Abstract
Objective
A quarter of the world’s population suffers from metabolic syndrome (MetS). MetS prevalence stratifies by socioeconomic status (SES), such that low SES is associated with higher MetS risk. The present study examined the relative roles of early-life SES and current SES in explaining MetS risk.
Methods
Participants (N = 354, ages 15–55, M = 36.5 years, SD = 10.7; 55% female; 72.9% White, 16.9% Asian, 10.2% other) were evaluated for SES and MetS. All were in good health, defined as free of chronic medical illness and acute infectious disease. Using occupational status as a proxy for SES, we recruited roughly equal numbers of participants with low-low, low-high, high-low and high-high combinations of early-life and current SES. We used the International Diabetes Federation definition for MetS using race- and sex-specific cut-offs for waist circumference, triglyceride levels, HDL cholesterol, blood pressure, and HbA1c levels.
Results
Analyses revealed a main effect of low early-life SES on increased MetS risk according to three separate definitions. They included the traditional MetS diagnosis (OR=1.53, CI=1.01–2.33, p=.044), the number of MetS components for which diagnostic thresholds were met (OR=1.61, CI=1.10–2.38, p=.015), and a continuous indicator of metabolic risk based on factor analysis, F(1,350)=6.71, p=.010, partial η2=.019. There was also a significant interaction of early-life SES and current SES in predicting MetS diagnosis (OR=1.54, CI=1.02–2.34). Main effects of current SES were non-significant in all analyses.
Conclusions
These findings suggest MetS health disparities originate in childhood, which may be an opportune period for interventions.
Keywords: metabolic syndrome, socioeconomic status, social mobility, early-life adversity
INTRODUCTION
Approximately a quarter of the world’s population suffers from metabolic syndrome (MetS) (1–3), a clustering of abdominal obesity, insulin resistance, dyslipidemia, and elevated blood pressure (1,4). MetS is associated with a two-fold increased risk of cardiovascular events or death (5) and a five-fold increased risk of incident type 2 diabetes mellitus (1). With MetS prevalence rates rising globally (6,7), it is increasingly important to understand its distribution and pathogenesis. Better insights around these issues could improve risk stratification and identify intervention targets.
Most research on the pathogenesis of MetS has focused on interrogating proximal causal factors such as energy imbalance, adiposity, insulin resistance, and physical inactivity (3,4). Much less is known about the distal social and economic contexts that shape these proximal causes across the lifespan. For instance, one observation that has been replicated in several countries is that the prevalence of MetS patterns by concurrent adult socioeconomic status (SES) measured through several indices (e.g., educational attainment, income, subjective social status), such that individuals with lower SES have a higher likelihood of diagnosis (1,8), above and beyond the effects of race/ethnicity and lifestyle factors (2,8–10). The stratification of MetS risk by current SES has been noted not only in adults, but also in children and adolescents (11,12). These socioeconomic disparities spark a number of questions that have been so far largely overlooked in studying the association between low SES and risk of MetS. For instance, is the increased MetS risk in low SES populations a result of exposures in adulthood? Or, since low SES is relatively stable across the life-course, is it the accumulation of living in disadvantaged environments from childhood onwards? Does the timing of exposure to low SES (i.e., childhood versus adulthood) matter in explaining risk of MetS? And for those who experience socioeconomic mobility – either upward or downward –does the risk of MetS change accordingly?
Epidemiological studies are revealing that the global prevalence of MetS is not only rising in adults, but also steadily increasing in children and adolescents worldwide (13). Furthermore, children and adolescents who experience socioeconomic disadvantage and other psychosocial adversities are at increased risk of metabolic dysfunction later in life (14–19). These findings raise questions about the developmental origins of adult MetS, and challenge us to find ways to understand when demographic and psychosocial risk factors for MetS might begin to influence health. Early-life conditions are thought to shape risk of obesity and MetS in adulthood through a number of pathways (13,15,20). Chronically stressful environments during fetal life and childhood are associated with low birthweight, altered childhood growth patterns and earlier pubertal onset (20), and with endocrine, autonomic and metabolic patterns that promote visceral adiposity (13,20). Additionally, pro-inflammatory signaling (21) and altered threat, reward, and executive control processes (22) that shape health behaviors such as food intake and physical exercise likely further exacerbate metabolic risk. Together, all these sources of influence are thought to contribute to enhancing metabolic risk in adulthood.
However, what is not well understood is whether the MetS risk conferred by early-life conditions in general and low early-life SES in particular is independent of adult experiences. This is important to consider, given that the majority of poor children grow up to be poor adults (23). Empirically, the association between childhood SES and risk of type 2 diabetes and obesity in adulthood is attenuated when adjusting for adult SES (15), but there is limited evidence on this issue available and the results are inconclusive. Indeed, in studies where childhood SES and adult SES are modeled together, the findings are mixed. Some studies observe that childhood but not adult SES predicts metabolic risk (16,17,24,25). Others find the reverse (26), or independent roles for both childhood and adult SES (27–29). This question is inherently difficult to address in the general population because childhood and adult SES are typically correlated due to high degree of continuity in SES across the lifespan for many individuals (15). The innovative aspect of the present study is that it explicitly addressed this challenge by disentangling the roles of early-life and current SES by study design.
Studying upward and downward social mobility can also shed light on independent contributions of early-life and adult SES. There is very limited research on associations between social mobility and MetS risk, but emerging patterns suggest that low childhood SES is associated with greater odds of MetS in later life, and that upward social mobility does not offset this risk (28,30,31). These results contrast with findings showing similar risk of diabetes for those with low-high combinations of childhood and adult SES compared to individuals with continuously high SES (32), suggesting that more work is needed to clarify these patterns. Proposing clearer life-course models of links between SES and MetS could inform interventions regarding the ideal developmental stages when intervening would have maximal health benefits.
The Present Study
The present study aimed to examine the independent and interactive roles of early-life and current adult SES in explaining variability in adult metabolic risk, indexed by three types of outcomes: (a) diagnosis of MetS according to the criteria specified by the International Diabetes Federation (IDF) definition; (b) a count of the number of MetS components on which the participant met IDF criteria; and (c) a continuous factor score indexing the common variance of the five standardized MetS components: waist circumference, blood pressure, triglycerides, HDL (reversed), and glycosylated hemoglobin. The rationale for using several indices of metabolic risk is that, even though IDF’s MetS diagnosis is clinically useful and has generalizability by providing information that is comparable globally (4), there is also some recognition in the literature that continuing to examine the individual risk factors embedded in MetS and the way their cluster together is a useful avenue for understanding subclinical disease processes and their etiology (33,34). Furthermore, there has been some debate about whether MetS is best viewed and analyzed as a dichotomous diagnosis or a spectrum of risk including continuous numeric components for each of the risk factors (34). For this reason, the present study examined not only a dichotomous indicator of MetS diagnosis according to IDF criteria, but also sought to descriptively characterize the prevalence of individual risk factors and to additionally examine metabolic risk across a continuum, captured in two ways (count of components and factor scores), as described in more depth below.
Methods
Participants
The study recruited 360 participants from Vancouver, BC, Canada through postings in local media and public transit. Recruitment and testing was conducted between February 2009 and May 2012. Only N = 6 participants had missing data for MetS components, thus primary analyses were conducted on N = 354. These 354 participants were between the ages of 15 and 55 (M = 36.5 years, SD = 10.7, 55% female) and recruited to fit into one of four groups defined by early-life (low vs. high) and adulthood (low vs. high) SES (see operational definitions under Measures and participant characteristics in Table 1). To minimize confounding by health status, participants had to be (a) free of infectious disease in the two weeks before testing, as evidenced by self-report and a normal complete blood count, and (b) without a history of serious and chronic medical illnesses including, but not limited to, cancer, diabetes mellitus, heart disease, stroke, autoimmune disease, HIV/AIDS, hepatitis, chronic obstructive pulmonary disorder, asthma, schizophrenia, bipolar disorder, and dementia. Participants who presented with acute infections were rescheduled after the signs had resolved. Candidates were also screened out if they were not fluent in English, if they were pregnant or had been pregnant in the prior year.
Table 1.
Characteristics of the sample (N = 354).
| Life course SES Trajectory1 | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Low-low SES (n=97) |
Low-high SES (n=93) |
High-low SES (n=72) |
High-high SES (n=92) |
Differs by Early SES |
Differs by Current SES |
|
| Age | 35.5 (10.7) | 39.5 (9.5) | 34.9 (10.6) | 35.6 (11.5) | .054 | .04 |
| Sex, Female, n(%) | 47(48.5%) | 55(59.1%) | 42(58.3%) | 50(54.3%) | .65 | .44 |
| Descent, European, n(%) | 65(67%) | 59(63.4%) | 59(81.9%) | 75(81.5%) | <.001 | .84 |
| Parental education (household max), years |
11.8 (2.7) | 12.4 (2.6) | 15.4 (2.1) | 16.1 (2.6) | <.001 | .01 |
| Current education (household max), years |
15.1 (2.8) | 15.7 (2.5) | 14.1 (2.3) | 16.6 (2.5) | .03 | <.001 |
| Current annual household income | $25K–34.9K | $50K–74.9K | $25K–34.9K | $50K–74.9K | .39 | <.001 |
| Waist circumference (cm) | 89.7 (16.3) | 86.9 (15.9) | 84.4 (13.3) | 83.4 (12.6) | .005 | .17 |
| Systolic blood pressure | 108.9 (13.3) | 107.8(11.7) | 105.02(11.2) | 108.1 (10.8) | .21 | .60 |
| Diastolic blood pressure | 69.0 (10.8) | 70 (9.5) | 67.53(9.6) | 69.7 (7.9) | .44 | .16 |
| Triglycerides | 1.2 (.74) | 1.1(.60) | .97(.51) | 1.01(.59) | .073 | .61 |
| HDL cholesterol | 1.4 (.34) | 1.5(.39) | 1.46(.37) | 1.5 (.42) | .32 | .085 |
| HbA1c | 5.4 (.39) | 5.4(.39) | 5.33(.29) | 5.3 (.33) | .26 | .79 |
| BMI (kg/m2) | 26.7 (6.3) | 26.6 (6.8) | 25.4 (5.8) | 24.4 (3.9) | .003 | .28 |
| Heavy smoker, >=10 cig./day, n(%) | 14 (15.9%) | 2 (2.3%) | 10 (14.5%) | 3 (3.3%) | .75 | <.001 |
| Alcohol user, >=10 drinks/week, n (%) |
9 (10.2%) | 10 (11.2%) | 12 (17.4%) | 16 (17.6%) | .07 | .78 |
| Physical activity, hours/week | 2.5 (3.0) | 2.6 (2.4) | 3.1 (2.9) | 2.99 (2.9) | .11 | .97 |
Data are presented as Mean (SD) unless noted otherwise.
The study included four groups of participants, with low-low, low-high, high-low and high-high combinations of early-life and current SES.
Of the 2880 individuals who responded to study advertisements, 417 met all of the eligibility criteria described above, and volunteered to participate after learning about the study details. 360 of them subsequently attended their scheduled laboratory session. The project was approved by the University of British Columbia’s Research Ethics Board and all participants gave written informed consent.
Procedure
All participants completed laboratory sessions between 8:00 a.m. and 10:00 a.m. following an overnight fasting period. After study details were explained, participants gave written consent, and had blood drawn by antecubital venipuncture to measure MetS components (see below). Later in the session, participants completed a battery of self-report measures and behavioral tasks, and had their height, weight, and waist circumference measured (see details in Measures).
Measures
Socioeconomic status
Participants were recruited based on their early-life and current SES, as defined by occupational status ratings derived from the United Kingdom’s National Statistics Socio-economic Classification (NS-SEC). This system is used widely in epidemiology and has been updated regularly since 1911, allowing for comparability to previous research. It is also well-suited to the Canadian social structure. The NS-SEC system codes occupational status using industry, occupation title, occupation description, self-employment status, supervisor status, and number of employees in the company/organization. It also provides specific procedures in case one piece of information among these is unavailable. All recruiters received extensive training prior to using this system. Coding consistency was regularly checked, and discrepancies were resolved by consensus. If an applicant did not clearly fall into one of the designated categories, s/he was not invited to participate in the study. Candidates whose life-course SES fell into one of four categories, as defined by early-life and current circumstances, were enrolled in the study. The categories were low early/low current (n = 97); low early/high current (n = 93); high early/low current (n = 72); and high early/high current SES (n = 92). Participants were recruited into the four groups based on occupational status because it is often a more visible aspect of SES than educational attainment, and because people can more reliably recall their parents’ occupations than income or education. Occupations were graded on an 8-point scale, which was reduced into three superordinate categories of low, middle, and high SES. Volunteers from either low or high SES categories for both childhood and current SES were enrolled, whereas those from middle categories were ineligible (e.g., small employers and own account workers, lower supervisory and technical occupations. Low SES included routine and manual occupations, those who never worked and the long-term unemployed. This included positions such as cleaners, laborers, and transportation operatives. High SES included higher managerial and professional occupations, such as architects, engineers, and medical practitioners. These definitions were used to categorize both early-life and current SES. For early-life SES, we coded parental occupational status during their first 5 years of life, using the higher of mother’s vs. father’s ratings. To classify the current SES of prospective participants, we coded their occupational status over the past 5 years, as well as that of their romantic partner (the higher of the two ratings was used). A small minority of the participants in our study were ages 15–23 (10.5%) and were full-time students, financially supported by their families. For these cases, we used parental occupation to categorize current SES, unless the participant was financially independent. However, results did not differ when including or excluding this age group.
Metabolic outcomes
A trained phlebotomist collected overnight fasting blood samples via antecubital venipuncture into Serum Separator and EDTA-treated tubes (Becton-Dickinson, Oakville, ON, Canada). Serum separator tubes were left standing for 60 minutes prior to being centrifuged at 1000g for 10 minutes, as per manufacturer’s instructions. After the serum was harvested, it was frozen at −30 C until assayed in batch at St. Paul’s Hospital Clinical Trials Laboratory, Vancouver, Canada. Fasting HDL was assayed in serum. Standard enzymatic techniques using cholesterol esterase and cholesterol oxidase were used, after low-density, intermediate density, and very-low density lipoproteins had been precipitated through centrifugation. Assays were done on a Hitachi 911 instrument (Kyowa Medex, Japan). The interassay coefficient of variation was 5.1% and the lower limit of detection was .189 mmol/L. Glycosylated hemoglobin was measured in whole blood from EDTA-treated tubes, at the same laboratory, via a Bio Rad D-10 ion exchange chromatography method (Bio-Rad Laboratories Inc., Hercules, CA). Assays yield the percentage of hemoglobin that is glycosylated out of total hemoglobin, with greater values indicating higher blood glucose exposure over the past 3 months. The lower range of detection was 1.2%, with an interassay coefficient of variation of 1.2%. Triglycerides were determined enzymatically on a Hitachi 747 (KyowaMedex, Japan) after hydrolysis to glycerol. This method has an interassay coefficient of variation of 1.1%.
We used the worldwide definition of metabolic syndrome provided by the International Diabetes Federation (IDF, (1,4), which uses racial/ethnic and sex-specific cutoffs, to evaluate the presence of MetS components and overall syndrome diagnosis. The five MetS components (central adiposity, raised blood pressure, triglyceride and glucose levels, and low HDL levels) were assessed during laboratory visits. Waist circumference was read at the midpoint between the upper iliac crest and lower costal margin at the midaxillary line. As recommended by IDF, waist circumference cutoffs for central adiposity were ≥ 94 cm for men and ≥ 80 cm for women of Europid and African descent, and ≥ 90 cm for men and ≥ 80 cm for women of Asian (including Asian-Indian) descent, for Ethnic South and Central Americans, and Aboriginal Canadians. There were 18 participants reporting mixed racial backgrounds, however their measurements were sufficiently high or low that they fit the same classification irrespective of which racial origin was used for the cutoff. Blood pressure was assessed with an automated oscillometric device (BpTRU, VSM Medtech; Burnaby, BC) while participants were seated in a comfortable chair. An initial reading was taken to acclimate participants to the procedure, but these data were not used in analysis. Three subsequent readings were taken at two-minute intervals, and their average was used for analysis (alpha’s for SBP and DBP were .93 and .95, respectively). Following IDF criteria, the cutoff for raised blood pressure was systolic readings ≥ 130 or diastolic readings ≥ 85 mm Hg. From fasting blood samples taken in the morning, triglyceride levels, high-density lipoprotein (HDL) levels and glycosylated hemoglobin (HbA1c) were measured. Again, following IDF criteria, we used the following cutoffs to define the presence of MetS components: triglyceride levels ≥ 1.7 mmol/L, HDL levels < 1.03 mmol/L in males and < 1.29 mmol/L in females. The fifth criterion specified by IDF is raised plasma glucose (FPG ≥ 100) or a diagnosis of diabetes. We deliberately excluded individuals with diabetes from participation, and fasting plasma glucose was not measured. In its place, we use glycosylated hemoglobin, which research shows is an accurate surrogate when a cutoff of ≥ 5.7% is used (35).
For analyses, we considered three sets of outcome variables: a binary variable indicating whether the participant met the IDF case definition for MetS; a count of the MetS components for which the participant met IDF cutoffs; and a continuous measure of metabolic function obtained by extracting factor scores reflecting the common variance of the five continuous metabolic measures. Maximum likelihood factor analysis (conducted with SPSS Statistics 22) indicated that the five component measures loaded highly on a single factor (eigenvalue: 2.24; explained 44.9 % of variance). Factor loadings for waist circumference, blood pressure (an average of z-scored systolic and diastolic blood pressure), triglyceride levels, HDL (reversed) and HbA1c levels were .74, .59, .62, .41 and .41, respectively. Using these different outcome measures also allows comparability with prior literature which sometimes uses the sum of MetS components (e.g., Davis et al., 2014) and in other instances uses factor analysis to capture multi-system dysregulation (e.g., Wiley, Gruenewald, Karlamangla, & Seeman, 2016).
Covariates
Participants completed questionnaires about basic demographic information (age, sex, and race/ethnicity) and about lifestyle including smoking, drinking, and exercise. Given the racial/ethnic distribution of the sample (72.9% European descent, 13.8% Asian descent, 5% or less in any other ethnic group), the variable was recoded (1= European descent, 0=non-European) to maximize statistical power for race/ethnicity effects. Using a previously validated instrument (38), we collected information on daily smoking and alcohol use. Specifically, participants reported the number of cigarettes they smoked each day and the number of alcoholic drinks they consumed per week. A drink was considered a bottle or can of beer, a glass of wine, or a shot of hard liquor (39). Lastly, regular physical activity was measured with the well-validated Paffenbarger Activity Scale (40), which estimates weekly hours of brisk physical activity.
Data Analysis
Data preparation
Variables were examined for outliers and their approximation of the normal distribution before analyses. Values that exceeded four standard deviations from the mean were Winsorized and replaced with the value at the 99.9th percentile (waist circumference: n = 3; triglyceride levels: n = 4; HbA1c: n = 1; diastolic blood pressure: n = 1). Most variables approximated the normal distribution. In contrast, variables indexing the count of MetS components, daily cigarette smoking, alcohol use, and weekly physical activity had a pronounced right skew. MetS components count, cigarette use and alcohol consumption variables were too skewed to be corrected with mathematical transformations, and were therefore converted into ordinal scales. For smoking, the new variable was coded as 0 = non-smoker, 1 = less than 10 daily cigarettes, and 2 = 10 or more cigarettes per day. For alcohol use, it was 0 = zero drinks per week, 1 = less than 10 drinks per week, and 2 = 10 or more drinks per week. For MetS component counts, meeting criteria for 3 or more symptoms was coded as 3 and ordinal regression was used instead of linear regression in analyses that had this measure as an outcome. For the Paffenbarger index of physical activity, a square-root transformation of the total number of hours of brisk physical activity per week was sufficient to reduce skewness and kurtosis and normalize the distribution, thus the square-root of the variable was used in analyses.
Missing data
Only 1.7% (N = 6) of participants were missing data in analyses without covariate adjustment, and 8.89% (N = 32) in covariate-adjusted analyses. Thus, data imputation was not necessary given that estimates are unlikely to be biased when the rate of missingness is less than 10% (53).
Statistical analyses
To examine SES disparities in the prevalence of MetS diagnoses, we conducted logistic regression analyses predicting the binary diagnosis outcome. Effect coding was used for estimating early-life and current SES main effects and their interaction in initial analyses. To probe interactions and test social mobility effects, follow-up logistic regressions using dummy-coding were used to examine differences between the four SES subgroups (low-low, low-high, high-low and high-high). Then, we used ordinal regression analyses to test the effects of early-life SES, current SES and their interaction on the count of MetS components, with follow-up ordinal regression analyses used to compare the low-low, low-high, high-low and high-high groups for probing social mobility effects. Finally, ANCOVAs were used to test the main effects of early-life and current SES and their interaction on MetS factor scores. To examine the role of social mobility, ANCOVAs examining the main effect of SES group (low-low, low-high, high-low or high-high) was also tested in these analyses. If the main effect of SES was significant, the analysis was followed up by comparisons of marginal means for the low-low versus low-high SES groups (corresponding to the effect of upward social mobility), and comparisons of the high-high versus high-low groups (corresponding to downward social mobility). All results are presented with and without adjustment for covariates (age, sex, race/ethnicity, alcohol consumption, cigarette smoking, and physical activity levels). Age did not moderate the effects of early-life SES, current SES, or their interaction in any of the analyses (all p’s > .22) thus these interaction terms were not included in the findings reported here.
Results
Table 1 presents sample characteristics. As can be seen, the recruitment strategy resulted in the expected differences in parental education, current education, and current income based on early-life SES and current SES. Participants with high current SES were also somewhat older and less likely to be heavy cigarette smokers than those with low current SES, thus we statistically adjusted for these covariates in all analyses, while also presenting unadjusted results. Participants who experienced high early-life SES were more likely to be of European descent than those with low early-life SES, thus again we also used this measure as a covariate for each of the questions we addressed. Interestingly, in these preliminary analyses there was a main effect of early-life SES on waist circumference and BMI, with higher values for those with low early-life SES, but no main effect of current SES (Table 1).
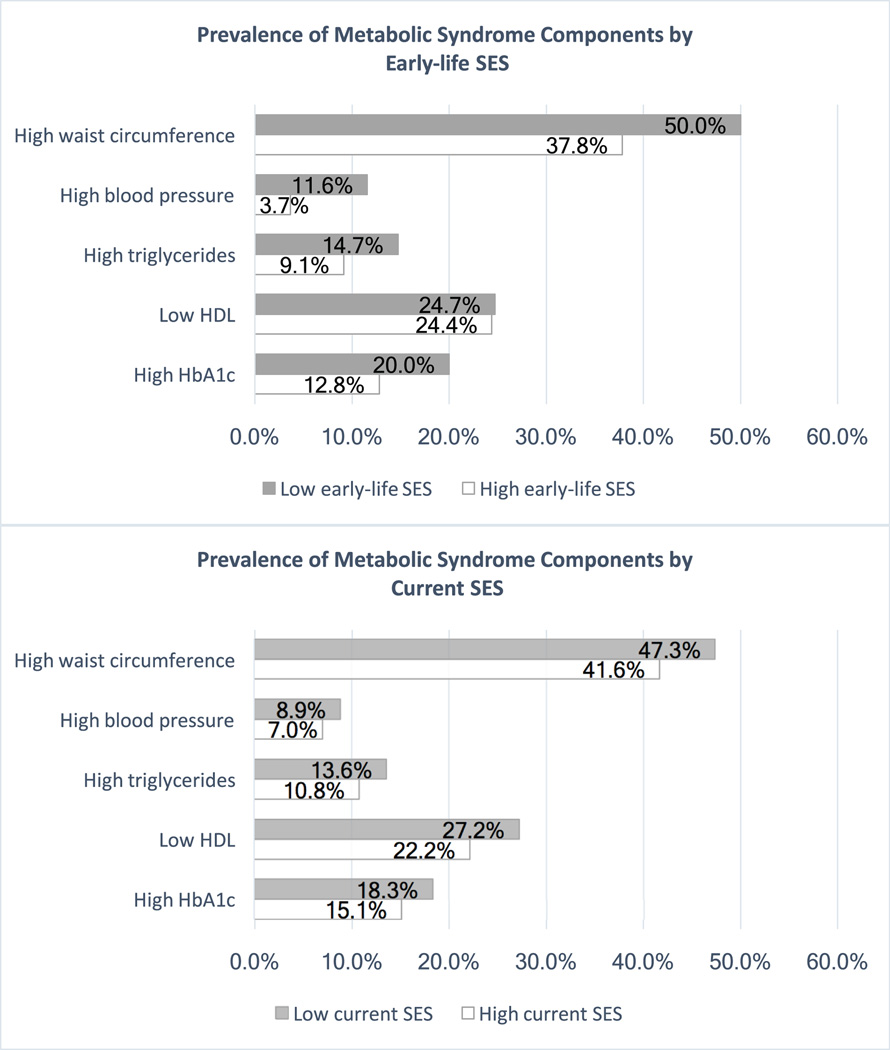
Descriptive statistics revealed that metabolic risk disparities by SES were starker when considering early-life SES versus current SES. As Figure 1 shows, participants with low early-life SES were more likely to meet IDF criteria for central adiposity (χ2 (1) = 5.3, p = .021), blood pressure (χ2 (1) = 7.58, p = .006), triglycerides (χ2 (1) = 2.58, p = .11) and HbA1c (χ2 (1) = 3.28, p = .070) compared to participants with high early-life SES. These groups had similar rates of low HDL (χ2 (1) = .006, p = .94). By contrast, none of the MetS components varied significantly according to current SES (χ2 (1) > 1.22, p’s > .27), though the pattern of values favored those with higher current SES. The bivariate correlations among early-life SES, current SES and each of the MetS summary measures or individual components (Table S1, Supplemental Digital Content 1) also revealed stronger associations of these MetS risk indices with early-life SES compared to current SES.
Figure 1.
MetS components prevalence by early-life SES and current SES.
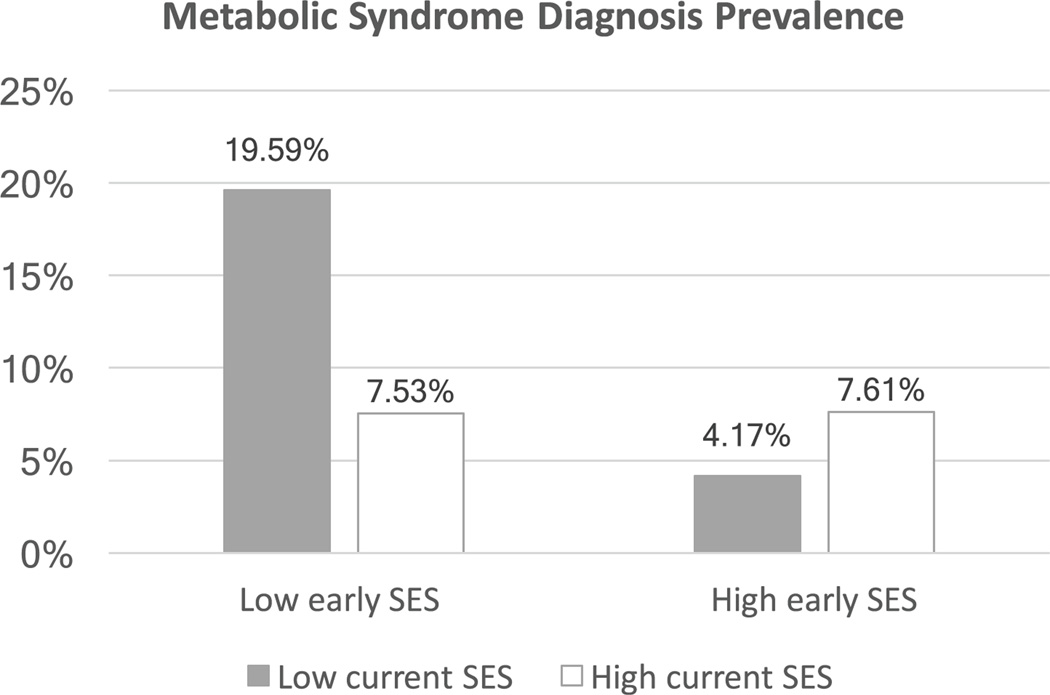
Using logistic regression, we next examined whether the prevalence of MetS diagnoses varied according to early-life SES, current SES, and/or their interaction. Table 2 presents the results of these analyses, in both crude and covariate-adjusted models. In both cases there was a significant main effect of early-life SES (p = .015), such that participants from low SES backgrounds had 1.83 greater odds (CI: 1.12 –2.96) of meeting IDF criteria for MetS diagnosis compared to those with high SES backgrounds. In both models, the main effect of current SES was non-significant (OR = .96, CI = .59 –1.57), and there was a significant early by current SES interaction (OR = 1.78, CI = 1.1 –2.87). To decipher the interaction, we conducted follow-up logistic regression analyses using dummy coding for the four SES subgroups (low-low, low-high, high-low and high-high combinations of early-life and current SES). When using the low-low SES group as the reference, analyses revealed that the other three groups all had significantly lower odds of meeting the diagnostic criteria (low-high: OR = .34, CI =.13 –.94; high-low: OR = .10, CI = .02 –.45; and high-high: OR = .33, CI = .12 –.89). When the high-high group was used as the reference, MetS prevalence rates did not differ statistically from the low-high and high-low groups (OR = 1.05, CI = .34 –3.26 and OR = .29, CI = .06 –1.54, respectively). Together, these analyses suggest that consistent exposure to low SES across the life-course is associated with the highest likelihood of adult MetS, and upward mobility may offset the risk of MetS diagnosis (please see Figure 2).
Table 2.
Logistic regression results predicting MetS diagnosis (binary outcome).
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| B | S.E. | Wald | p | Exp (B) | Lower | Upper | |
| Model 1 (N = 354) | |||||||
| Constant | −2.29 | .19 | 138.63 | <.001* | .10 | ||
| Early-life SES | .45 | .19 | 5.27 | .022* | 1.56 | 1.07 | 2.29 |
|
| |||||||
| Model 2 (N = 354) | |||||||
| Constant | −2.20 | .18 | 149.59 | <.001* | .11 | ||
| Current SES | .30 | .18 | 2.81 | .094Δ | 1.35 | .95 | 1.92 |
|
| |||||||
| Model 3 (N = 354) | |||||||
| Constant | −2.39 | .21 | 126.33 | .001* | .09 | ||
| Early-life SES | .43 | .21 | 4.06 | .044* | 1.53 | 1.01 | 2.33 |
| Current SES | .11 | .21 | .29 | .59 | 1.12 | .74 | 1.70 |
| Early-life SES × Current SES | .43 | .21 | 4.17 | .041* | 1.54 | 1.02 | 2.34 |
|
| |||||||
| Model 4 (N = 328) | |||||||
| Constant | −4.79 | 1.05 | 2.87 | .001* | .01 | ||
| Age | .05 | .02 | 6.72 | .010* | 1.05 | .52 | 2.50 |
| Sex | .13 | .40 | .11 | .74 | 1.14 | .64 | 4.79 |
| Race/ethnicity | .56 | .51 | 1.21 | .27 | 1.76 | .57 | 1.75 |
| Alcohol use | .00 | .28 | .00 | 1.00 | 1.00 | .98 | 2.81 |
| Cigarette smoking | .51 | .27 | 3.61 | .058Δ | 1.66 | .91 | 1.02 |
| Physical activity | −.04 | .03 | 1.95 | .16 | .96 | .52 | 2.50 |
| Early-life SES | .60 | .25 | 5.93 | .015* | 1.83 | 1.12 | 2.96 |
| Current SES | −.04 | .25 | .03 | .87 | .96 | .59 | 1.57 |
| Early-life SES × Current SES | .58 | .24 | 5.58 | .018* | 1.78 | 1.10 | 2.87 |
Df = degrees of freedom. CI = confidence interval for Exp (B).
p< .05.
p < .10.
Early-life SES and current SES are effect-coded such that coefficients represent the effect of being low SES.
Figure 2.
MetS prevalence by SES grouping.
The next sets of analyses explored metabolic risk using ordinal and continuous outcomes. We started by defining risk as a simple count of the number of MetS components each participant had. Table 3 presents the results of these analyses in both crude- and covariate-adjusted ordinal regression models. In the unadjusted model, there was a significant main effect of early-life SES (OR = 1.61, CI = 1.10 –2.38), such that participants from low-SES backgrounds exhibited a higher number of MetS components (on average, M = 1.15, SE = .08) compared to participants from high-SES backgrounds (on average, M = .87, SE = .07) (see Fig. S1, Supplemental Digital Content 1, for prevalence). As with the binary diagnostic outcome, the main effect of current SES was non-significant (OR = 1.31, CI = .89 –1.92), and in these models so was the interaction (OR = .78, CI = .53 –1.14). The same pattern emerged in the adjusted model, where there was a main effect of early-life SES (OR = 1.51, CI = .99 –2.29), but not current SES (OR = 1.22, CI = .80 –1.85) or an interaction (OR = .74, CI = .50 –1.12). Given our interest in social mobility, we conducted a follow-up ordinal regression comparing the four SES groups using dummy coding. When using the low-low SES group as the reference, analyses revealed that the high-low and high-high SES groups had significantly lower counts of MetS components (high-low: OR = .49, CI = .27 –.90; and high-high: OR = .54, CI = .31 –.96), whereas the low-high group had marginally (p = .091) fewer symptom counts than the low-low group (OR = .61, CI = .35 –1.08), suggesting a trend-level beneficial effect of upward social mobility. When the high-high group was used as the reference, the count of MetS components in this group did not differ statistically from that in the low-high and high-low groups (OR = 1.12, CI = .64 –1.96 and OR = .91, CI = .50 –1.65, respectively) –i.e., there was no evidence for effects of downward social mobility.
Table 3.
Results of ordinal regression predicting count of MetS components.
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| B | S.E. | Wald | p | Exp(B) | Lower | Upper | |
| Model 1 (N = 354) | |||||||
| Early-life SES | .48 | .20 | 5.88 | .015* | 1.61 | 1.10 | 2.36 |
|
| |||||||
| Model 2 (N = 354) | |||||||
| Current SES | .31 | .19 | 2.48 | .12 | 1.36 | .93 | 1.99 |
|
| |||||||
| Model 3 (N = 354) | |||||||
| Early-life SES | .48 | .20 | 5.90 | .015* | 1.61 | 1.10 | 2.38 |
| Current SES | .27 | .20 | 1.90 | .17 | 1.31 | .89 | 1.93 |
| Early-life SES × Current SES | −.25 | .20 | 1.63 | .20 | .78 | .53 | 1.14 |
|
| |||||||
| Model 4 (N = 328) | |||||||
| Age | .03 | .01 | 11.29 | .001 | 1.04 | 1.01 | 1.06 |
| Sex | .16 | .21 | .57 | .45 | 1.17 | .78 | 1.76 |
| Race/ethnicity | .04 | .25 | .03 | .87 | 1.04 | .64 | 1.69 |
| Alcohol use | −.25 | .15 | 2.74 | .098 | .78 | .58 | 1.05 |
| Cigarette smoking | .33 | .18 | 3.39 | .066 | 1.39 | .98 | 1.98 |
| Physical activity | −.02 | .02 | 1.22 | .27 | .98 | .95 | 1.01 |
| Early-life SES | .41 | .21 | 3.72 | .054Δ | 1.51 | .99 | 2.29 |
| Current SES | .20 | .21 | .84 | .36 | 1.22 | .80 | 1.85 |
| Early-life SES × Current SES | −.30 | .21 | 2.03 | .15 | .74 | .50 | 1.12 |
Df = degrees of freedom. CI = confidence interval for Exp(B).
p < .05.
p < .10.
Early-life SES and current SES are effect-coded such that coefficients represent the effect of being low SES.
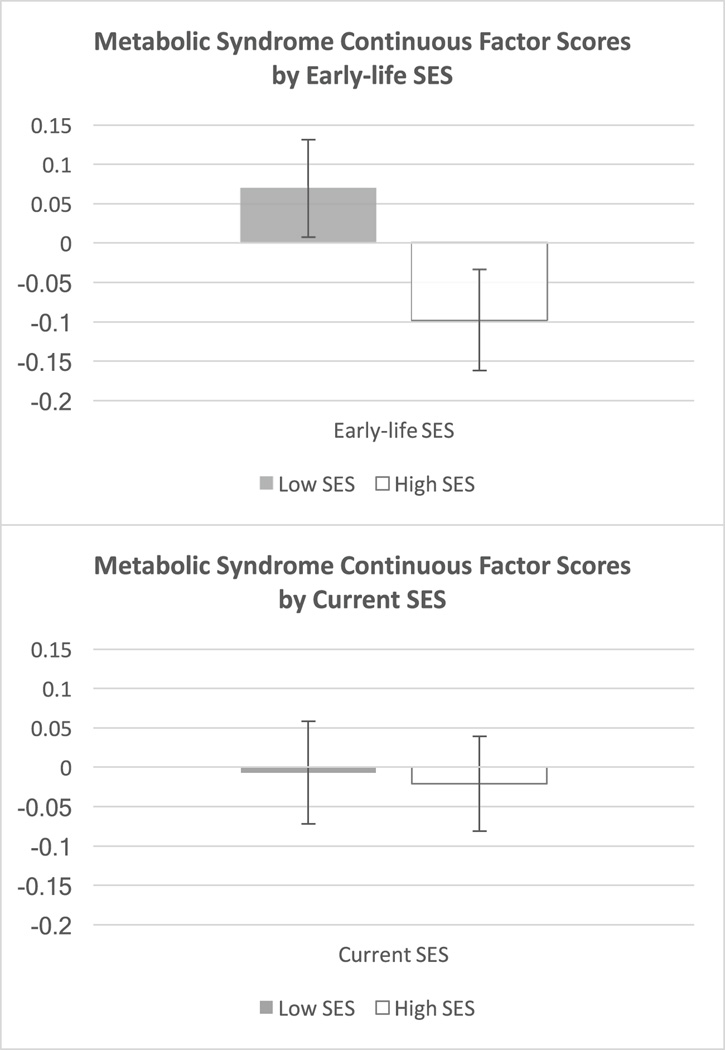
Next, we used factor analysis to derive a continuous metabolic risk score for each participant based on the severity of his/her signs. These factor scores reflected the common variance of the five continuous metabolic measures (see details in the Methods section). As Table 4 shows, the pattern of results mirrored those above. There was a significant main effect of early-life SES (p = .010) in the crude model, such that participants from low-SES backgrounds had higher composite scores (M = .11, SE = .06) than participants from high-SES backgrounds (M = −.13, SE = .07). This difference became marginal (adjusted p = .063) with the introduction of covariates (see Figure 3 for estimated marginal means in this adjusted analysis). Neither the current SES main effect nor the interaction were significant (F(1,350) = .37, p = .54, and F(1,350) = 1.03, p = .31, respectively). With respect to social mobility effects, the main effect of SES in a one-way ANOVA was statistically significant in unadjusted analyses, F(3, 350) = 2.74, p = .043, but was no longer significant with covariate adjustment, F(3, 318) = 1.34, p = .26, thus we did not probe pairwise comparisons between the four SES groups any further.
Table 4.
The association between early-life and adult SES with continuous MetS factor scores.
| F | Df | p | Partial η2 | |
|---|---|---|---|---|
| Model 1 (N = 354) | ||||
| Early-life SES | 6.73 | 1, 352 | .010* | .019 |
|
| ||||
| Model 2 (N = 354) | ||||
| Current SES | .76 | 1, 352 | .39 | .002 |
|
| ||||
| Model 3 (N = 354) | ||||
| Early-life SES | 6.71 | 1, 350 | .010* | .019 |
| Current SES | .37 | 1, 350 | .54 | .001 |
| Early-life SES × Current SES | 1.03 | 1, 350 | .31 | .003 |
|
| ||||
| Model 4 (N = 328) | ||||
| Age | 26.5 | 1, 318 | <.001* | .077 |
| Sex | 35.54 | 1, 318 | <.001* | .101 |
| Race/ethnicity | .009 | 1, 318 | .92 | <.001 |
| Alcohol use | .70 | 1, 318 | .40 | .002 |
| Cigarette smoking | 1.35 | 1, 318 | .25 | .004 |
| Physical activity | 4.19 | 1, 318 | .041* | .013 |
| Early-life SES | 3.49 | 1, 318 | .063Δ | .011 |
| Current SES | .02 | 1, 318 | .88 | <.001 |
| Early-life SES × Current SES | .60 | 1, 318 | .44 | .002 |
Df = degrees of freedom. Partial η2 is reported as a measure of effect size.
p < .05.
p < .10.
Figure 3.
Estimated marginal means for continuous MetS factor scores. Marginal means are adjusted for covariates (age, sex, race/ethnicity, cigarette smoking, alcohol consumption, and physical activity levels). Error bars are SEMs.
Discussion
The prevalence rates of MetS have been rising globally (6) and its distribution and pathogenesis need to be better understood (1,41). Epidemiological studies are increasingly showing socioeconomic stratification of MetS risk (1), which may in turn contribute to explaining social gradients in coronary heart disease morbidity and mortality (10,42). The present study aimed to disentangle the roles of early-life and current SES in explaining MetS risk in a sample of healthy Canadian adults. Secondly, we aimed to test whether there was any evidence consistent with effects of upward or downward social mobility on MetS risk.
We found a consistent pattern across all three metabolic outcomes used: diagnosis according to IDF criteria, count of MetS components present, and continuous factor scores. In each case there was a main effect of early-life SES, indicating greater metabolic risk among participants from disadvantaged backgrounds, and a non-significant main effect of current SES; additionally, there was a significant interaction of early-life SES and current SES in predicting MetS diagnosis. On average, participants with low childhood SES had 1.83 greater odds of meeting IDF criteria for MetS diagnosis compared to those with high early-life SES. Is this difference clinically meaningful? One way to address this question is to compare its magnitude to that of other established risk factors. In this regard, the 83% higher likelihood of MetS diagnosis in those from low early-life SES backgrounds is comparable to the MetS risks associated with cigarette smoking in this sample, and the risks associated with 16 years of aging. Furthermore, those meeting criteria for MetS diagnosis have a 2-fold increased risk of cardiovascular events or death (5) and a 5-fold increased risk of diabetes (1), which would translate to substantially higher mortality levels at the population level.
The nonsignificant main effect of current SES may seem puzzling in light of the prior literature revealing concurrent associations between adult SES and MetS risk (1). However, many individuals who experience low SES in adulthood are exposed to socioeconomic disadvantage throughout their lifespan (23), so it is unclear whether adult SES in any of these studies is an independent risk factor or reflects lifelong disadvantage. Only a few studies have explicitly tested the relative roles of childhood and adult SES against each other, and among these reports, the evidence is quite mixed. When modeling childhood and adult SES together, some studies with large samples have found that only childhood SES retains explanatory power (16,17,24,25), consistent with our findings. Some have found that only adult SES is significant (26) or that both childhood and adult SES are significant (27–29). Prospective studies found a prevailing role of childhood SES over adult SES (17,24,25), whereas retrospective studies (26) tended to find a stronger role for concurrent SES, suggesting that, when precisely measured, childhood SES might play a stronger role in shaping MetS risk than adult SES. The novel contribution of the present study is that it isolates the effects of early and current SES from each other. From a scientific perspective, this is a useful approach. But since life-course continuity in SES is the norm in many countries – particularly the United States – the patterns seen here may not reflect trends at the population level. (In other words, upwardly and downwardly mobile people are over-represented in our sample relative to the population at large.)
The lack of a main effect of current SES in the present study could also be interpreted in light of the statistical interaction observed. Low current SES was only associated with increased MetS diagnosis for individuals who also experienced low early SES, and there was no evidence of metabolic risk associated with downward mobility. These patterns suggest the possibility that current low SES shapes MetS risk, but may require sensitization by adverse early-life experiences. There is certainly some suggestive evidence from nonhuman animals that early-life conditions may have developmental programming effects that affect lifelong metabolism (13,15,20). Perhaps with the closure of putative sensitive periods for shaping neuroendocrine and metabolic function, the role of environmental adversity becomes progressively weaker across the lifespan. Or perhaps it is simply an issue of duration of exposure, and the metabolic risk associated with current low SES is contingent on a sufficient prior experience of disadvantage. Future studies will have to test these competing explanations.
An additional consideration in interpreting the findings is that the present sample was Canadian. Socioeconomic gradients in MetS components like obesity are less steep in Canada, when compared to countries that are culturally similar like the United States (43). It is possible that in societies with less pronounced health disparities, adverse experiences need to occur during sensitive or vulnerable periods for these effects to become manifest, but more cross-national research will be needed to test this hypothesis.
Turning to the interaction between early-life and current SES, we found varying patterns when examining the three MetS outcomes. Namely, there was a significant interaction between early-life and current SES in predicting the binary MetS variable, such that diagnosis rates were higher among participants with a low-low pattern relative to individuals with other trajectories. This interaction was non-significant in predicting the ordinal and continuous metabolic outcomes. This is unlikely to be an artifact of differential statistical power, as binary outcomes generally require greater power to detect than continuous outcomes. One possibility is that the MetS diagnosis may reflect an underlying pathological process, which is more than the sum of its parts, as has been discussed (33,34). This putative disease state might be less well-measured by the component count and factor scores, thus diluting some of the associations with SES indices.
Nevertheless, the conflicting patterns of findings make it difficult to draw firm conclusions about the importance of mobility. Based on the binary diagnostic outcome, it appears that upward mobility can offset the MetS risks associated with low early SES. Indeed, in the upwardly mobile group (low-high trajectory), prevalence of MetS was statistically identical to the high-high and high-low groups, a pattern that is consistent with at least one prior study (32). However, the non-significant interactions in the models with ordinal and continuous metabolic outcomes suggest that these offsetting influences of mobility are not especially robust. These patterns are consistent with an emerging developmental literature, which suggests that upward mobility may entail some cost to health, particularly for individuals from highly disadvantaged backgrounds (44–46). Replication will also be needed to rule out the possibility of Type I error.
The present study also found no evidence that downward mobility was associated with detrimental effects on metabolic health. This suggests that high early-life SES may confer resilience against later disruptions, supporting the notion that intervening early in the lifespan may be beneficial. Nevertheless, we cannot rule out the possibility that the negative effects of downward mobility in adulthood might simply take longer to manifest, as discussed above, or that we had limited statistical power to detect these effects given that the sample size for individuals in the high-low group was slightly smaller than the others. Furthermore, given the correlational nature of these analyses, conclusions regarding social mobility are merely suggestive of patterns that should be explored using experimental (e.g., cash transfer interventions) or quasi-experimental (e.g., adoption studies) methodology.
The present study’s design provides a novel contribution to our inferences about the relative roles of early-life versus later SES, by disentangling two SES dimensions that typically co-vary in the population. The inevitable tradeoff of the recruitment strategy in this study was that the sample was not designed to be a representative section of the Canadian population, which may limit the generalizability of the findings. Participants studied here were also younger than the general adult population (only those 55 years or younger were recruited), healthier (no history of chronic disease), and participants who experienced upward or downward mobility were oversampled, which allowed us enough participants to begin to model these effects that have generated mixed findings in the literature (28,30–32). It is not surprising then that the average rate of MetS in our sample (10.2%) was slightly lower than that of the adult Canadian population (19.1%, 47), suggesting that the effects we report here might be even stronger in the general population. Other limitations include the retrospective assessment of early-life SES, and the absence of measures of childhood metabolic health. Given that poor health in childhood is associated with lower occupational status in adulthood (48), multi-wave prospective longitudinal studies that monitor changes in both SES and health are an important next step for testing social causation versus social selection hypotheses about the links between low SES and health. Additionally, these prospective studies should shed light on potential mediators and moderators explaining the relation between low SES and current MetS risk, which were not examined here –e.g., positive affect (49), sleep (50), work stress (51), practice of relaxation techniques (52) etc.
Despite these limitations, the current study provides new insights into the associations between socioeconomic disadvantage and risk of MetS, which seemed to be more strongly associated with early-life SES than current adult SES. If this observation is supported by future studies, one implication may be that early childhood is an opportune period for targeting interventions to reduce the risk of MetS across the lifespan.
Supplementary Material
Acknowledgments
We thank the participants for their contribution to this project. This research was supported by NIH Grants R01 HD058502 and F32 HD078048.
Footnotes
Conflicts of Interest: Authors have no conflicts of interest to declare.
References
- 1.Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The Metabolic Syndrome. Arch Intern Med. 2003;163:1988–1994. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364–373. doi: 10.1016/j.tcm.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome -A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami La, Somers VK, et al. Metabolic Syndrome and risk of incident cardiovascular events and death. J Am Coll Cardiol. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- 7.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world — A growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 8.Manuck S, Phillips J, Gianaros P, Flory J, Muldoon M. Subjective socioeconomic status and presence of the Metabolic Syndrome in midlife community volunteers. Psychosom Med. 2010;72(1):35–45. doi: 10.1097/PSY.0b013e3181c484dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz MS, Myers HF, Schetter CD, Rodriguez CJ, Seeman TE. Psychosocial predictors of metabolic syndrome among Latino groups in the Multi- Ethnic Study of Atherosclerosis (MESA) PLoS One. 2015;10(4):e0124517. doi: 10.1371/journal.pone.0124517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silventoinen K, Pankow J, Jousilahti P, Hu G, Tuomilehto J. Educational inequalities in the metabolic syndrome and coronary heart disease among middle-aged men and women. Int J Epidemiol. 2005;34(2):327–334. doi: 10.1093/ije/dyi007. [DOI] [PubMed] [Google Scholar]
- 11.Chun S, Lee S, Son H, Noh H, Oh H, Jang HB, Lee HJ, Kang JH, Song HJ, Paek YJ, Park KH. Clinical characteristics and metabolic health status of obese Korean children and adolescents. Korean J Fam Med. 2015;36:233–238. doi: 10.4082/kjfm.2015.36.5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saland JM. Update on the metabolic syndrome in children. Curr Opin Pediatr. 2007;19(2):183–191. doi: 10.1097/MOP.0b013e3280208519. [DOI] [PubMed] [Google Scholar]
- 13.Faienza MF, Wang DQH, Fruhbeck G, Garruti G, Portincasa P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med. 2016;11(2):175–182. doi: 10.1007/s11739-015-1382-6. [DOI] [PubMed] [Google Scholar]
- 14.Gustafsson PE, Persson M, Om AH. Life course origins of the Metabolic Syndrome in middle-aged women and men: The role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Ann Epidemiol. 2011;21:103–110. doi: 10.1016/j.annepidem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. 2010 Jan;10:525. doi: 10.1186/1471-2458-10-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehman B, Taylor S, Kiefe C, Seeman T. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosom Med. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- 17.Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, Appleton AA, Román JC, Buka SL, Kubzansky LD. Childhood social disadvantage, cardiometabolic risk, and chronic disease in adulthood. Am J Epidemiol. 2014;180(3):263–271. doi: 10.1093/aje/kwu127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi B, Lee D, Chun E, Lee J. The relationship between Metabolic Syndrome and childhood maternal education level, job status. Findings from the Korean National Health and Nutrition Examination, 2007–2009. Korean J Fam Med. 2014;35(4):207–215. doi: 10.4082/kjfm.2014.35.4.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson PE, Hammarström A. Socioeconomic disadvantage in adolescent women and metabolic syndrome in mid-adulthood: An examination of pathways of embodiment in the Northern Swedish Cohort. Soc Sci Med. 2012;74(10):1630–1638. doi: 10.1016/j.socscimed.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61(5):611–619. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011 Nov;137(6):959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biol Psychiatry. 2016;80(1):23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagmiller R, Adelman R. Childhood and intergenerational poverty: The long-term consequences of growing up poor. Natl Cent Child Poverty Reports. 2009:1–7. [Google Scholar]
- 24.Langenberg C, Kuh D, Wadsworth MEJ, Brunner E, Hardy R. Social circumstances and education: life course origins of social inequalities in metabolic risk in a prospective national birth cohort. Am J Public Health. 2006;96(12):2216–2221. doi: 10.2105/AJPH.2004.049429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker L, Lamont DW, Unwin N, Pearce MS, Bennett SMA, Dickinson HO, White M, Mathers JC, Alberti KG, Craft AW. A lifecourse study of risk for hyperinsulinaemia, dyslipidaemia and obesity (the central metabolic syndrome) at age 49 – 51 years. Diabet Med. 2003;20:406–415. doi: 10.1046/j.1464-5491.2003.00949.x. [DOI] [PubMed] [Google Scholar]
- 26.Lucove JC, Kaufman JS, James SA. Association between adult and childhood socioeconomic status and prevalence of the Metabolic Syndrome in African Americans : The Pitt County Study. Am J Public Health. 2007;97(2):234–236. doi: 10.2105/AJPH.2006.087429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, Heiss G. Life course socioeconomic conditions and Metabolic Syndrome in adults: The Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol. 2009;19(12):875–883. doi: 10.1016/j.annepidem.2009.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawlor DA, Ebrahim S, Smith GD. Socioeconomic position in childhood and adulthood and insulin resistance: Cross sectional survey using data from British women’s heart and health study. BMJ. 2002;325:1–5. doi: 10.1136/bmj.325.7368.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schooling CM, Jiang CQ, Lam TH, Zhang WS, Cheng KK, Leung GM. Life-course origins of social inequalities in metabolic risk in the population of a developing country. Am J Epidemiol. 2008;167(4):419–428. doi: 10.1093/aje/kwm329. [DOI] [PubMed] [Google Scholar]
- 30.Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to resilience: Maternal nurturance as a buffer against the effects of childhood poverty on metabolic syndrome at midlife. Psychol Sci. 2011 Dec;22(12):1591–1599. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lidfeldt J, Li TY, Hu FB, Manson JE, Kawachi I. A prospective study of childhood and adult socioeconomic status and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165(8):882–889. doi: 10.1093/aje/kwk078. [DOI] [PubMed] [Google Scholar]
- 32.Stringhini S, Batty GD, Bovet P, Shipley MJ, Marmot MG. Association of lifecourse socioeconomic status with chronic inflammation and type 2 diabetes risk: The Whitehall II prospective cohort study. PLoS Med. 2013;10(7):1–15. doi: 10.1371/journal.pmed.1001479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis. Diabetes. 2002;51(18):3069–3076. doi: 10.2337/diabetes.51.10.3069. [DOI] [PubMed] [Google Scholar]
- 34.Beaser RS, Levy P. Metabolic syndrome: A work in progress, but a useful construct. Circulation. 2007;115:1812–1818. doi: 10.1161/CIRCULATIONAHA.106.673616. [DOI] [PubMed] [Google Scholar]
- 35.Ong KL, Tso AW, Lam KS, Cherny SS, Sham PC, Cheung BM. Using glycosylated hemoglobin to define the metabolic syndrome in United States adults. Diabetes Care. 2010;33:1856–1858. doi: 10.2337/dc10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis CR, Usher N, Dearing E, Barkai A, Crowell-Doom C, Neupert S, Mantzoros CS, Crowell JA. Attachment and the Metabolic Syndrome in midlife: The role of interview-based discourse patterns. Psychosom Med. 2014;76(8):611–621. doi: 10.1097/PSY.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiley JF, Gruenewald TL, Karlamangla AS, Seeman TE. Modeling multisystem physiological dysregulation. Psychosom Med. 2016;78(3):1–12. doi: 10.1097/PSY.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller GE, Cohen S, Rabin BS, Skoner DP, Doyle WJ. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain Behav Immun. 1999;123:109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- 39.Cohen S, Tyrrell D, Russell M, Jarvis M, Smith A. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. doi: 10.2105/ajph.83.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paffenbarger RS, Blair SN, Lee I-M, Hyde RT. Measurement of physical activity to assess health effects in free-living populations. Med Sci Sports Exerc. 1993;25(1):60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Abraham NG, Brunner EJ, Eriksson JW. Metabolic syndrome: Psychosocial, neuroendocrine, and classical risk factors in type 2 diabetes. Ann N Y Acad Sci. 2007;275:256–275. doi: 10.1196/annals.1391.015. [DOI] [PubMed] [Google Scholar]
- 42.Marmot MG, Shipley MJ, Hemingway H, Head J, Brunner EJ. Biological and behavioural explanations of social inequalities in coronary heart disease: the Whitehall II study. Diabetologia. 2008 Nov;51(11):1980–1988. doi: 10.1007/s00125-008-1144-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddiqi A, Brown R, Nguyen QC, Loopstra R, Kawachi I. Cross-national comparison of socioeconomic inequalities in obesity in the United States and Canada. Int J Equity Health. International Journal for Equity in Health. 2015;14:1–10. doi: 10.1186/s12939-015-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brody GH, Yu T, Chen E, Miller GE, Kogan SM, Beach SRH. Is resilience only skin deep? Rural african americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychol Sci. 2013 Jul 1;24(7):1285–1293. doi: 10.1177/0956797612471954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen E, Miller GE, Brody GH. Neighborhood poverty, college attendance, and diverging profiles of substance use and allostatic load in rural African American youth. Clin Psychol Sci. 2015;3(5):675–685. doi: 10.1177/2167702614546639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller GE, Yu T, Chen E, Brody GH. Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proc Natl Acad Sci. 2015;112(33):10325–10330. doi: 10.1073/pnas.1505063112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riediger ND, Clara I. Prevalence of metabolic syndrome in the Canadian adult population. Can Med Assoc J. 2011;183(15):1127–1134. doi: 10.1503/cmaj.110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elovainio M, Ferrie JE, Singh-Manoux A, Shipley M, Batty GD, Head J, Hamer M, Jokela M, Virtanen M, Brunner E, Marmot MG, Kivimäki M. Socioeconomic differences in cardiometabolic factors: Social causation or health-related selection? Evidence from the Whitehall II Cohort Study, 1991 – 2004. Am J Epidemiol. 2011;174(19):779–789. doi: 10.1093/aje/kwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boylan JM, Ryff CD. Psychological well-being and metabolic syndrome: Findings from the Midlife in the United States national sample. Psychosom Med. 2015;77(5):548–558. doi: 10.1097/PSY.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talbot LS, Rao MN, Cohen BE, Richards A, Inslicht SS, Donovan AO, Maguen S, Metzler TJ, Neylan TC. Metabolic risk factors and posttraumatic stress disorder: The role of sleep in young, healthy adults. Psychosom Med. 2015;77(4):383–391. doi: 10.1097/PSY.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dich N, Lange T, Head J, Rod NH. Work stress, caregiving, and allostatic load: Prospective results from the Whitehall II cohort study. Psychosom Med. 2015;77(5):539–547. doi: 10.1097/PSY.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Younge JO, Leening MJG, Tiemeier H, Franco OH, Jong JK, Hofman A, Roos-Hesselink JW, Hunink MG. Association between mind-body practice and cardiometabolic risk factors: The Rotterdam Study. Psychosom Med. 2015;77(7):775–783. doi: 10.1097/PSY.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 53.Bennett DA. How can I deal with missing data in my study? Australian and New Zealand Journal of Public Health. 2001;25(5):464–469. http://doi.org/10.1111/j.1467-842X.2001.tb00294.x. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.