Abstract
OBJECTIVE
This study aimed to examine the feasibility, acceptability and initial validity of using smartphone-based ecological momentary assessment (EMA) to assess daily functioning and other behavioral factors among older HIV+ adults.
METHOD
Twenty older HIV+ adults (mean age = 59 years) completed laboratory-based neurobehavioral and functional assessments then completed EMA surveys via smartphones five times per day for one week.
RESULTS
Excellent EMA adherence (86.4%) was found, and participants rated their experience with EMA methods positively. Time use data indicated participants were spending 74% of their waking-sampled time at home, 63% of their time alone, and 32% of their time engaged in passive leisure activities (e.g., watching TV). Better neurocognitive and functional capacity abilities were correlated with less time spent in passive leisure activities. Lastly, mood and cognitive symptom data collected via EMA were significantly associated with scores from laboratory-based assessments of these same constructs.
CONCLUSIONS
EMA via smartphones is a feasible and acceptable data collection method among older HIV+ adults and appears to be a promising mobile tool to assess daily functioning behaviors in HIV. These preliminary findings indicate older HIV+ adults are spending a considerable amount of time at home, alone, and engaged in passive leisure activities, primarily watching TV. EMA may contribute to future research examining functional disability among the growing population of older HIV+ adults.
Keywords: ecological momentary assessment, mHealth, HIV/AIDS, disability, daily functioning, aging
Introduction
Older adults with HIV-infection (HIV+) can experience substantial disability(1, 2), and the costs associated with disability impairments in daily functioning place a significant burden on society. Given that HIV is now a chronic illness and due to the rapid growth in the number of older HIV+ adults, the societal impact of disability will likely be exacerbated over the coming years(3). As such, the NIH Office of AIDS Research and the U.S. Senate Special Committee on Aging have emphasized the significant public health priority for research assessing and improving functioning in those aging with HIV(4, 5).
Beyond summative self-reports, it is unclear how older adults with HIV are getting along in their daily lives, who may need the most assistance, and which modifiable factors might be targeted to enhance real-world daily functioning. To date, much of the research on aging with HIV has focused on HIV-associated neurocognitive impairment. While age and HIV-associated neurocognitive impairment are established predictors of decline in daily functioning(1-3, 6, 7), daily functioning impairments have been observed in older HIV+ adults without HIV-associated neurocognitive impairment, and not all those with neurocognitive impairment experience daily functioning impairment(8-11). Additional determinants of disability include depressed mood, although this relationship is complex because mood also influences the validity of self-report assessments. For example, Thames et al.(8) found that higher levels of depression related to over-reporting of cognitive and functional impairments, while lower levels of depressive symptoms were related to the under-reporting of these difficulties. Research has also demonstrated that self-reports of instrumental activities of daily living (IADL) among persons living with HIV can demonstrate impairment despite normal laboratory-based performance of functional ability(12-14).
While direct, performance-based functional tests are reasonably sensitive to HIV-associated IADL declines (e.g., 12), they only take a “snapshot” of functioning at one time point and under controlled conditions (i.e., laboratory). Therefore, questions remain regarding the extent to which performance-based tests fully capture real-world daily functioning. Self-report, proxy report, and clinician-administered measures are the most common assessment approaches to detailing daily functioning. Self-report measures, however, may be heavily influenced by retrospective recall errors, social desirability effects, lack of insight, cognitive and metacognitive deficits(15), or other state-dependent bias(16, 17). Proxy reports and clinician-administered measured may be affected by limited opportunities to observe the rated skills or activities(17). Additionally, these measures are limited in that they use a narrow definition of “daily functioning” to describe difficulty or dependency in engaging in ADLs or IADLs, and do not capture other important domains of daily functioning in real-world contexts, such as physical and socially-engaging activities.
Ecological momentary assessment (EMA) permits the measurement of daily functioning in real-world settings, which could be especially useful for studies of older HIV+ individuals. EMA is an ambulatory data collection technique that relies on repeated intra-day assessments in naturalistic contexts to assess functional behaviors in real-time, including engagement in self-maintenance (ADLs) as well as intellectual, socially-engaging, physical, and passive leisure activities. It also captures contextual influences on behavior, such as with whom these activities are performed and concurrent mood symptoms and cognitive appraisals of the activities. A novel measurement of daily functioning via EMA could greatly enhance the accuracy and detail of measurement of daily functioning in adults who are aging with HIV, and help identify the potential mediators and moderators by which neurocognitive impairment and other risk and protective factors impact daily functioning. Prior studies have found strong support for the feasibility and application of EMA use in older samples of healthy adults and people with clinical disorders(18).
There are several reasons to believe that adherence and tolerance to such a method, however, might be lower among older HIV+ adults compared to other populations with which it has been used. For example, EMA is typically administered via smartphones, and some older HIV+ adults may be inexperienced with carrying a phone and using a smartphone platform, which could make EMA-based studies more challenging in older adults living with HIV and reduce adherence (19, 20). Additionally, it is unknown whether people already burdened with potential medical complications and complex daily medication regimens will tolerate an additional schedule of multiple daily smartphone-based queries. The aim of this study was therefore to test the feasibility and acceptability of using EMA methods delivered via smartphones to assess daily functioning and other behavioral factors (e.g., mood, cognitive symptoms) among older HIV+ adults. A second aim was to test the initial convergent and discriminant validity of EMA assessments of daily functioning in this population by examining the relationship between EMA-measured daily functioning data with traditional measures of neuropsychological performance and functional capacity. While we expect EMA surveys to more accurately access the nuances of real world, day-to-day functioning, initial validation of EMA methods (at an aggregate level) compared to paper-and-pencil methods are warranted at this relatively early stage of the current literature.
Method
Participants
Twenty HIV+ participants aged 51-67 were recruited from ongoing studies at the University of California, San Diego (UCSD) HIV Neurobehavioral Research Program (HNRP). Patients with normal or mild neurocognitive impairment were recruited (based on their most recent neuropsychological assessment at the HNRP). Inclusion criteria were: HIV seropositive; age at enrollment 50 years or older; English fluency; and ability to provide written informed consent. Exclusion criteria were: psychotic disorders (e.g., schizophrenia); neurological disease (e.g., head injury with loss of consciousness >30 minutes, seizure disorder, stroke); or a positive toxicology test for substances of abuse at day of testing.
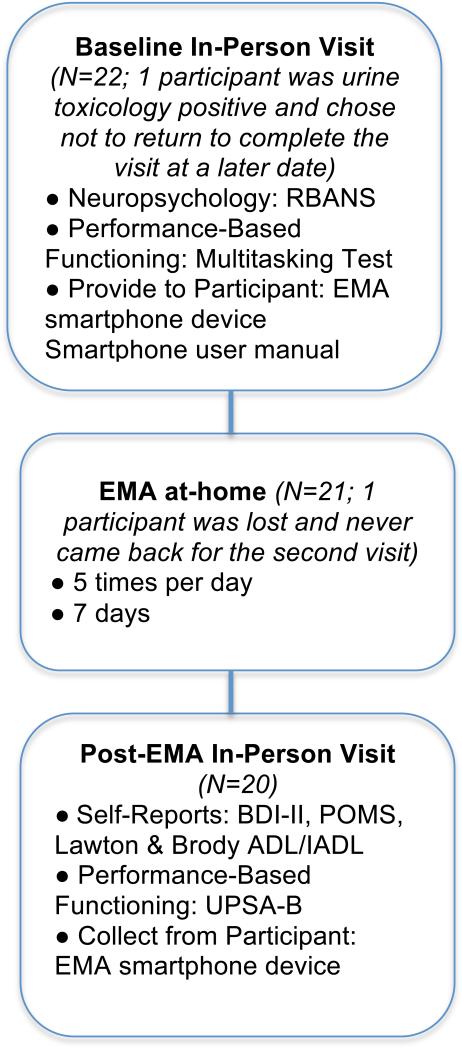
The UCSD Human Research Protections Program approved the study, and all participants completed an assessment of capacity to consent(21) prior to providing written informed consent. A study flowchart is presented in Figure 1.
Figure 1.
Timing and frequency of EMA and laboratory assessments.
Measures and Procedure
HIV Disease Characteristics
To assess HIV disease characteristics, the participants completed a neuromedical examination that consisted of a clinical interview and laboratory testing. HIV serostatus was determined by MedMira Miriad rapid test (Nova Scotia, Canada) or by enzyme-linked immunosorbent assays and confirmatory Western blot test. Acquired immune deficiency (AIDS) status was based on 1993 Centers for Disease Control classification(22). Unless a participant's laboratory CD4 count was lower than the self-reported value, Nadir CD4+ count was self-reported. Plasma HIV viral load was considered “undetectable” below 40 copies/mL.
Neurocognitive Ability
Participants were recruited for this study using recent results on a standardized neuropsychological battery, which had been designed in accordance with international consensus conference recommendations and had published norms that correct for demographic effects of age, education, sex and (where appropriate) race/ethnicity(23). Cognitive domains assessed in this battery include: verbal fluency, attention/working memory, abstraction/executive functioning, learning, memory (delayed recall), speed of information processing, and complex motor skills(9). In order to assess feasibly of the EMA methods in neurocognitively normal to mildly/moderately impaired participants, individuals with Global Deficit Scores (GDS > 1; 24) were excluded. The GDS approach was developed to assess cognitive impairment that may be relatively mild and variable with respect to affective cognitive domains, and brain substrates, such as milder forms of HIV-associated neurocognitive disorders (HAND; 24, 25, 26). Briefly, this approach considers the number and severity of deficits in performance on a standard neuropsychological test battery, and provides an overall deficit scores ranging from zero (no impairment) to five (severe impairment). Individuals who receive GDS scores <0.5 are considered to have normal cognitive functioning, and 0.5-1.0 reflects mild to moderate HAND. Thus, prior to a large-scale study using these methods, we aimed to test the feasibility and validity of our EMA methods in a sample without cognitive severe impairment using a standardized measure of overall cognitive status. To assess current neurocognitive status, and because the participants had had prior examinations with the more comprehensive test battery, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; 27) was administered at this baseline visit. The RBANS is a brief screening battery that assesses neurocognition in five cognitive domains: immediate memory, visuospatial/constructional skills, language, attention, and delayed memory. Age-specific normative data was used to calculate scores in each domain, and a total score is devised by summing the five domain-level scores. A total score of 100 (standard deviation=15) is considered average.
Laboratory-Based Functional Capacity
Participants completed two performance-based measures of functional capacity: the UCSD Performance-Based Skills Assessment (UPSA-B; 28) and the Everyday Multitasking Test(29). The UPSA-B measures a participant's ability to perform common life tasks related to financial (i.e., counting and making change, writing a check) and communication skills (calling emergency services, information to obtain a phone number, and a physician). Each domain yields a score ranging from 0-50 based on percent of correct tasks and the two domains are added to yield a summary score ranging from 0-100. The multitasking test asks participants to complete four tasks (i.e., cooking, advanced finances, medication management, and telephone communication) over a 12-minute time interval. Points are awarded for completing tasks accurately for a total score ranging from 0-70 (higher scores indicate better multitasking skills). The administration and scoring of these tasks is described elsewhere(12, 29).
Mood and Cognitive Symptoms
Participants were administered the Beck Depression Inventory-II (BDI-II; 30), the Profile of Mood States (POMS; 31), and a modified version of the Lawton and Brody (32) ADL/IADL questionnaire (see Heaton et al., 2004) at the follow-up visit. The ADL/IADL questionnaire details the degree to which people have decreased independence (compared to their highest-level of functioning) in the following domains: finances, social activities, buying groceries, using the telephone, transportation, child care, understanding written material/TV, shopping, cooking, medication management, and working. Participants were classified as IADL-dependent or IADL-independent based on a classification approach that has been validated in a normative sample compared to one with cognitive impairment(12). In this approach, participants are deemed IADL-dependent if their self-reported current level of functioning was lower than their self-reported highest level of functioning in at least two functional domains. A post-EMA feedback survey asking about experiences participating in the study was also administered at the end of the week.
EMA Functioning Survey
We developed an EMA functioning survey tailored to the unique needs of older HIV+ adults by: 1) modifying a validated EMA functioning survey(33); 2) vetting our survey questions through a consensus process with four experts in the fields of daily functioning in HIV and daily functioning via EMA; and 3) conducting a focus group of eight persons (mean age = 69 years, SD = 9; males: N = 7; race/ethnicity: 88% Caucasian; mean education = 14 years). Three members of the focus group met criteria for neurocognitive impairment. The focus group was audio-recorded, transcribed in a de-identified fashion and themes were reviewed. Additionally, focus group members made recommendations made to improve the survey questions around areas of daily functioning we had not previously included (e.g., spending time with pets; spiritual/meditative activities).
The EMA survey captured data about: 1) time use (e.g., Where are you?; What are you doing?); 2) ratings around individual behavior (e.g., How productive are you?; How difficult is this activity for you?); 3) mood and physical symptoms (e.g., What is your pain level right now?; How happy do you feel right now?); and 4) antiretroviral use and side effects, as well as substance use since the last EMA assessment. Items used a 7-point Likert scale for ratings. Branching was used for some experiences in order to reduce survey length by skipping irrelevant questions (e.g., if the participant reported they were alone, questions about appraisals of social interactions were skipped).
EMA Protocol/Procedures
All participants were provided with an Android Operating System smartphone, which included unlimited data and text messaging. At the initial study visit the examiner reviewed how to access and respond to the survey questions on the smartphone with the participant. Participants were given an informational manual on how to use the smartphone and complete the surveys. Additionally, participants completed a practice survey monitored by the examiner to assess any difficulties they had with the phone or survey questions. On the first day of the study, the examiner called each participant to verify if they had any questions or difficulties completing the surveys at home. Participants completed EMA-based surveys of daily functioning on a research-provided smartphone five times a day for one week, providing 35 data points per person. This frequency of assessment has been previously associated with high rates of survey adherence in psychiatric patients(34). Timing of the surveys was adjusted to accommodate each participant's sleep-wake schedules. Time-stamped, de-identified and encrypted responses were instantly transferred to our password-protected server. At the completion of the EMA assessment period, participants returned the smartphones and completed a follow-up interview regarding their experience carrying and operating the smartphone, as well as their opinion regarding the frequency and duration of the assessments.
Statistical Analyses
Data were analyzed using IBM SPSS Statistics Version 23(35). All participants included sufficient EMA data points (>50%) to be included in analyses. The EMA data on time use was calculated by the per-person number of samples at home, sampled time alone, and sampled time engaged in intellectual activities, IADLs, socialization, and passive leisure activities divided by the total number of samples for that person. An exception to this approach was the calculation of the socialization activity subscale, in which scores for each participant were tabulated as a sum of “who are you with at the moment” (alone=0, with unknown people or pets=1, spouse/partner, friends, other family members, healthcare provider, or other known people=2) plus “since the last alarm, how many times did you socialize with someone else (e.g., spent more than 5 minutes talking/communicating with someone else; no interactions=0; 1 interaction=1; 2-3 interactions=2; 4+ interactions=4), with total scores ranging from 0-6. For the EMA mood and cognitive difficulties variables, mean scores across the 7-day study period were aggregated for each individual. Pearson correlations between EMA items and laboratory-based measures were examined.
Results
Feasibility and Adherence
The participants’ demographics and clinical characteristics are presented in Table 1. Four participants met criteria for neurocognitive impairment (GDS ≥0.5) based on their prior comprehensive neuropsychological evaluation. Results demonstrated an excellent EMA average adherence per person rate of 86.4% (i.e., completing approximately 30 out of 35 surveys; SD=4 surveys; range=19-35 surveys), resulting in an overall total of 605 EMA data points. The mean satisfaction rating to the post-EMA feedback question, “I enjoyed the experience” was 3 on a scale from 0=Not at all to 4=Very much. No participants reported difficulty operating the smartphone or understanding the questions. Additionally, 60% of the participants stated that the smartphone did not interfere at all with their activities, while the remaining participants said it interfered “a little bit.”
Table 1.
Participant Demographics and Clinical Characteristics (N=20)
| M (SD) unless otherwise specified | Range | |
|---|---|---|
| Demographics and HIV Disease Characteristics | ||
| Age | 58.8 (4.3) | 51-67 |
| % Female | 15 (n=3) | -- |
| Race/Ethnicity (%) | -- | |
| Caucasian | 70 (n=14) | |
| African American | 25 (n=5) | |
| Native American | 5 (n=1) | |
| Education (years) | 13.4 (2.7) | 8-20 |
| Receiving Disabilitya (%) | 64 (n=7) | -- |
| Estimated duration of HIV (years) | 20.4 (7.8) | 4.8-29.8 |
| AIDS (%) | 70 | -- |
| cART(%) | 90 | -- |
| Nadir CD4b (cell/μl) | 128.6 (106.5) | 7-350 |
| Current CD4b (cell/μl) | 476.2 (167.2) | 108-750 |
| Plasma Viral Load Detectable (number of participants) | 1 | -- |
| Clinical Characteristics | ||
| Beck Depression Inventory-II (BDI-II) | 7.4 (9.5) | 0-38 |
| POMS Confusion-Bewilderment Scale | 5.3 (5.7) | 0-19 |
| Global Deficit Score (GDS)c | 0.3 (0.2) | 0-1 |
| RBANS Total Score | 98.9 (13.0) | 78-122 |
| Multitasking Total Score | 27.5 (5.7) | 4-41 |
| UPSA-B Total Score | 83.0 (5.7) | 73-89 |
| IADL Dependent (%) | 15 (n=3) | -- |
| Phone Ownership | ||
| Own a cell phone (%) | ||
| Smartphone | 55 (n=11) | |
| Other type of cell phone | 25 (n=5) | |
| EMA Surveys | ||
| Number of EMA surveys completed | 30.3 (4.2) | 19-35 |
| EMA Mood and Cognitive Difficultiesd | ||
| Sad | 1.9 (1.1) | 1-7 |
| Stress | 2.2 (1.2) | 1-7 |
| Happy | 4.5 (1.1) | 1-7 |
| Tired | 2.7 (0.9) | 1-7 |
| Pain Level | 2.0 (1.4) | 1-10 |
| Forgetfulness | 1.6 (1.0) | 1-7 |
| Problems Concentrating | 1.6 (0.9) | 1-7 |
Note.
Data on disability for 9 participants were missing
Data represent medians with interquartile ranges in parentheses
GDS scores based on most recent neuropsychological evaluation within the last year
Within-person means from EMA data
POMS = Profile of Mood State; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; UPSA-B = UCSD Performance-Based Skills Assessment-Brief; IADL = Instrumental Activities of Daily Living
Time Use
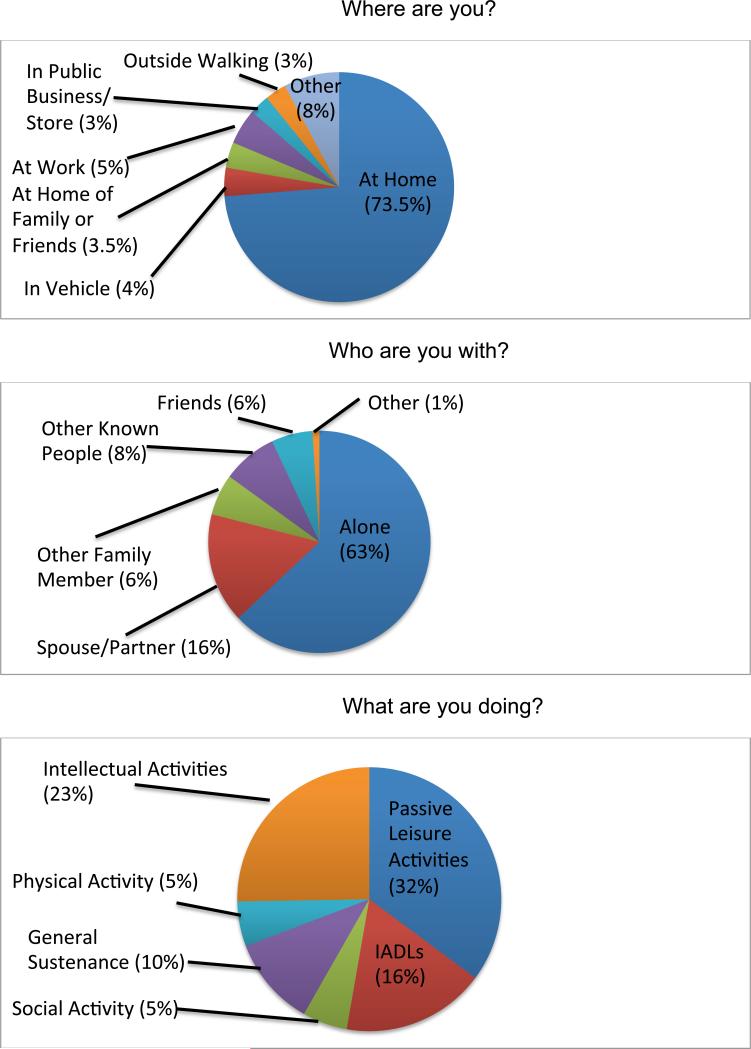
Time use data indicated that participants were spending 74% (SD = 19%; range = 29-100%) of their waking sampled time at home, 63% of their time alone (SD = 31%; range = 10-100%), and 32% of their time engaged in passive leisure activities (e.g., watching TV; SD = 16%; range = 0-63%; see Figure 2). Twenty-three percent of their time was spent involved in intellectual activities (SD = 20%; range = 0-62%), and only 5% of their time was engaged in physical activity (SD = 6%; range = 0-17%). EMA time use data were unrelated to HIV disease characteristics (i.e., duration of HIV, Nadir or current CD4, AIDS status, or antiretroviral [ART] use) (see Table 2).
Figure 2.
Percent of time spent in various daily activities.
Note. Where are you? Other includes: outpatient medical, community center, class/educational setting, inside other, outside other. Who are you with? Other includes: healthcare provider; unknown people. What are you doing? Intellectual Activities includes: Working (paid or unpaid), volunteering, schoolwork, computer/tablet use, non-physical leisure (e.g., reading); Passive Leisure Activities includes: watching TV, listening to music, resting, smoking, nothing; Instrumental Activities of Daily Living (IADLs) include: Household chores, preparing food, shopping, traveling.
Table 2.
Correlations between HIV disease characteristics and time use data (N=20)
| Time Use Data | |||
|---|---|---|---|
| At home vs not at home | Alone vs not alone | Passive leisure vs non-passive activates | |
| HIV Disease Characteristics | |||
| Current CD4 (cell/μl) | −0.36 | −0.33 | −0.04 |
| Nadir CD4 (cell/μl) | −0.13 | −0.07 | −0.09 |
| AIDS Status (yes or no) | 0.33 | 0.22 | −0.17 |
| Duration of HIV (years) | 0.42 | 0.02 | 0.05 |
| HAART status (yes or no) | 0.00 | 0.35 | 0.37 |
Note. All p-values >0.05. Time Use Data is derived from the following Ecological Momentary Assessment (EMA) questions: Where are you? = At home vs not at home; Who are you with? = Alone vs not alone; What are you doing? = Passive leisure vs non-passive activities.
Clinical Correlates of EMA Daily Functioning
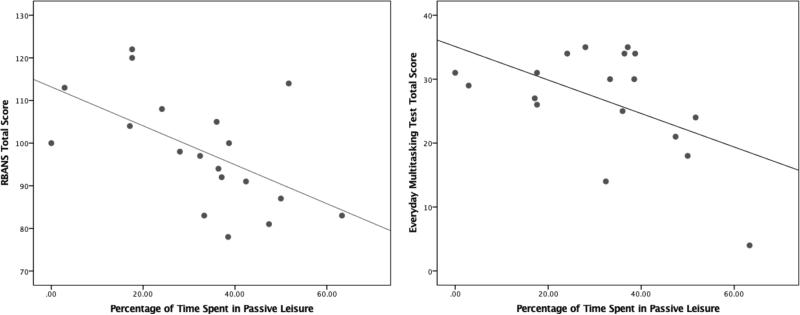
Next, we examined the associations between age, neurocognition, and functional capacity with EMA daily functioning domains: 1) percent of time spent engaged in intellectual activities; 2) IADLs; 3) socialization; and 4) passive leisure activities (see Table 3 for intercorrelations among EMA and in-lab tasks). Older age was related to more time spent engaged in passive leisure activities and was unrelated to the other EMA functioning domains. Worse neurocognitive ability on the age-corrected RBANS total score was related to more time spent in passive leisure activities (see Figure 3), while RBANS scores were not significantly related to percent of time spent in intellectual, IADLs, or social activities. Additionally, better multitasking was related to less passivity and more intellectual activity (Figure 3); multitasking was unrelated to IADLs or social activity. No relationships were found between UPSA-B performance and EMA functioning domains.
Table 3.
Pearson correlations between EMA daily functioning variables, age, and laboratory assessments (N=20)
| EMA Daily Functioning Domains | ||||
|---|---|---|---|---|
| Intellectual Activities | IADLs | Socialization | Passive Leisure Activities | |
| Age | −0.34 | −0.25 | 0.27 | 0.47* |
| RBANS Total Score | 0.37 | 0.05 | 0.11 | −0.57** |
| UPSA-B | 0.13 | 0.08 | 0.43 | −0.37 |
| Multitasking Test | 0.64** | −0.19 | 0.21 | −0.53* |
| EMA Mood and Cognitive Symptoms | |||||||
|---|---|---|---|---|---|---|---|
| Sadness | Stress | Happiness | Tired | Pain | Forgetfulness | Problems Concentrating | |
| Age | −0.06 | −0.03 | −0.11 | −0.01 | −0.06 | 0.10 | 0.18 |
| BDI-II | 0.57** | 0.55* | −0.40 | 0.68** | 0.15 | 0.67** | 0.73** |
| POMS Confusion/Bewilderment Scale | 0.65** | 0.61** | −0.54* | 0.69** | 0.27 | 0.93** | 0.78** |
Note.
p<0.05
p<0.01.
Figure 3.
Better neurocognitive ability and better everyday multitasking are related to less time spent in passive leisure activities.
Note. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status
Clinical Correlates of EMA Mood and Cognitive Symptoms
Lastly, we examined the relationships between paper-and-pencil and EMA-measured mood and cognitive symptoms. Average EMA mood ratings across the week were created (see Table 1 for means and standard deviations). Scores on the BDI-II were related to greater EMA-measured sadness, tiredness, forgetfulness, and problems concentrating, and unrelated to EMA-measured stress, happiness and pain. Scores on the POMS Confusion/Bewilderment scale were strongly related to greater EMA-measured forgetfulness and problems concentrating, and also related to greater EMA-measured sadness, stress, and tiredness.
Discussion
Although the health status of HIV-infected adults has greatly improved over the past 15 years, HIV is still associated with a variety of adverse real-world outcomes. There has been a focus in the field on HIV-associated neurocognitive impairment as an important driver of these functional impairments(2, 6, 8). However, a fair number of older HIV+ adults without neurocognitive impairment report declines in daily functioning, highlighting the need for enhanced understanding of everyday functioning difficulties among older HIV+ individuals(9-11).
Here, we tested the feasibility and acceptability of using EMA methods via smartphone to get a more detailed and extended assessment of daily functioning behaviors among older HIV+ adults. Findings revealed that older HIV+ adults were able to engage with smartphones and predominately enjoyed completing the EMA surveys. In this sample of older HIV+ adults with normal and mildly-moderately impaired neurocognition, real-time assessment data indicated that they are spending a considerable amount of time at home, alone, and engaged in passive leisure activities, primarily watching TV. Comparatively, data from a large normative sample of older adults (American Time Use (ATU) survey; 36) indicate Americans aged 65 and older spend 23-42% of their time alone (compared to 63% in the current sample). Additionally, the percentage of time participants spent in leisure activities was comparable to much older (≥75 years old) people in this normative sample. One caveat when comparing our sample to the aforementioned ATU survey sample is the ATU data was collected via a different sampling method, so our comparison with this data is imperfect. The ATU data were obtained via participant's reports of how much time they spent doing specific activities “yesterday”. Each ATU participant only reports on one day of his or her life. Therefore, the ATU data may be subject to common errors and biases associated with retrospective self-report data, as well as limited to a “snapshot” of functioning over a single day. For further (and perhaps a more accurate) comparison, other EMA data by our group indicates adults with bipolar disorder spend 65% of their time at home(37) and adults with schizophrenia spend 75% of their time at home(38). Thus, the functional profile is in some respects similar to that of much older adults or persons with serious mental illness, yet the data suggest the cohort is physically healthy enough to be more engaged (i.e., only one participant with a detectable viral load; average current CD4 counts in the average range; only three participants met lab-based criteria for functional dependence).
Construct validity of the EMA daily functioning variables was assessed by first examining the clinical correlates of EMA daily functioning with laboratory-based measures. We observed an association between greater passivity, predominately watching television, and worse cognition and multitasking ability. We also observed an association between greater engagement in intellectual activities and multitasking ability. In-lab measures of cognition and functional capacity (UPSA-B and multitasking) were unrelated to engagement in IADLs or social activity. These findings have implications for future lifestyle modification interventions to promote optimal cognitive aging. Among older adults, cognitive impairment and dementia have been found to be associated with high levels of sedentary behavior and low levels of physical activity(39-41). There is some evidence in the aging literature that television watching is a risk factor for cognitive impairment(42-45), although studies to-date have been reliant on traditional assessment methods. The mechanisms by which high levels of television watching may impact (or simply be associated with) lower cognition are unknown, although it may be that both extensive television watching and lower cognition are associated with negative lifestyle factors, such as social isolation, depression, physical inactivity, poor dietary patterns, and low cognitive engagement(46-48). It may also be that the direction of the relationship between cognition and daily functioning is bidirectional: cognitive impairment leads to poor functioning, and/or passivity and isolation leads to worse cognition. A combination of EMA surveys and assessing cognitive performance in vivo using EMA paradigms could be useful in teasing out this relationship(49). A unique advantage of this approach is that it provides simultaneous assessment of real-time functional and leisure time behaviors and cognitive ability, allowing for greater ecological validity of data and for the examination of within-person trends over time and directional effects.
Disability status is high among adults aging with HIV, and limited employment is often associated with reduced functional resources needed to engage in intellectually stimulating and social activities, which increase the likelihood of staying at home and engaging in inexpensive, passive leisure activities, such as watching television(50). Future prospective studies should consider how a composite of sedentary, solitary activity combined with socioeconomic status predict real-time change in cognition and well-being outcomes.
The clinical correlates of EMA-measured mood and cognitive symptoms with laboratory-assessed measures were also examined for expected associations between EMA-measures with traditional lab-based measures. Laboratory-assessed depressive symptomatology was related to EMA-measured sadness, tiredness, forgetfulness and problems concentrating averaged over the one-week period; all of these symptoms go into the total depressive score on the BDI-II and findings thus support the construct validity of the EMA methods. Laboratory-based cognitive symptoms were related to EMA-measured forgetfulness, problems concentrating, greater sadness, greater stress, and tiredness. Depression and cognitive difficulties are known to be highly correlated in the HIV literature (e.g., 8), so these results are unsurprising. EMA-measured happiness and pain were unrelated to in-lab measured depression or cognitive difficulties, which supports the discriminant validity of our EMA methods. These preliminary data show concurrent validity for our aggregated EMA variables. Future studies examining the relationship between intra- and inter-daily variability in mood with daily functioning behaviors could be clinically useful to identify future treatment targets to improve functioning in this population.
While our pilot study addressed the feasibility and provides some preliminary validity data of EMA daily functioning among older HIV+ adults, the small sample size limits us from an in-depth exploration of the validity of our methods. Only three participants met criteria for functional dependence, so the study was not able to examine associations of paper-and-pencil IADL-dependence vs. EMA reported independence in performing daily activities. We also did not administer a lab-based measure of leisure time activities, so we were unable to examine associations of traditional paper-and-pencil leisure activities with our EMA reported activities. In addition, this study did not contain an HIV− comparison sample, so we were unable to directly assess factors that differentiate older HIV+ from HIV− adults in terms of daily functioning or address reasons for functional disability. Further, we did not obtain proxy reports or clinical-administered measures of daily functioning to compare with our EMA measures, which will be crucial to include in future studies of the clinical utility of EMA methods. In regard to our EMA survey, we chose to include computer and tablet use as “intellectual activities,” when in fact they may have been passively engaged with these devices (e.g., watching TV or movies). Additionally, the low prevalence rates of physical activity may have been influenced by the survey methodology (for example, if a participant was jogging, they may have waited until it was completed to respond to the survey). Future studies would benefit from incorporating accelerometry-based methods for physical activity tracking (e.g., wrist-worn activity trackers) into EMA functioning studies as well as other connected devices that provide psychophysiological data. Such a combination would provide better estimates of physical activity and allow for the examination of independent and interdependent relationships between physical activity, health-related variables and EMA-measured engagement in daily activities. Lastly, there was a restricted range of cognitive functioning within our sample, so we were unable to address if patients with the poorest cognition are at greatest risk for poor EMA daily functioning. Future studies with larger sample sizes and both HIV+ and HIV− participants with a wide-range of cognitive functioning would help to identify patients with the greatest need for clinical services.
Despite these limitations, this study demonstrates the feasibility, acceptability, and initial validity of EMA for assessing daily functioning among older HIV+ adults. Moreover, it represents the first step in using EMA to determine which facets of daily functioning are most affected in older adults with HIV and how neurocognitive functioning may relate to these deficits. One area of future application would be to focus on using EMA methods to deliver just-in-time interventions to promote engagement in active lifestyle behaviors. Ultimately, EMA may emerge as a useful clinical and research tool for both assessment and delivering of interventions to promote successful functional aging among this growing population.
Acknowledgments
* The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes: Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D.; (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government.
Funding: This research was supported by the National Institute of Mental Health (R.C.M., K23 MH107260) and the HIV Neurobehavioral Research Center (HNRC). The HNRC is supported by Center award P30MH062512 from NIMH. Dr. Scott's participation was supported by a Department of Veterans Affairs Career Development Award (IK2CX000772).
Footnotes
Previous Presentation: These data were presented during an oral presentation at the 6th International HIV and Aging workshop, Washington DC, October, 2015.
No disclosures to report.
References
- 1.Morgan EE, Iudicello JE, Weber E, et al. Synergistic effects of HIV infection and older age on daily functioning. Journal of Acquired Immune Deficiency Syndromes (1999) 2012;61:341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vance DE, Fazeli PL, Gakumo CA. The impact of neuropsychological performance on everyday functioning between older and younger adults with and without HIV. The Journal of the Association of Nurses in AIDS Care : JANAC. 2013;24:112–125. doi: 10.1016/j.jana.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HIV/AIDS among persons 50 and older. Center for Disease Control; 2008. http://www.cdc.gov/hiv/topics/over50/resources/factsheets/pdf/over50.pdf. [Google Scholar]
- 4.High KP, Brennan-Ing M, Clifford DB, et al. HIV and Aging: State of knowledge and areas of critical need for research. A report to the NIH office of AIDS research by the HIV and aging working group. Journal of Acquired Immune Deficiency Syndrome. 2012;60:S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Special Committee on Aging . United States Senate: Hearing: Older Americans: The Changing Face of HIV/AIDS in America. Washington, D.C.: 2013. [Google Scholar]
- 6.Vance DE, Mugavero M, Willig J, et al. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. The Journal of the Association of Nurses in AIDS Care : JANAC. 2011;22:17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith G. Aging Hearing: HIV over fifty, exploring the new threat. Washington, DC: 2006. [Google Scholar]
- 8.Thames AD, Becker BW, Marcotte TD, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. The Clinical Neuropsychologist. 2011;25:224–243. doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaton RK, Clifford DB, Franklin DR, Jr., et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albert SM, Marder K, Dooneief G, et al. Neuropsychologic impairment in early HIV infection. A risk factor for work disability. Archives of Neurology. 1995;52:525–530. doi: 10.1001/archneur.1995.00540290115027. [DOI] [PubMed] [Google Scholar]
- 11.Hinkin CH, Castellon SA, Durvasula RS, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. Journal of the International Neuropsychological Society : JINS. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 13.Burgess PW, Alderman N, Evans J, et al. The ecological validity of tests of executive function. Journal of the International Neuropsychological Society : JINS. 1998;4:547–558. doi: 10.1017/s1355617798466037. [DOI] [PubMed] [Google Scholar]
- 14.Blackstone K, Moore DJ, Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. Journal of the International Neuropsychological Society : JINS. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casaletto KB, Doyle KL, Weber E, et al. Self-predictions of prospective memory in HIV-associated neurocognitive disorders: evidence of a metamemory deficit. Archives of Clinical Neuropsychology : The Official Journal of the National Academy of Neuropsychologists. 2014;29:818–827. doi: 10.1093/arclin/acu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz N. Self-reports: how the questions shape the answers. American Psychologist. 1999;54:93–105. [Google Scholar]
- 17.Moore DJ, Palmer BW, Patterson TL, et al. A review of performance-based measures of functional living skills. Journal of Psychiatric Research. 2007;41:97–118. doi: 10.1016/j.jpsychires.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Cain AE, Depp CA, Jeste DV. Ecological momentary assessment in aging research: a critical review. Journal of Psychiatric Research. 2009;43:987–996. doi: 10.1016/j.jpsychires.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson M. For vast majority of seniors who own one, a smartphone equals ‘freedom’. Pew Research Center; 2015. [Google Scholar]
- 20.Smith A. Older adults and technology use. Pew Research Center; 2014. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use/ [Google Scholar]
- 21.Jeste DV, Palmer BW, Appelbaum PS, et al. A new brief instrument for assessing decisional capacity for clinical research. Archives of General Psychiatry. 2007;64:966–974. doi: 10.1001/archpsyc.64.8.966. [DOI] [PubMed] [Google Scholar]
- 22.Castro K, Ward J, Slutsker L, et al. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. 1993 [PubMed] [Google Scholar]
- 23.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings. The Clinical Neuropsychologist. 2012;26:894–908. doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez R, Heaton RK, Moore DJ, et al. Computerized reaction time battery versus a traditional neuropsychological battery: detecting HIV-related impairments. Journal of the International Neuropsychological Society : JINS. 2003;9:64–71. doi: 10.1017/s1355617703910071. [DOI] [PubMed] [Google Scholar]
- 26.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randolph C, Tierney MC, Mohr E, et al. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 28.Mausbach BT, Harvey PD, Pulver AE, et al. Relationship of the Brief UCSD Performance-based Skills Assessment (UPSA-B) to multiple indicators of functioning in people with schizophrenia and bipolar disorder. Bipolar Disorders. 2010;12:45–55. doi: 10.1111/j.1399-5618.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott JC, Woods SP, Vigil O, et al. A neuropsychological investigation of multitasking in HIV infection: implications for everyday functioning. Neuropsychology. 2011;25:511–519. doi: 10.1037/a0022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: 1996. [Google Scholar]
- 31.McNair D, Loor M, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1981. [Google Scholar]
- 32.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 33.Granholm E, Ben-Zeev D, Fulford D, et al. Ecological momentary assessment of social functioning in schizophrenia: impact of performance appraisals and affect on social interactions. Schizophrenia Research. 2013;145:120–124. doi: 10.1016/j.schres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson EI, Grondin O, Barrault M, et al. Computerized ambulatory monitoring in psychiatry: a multi-site collaborative study of acceptability, compliance, and reactivity. International Journal of Methods in Psychiatric Research. 2009;18:48–57. doi: 10.1002/mpr.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.SPSS . SPSS Statistics Base 23.0. IBM Corporation; Chicago, IL: 2010. [Google Scholar]
- 36.United States Department of Labor Bureau of Labor Statistics: American Time Use Survey. 2015 http://www.bls.gov/TUS/CHARTS/OLDER.HTM.
- 37.Depp CA, Ceglowski J, Wang VC, et al. Augmenting psychoeducation with a mobile intervention for bipolar disorder: a randomized controlled trial. Journal of Affective Disorders. 2015;174:23–30. doi: 10.1016/j.jad.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granholm E, Loh C, Swendsen J. Feasibility and validity of computerized ecological momentary assessment in schizophrenia. Schizophrenia Bulletin. 2008;34:507–514. doi: 10.1093/schbul/sbm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes DE, Blackwell T, Stone KL, et al. Cognition in older women: the importance of daytime movement. Journal of the American Geriatrics Society. 2008;56:1658–1664. doi: 10.1111/j.1532-5415.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Luo X, Barnes D, et al. Physical activity and risk of cognitive impairment among oldest-old women. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2014;22:1149–1157. doi: 10.1016/j.jagp.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kesse-Guyot E, Charreire H, Andreeva VA, et al. Cross-sectional and longitudinal associations of different sedentary behaviors with cognitive performance in older adults. PloS One. 2012;7:e47831. doi: 10.1371/journal.pone.0047831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JY, Zhou DH, Li J, et al. Leisure activity and risk of cognitive impairment: the Chongqing aging study. Neurology. 2006;66:911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- 43.Lindstrom HA, Fritsch T, Petot G, et al. The relationships between television viewing in midlife and the development of Alzheimer's disease in a case-control study. Brain and Cognition. 2005;58:157–165. doi: 10.1016/j.bandc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 44.Geda YE, Topazian HM, Roberts LA, et al. Engaging in cognitive activities, aging, and mild cognitive impairment: a population-based study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:149–154. doi: 10.1176/appi.neuropsych.23.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoang TD, Reis J, Zhu N, et al. Effect of early adult patterns of physical activity and television viewing on midlife cognitive function. JAMA Psychiatry. 2016;73:73–79. doi: 10.1001/jamapsychiatry.2015.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costigan SA, Barnett L, Plotnikoff RC, et al. The health indicators associated with screen-based sedentary behavior among adolescent girls: a systematic review. The Journal of Adolescent Health : Official Publication of the Society for Adolescent Medicine. 2013;52:382–392. doi: 10.1016/j.jadohealth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Teychenne M, Ball K, Salmon J. Sedentary behavior and depression among adults: a review. International Journal of Behavioral Medicine. 2010;17:246–254. doi: 10.1007/s12529-010-9075-z. [DOI] [PubMed] [Google Scholar]
- 48.Sisson SB, Shay CM, Broyles ST, et al. Television-viewing time and dietary quality among U.S. children and adults. American Journal of Preventive Medicine. 2012;43:196–200. doi: 10.1016/j.amepre.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Allard M, Husky M, Catheline G, et al. Mobile technologies in the early detection of cognitive decline. PloS One. 2014;9:e112197. doi: 10.1371/journal.pone.0112197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vance DE, Cody SL, Yoo-Jeong M, et al. The role of employment on neurocognitive reserve in adults with HIV: A review of the literature. The Journal of the Association of Nurses in AIDS Care : JANAC. 2015;26:316–329. doi: 10.1016/j.jana.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]