Abstract
Abuse of synthetic psychostimulants like synthetic cathinones has risen in recent years. 3,4-methylenedioxypyrovalerone (MDPV) is one such synthetic cathinone that demonstrates a mechanism of action similar to cocaine. Compared to cocaine, MDPV is more potent at blocking dopamine and norepinephrine reuptake and is readily self-administered by rodents. The present study compared the rewarding and reinforcing properties of MDPV and cocaine using systemic injection dose-response and self-administration models. Fifty-kHz ultrasonic vocalizations (USVs) were recorded as an index of positive affect throughout experiments. In Experiment 1, MDPV and cocaine dose-dependently elicited 50-kHz USVs upon systemic injection, but MDPV increased USVs at greater rates and with greater persistence relative to cocaine. In Experiment 2, latency to begin MDPV self-administration was shorter than latency to begin cocaine self-administration, and self-administered MDPV elicited greater and more persistent rates of 50-kHz USVs versus cocaine. MDPV-elicited 50-kHz USVs were sustained over the course of drug load-up whereas cocaine-elicited USVs waned following initial infusions. Notably, we observed a robust presence of context-elicited 50-kHz USVs from both MDPV and cocaine self-administering rats. Collectively, these data suggest that MDPV has powerfully rewarding and reinforcing effects relative to cocaine at one-tenth doses. Consistent with prior work, we additionally interpret these data in supporting that MDPV has significant abuse risk based on its potency and subjectively positive effects. Future studies will be needed to better refine therapeutic strategies targeted at reducing the rewarding effects of cathinone analogs in efforts to ultimately reduce abuse liability.
Keywords: 3,4-methylenedioxypyrovalerone; Affect; Cocaine; Self-Administration; Synthetic Cathinone; Ultrasonic Vocalizations
Introduction
The usage of novel psychoactive substances, including cathinone analogs (synthetic cathinones), has brought into focus a developing drug abuse epidemic receiving international attention. Synthetic cathinones, derived from the parent compound cathinone in the Catha edulis plant native to North Africa and the Arabian Peninsula, are popularized and marketed as “legal high” alternatives structurally designed to mimic the effects of popular illicit psychostimulants. While many synthetic drug manufacturers have been able to avoid law enforcement detection by labeling drug mixtures as “not for human consumption” and branding mixtures to street names like “bath salts”, “Ivory Wave”, and “Vanilla Sky”, the United States and 6 countries worldwide have passed legislation designating synthetic cathinones as illegal (Ross et al, 2011; Carroll et al, 2012).
Of the abused synthetic cathinones, 3,4-methylenedioxypyrovalerone (MDPV) can induce powerfully subjective effects including euphoria, hallucinatory delirium and paranoid psychosis (Antonowicz et al, 2011; Penders and Gestring, 2011; Penders et al, 2012). (Ross et al, 2011; Borek and Holstege, 2012; Murray et al, 2012; Beck et al, 2015). (Wojcieszak et al, 2016). The pharmacological and behavioral profiles of MDPV delineated from preclinical investigation support an abuse liability similar to that of cocaine and amphetamine (for review, see Glennon and Young, 2016). Similar to other pyrovalerone-derived synthetic cathinones, MDPV is a potent, lipophilic blocker of pre-synaptic dopamine (DA) and norepinephrine (NE) transporters (Meltzer et al, 2006). Baumann and colleagues (2013) additionally found that MDPV produces comparable elevations in extracellular DA within the ventral striatum at one-tenth the dose of cocaine. Comparably to cocaine, MDPV primes brain reward thresholds in intracranial self-stimulating rodents (Bonano et al, 2014; Watterson et al, 2014), and methylenedioxy-containing synthetic cathinones were found to possess both rapid onset and persistent facilitations of self-stimulation lasting as long as 300 minutes (Bonano et al, 2014). MDPV enhances locomotor activity that sensitizes following chronic administration (Huang et al, 2012; Aarde et al, 2013; Gregg et al, 2016) and cross-sensitizes with both methamphetamine and cocaine (Berquist et al, 2016; Watterson et al, 2016). Indeed, MDPV is self-administered by rodents at rates comparable to or exceeding those reached during methamphetamine self-administration (Aarde et al, 2013; Watterson et al, 2014; Schindler et al, 2016).
Interoceptive stimuli, such as euphoria and dysphoria, underlie drug-seeking behavior and contribute towards the escalation to addiction. Positive and negative affective states can be non-invasively probed in rats by recordings ultrasonic vocalizations (USVs); rewarding stimuli evoke 50-kHz USVs whereas aversive stimuli evoke 22-kHz USVs (e.g., Blanchard et al, 1991; Sanchez, 1993; Brudzynski and Pniak, 2002). In models of substance use disorders, 50-kHz USVs consistently associate with systemic- and self-administration of cocaine and amphetamine whereas 22-kHz USVs associate with corresponding drug withdrawal states (e.g., Barros and Miczek, 1996; Wintink and Brudzynski, 2001; Barker et al, 2010; for review, see Barker et al, 2015). Production of positive 50-kHz USVs are supported by mesolimbic dopamine (DA) transmission, as direct infusion of amphetamine into the nucleus accumbens (NAcc), a principal target structure of DA-producing neuronal afferents from ventral tegmental area (VTA), readily evokes 50-kHz USVs (Burgdorf et al, 2001; Thompson et al, 2006). Additionally, photostimulation of DA terminals within NAcc elicits 50-kHz USVs (Scardochio et al, 2015). Collectively, the underlying neurobiology of 50-kHz USV production positions it as an attractive measure for assessing subjective effects in preclinical models of psychostimulant abuse.
The rich literature supporting MDPV as a potent, reinforcing monoamine transport blocker provided strong rationale for its ability to induce a positive subjective state in rats and, in turn, evoke high rates of 50-kHz USVs. The present set of studies was conducted to more completely characterize the rewarding and reinforcing effects mediated by MDPV as they relate to cocaine using systemic injection dose-response and self-administration rodent models. Collectively, our data demonstrate that: (1) MDPV dose-dependently elevates 50-kHz USVs upon systemic injection, (2) the anticipation to self-administer either MDPV or cocaine is met with a strong elicitation of 50-kHz USVs, (3) the latency to begin MDPV self-administration is shorter than that to begin cocaine self-administration, (4) positive affective 50-kHz USVs remain sustained following initial MDPV infusions relative to the decline observed following cocaine infusions, and (5) 50-kHz USVs eventually decline to near-zero levels after drug load-up from both MDPV and cocaine self-administering rats. These results, consistent with a growing literature, support a critically high abuse risk of MDPV and position USVs as a means to study MDPV-associated affective changes.
Materials and Methods
Animals
Adult male Sprague Dawley rats (Charles Rivers Laboratories) weighing 250-275 g were pair-housed and cared for in a vivarium of the Lewis Katz School of Medicine at Temple University. For Experiment 2, rats became singly-housed following jugular vein catheterization surgery. Rats were kept on a reverse light cycle (lights turned off at 9:00 AM) and had access to food and water ad libitum except during behavioral sessions. All surgical and behavioral procedures were approved by the Institutional Animal Care and Use Committee of the Lewis Katz School of Medicine at Temple University.
Drugs
For all experiments, MDPV and cocaine hydrochloride were dissolved in 0.9% saline. Cocaine was received as a generous gift from the Drug Supply Program of the National Institute on Drug Abuse and MDPV was synthesized by Dr. Allen Reitz as described in prior work (Abiedalla et al, 2012).
USV recording and analysis
Ultrasonic condenser microphones (Avisoft Bioacoustics; Dodotronic) were positioned atop Plexiglas chambers which were themselves contained within larger, sound-attenuating wooden chambers for recording sessions in both experiments. USVs were manually detected and quantified using Raven Pro software (Cornell Lab of Ornithology, Bioacoustics Research Program) or automatically detected using a MATLAB-based program (Barker et al, 2014). Rats emit USVs around 22-kHz; however, 22-kHz USVs were sparsely observed. Coupled with experimental aims of assessing positive effects of systemic- or self-administered psychostimulants, only putative 50-kHz USVs were included in analyses.
Experiment 1: Systemic injection dose-response
Separate groups of rats were given daily injections of either cocaine (5.0, 10.0, 30.0 mg/kg, i.p.), MDPV (0.5, 1.0, 3.0 mg/kg, i.p.) or saline for 7 days. Doses were selected based in part on prior evidence demonstrating ~10-fold potency differences between MDPV- and cocaine-evoked elevations in ventral striatal dopamine (Baumann et al, 2013). On day 7, rats were placed in a Plexiglas recording chamber for 30-minute USV recordings immediately following injection. All injections and recordings took place between 10:00 AM and 5:00 PM.
Experiment 2: Intravenous drug self-administration
Jugular vein catheterization surgery
Rats were anesthetized with isoflurane gas (5% induction, 2-3% maintenance) and prepared for surgery. The mid-scapular region of the dorsal surface of the rat and the right neck region on the rat's ventral surface were shaved followed by cutaneous wiping with alcohol and betadine. Rats were then subcutaneously injected with buprenorphine (0.05 mg/kg, s.c.) for pre-operative analgesia. Thereafter, small incisions were made on both dorsal and ventral surfaces. Connective tissue was cleared following ventral surface incision and the jugular vein was isolated. A permanent indwelling catheter (CamCaths) was inserted into the right jugular vein of the rat and secured with suture thread. Catheter tubing was threaded subcutaneously from the right jugular vein to the incision made on the rat's dorsal surface where it connected to a stainless steel exit port and was secured to the rat using biologically-inert mesh. Incisions were secured with 9 mm surgical staples. Following surgical procedures, triple antibiotic ointment was applied on incision areas, and 100 μL of heparinized saline containing antibiotic (gentamycin) was administered intravenously. Finally, rats were post-operatively administered buprenorphine (0.05 mg/kg, s.c.) and returned to their home cage upon waking from anesthesia.
Drug self-administration
Following ~7 days of recovery, separate groups of rats were trained to self-administer either cocaine (0.56 mg/kg/infusion) or MDPV (0.056 mg/kg/infusion). For each training session, rats were placed into self-administration chambers (Med Associates), and tubing encased by a spring leash was fitted onto the surgically-implanted catheters to allow drug delivery from a nearby syringe pump. Rats were trained to lever press for drug infusions under a fixed-ratio 1 schedule of reinforcement with a 20-second inter-trial interval during daily 2-hour sessions for 14 days. Lever presses on the active lever resulted in a 2-second intravenous drug delivery paired with a ~6-kHz tone (Mallory-Sonalert; Indianapolis, IN). All inputs and outputs were controlled from a local computer running MED-PC IV software (Med Associates). All self-administration sessions took place between 10:00 AM and 5:00 PM.
For USV analyses, individual rats were recorded from single randomly selected sessions throughout the second week of training (i.e. days 8-14) as has been conducted in prior work (e.g., Barker et al. 2014). Recordings were initiated immediately after rats were placed in the self-administration chamber. Following a 5-minute “anticipation” period, MED-PC IV programs were initiated and levers were presented to allow rats to begin drug self-administration.
Statistical analyses
For Experiment 1, 50-kHz USVs were analyzed using one-tailed Mann-Whitney U-tests to handle violations in normality. An outlier analysis (± 2 standard deviations from group mean in 5-minute time bins) led to the omission of two rats from the saline group. Further, a temporal analysis using 10-minute time bins across the 30-minute recording session was conducted for each drug type using mixed-model ANOVAs with Dose as the between-subjects factor (i.e. MDPV: 0.0, 0.5, 1.0, 3.0 mg/kg; cocaine: 0.0, 5.0, 10.0, 30.0 mg/kg) and Time (0-10, 10-20, 20-30 min) as the within-subjects factor. Greenhouse-Geisser corrections were applied when violations in sphericity were found. For Experiment 2, infusion latencies during drug ‘load-up’ (i.e. first ten infusions) and 50-kHz USVs were analyzed using mixed-model ANOVAs with Drug Type as the between-groups factor and Infusion Number as the within-groups factor. A student's t-test was conducted to compare the onset to self-administer either MDPV or cocaine (i.e. latencies to infusion 1). Finally, student's t-tests were used to assess differences between 50-kHz USVs occurring during the 5-minute anticipation period versus the 120-minute administration period for each of the two drug types. For all analyses, type I error rate (α) was adjusted to 0.05 following Bonferroni correction for multiple familywise comparisons.
Results
Systemic MDPV elicits 50-kHz USVs (Figure 1)
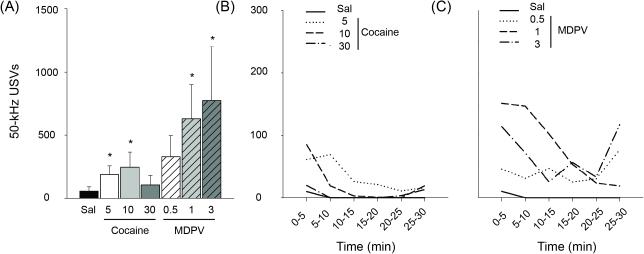
Figure 1.
Systemic injection dose-response. (A) 50-kHz USVs across 30-minute recording session on day 7 of cocaine (solid bars) or MDPV (hashed bars) administration. (B-C) Time course of cocaine- or MDPV-elicited 50-kHz USVs in 10-minute time bins. * p < 0.05 against saline control group, Mann Whitney tests. Data for (A) are means ± S.E.M., and data for (B-C) are medians. n's=5-8.
Data from Experiment 1 showed that MDPV and cocaine both evoked 50-kHz USVs with greater elevations observed from rats receiving MDPV (Figure 1A). Specifically, independent samples Mann-Whitney U-tests further showed that 5.0 and 10.0 mg/kg of cocaine as well as 1.0 and 3.0 mg/kg MDPV evoked significantly greater 50-kHz USVs relative to saline-elicited USVs (all p < 0.05). A mixed-model ANOVA examining 50-kHz USVs by Dose and Time within the MDPV group revealed a significant main effect of Time [F(1.296, 27.224) = 11.813, p < 0.001]. Comparisons within each Time point revealed that 1.0 mg/kg of MDPV produced marginally greater 50-kHz USVs at minutes 0-10 (p = 0.09), and that 3.0 mg/kg of MDPV produced significantly greater 50-kHz USVs at minutes 20-30 (p < 0.05) relative to respective saline-evoked USVs. For the cocaine groups, a significant main effect of Time emerged [F(2, 46) = 15.347, p < 0.001], but comparisons between Dose within each Time point did not reveal any significant test results. For depiction of median 50-kHz USVs by Dose across Time for each drug type, see Figure 1B-C.
Anticipation and self-administration of MDPV elicit 50-kHz USVs (Figures 2 and 3)
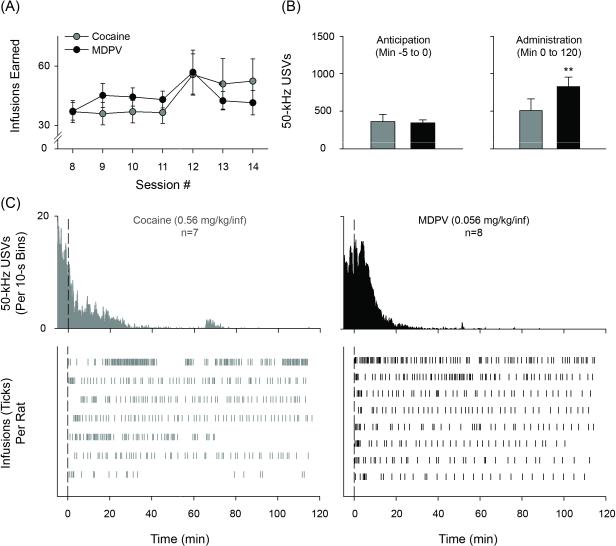
Figure 2.
Self-administration of cocaine or MDPV, whole session. (A) Number of infusions earned during second week of self-administration. Mean number of 50-kHz USVs during anticipation (B) or administration (C) of either cocaine (grey bars) or MDPV (black bars). (D, upper) 50-kHz USVs throughout 2-hours of drug self-administration in 10-s time bins. (D, lower) Drug infusion vertical line plots for individual rats. Vertical dashed line represents lever extension. ** p < 0.01 against respective anticipation data. Data in A, B and C are mean ± S.E.M. n's=7-8.
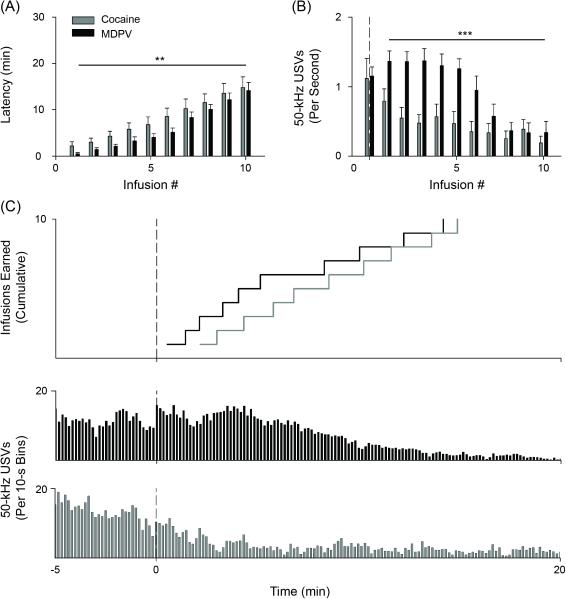
Figure 3.
Self-administration of cocaine or MDPV, load-up. (A) Latency to receive infusions 1-10 of cocaine (grey bars) or MDPV (black bars) relative to lever extension. (B) 50-kHz USVs per infusion where infusion “0” represents USV rate prior to infusion 1. (C, upper) Cumulative time to earn infusions 1-10 plotted using horizontal lines with corresponding 50-kHz USVs across anticipation and load-up (C, lower). Vertical dashed line represents lever extension. * p < 0.05, ** p < 0.01, *** p < 0.001 main effect of Drug Type. Data in A and B are mean ± S.E.M. n's=7-8.
From analyzing sessions during the second week of training, MDPV self-administering rats elicited significantly more 50-kHz USVs during minutes 0 to 120 relative to USVs observed during a 5-minute anticipatory period (p < 0.01; Figure 2B)—this effect was not observed from sessions recorded during cocaine self-administration. As is depicted in Figure 2C, nearly all 50-kHz USVs in both drug groups occurred early in self-administration sessions (i.e. during anticipation and load-up) and declined to near-zero levels following load-up. A significant Drug Type × Infusion interaction was found when examining 50-kHz USVs across load-up [F(9, 130) = 2.443, p < 0.05]. Follow-up analyses revealed a main effect of Drug Type [F(1, 130) = 49.445, p < 0.001] and demonstrated that, while cocaine self-administering rats decreased emission of 50-kHz USVs, MDPV-evoked USVs remained relatively high (Figure 3B and C [lower panels]). Finally, the anticipation to self-administer either cocaine or MDPV was marked by a robust presence of 50-kHz USVs (Figure 3B and C [lower panels]).
MDPV infusions are earned more quickly relative to cocaine during drug load-up (Figure 3)
In analyzing latency to earn drug infusions during load-up, a significant main effect of Drug Type across Infusions was found [F(1, 140) = 11.713, p < 0.01] (Figure 3A and C [upper panel]). In examining the time needed to earn the first drug infusion, rats tended to take less time to initiate MDPV self-administration relative to time taken to initiate cocaine self-administration (p = 0.07; Figure 3A).
Discussion
In the present report, we observed that the synthetic cathinone MDPV elicits positive affective 50-kHz USVs in systemic- and self-administration designs. To our surprise, results from Experiment 2 suggest that the robust context-evoked positive affect elicited prior to cocaine or MDPV administration overshadowed the positive effects of drug infusions—though this effect was most prominent during sessions recorded from cocaine self-administering rats. Put another way, the anticipation to self-administer psychostimulants was marked by the greatest rate of 50-kHz USVs relative to drug intoxication. We additionally observed that MDPV self-administering rats receive their first drug infusion more quickly than cocaine self-administering rats. Taken together, our report demonstrates that: (1) like cocaine, MDPV dose-dependently evokes 50-kHz USVs in rats but with greater persistence, (2) context-evoked anticipation to self-administer either MDPV or cocaine is marked by a robust signature of 50-kHz USVs, (3) MDPV self-administration is initiated more quickly than cocaine self-administration in well-trained rats, (4) 50-kHz USVs remain sustained following self-administered MDPV but wane following self-administered cocaine relative to pre-drug calling rates, and (5) 50-kHz USVs eventually decline to near-zero levels following drug load-up.
MDPV possesses euphorogenic properties that support its reinforcing value as has been shown in present and past self-administration studies. These experiments were designed to expand on previous work characterizing the reinforcing effects of MDPV and is the first demonstration of the effects of MDPV on positive affective USVs. Previous work shows that cocaine elicits 50-kHz USVs in both systemic and self-administration designs (Barker et al, 2010; Williams and Undieh, 2010). Further, cocaine-induced 50-kHz USVs are supported by mesolimbic DA transmission as these calls can be diminished by local (within nucleus accumbens [NAcc]) or systemic pre-treatment with D1 or D2 receptor antagonists (Williams and Undieh, 2010). In support of a functional link between mesolimbic DA transmission and production of positive affective 50-kHz USVs, prior studies demonstrate that elevations in 50-kHz USVs occur following direct infusion of amphetamine into the ventral striatum (Burgdorf et al, 2001) as well as following photostimulation of DA afferents within NAcc (Scardochio et al, 2015). Interestingly, photostimulation of DA afferents within NAcc evoked an initial, transient bout of 50-kHz USVs that succumbed to a tolerance-like effect much like the decay in 50-kHz USVs we observed in the present report as well as in prior work with cocaine self-administering rats (Barker et al, 2014). Combined, these reports support that positive affective USVs are generated in part through mesolimbic DA transmission. It should be noted that a constellation of other transmitter systems have been individually implicated in the production of 50-kHz USVs including glutamatergic, noradrenergic, serotoninergic, adenosinergic and dynorphinergic transmission (Sadananda et al, 2012; Wright et al, 2012; Costa et al, 2015; Hamed et al, 2015; Wohr et al, 2015; Simola et al, 2016). As MDPV functions as both a DA and NE transport blocker, the possibility remains that positive affect elicited following MDPV administration is supported by enhanced signaling along both dopaminergic and noradrenergic transmitter systems. Considering the mechanistic similarity between cocaine and MDPV, it was not altogether surprising that MDPV also evoked 50-kHz USVs in a similar manner as cocaine.
Experiment 2 demonstrated that rats self-administering MDPV were quicker to receive an initial drug infusion relative to cocaine self-administering rats. This is consistent with prior reports showing that rats readily self-administer MDPV and that rats self-administer MDPV at greater infusion rates than methamphetamine self-administration (Huang et al, 2012; Watterson et al, 2014). These data suggest that MDPV confers a significant abuse potential. Interestingly, the number of infusions earned during the second week of self-administration training remained comparable between drug groups. In a qualitative temporal analysis of self-administration infusion data, it appeared that MDPV infusions were characterized by well-timed/spaced infusions relative to drug-taking behavior during cocaine self-administration which appeared more dysregulated/binge-like. The timing of MDPV infusions may relate in part to its ability to cause more sustained elevations in extracellular DA at one-tenth the dose of cocaine (Baumann et al, 2013)—however, future studies using site-specific pharmacology or optical silencing methods will better untangle the transmitter systems and pathways underlying MDPV reinforcement.
Our data also showed that MDPV-elicited 50-kHz USVs were sustained across drug load-up whereas cocaine-elicited 50-kHz USVs waned with subsequent drug infusions. The prolonged rate of 50-kHz USV calling may suggest a prolonged positive state that is not experienced following cocaine intake during self-administration. Eventually, the sustained rate of 50-kHz USVs during MDPV self-administration declined to near-zero levels similarly to cocaine-induced USVs. An earlier study described that cocaine-elicited 50-kHz USVs decline with repeated intravenous delivery and are thus subject to tolerance (Maier et al, 2012). In our prior report (Barker et al, 2014), we conceived the decline in 50-kHz USVs following cocaine load-up as the termination of a positive ‘A’-state in accordance to the opponent-process model of drug addiction (i.e. Solomon and Corbit, 1974). Indeed, in that report, we observed an emergence of negative affective 22-kHz USVs (the ‘B’-state of the opponent-process) when rats were affixed at drug levels below individually-defined satiety points. In the present study, we did not observe an appreciable quantity of 22-kHz USVs for analysis. One possible explanation could relate to reinforcement schedule—the present report employed a fixed 20-s inter-trial interval schedule with 2-s drug infusions whereas a variable 1-to-6 minute interval schedule with 7-s drug infusions was used in our prior report. The present schedule of reinforcement could have prevented the emergence of a negative affective state by allowing desired drug levels to be maintained throughout self-administration. In support of this notion, prior work has indicated that low-dose cocaine self-administration, whereby cocaine satiety levels are not believed to be met, is marked by an increase in negative affective 22-kHz USVs (Barker et al, 2010). Further, decreasing sucrose reward probability from 100% to 25% in a Pavlovian approach behavior task is met with increased 22-kHz USVs (Coffey et al, 2013).
Results from Experiment 2 further demonstrate that positive anticipation for drug was context-elicited. In Experiment 2, both drug groups’ anticipatory 50-kHz calling rates were comparable to the elevated calling rates observed during the first several infusions of drug. Indeed, an original finding described pre-drug anticipatory 50-kHz USVs when rats are exposed to an environment predicting cocaine infusions (Ma et al, 2010). Pre-drug 50-kHz USVs have additionally been captured from amphetamine-experienced rats suggesting context-driven positive affect across multiple psychostimulants (Mahler et al, 2013; Simola et al, 2014; Simola and Morelli, 2015). Interestingly, our prior published results revealed a very low calling rate during the anticipatory recording period. In this study, however, rats both self-administered and lived in the recording environment, suggesting that the salience of the context may have been diminished and anticipation to self-administer consequently attenuated, whereas rats in the present study only self-administered in the recording chamber. In support of context facilitating in part the positive response following cocaine administration, a recent report showed that rats for whom the self-administration context was distinguished (i.e. Non-Resident) emitted significantly greater 50-kHz USVs during cocaine self-administration relative to Resident rats (Avvisati et al, 2016). These prior reports and our current findings support that psychostimulant-paired contexts elicit a strong subjectively-positive anticipatory response.
Taken together, the present study demonstrated synthetic cathinone MDPV elicits positive affective 50-kHz USVs at high rates compared to cocaine in systemic- and self-administration designs. These results corroborate with a growing literature on significant abuse liability associated with the use of novel psychoactive substances including synthetic cathinones. Future studies should be designed to identify specific neuronal pathways and circuits underlying the positive subjective effects of MDPV which in turn modulate drug-seeking behavior.
Acknowledgements
The authors acknowledge generous grant support from the National Institute on Drug Abuse (T32 DA007237 – SJS, TAG; R01 DA039139 – SMR; R00 DA031767 – JWM). SJS conceived research questions under advisory of SMR and JWM. SJS collected/analyzed data and wrote/edited this manuscript. RAG and LRW collected data while FHT, LM, EVW, DJB and TAG participated in data analysis.
Footnotes
All authors have read and approved this manuscript's content and collectively declare no competing financial interests.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiedalla YF, Abdel-Hay K, DeRuiter J, Clark CR. Synthesis and GC-MS analysis of a series of homologs and regioisomers of 3,4-methylenedioxypyrovalerone (MDPV). Forensic Sci Int. 2012;223:189–197. doi: 10.1016/j.forsciint.2012.08.040. [DOI] [PubMed] [Google Scholar]
- Antonowicz JL, Metzger AK, Ramanujam SL. Paranoid psychosis induced by consumption of methylenedioxypyrovalerone: two cases. Gen Hosp Psychiatry. 2011;33:640, e645–646. doi: 10.1016/j.genhosppsych.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Avvisati R, Contu L, Stendardo E, Michetti C, Montanari C, Scattoni ML, et al. Ultrasonic vocalization in rats self-administering heroin and cocaine in different settings: evidence of substance-specific interactions between drug and setting. Psychopharmacology (Berl) 2016;233:1501–1511. doi: 10.1007/s00213-016-4247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Herrera C, West MO. Automated detection of 50-kHz ultrasonic vocalizations using template matching in XBAT. J Neurosci Methods. 2014;236:68–75. doi: 10.1016/j.jneumeth.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, West MO. Ultrasonic Vocalizations as a Measure of Affect in Preclinical Models of Drug Abuse: A Review of Current Findings. Curr Neuropharmacol. 2015;13:193–210. doi: 10.2174/1570159X13999150318113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, et al. Dose-dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology (Berl) 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Simmons SJ, Servilio LC, Bercovicz D, Ma S, Root DH, et al. Ultrasonic vocalizations: evidence for an affective opponent process during cocaine self-administration. Psychopharmacology (Berl) 2014;231:909–918. doi: 10.1007/s00213-013-3309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology (Berl) 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck O, Franzen L, Backberg M, Signell P, Helander A. Intoxications involving MDPV in Sweden during 2010-2014: Results from the STRIDA project. Clin Toxicol (Phila) 2015;53:865–873. doi: 10.3109/15563650.2015.1089576. [DOI] [PubMed] [Google Scholar]
- Berquist MD, 2nd, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3,4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. J Comp Psychol. 2002;116:73–82. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behav Neurosci. 2001;115:940–944. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: a medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18–38. doi: 10.1111/j.1749-6632.2011.06199.x. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Barker DJ, Ma S, Root DH, Martinez L, Horvitz JC, et al. Effects of varying reinforcement probability on pavlovian approach behavior and ultrasonic vocalizations in rats. Behav Brain Res. 2013;237:256–262. doi: 10.1016/j.bbr.2012.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa G, Morelli M, Simola N. Involvement of Glutamate NMDA Receptors in the Acute, Long-Term, and Conditioned Effects of Amphetamine on Rat 50 kHz Ultrasonic Vocalizations. Int J Neuropsychopharmacol. 2015;18:pyv057. doi: 10.1093/ijnp/pyv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Neurobiology of 3,4-methylenedioxypyrovalerone (MDPV) and alpha-pyrrolidinovalerophenone (alpha-PVP). Brain Res Bull. 2016 doi: 10.1016/j.brainresbull.2016.04.011. 10.1016/j.brainresbull.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, et al. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology. 2016;108:111–119. doi: 10.1016/j.neuropharm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed A, Szyndler J, Taracha E, Turzynska D, Sobolewska A, Lehner M, et al. kappa-opioid receptor as a key mediator in the regulation of appetitive 50-kHz ultrasonic vocalizations. Psychopharmacology (Berl) 2015;232:1941–1955. doi: 10.1007/s00213-014-3824-7. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212:109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, et al. A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behav Brain Res. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology (Berl) 2012;219:1141–1152. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin- 1-yl-pentan-1-one (Pyrovalerone) analogues: a promising class of monoamine uptake inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders TM, Gestring R. Hallucinatory delirium following use of MDPV: “Bath Salts”. Gen Hosp Psychiatry. 2011;33:525–526. doi: 10.1016/j.genhosppsych.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Penders TM, Gestring RE, Vilensky DA. Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry. 2012;34:647–650. doi: 10.1016/j.genhosppsych.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365:967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- Sadananda M, Natusch C, Karrenbauer B, Schwarting RK. 50-kHz calls in rats: effects of MDMA and the 5-HT(1A) receptor agonist 8-OH-DPAT. Pharmacol Biochem Behav. 2012;101:258–264. doi: 10.1016/j.pbb.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Sanchez C. Effect of serotonergic drugs on footshock-induced ultrasonic vocalization in adult male rats. Behav Pharmacol. 1993;4:269–277. [PubMed] [Google Scholar]
- Scardochio T, Trujillo-Pisanty I, Conover K, Shizgal P, Clarke PB. The Effects of Electrical and Optical Stimulation of Midbrain Dopaminergic Neurons on Rat 50-kHz Ultrasonic Vocalizations. Front Behav Neurosci. 2015;9:331. doi: 10.3389/fnbeh.2015.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, et al. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233:1981–1990. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simola N, Morelli M. Repeated amphetamine administration and long-term effects on 50-kHz ultrasonic vocalizations: possible relevance to the motivational and dopamine-stimulating properties of the drug. Eur Neuropsychopharmacol. 2015;25:343–355. doi: 10.1016/j.euroneuro.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Simola N, Costa G, Morelli M. Activation of adenosine A(2)A receptors suppresses the emission of pro-social and drug-stimulated 50-kHz ultrasonic vocalizations in rats: possible relevance to reward and motivation. Psychopharmacology (Berl) 2016;233:507–519. doi: 10.1007/s00213-015-4130-8. [DOI] [PubMed] [Google Scholar]
- Simola N, Frau L, Plumitallo A, Morelli M. Direct and long-lasting effects elicited by repeated drug administration on 50-kHz ultrasonic vocalizations are regulated differently: implications for the study of the affective properties of drugs of abuse. Int J Neuropsychopharmacol. 2014;17:429–441. doi: 10.1017/S1461145713001235. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Thompson B, Leonard KC, Brudzynski SM. Amphetamine-induced 50 kHz calls from rat nucleus accumbens: a quantitative mapping study and acoustic analysis. Behav Brain Res. 2006;168:64–73. doi: 10.1016/j.bbr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF. Sensitization to the motor stimulant effects of 3,4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. J Drug Alcohol Res. 2016;5 doi: 10.4303/jdar/235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Addict Biol. 2014;19:165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Brain-derived neurotrophic factor signaling modulates cocaine induction of reward-associated ultrasonic vocalization in rats. J Pharmacol Exp Ther. 2010;332:463–468. doi: 10.1124/jpet.109.158535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintink AJ, Brudzynski SM. The related roles of dopamine and glutamate in the initiation of 50-kHz ultrasonic calls in adult rats. Pharmacol Biochem Behav. 2001;70:317–323. doi: 10.1016/s0091-3057(01)00615-3. [DOI] [PubMed] [Google Scholar]
- Wohr M, Rippberger H, Schwarting RK, van Gaalen MM. Critical involvement of 5-HT2C receptor function in amphetamine-induced 50-kHz ultrasonic vocalizations in rats. Psychopharmacology (Berl) 2015;232:1817–1829. doi: 10.1007/s00213-014-3814-9. [DOI] [PubMed] [Google Scholar]
- Wojcieszak J, Andrzejczak D, Woldan-Tambor A, Zawilska JB. Cytotoxic Activity of Pyrovalerone Derivatives, an Emerging Group of Psychostimulant Designer Cathinones. Neurotox Res. 2016;30:239–250. doi: 10.1007/s12640-016-9640-6. [DOI] [PubMed] [Google Scholar]
- Wright JM, Dobosiewicz MR, Clarke PB. alpha- and beta-Adrenergic receptors differentially modulate the emission of spontaneous and amphetamine-induced 50-kHz ultrasonic vocalizations in adult rats. Neuropsychopharmacology. 2012;37:808–821. doi: 10.1038/npp.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]