Abstract
Objective
To inform the first-line treatment choice between cognitive behavior therapy (CBT) or an antidepressant medication for treatment-naïve adults with major depressive disorder by defining a neuroimaging biomarker that differentially identifies the outcomes of remission and treatment failure to these interventions.
Method
Functional magnetic resonance imaging resting state functional connectivity analyses using a bilateral subcallosal cingulate cortex (SCC) seed was applied to 122 patients from the Prediction of Remission to Individual and Combined Treatments (PReDICT) study who completed 12 weeks of randomized treatment with CBT or antidepressant medication. Of the 122, 58 achieved remission (Hamilton Depression Rating Scale, HDRS) ≤7 at weeks 10 and 12); 24 were treatment failures (HDRS <30% decrease from baseline). A 2×2 ANOVA using voxel-wise subsampling permutation tests compared the interaction of treatment and outcome. ROC curves constructed using brain connectivity measures were used to determine possible classification rates for differential treatment outcomes.
Results
The resting state functional connectivity of three regions with the SCC was differentially associated with outcomes of remission and treatment failure to CBT and antidepressant medication, and survived application of the subsample permutation tests: left anterior ventrolateral/insula prefrontal cortex, dorsal midbrain, and left ventromedial prefrontal cortex. Using the summed SCC functional connectivity scores for these three regions, we demonstrated overall classification rates of 72–78% for remission and 75–89% for treatment failure. Positive summed functional connectivity was associated with remission with CBT and treatment failure with medication, whereas negative summed functional connectivity scores were associated with remission to medication and treatment failure with CBT.
Conclusions
Imaging-based depression subtypes defined using resting state functional connectivity differentially identified an individual’s probability of remission or treatment failure with first-line treatment options for major depression. This biomarker should be explored in future research through prospective testing and as a component of multivariate treatment prediction models.
INTRODUCTION
The syndrome of major depressive disorder, a highly heterogeneous clinical condition, has largely defied meaningful subtyping (1). First-line treatments for major depression include an evidence-based psychotherapy, such as cognitive behavior therapy (CBT), or antidepressant medication (2). Both treatments have roughly equivalent efficacy, on average, for outpatients with major depression, though the remission rates of 30–40% with either treatment alone are low (2). Combination treatment with psychotherapy and antidepressant medication improves remission rates, but barriers such as cost, time, and patient preference preclude this option for many patients (3). Importantly, some patients who do not respond to one treatment intervention exhibit an excellent response when switched to the alternative (4,5). This observation strongly suggests that biological or psychological variability may be identified and, thereby, improve the precision of treatment selection for individual depressed patients (6).
Despite extensive efforts, work to identify clinical predictors of outcomes to treatments in non-psychotic major depression has been disappointing. Depressive symptom severity has received the most attention among the clinical predictors but the largest patient-level meta-analyses found no difference in outcome among patients treated with CBT or antidepressant medication based on severity (7) or depressive clinical subtype (8). The consistent failure of clinical features meaningfully to inform treatment selection serves as an impetus to identify biomarkers predictive of treatment outcomes (9,10). Unfortunately, genetic testing, neuroimaging, and psychophysiological approaches, though promising, have not yet proven sufficiently accurate or replicable to warrant clinical application to individual patients (11).
Neuroimaging using positron emission tomography (PET) or functional magnetic resonance imaging (fMRI) has been used extensively to characterize brain states of depressed patients. Among patients with major depression compared to healthy controls, relative hyperactivity of limbic brain regions, including the amygdala, insula, and subcallosal cingulate cortex (SCC) are among the most consistently reported; hypoactivity in the dorsolateral prefrontal cortex is another replicated finding (12). However, average differences between groups may mask important heterogeneity between individuals (13), with some patients failing to show these changes, or even demonstrating opposite patterns (e.g., increased metabolism in the dorsolateral prefrontal cortex) (14). This variability in brain states across patients likely has important implications for treatment responsiveness.
Recent reviews have found inconsistent results for potential neuroimaging predictors of treatment outcomes in major depression, (15,16). Many factors contribute to this inconsistency, including differences in imaging modality and analytic approaches, patient sample characteristics, treatment type and duration, and treatment outcome definitions (11). Most studies of neuroimaging moderators of outcome have employed a single type of treatment, with testing for baseline imaging differences between patients who did and did not respond to the intervention.
Several studies have reported that fMRI activity patterns can predict outcomes to CBT (17–19). In contrast, studies using medication treatments have identified different patterns of neural activity and connectivity associated with acute treatment outcomes (20–23). These findings suggest that brain states may differ between patients benefiting from one treatment modality versus an alternative. Without an active comparison treatment group, however, these studies could not conclude whether the identified imaging biomarkers moderated outcomes specifically for the treatment studied, or simply predicted outcomes across all potential treatments (i.e., a non-specific predictor). Consequently, extant biomarker studies employing single forms of treatment can inform response signatures, but they are unable to achieve the precision medicine goal of selecting the optimal type of treatment for a given individual.
Optimal application of precision medicine in depression should involve the prediction of both the desired outcome, remission, and of the most undesired outcome, treatment failure. Remission is the goal of treatment because long-term wellness and overall functioning are greater among patients who fully remit from treatment compared to those who respond to a lesser degree or show no response (2). However, avoiding treatment failure is also a vitally important outcome (24). Because treatment efficacy can only be known after 6–12 weeks of treatment, application of an ineffective treatment prolongs patient suffering and role dysfunction, potentially increasing feelings of hopelessness and interpersonal strife, with persistence of suicidal ideation. These severe consequences from choosing the “wrong” treatment for a patient underscore the need for biomarkers predictive of both remission and treatment failure (25). Furthermore, because combination treatment with both psychotherapy and medication for major depression is often not required or not feasible, selection of the initial treatment is typically a forced choice between psychotherapy and medication (5). An optimal biomarker could identify whether failure to improve with one treatment modality could simultaneously predict improvement with the alternative modality.
Recently, resting state metabolic activity assessed by 18F-fluorodeoxyglucose PET found six brain regions that were differentially associated with the outcomes of remission and treatment failure among major depression patients randomized to treatment with CBT or escitalopram. Metabolic activity in the right anterior insula emerged as the best candidate for use as a treatment selection biomarker (26), with support provided by the finding that, among non-remitters to monotherapy, the same biomarker predicted eventual remission after addition of the alternative treatment (27). Due to the cost and radiation exposure involved in PET imaging, more readily available and less expensive fMRI methods have appeal as an alternative approach for assessing regional brain activity. Resting state functional connectivity is an fMRI technique that measures the degree to which separate brain regions demonstrate temporal correlations in the low-frequency components of the blood-oxygen-level dependent (BOLD) signal. The resting state functional connectivity signal has identified brain networks involved in several aspects of mental functioning in healthy subjects, and resting state functional connectivity in these networks differed between healthy controls and major depression patients in several studies (28).
Of the many important frontal and limbic regions identified using fMRI studies of major depression, activity in the subcallosal cingulate cortex (SCC) has consistently emerged as a core component of major depression pathophysiology (29). The SCC is an extensively connected component of the limbic system that modulates emotional behavior and is particularly involved in feelings of sadness (30,31). Greater functional connectivity between the SCC and the default mode network is present in patients with treatment resistant depression (32). Elevated pre-treatment SCC metabolism has been associated with poorer outcomes to treatment with antidepressant medication (33–35), CBT (17,35), and the combination of antidepressant medication and CBT (36). Finally, deep brain stimulation to the SCC and its cortical and subcortical connections may be efficacious in highly treatment-resistant patients (37,38).
The aim of the present study was to identify resting state functional connectivity differential predictors of outcomes among adults with treatment-naïve major depression randomly assigned to receive 12 weeks of treatment with either CBT or an antidepressant medication. Based on prior work (26,27,36), we hypothesized that pre-treatment levels of SCC resting state functional connectivity to other cortical and limbic regions would differentially predict the clinical outcomes of the CBT and antidepressant medication treatments. To minimize any dilution of the biomarker signal by patients with ambiguous outcomes, we made the a priori decision to analyze the imaging data using the clearly defined outcomes of remission and treatment failure (defined as a <30% improvement from baseline), in the same manner as our previous work using PET imaging in depressed patients (26).
METHODS
Studies
The design of the Emory PReDICT study has been published previously (39), and the clinical results of the trial are published elsewhere (10). The overarching goal of PReDICT was to identify clinical and biological moderators of outcomes to CBT and antidepressant medication. The study was conducted through the Mood and Anxiety Disorders Program at Emory University, including a purely Spanish-language location at Grady Hospital. The Emory Institutional Review Board and the Grady Hospital Research Oversight Committee approved the study. All patients provided written informed consent prior to beginning study procedures.
Patients
Adults aged 18–65 years were eligible to participate if they met DSM-IV criteria for a primary current diagnosis of non-psychotic major depression as assessed by the Structured Clinical Interview for DSM-IV (40) and a psychiatrist’s evaluation, and if they scored ≥18 on the 17-item Hamilton Depression Rating Scale (HDRS) (41). Additionally, patients were required to be treatment naïve, defined as having never previously received a minimally adequate course of treatment with an antidepressant medication or evidence-based psychotherapy for a mood disorder. Exclusion criteria included a lifetime history of bipolar disorder, primary psychotic disorder, or dementia, or meeting DSM-IV criteria for any of the following in the past 12 months: obsessive compulsive disorder, eating disorder, substance dependence (except for nicotine and caffeine), or dissociative disorder. Meeting DSM-IV criteria for substance abuse within 3 months, or a positive urine test for drugs of abuse at the screening visit were also exclusionary. Pregnant or breast-feeding women and patients with a medical condition that could interfere with the study or the interpretation of the study results were excluded.
Randomization and Treatment
Patients scoring ≥15 on the HDRS at the baseline visit were randomly assigned 1:1:1 to 12 weeks of treatment with one of three treatments: 1) a selective serotonin reuptake inhibitor (SSRI), escitalopram, 10–20 mg/d; 2) a serotonin norepinephrine reuptake inhibitor (SNRI), duloxetine, 30–60 mg/d; or 3) CBT, 16 individual 50-minute sessions. The medications were dispensed in a double-blind manner in compounded purple capsules and were dosed flexibly based on patient tolerability and response. CBT was delivered in accord with Beck and colleagues’ manual (42). Symptom severity using the HDRS, Hamilton Anxiety Rating Scale (43), and Beck Depression Inventory (44) was assessed weekly by blinded raters for the first 6 weeks after randomization and then every other week until week 12. In addition, the Childhood Trauma Questionnaire (45) was completed prior to randomization. Patients were not permitted to use benzodiazepines, antipsychotics, anxiolytics, chronic opiates, or any other psychoactive medication, with the exception of hypnotics up to three times per week, though not on the night before MRI scans or ratings assessments. PReDICT also included a second treatment phase for non-remitters. Patients failing to remit after 12 weeks with single modality treatment were offered combination treatment for 12 more weeks, in which CBT was added to medication non-remitters and escitalopram was added to CBT non-remitters (39).
Clinical Outcomes
Remission was defined as HDRS score ≤7 at both weeks 10 and 12. Treatment failure was defined as <30% reduction from baseline HDRS score at week 12 (26). Response without remission was defined as non-remitters with a week 12 HDRS score ≥50% reduction from baseline, and Partial Response was defined as a week 12 HDRS score with 30–49% reduction from baseline.
MRI Data Acquisition and Preprocessing
After screening and during the week prior to randomization, resting state fMRI scanning was performed with patients’ eyes closed for 7.4 minutes in a 3 Tesla Siemens TIM Trio (Siemens Medical Systems, Erlangen, Germany). The anatomical data were acquired using Siemens’ magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence: (TR/TI/TE = 2600/900/3.02 ms; flip angle 8°, voxel resolution = 1×1×1 mm; number of slices = 176; matrix = 224×256). Resting state fMRI data were acquired using a Z-SAGA sequence (46) to recover areas affected by susceptibility artifact, with the following parameters: 150 measurements; 30 axial slices; voxel resolution=3.4*3.4*4mm; matrix=64*64, TR/TE=2950/30ms. Echo planar images were corrected for motion and slice-time acquisition and smoothed using an isotropic Gaussian kernel of 8 mm FWHM. Scans with head motion > 2mm in any direction were removed from the analysis. Mean head motion of included subjects, assessed by framewise displacement (47), did not significantly differ between the treatment outcome groups. Scans were corrected for motion with rigid body registration to the first volume using AFNI’s 3dvolreg (48). Motion parameters, eroded white matter, and cerebral spinal fluid nuisance regressors were removed and the data were simultaneously band pass filtered at 0.01 to 0.1 Hz. In scans meeting these criteria the imaging anatomical and functional datasets were co-registered (with visual confirmation) and normalized to standard Montreal Neurological Institute (MNI) 1 mm voxel space.
FC Analysis
Image analysis was conducted using AFNI (48). A region of interest seed-based approach was used to assess the resting state functional connectivity of the SCC. The SCC volume was defined using the Harvard-Oxford atlas (49), and the SCC was thresholded at 50% probability centered on MNI coordinates ±6, 24, −11 consistent with seeds used in our other studies of SCC (38,50). The seeds comprised two 5mm radius spheres with a final volume of 485 microliters each. Utilizing 3dNetCorr (51), the mean time course of the bilateral seed was correlated voxel-wise with the rest of the brain. The voxel-wise correlation coefficients were then z-scored by calculating the inverse hyperbolic tangent yielding the seed-based resting state functional connectivity maps for analysis.
Group Analysis
Because a specific response effect was targeted (i.e., one that would allow choice between treatments) intermediate responders (i.e., those with a change in HDRS score ≥30% but not achieving remission) were not included in the voxel-level analysis. More specifically, the extremes of the response distribution were used to screen for a particular pattern of interaction (remission to one treatment but treatment failure with the other) at the voxel level (26). Using all completers within each treatment group, the results were then verified by comparing the correlation between the percent change in HDRS score from baseline to week 12 and the functional connectivity of the three regions identified from the voxel-level analysis.
The rationale for combining the medication arms was supported by the results of an ex-vivo assay, which found that both medications blocked between 60–70% of the serotonin transporters in the PReDICT patients (52); this indicated that the medications shared a primary mechanism of action. In addition, the separate contrasts between escitalopram vs CBT and duloxetine vs CBT identified the same regions as the combined medication vs CBT contrast (see Results). Contrasts were performed using a whole brain voxel-wise 2×2 ANOVA (3dMVM) (53) with treatment (medication or CBT) and 12-week outcomes (remission or treatment failure). This approach generated four comparisons of interest for determining the predictive value of each SCC FC brain region: remission versus treatment failure within each treatment (medication or CBT), and the medication versus CBT treatments within each outcome group (remission or treatment failure). In order to calculate the effect sizes of the group differences (54), post hoc evaluations of each region functionally connected to the SCC identified from the ANOVA were conducted; this allowed evaluation of the potential value of each region as a biomarker of treatment outcomes. To avoid excluding small regions with potentially relevant functional connectivity to the SCC, all identified clusters exceeding a minimum threshold of 300 voxels were evaluated.
To evaluate the robustness of the ANOVA results and reduce the impact of outliers, whole brain, voxel-wise subsampling permutation tests were run with 70% random subsamples. To keep the relative number of patients in each group the same, group assignments of each subsample were proportional to the full cohort groups. The voxel-wise ANOVA was repeated 1000 times with the 70% subsamples. The resulting F-maps for each ANOVA utilized an alpha threshold of p<0.005, and a beta of 0.80 was selected to retain voxels with at least 80% power. As discussed in the Results, this analysis identified three brain regions with significant resting state functional connectivity with the SCC. Subsequently, regions were extracted for each subject and used for post-hoc evaluation of the possible predictive validity of the imaging markers. Because the three regions were highly correlated, we also tested the internal predictive value of the summed connectivity score biomarker, calculated by adding the individual SCC resting state functional connectivity z-scores for each of the three identified regions identified from the ANOVA results displayed in Figure 1.
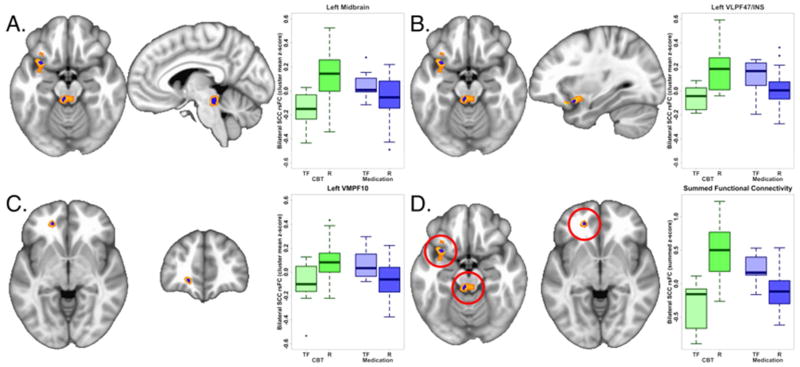
Figure 1. Differential functional connectivity of subcallosal cingulate cortex between remitters and treatment failures with antidepressant medication or cognitive behavior therapy.
A–C: Representative brain region and box plot of the z-score of the resting state functional connectivity with subcallosal cingulate cortex (SCC) between remitters (R) and treatment failures (TF) with each treatment type. The voxels identified by the subsample permutation testing (blue) are shown superimposed over voxels identified by the original ANOVA (yellow scale, see Methods). Box plots reflect contrasts using the permuted data. In all regions, the functional connectivity with the SCC seed is positive in CBT remitters and anti-correlated in CBT-treatment failures, whereas the inverse is true for antidepressant medication remitters and treatment failures. A, Dorsal midbrain, B, Ventrolateral prefrontal cortex BA 47/Insula (VLPF47/INS), C, Ventromedial prefrontal cortex BA10 (VMPF10). D: Box plots of the z-scores of the sum of the functional connectivity of the SCC with the three regions. The treatment by response interaction was significant at p=5e-10.
CBT: Cognitive behavior therapy.
Subject-level Predictive ability (post-hoc)
The precision medicine goal of the subject-level fMRI evaluation was to examine the possible predictive value of each region as well as the summed functional connectivity z-score. For each of these measures, we used receiver operating characteristic (ROC) curves to examine the sensitivity and specificity of using various levels of connectivity to dichotomize the entire sample (N=122) of patients into outcome groups. In each case, we looked at the classification rates for both remission (compared to non-remission), as well as treatment failure (compared to response of any kind) in order to illustrate the use of the imaging measures to identify the key clinical targets. To determine the level of connectivity that resulted in the highest combination of sensitivity and specificity we chose the maximum Youden index (55). Notably, these evaluations of predictive ability are post-hoc and thus reflect an examination of the classification capabilities of the identified regions in this sample; they are not independent validations.
RESULTS
Demographic and Clinical Measures
Of the 234 per-protocol completers, 122 had MRIs of adequate quality for analysis. The majority of patients in both treatment groups were women (CBT: 20/37, 54.1%; medication: 45/85, 52.9%) and experiencing their first major depressive episode (CBT: 21/37, 56.8%; medication: 55.3%). Table 1 presents additional clinical and demographic characteristics of the sample. Of the 122 patients, 82 had clear clinical outcomes: 58 achieved remission (CBT:17, medication:41) and 24 experienced treatment failure (CBT:10, medication:14); forty patients had intermediate outcomes (CBT:10, medication: 30). The mean percent change at week 12 did not significantly differ between treatments (CBT: 50.9±39.6%, medication: 60.7±28.0%, p=.178).
Table 1.
Baseline clinical and demographic characteristics (N=122)
| Medication-Treated (n=85) | CBT-Treated (n=37) | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | p | |
| Age | 38.7 | 11.0 | 38.5 | 10.6 | .912 |
| Length of current episode (wks) | 129.0 | 239.3 | 79.9 | 84.6 | .100 |
| HDRS baseline | 19.0 | 3.1 | 17.9 | 2.4 | .028 |
| BDI total baseline | 22.6 | 6.3 | 20.2 | 6.6 | .078 |
| HAMA total baseline | 15.4 | 4.6 | 14.5 | 3.9 | .272 |
| CTQ total | 46.0 | 15.7 | 43.2 | 12.6 | .291 |
BDI, Beck Depression Inventory; CBT, Cognitive Behavior Therapy; CTQ, Childhood Trauma Questionnaire; HAMA, Hamilton Anxiety Rating Scale; HDRS, Hamilton Depression Rating Scale.
Treatment x Outcome ANOVA
Table 2 shows the effect sizes (Cohen’s d) of the mean of all voxels in each region identified in the primary ANOVA; they are ordered by cluster size and the overall marginal effect size. Differential outcomes to antidepressant medication or CBT were associated with SCC resting state functional connectivity with 6 regions: (1) left dorsal midbrain (appearing to include areas of the periaqueductal grey and dorsal raphe), (2) left frontal operculum (incorporating Brodmann Area (BA) 47 of the ventrolateral prefrontal cortex and parts of the anterior insula (VLPF47/INS), (3) right posterior cingulate (BA 7), (4) cerebellar vermis, (5) right superior frontal pole (BA 10), and (6) left ventromedial prefrontal cortex (BA 10) (VMPF10).
Table 2.
Cluster maxima differentiating remitters and treatment failures by treatment type
| Region | BA | MNI Coordinates, Peak | Cluster Size (1 mm Voxels) | Remitters -- Treatment Failures with CBT | Remitters -- Treatment Failures with Medication | CBT – Medication in Treatment Failures | CBT -- Medication in Remitters | Average Marginal ES | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | Side | |||||||||
| Original ANOVA | ||||||||||||
|
| ||||||||||||
| Dorsal midbrain | -- | −5 | −32 | −17 | L | 1383 | p | < 0.001 | 0.371 | 0.051 | < 0.001 | |
| ES | 1.45 | 0.57 | 1.29 | 1.13 | 1.11 | |||||||
|
| ||||||||||||
| VLPF47/INS | 47 | −31 | 12 | −17 | L | 1041 | p | < 0.001 | 0.143 | 0.06 | < 0.001 | |
| ES | 1.58 | 0.75 | 1.16 | 1.45 | 1.24 | |||||||
|
| ||||||||||||
| Posterior Cingulate | 7 | 18 | −47 | 37 | R | 603 | p | < 0.001 | 0.649 | 0.016 | 0.044 | |
| ES | 1.79 | 0.35 | 1.12 | 0.82 | 1.02 | |||||||
|
| ||||||||||||
| Cerebellar vermis | -- | 1 | −53 | −3 | -- | 477 | p | 0.009 | 0.634 | 0.044 | 0.228 | |
| ES | 1.34 | 0.36 | 1.20 | 0.54 | 0.86 | |||||||
|
| ||||||||||||
| Superior frontal pole | 10 | 12 | 65 | 20 | R | 357 | p | 0.084 | 0.010 | 0.003 | 0.366 | |
| ES | 1.03 | 0.96 | 1.41 | 0.48 | 0.86 | |||||||
|
| ||||||||||||
| VMPF10 | 10 | −19 | 44 | −5 | L | 342 | p | 0.005 | 0.024 | 0.033 | 0.001 | |
| ES | 1.06 | 1.09 | 1.09 | 1.14 | 1.10 | |||||||
|
| ||||||||||||
| Permuted Regions | ||||||||||||
|
| ||||||||||||
| Dorsal midbrain | -- | −5 | −32 | −17 | L | 123 | p | < 0.001 | 0.192 | 0.015 | < 0.001 | |
| ES | 1.54 | 0.70 | 1.71 | 1.11 | 1.26 | |||||||
|
| ||||||||||||
| VLPF47/INS | 47 | −31 | 12 | −17 | L | 77 | p | < 0.001 | 0.119 | 0.064 | < 0.001 | |
| ES | 1.49 | 0.75 | 1.13 | 1.30 | 1.17 | |||||||
|
| ||||||||||||
| VMPF10 | 10 | −18 | 44 | −5 | L | 42 | P | 0.003 | 0.021 | 0.022 | 0.001 | |
| ES | 1.15 | 1.06 | 1.14 | 1.16 | 1.13 | |||||||
|
| ||||||||||||
| Sum of Permuted Regions | p | < 0.001 | 0.003 | < 0.001 | < 0.001 | |||||||
| ES | 1.89 | 1.36 | 1.95 | 1.77 | 1.74 | |||||||
BA, Brodmann Area; CBT, Cognitive behavior therapy; ES: Effect size; VLPF47/INS, Ventrolateral prefrontal cortex Brodmann Area 47/Insula; MNI: Montreal Neurological Institute; VMPF10, Ventromedial prefrontal cortex, Brodmann Area 10
Non-permuted regions were thresholded at p<.005.
After application of the subsampling permutation testing, only three of the six regions retained significance: left midbrain, left VLPF47/INS and left VMPF10 (Table 2). The three regions (BA 7, cerebellar vermis, superior frontal pole) that failed to survive the permutation testing likely represent effects that were due to a small number of subjects, and thus are less generalizable. Figure 1A–C shows the three regions identified by the permutation testing superimposed over the regions identified from the original primary ANOVA. The peak voxels for each of the three identified regions are exactly the same in the primary and permutated analyses (Table 2). As shown by the boxplots of the permuted data for each region (Figure 1A–C), greater positive functional connectivity with the SCC was associated with remission to CBT and treatment failure with medication, with absent or negative connectivity associated with the opposite pattern of outcomes for the two treatments. The box plots for the permuted summed functional connectivity of the three regions and the four outcomes are shown in Figure 1D.
Individual Medications vs CBT
To evaluate whether either drug individually was driving the results, and to test possible effects of sample size imbalance on the results reported in Table 2 and Figure 1, additional separate 2×2 ANOVAs were conducted using escitalopram vs CBT and duloxetine vs CBT for the remission and treatment failure outcomes. Figure S1 demonstrates that the individual drug contrasts map very closely onto the same regions identified in the original ANOVA of the two drugs combined, indicating that the resting state functional connectivity patterns differentiating CBT and medication outcomes are consistent for both the SSRI and SNRI.
Subject Level Prediction of Outcome
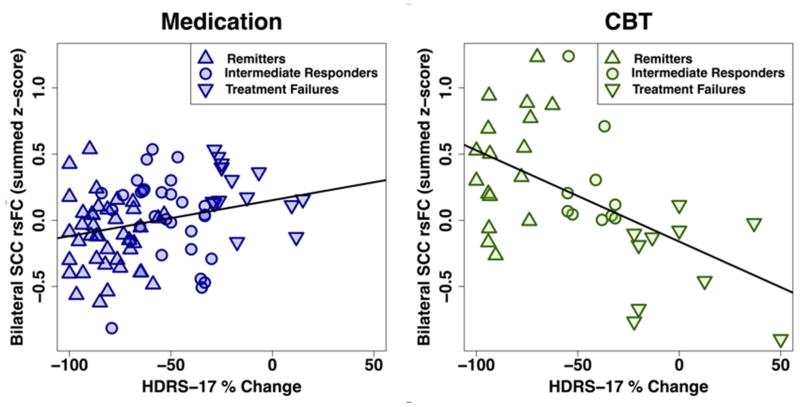
Figure 2 shows the relationship between summed functional connectivity scores and the individual percent HDRS change for all subjects (N=122) by treatment. The Pearson correlations were significant for both treatments, though the strength of the correlation was stronger among CBT-treated patients (r= −0.539, p<.001) than among medication-treated patients (r=0.258, p<.017).
Figure 2. Correlation between percent change in depression severity and the summed functional connectivity of subcallosal cingulate cortex across all patients by treatment type.
Correlation between summed functional connectivity and subjects’ percent change in HDRS score across all 122 patients with analyzable fMRIs. The left panel with blue symbols show the data for the medication-treated patients; the right panel with green symbols shows that for the CBT-treated patients. The correlations between Summed Functional Connectivity Scores and the percent HDRS change were significant for both treatments, though the strength of the correlation was stronger among CBT treated patients (r= −0.539, p<.001) than among medication-treated patients (r=0.258, p<.017). Summed functional connectivity reflects the added scores of the functional connectivity of the cingulate cortex resting state with each of the 3 regions identified in Figure 1. “Intermediate response” subjects had ≥30% improvement in HAMD-17 score, but did not meet criteria for remission.
HDRS-17: 17-item Hamilton Depression Rating Scale
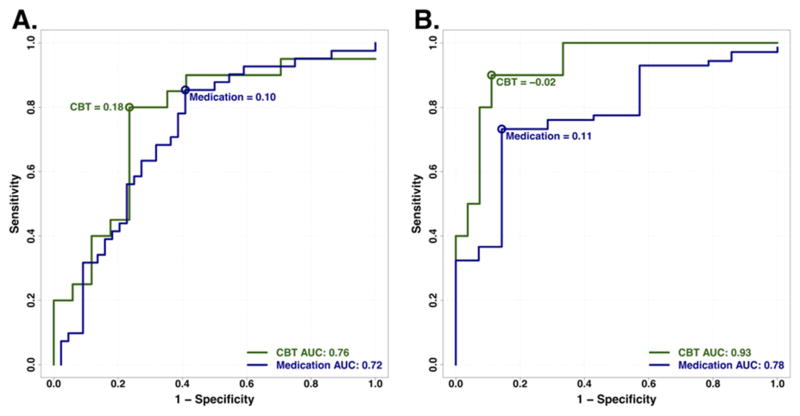
ROC curves were constructed to characterize the overall predictive value of the brain connectivity measures among all subjects (N=122). As expected, all 3 individual region measures had significant predictive value for remission above chance, but the area under the curve was highest for the summed score when compared to the individual regions. Thus, only the summed score was pursued further.
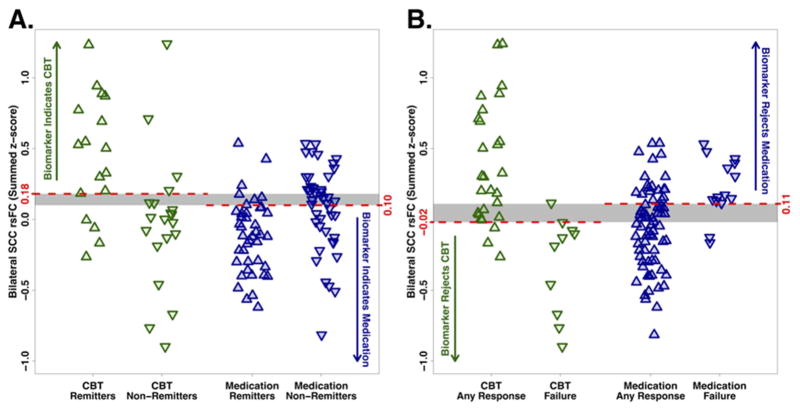
Evaluation of the predictive ability of the summed connectivity score was performed within the two treatment groups. For CBT, the maximum index for remission occurred at a summed connectivity score of approximately 0.18 or higher, and resulted in a classification rate of 78% (Figure 3A). In contrast, the maximum index for treatment failure with CBT was approximately −0.02 or less, which resulted in a higher classification rate of 89% (Figure 3B). For medication, the maximum index for remission occurred at a summed score of 0.10 or lower (classification rate of 72%, Figure 3A), and for treatment failure at 0.11 or higher (classification rate of 75%, Figure 3B). The distribution of the individual subject summed connectivity scores, along with the maximum indexes for remission and treatment failure for each treatment, are displayed in Figure 4A and 4B. Taken together, these results indicate that 1) differential response to CBT is the stronger signal and, 2) positive connectivity in these regions is associated with a recommendation for CBT, while negative connectivity in these regions would suggest medication as the better choice. Given there is some overlap in these cutoffs, it appears that the region where there is very little connectivity in either direction may not provide adequate evidence for a choice.
Figure 3. Receiver operating characteristic curves for classification of remission and treatment failure outcomes with CBT and medication treatment.
ROC curves for A) remission, and B) treatment failure, showing optimal summed functional connectivity values for classifying these respective outcomes for each treatment. Blue color represents medication, green represents CBT.
Figure 4. Individual subjects’ summed functional connectivity scores grouped by treatment outcome.
Strip charts demonstrating individual subjects’ summed functional connectivity scores. Green triangles represent CBT-treated patients; blue triangles represent medication-treated patients. A) Upward-pointing triangles represent remission; downward-pointing triangles represent non-remission. For maximizing remission outcomes, z-scores >0.18 indicate CBT should be selected; z-scores <0.10 indicate medication should be chosen. B) Upward-pointing triangles represent any level of response (partial response, response, or remission); downward-pointing triangles represent treatment failure. To minimize treatment failure, z-scores <−0.02 indicate CBT should be avoided; z-scores >0.11 indicate medication should be avoided.
Among the 122 patients with adequate baseline fMRI data, there were 5 treatment failures with CBT and 7 treatment failures with medication from Phase 1 who completed the Phase 2 combination treatment. Although the numbers are too low for statistical analysis, Figure S2 displays the outcomes of patients who completed the 12-week combination treatment. The figure demonstrates that 50% of the treatment failure patients remitted (defined as an HDRS score ≤7 at both weeks 22 and 24) when the alternative treatment was added; those patients whose summed functional connectivity measure was most strongly categorized as a likely remitter to CBT or to medication were particularly likely to benefit from the second treatment.
Clinical Correlates of Imaging Subgroups
In order to evaluate whether the imaging subtypes were simply reflecting a demographic or clinical characteristic, we conducted exploratory analyses assessing whether any of the characteristics listed in Table 1 were associated with the summed functional connectivity measure. No significant associations were found, indicating that there were no demographic or clinical surrogates of the imaging biomarker.
Non-Specific Imaging Predictors of Response
To identify regions predictive of outcomes regardless of treatment modality, we conducted whole-brain t-tests of SCC resting state functional connectivity contrasting all responders and non-responders regardless of treatment type as well as all remitters versus all treatment failures. Responders showed significantly greater SCC functional connectivity with the right post-central gyrus, and significantly lower functional connectivity with the right superior frontal gyrus. Remitters demonstrated significantly lower SCC functional connectivity with both the right pre-central gyrus and right posterior putamen (Table S1).
DISCUSSION
In this study of previously untreated adults with major depression, outcomes after 12 weeks of treatment with randomly-assigned medication or CBT were associated with the degree of resting state functional connectivity between brain regions involved in mood regulation—specifically the SCC and: 1) the left frontal operculum (incorporating VLPFC, BA47 and anterior insula); 2) the left VMPFC, BA10, and 3) the dorsal midbrain. By examining the summed z-score of the functional connectivity of the SCC with these three regions, it was demonstrated that the summed value, when applied to all individual subjects, provides reasonable measures of internal validity (72–78% for remission; 75–89% for treatment failure), exceeding the value of any clinical measure. Overall, negative connectivity scores were associated with remission to medication and treatment failure with CBT, whereas positive connectivity scores were associated with remission to CBT and treatment failure with medication. These robust findings indicate that neuroimaging may have an important role in the application of precision medicine for depression by identifying neural signatures of brain states that are differentially responsive to treatments with differing mechanisms of action.
Potential clinical applications of the summed functional connectivity z-score biomarker may depend on the clinical status of the patient and the treatment options available. For patients with profound functional impairment or high suicidality, avoidance of treatment failure may be the treatment priority, whereas for other patients the primary goal may be remission. When remission is the goal, a summed connectivity score >0.18, indicates CBT should be used, whereas a score <0.10 suggests medication is indicated. Patients with scores between 0.10–0.18 fall into a grey zone, where the biomarker does not suggest a specific treatment (Figures 3A, 4A). Alternatively, in situations where avoiding treatment failure is the goal, a summed connectivity score of −0.02 or lower indicates CBT should not be the initial treatment. Scores >0.11 suggest medication is not the better choice. Scores between zero and 0.11 do not clearly indicate one treatment would be superior to the alternative (Figure 3B, 4B); treatment selection for these patients may be informed by other markers of likely treatment outcomes. Although these findings are encouraging, attempts at replication of the classification value of these indicators in existing datasets or thorough prospective testing should be undertaken before this imaging-based treatment selection approach is incorporated into routine clinical care.
The current findings are broadly consistent with prior neuroimaging prediction studies in major depression (15). We have previously proposed that psychotherapy-responsive depression may represent a brain state with sufficiently adequate connectivity in mood-regulating systems such that engagement of these systems via psychotherapy can reduce negative emotional states (25). Several studies support the conclusion that, on average, patients with major depression have reduced prefrontal control over emotion-generating limbic structures (29). Greater SCC reactivity (not functional connectivity) to presentations of negative, self-relevant words is associated with poorer outcomes to treatment with CBT (17). Others have found better response to CBT among major depressive disorder patients who were closest to healthy controls in terms of reactivity to emotional stimuli in the ventromedial prefrontal cortex (56), ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex. and dorsal anterior cingulate cortex (57).
VLPFC activity in healthy controls has been linked repeatedly to emotion regulation (58,59) and sustained attention (60). The VLPFC is involved in stimulus selection, and reduced VLPFC activation in response to stimuli is associated with inability to disengage from negative stimuli (61). Impaired emotion regulation is linked to activity in the VLPFC among patients with major depression (62), and rumination is associated with VLPFC activity (63,64) and volume (65). Greater resting state functional connectivity between the SCC and VLPF47 may reflect the availability of this system to be recruited for mood regulation, and therefore ability to respond to CBT.
The operculo-insular cortex (VLPF47/INS region) is important for interoceptive and emotional processing (66,67). In our previous report using PET, resting state metabolic activity in the right anterior insula (with the cluster extending into the frontal operculum) differentially predicted remission and treatment failure with CBT and escitalopram (27,28). Although the prior PET study differed from the current study in its methodology and the amount of prior treatment of the evaluated patients, and although the findings differed by side (right vs left), taken together the studies suggest that abnormal metabolic activity in regions associated with interoception and mood regulation may be an important predictor of outcomes to differing forms of treatment. From a clinical perspective, cases of major depression more associated with signals from the body (“gut feelings”) may be more resistant to pure psychotherapy approaches, whereas major depression that does not involve strong interoceptive experiences may be particularly responsive to CBT (68).
Functional connectivity of frontal brain regions with the midbrain has not emerged in prior fMRI analyses of major depression or its treatment, though an FDG-PET study found lower pre-treatment resting state metabolism in the midbrain did predict remission to standard antidepressant medications (69). The coordinates of the midbrain signal in the present analyses indicated potential involvement of the periaqueductal grey, which is involved in coordinated autonomic and behavioral responses to emotional stimuli. Neuroanatomical analyses of periaqueductal grey connection studies in macaques demonstrated that the subcallosal (BA25) and pregenual (BA32) frontal regions provided the strongest direct input to the dorsolateral column of the periaqueductal grey identified here (70). Further, serotonin transporter concentrations may be elevated in the periaqueductal grey of major depression patients versus healthy controls (71). In healthy controls, periaqueductal grey activity can be modulated by placebo-induced expectation of pain relief, and functional connectivity between SCC and periaqueductal grey is increased during a cold pressor task (72). This ability to regulate the periaqueductal grey may be diminished in the subset of patients with lower periaqueductal grey -SCC functional connectivity.
Sufficient connectivity between the SCC and the midbrain may also reflect the importance of the structural connections between the ventromedial prefrontal cortex and the dorsal raphe (73). The ventromedial prefrontal cortex (incorporating the SCC) is crucial for regulating an organism’s response to both controllable and uncontrollable stressors, mediated in part by its regulation of dorsal raphe activity in response to stress (73,74). Furthermore, in a chronic social defeat model of depression in mice, deep brain stimulation to the ventromedial prefrontal cortex induces neuroplastic changes in the serotonergic neurons in the dorsal raphe (75). Weak or absent connectivity between the SCC and dorsal raphe may reflect a biological inability of a patient to achieve effortful control over stress responses, and thus indicate the need for a direct effect on the serotonergic transporters and autoreceptors of the raphe by antidepressant medication (76).
Several prior studies have implicated dysregulation in the medial portion of BA 10 in patients with major depression (77,78), and the polar components of VMPF10 may be smaller in patients with major depression than healthy controls (79). The ventromedial portion of BA10 in the PFC is an important component of the default mode network (80) and is extensively connected to the SCC (via the fronto-medial extent of the uncinate fasciculus) (38), as well as the periaqueductal grey and hypothalamus (81,82). Moreover, VMPF10 and VLPF47 are bidirectionally connected by lateral branches of the uncinate fasciculus, disruptions of which are associated with impairments in use of memory to guide decision-making and socio-emotional difficulties (83). Intriguingly, metabolic activity in the anterior insula and periaqueductal grey of rhesus monkeys correlates positively with anxious temperament behaviors in animals exposed to threat (84). Taken together, the three regions identified in the current analyses are consistent with an interactive network of regions involved in processing and regulating emotional states. Beyond the findings related to the association with treatment outcomes, these results provide further information regarding the pathophysiology of major depression. It would also be informative to examine how the functional connectivity patterns associated with treatment outcomes in the present analysis compare to the functional connectivity of the SCC in age- and gender-matched healthy control subjects and in patients with remitted major depression.
An important strength of the PReDICT study is that all patients were treatment-naïve. Prior studies have shown that antidepressant medication treatment alters reactivity of the SCC, VLPFC, and insula (85), as well as the functional connectivity between cortical and limbic regions in major depression patients (86). These findings indicate the potential for confounding in neuroimaging studies using patients on antidepressant medications at baseline. PReDICT’s treatment-naïve sample indicates that treatment-related subtypes are not driven by prior treatment exposures.
Studying treatment-naïve patients without substantial comorbidity controlled for variables that could have impaired detection of between-group differences. The treatment-naïve sample potentially limits generalizability, but our prior work demonstrated that pre-treatment anterior insula metabolism was associated with differential treatment outcomes to medication and CBT in a previously-treated, predominantly recurrent, sample of depressed patients (26). Similar to other searches for predictors, another limitation of the current study is that scans were conducted at a single time point, and thus reflect only a cross-sectional (“state”) view into depression pathophysiology. Finally, a placebo control treatment arm could have helped interpretation of the treatment-specific effects of the imaging findings.
The present results, in conjunction with our prior CBT vs antidepressant medication study using FDG-PET (26,27), argue strongly that brain state subtypes of heterogeneous major depressive disorder patients may reflect their biological capacity to benefit differentially from treatments with differing mechanisms of action. Brain-based measures of major depression are proving superior to clinical measures and patient preferences in signifying differential outcomes to depression treatments (7,8,10). Such measures may provide a basis for possible future algorithms for triaging subjects to the appropriate treatment, likely as a component within a multivariate approach to prediction. Further development of treatment selection biomarkers using replication and prospective testing can be expected to contribute meaningfully to the clinical goals of precision medicine approaches for patients with major depressive disorder.
Supplementary Material
Acknowledgments
This study was supported by the following National Institutes of Health grants: P50 MH077083; RO1 MH080880; UL1 RR025008; M01 RR0039 and K23 MH086690. Forest Labs and Elli Lilly Inc. donated the study medications, escitalopram and duloxetine, respectively, but were otherwise uninvolved in study design, data collection, data analysis, or interpretation of findings.
Footnotes
Clinicaltrials.gov listing: NCT00360399
Disclosures
BWD has received research support from Assurex, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, NIMH, Otsuka, Pfizer, and Takeda. He has served as a consultant to Pfizer and Medavante.
WEC is a board member of Hugarheill ehf, an Icelandic company dedicated to the prevention of depression, receives book royalties from John Wiley & Sons, is supported by the Mary and John Brock Foundation and the Fuqua Family Foundations, is a consultant to the George West Mental Health Foundation, and is a member of the Scientific Advisory Board of ADAA.
CBN has received research support from the NIH and in the past three years he has served as a consultant to Xhale, Takeda, SK Pharma, Lilly, Allergan, Mitsubishi Tanaba Pharma Development America, Taisho Pharmaceutical Inc., Lundbeck, Prismic Pharmaceuticals, Clintara/Bracket, Sunovion and Total Pain Solutions. He is a stockholder in Xhale, Celgene, Seattle Genetics, Abbvie, and Titan Pharmaceuticals. He has served on the scientific advisory boards of American Foundation for Suicide Prevention (AFSP), Laureate Institute for Brain Research, Brain and Behavior Research Foundation (BBRF), Xhale, Anxiety Disorders Association of America (ADAA), Skyland Trail, Clintara/Bracket, and RiverMend Health LLC. He has served on the Board of Directors for the AFSP, Gratitude America, and the ADAA. He has had income sources or equity of $10,000 or more from American Psychiatric Publishing, Xhale, and Clintara/Bracket. He holds a patent on the method and devices for transdermal delivery of lithium (US 6,375,990 B1) and the method of assessing antidepressant drug therapy via transport inhibition of monoamine neurotransmitters by ex vivo assay (US 7,148,027B2).
HSM has received consulting fees from St. Jude Medical Neuromodulation and Eli Lilly (2013 only) and intellectual property licensing fees from St. Jude Medical Neuromodulation.
JKR, CLM, MEK, and BK report no competing interests
References
- 1.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for depression. Neuropsychopharmacol. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. 3. Arlington, VA: American Psychiatric Association; 2010. [Google Scholar]
- 3.Craighead WE, Dunlop BW. Combination psychotherapy and antidepressant medication for depression: For whom, when and how. Ann Rev Psychol. 2014;65:267–300. doi: 10.1146/annurev.psych.121208.131653. [DOI] [PubMed] [Google Scholar]
- 4.Schatzberg AF, Rush AJ, Arnow BA, Banks PL, Blalock JA, Borian FE, Howland R, Klein DN, Kocsis JH, Kornstein SG, Manber R, Markowitz JC, Miller I, Ninan PT, Rothbaum BO, Thase ME, Trivedi MH, Keller MB. Chronic depression: medication (nefazodone) or psychotherapy (CBASP) is effective when the other is not. Arch Gen Psychiatry. 2005;62:513–520. doi: 10.1001/archpsyc.62.5.513. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop BW. Evidence-based applications of combination psychotherapy and pharmacotherapy for depression. FOCUS. 2016;14:156–173. doi: 10.1176/appi.focus.20150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weitz E, Hollon SD, Twisk J, van Straten A, David D, DeRubeis RJ, Dimidjian S, Dunlop BW, Faramarzi M, Hegerl U, Jarrett RB, Kheirkhah F, Kennedy SJ, Mergl R, Miranda J, Mohr DC, Rush AJ, Segal ZV, Siddique J, Simmons AD, Vittengl JR, Cuijpers P. Does baseline depression severity moderate outcomes between CBT and pharmacotherapy? An individual participant data meta-analysis. JAMA Psychiatry. 2015;72:1102–1109. doi: 10.1001/jamapsychiatry.2015.1516. [DOI] [PubMed] [Google Scholar]
- 8.Cuijpers P, Weitz E, Lamers F, Penninx B, Twisk J, DeRubeis R, Dimidjian S, Dunlop BW, Jarrett R, Segal Z, Hollon S. Melancholic and atypical depression as predictor and moderator of outcome in cognitive behavior therapy and pharmacotherapy for adult depression. Depress Anxiety. 2016 doi: 10.1002/da.22580. in press. [DOI] [PubMed] [Google Scholar]
- 9.Paulus M. Pragmatism instead of mechanism. JAMA Psychiatry. 2015;72:631–632. doi: 10.1001/jamapsychiatry.2015.0497. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop BW, Kelley ME, Aponte-Rivera V, Kinkead B, Mletzko-Crowe T, Ritchie JC, Nemeroff CB, Craighead WE, Mayberg HS. Effects of patient preferences on outcomes in the Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT) study. doi: 10.1176/appi.ajp.2016.16050517. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunlop BW. Prediction of treatment outcomes for major depressive disorder. Expert Rev Clin Pharmacol. 2015;8:669–672. doi: 10.1586/17512433.2015.1075390. [DOI] [PubMed] [Google Scholar]
- 12.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016 doi: 10.1016/S2215-0366(15)00579-9. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 15.Fu CH, Steiner H, Costafreda SG. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis. 2012;52:75–83. doi: 10.1016/j.nbd.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Chi KF, Korgaonkar M, Grieve SM. Imaging predictors of remission to anti-depressant medications in major depressive disorder. J Affect Disord. 2015;186:134–144. doi: 10.1016/j.jad.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Siegle GJ, Thompson WK, Collier A, Berman SR, Feldmiller J, Thase ME, Friedman ES. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69:913–924. doi: 10.1001/archgenpsychiatry.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;154:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Dichter GS, Gibbs D, Smoski MJ. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milak MS, Parsey RV, Lee L, Oquendo MA, Olvet DM, Eipper F, Malone K, Mann JJ. Pretreatment regional brain glucose uptake in the midbrain on PET may predict remission from a major depressive episode after three months of treatment. Psychiatry Res. 2009;173:63–70. doi: 10.1016/j.pscychresns.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, Grieve SM, Harris AW, Usherwood T, Etkin A. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacol. 2015;40:2398–2408. doi: 10.1038/npp.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samson AC, Meisezahl E, Scheuerecker J, Rose E, Schoepf V, Wiesmann M, Frodl T. Brain activation predicts treatment improvement in patients with major depressive disorder. J Psychiatr Res. 2011;35:1214–1222. doi: 10.1016/j.jpsychires.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Rush AJ. Narrowing the gaps between what we know and what we do in psychiatry. J Clin Psychiatry. 2015;76:1366–1372. doi: 10.4088/JCP.15ac10309. [DOI] [PubMed] [Google Scholar]
- 25.Dunlop BW, Mayberg HS. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin Neurosci. 2014;16:507–518. doi: 10.31887/DCNS.2014.16.4/bdunlop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR, Craddock RC, Mayberg HS. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunlop BW, Kelley ME, McGrath CL, Craighead WE, Mayberg HS. Preliminary findings supporting insula metabolic activity as a predictor of outcome to psychotherapy and medication treatments for depression. J Neuropsychiatry Clin Neurosci. 2015;27:237–239. doi: 10.1176/appi.neuropsych.14030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Salerian AJ, Altar CA. The prefrontal cortex influence over subcortical and limbic regions governs antidepressant response by N=H/(M+R) Psychiatry Res. 2012;204:1–12. doi: 10.1016/j.pscychresns.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 31.Smith R, Fadok RA, Purcell M, Liu S, Stonnington C, Spetzler RF, Baxter LC. Localizing sadness activation within the subgenual cingulate in individuals: a novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging Behav. 2011;5:229–239. doi: 10.1007/s11682-011-9127-2. [DOI] [PubMed] [Google Scholar]
- 32.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Little JT, Ketter TA, Kimbrell TA, Danielson A, Benson B, Willis MW, Post RM. Venlafaxine or bupropion responders but not nonresponders show baseline prefrontal and paralimbic hypometabolism compared with controls. Psychopharmacol Bull. 1996;32:629–635. [PubMed] [Google Scholar]
- 34.Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 35.Konarski JZ, Kennedy SH, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, Mayberg HS. Predictors of nonresponse to cognitive behavioural therapy or venlafaxine using glucose metabolism in major depressive disorder. J Psychiatry Neurosci. 1009;34:175–180. [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath CL, Kelley ME, Dunlop BW, Holtzheimer PE, Craighead WE, Mayberg HS. Pretreatment brain states identify likely nonresponse to standard treatments for depression. Biol Psychiatry. 2014;76:527–535. doi: 10.1016/j.biopsych.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, Crowell AL, Garlow SJ, Rajendra JK, Mayberg HS. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–969. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunlop BW, Binder EB, Cubells JF, Goodman MG, Kelley ME, Kinkead B, Kutner M, Nemeroff CB, Newport DJ, Owens MJ, Pace TWW, Ritchie JC, Aponte Rivera V, Westen D, Craighead WE, Mayberg HS. Predictors of remission in depression to individual and combined treatments (PReDICT): Study protocol for a randomized controlled trial. Trials. 2012;13:106. doi: 10.1186/1745-6215-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beck AT, Rush AJ, Shaw BF, Emery G. Cognitive Therapy of Depression. New York, NY: Guilford; 1979. [Google Scholar]
- 43.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 44.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein DP, Fink L. Childhood trauma questionnaire manual. San Antonio, TX: Psychological Corporation; 1998. [Google Scholar]
- 46.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn Reson Med. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 47.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systemic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox RW, Hyde JS. Software tools for analysis and visualization of FMRI Data. NMR in Biomedicine. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 49.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS. A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry. 2009;65:276–282. doi: 10.1016/j.biopsych.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor PA, Saad ZS. FATCAT: (An Efficient) Functional and Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3:523–535. doi: 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens MJ, Dunlop BW, Plott S, Zejnelovik F, Craighead WE, Mayberg HS, Nemeroff CB. Estimates of serotonin or norepinephrine transporter occupancy do not predict efficacy in a 12 week trial. Poster presentation. American College of Neuropsychopharmacology annual meeting; 2015. [Google Scholar]
- 53.Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. NeuroImage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associated; 1988. [Google Scholar]
- 55.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 56.Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–587. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;154:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Curr Dir Psychol Sci. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Exp Brain Res. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 61.Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 62.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 64.Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiao L, Wei DT, Li WF, Chen QL, Che XW, Li BB, Li YD, Qiu J, Zhang QL, Liu YJ. Rumination mediates the relationship between structural variations in ventrolateral prefrontal cortex and sensitivity to negative life events. Neuroscience. 2013;255:255–264. doi: 10.1016/j.neuroscience.2013.09.053. [DOI] [PubMed] [Google Scholar]
- 66.Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.zu Eulenburg P, Baumgärtner U, Treede RD, Dieterich M. Interoceptive and multimodal functions of the operculo-insular cortex: tactile, nociceptive and vestibular representations. Neuroimage. 2013;83:75–86. doi: 10.1016/j.neuroimage.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 68.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milak MS, Parsey RV, Lee L, Oquendo MA, Olvet DM, Eipper F, Malone K, Mann JJ. Pretreatment regional brain glucose uptake in the midbrain on PET may predict remission from a major depressive episode after three months of treatment. Psychiatry Res. 2009;173:63–70. doi: 10.1016/j.pscychresns.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An X, Bandler R, Ongür D, Price JL. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401:455–479. [PubMed] [Google Scholar]
- 71.Cannon DM, Ichise M, Rollis D, Klaver JM, Gandhi SK, Charney DS, Manji HK, Drevets WC. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11C]DASB; comparison with bipolar disorder. Biol Psychiatry. 2007;62:870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 72.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 73.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492(7429):428–32. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol Psychiatry. 2014;76:203–212. doi: 10.1016/j.biopsych.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinyero G, Blier P. Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev. 1999;51:533–591. [PubMed] [Google Scholar]
- 77.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimized treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 78.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bludau S, Bzdok D, Gruber O, Kohn N, Riedl V, Sorg C, Palomero-Gallagher N, Müller VI, Hoffstaedter F, Amunts K, Eickhoff SB. Medial prefrontal abberations in major depressive disorder revealed by cytoarchitectonically informed voxel-based morphometry. Am J Psychiatry. 2016;173:291–298. doi: 10.1176/appi.ajp.2015.15030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carmichael S, Price J. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 82.Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 83.von der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinated fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox AS, Oler JA, Shackman AJ, Shelton SE, Raveendran M, McKay DR, Converse AK, Alexander A, Davidson RJ, Blangero J, Rogers J, Kalin NH. Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci USA. 2015;112:9118–9122. doi: 10.1073/pnas.1508593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delaveau P, Jabourian M, Lemogne C, Guionnet S, Bergouignan L, Fossati P. Brain effects of antidepressants in major depression: a meta-analysis of emotional processing studies. J Affect Disord. 2011;130:66–74. doi: 10.1016/j.jad.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 86.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an FMRI study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.