Abstract
Objective
In clinical trials, omega-3 fatty acid supplementation improves symptoms in psychiatric disorders involving dysregulated mood and impulse control, yet it is unclear whether in healthy adults omega-3 fatty acid supplementation affects mood, impulse control and the brain systems supporting these processes. Accordingly, this study tested the hypotheses that eciosapentaenoic (EPA) and docosahexaenoic (DHA) acid supplementation reduces negative affect and impulsive behaviors in healthy adults and that these changes correspond to alterations in corticolimbic and corticostriatal brain systems which support affective and impulsive processes.
Methods
Healthy volunteers (N = 272) consuming 300 mg/day or less of EPA and DHA were enrolled in a double-blind, randomized, placebo controlled clinical trial. Participants received either capsules providing 1000 mg of EPA and 400 mg of DHA versus identical appearing soybean oil capsules per day for 18 weeks. Negative affect and impulsivity were measured by questionnaire and ecological momentary assessment (EMA), as well as functional alterations in corticolimbic and corticostriatal brain systems evoked by standardized fMRI tasks.
Results
There were no group-by-time interactions for any questionnaire or EMA measures of mood and impulsivity. Likewise, no group-by-time interactions were observed for fMRI responses evoked within corticolimbic and corticostriatal systems.
Conclusions
In healthy adults with low intake of omega-3 fatty acids, moderate-dose supplementation for 18 weeks did not alter affect or impulsive behaviors, nor alter corticolimbic and corticostriatal brain functionality.
Trial Registration
Trial number NCT00663871, URL: https://www.clinicaltrials.gov/ct2/show/NCT00663871?term=NCT00663871&rank=1
Keywords: omega-3 fatty acids, clinical trial, negative affect, fMRI, impulsivity
Introduction
Although omega-3 (ω-3) polyunsaturated fatty acids are essential in the human diet, Americans consume these nutrients in minimal quantities1. There is cross-sectional evidence that deficiency of ω-3 fatty acids associates with depression, hostility, aggressive behavior, and impulsivity in both psychiatric and non-psychiatric populations2–13. Clinical-trials also show ω-3 fatty acids to have efficacy in some psychiatric disorders involving dysregulated mood and impulse control, including bipolar disorder14, borderline personality disorder15, major depressive disorder16–17, and attention deficit hyperactivity disorder18–19. However, meta-analyses of ω-3 fatty acid clinical trials in psychiatric populations report the behavioral effects to be relatively small20–22, and ω-3 fatty acid clinical trials assessing affect changes in non-psychiatric populations have produced mixed results. In some studies, for example, ω −3 supplementation has been related to decreases in negative affect23–24, whereas other studies have found no effect of ω −3 supplementation on negative affect or impulsivity25–28.
In view of existing evidence, it appears possible that ω-3 fatty acids may alter brain functionality prior to emergent or reliably detectable behavioral changes. In support of this possibility, eciosapentaenoic (EPA) or docosahexaenoic (DHA) acids constitute 14–30% of fatty acids in the phospholipids of brain tissue, particularly neuronal gray matter, compared to less than 4% of fatty acids in plasma29–30. The types and amounts of dietary fatty acids affect neural phospholipid concentration, with exchange between serum and brain phospholipids in humans occurring by diffusion or active transport proteins31–34. Further, placebo-controlled human neuroimaging studies show that ω-3 fatty acid supplementation alters neural activity during cognitively demanding tasks in children and adults35–37. In these studies, ω-3 fatty acid supplementation also improved cognitive performance, indicating that alterations in neural functionality could link ω-3 fatty acids with behavioral change35–37.
Accordingly, ω-3 fatty acids may relate to mood, impulsivity, and related behavioral processes, in part, by affecting brain systems supporting these processes; namely, corticolimbic and corticostriatal systems. Dysfunction of corticolimbic brain regions is associated with mood disorders, such as major depressive disorder and bipolar disorder38. Corticostriatal regions are also associated with appetitive, reward-dependent behavior and are strongly modulated by dopaminergic input39–40. Moreover, corticostriatal dysregulation is associated with disorders such as attention deficit hyperactivity disorder38. Animal studies have shown that fatty acid supplementation enhances production of neurotransmitters (e.g., serotonin and dopamine) that modulate corticolimbic and corticostriatal circuit function 41–42. Additionally, cross-sectional human research has shown higher ω-3 fatty acid intake to associate with greater corticolimbic gray matter volume in healthy adults43–46.
Despite long-chain ω-3 being concentrated in the brain and appearing to have treatment efficacy for several psychiatric disorders, no human placebo-controlled study has yet examined whether the effects of EPA and DHA on negative affect and impulsive decision-making are accompanied, and potentially accounted for, by longitudinal alterations in corticolimbic and corticostriatal functionality. Nor have any placebo-controlled studies examined whether EPA and DHA affect corticolimbic and corticostriatal functionality in the absence of observable effects on affective and impulsive processes. The present study tested for these effects in a double-blind, randomized placebo-controlled design in a healthy adult sample by measuring pre- and post-intervention corticolimbic and corticostriatal circuit activity changes that were evoked by standardized affect and reward-based functional magnetic resonance imaging (fMRI) tasks in conjunction with questionnaire and ecological momentary assessment of negative affect and impulsivity.
Methods and Materials
Participants
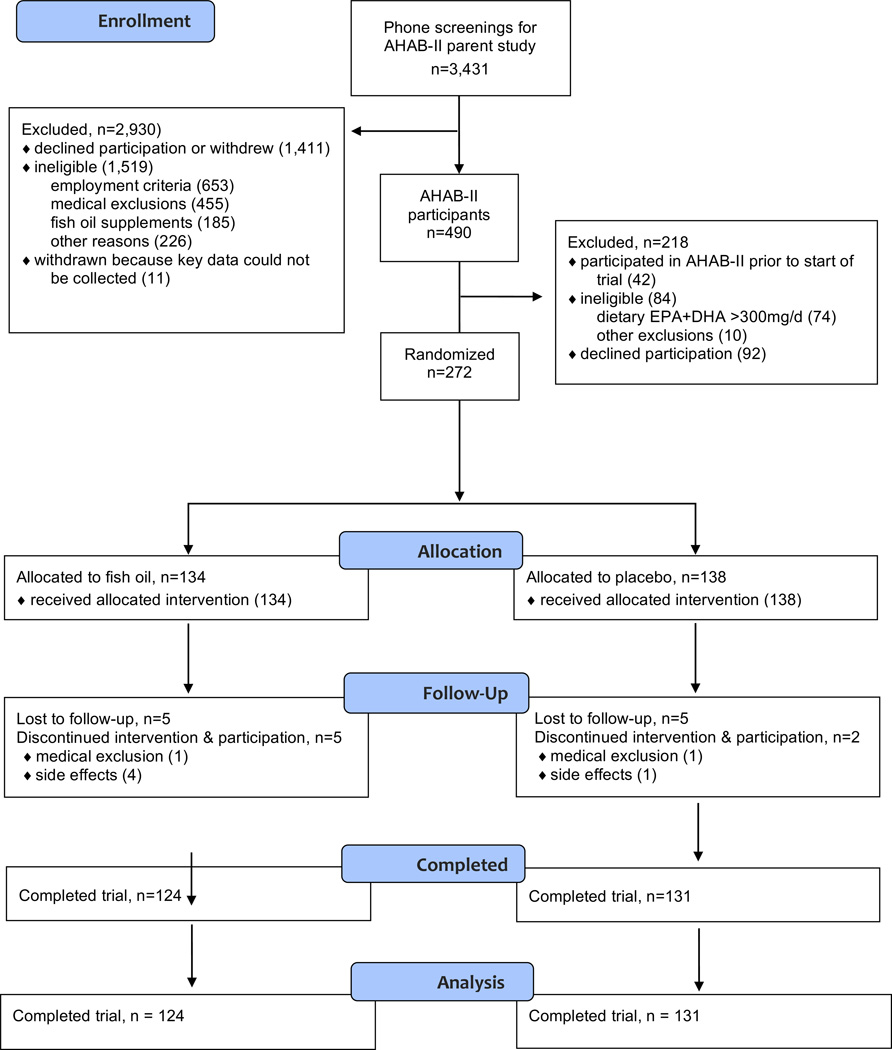
Participants were 134 men and 138 women between 30 and 54 years of age (see Figure 1 for Consort flow chart). A subset of participants (n = 121) participated in a neuroimaging protocol at baseline and post-supplementation. All participants were drawn from the Adult Health and Behavior Project – Phase 2 (AHAB-II) project. AHAB-II recruited volunteers through mass mailings of recruitment letters to individuals selected from voter registration and other public domain lists from the greater Pittsburgh metropolitan area. Participants were free of major chronic medical disorders and consumed ≤300 mg/day of EPA+DHA, as estimated from a food frequency questionnaire, had no seafood allergies, and were not currently taking fish oil supplementation. Participants were also screened to exclude those with current diagnoses of DSM-IV Axis-I disorders using the structured Mini International Neuropsychiatric Interview47. Further details regarding screening criteria for the AHAB-II study are provided in the text of Supplemental Digital Content 1.
Figure 1.
Consort flow diagram of trial.
Study Design
Data were generated from an exploratory randomized, double-blind and placebo-controlled trial at a single site using supplemented dietary intake of long-chain ω-3 polyunsaturated fatty acids in healthy mid-life adults. The trial was designed to test several putative primary prevention mechanisms, each linked to low dietary intake in published research: a) chronic systemic inflammation, b) low cardiac autonomic control, c) subtle cognitive functioning, and d) behavioral measures of reward-related impulsivity and negative affect. The trial’s first published report presented findings related to chronic inflammation, along with details of the protocol and study design, adverse events and study blinding48. The current report describes affect outcomes and substudy functional MRI results. The trial is registered on ClinicalTrials.gov (RCT00663871). The investigation was approved by the Institutional Review Board of the University of Pittsburgh, and was conducted between June 2008 and December 2011. All participants provided written informed consent and were paid for their participation.
Intervention
Enrolled participants were randomized to one of two treatment conditions using R-Track, a secure web based program-wide clinical trial management system. Through minimization the marginal treatment distribution was balanced within levels of stratification factors of race (white vs. nonwhite), age (< 45 years, > 45 years), and sex (male, female). Participants in the fish oil condition (n = 134) received a daily dose of two 1000 mg fish oil capsules, together providing 1000 mg EPA and 400 mg DHA. This dose was chosen because it has been shown to substantially increase EPA and DHA levels in serum or plasma49. Participants in the placebo condition (n = 138) received a daily dose of two identical appearing 1000 mg soybean oil capsules. The placebo capsules contained 1% fish oil, and both supplements contained mint flavor to help maintain participant blinding. Capsules were distributed in weekly blister packs to assist with adherence, each labeled with the week number and a code for treatment assignment. The assigned supplements were distributed by a study nurse blinded to condition immediately after randomization. Through this standardized procedure, treatment allocation concealment was maintained. The treatment period was 18 weeks. Participants completed Ecological Momentary Assessment (EMA) recording days approximately two weeks before beginning supplementation. Due to scheduling reasons, follow-up EMA measures were completed approximately 16 weeks into supplementation, two weeks before the trial ended. Participants in the fMRI subsample began the fish oil trial on average two weeks after their baseline fMRI scan. Follow-up fMRI scans took place 16 weeks after beginning supplementation, two weeks before the trial ended.
Fatty Acid Composition of Red Blood Cells (RBCs)
Fatty acid composition of red blood cells (RBCs) was determined pre- and post- supplementation by first preparing hemoglobin-free RBC ghost membranes as previously described and stored at −70°C until further analysis50. Methods for lipid extraction and quantitative determination of fatty acid distribution have been reported elsewhere47. The intra- and inter-assay coefficients were 1.98 % and 3.88 %, respectively, for fatty acid at mean concentration of >300 nmol/mL, and 3.57% and 8.62%, respectively, for fatty acid at mean concentration of <150 nmol/mL.
Affect Measures
Negative affect
Negative affect was assessed using measures of depressive symptomatology and hostility. The original Beck Depression Inventory (BDI)51 is a 21-item self-report measure having adequate discriminant validity52, test-retest reliability (test-retest reliability = .73 to .90)53, and internal consistency (Chronbach’s alpha = .86) in clinical and non-clinical populations. Participants were asked to fill out the BDI reflective of their mood in the past week, including the day of testing. The cognitive, affective, and behavioral components of hostility were assessed using the 39-item version of the Cook-Medley Hostility scale54–56. This measure has reasonable test-retest reliability (10-year test-retest reliability 0.74), internal consistency (Chronbach’s alpha = .83) and construct validity56–57.
Impulsivity and aggression
The Barratt Impulsiveness Scale (BIS) is scored on a 4-point likert scale and consists of 30-items designed to assess control of thoughts and behavior (e.g., acts without thinking)58–60. The BIS has high internal consistency (alpha coefficients 0.79–0.83) and high reproducibility (reliability coefficient 0.85)59–60. Aggression was measured using the total score of the Buss and Perry Aggression Questionnaire (BPAQ)61, which demonstrates good test-retest reliability (test-retest reliability = 0.80) and internal consistency (Chronbach’s alphas = .72-.85)61.
Average Ecological Momentary Assessments
Ecological momentary assessments (EMA) allowed for measurements of negative affect and impulsive behavior, throughout the daily course of living; providing a more comprehensive measure of the intensities of different emotions, rather than relying on a summary (i.e., by questionnaires)62. EMA assessments were completed using a 4-day monitoring protocol (3 working days and 1 nonworking day). The monitoring protocol consisted of two 2-day monitoring periods, usually one period at the beginning of the workweek and another at the end of the workweek, with at least one non-monitoring day in between. The mean (standard deviation) number of observations for each item pre- and post- supplementation were 54.4 (8.64) and 55.53 (6.49), respectively. Participants carried a PDA (palm Z22, software: Satellite Forms) and answered a 43-item EMA questionnaire hourly on the PDA63–64. Each item asked participants to rate to what extent they were feeling the emotion. Answers corresponded with a 6-point Likert scale, 1 = strong “no” and 6 = strong “yes”. Participants received extensive training and practice on the use of the PDA as well as feedback on compliance after completing a practice day. For this study, the negative affect (consisting of upset, hostile, nervous, afraid, angry, lonely, sad) and anger expression (consisting of annoyed, yelled) scores were used.
Neuroimaging Tasks and Measures
Participants engaged in two standardized affective and reward-based fMRI tasks designed to elicit activity changes in corticolimbic and corticostriatal brain systems65–67 (for task details see Supplementary Text). In brief, the affective processing task required participants to match emotional facial expressions or simple geometric shapes to a target presented on a screen. The reward-based task required participants to make guesses that were rewarded or not rewarded with money.
Image Acquisition and Preprocessing
Functional blood oxygenation level-dependent (BOLD) images were collected on a 3T Trio TIM whole-body scanner (Siemens, Erlangen, Germany) using a 12 –channel phased-arrayed head coil. A small mirror was attached to the head coil to allow the participants to see the projector placed behind her or him while in the scanner. The functional BOLD image acquisition parameters can be found in the Supplementary Text.
The functional BOLD images were processed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK). Before analyses, BOLD images were realigned to the first image of the series by a 6-parameter rigid-body transformation, with the unwarp procedure in SPM being applied to adjust for geometric distortion due to movement. Realigned images were co-registered to each participant’s T2-weighted structural image. Co-registered images were normalized by a 12-parameter nonlinear and affine transformation to the International Consortium for Brain Mapping 152 template (Montreal Neurological Institute; MNI). Normalized images were smoothed by a 6mm full-width-at-half-maximum (FWHM) Gaussian kernel.
Data Analysis
Sample Size
This study had a target sample size of 250 subjects (125 per treatment group) in order to achieve at least 0.80 power to detect differences in mean changes in negative affect and impulsivity from baseline to post-supplementation in terms of the standardized mean difference (d) as small as d = 0.4 between treatment groups. Sample size determination assumed a test-wise significance level of .01 when conducting two-sided hypothesis testing using linear contrasts within a repeated measures framework. ω −3 supplementation clinical trials using fMRI are limited and have mainly been focused on cognitive function, however, positive effects in these studies were seen with relatively small sample sizes (largest sample, total N = 36)67.
Demographics
Baseline characteristics between groups were compared using one-way ANOVA for continuous data and chi-squared for categorical variables. Adherence to treatment was quantified as counts of returned pills and change in blood levels of EPA and DHA. Group differences in EPA and DHA over time were assessed using group (ω-3 fatty acid, placebo) by time (baseline, follow-up) ANOVAs. Of the 255 participants who completed the full trial there were missing data on the following outcome measures: BDI (n = 1), CMH (n = 6), BIS (n = 3), BPAQ (n = 6), all EMA measures (n = 10). An earlier paper from this trial demonstrated no group differences in adherence48 between the two groups.
Affect and impulsivity analyses
Total scores for each of the questionnaires was used. Averages were created for EMA by creating an average for each day of assessment. Treatment differences between groups for all affect measures were examined using a series of group (ω-3 fatty acid, placebo) by time (baseline, follow-up) ANOVAs. Intention-to-treat (ITT) analyses, including all participants randomized into the trial, were conducted using linear mixed effects modeling where condition was included as a fixed factor19. These methods handle missing data points for the dependent variable without the use of listwise deletion.
Post-hoc sensitivity analyses
Mixed design ANOVAs were conducted examining if there were gender x treatment group x time interactions. Additionally, a series of bivariate regressions were run between change in DHA and EPA blood levels (level at follow-up minus baseline level) and affect variables (level at follow-up minus baseline level). Lastly, additional sensitivity analyses using the last observation carried forward method examining the effects of the trial on the main outcome variables were conducted. All affect related statistical analyses were performed using SPSS 21 (IBM Corp., Armonk, NY).
fMRI analysis
One-way ANOVAs were conducted to examine differences between the participants who had both fMRI and affect measures (n = 121) compared to those who just had affect measures (n = 146). After preprocessing, linear contrast images reflecting relative BOLD signal changes (i.e. Faces vs. Shapes; Reward vs. No Reward) were estimated for each participant. To this end, task conditions were modeled with rectangular waveforms convolved with the default SPM hemodynamic response function (HRF). Contrast images were then generated by general linear model (GLM) estimation using an explicit brain mask and incorporating outlier weighting using the Robust Weighted Least Squares toolbox (v3.1; http://www.icn.ucl.ac.uk/motorcontrol/imaging/robustWLS.html). Before estimation, low-frequency BOLD signal noise was removed by high-pass filtering (128sec cut-off). Finally, regression vectors derived from the realignment step were included in the GLMs to account for BOLD signal variance attributable to head movement. Individual contrast images were then submitted to group-level, one-sample t-tests. Main effects for task at each visit were conducted using one-sample T-tests comparing the conditions (Faces vs. Shapes; Reward vs. No Reward). Group (ω-3 fatty acid; placebo) x time (visit 1; visit 2) interactions were examined using a mixed design ANOVA for both tasks. We first performed a priori region of interest analyses focusing on the amygdala for the affective task and the ventral striatum for the reward-based task. The amygdala and ventral striatum are core components of the corticolimbic and corticostriatal circuits, respectively, and are most reliably engaged by these tasks65–66. These a priori analyses were supplemented with whole brain voxel wise analyses to examine corticolimbic and corticostriatal responses outside of our ROIs. See Supplemental Digital Content 2 for description of ROIs. Family-wise error rate (FWER) threshold of 0.05 and cluster threshold of k ≥ 20 were employed in all imaging analyses.
Participant blinding and side effects
Study staff assessed side effects by calling participants during Week 2 and Week 12, and at a brief appointment during week 7. At the end of the trial participants were asked to guess what group they were assigned to. Differences between groups in reported side-effects and correctly guessing their assigned group were analyzed using chi-square tests.
Results
Of the original 272 participants, 255 completed the full trial. There were no significant differences between groups in demographic characteristics (Table 1). There were significant group (ω-3 fatty acid, placebo) by time (pre-, post-) interaction for red blood cell content of DHA and EPA. Specifically, supplementation increased EPA by 322% and DHA by 41% while levels did not change in the placebo group (Table 2).
Table 1.
Baseline characteristics of randomized participants (N = 255)
| Characteristic | Fish Oil | Placebo | Difference Statistic |
|---|---|---|---|
| Age | 43.09 (7.54) | 42.47 (7.03) | F (1,270) = 0.49, p = .48 |
| Sex | χ2 (1) = 0.001, p = .97 | ||
| Male | 58 | 76 | |
| Female | 60 | 78 | |
| Ethnicity | χ2 (4) = 4.18, p = .38 | ||
| White | 109 | 116 | |
| Black | 21 | 22 | |
| Asian | 1 | 0 | |
| Multi-racial | 1 | 0 | |
| Other | 2 | 0 | |
| BMI | 27.53 (5.92) | 26.71 (4.60) | F (1,270) = 1.61, p = .21 |
| Years of school completed |
16.54 (2.77) | 16.80 (2.68) | F (1,270) = .653, p = .42 |
| WASI IQ | 112.50 (12.61) | 112.89 (12.55) | F (1,270) = .066, p = .80 |
Table 2.
Blood levels of EPA and DHA pre- and post-intervention
| Placebo | Omega-3 | Main group effect, F |
Main time effect, F |
Group x time interaction, F |
|||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| EPA | 0.44 (0.24) | 0.41 (0.18) | 0.45 (0.25) | 1.45 (0.75) | 169.59* | 173.49* | 199.44* |
| DHA | 2.42 (0.89) | 2.43 (0.98) | 2.48 (0.87) | 3.49 (1.19) | 26.75* | 57.62* | 55.01* |
p < .05
Measures of affect, impulsivity, and aggression
Relative to placebo, the ω-3 fatty acid group did not show any significant changes in questionnaire or EMA measures of negative affect or impulsivity/aggression, p’s > .20 (Table 3). There were main effects of time for BDI and EMA hostility scores, wherein participants in both groups reported less hostility and depressive symptomology in post-test compared to pre-test values. The ITT, utilizing the entire sample who enrolled in the study (N = 272), produced similar results for all dependent variables.
Table 3.
Measures of negative affect and impulsivity/aggression pre- and post-intervention.
| Placebo | Omega-3 | Main group effect, F |
Main time effect, F |
Group x time interaction, F |
|||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||||
| Negative Affect | |||||||
| BDI scorea | 4.47 (3.86) | 3.79 (3.43) | 5.01 (4.87) | 4.21 (3.81) | 1.10 | 13.40* | 0.07 |
| CMH score | 16.47 (7.34) | 16.54 (7.21) | 17.77 (7.92) | 17.44 (7.59) | 1.47 | 0.21 | 0.51 |
| EMA
negative affect |
1.88 (0.70) | 1.82 (0.73) | 1.86 (0.71) | 1.80 (0.68) | 0.03 | 3.49 | 0.01 |
| Impulsivity/Aggression | |||||||
| BIS total score | 59.51 (8.44) | 59.38 (8.86) | 59.23 (8.50) | 60.10 (9.39) | 0.29 | 0.004 | 0.20 |
| BPAQ total score | 56.07 (14.21) | 55.51 (14.17) | 58.33 (16.27) | 57.63 (17.31) | 1.35 | 1.23 | 0.02 |
| EMA
anger expression |
1.50 (0.64) | 1.53 (0.69) | 1.52 (0.66) | 1.49 (0.66) | 0.90 | 0.02 | 1.13 |
p > .05
Original version of BDI used
Sensitivity Analyses
Additional post-hoc analyses examining potential interactions with gender for each of the measures of affect and impulsivity/aggression were conducted. There were no significant interactions. Change scores were calculated for both EPA and DHA blood levels and all questionnaire and EMA measures. Bivariate correlations between EPA and DHA change scores and change scores of all questionnaire and EMA measures were run. There were no significant correlations between EPA and DHA blood levels and any of the affective measurements. All results remained the same for main trial outcomes when using the last observation carried forward method.
Functional measures
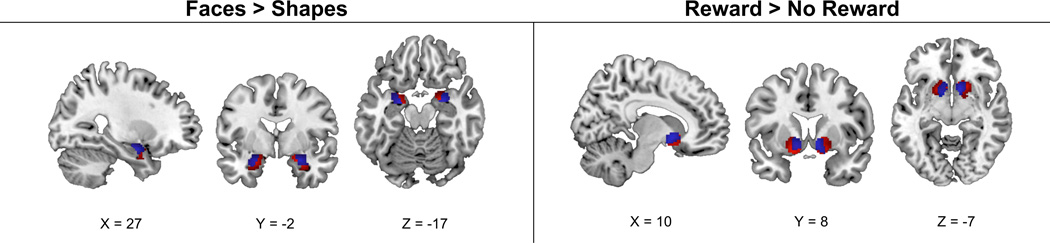
There were no differences on any demographic variables or affective measures at either pre- or post- intervention between the sub-sample participating in the fMRI protocol and those with only affective measures (p’s > .20). Both fMRI tasks evoked the expected main effects (groups combined) on functional activity changes pre- and post-intervention (see Figure 2, Supplement Table 1). Relative to placebo, ω-3 fatty acid supplementation did not change corticolimbic activity in response to the affective task in either ROI analyses or supplemental whole brain analyses. Similarly, there were no group-by-time differences in corticostriatal activity changes in response to the reward-based task in either ROI analyses or supplemental whole brain analyses.
Figure 2.
Clusters of relative BOLD activation at time 1 (in red) and time 2 (in blue) within the amygdala and ventral striatum regions-of-interest, as revealed by the Faces vs. Shapes (affective task) contrast and Reward > No Reward (reward task) contrast at FWE ≤ 0.05 and k ≥ 20 voxels. FWE, family-wise error rate. Coordinates are displayed in MNI space.
Participant blinding and side effects
Overall, 33% in the fish oil group and 28% in the placebo group guessed their treatment assignment correctly (p = 0.58). Compared to those on placebo participants receiving fish oil reported somewhat more “fishy belch or aftertaste” and “loose stool, bloating or gas pains,” but less “minty belch or aftertaste.” No serious adverse events were reported by any participants enrolled in the trial (completed or not). Additional detail on these analyses have been reported previously48.
Discussion
Low dietary intake and low blood levels of ω −3 fatty acid consumption are associated with negative affect and impulsivity in both psychiatric and non-psychiatric populations2–13. Placebo controlled clinical trials demonstrate that fish oil supplementation can reduce symptoms in a number of psychiatric illnesses (e.g., depressive symptomology, aggression in borderline personality disorder)14–18. However, no study has yet examined if such supplementation reduces negative affect and impulsive decision-making in parallel with changes in the functionality of brain systems implicated in affect regulation and appetitive or reward motivation. This study aimed to address this possibility in a non-psychiatric sample of adult community volunteers not currently taking medication that could alter neural activity. The present study further used comprehensive measures, both questionnaire based and EMA, to assess affect and impulsivity before and after the intervention. While the ω −3 fatty acid group had significant increases in levels of both EPA and DHA as part of the trial, there were no significant changes in negative affect or impulsivity. Additionally, there were no intervention-related alternations in task-evoked corticolimbic or corticostrital activity in the ω-3 fatty acid group compared to placebo group.
To date, the current study is the largest ω −3 fatty acid supplementation trial to assess neural functionality pre- and post-intervention and the only such trial to our knowledge to use fMRI with affective-related changes in a non-psychiatric, adult population67. In a randomized, placebo-controlled study, children (N = 33) who received low (400 mg) or high (1200 mg) DHA supplementation increased functional activity in the frontal and occipital lobes during a cognitive task relative to children in the placebo group35. ω −3 fatty acid supplementation increased bold signal activity during a cognitively demanding task in older adults (N = 21) with memory impairment36. In a double-blind, counterbalanced, cross-over study of 13 young adults EPA-rich supplementation was associated with faster reaction times on a Stroop task and less functional activation in the anterior cingulate cortex and increased activation in the precentral gyrus during the task37. These initial studies suggest ω −3 supplementation can alter neural function during cognitively demanding tasks. However, a recent study demonstrated that in children with ADHD and age-matched controls ω −3 supplementation improved parent-rated attention, but did not alter measures of brain activity or cognitive control19. None of these studies assessed measures of affect or function of the corticolimbic and corticostriatal circuits.
It is somewhat surprising that previous studies have not sought to examine the neural correlates of ω −3 fatty acid related changes in affect. Several sources have linked fatty acid deficiency to negative affect2–7. Accordingly, the current study attempted to extend these observational and preclinical studies. Cross-nationally, low seafood consumption is associated with a high lifetime risk of depression2. Within populations, low fish consumption is associated with heightened odds of both depression3–4 and hostility5. Low concentrations of specific ω −3 fatty acids in plasma and red blood cell membranes are found in those with clinically significant depression or depressive symptoms6–7 and violent or aggressive behavioral tendencies8, 68–69. In two different cohorts of approximately 100 generally healthy volunteers, serum EPA and DHA concentrations co-varied with normative variability in depressive symptoms and self-reported impulsivity13, 70. Clinical trials assessing affect changes in non-psychiatric participants have produced mixed results. In some studies, ω −3 supplementation has reduced anger23, anxiety23–24, and depression23, while other studies have found no effect of on negative affect or impulsivity25–28. The current randomized and placebo-controlled ω −3 supplementation assessing negative affect impulsivity in a healthy population is the largest to date, and the first trial to assess both negative affect and impulsive behavior throughout the course of daily living pre- and post-intervention by EMA.
The trial is not without limitations. Participants consumed some ω −3 fatty acids before the trial began. They had a mean EPA+DHA intake near the US adult average of about 100 mg/day45. However, this amount is well below the recommended minimum consumption of 250 or 500 mg/day and did not differ between placebo and intervention group. It is possible that our observations were influenced by the exact dosage of EPA and DHA chosen here, as well as the duration of supplementation. There is some evidence that EPA and DHA have differential effects on different biological and behavioral outcomes71. Meta-analyses of trials of ω −3 on depression, for example, have suggested that EPA may specifically have therapeutic benefits for depression72. The current trial provided 1000mg/d of EPA and 400 mg/d DHA. The quantity was chosen to approximate the upper limit of what might be consumed through dietary measures (with greater amounts constituting “pharmacologic” doses), and the ratio reflecting that of fish oil capsules widely used internationally. We also note that ω −3 acid supplementation has been reported to have measureable mood effects in patient populations72 . It is possible that due to slow brain turnover ω −3 acid supplementation may also result in similar outcomes among healthy adults if they given in larger doses for longer periods of time. In addition, mean scores for depressive symptomology were low and it could be argued that a “floor effect” had been reached. However, there was a range in total BDI scores at baseline (Range: 0–24) and follow-up (Range: 0–19) and in BDI change from baseline to follow-up (Range: −14 – 9). Similar ranges were seen for other main variable outcomes. Additionally, the concept of impulsivity could be seen as trait rather than state. Therefore, it could be argued that one cannot directly reduce impulsivity73-74. However, recent short-term interventions have shown that certain interventions are able to reduce impulsivity. Finally, as study participants had to meet a large number of enrollment criteria, the findings may not be completely generalizable to the average adult population.
In summary, this randomized and placebo-controlled trial in healthy adults found no effect of 1400 mg per day of EPA+DHA supplementation for 18 weeks on corticolimbic or corticostriatal brain activity in healthy adults. Additionally, in line with some recent literature, there was no effect of supplementation on negative affect or impulsivity25–28. Future research may be needed to examine higher doses of supplementation and possibly psychiatric populations exhibiting clinical alternations in neural activity linked to dysregulated mood and impulsivity.
Supplementary Material
Acknowledgments
We acknowledge the efforts of Dr. François Lespérance, MD, who served as the trial data safety monitor. Drs. Matthew Muldoon and Annie Ginty had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest and source funding: The study was funded by US Public Health Service Awards P01 HL40962, R01 HL101421, R21 HL081282, and T32 HL07560. The US Public Health Service had no role in the study design or implementation, data collection, statistical analysis, interpretation or manuscript composition.
Acronyms used in text
- EPA
Eciosapentaenoic acid
- DHA
Docosahexaenoic acid
- EMA
Ecological momentary assessment
- ω-3
Omega-3
- fMRI
functional Magnetic Resonance Imaging
- BDI
Beck Depression Inventory
- BIS
Barratt Impulsiveness Scale
- BPAQ
Buss and Perry Aggression Questionnaire
- BOLD
Blood oxygenation level-dependent
- FWER
Family-wise error rate
- GLM
General linear model
- HRF
Hemodynamic response function
Footnotes
The authors have no conflict of interests or disclosures.
References
- 1.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 2.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351:1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 3.Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Vinamaki H. Fish consumption, depression, and suicidality in a general population. Arch Gen Psychiatry. 2001;58:512–513. doi: 10.1001/archpsyc.58.5.512. [DOI] [PubMed] [Google Scholar]
- 4.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Rasanen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. J Affect Disord. 2004;82:447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Iribarren C, Markovitz JH, Jacobs DR, Jr, Schreiner PJ, Daviglus M, Hibbeln JR. Dietary intake of n-3, n-6 fatty acids and fish: relationship with hostility in young adults - the CARDIA study. Eur J Clin Nut. 2004;58:24–31. doi: 10.1038/sj.ejcn.1601739. [DOI] [PubMed] [Google Scholar]
- 6.Frasure-Smith N, Lesperance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Bio Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 7.McNamara RK. Role of omega-3 fatty acids in the etiology, treatment, and prevention of depression: Current status and future directions. JNIM. doi: 10.1016/j.jnim.2016.04.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallahan B, Garland MR. Essential fatty acids and their role in the treatment of impulsivity disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;7:211–216. doi: 10.1016/j.plefa.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Richardson AJ, Ross MA. Fatty acid metabolism in neurodevelopmental disorder: a new perspective on associations between attention-deficit/hyperactivity disorder, dyslexia, dyspraxia and the autistic spectrum. Prostaglandins Leukot Essent Fatty Acids. 2000;63:1–9. doi: 10.1054/plef.2000.0184. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LJ, Zentall SS, Deck JL, Abate ML, Watkins BA, Lipp SR, Burgess JR. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–768. doi: 10.1093/ajcn/62.4.761. [DOI] [PubMed] [Google Scholar]
- 11.Young GS, Maharaji NJ, Conquer JA. Blood phospholipid fatty acid analysis of adults with and without attention Deficit/Hyperactivity Disorder. Lipids. 2004;39:117–123. doi: 10.1007/s11745-004-1209-3. [DOI] [PubMed] [Google Scholar]
- 12.De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in poly-unsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins Leukot Essent Fatty Acids. 2004;71:13–18. doi: 10.1016/j.plefa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Conklin SM, Harris JI, Manuck SB, Yao JK, Hibbeln JR, Muldoon MF. Serum omega-3 fatty acids are associated with variation in mood, personality and behavior in hypercholesterolemic community volunteers. Psychiatry Res. 2007;152:1–10. doi: 10.1016/j.psychres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo controlled trial. Arch Gen Psychiatry. 1999;56:407–412. doi: 10.1001/archpsyc.56.5.407. [DOI] [PubMed] [Google Scholar]
- 15.Zanarini MC, Frankenburg FR. Omega-3 fatty acid treatment of women with borderline personality disorder: a double blind, placebo-controlled pilot study. Am J Psychiatry. 2003;160:167–169. doi: 10.1176/appi.ajp.160.1.167. [DOI] [PubMed] [Google Scholar]
- 16.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch Gen Psychiatry. 2002;59:913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 17.Su KP, Huang SY, Chiu CC, Shen WW. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo controlled trial. Eur. Neuropsychopharmacol. 2003;13:267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 18.Sinn N. Nutritional and dietary influences on attention deficit hyperactivity disorder. Nutr Rev. 2008:66558–568. doi: 10.1111/j.1753-4887.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- 19.Bos DJ, Oranje B, Veerhoek ES, Van Diepen RM, Weusten JM, Demmelmair H, Koletzko B, de Sain-van der Velden MG, Eilander A, Hoeksma M, Durston S. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2015;40:2298–2306. doi: 10.1038/npp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocking RJ, Harmsen I, Assies J, Koeter MW, Ruhe HG, Schene AH. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. doi: 10.1038/tp.2016.29. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 22.Cooper RE, Tye C, Kuntsi J, Vassos E, Asherson P. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behavior and conduct problems in ADHD: A systematic review and meta-analysis. J Affect Disord. doi: 10.1016/j.jad.2015.09.053. In press. [DOI] [PubMed] [Google Scholar]
- 23.Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest. 2005;35:691–699. doi: 10.1111/j.1365-2362.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 24.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: r randomized controlled trial. Brain Behav Immun. 2011;25:1725–34. doi: 10.1016/j.bbi.2011.07.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, Heatherley SV, Christian LM, McNaughton SA, Ness AR. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99:421–421. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 26.Jackson PA, Deary ME, Reay JL, Scholey AB, Kennedy DO. No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18–25 years. Br J Nutr. 2012;107:1232–1243. doi: 10.1017/S000711451100403X. [DOI] [PubMed] [Google Scholar]
- 27.Antypa N, Van der Does AJ, Smelt AH, Rogers RD. Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol. 2000;23:831–840. doi: 10.1177/0269881108092120. [DOI] [PubMed] [Google Scholar]
- 28.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Hwang BS, Glaser R. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26:988–995. doi: 10.1016/j.bbi.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien JS, Fillerup DL, Mead JF. Quantification and fatty acid and fatty aldehyde composition of ethanolamine, choline, and serine glycerophosphatides in human cerebral grey and white matter. J Lipid Res. 1964;5:329–338. [PubMed] [Google Scholar]
- 31.Jia Z, Pei Z, Maiguel D, Toomer CJ, Watkins PA. The fatty acid transport protein (FATP) family: very long chain acyl-CoA synthetases or solute carriers? J Mol Neurosci. 2007;33:25–31. doi: 10.1007/s12031-007-0038-z. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport SI, Purdon D, Shetty HU, Grange E, Smith Q, Jones C, Chang MC. In vivo imaging of fatty acid incorporation into brain to examine signal transduction and neuroplasticity involving phospholipids. Ann. N. Y. Acad. Sci. 1997;30:820–856. doi: 10.1111/j.1749-6632.1997.tb46189.x. [DOI] [PubMed] [Google Scholar]
- 33.Puri BK, Counsell SJ, Hamilton G, Richardson AJ, Horrobin DF. Eicosapentaenoic acid treatment-resistant depression associated with symptom remission, structural brain changes and reduced neuronal phospholipid turnover. Int J Clin Pract. 2001;55:560–563. [PubMed] [Google Scholar]
- 34.Wahle KWJ, Rotondo D, Heys SD. Polyunsaturated fatty acids and gene expression in mammalian systems. Proc Nutr Soc. 2003;62:349–360. doi: 10.1079/pns2003249. [DOI] [PubMed] [Google Scholar]
- 35.McNamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, Alferi D, Weber W, Jarvis K, DelBello MP, Strakowski SM, Adler CM. Docosahexaenoic acid supplementation increases prerontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nut. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boespflug EL, McNamara RK, Eliassen JC, Schidler MD, Krikorian R. Fish oil supplementation increases event-related posterior cingulate activation in older adults with subjective memory impairment. J Nutr Health Aging. 2016;20:161–169. doi: 10.1007/s12603-015-0609-6. [DOI] [PubMed] [Google Scholar]
- 37.Bauer I, Hughes M, Rowsell R, Cockerell R, Pipingas A, Crewther S, Crewther D. Omega-3 supplementation improves congition and modifies brain activation in young adults. Hum Psychopharmacolo. 2014;29:133–144. doi: 10.1002/hup.2379. [DOI] [PubMed] [Google Scholar]
- 38.Hariri AH. Looking Inside the Disordered Brain. Sunderland, MA: Sinaur Associates, Inc; 2015. [Google Scholar]
- 39.Berridge KC, Robinson TE. Parsing Reward. Trends Neurosci. 2003;26:507–512. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 40.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 41.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Postaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Levant B, Ozias MK, Davis PF, Winter M, Russell KL, Carlson SE, Reed GA, McCarson KE. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33:1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conklin SM, Gianaros PJ, Brown SM, Yao JK, Hariri AR, Manuck SB, Muldoon MF. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neurosci Lett. 2007;421:209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 44.Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, Debette S, Pikula A, Decarli C, Wolf PA, Vasan RS, Robins SJ, Seshadri S. Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology. 2012;78:658–664. doi: 10.1212/WNL.0b013e318249f6a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titova OE, Sjogren P, Brooks SJ, Kullberg J, Ax E, Kilander L, Riserus U, Cederholm T, Larrson EM, Johansson L, Ahlstrom H, Lind L, Schioth HB, Benedict C. Dietary intake of eciosapentaenoic and docosahexaenoic acids linked to gray matter volume and cognitive function in the elderly. Age. 2013;35:1495–1505. doi: 10.1007/s11357-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pottala JV, Yaffe K, Robinson JG, Espeland MA, Wallace R, Harris WS. Higher RBC EPA+DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology. 2014;82:435–442. doi: 10.1212/WNL.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sheehan DV, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Keskiner A, Schinka J, Knapp E, Sheehan MF, Dunbar GC. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12:232–241. [Google Scholar]
- 48.WAS 47 NOW48. Muldoon MF, Laderian B, Kuan DC, Sereika SM, Marsland AL, Manuck SB. Fish oil supplementation does not lower C-reactive protein or interleukin-6 levels in healthy adults. J Intern Med. 2016;279:98–109. doi: 10.1111/joim.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blonk MC, Bilo HJ, Nauta JJ, Popp-Snijders C, Mulder C, Donker AJ. Dose-response effects of fish-oil supplementation in healthy volunteers. Am J Clin Nutr. 1990;52:120–127. doi: 10.1093/ajcn/52.1.120. [DOI] [PubMed] [Google Scholar]
- 50.Dodge JT, Mitchell C, Hanahan DJ. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963;100:119–3. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- 51.Beck AT, Steer RA, Brown GA. Beck Depression Inventory: Second Edition Manual. San Antonio (TX): The Psychological Corporation; 1996. [Google Scholar]
- 52.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1998;8:77–100. [Google Scholar]
- 53.Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Piquiatr. 2003;35:416–431. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- 54.Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- 55.Barefoot JC, Larsen S, von der Lieth L, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiology. 1995;142:477–484. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- 56.Barefoot JC. Depression and coronary heart disease. Cardiologia. 1997;42:1245–1250. [PubMed] [Google Scholar]
- 57.Contrada RJ, Jussim L. What does the Cook-Medley Hostility Scale measure? In search of an adequate measurement model. J Appl Soc Psychol. 1992;8:615–627. [Google Scholar]
- 58.Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion, and Personality. North-Holland: Elsevier Science; [Google Scholar]
- 59.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 60.Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsitivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- 61.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 62.Kamarck TW, Shiffman S, Muldoon MF, Sutton-Tyrrell K, Gwaltney CH, Janicki DL, Schwartz JE. Ecological momentary assessment as a resource for social epidemiology. In: Stone AA, Shiffman S, Atienza AA, Nebeling L, editors. The Science of Real-Time Data Capture. Oxford: Oxford UP; [Google Scholar]
- 63.Janicki DL, Kamarck TW, Shiffman S, Sutton-Tyrrell K, Gwaltney CJ. Freuqency of spousal interaction and 3-year progression of carotid artery intima media thickness: the Pittsburgh Healthy Heart Project. Psychosom Med. 2005;67:889–896. doi: 10.1097/01.psy.0000188476.87869.88. [DOI] [PubMed] [Google Scholar]
- 64.Gump BB, Polk DE, Kamarck TW, Shiffman SM. Partner interactions are associated with reduced blood pressure in the natural environment: ambulatory monitoring evidence from a healthy, multiethnic adult sample. Psychosom Med. 2001;63:423–433. doi: 10.1097/00006842-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26:13212–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carre JM, Fisher PM, Manuck SB, Hariri AR. Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Soc Cogn Affect Neurosci. 2012;7:213–221. doi: 10.1093/scan/nsq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bos DJ, van Montfort SJT, Oranje B, Durston S, Smeets PAM. Effects of omega-3 polyunsaturated fatty acids on human brain morphology and function: What is the evidence? Eur Neuropsychopharmacol. 2016;26:546–561. doi: 10.1016/j.euroneuro.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 68.Buydens-Branchey L, Branchey M, McMakin DL, Hibbeln JR. Polyunsaturated fatty acid status and aggression in cocaine addicts. Drug Alcohol Depend. 2003;71:319–323. doi: 10.1016/s0376-8716(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 69.Virkkunen ME, Horrobin DF, Jenkins DK, Manku MS. Plasma phospholipid essential fatty acids and prostaglandins in alcoholic, habitually violent, and impulsive offenders. Biol Psychiatry. 1987;22:10871096. doi: 10.1016/0006-3223(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 70.Conklin SM, Manuck SB, Yao JK, Flory JD, Hibbeln JR, Muldoon MF. High omega-6 and low omega-3 fatty acids are associated with depressive symptoms and neuroticism. Psychosom Med. 2007;69:932–934. doi: 10.1097/PSY.0b013e31815aaa42. [DOI] [PubMed] [Google Scholar]
- 71.Mori TA. Omega-3 fatty acids and cardiovascular disease: epidemiology and effects of cardiometabolic risk factors. Food Funct. 2014;5:2004–219. doi: 10.1039/c4fo00393d. [DOI] [PubMed] [Google Scholar]
- 72.Hallahan B, Ryan T, Hibbeln JR, Murray IT, Glynn S, Ramsden CE, San Giovanni JP, Davis JM. Efficacy of omega-3 highly unsaturated fatty acids in the treatment of depression. Br J Psychiatry. 2016;209:192–201. doi: 10.1192/bjp.bp.114.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tarrega S, Castro-Carreras L, Fernandez-Aranda F, Granero R, Giner-Bartolome C, Aymami N, Gomez-Pena M, Santamaria JJ, Forcano L, Steward T, Menchon JM, Jimenez-Murica S. A serious videogame as an additional therapy tool for training emotion regulation and impulsivity control in severe gambling disorder. Front Psychol. 2015;12:1721. doi: 10.3389/fpsyg.2015.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gruber SA, Sagar KA, Dahlgren MK, Gonec A, Conn NA, Winer JP, Penetar D, Lukas SE. Citicoline treatment improves measures of impulsivity and task performance in chronic marijuana smokers: A pilot BOLD fMRI study. Int J Neurol Neurother. 2015;30:1–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.