Exp Eye Res. 2014;127:14–19. doi: 10.1016/j.exer.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Schinhan M, Gruber M, Vavken P, et al. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2011;30:214–220. doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 45.Smith G, Jr., Myers SL, Brandt KD, Mickler EA, Albrecht ME. Effect of intraarticular hyaluronan injection on vertical ground reaction force and progression of osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 2005;32:325–334. [PubMed] [Google Scholar]
- 46.Stein T, Mehling AP, Welsch F, von Eisenhart-Rothe R, Jager A. Long-term outcome after arthroscopic meniscal repair versus arthroscopic partial meniscectomy for traumatic meniscal tears. Am J Sports Med. 2010;38:1542–1548. doi: 10.1177/0363546510364052. [DOI] [PubMed] [Google Scholar]
- 47.Teeple E, Elsaid KA, Jay GD, et al. Effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of posttraumatic arthritis in the anterior cruciate ligament-deficient rat knee. Am J Sports Med. 2011;39:164–172. doi: 10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal Models of Osteoarthritis: Challenges of Model Selection and Analysis. AAPS J. 2013;15:438–446. doi: 10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Hall S, Hanna F, et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord. 2011;12:195. doi: 10.1186/1471-2474-12-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M, Mani SB, He Y, Hall A, Xu L, Li Y. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine post-traumatic osteoarthritis. J Clin Invest. 2016;126:2893–2902. doi: 10.1172/JCI83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.