Abstract
Background
Emerging evidence suggests that implant therapy may be a viable option for diabetic individuals with elevated glycemic levels.
Purpose
The purpose of this two year observational study was to evaluate survival and clinical complications of dental implants following placement in type 2 diabetes individuals having poor glycemic control.
Materials and Methods
Adult participants (n=24) with poorly controlled type 2 diabetes (8.0% ≤ HbA1c ≤ 12.0%) received two or more transgingival dental implants. Survival was evaluated after one (23 participants, 72 implants) and two (20 participants, 59 implants) years. Clinical complications were evaluated in 18 participants (52 implants) after 21–34 months. Relationships between complications and stratified HbA1c levels were assessed using Pearson’s correlation test.
Results
Survival rates were 98.6% (71/72 implants) after 1 year and 96.6% (57/59 implants) after 2 years. Complications were identified in 29% of participants with peri-implant mucositis, the most common event. Complications correlated directly with number of implants across HbA1c strata (0.42, R2=0.66). There was no correlation between HbA1c and the occurrences of complications or mucositis.
Conclusions
This 2-year evaluation supports the broader application of implant therapy in type 2 diabetes individuals with poor glycemic control in demonstrating high survival rates with limited complications.
Keywords: Hyperglycemia, Diabetes mellitus, Type 2 diabetes, Implant success, Implant survival
Introduction
Diabetes mellitus, a chronic metabolic disorder affecting over 20 million individuals, or 8.5% of the U.S. adult population, is considered one of the most commonly encountered contraindications to dental implant therapy.1, 2 As a result, well-controlled diabetic individuals are often considered appropriate for implant therapy, while those lacking good glycemic control may be denied the benefits of implant therapy. 3
Consistent with this concern, animal studies have repeatedly shown poor bone-implant healing with delays in osseointegration directly related to inadequate glycemic control.4,5,6,7,8,9 Similarly, a previous clinical report correlated delays in implant integration with increased HbA1c levels.10 Nevertheless, the clinical evidence supporting the consideration of glycemic control as a relative contraindication to implant therapy remains limited.
A recent review of the literature concerning dental implant survival in individuals with diabetes identified 17 primary studies.11 The large variation in rates of implant failures (0 to 14.3%) and individuals experiencing implant failure (0 to 31.8%) found in these studies highlight the uncertainties that persist in the literature. Looking more closely, the majority of the studies (13 of 17), while intending to include only those individuals thought to have good glycemic control, did not clearly assess or report glycemic levels, limiting their applicability toward clinical practice. The remaining 4 identified studies had documentation of glycemic levels using HbA1c and reported a narrower range of implant failure rates (0 to 9.1%) and individuals experiencing implant failure (0 to 4.1%).12,13,14,15 Only one of these studies extended beyond 1 year, and this study evaluated just one individual with an elevated HbA1c over 9%.13 This study found no significant difference comparing the overall non-diabetic group survival rate (98.8%) and the diabetic group survival rate (97.2%). A more recent cohort study included 117 individuals with and without type 2 diabetes. This study failed to identify any increased risk for implant failure after 1 year in function. 16 However, this study relied on edentulous individuals receiving two mandibular implants supporting removable dental prostheses, for individuals with a wide range of glycemic control.
It is these individuals with poor glycemic control that may have an enhanced vulnerable to implant-related biologic complications consistent with their increased vulnerability to periodontal disease. 17,18,19,20 However, the evidence for increased risk of peri-implant disease with poor glycemic control remains limited.,21,22,23,24
Therefore, purpose of this study was to extend the observational period following a 4 month comparative trial of dental implant therapy for this unique group of poorly-controlled type 2 diabetic individuals to examine the potential for poor glycemic control to compromise implant performance, including biologic complications and implant failures, after two years.
Materials and Methods
This observational, cohort study is a long-term follow-up of participants with poorly-controlled type 2 diabetes mellitus that received dental implants as part of a randomized comparative study of implant surfaces (NCT01142297).15 This study was conducted in accordance with approval by the University of Texas Health Science Center at San Antonio Institutional Review Board. While the initial study focused on short-term healing (4 months) following implant placement, the goal of this longitudinal study was to evaluate the consequences of type 2 diabetes on implant success in relation to biologic and restorative complications after 2 years. Study participants were recruited into the initial comparative study from among individuals seeking dental treatment within the University of Texas Health Science Center at San Antonio (UTHSCSA) Dental School. All participants were provided informed consent as approved by Institutional Review Board at UTHSCSA. The study population included 24 adult participants who were missing at least 2 posterior mandibular teeth. Implant sites were required to have at least 4 months of healing following tooth extraction, no previous ridge augmentation with bone grafting, and a clinical indication for an implant-supported fixed dental prosthesis for tooth replacement. The study enrolled individuals over 18 years of age with a diagnosis of type 2 diabetes of over one year duration and baseline glycated hemoglobin (HbA1c; Quest Diagnostics Laboratory, San Antonio, TX, USA) levels between 8.0% and 12.0% at the time of enrollment. Physician consultations were used to confirm medical history, diabetes status, and medications as appropriate. Individuals having a history of treatment for microvascular or macrovascular complications of diabetes were excluded. These exclusion criteria included conditions requiring chronic and routine use of antibiotics, diabetic neuropathy or nephropathy of sufficient severity that may require treatment or surgical intervention. Additional clinical findings consistent with exclusion included serum creatinine ≥ 1.6mg/dL, prolonged use of steroids, AST/ALT values >2 times the normal levels, leukocyte dysfunction/deficiencies, hypertension having systolic pressure at >185mmHg and diastolic pressure at > 105mmHg with or without medications, untreated oral or inflammatory lesions, prolonged use of steroids, bleeding disorders, metabolic bone disorders, alcoholism or drug abuse or smoking more than 10 cigarettes per day. Local exclusion factors included untreated oral or inflammatory lesions such as untreated periodontitis or erosive lichen planus, bone surgery in less than 6 months in the implant site, unhealed extraction sites, presence of bone defects or persistent intra-oral infections. HbA1c levels were assessed at the time of implant placement and thereafter at 2 months, 4 months, 1 year, and 2 years.
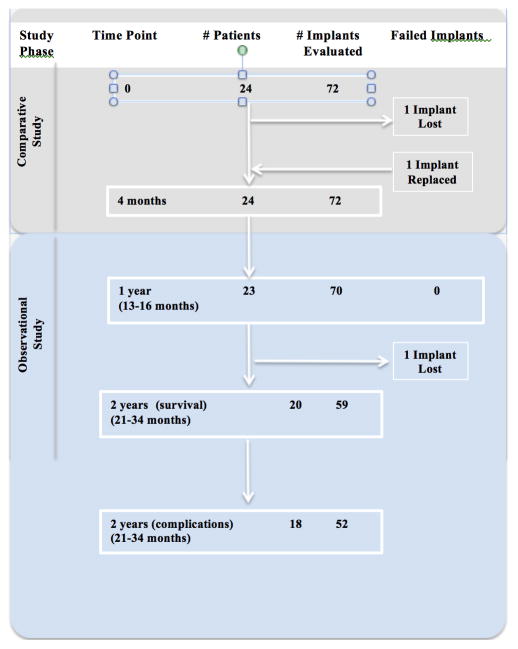
The participants each received two Straumann® Standard Plus (Institut Straumann AG, Basel, Switzerland) 4.1 mm diameter implants, one control with a hydrophobic sand-blasted and acid-etched implant surface (SLA) and one test with a hydrophilic sand-blasted and acid-etched implant surface (SLActive) implant, in the posterior mandible, totaling 48 implants placed as part of the comparative study. In addition, many participants received an additional 1–3 implants at the same surgical visit to meet their overall treatment needs. While these additional implants were not considered for analysis in the comparative study15, their evaluation is included in the present report. Implants were placed as per manufacturer’s protocols and covered using transgingival healing cap. Participants were prescribed antibiotics for one week post-surgically, analgesics given as required and chlorhexidine-digluconate 0.12% oral rinse (Peridex®) for 7–14 days. After a minimum of 16 weeks of healing, the participants received implant-supported fixed dental prostheses. Maintenance was offered by providers outside of the study and was not required for participants to remain in this study. A total of seventy-two implants were placed in these 24 participants between March 2009 and January 2010. (Figure 1) All implants were solid, one-piece implants of 8mm or 10mm in length with a 1.8mm polished collar.
Figure 1.
Participants were seen 13 to 16 months after implant placement to evaluate implant complications and HbA1c levels, and were then recalled for a long-term follow up which occurred between 21 and 34 months post-surgery to assess implant survival and success by a single examiner. During follow-up visits, medical history was reviewed. Any adverse event or symptom related to implant treatment was recorded, and signs or symptoms of clinical complications were noted. Survival was defined as the implant remaining in situ throughout the observation period. Implant evaluation included each of the following: implant stability (as detected by sound on percussion or by visual/tactile evidence), pain, peri-implant mucositis (presenting as bleeding and/or suppuration on light probing), and radiographic findings (i.e. peri-implant radiolucencies or clinically-evident changes in crestal bone loss of ≥2mm). Peri-implantitis was diagnosed if peri-implant mucositis existed in combination with signs of crestal bone loss occurring after the 4 month healing period to allow for osseous remodeling following surgical placement. Additional adverse events or restorative complications were noted, such as prosthetic material fracture or screw loosening. 25,26,27
Correlations between HbA1c levels and the ratio of complications/number of implant were determined using Pearson ‘s correlation with the HbA1c levels for both the average HbA1c during the 4-month comparative study and the value at the 2 year visit stratified using 1% increments. The overall ratio of complications and the ratio of cases of mucositis were determined relative to the number of implants placed within each stratification group.
Results
At the one-year clinical evaluation, 23 of the 24 participants returned, representing a total of 70 of the original 72 implants placed. Of the 70 implants evaluated, 69 (98.6%) implants survived through the first year. The one implant failure was an early failure, occurring between weeks 4 and 6 following implant placement. This implant was successfully replaced and restored without further complications, and was evaluated at the one and two year assessments. Most conservatively assuming the two implants on the one participant who did not return for the 1-year evaluation were failures, the implant survival after one year was 95.8%.
Twenty of the initial 24 participants, with 59 implants, were evaluated for survival after 2 years. Of the 59 implants evaluated, 58 implants survived through two years. These implants represent 58 of the 60 (96.7%) implants originally placed, including the one early failure that was replaced successfully.
The 20 returning participants included 7 males and 13 females with an average age of 59.9 years. At the time of the 2-year follow-up, HbA1c levels were between 6.3% and 13.1%, with a mean value of 8.9 ± 2.0%. This mean HbA1c level had decreased from the baseline level of 9.70 ± 0.83% (Table 1). Twelve participants had HbA1c values at this long-term evaluation visit that decreased from their initial levels, with 9 of theses 12 participants showing decreased HbA1c of more than 1%. Five participants had increased HbA1c levels, with 2 having an increase ≥1%.
Table 1.
Baseline Patient Characteristics (mean ± sd) for Patients Evaluated in Study Phases or Lost to Follow Up
| Study Phase | Time Of Assessment (months) | Number of Patients/Male | Mean Age (years) | BMI | Duration of Diabetes (years) | Using Insulin (%) | Mean HbA1c (%) |
|---|---|---|---|---|---|---|---|
| Comparative | 0–4 | 24/9 | 59.7±9.6 | 36.6±6.8 | 14.2±7.7 | 60 | 9.55±1.00 |
| Observational | 21–34 | 20/8 | 59.9±9.4 | 37.7±6.8 | 13.7±6.7 | 58 | 9.70±0.83 |
| Patients Lost to Follow up | 0–4 | 4/1 | 59.0±11.0 | 33.5±6.5 | 16.7±9.9 | 67 | 8.85±1.56 |
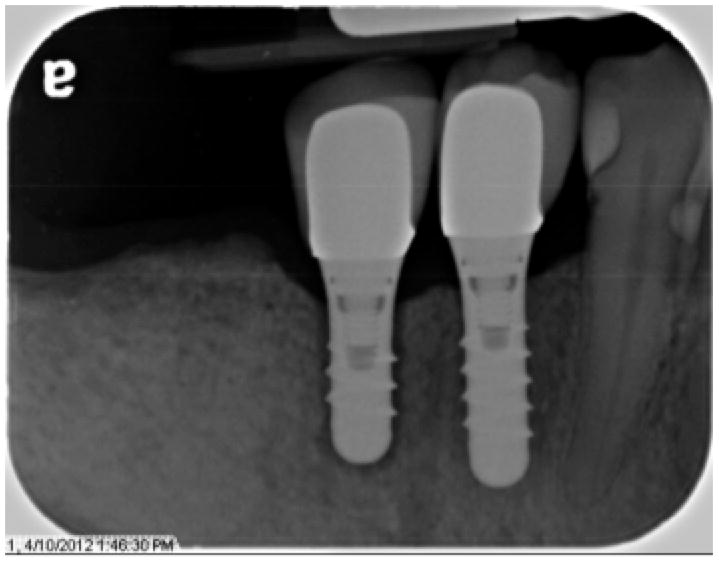
Clinically, all but two implants evaluated over the course of the study survived. (Table 2) In addition to the one early implant failure noted above, one implant was lost after 31 months (26 months post-restoration). This participant reported pain on chewing for 4 weeks, and upon evaluation the implant exhibited mobility and radiographic bone loss circumferentially (Figure 2). This participant had four additional implants placed and restored as part of the study, including two in the posterior maxilla and two in the posterior mandible, all of which showed no signs of clinical complications over this time period.
Table 2. Implant Survival.
Overall implant survival rates based on HbA1c stratification levels at 2-year evaluation.
| HbA1c (%) Stratification | Number of Patients Evaluated | Number of Implants Evaluated | Number of Failed Implants | % Implant Survival |
|---|---|---|---|---|
| 6.0–6.9 | 3 | 8 | 0 | 100 |
| 7.0–7.9 | 6 | 13 | 0 | 100 |
| 8.0–8.9 | 4 | 13 | 1 | 92.3 |
| 9.0–9.9 | 1 | 4 | 0 | 100 |
| 10.0–10.9 | 3 | 9 | 0 | 100 |
| 11.0–11.9 | 1 | 5 | 0 | 100 |
| 12.0–12.9 | 1 | 5 | 1 | 80 |
| 13.0–13.9 | 1 | 2 | 0 | 100 |
| TOTAL | 20 | 59 | 2 | 96.6 |
Figure 2.
Assessment of complications after two years was completed on 18 of these 20 participants, representing 52 implants, with a mean follow-up time of 28.0 ± 3.8 months after implant placement. (Figure 1) The four participants not included in the 2 year follow up were unable to be contacted or declined further participation, with two others not completing the clinical evaluation for complications, but no participants reported any implant-related complications.
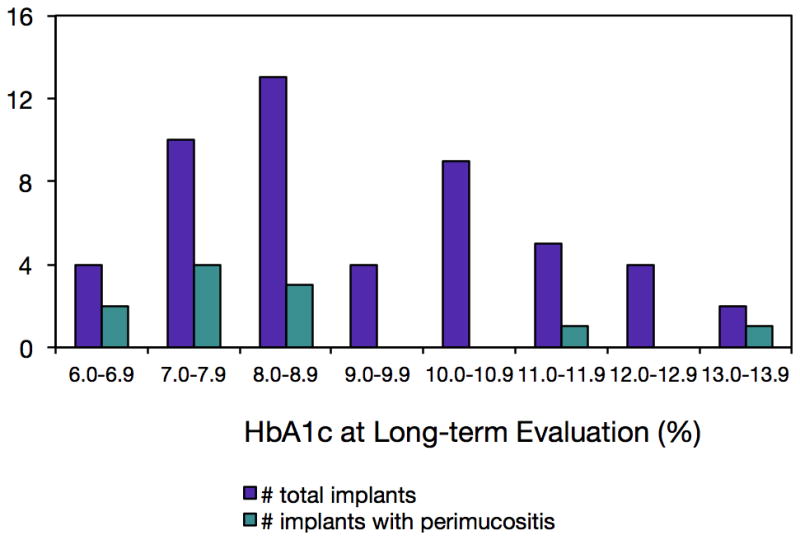
In the 18 participants with 52 implants, 15 biologic complications (29.4% of implants) were encountered during the course of the long-term follow-up, with the most frequent being peri-implant mucositis (21.6% of implants). While the total number of implants per HbA1c category was positively correlated with number of complications (0.42, R2=0.66), there were minimal correlations between HbA1c levels and the percentages of implants with number of complications (−0.028, R2=0.11) or occurrences of mucositis (−0.026, R2=0.09; Figures 3 and 4).
Figure 3.
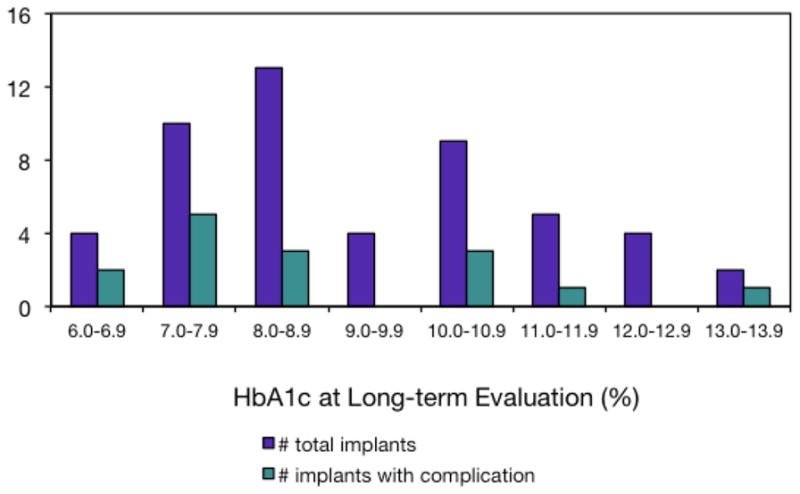
Figure 4.
Ten of the 18 participants experienced a biologic complication on at least one implant. The majority of implants showed no signs of bone loss, peri-implant mucositis, or restorative complications (Table 3). The participants required no additional treatment other than standard maintenance procedures to address these complications. The most common adverse event was mild to moderate soft tissue inflammation, or peri-implant mucositis, identified through demonstration of bleeding on probing (11 implants in 8 subjects; Table 3). Additional adverse events included gingival hyperplasia (2 implants in 1 subject), gingival recession (1 implant), and radiographic peri-implant crestal bone loss (3 implants in 2 subjects). The crestal bone loss was seen to the level of the first thread of the implants. However, the bone loss seen on all three of these implants was consistent with that evident in radiographs of these implants taken 4 months after placement, suggesting that the bone loss occurred during the first few months following placement and with little change over the following 2 years. Additionally, no signs of gingival inflammation, as suggestive of peri-implantitis, were seen around these implants, and stability of the implants had not been affected.
Table 3.
Rate of occurrence of biologic complications for implants (n=52) in 18 patients.
| Biologic Complication | Number (%) of Subjects (n=18) | Number (%) of Implants (n=52) |
|---|---|---|
| Peri-implant mucositis | 8 (44) | 11 (22) |
| Mucosal hyperplasia | 1 (6) | 2 (4) |
| Mucosal recession | 1 (6) | 1 (2) |
| Crestal bone loss | 2 (11) | 3 (6) |
| Mobility | 0 | 0 |
| Paresthesia | 0 | 0 |
| Pain | 0 | 0 |
| Peri-implantitis | 1 (6)* | 1 (2)* |
Represents implant lost after 2 years
Discussion
The goal of this study was to extend our understanding of the relationship between glycemic control for individuals with type 2 diabetes and dental implant therapy. This group of poorly-controlled diabetic participants with glycated hemoglobin levels as high as 13.1% was re-evaluated at an intermediate time point approximately one year following implant placement and again two years after implant placement. While this study had 25% attrition over the two-year period, there were minimal differences in the participant characteristic between the participants seen versus those lost to follow up, offering a valuable perspective on a unique group of individuals with type 2 diabetes receiving dental implants. Evaluation of implant-related outcomes over this 2-year period is important as there is little direct information available in the literature to guide this process. In considering the value in examining the effects of elevated glycemic levels over a longer time period as done in this study, it must be remembered that the majority of individuals with diabetes lack good glycemic control. In fact, it has been reported that as many as 60% of type 2 diabetes individuals under physician care have HbA1c levels greater than 8%, and 39% of US diabetes individuals were reported as having HbA1c levels over 10%.28,29 In this light, the use of glycemic levels to determine the appropriateness of implant therapy relative to the benefits derived from this treatment must be considered carefully.
There is no doubt that these elevated glycemic levels are directly associated with increased risk of numerous systemic co-morbidities, including periodontal disease. Given the documented risks of diabetic subjects for inflammatory periodontal disease, compromised wound healing, and infection, the potential for long-term peri-implant complications may represent a similar vulnerability.30,31,32,33,34 Previous studies have shown that glycemic control can affect bone physiology, and impaired osseous healing has been demonstrated in animal models.4,5,6,7,8,9 These studies also suggest concerns with establishing and maintaining the health of the supporting tissues for implants.
Importantly, this observational study documented little relationship between glycemic levels and implant-related clinical complications for type 2 diabetes patients having mean glycemic levels (HbA1c) between 6.3 and 13.1% over two years after implant placement, and above 8% at the time of implant placement. These findings reinforce recent examinations of the literature questioning our understanding of the role of glycemic control as a risk factor for implant failure along with a recent one-year prospective study.11,16,
The findings from this study begin to offer some clarity to the deficiencies documented in previous reports in the literature (for review see Oates 2012).11 The 96.2% survival rate found after two years in this study is consistent with previous reports of implants in non-diabetic subjects. A recent review of the literature identified 5 previous studies that directly compared failure rates between diabetic and non-diabetic individuals, with none of these studies finding a significant difference in implant survival for diabetic individuals35. The success rates in these studies ranged from 92.2–100% for diabetic individuals and 93.2–100% for non-diabetic individuals.
In the current study, one implant failed during the integration phase, and one implant failed 31 months after placement, giving a cumulative survival rate of 96.2% for all implants. Interestingly, at the time of this 31-month failure, the participant’s HbA1c was 12.4%, whereas his mean HbA1c throughout the observational period was 10.5%. This difference highlights the inconsistencies in glycemic control in many diabetic subjects with poor control, and may suggest a more subtle relationship between glycemic levels and inflammatory conditions. While the patient did experience one implant failure, he had four successful implants as well, suggesting that local factors rather than systemic factors may have had a contribution to the failure.
Although our knowledge of the ways in which HbA1c values effect implant osseointegration are limited, this study demonstrates the potential for implants to be a successful treatment option for poorly controlled diabetic patients within the clinical conditions provided within this study design. This study protocol did include the use of post-operative antibiotics, an oral antimicrobial rinse, and an extended integration period prior to restoration. The value of these modifications individually or collectively cannot be determined from the current investigation, but may form the basis for further study. Additionally, these patients did not participate in a structured maintenance program. Given the positive outcomes of the study, this reinforces the applicability of the findings to a broader group of patients.
Understanding the potential for successful implant therapy independent of glycemic control may be critical in supporting the overall management of individuals’ oral health. The findings of biologic complications at a frequency of 29.4%, with the most frequent being peri-implant mucositis, are consistent with reports in the literature for non-diabetic patients35.
Numerous studies have shown that patients with diabetes have a significantly increased risk of periodontal disease with resultant tooth loss. 18,24,37 The risks of partial or complete edentulism in the diabetic population is of concern because it may affect the individual’s ability to maintain a healthy diet due to a decreased chewing efficiency. These compromises in diet may have a negative impact on glycemic control. 38,39 Ultimately, these patients may benefit from tooth replacement, and the appropriate application of implant therapy may become an important contribution to the health and wellbeing of diabetic patients looking to improve glycemic control.
In conclusion, this study evaluated dental implants for a small but unique group of individuals with poorly controlled diabetes after a two-year period. While the impact of glycemic levels on long-term dental implant therapy remains in question, this study demonstrates encouraging results in building our understanding of implant therapy among our treatment options for these patients. As we continue to clarify both the risks and benefits of implant therapy for patients with diabetes, long-term longitudinal studies with large sample sizes will be necessary to confirm the results of the present study. We look forward to clarifying the role of the dental care for these patients in the future.
Acknowledgments
This study was supported by Institut Straumann AG (Basel, Switzerland) and National Institute of Dental and Craniofacial Research (NIDCR) grant R01 DE023518, National Institutes of Health.
Role of the Sponsors: The National Institutes of Health and Institut Straumann had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data; the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Authors have no conflicts of interest.
All authors meet the 4 criteria for their contributions, with primary responsibilities as noted:
Thomas Oates-concept, design, interpretation, regulatory management, funding Caroline Eskow-data acquisition, drafted article
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Oikarinen K, Raustia AM, Hartikainen M. General and local contraindications for endosseal implants - an epidemiological panoramic radiograph study in 65-year-old subjects. Community Dent Oral Epidemiol. 1995;23(2):114–8. doi: 10.1111/j.1600-0528.1995.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 3.Marchand F, Raskin A, Dionnes-Hornes A, et al. Dental implants and diabetes: conditions for success. Diabetes Metab. 2012;38(1):14–19. doi: 10.1016/j.diabet.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Fiorellini JP, Nevins ML, Norkin A, et al. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin Oral Implants Res. 1999;10:362–368. doi: 10.1111/j.1600-0501.1999.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 5.Gerritsen M, Lutterman JA, Jansen JA. Wound healing around bone-anchored percutaneous devices in experimental diabetes mellitus. J Biomed Mater Res A. 2000;53:702–709. doi: 10.1002/1097-4636(2000)53:6<702::aid-jbm13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa H, Ozawa S, Hashimoto K, et al. Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Surg. 2008;23(2):237–246. [PubMed] [Google Scholar]
- 7.Goodman WG, Hori MT. Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes. 1984;33(9):825–831. doi: 10.2337/diab.33.9.825. [DOI] [PubMed] [Google Scholar]
- 8.Nevins ML, Karimbux NY, Weber HP, et al. Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Surg. 1998;13:620–629. [PubMed] [Google Scholar]
- 9.Takeshita F, Iyama S, Ayukawa Y, et al. The effects of diabetes on the interface between hydroxyapatite implants and bone in rat tibia. J Periodontol. 1997;68:180–185. doi: 10.1902/jop.1997.68.2.180. [DOI] [PubMed] [Google Scholar]
- 10.Oates TW, Dowell S, Robinson M, et al. Glycemic control and implant stabilization in type 2 diabetes mellitus. J Dent Res. 2009;88:367–71. doi: 10.1177/0022034509334203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oates TW, Huynh-Ba G, Vargas A, Alexander P, Feine J. A critical review of diabetes, glycemic control, and dental implant therapy. Clin Oral Implants Res. 2013 Feb;24(2):117–27. doi: 10.1111/j.1600-0501.2011.02374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell S, Oates TW, Robinson M. Implant success in people with type 2 diabetes mellitus with varying glycemic control: A pilot study. J Am Dent Assoc. 2007;138:355–61. doi: 10.14219/jada.archive.2007.0168. [DOI] [PubMed] [Google Scholar]
- 13.Tawil G, Younan R, Azar P, et al. Conventional and advanced implant treatment in the type II diabetic patient: Surgical protocol and long-term clinical results. Int J Oral Maxillofac Surg. 2008;23:744–752. [PubMed] [Google Scholar]
- 14.Turkyilmaz I. One-year clinical outcome of dental implants placed in patients with type 2 diabetes mellitus: a case series. Implant Dent. 2010;19(4):323–326. doi: 10.1097/ID.0b013e3181e40366. [DOI] [PubMed] [Google Scholar]
- 15.Khandelwal N, Oates TW, Vargas A, et al. Conventional SLA and chemically modified SLA implants in patients with poorly controlled type 2 Diabetes mellitus – a randomized controlled trial. Clin Oral Implants Res. 2013;24:13–19. doi: 10.1111/j.1600-0501.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 16.Oates TW, Jr, Galloway P, Alexander P, Vargas Green A, Huynh-Ba G, Feine J, McMahan CA. The effects of elevated hemoglobin A(1c) in patients with type 2 diabetes mellitus on dental implants: Survival and stability at one year. J Am Dent Assoc. 2014 Dec;145(12):1218–26. doi: 10.14219/jada.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinane D, Bouchard P on behalf of group E of the European Workshop on Periodontology. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 18.Salvi GE, Carollo-Bittel B, Lang NP. Effects of diabetes mellitus on periodontal and peri-implant conditions: update on associations and risks. J Clin Periodontol. 2008;35(8 Suppl):398–409. doi: 10.1111/j.1600-051X.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 19.Javed F, Näsström K, Benchimol D, et al. Comparison of periodontal and socioeconomic status between subjects with type 2 diabetes mellitus and non-diabetic controls. J Periodontol. 2007;78(11):2112–2119. doi: 10.1902/jop.2007.070186. [DOI] [PubMed] [Google Scholar]
- 20.Emrich LJ, Shlossman M, Genco RJ. Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62(2):123–31. doi: 10.1902/jop.1991.62.2.123. [DOI] [PubMed] [Google Scholar]
- 21.Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. J Periodontol. 2009;80:1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 22.Fiorellini JP, Chen PK, Nevins M, et al. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000;20:366–373. [PubMed] [Google Scholar]
- 23.Kwon SY, Kim SS, Kwon OS, et al. Prognostic significance of glycaemic control in patients with HBV and HCV-related cirrhosis and diabetes mellitus. Diabet Med. 2005;22:1530–1535. doi: 10.1111/j.1464-5491.2005.01687.x. [DOI] [PubMed] [Google Scholar]
- 24.Pacios S, Kang J, Galicia J, et al. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 2012;26(4):1423–1430. doi: 10.1096/fj.11-196279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.
- 26.Albrektsson T, Zarb G, Worthington P, et al. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Surg. 1986;1(1):11–25. [PubMed] [Google Scholar]
- 27.Quirynen M, Assche NV, Botticelli D, et al. How does the timing of implant placement to extraction affect outcome? Int J Oral Maxillofac Surg. 2007;22(suppl):203–226. [PubMed] [Google Scholar]
- 28.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35:286–291. doi: 10.1111/j.1600-051X.2008.01274.x. [DOI] [PubMed] [Google Scholar]
- 29.van den Arend IJ, Stolk RP, Krans HM, Grobbee DE, Schrijvers AJ. Management of type 2 diabetes: a challenge for patient and physician. Patient Educ Couns. 2000 May;40(2):187–94. doi: 10.1016/s0738-3991(99)00067-1. [DOI] [PubMed] [Google Scholar]
- 30.Peters AL, Legorreta AP, Ossorio RC, Davidson MB. Quality of outpatient care provided to diabetic patients. A health maintenance organization experience. Diabetes Care. 1996;19:601–606. doi: 10.2337/diacare.19.6.601. [DOI] [PubMed] [Google Scholar]
- 31.McMahon MM, Bristrian BR. Host defenses and susceptibility in patients with diabetes mellitus. Infect Dis Clin North Am. 1995;9:1–10. [PubMed] [Google Scholar]
- 32.Gallacher SJ, Thomson G, Fraser WD, et al. Neutrophil bactericidal function in diabetes mellitus: Evidence for association with blood glucose control. Diabet Med. 1995;12:916–920. doi: 10.1111/j.1464-5491.1995.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 33.Shurtz-Swirski R, Sela S, Herskovits AT, et al. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001;24:104–110. doi: 10.2337/diacare.24.1.104. [DOI] [PubMed] [Google Scholar]
- 34.Delamaire M, Maugendre D, Moreno M, et al. Impaired leukocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Pearl SH, Kanat IO. Diabetes and healing: A review of literature. J Foot Surg. 1998;27:268–70. [PubMed] [Google Scholar]
- 36.Oates TW, Huynh-Ba G. Diabetes effects on dental implant survival. Forum Implantol. 2012;8(2):78–87. [PMC free article] [PubMed] [Google Scholar]
- 37.Atieh MA, Alsabeeha NH, Faggion CM, Jr, Duncan WJ. The Frequency of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. J Periodontol. 2012 Dec 13; doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 38.Genco RJ. Current view of risk factors for periodontal diseases. J Periodontol. 1996;67:1041–1049. doi: 10.1902/jop.1996.67.10.1041. [DOI] [PubMed] [Google Scholar]
- 39.Kawamura T, Egusa G, Fujikawa R, et al. Gln27Glu variant of the beta2-adrenergic receptor gene is not associated with obesity and diabetes in Japanese-Americans. Metab. 2001;50(4):443–446. doi: 10.1053/meta.2001.21695. [DOI] [PubMed] [Google Scholar]
- 40.Nuttall FQ, Gannon MC, Saeed A, et al. The metabolic response of subjects with type 2 diabetes to a high-protein, weight-maintenance diet. Endocrine Care. 2003;88(8):3577. doi: 10.1210/jc.2003-030419. [DOI] [PubMed] [Google Scholar]
- 41.Abdulwassie H, Dhanrajani PJ. Diabetes mellitus and dental implants: A clinical study. Implant Dent. 2002;11:83–6. doi: 10.1097/00008505-200201000-00019. [DOI] [PubMed] [Google Scholar]