Abstract
Fear of gaining weight after quitting cigarette smoking is a major barrier to smoking cessation among women. Distress tolerance, which refers to one’s ability and willingness to tolerate physical and emotional discomfort, predicts successful behavior change. Novel interventions rooted in Acceptance and Commitment Therapy (ACT) have emerged that aim to increase distress tolerance and engagement in values-oriented behavior. In this study, we developed a 9-week, group-based distress tolerance intervention for weight concern in smoking cessation among women (DT-W). Using an iterative process, we piloted DT-W with two small groups (n = 4 and n = 7) of female weight-concerned smokers. Results indicated that we successfully established the feasibility and acceptability of DT-W, which was well-attended and well-received. Biochemically verified 7-day point-prevalence abstinence rates at post-intervention, 1, 3, and 6 months were 64%, 36%, 27%, and 27%, respectively. We are now evaluating DT-W in a randomized controlled trial.
Keywords: smoking cessation, tobacco cessation, Acceptance and Commitment Therapy, distress tolerance, weight
Introduction
Cigarette smoking cessation is accompanied by weight gain for about 80% of quitters. The mean weight gain is 10 lb within the first year after quitting, with most of this weight gained within the first 6 months (Aubin, Farley, Lycett, Lahmek, & Aveyard, 2012). This weight is not typically lost; rather, weight gain continues at a slower rate. One study found that after 8 years, quitters had gained 19 lb compared with 5 lb for continuing smokers (Lycett, Munafò, Johnstone, Murphy, & Aveyard, 2011).
Women are much more likely than men to report that they smoke cigarettes as a means of weight control (Pinto et al., 1999; Weekley, Klesges, & Reylea, 1992; White, McKee, & O’Malley, 2007) and to be concerned about gaining weight after smoking cessation (Clark et al., 2006; Levine, Perkins, & Marcus, 2001). The majority of female smokers are concerned about post-cessation weight gain (Beebe & Bush, 2015; Jeffery, Hennrikus, Lando, Murray, & Liu, 2000; Pirie, Murray, & Luepker, 1991; Pomerleau, Zucker, & Stewart, 2001), with 75% reporting that they are unwilling to gain more than 5 lb, and 90% unwilling to gain more than 10 lb (Levine et al., 2001; Pomerleau & Kurth, 1996). In other studies, smokers were asked whether they would relapse if they gained weight up to 20 lb. Half of women said they would relapse, compared with only 25% of male smokers (Clark et al., 2004; Cooper, Dundon, Hoffman, & Stoever, 2006). Caucasian and obese female smokers are most likely to be weight-concerned, but weight concern affects the majority of female smokers in all body mass index (BMI) categories except underweight and a significant proportion from all races and ethnicities (Beebe & Bush, 2015; Pomerleau, Zucker, Namenek Brouwer, Pomerleau, & Stewart, 2001; Pomerleau, Zucker, & Stewart, 2001). A national random-digit-dialing survey revealed that compared with female smokers who were “not at all” or “somewhat” weight-concerned, those who were “very” weight-concerned were less likely to have post–high school education, but did not differ in age or household income (Pomerleau, Zucker, & Stewart, 2001).
Female smokers report that their fear of gaining weight deters them from initiating quit attempts (Jeffery et al., 2000; Pomerleau, Zucker, & Stewart, 2001; Weekley et al., 1992) and decreases their confidence in their ability to quit (Bowen, McTiernan, Powers, & Feng, 2000). They also attribute past relapses to anticipated or actual weight gain (Jarry, Coambs, Polivy, & Herman, 1998; Pisinger & Jorgensen, 2007; Pomerleau, Zucker, & Stewart, 2001; Swan, Ward, Jack, & Javitz, 1993). Consistent with women’s retrospective self-reports, weight concern is indeed associated with poor smoking cessation treatment outcomes, including more severe self-reported withdrawal symptoms (Pinto et al., 1999), failure to attend the first treatment session and treatment dropout (Copeland, Martin, Geiselman, Rash, & Kendzor, 2006; Mizes et al., 1998; Namenek Brouwer & Pomerleau, 2000), and relapse (Clark et al., 2006; Jeffery et al., 2000; Meyers et al., 1997).
Researchers have previously tested two types of adjunctive interventions to address weight concern in smoking cessation among women. The first type aims to prevent weight gain directly; approaches have included behavioral weight management treatment (e.g., reduced calorie diet) and pharmacotherapy. Reviews support a short-term benefit of behavioral weight management treatment in some studies but no long-term effects on smoking or weight outcomes (Farley, Hajek, Lycett, & Aveyard, 2012; Parsons, Shraim, Inglis, Aveyard, & Hajek, 2009; Spring et al., 2009). Regarding pharmacotherapy, a recent meta-analysis revealed that nicotine replacement therapies, bupropion, and varenicline did not produce long-term effects on weight outcomes (Aubin et al., 2012). Furthermore, weight gain trajectories were similar regardless of use or non-use of medication (Aubin et al., 2012).
Perkins and colleagues developed an alternative cognitive-behavioral therapy (CBT) approach for weight-concerned female smokers intended to reduce negative affect associated with fear of weight gain and body dissatisfaction rather than to prevent weight gain directly. Women were encouraged to challenge their negative thoughts about weight and body image, whereas dieting was discouraged (Levine, Marcus, & Perkins, 2003; Levine et al., 2010; Perkins et al., 2001). An initial trial, which did not include pharmacotherapy, demonstrated a positive effect of this CBT relative to behavioral weight management treatment or smoking treatment only on both smoking and weight outcomes. Unexpectedly, however, no impact on weight concern was found, indicating that the mechanism of treatment was unclear (Perkins et al., 2001). A second trial revealed only a marginal effect of CBT relative to bupropion (Levine et al., 2010).
Perkins and colleagues’ approach, which targeted negative affect associated with weight and body image concerns in the context of smoking cessation rather than actual weight, is consistent with theory and research implicating negative affect as a key factor in the maintenance of tobacco use (e.g., Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Khantzian, 1997; Leventhal & Zvolensky, 2015), a primary precipitant of relapse (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996), and a mediator of the relationship between body image dissatisfaction and craving to smoke (Lopez Khoury, Litvin, & Brandon, 2009). It might be expected that the severity or magnitude of negative affect experienced during a quit attempt would be a primary predictor of relapse. However, recent research has revealed that the extent of an individual’s willingness and ability to tolerate aversive experiences such as physical or emotional distress (i.e., distress tolerance; Leventhal & Zvolensky, 2015) is an important contributor to outcomes in smoking cessation above and beyond the absolute severity or magnitude of distress (Brandon et al., 2003; Brown, Lejuez, Kahler, & Strong, 2002; Brown et al., 2009; Trujillo et al., 2015). A framework by Leventhal and Zvolensky posits that distress tolerance is a transdiagnostic emotional vulnerability such that individuals who are low in distress tolerance are particularly motivated to smoke to relieve or escape distress (Leventhal & Zvolensky, 2015) and therefore may be more likely to relapse when experiencing distress (e.g., nicotine withdrawal, cravings, general life stress). Similar models have been proposed to explain the influence of distress tolerance on weight management (Forman & Butryn, 2015; Lillis & Kendra, 2014).
Supporting these models, studies have shown that smokers are less persistent than non-smokers on physically or emotionally aversive tasks (e.g., breath-holding, mental arithmetic, tracing geometric figures from the perspective of a mirror; Brown et al., 2002; Quinn, Brandon, & Copeland, 1996) and that the duration of persistence on these tasks prospectively predicts successful cessation (Brandon et al., 2003; Brown et al., 2009). Furthermore, low distress tolerance is positively associated with indices of nicotine dependence after controlling for symptoms of negative affect (Trujillo et al., 2015), and smokers who are low in self-reported distress tolerance believe that quitting smoking will be difficult because smoking serves as a primary method of affect regulation (Kraemer, McLeish, Jeffries, Avallone, & Luberto, 2013). Other recent research has focused on the related concept of experiential avoidance, which refers to efforts to control, suppress, or escape distress (e.g., nicotine withdrawal, craving). Reduction of experiential avoidance has been implicated in successful smoking cessation as well as weight management (Lillis, Hayes, & Levin, 2011; Minami, Bloom, Reed, Hayes, & Brown, 2015; Niemeier, Leahey, Palm Reed, Brown, & Wing, 2012), suggesting a common clinical pathway for smoking cessation and weight control (see Gifford & Lillis, 2009).
These findings have influenced the development of novel treatments intended to increase distress tolerance (e.g., Brown et al., 2013). Distress tolerance skills have been primarily derived from, and share many common elements with, Acceptance and Commitment Therapy (ACT; Hayes, Luoma, Bond, Masuda, & Lillis, 2006). The central focus of ACT is on teaching the practice of acceptance, defined as “the active and aware embrace” of uncomfortable or aversive thoughts and feelings “without unnecessary attempts to change their frequency or form” for the purpose of “increasing values-based action” (Hayes et al., 2006, pp. 7–8). The theoretical rationale for incorporating these skills is that behaviors such as smoking or eating are often used to reduce, avoid, escape, and/or cope with negative thoughts and feelings and interfere with engagement in values-oriented behaviors; individuals are taught that values-oriented behavior can be engaged in regardless of the presence or severity of distress (Hayes et al., 2006).
Promising results are now emerging for interventions that incorporate acceptance-based techniques in the areas of weight management (e.g., Forman et al., 2013; Katterman, Goldstein, Butryn, Forman, & Lowe, 2014), improving disordered eating and body image (e.g., Pearson, Follette, & Hayes, 2012), and increasing physical activity (e.g., Butryn, Forman, Hoffman, Shaw, & Juarascio, 2011), as well as in smoking cessation (e.g., Bricker, Mann, Marek, Liu, & Peterson, 2010; Hernández-López, Luciano, Bricker, Roales-Nieto, & Montesinos, 2009). The use of acceptance-based interventions for addressing negative affect associated with body dissatisfaction among non-eating disordered women is particularly compelling because the “pervasive yet subclinical nature of the problem” (Pearson et al., 2012, p. 182) across the life span may make it particularly resistant to change-based strategies (see also Juarascio, Forman, & Herbert, 2010). Furthermore, there is considerable support for theorized mechanisms of action for acceptance-based approaches and for a general relationship between distress tolerance and related constructs and mental and physical health (Ciarrochi, Billich, & Godsell, 2010). Among weight-concerned female smokers, two specific behaviors may be conceptualized as evidence of limited distress tolerance: (a) continuing to smoke (i.e., not initiating a quit attempt) to avoid fear of post-cessation weight gain and negative affect associated with body dissatisfaction more generally, and (b) after quitting, eating instead of smoking to reduce, avoid, escape, or cope with stress (Hudmon, Gritz, Clayton, & Nisenbaum, 1999).
In the current study, we developed and piloted a group-based intervention for weight-concerned female smokers that retained Perkins and colleagues’ focus on negative affect associated with fear of weight gain and body dissatisfaction but used an alternative distress tolerance-based orientation. Our distress tolerance intervention for weight concern in smoking cessation among women (DT-W) promoted acceptance of negative weight-related thoughts and feelings, reduction of eating triggered by external and/or emotional cues, and increased engagement in values-oriented behaviors. DT-W also included all components of standard cognitive-behavioral smoking cessation treatment (Standard Treatment, ST; Brown, 2003) and an 8-week course of transdermal nicotine patch (TNP). The purpose of this pilot study was to establish the feasibility and acceptability of DT-W. We also piloted the assessment procedures for a planned future randomized controlled trial (RCT), including administration of primary outcome measures and several process measures that we hypothesized would be potential mechanisms of treatment efficacy.
Method
Participants
Two small groups of adult female smokers were recruited from the local community via online advertisements on Craigslist and Facebook, and paper flyers and brochures displayed throughout the study site and other public locations (e.g., grocery stores, mall). Advertisements for the study specifically targeted women who wanted to quit smoking and were concerned about gaining weight. The study was branded as the “WE QUIT” Study (Women Engaging in Quitting Smoking Together) in advertisements and participant materials (see Figure 1 for the logo that appeared on all advertisements and participant materials). All staff including group leaders referred to the study as “WE QUIT” and the treatment program as the “WE QUIT program” when communicating with participants. The term DT-W was not used with participants.
Figure 1.

The WE QUIT study logo that was used in advertisements and on participant materials.
Eligible women were 18 to 65 years old, had smoked ≥10 cigarettes per day for ≥1 year, were motivated to quit, and were concerned about post- cessation weight gain, defined as a rating of at least 50 on at least one of two 100-point rating scales: “How concerned are you about gaining weight after quitting smoking” and “How concerned would you be if quitting smoking caused you to permanently gain 10–15 pounds?” (Levine et al., 2010, p. 544; Perkins et al., 2001, p. 605). Women were excluded if they were currently using other smoking cessation or weight loss treatments; used other tobacco products at least weekly; were pregnant or breast-feeding; had a medical condition that was a contraindication for the use of TNP; self-reported a current diagnosis of non-nicotine substance use disorder (SUD) or a lifetime diagnosis of eating, bipolar, or psychotic disorder; were taking psychotropic medication except for selective serotonin reuptake inhibitors (SSRIs); or scored above standard cutoffs on self-report screening measures of depression, eating disorder, or SUD symptoms (see “Measures” section below). Initially, we also excluded women with a BMI of >35 (Obesity Class II), with the rationale that a weight loss program was more appropriate (i.e., medically advisable) and desirable for these women. However, shortly after beginning recruitment for the first group, we eliminated this criterion after observing that women were interested who were otherwise eligible except for having a BMI >35. We realized that although it would be medically advisable for women with a BMI >35 to lose weight, other smoking cessation trials have not typically had BMI restrictions. Therefore, women with a BMI >35 would not typically receive a weight loss program as part of smoking cessation treatment. Furthermore, we believed that the risk of our experimental intervention resulting in significantly greater weight gain than standard treatment (which could present a more significant health risk for women with BMI >35) was minimal. The participant flow diagram is shown in Figure 2. Sixteen (n = 16) women completed a baseline assessment, of whom 11 attended at least one group session of DT-W. The first group (n = 4) received DT-W in the spring of 2014 and the second group (n = 7) in the fall of 2014.
Figure 2.
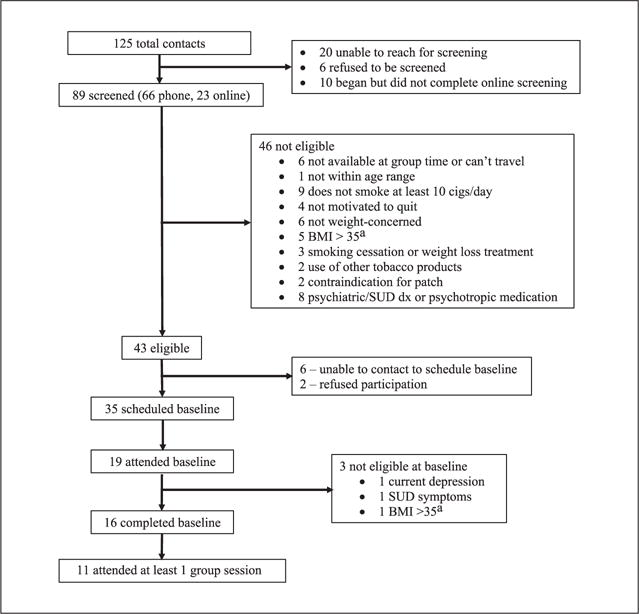
Participant flow diagram.
Note. BMI = body mass index; SUD = substance use disorder.
aThis criterion was eliminated partway through recruitment of the first group.
Procedure
Interested women were screened for preliminary eligibility via telephone by a research assistant or by completing an online survey created using Research Electronic Data Capture (REDCap; Harris et al., 2009). Those who met preliminary criteria were scheduled for an in-person baseline assessment during which they provided written informed consent and completed additional screening to confirm their eligibility, including a urine pregnancy test. When enough women had completed the baseline assessment to form a small group, their group began the intervention. Piloting of DT-W followed an iterative process, in which the original DT-W intervention was revised based on feedback received from participants in the first group, with additional revisions made based on the second group’s feedback. This feedback is described in the “Results” section below.
DT-W intervention
Overview: Structure, clinicians, and treatment components
DT-W consisted of a 60-min individual counseling session followed by eight weekly, 90-min group counseling sessions, with quit date at Group Session 4, and a brief 20-min individual phone session between Group Sessions 4 and 5. Groups were co-led by two clinicians who followed a structured manual to ensure standardized intervention delivery. The spring 2014 (n = 4) group was co-led by a licensed clinical psychologist (E.L.B.) and a clinical psychology postdoctoral fellow (H.M.); the fall 2014 (n = 7) group was co-led by E.L.B. and a master’s level nurse (J.H.). E.L.B. conducted weekly supervision with the other clinicians, both of whom had past experience in delivering similar ACT-based interventions. All sessions were audiotaped or videotaped to facilitate supervision. DT-W included three main components as described below.
ACT-based component of DT-W
Table 1 provides a description of the ACT-based treatment elements in DT-W and the sessions in which they appear in the final version. The ACT-based elements of DT-W were adapted from the following previous ACT-based treatment manuals, with permission from their authors: (a) distress tolerance treatment for smokers with a history of early lapse (Brown et al., 2008; Brown et al., 2013), (b) distress tolerance treatment for smoking cessation in a general population of smokers (manuscript under review, principal investigator [PI]: Richard A. Brown, PhD), (c) acceptance-based behavioral intervention for weight loss (Lillis et al., 2015), (d) ACT for body image dissatisfaction (Pearson et al., 2012; Pearson, Heffner, & Follette, 2010), and (e) Mindfulness and Acceptance-Based Group Therapy for Social Anxiety Disorder: A Treatment Manual (Fleming & Kocovski, 2009). The ACT-based content also incorporated concepts from The Appetite Awareness Workbook (Craighead, 2006), which was also used by Pearson et al. (2012).
Table 1.
Description of ACT-Based DT-W Elements Targeting Weight Concerns.
| Sessions | Description | |
|---|---|---|
| Psychoeducation | 1 | History of the promotion of smoking as a weight control strategy, physiological effects of nicotine on weight, reasons for post-cessation weight gain |
| Distress tolerance skills | ||
| Values | I, 1, 4, 7, 8 | Identify and clarify life values as they relate to smoking vs. weight, differentiate values vs. goals |
| Weight concerns as triggers for smoking | I, 1 | Differentiate external (e.g., people, places, situations) vs. internal (e.g., thoughts, feelings) triggers for smoking and eating, identify weight and body image concerns as internal triggers, discuss the role of these concerns in smoking cessation and relapse |
| Problems with efforts to control weight concerns | I, 2 | Discuss past unsuccessful efforts to control or avoid weight concerns that led to resumption of smoking; understand why efforts to control/avoid weight and body image concerns are likely to maintain smoking |
| Acceptance | 2, 6 | Understand acceptance as an alternative to control/avoidance of weight concerns; participate in a mirror exposure exercise to experience these concerns fully without acting on them by smoking |
| Cognitive defusion | 2, 6, 7 | Learn to defuse weight concerns by viewing negative weight and body image thoughts as what they are (cognitive constructs that do not have to be reacted to or believed), with emphasis on weight-related rationalizations for smoking |
| Self as context | 3 | Practice taking a non-judgmental, observer perspective toward weight and body image concerns via exercises in which participants focused on breathing while observing thoughts and feelings |
| Values-oriented living skills | ||
| Willingness | 3 | Commit to non-judgmental acceptance of negative thoughts and feelings related to weight and body image concerns; address tendency to set limits on weight gain tolerance. Commit to engaging in cessation-promoting, values-oriented behavior regardless of the presence of weight and body image concerns |
| Being present— Appetite awareness training |
4–7 | Identify that during past quit attempts, eating may have replaced smoking as a strategy to control or avoid negative emotions; practice increasing awareness and use of physiological hunger and satiety cues rather than external or emotional triggers to guide when and how much to eat; provide forms to practice monitoring appetite at home (Craighead, 2006) |
| Being present— Mindful eating |
5–7 | Practice (with a piece of candy) paying attention to the smell, taste, and texture of food while eating; eating slowly and noticing physical sensations of hunger and fullness; removing distractions while eating (e.g., TV) |
| Committed action | 4, 8 | Identify goals and barriers with respect to quitting smoking; link values to goals; make daily commitment to values-oriented healthy living behaviors. Participants generate their own behavioral goals, which could include activities such as increasing physical activity, increasing consumption of fruits and vegetables, self-esteem building hobbies, and so forth |
Note. ACT = Acceptance and Commitment Therapy; I = individual session.
The ACT-based content in DT-W specifically targeted weight concerns in the context of smoking cessation. This ACT-based content was “front-loaded” to motivate treatment retention, such that the first two group sessions included only ACT-based content targeting weight concerns, whereas the individual session and remaining group sessions (3–8) were split evenly between ACT-based content and Standard Treatment (ST) content (see description of ST components below). The ACT-based content covered all six core processes of ACT: values, acceptance, defusion, self as context, being present, and committed action (Hayes et al., 2006). ACT-based content in group sessions prior to quit date (Weeks 1–3) focused on “distress tolerance skills” for coping with the fear of anticipated weight gain. During the quit date (Week 4) and post-quit (Weeks 5–8) sessions, these distress tolerance skills were reinforced while new “values-oriented living skills” were introduced.
The distress tolerance skills content culminated in a mirror exposure exercise in Session 6 (Pearson et al., 2012). Participants were paired and each pair was sent to a different corner of the room where there was a full-length mirror on the wall. One member of each pair was asked to stand in front of the mirror and verbalize her thoughts about her body, starting at her head and moving down to her feet, while her partner wrote down each thought on an individual piece of paper. Then they switched roles. After all pairs had finished the exercise and returned to the table in the middle of the room, the group leaders collected all of the pieces of paper, shuffled them, and then distributed them evenly among the group. One at a time, each group member was asked to read the thoughts on her piece of paper to the rest of the group. After hearing all thoughts, the group was asked to discuss their reactions and also whether they could determine to whom each thought belonged. The goals of this exercise were practice in acceptance via exposure, and to notice the similarities among the thoughts, as well as how judgmental the thoughts were. Finally, participants were asked to rip up the pieces of paper, pick up a handful of the pieces, and “hold them lightly” (see Pearson et al., 2012, p. 192) as an exercise in cognitive defusion and self as context.
Values-oriented living skills in the post-quit date sessions focused on reducing eating triggered by external and/or emotional cues, a primary cause of excessive post-cessation weight gain (Hudmon et al., 1999). Participants were taught to become more aware of physiological sensations of hunger and fullness and to use those sensations rather than external or emotional triggers to guide decisions about when and how much to eat, following the appetite awareness training methods described in the Appetite Awareness Workbook (Craighead, 2006).
Standard smoking cessation treatment component of DT-W
Standard Treatment (ST) content in DT-W was based on a manual used in previous research (Brown, 2003), and was consistent with the most recent clinical practice guideline from the U.S. Department of Health and Human Services, Treating Tobacco Use and Dependence: 2008 Update (Fiore et al., 2008). The pre-quit date and quit date group sessions (Weeks 3 and 4) focused on preparation for quit date, reinforcement and support for quitting, discussion of past quit experiences, initiation of self-monitoring, identification of triggers and high-risk situations, development of coping strategies for triggers unrelated to weight and body image (three “As”: avoid, alter, alternative), enlisting social support, and instruction in use of TNP. During the quit date session (Week 4), participants engaged in extended discussion of quitting experiences and coping strategies. After quit date, remaining sessions (Weeks 5–8) consisted of providing support and relapse prevention, including ongoing discussion of quitting experiences, anticipation of high-risk situations, developing social support, and making lifestyle changes that supported abstinence.
TNP component of DT-W
All participants were educated about the proper use of the patch at the group session immediately prior to quit date. On quit date, participants began with the label-recommended dose for their current smoking level (21 mg if >10 cigarettes per day, 14 mg if ≤10) and gradually tapered to 7 mg over an 8-week period.
Assessments and compensation
All assessment data were collected and managed using REDCap electronic data capture tools (Harris et al., 2009). In addition to the baseline assessment and brief assessments at the beginning of each group DT-W session, participants completed individual assessments at “pre-quit” (the week prior to quit date, between Group Sessions 3 and 4), post-treatment (at last group session or within 1 week afterward if missed last session), and 1, 3, and 6 months post-treatment. Shortly after completing DT-W (within about 1 week), participants were also interviewed individually about their experience in DT-W. Interviews were conducted by a research assistant or a study clinician who was not one of the participants’ group leaders, using a semi-structured interview guide. All participants were compensated US$25 for the pre-quit assessment, US$25 for the post-intervention interview, US$25 for the 1-month assessment, and US$50 each for the 3- and 6-month assessments, for a total of US$175. All women in both groups were asked to provide breath samples for carbon monoxide (CO) testing to verify self-reported smoking abstinence (see “Measures” section below). Women in the first group who self-reported abstinence received an extra US$25 at the 1-, 3-, and 6-month assessments for providing breath samples; we did not provide these extra payments to Group 2 because we realized they were unnecessary.
Measures
Screening to confirm eligibility
At the baseline assessment, after providing written informed consent, participants reported their demographics and completed additional screening to confirm their eligibility, including two items to screen for current major depressive disorder (MDD), the Center for Epidemiological Studies–Depression Scale (CES-D; excluded if ≥23; Radloff, 1977), the behavior items from the Eating Attitudes Test (EAT-26) to screen for eating disorder (excluded if above the cutoff for any behavior, available at www.eat-26.com; Garner, Olmsted, Bohr, & Garfinkel, 1982), the Alcohol Use Disorders Identification Test (AUDIT; excluded if ≥8; Babor, Higgins-Biddle, Saunders, & Monteiro, 2001), and the Drug Abuse Screening Test (DAST; excluded if ≥3; Skinner, 1982). Women who were not eligible based on these screenings were provided with referrals to other local smoking cessation programs; eligible women completed the rest of the baseline assessment.
Other baseline-only measures
Smoking History included current smoking pattern and quit attempt history and the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
The Smoking-Related Weight and Eating Episodes Test (SWEET; Adams, Baillie, & Copeland, 2011) is a 10-item measure that assessed the extent to which participants used smoking to manage weight and body image concerns.
The Weight Concern Scale (WCS; Borelli & Mermelstein, 1998) is a six-item measure that assessed concern about post-cessation weight gain, perceived likelihood of gaining weight after cessation, and perceived likelihood of resuming smoking if too much weight gain occurs.
Weight Gain Tolerance referred to how much post-cessation weight gain (in pounds) participants would be willing to tolerate.
DT-W program evaluations
Participants in both DT-W groups completed brief evaluations at the end of each group session on which they rated how helpful they perceived the session with regard to quitting smoking and how well they understood the session content on 5-point scales (from 1 = not at all to 5 = extremely). The second group additionally completed an end-of-treatment evaluation at the last group session that included helpfulness and comprehension ratings for DT-W as a whole, and helpfulness ratings for each of the major DT-W concepts and activities.
Post-treatment semi-structured interview
Interviewers first asked participants’ for general feedback, including most and least useful concepts/activities, and participants’ opinion about the program’s structure, including the number, duration, and frequency of sessions, order of concepts/activities, proportion of content dedicated to coping with weight concerns versus general smoking cessation strategies, homework assignments, and the group leaders’ style and skill. During the second portion of the interview, interviewers asked for participants’ reactions to specific concepts and activities that they had not already mentioned spontaneously.
Primary outcomes
Smoking behavior was tracked from baseline through the 6-month follow-up using the Timeline Followback (TLFB) procedure at each assessment (Brown et al., 1998). The TLFB included assessment of e-cigarette and other tobacco product use. At follow-ups, we also assessed use of other smoking cessation treatments not provided by the study, including medications and counseling. At all time points, reports of past-week abstinence from smoking (i.e., 7-day point-prevalence abstinence) were biochemically verified by expired CO (4 ppm cutoff; Cropsey et al., 2014). At the 3- and 6-month follow-ups, self-reported past-week abstinence from nicotine (including cigarettes and other nicotine-containing products) was additionally verified by saliva cotinine levels (15 ng/ml cutoff; SRNT Subcommittee on Biochemical Verification, 2002).
Participants’ height was measured at baseline. Weight was measured at baseline, the last group session (Week 8), and follow-ups using a calibrated medical scale. Height and weight measurements were used to calculate BMI (weight [lb]/(height)2 [in.] × 703).
Process measures
Depressive symptoms and body dissatisfaction
The CES-D (Radloff, 1977; 20 items, score range = 0–60) and two brief 100-point Visual Analogue Scales (VAS) were administered at baseline, weekly during DT-W, and follow-ups to monitor depressive symptoms and state dissatisfaction with weight/size and body shape (0 = none to 100 = extreme dissatisfaction), respectively.
The Three-Factor Eating Questionnaire (TFEQ-R21; Karlsson, Persson, Sjöström, & Sullivan, 2000; Stunkard & Messick, 1985) was administered at baseline, end of treatment, and follow-ups to assess cognitive restraint (CR; tendency to consciously restrict food intake to control body weight; six items, score range = 6–24), emotional eating (EE; tendency to eat when experiencing negative mood states; six items, score range = 6–24), and uncontrolled eating (UE; tendency to overeat because of a perceived loss of control or hunger; nine items, score range = 9–36).
The Body Image–Acceptance and Action Questionnaire (BI-AAQ; Sandoz, Wilson, Merwin, & Kellum, 2013; 12 items, score range = 7–84) was administered at baseline, end of treatment, and follow-ups to assess self-reported cognitive flexibility (e.g., acceptance, willingness, commitment to values-oriented behavior) specific to body image concerns. The BI-AAQ is reverse scored, such that higher scores indicate less flexibility.
Results
Participant Characteristics
Demographics and baseline characteristics for the 11 participants who attended at least one DT-W group session versus the five dropouts (four dropped out after baseline and were never exposed to DT-W, one dropped out after the individual session and did not attend any group sessions) are in Table 2. Participants who attended at least one group session were significantly more likely to have a 4-year college degree or higher and had significantly higher baseline scores on the WCS than dropouts (ps < .05), but there were no other significant differences.
Table 2.
Demographic and Baseline Characteristics.
| Attended DT-W (n = 11) |
Dropped out (n = 5) |
|
|---|---|---|
| Age | 46.45 (9.46) | 43.00 (17.51) |
| Caucasian (n) | 9 | 4 |
| Married (n) | 4 | 2 |
| 4-year degree or higher (n)* | 7 | 0 |
| Household income < US$25,000 | 5 | 2 |
| Cigarettes per day | 16.09 (3.86) | 18.60 (5.46) |
| Years smoked | 25.00 (9.52) | 24.80 (16.95) |
| FTND | 5.55 (2.07) | 4.60 (1.52) |
| BMI | 29.87 (6.64) | 31.89 (4.28) |
| SWEET | 2.51 (1.04) | 2.14 (0.38) |
| Weight gain tolerance (lb) | 6.36 (4.39) | 7.40 (2.51) |
| WCS** | 7.55 (2.00) | 5.07 (1.76) |
| CES-D | 3.64 (3.41) | 2.40 (2.30) |
Note. Means and standard deviations unless specified. FTND = Fagerström Test for Nicotine Dependence; BMI = body mass index; SWEET = Smoking-Related Weight and Eating Episodes Test; WCS = Weight Concern Scale; CES-D = Center for Epidemiological Studies–Depression Scale.
p = .03.
p = .03.
Group Attendance and Retention
Participants (n = 11) attended a mean of 6.27 (SD = 2.00) of the eight group sessions. One participant attended only the first group session, while the other 10 attended at least five sessions. Follow-up retention was 91% (10/11) for the post-treatment (Week 8) assessment, including the interview, and for the 1-month and 3-month assessments, and 82% (9/11) for the 6-month assessment.
DT-W Program Evaluations
The mean of all individual session helpfulness ratings was 4.05 (SD = 0.48; 1–5 scale with 5 being most helpful), while the mean of all individual session comprehension ratings was 4.58 (SD = 0.41; 1–5 scale with 5 being most well understood). On the end-of-treatment evaluation (completed by n = 6 from the second group), the mean helpfulness rating for DT-W as a whole was 4.83 (SD = 0.41) and the mean rating for comprehension for DT-W as a whole was 4.80 (SD = 0.45). Ratings for individual treatment components are shown in Table 3.
Table 3.
Helpfulness Ratings for DT-W Treatment Components (Means and Standard Deviations).
| Treatment component | M rating (SD) |
|---|---|
| Values | 4.83 (0.41) |
| Cognitive defusion | 4.67 (0.82) |
| Acceptance | 3.83 (0.75) |
| Willingness | 3.83 (0.98) |
| Appetite awareness training | 4.17 (0.41) |
| Mindful eating | 3.83 (0.75) |
| Mirror exposure exercise | 4.00 (0.89) |
Note. All items were on 5-point scales with 5 being the most helpful.
Common Themes From Post-Treatment Interviews (Groups 1 and 2)
Ten participants (four in Group 1, six in Group 2) completed the post-intervention interview. Both groups unanimously praised DT-W and said they would recommend it to other women. One common theme that emerged from their responses was that DT-W was more effective than and different from other treatments. Specifically, participants described DT-W as effective (n = 3) or a similar word or phrase (e.g., instrumental in helping me quit, seems to be working). In addition, participants described DT-W as different, in a positive way, from other smoking cessation treatments they had tried:
It was something kind of out of my comfort zone, but that was okay. I think that’s what I needed to actually quit.
… the whole program is so totally different from anything else I’ve ever tried … it was more about you and … it made you stop and experience who you are and what you want.
I think this study by far was superior to anything I’ve experienced … everything as regards to the paying attention to your life values—that was completely new to me. Explained in the way that it was. I had never thought to put the two together.
Another theme was that ACT-based content was applicable beyond weight and smoking. Although the ACT-based content was applied specifically to weight concern in the context of smoking cessation, participants in both groups readily applied this content more generally:
It was helpful, I mean, not just for quitting smoking but for kind of managing so many things … about, as women, how quickly we are to judge ourselves. How many thoughts we have in our head about what’s not right, what’s not perfect, what we might want to be better, what we might want to change. And understanding that those are just thoughts, and that they’re not, in fact, facts, can really—I think this is the beginning of paving the way of just doing something differently. So I found it really, you know, profound.
Prior to this program, having those negative self-thoughts, I almost gave it so much power over how I would react [to] or handle situations. And it’s only because I gave it that much power that it, I don’t want to say controlled my life, but in a way, it did. So knowing that those negative self-thoughts don’t have to control our lives was awesome.
And it’s a change—not just the smoking part—but pretty much how you do everything else.
Participants did not complete written homework
Participants praised the homework worksheets and appreciated having them for future reference, but most admitted that they did not complete them.
Group 1 Feedback and Manual Revisions for Group 2
Overall, participants in Group 1 were satisfied with DT-W’s structure, content, and group leaders’ style. They noted that group leaders often had to rush to complete all exercises within the allotted session time and suggested adding additional sessions or reducing redundancy to provide more time for unstructured discussion time and facilitation of general social support. Participants described the quantity of handouts and homework assignments as appropriate. They reported that the most helpful and useful components were strategies for thought defusion and appetite awareness training. They were less enthusiastic about mindful eating (strange) and the mirror exposure exercise (neutral), but one participant noted the mirror exercise may have been more helpful in a larger group. For the second group, we streamlined the manual to reduce redundancy and detail instead of adding additional sessions, as DT-W was already lengthy at eight sessions and research suggests no additional benefit beyond eight sessions with respect to abstinence rates (Fiore et al., 2008). We retained mindful eating and the mirror exercise given the small number of participants who provided feedback on these components.
Group 2 Feedback and Manual Revisions for RCT
As with the first group, the second group provided positive feedback on the program structure, content, group leaders, handouts, and homework assignments. In this group, most participants thought that the eight-session length was sufficient. Concepts and activities most frequently named spontaneously as the most helpful or useful were an exercise in which participants practiced envisioning their thoughts and feelings as “leaves on a stream” that float in and out of consciousness, the mirror exposure exercise (necessarily painful, powerful), an ACT metaphor for thought defusion in which participants envisioned their thoughts and feelings as “passengers on a bus” for which they retained the power to determine the destination regardless of “passenger” requests, and appetite awareness training and mindful eating (eye-opening). There was no consensus about the least helpful components and very few suggestions for content to remove or add. Given that participants in the second group were very satisfied with the program, we made few additional changes to DT-W. We further streamlined the content, made some changes to the Appetite Monitoring forms that were intended to make them easier to understand and use, and instructed group leaders to put more emphasis on the option to complete monitoring forms electronically (e.g., on their smart-phone) instead of on the provided paper forms if it was more convenient.
Smoking Cessation and Weight Outcomes
CO-verified (and cotinine-verified when applicable) 7-day point-prevalence abstinence rates at end-of-treatment (last group session), 1, 3, and 6 months were 64% (7/11), 36% (4/11), 27% (3/11), and 27% (3/11), respectively. The same three participants were abstinent at 3- and 6-month follow-up. Notably, they were all from the first group and all self-reported continuous abstinence since quit date. The second group ended in mid-December, and at 1-month follow-up, some participants in that group attributed lapses to holiday-related events. Although none of the participants in Group 2 met our criteria for biochemically verified point-prevalence abstinence at 3- or 6-month follow-up, some were close (isolated lapses only). Many remained actively engaged in efforts to quit smoking; at 6-month follow-up, only two reported returning to baseline smoking levels.
At the last group session, all nine participants who were weighed had gained weight since the baseline assessment (M = 5.78 lb). By the 6-month follow-up, six of the nine participants who were weighed had a higher weight than at baseline; their mean weight gain between baseline and 6-month follow-up was 11.67 lb. Among the three participants who self-reported continuous abstinence between quit date and the 6-month follow-up (all from the first group), the mean weight gain at 6 months was 12.17 lb.
Six of the 10 participants who completed at least one follow-up assessment reported use of another smoking cessation method or treatment not provided by the study at some point during their DT-W intervention or the follow-up period. One participant reported use of nicotine patches obtained from another source (not the patches we provided), four reported e-cigarette use, and one used an Internet program.
Process Measures
Formal statistical analyses were not conducted on process measures given the small sample size. However, a visual inspection suggests that depressive symptoms (CES-D) were low and relatively stable (see Figure 3). State body dissatisfaction (VAS) declined sharply on quit date but then gradually returned to baseline levels (see Figure 4). Regarding TFEQ scores, CR and EE were stable, but UE increased slightly after quit date and was stable thereafter (see Figure 5). Contrary to expectation, cognitive flexibility with respect to body image concerns (BI-AAQ) decreased (BI-AAQ is reverse scored) between baseline and quit date, but remained stable thereafter (see Figure 6).
Figure 3.
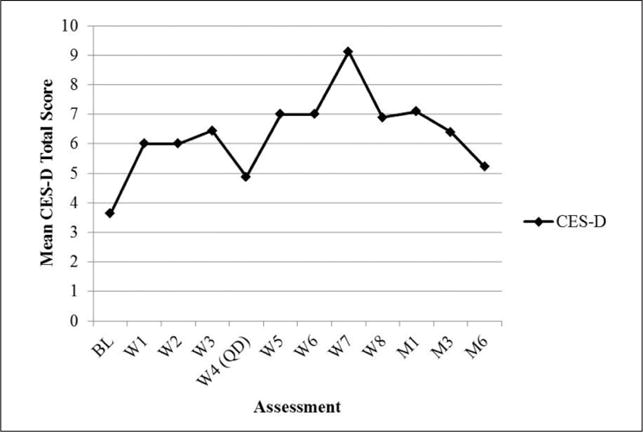
Mean score on CES-D over time.
Note. CES-D = Center for Epidemiological Studies–Depression Scale; BL = baseline; W = week; QD = quit date; M1 = 1-month follow-up; M3 = 3-month follow-up; M6 = 6-month follow-up.
Figure 4.
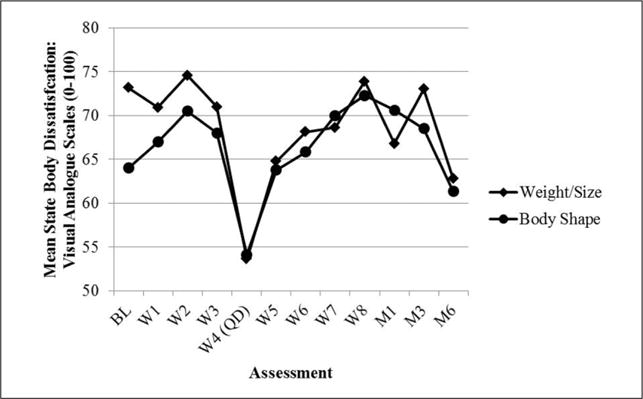
Mean score on State Body Dissatisfaction over time.
Note. BL = baseline; W = week; QD = quit date; M1 = 1-month follow-up; M3 = 3-month follow-up; M6 = 6-month follow-up.
Figure 5.
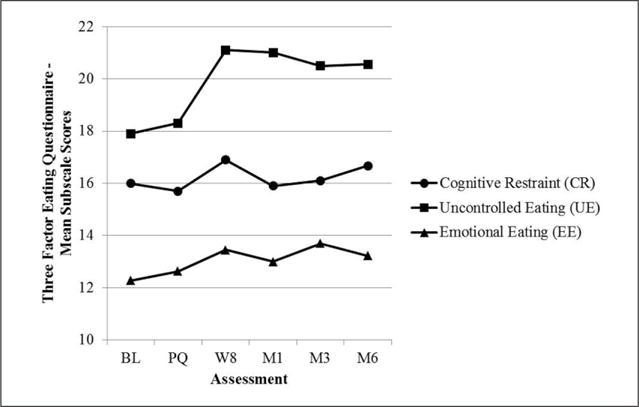
Mean score on Three Factor Eating Questionnaire (TFEQ) over time.
Note. BL = baseline; PQ = pre-quit (between Weeks 3 and 4); W = week; M1 = 1-month follow-up; M3 = 3-month follow-up; M6 = 6-month follow-up.
Figure 6.
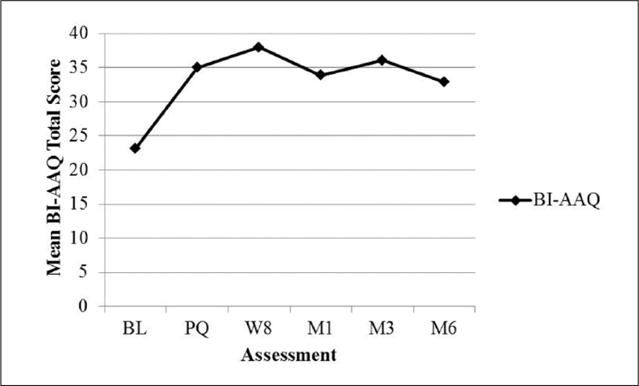
Mean score on BI-AAQ over time.
Note. BI-AAQ = Body Image–Acceptance and Action Questionnaire (reverse scored, higher scores indicate greater inflexibility); BL = baseline; PQ = pre-quit (between Weeks 3 and 4); W = week; M1 = 1-month follow-up; M3 = 3-month follow-up; M6 = 6-month follow-up.
Discussion
In the current study, we developed and piloted a novel group-based intervention to address women’s concerns about gaining weight after smoking cessation called Distress Tolerance Treatment for Weight Concern in Smoking Cessation Among Women (DT-W). The goal of DT-W was to increase distress tolerance with respect to fear of weight gain prior to quitting and to reduce eating triggered by external and emotional cues that promotes weight gain after quitting, using concepts and metaphors derived primarily from ACT (Hayes et al., 2006) and the Appetite Awareness Workbook (Craighead, 2006). We were successful at establishing the feasibility and acceptability of DT-W. The 11 participants who initiated DT-W unanimously praised the intervention, describing it as more effective yet different from other smoking cessation treatments they had tried. On average, participants rated program sessions as “very” helpful on a quantitative evaluation, and five of six who completed the end-of-program evaluation rated the program as a whole as “extremely helpful,” which compares favorably with past research in which similar rating scales were used (e.g., Brown et al., 2013; Napolitano, Lloyd-Richardson, Fava, & Marcus, 2011; Perkins et al., 2001). Based on participants’ feedback, only minor changes were made to DT-W. We are now evaluating the finalized version of DT-W in an RCT.
Previous research has indicated that weight-concerned female smokers have high rates of failure to initiate smoking cessation treatment and treatment dropout (Copeland et al., 2006; Namenek Brouwer & Pomerleau, 2000). In our study, five of 16 participants who completed a baseline assessment for one of the two DT-W groups did not attend any group treatment sessions. However, the 11 women who initiated treatment had more severe weight concerns on the WCS than the five who dropped out. Therefore, we believe that we successfully retained weight-concerned participants. We speculate that the relatively high dropout rate may be partially attributable to loss of motivation and interest in quitting associated with a lengthy waiting time (1–2 months) for some participants between the completion of their baseline assessment and the beginning of their DT-W intervention.
Although this pilot study was not designed or powered to evaluate efficacy with respect to smoking or weight outcomes, smoking abstinence rates were comparable to other intensive treatments (Fiore et al., 2008) and promising, given that weight-concerned female smokers are considered a particularly treatment-resistant population. Weight gain among participants varied but was generally consistent with population means (Aubin et al., 2012). We also administered several process measures that could be potential mechanisms of treatment efficacy, including depressive symptoms, dissatisfaction with weight/size and body shape, cognitive flexibility with respect to body image concerns, and eating behavior (restrained, uncontrolled, and emotional). We would expect that in the absence of an intervention targeting weight concerns, depressive symptoms, body dissatisfaction, cognitive inflexibility related to body image, and all types of eating behavior would be likely to increase during the immediate pre-quit and/or post-quit period as weight concerns become activated and salient. The general pattern of scores in DT-W suggests that depressive symptoms, and restrained and emotional eating were fairly stable from baseline through the follow-up period. While cognitive inflexibility related to body image did increase between baseline and the pre-quit assessment, it remained stable thereafter. Uncontrolled eating increased slightly after quit date, as might be expected given that metabolism slows, appetite increases, and sense of smell and taste improve upon cessation of smoking (Audrain-McGovern & Benowitz, 2011). A randomized trial will be needed to determine whether DT-W has different effects on these process measures relative to standard treatment; for example, DT-W may prevent increases in depressive symptoms, body dissatisfaction, eating behaviors, and cognitive inflexibility.
Interestingly, four of the 10 participants who completed at least one follow-up assessment reported some use of e-cigarettes during the follow-up period, including all three participants from the first group who attended more than one group session and self-reported continuous abstinence from cigarettes between quit date and 6-month follow-up. Participant-initiated discussion of e-cigarette use, which occurred during the first group, may have influenced participants’ behavior in that group. Although we did not assess reasons for e-cigarette use, we speculate that some women may try e-cigarettes because they believe that e-cigarette use could help prevent or minimize weight gain after smoking cessation. However, additional research is needed to determine the validity of this belief (Russo et al., 2016).
This study has several limitations. The purpose of this study was to establish the feasibility and acceptability of DT-W and pilot the assessment procedures for a future RCT. Although we have reported the primary outcomes of smoking status and weight change and means on process measures, given the small sample size and lack of control group, we cannot make any conclusions about the efficacy of DT-W or the mechanisms of treatment efficacy. Second, the sample was primarily Caucasian, well-educated, and healthy (i.e., few physical or psychiatric comorbidities). Future research should evaluate the feasibility and acceptability of this treatment for a more diverse sample with regard to demographics and comorbidities.
In conclusion, we established the acceptability and feasibility of a novel intervention for weight-concerned female smokers intended to increase distress tolerance with respect to weight and body image concerns, and reduce eating triggered by external and emotional cues after quit date. Future research will evaluate the efficacy of this intervention in an RCT, as well as examine potential treatment mechanisms such as trajectories of depressive symptoms, body image dissatisfaction, cognitive inflexibility related to body image, and eating behavior.
Acknowledgments
The authors thank Caitlin Melvin for her contributions to this research including assistance with recruitment and conducting assessments.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by Grant K23DA035288 from the National Institute on Drug Abuse (Principal Investigator [PI]: Erika L. Bloom, PhD).
Biographies
Erika Litvin Bloom, PhD, is Assistant Professor (Research) of Psychiatry and Human Behavior and Medicine in the Alpert Medical School at Brown University and Rhode Island Hospital. Previously, she was at Butler Hospital. Her research interest is in treatment development for addictive behaviors.
Rena R. Wing, PhD, is Professor of Psychiatry and Human Behavior in the Alpert Medical School at Brown University and the Director of the Weight Control and Diabetes Research Center at The Miriam Hospital. Her research has focused on interventions for long-term weight loss and weight gain prevention.
Christopher W. Kahler, PhD, is Professor and Chair of the Department of Behavioral and Social Sciences at the Brown University School of Public Health. His work focuses on developing smoking cessation interventions, including addressing heavy alcohol use in the context of smoking cessation, as well as HIV prevention and treatment.
J. Kevin Thompson, PhD, is Professor of Psychology and director of the Body Image Research Group (BIRG) at the University of South Florida. In his research, he is committed to extending and clarifying understanding of the psychology of physical appearance.
Sari Meltzer is pursuing her master’s degree in social work (MSW) at New York University. Prior to beginning her MSW program, she was a research assistant in addictions research in Butler Hospital.
Jacki Hecht, RN, MSN, is managing director of the Center for Transdisciplinary Collaborative Research in Self-Management Science (TCRSS) in the School of Nursing at the University of Texas at Austin. Previously, she was a senior research associate in Butler Hospital. Her focus is on the development of behavior change interventions.
Haruka Minami, PhD, is Assistant Professor of Psychology at Fordham University. Previously, she was a post-doctoral research fellow in clinical psychology in the Alpert Medical School at Brown University and Butler Hospital.
Lawrence H. Price, MD, is Professor of Psychiatry and Human Behavior in the Alpert Medical School at Brown University and president and COO of Butler Hospital.
Richard A. Brown, PhD, is Research Professor in the School of Nursing at the University of Texas at Austin. Previously, he was Professor of Psychiatry and Human Behavior in the Alpert Medical School at Brown University and Butler Hospital. His research focus is on interventions for tobacco dependence and other substance use disorders.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Adams CE, Baillie LE, Copeland AL. The Smoking-Related Weight and Eating Episodes Test (SWEET): Development and preliminary validation. Nicotine & Tobacco Research. 2011;13:1123–1131. doi: 10.1093/ntr/ntr162. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: Meta-analysis. British Medical Journal. 2012;345 doi: 10.1136/bmj.e4439. Article e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Benowitz NL. Cigarette smoking, nicotine, and body weight. Clinical Pharmacology and Therapeutics. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for use in primary care. 2nd. Geneva, Switzerland: Department of Mental Health and Substance Dependence, World Health Organization; 2001. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Beebe LA, Bush T. Post-cessation weight concerns among women calling a state tobacco quitline. American Journal of Preventive Medicine. 2015;48(1, Suppl 1):S61–S64. doi: 10.1016/j.amepre.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Borelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addictive Behaviors. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Bowen DJ, McTiernan A, Powers D, Feng Z. Recruiting women into a smoking cessation program: Who might quit? Women & Health. 2000;31:41–58. doi: 10.1300/j013v31n04_03. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Herzog TA, Juliano LM, Irvin JE, Lazev AB, Simmons VN. Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology. 2003;112:448–456. doi: 10.1037/0021-843x.112.3.448. [DOI] [PubMed] [Google Scholar]
- Bricker JB, Mann SL, Marek PM, Liu J, Peterson AV. Telephone-delivered Acceptance and Commitment Therapy for adult smoking cessation: A feasibility study. Nicotine & Tobacco Research. 2010;12:454–458. doi: 10.1093/ntr/ntq002. [DOI] [PubMed] [Google Scholar]
- Brown RA. Intensive behavioral treatment. In: Abrams DB, Niaura RS, Brown RA, Emmons KM, Goldstein MG, Monti PM, editors. The tobacco dependence treatment handbook: A guide to best practices. New York, NY: The Guilford Press; 2003. pp. 118–177. [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12:101–112. [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11:493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV. Distress tolerance treatment for early-lapse smokers: Rationale, program description, and preliminary findings. Behavior Modification. 2008;32:302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Reed KM, Bloom EL, Minami H, Strong DR, Lejuez CW, Hayes SC. Development and preliminary randomized controlled trial of a distress tolerance treatment for smokers with a history of early lapse. Nicotine & Tobacco Research. 2013;15:2005–2015. doi: 10.1093/ntr/ntt093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Forman E, Hoffman K, Shaw J, Juarascio A. A pilot study of acceptance and commitment therapy for promotion of physical activity. Journal of Physical Activity & Health. 2011;8:516–522. doi: 10.1123/jpah.8.4.516. [DOI] [PubMed] [Google Scholar]
- Ciarrochi J, Billich L, Godsell C. Psychological flexibility as a mechanism of change in acceptance and commitment therapy. In: Baer R, editor. Assessing mindfulness and acceptance processes in clients: Illuminating the theory and practice of change. Oakland, CA: New Harbinger; 2010. pp. 51–76. [Google Scholar]
- Clark MM, Decker PA, Offord KP, Patten CA, Vickers KS, Croghan IT, Dale LC. Weight concerns among male smokers. Addictive Behaviors. 2004;29:1637–1641. doi: 10.1016/j.addbeh.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Clark MM, Hurt RD, Croghan IT, Patten CA, Novotny P, Sloan JA, Loprinzi CL. The prevalence of weight concerns in a smoking abstinence clinical trial. Addictive Behaviors. 2006;31:1144–1152. doi: 10.1016/j.addbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Cooper TV, Dundon M, Hoffman BM, Stoever CJ. General and smoking cessation related weight concerns in veterans. Addictive Behaviors. 2006;31:722–725. doi: 10.1016/j.addbeh.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Predictors of pretreatment attrition from smoking cessation among pre-and postmenopausal, weight-concerned women. Eating Behavior. 2006;7:243–251. doi: 10.1016/j.eatbeh.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Craighead LW. The appetite awareness workbook: How to listen to your body & overcome bingeing, overeating & obsession with food. Oakland, CA: New Harbinger; 2006. [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine & Tobacco Research. 2014;16:1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley AC, Hajek P, Lycett D, Aveyard P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2012;1:CD006219. doi: 10.1002/14651858.CD006219.pub3. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Wewers ME. Treating tobacco use and dependence: 2008 update—Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Fleming JE, Kocovski NL. Mindfulness and acceptance-based group therapy for social anxiety disorder: A treatment manual (Unpublished treatment manual) 2009 Available from http://actonsocialanxiety.com/pdf/Treatment_Manual.pdf.
- Forman EM, Butryn ML. A new look at the science of weight control: How acceptance and commitment strategies can address the challenge of self-regulation. Appetite. 2015;84:171–180. doi: 10.1016/j.appet.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EM, Butryn ML, Juarascio AS, Bradley LE, Lowe MR, Herbert JD, Shaw JA. The mind your health project: A randomized controlled trial of an innovative behavioral treatment for obesity. Obesity (Silver Spring) 2013;21:1119–1126. doi: 10.1002/oby.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The eating attitudes test: Psychometric features and clinical correlates. Psychological Medicine. 1982;12:871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- Gifford EV, Lillis J. Avoidance and inflexibility as a common clinical pathway in obesity and smoking treatment. Journal of Health Psychology. 2009;14:992–996. doi: 10.1177/1359105309342304. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: Model, processes, and outcomes. Behaviour Research and Therapy. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hernández-López M, Luciano MC, Bricker JB, Roales-Nieto JG, Montesinos F. Acceptance and commitment therapy for smoking cessation: A preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors. 2009;23:723–730. doi: 10.1037/a0017632. [DOI] [PubMed] [Google Scholar]
- Hudmon SG, Gritz ER, Clayton S, Nisenbaum R. Eating orientation, postcessation weight gain, and continued abstinence among female smokers receiving an unsolicited smoking cessation intervention. Health Psychology. 1999;18:29–36. doi: 10.1037//0278-6133.18.1.29. [DOI] [PubMed] [Google Scholar]
- Jarry JL, Coambs RB, Polivy J, Herman CP. Weight gain after smoking cessation in women: The impact of dieting status. International Journal of Eating Disorders. 1998;24:53–64. doi: 10.1002/(sici)1098-108x(199807)24:1<53::aid-eat5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychology. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- Juarascio A, Forman S, Herbert JD. Acceptance and commitment therapy versus cognitive therapy for the treatment of comorbid eating pathology. Behavior Modification. 2010;34:175–190. doi: 10.1177/0145445510363472. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Persson LO, Sjöström L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. International Journal of Obesity and Related Metabolic Disorders. 2000;24:1715–1725. doi: 10.1038/sj.ijo.0801442. [DOI] [PubMed] [Google Scholar]
- Katterman SN, Goldstein SP, Butryn ML, Forman EM, Lowe MR. Efficacy of an acceptance-based behavioral intervention for weight gain prevention in young adult women. Journal of Contextual Behavioral Science. 2014;3:45–50. [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Review of Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Kraemer KM, McLeish AC, Jeffries ER, Avallone KM, Luberto CM. Distress tolerance and perceived barriers to smoking cessation. Substance Abuse. 2013;34:277–282. doi: 10.1080/08897077.2013.771597. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141:176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Marcus MD, Perkins KA. Women, weight, and smoking: A cognitive behavioral approach to women’s concerns about weight gain following smoking cessation. Cognitive and Behavioral Practice. 2003;10:105–111. [Google Scholar]
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, Marcus MD. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Archives of Internal Medicine. 2010;170:543–550. doi: 10.1001/archinternmed.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Marcus MD. The characteristics of women smokers concerned about postcessation weight gain. Addictive Behaviors. 2001;26:749–756. doi: 10.1016/s0306-4603(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Lillis J, Hayes SC, Levin ME. Binge eating and weight control: The role of experiential avoidance. Behavior Modification. 2011;35:252–264. doi: 10.1177/0145445510397178. [DOI] [PubMed] [Google Scholar]
- Lillis J, Kendra KE. Acceptance and Commitment Therapy for weight control: Model, evidence, and future directions. Journal of Contextual Behavioral Science. 2014;3:1–7. doi: 10.1016/j.jcbs.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis J, Niemeier HM, Ross KM, Thomas JG, Leahey T, Unick J, Wing RR. Weight loss intervention for individuals with high internal disinhibition: Design of the Acceptance Based Behavioral Intervention (ABBI) randomized controlled trial. BMC Psychology. 2015;3 doi: 10.1186/s40359-015-0075-2. Article 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Khoury EN, Litvin EB, Brandon TH. The effect of body image threat on smoking motivation among college women: Mediation by negative affect. Psychology of Addictive Behaviors. 2009;23:279–286. doi: 10.1037/a0014291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett D, Munafò M, Johnstone E, Murphy M, Aveyard P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction. 2011;106:188–196. doi: 10.1111/j.1360-0443.2010.03136.x. [DOI] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. Journal of Consulting and Clinical Psychology. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- Minami H, Bloom EL, Reed KM, Hayes SC, Brown RA. The moderating role of experiential avoidance in the relationships between internal distress and smoking behavior during a quit attempt. Psychology of Addictive Behaviors. 2015;29:400–407. doi: 10.1037/adb0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizes J, Sloan DM, Segraves K, Spring B, Pingitore R, Kristeller J. The influence of weight-related variables on smoking cessation. Behavior Therapy. 1998;29:371–385. [Google Scholar]
- Namenek Brouwer RJ, Pomerleau CS. “Prequit attrition” among weight-concerned women smokers. Eating Behavior. 2000;1:145–151. doi: 10.1016/s1471-0153(00)00014-3. [DOI] [PubMed] [Google Scholar]
- Napolitano MA, Lloyd-Richardson EE, Fava JL, Marcus BH. Targeting body image schema for smoking cessation among college females: Rationale, program description, and pilot study results. Behavior Modification. 2011;35:323–346. doi: 10.1177/0145445511404840. [DOI] [PubMed] [Google Scholar]
- Niemeier HM, Leahey T, Palm Reed K, Brown RA, Wing RR. An acceptance-based behavioral intervention for weight loss: A pilot study. Behavior Therapy. 2012;43:427–435. doi: 10.1016/j.beth.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons AC, Shraim M, Inglis J, Aveyard P, Hajek P. Interventions for preventing weight gain after smoking cessation. Cochrane Database of Systematic Reviews. 2009;1:CD006219. doi: 10.1002/14651858.CD006219.pub2. [DOI] [PubMed] [Google Scholar]
- Pearson AN, Follette VM, Hayes SC. A pilot study of acceptance and commitment therapy as a workshop intervention for body dissatisfaction and disordered eating attitudes. Cognitive and Behavioral Practice. 2012;19:181–197. [Google Scholar]
- Pearson AN, Heffner M, Follette VM. Acceptance and commitment therapy for body image dissatisfaction: A practitioner’s guide to using mindfulness, acceptance, and values-based behavior change strategies. Oakland, CA: New Harbinger; 2010. [Google Scholar]
- Perkins KA, Marcus MD, Levine MD, D’Amico D, Miller A, Broge M, Shiffman S. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. Journal of Consulting and Clinical Psychology. 2001;69:604–613. [PubMed] [Google Scholar]
- Pinto BM, Borrelli B, King TK, Bock BC, Clark MM, Roberts M, Marcus BH. Weight control smoking among sedentary women. Addictive Behaviors. 1999;24:75–86. doi: 10.1016/s0306-4603(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Pirie PL, Murray DM, Luepker RV. Gender differences in cigarette smoking and quitting in a cohort of young adults. American Journal of Public Health. 1991;81:324–327. doi: 10.2105/ajph.81.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: The Inter99 study. Preventive Medicine. 2007;44:283–289. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Kurth CL. Willingness of female smokers to tolerate postcessation weight gain. Journal of Substance Abuse. 1996;8:371–378. doi: 10.1016/s0899-3289(96)90215-1. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Namenek Brouwer RJ, Pomerleau OF, Stewart AJ. Race differences in weight concerns among women smokers: Results from two independent samples. Addictive Behaviors. 2001;26:651–663. doi: 10.1016/s0306-4603(00)00148-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: Results from a national survey of women smokers. Nicotine & Tobacco Research. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- Quinn EP, Brandon TH, Copeland AL. Is task persistence related to smoking and substance use? Applying learned industriousness theory to addictive behaviors. Experimental and Clinical Psychopharmacology. 1996;4:186–190. doi: 10.1037/1064-1297.4.2.186. [DOI] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Russo C, Cibella F, Caponnetto P, Campagna D, Maglia M, Frazzetto E, Polosa R. Evaluation of post cessation weight gain in a 1-year randomized smoking cessation trial of electronic cigarettes. Scientific Reports. 2016;6:18763. doi: 10.1038/srep18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz EK, Wilson KG, Merwin RM, Kellum KK. Assessment of body image flexibility: The Body Image-Acceptance and Action Questionnaire. Journal of Contextual Behavioral Science. 2013;2:39–48. [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Spring B, Howe D, Berendsen M, McFadden HG, Hitchcock K, Rademaker AW, Hitsman B. Behavioral intervention to promote smoking cessation and prevent weight gain: A systematic review and meta-analysis. Addiction. 2009;104:1472–1486. doi: 10.1111/j.1360-0443.2009.02610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of Psychosomatic Research. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM, Javitz HS. Cardiovascular reactivity as a predictor of relapse in male and female smokers. Health Psychology. 1993;12:451–458. doi: 10.1037//0278-6133.12.6.451. [DOI] [PubMed] [Google Scholar]
- Trujillo MA, Khoddam R, Greenberg JB, Dyal SR, Ameringer KJ, Zvolensky MJ, Leventhal AM. Distress tolerance as a correlate of tobacco dependence and motivation: Incremental relations over and above anxiety and depressive symptoms. Behavioral Medicine. 2015:1–9. doi: 10.1080/08964289.2015.1110559. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekley CK, Klesges RC, Reylea G. Smoking as a weight-control strategy and its relationship to smoking status. Addictive Behaviors. 1992;17:259–271. doi: 10.1016/0306-4603(92)90031-p. [DOI] [PubMed] [Google Scholar]
- White MA, McKee SA, O’Malley SA. Smoke and mirrors: Magnified beliefs that cigarette smoking suppresses weight. Addictive Behaviors. 2007;32:2200–2210. doi: 10.1016/j.addbeh.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


