Abstract
PURPOSE: To assess the effect of adding neoadjuvant chemotherapy (NACT) to concurrent chemoradiotherapy (CCRT) in patients with locoregionally advanced nasopharyngeal carcinoma (NPC) and undetectable pretreatment Epstein-Barr virus (pEBV) DNA. MATERIALS AND METHODS: We enrolled 639 NPC patients with stage II to IVB and undetectable pEBV DNA to receive CCRT with or without NACT. Radiotherapy was 2.0 to 2.27 Gy per fraction with five daily fractions per week for 6 to 7 weeks to the primary tumor and 62 to 70 Gy to the involved neck area. NACT was cisplatin (80-100 mg/m2 day 1) and 5-fluorouracil (800-1000 mg/m2, 120-hour continuous intravenous infusion) every 3 weeks for two or three cycles. CCRT was cisplatin (80-100 mg/m2 day 1) every 3 weeks for three cycles. RESULTS: For all patients, the 5-year overall survival (OS), locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) rates were 91.9%, 92.2%, 95.0%, and 86.4%, respectively. There was no significant difference in OS (5-year OS 90.8% [NACT + CCRT group] vs 92.7% [CCRT alone]; hazard ratio [HR] 1.24; P = .486), LRFS (HR 1.13, 95% confidence interval [CI] 0.59-2.14, P = .715), DMFS (HR 0.78, 95% CI 0.34-1.78, P = .554), or PFS (HR 1.21, 95% CI 0.75-1.95, P = .472). CONCLUSION: CCRT with or without NACT produced a good treatment outcome in patients with locoregionally advanced NPC and undetectable pEBV DNA, but NACT before CCRT did not significantly improve survival rates.
Introduction
Nasopharyngeal carcinoma (NPC) is common in the Asian population, especially among the Southern Chinese, with an age-standardized incidence rate of 20 to 50 per 100,000 person-years [1]. Radiotherapy is the mainstay treatment modality for nondisseminated NPC because of the anatomical location and radiosensitivity. Control of early-stage disease with radiotherapy alone is usually successful; however, the response of locoregionally advanced NPC is unsatisfactory [2]. Several clinical trials and systematic reviews have confirmed that concurrent chemoradiotherapy (CCRT) improves survival outcomes for locoregionally advanced NPC [3], [4], [5], [6]. However, the role of adding neoadjuvant chemotherapy (NACT) to CCRT remains controversial. So far, only three trials have been published that compare NACT followed by CCRT with CCRT alone. The trial by Hui et al. [7] reported a significant increase in survival with NACT + CCRT, but the trials by Fountzilas et al. [8] and Tan et al. [9] did not find a significant increase. This inconsistency might be partly due to the tumor heterogeneity in their patients [10], [11].
It is well recognized that infection with the Epstein-Barr virus (EBV) is one of the main risk factors for NPC [12], and EBV DNA load has been found to be an indicator of tumor burden in patients with NPC [13]. Therefore, researchers conducting clinical trials for personalizing cancer therapies should pay attention to the EBV DNA load. An example is the ongoing NRG-HN001 trial, in which patients are randomized and assigned to adjuvant or no adjuvant chemotherapy depending on whether EBV DNA is detected. However, to the best of our knowledge, no study to date has investigated the value of adding NACT to CCRT in patients with locoregionally advanced NPC and undetectable pretreatment EBV (pEBV) DNA. We therefore undertook the current study to compare NACT + CCRT with CCRT alone and appraise the value of NACT in this set of patients.
Materials and Methods
Patient Selection
Between November 2009 and February 2012, 639 patients with biopsy-proven stage II to IVB NPC (according to the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control criteria) were enrolled in this study. The eligibility criteria were as follows: (1) presence of histologically confirmed NPC; (2) no evidence of distant metastases; (3) receiving intensity-modulated radiotherapy (IMRT); (4) treated with concurrent or/and neoadjuvant chemotherapy; (5) presence of undetectable (0 copy/ml) pEBV DNA; and (6) absence of secondary malignancy, pregnancy, or lactation.
All patients were evaluated by a complete physical examination, magnetic resonance imaging of the nasopharynx and neck, abdominal sonography, chest radiograph, electrocardiography, bone scan, fiberoptic nasopharyngoscopy, and complete blood sampling, including differential cell counts, biochemical profiling, and EBV serology. This study was approved by the Ethics Committee of the study institute, and written informed consent was obtained from all patients.
Radiotherapy
All patients were treated according to the principles of treatment for NPC patients at the Sun Yat-sen University Cancer Center [14]. Immobilization was carried out using a custom-made head-to-neck thermoplastic cast with the patient's neck resting on a support. A high-resolution planning computed tomographic scan with contrast was taken from the vertex to 2 cm below the sternoclavicular joint at a slice thickness of 3 mm. Target volumes were defined slice-by-slice using an individualized protocol that complies with the International Commission on Radiation Units and Measurements reports 50 and 62. The prescribed doses to the planning target volumes (PTVs) of the gross tumor volumes (GTVs) were as follows: 66 to 72 Gy at 2.12 to 2.27 Gy/fraction to the PTV of the primary GTV, 62 to 70 Gy to the PTV of the GTV of the involved lymph nodes, 60 Gy to the PTV of the high-risk clinical target volume, and 54 Gy to the PTV of the low-risk clinical target volume.
Chemotherapy
According to our institutional guidelines, we recommended radiotherapy alone for stage I disease, CCRT for stage II disease, and CCRT +/− neoadjuvant/adjuvant chemotherapy for stage III to IVA-B disease. Neoadjuvant or adjuvant chemotherapy consisted of cisplatin (80-100 mg/m2 on the first day) and 5-fluorouracil (800-1000 mg/m2, 120-hour continuous intravenous infusion) administered every 3 weeks for two or three cycles. Concurrent chemotherapy consisted of cisplatin (80-100 mg/m2 on the first day) given every 3 weeks for three cycles.
Quantification of Plasma EBV DNA
Before treatment, 3 ml of peripheral venous blood was collected, placed into EDTA-containing tubes, and centrifuged at 3000 rpm for 5 minutes. Total plasma DNA was extracted using a QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). A fluorescence polymerase chain reaction (PCR) was carried out using an EBV PCR quantitative diagnostic kit (Da-An Genetic Diagnostic Center, Guangzhou, China). Plasma EBV DNA levels were measured using real-time quantitative PCR of the BamHI-W region in the EBV genome. Undetectable plasma EBV DNA in the sample was set at 0 copy/ml. The experimental data were analyzed using Applied Biosystems 7300 SDS software.
Follow-Up
Patients were observed weekly during treatment, and the first assessment of tumor response was performed 1 month after completion of radiotherapy. Patients were then evaluated once every 3 months in the first 3 years, once every 6 months for the following 2 years, and once every year thereafter. Patients who did not meet follow-up requirements more than twice were excluded. Patients with residual or recurrent local disease were clinically diagnosed by physical examination (including endoscopic examination) and magnetic resonance imaging, and some underwent biopsy to confirm malignancy. Additional tests were ordered when indicated to evaluate for local or distant failure.
Statistical Analysis
We defined overall survival (OS), locoregional relapse-free survival (LRFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) as the first day of diagnosis to the date of death, locoregional failure, distant failure, or disease progression, respectively. Survival rate was estimated with the Kaplan-Meier method, and the difference between survival curves was assessed with a log-rank test. The hazard ratios (HRs) of the treatment effects were estimated using a univariate Cox proportional-hazard model with 95% confidence intervals (CIs). A planned multivariate Cox analysis of each end point was also performed to adjust the treatment effect for the stratification variable. The proportional-hazards assumption underlying each Cox model was verified according to the significance of the time-varying covariates in the model. The criterion for statistical significance was set at .05, and P values were based on two-sided tests.
Results
Patients and Treatment Characteristics
A total of 639 patients were enrolled in this study, with a median age of 46 years (range, 19-70 years). Of these patients, 296 (46.3%) received NACT + CCRT and 343 (53.7%) received CCRT alone. The median follow-up for the NACT + CCRT group was 58.5 months (range, 5-77 months), and for CCRT alone, it was 58.2 months (range, 4-76 months). The baseline characteristics of the two groups are summarized in Table 1. There were no differences in terms of age, sex, histology, history of smoking, T (tumor) stage, N (nodal) stage, clinical stage, primary tumor dose, or cervical dose (all P > .05).
Table 1.
Characteristics of Patients
| Characteristics | NACT + CCRT (n = 296) | CCRT alone (n = 343) | P Value |
|---|---|---|---|
| Age (years) | .861 | ||
| <50 | 212 (71.6) | 243 (70.8) | |
| ≥50 | 84 (28.4) | 100 (29.2) | |
| Sex | .519 | ||
| Male | 227 (76.7) | 255 (74.3) | |
| Female | 69 (23.3) | 88 (25.7) | |
| Histology | .722 | ||
| WHO II | 14 (4.7) | 19 (5.6) | |
| WHO III | 282 (95.3) | 324 (94.5) | |
| History of smoking | .110 | ||
| Never smoker | 176 (59.5) | 224 (65.3) | |
| Current or ex-smoker | 120 (40.5) | 119 (34.7) | |
| T stage | .109 | ||
| T1-2 | 75 (25.4) | 108 (31.4) | |
| T3-4 | 221 (74.6) | 225 (65.6) | |
| N stage | .266 | ||
| N0-1 | 235 (79.5) | 285 (83.1) | |
| N2-3 | 61 (20.5) | 59 (16.9) | |
| Clinical stage | .296 | ||
| II-III | 224 (75.7) | 272 (79.3) | |
| IVA-B | 72 (24.3) | 71 (20.7) | |
| Primary tumor dose | .427 | ||
| ≤68 Gy | 144 (48.6) | 155 (45.2) | |
| >68 Gy | 152 (51.4) | 188 (54.8) | |
| Cervical dose | .357 | ||
| ≤66 Gy | 218 (73.6) | 264 (77.0) | |
| >66 Gy | 78 (26.4) | 79 (23.0) |
WHO, World Health Organization.
Survival Outcomes
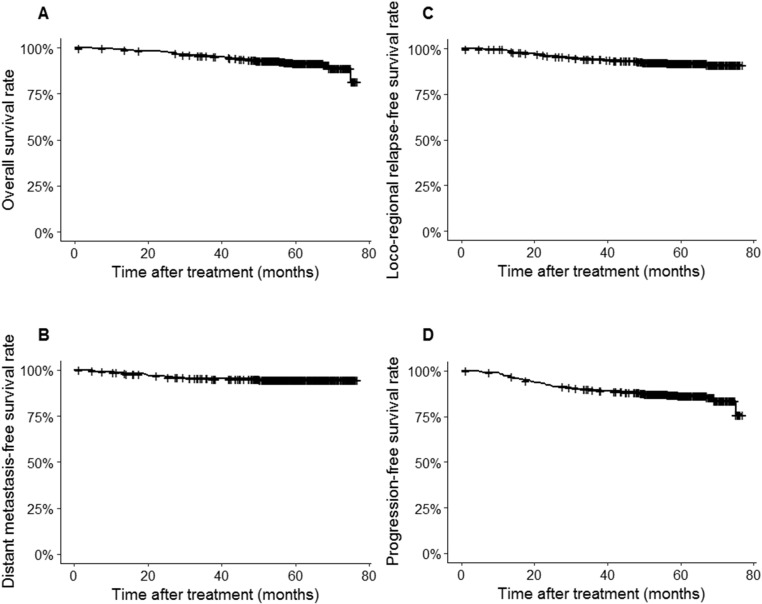
At the time of their final follow-up, there were 34 cases of local relapse, 19 cases of regional relapse, 32 cases of distant metastasis, and 11 cases with both distant and local/regional recurrences. Fifty-two patients were deceased at the time of analysis: 24 patients died from distant metastasis, 21 died from progression of locoregional disease after recurrence, 5 patients died from comorbidities unrelated to NPC, 1 died from a traffic accident, and there was 1 unreported cause. For all patients, the 5-year OS, LRFS, DMFS, and PFS rates were 91.9%, 92.2%, 95.0%, and 86.4%, respectively (Figures 1, A-D).
Figure 1.
Kaplan-Meier plot for the entire cohort for (A) OS, (B) LRFS, (C) DMFS, and (D) PFS.
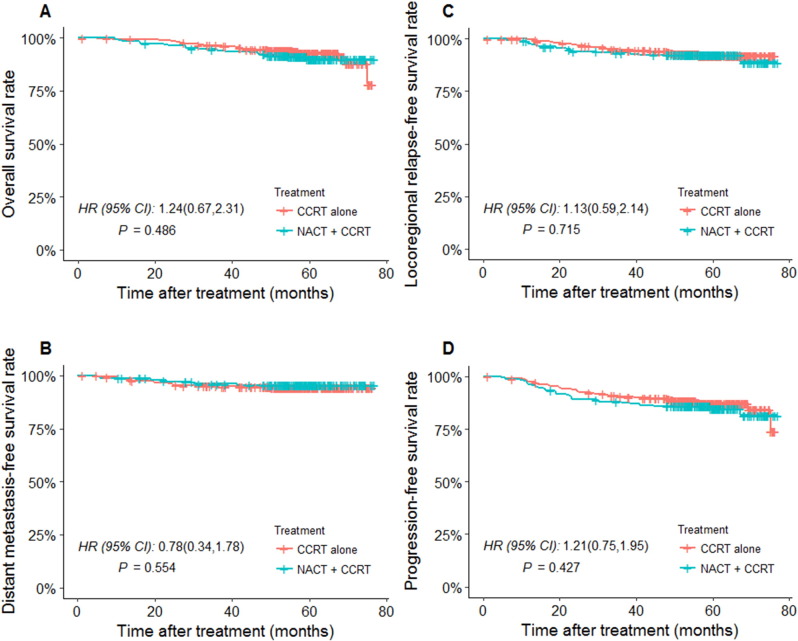
The 5-year OS for NACT + CCRT patients was 90.8% (95% CI 84.9%-95.2%), and for CCRT alone, it was 92.7% (95% CI 87.2%-97.5%). The HR for treatment effect was 1.24 (95% CI 0.67-2.31; P = .486, Figure 2A). The 5-year LRFS for NACT + CCRT was 91.9%, and for CCRT alone, it was 92.7%. The HR for treatment effect was 1.13 (95% CI 0.59-2.14; P = .715, Figure 2B). The 5-year DMFS for NACT + CCRT was 95.7%, and for CCRT alone, it was 94.5%. The HR for treatment effect was 0.78 (95% CI 0.34-1.78; P = .554, Figure 2C). The 5-year PFS for NACT + CCRT was 84.9%, and for CCRT alone, it was 87.5%. The HR for treatment effect was 1.21 (95% CI 0.75-1.95; P = .427, Figure 2D). Therefore, no significant differences were found in 5-year OS, LRFS, DMFS, or PFS rates between the NACT + CCRT group and the CCRT alone group (all P > .05).
Figure 2.
Kaplan-Meier plot for the entire cohort treated with NACT + CCRT versus CCRT alone for (A) OS, (B) LRFS, (C) DMFS, and (D) PFS.
Prognostic Factors
Factors including treatment protocol, age, sex, histology, history of smoking, T stage, N stage, clinical stage, primary tumor dose, and cervical dose were assessed for their ability to predict OS, LRFS, DMFS, and PFS. Univariate analysis by log-rank test showed that age, histology, and clinical stage were prognostic factors for OS (all P < .05). Clinical stage and N stage were prognostic factors for DMFS (P = .021 and 0.049, respectively). Age and clinical stage were significant factors for PFS (P = .033 and 0.029, respectively). No significant relationship was found between OS, LRFS, DMFS, or PFS and treatment protocols (all P > .05; Table 2).
Table 2.
Univariate Analysis of Prognostic Factors for the Whole Cohort
| End Points | HR (95% CI) | P⁎ | End Points | HR (95% CI) | P⁎ |
|---|---|---|---|---|---|
| OS | LRFS | ||||
| Age, ≤50 years vs >50 years | 2.72 (1.48-4.97) | .001 | Age, ≤50 years vs >50 years | 1.16 (0.59-2.28) | .671 |
| Sex, men vs women | 0.31 (0.11-0.86) | .025 | Sex, men vs women | 1.48 (0.76-2.86) | .248 |
| Histology, WHO II vs WHO III | 1.21 (0.29-5.02) | .794 | Histology, WHO II vs WHO III | 1.05 (0.25-4.35) | .947 |
| History of smoking, no vs yes | 2.17 (1.18-3.99) | .012 | History of smoking, no vs yes | 0.76 (0.39-1.50) | .427 |
| T stage, T1-2 vs T3-4 | 2.14 (0.95-4.82) | .066 | T stage, T1-2 vs T3-4 | 1.28 (0.63-2.63) | .493 |
| N stage, N0-1 vs N2-3 | 1.97(0.99-3.92) | .054 | N stage, N0-1 vs N2-3 | 0.96 (0.40-2.28) | .925 |
| Clinical stage, II-III vs IVA-B | 2.79 (1.50-5.17) | .001 | Clinical stage, II-III vs IVA-B | 1.05 (0.48-2.28) | .897 |
| Primary dose, ≤68 vs >68 Gy | 0.79 (0.43-1.47) | .455 | Primary dose, ≤68 vs >68 Gy | 0.82 (0.44-1.53) | .526 |
| Cervical dose, ≤66 vs >66 Gy | 1.80 (0.94-3.45) | .078 | Cervical dose, ≤66 vs >66 Gy | 1.13 (0.57-2.22) | .727 |
| Treatment, CCRT vs NACT + CCRT | 1.24 (0.67-2.31) | .979 | Treatment, CCRT vs NACT + CCRT | 1.13 (0.59-2.14) | .715 |
| DMFS | PFS | ||||
| Age, ≤50 years vs >50 years | 1.37 (0.61-3.05) | .443 | Age, ≤50 years vs > 50years | 1.68 (1.04-2.72) | .033 |
| Sex, men vs women | 0.51 (0.18-1.48) | .215 | Sex, men vs women | 0.82 (0.46-1.44) | .482 |
| Histology, WHO II vs WHO III | 0.70 (0.17-2.95) | .625 | Histology, WHO II vs WHO III | 0.91 (0.33,2.50) | .858 |
| History of smoking, no vs yes | 1.05 (0.48-2.29) | .904 | History of smoking, no vs yes | 1.38 (0.86-2.20) | .179 |
| T stage, T1-2 vs T3-4 | 1.21 (0.51-2.87) | .658 | T stage, T1-2 vs T3-4 | 1.48 (0.85-2.58) | .170 |
| N stage, N0-1 vs N2-3 | 2.29 (1.00-5.24) | .049 | N stage, N0-1 vs N2-3 | 1.59 (0.91-2.77) | .105 |
| Clinical stage, II-III vs IVA-B | 2.51 (1.15-5.49) | .021 | Clinical stage, II-III vs IVA-B | 1.76 (1.06-2.94) | .029 |
| Primary dose, ≤68 vs >68 Gy | 1.18 (0.55-2.51) | .668 | Primary dose, ≤68 vs >68 Gy | 0.83 (0.51-1.32) | .426 |
| Cervical dose, ≤66 vs >66 Gy | 2.09 (0.95-4.60) | .068 | Cervical dose, ≤66 vs >66 Gy | 1.40 (0.84-2.32) | .194 |
| Treatment, CCRT vs NACT + CCRT | 0.78 (0.34-1.78) | .555 | Treatment, CCRT vs NACT + CCRT | 1.21 (0.75-1.95) | .428 |
Values in bold are significant (P < .05).
P values were calculated with an adjusted Cox proportional-hazards model.
Multivariate analysis was performed to adjust for various prognostic factors. Consistent with the results of the univariate analysis, age and clinical stage were independent prognostic predictors of PFS and OS (all P < .05; Table 3). Although DMFS rate was higher for N0 to 1 disease (87.6%) than N2 to 3 disease (85.7%), we did not indicate a significant association between N stage and risk of distant metastasis in multivariate analysis (P = .285).
Table 3.
Multivariate Analysis of Prognostic Factors for the Whole Cohort
| End Points | HR (95% CI) | P Value⁎ |
|---|---|---|
| OS | ||
| Age, ≤50 years vs >50 years | 2.60 (1.36-4.96) | .004 |
| Sex, men vs women | 0.43 (0.14-1.31) | .137 |
| History of smoking, no vs yes | 1.35 (0.67-2.68) | .400 |
| T stage, T1-2 vs T3-4 | 1.68 (0.72-3.94) | .233 |
| N stage, N0-1 vs N2-3 | 1.52 (0.70-3.30) | .295 |
| Clinical stage, II-III vs IVA-B | 12.07 (1.02-4.20) | .043 |
| Cervical dose, ≤64 vs >64 Gy | 1.85 (0.95-3.59) | .071 |
| DMFS | ||
| N stage, N0-1 vs N2-3 | 1.65 (0.66-4.14) | .285 |
| Clinical stage, II-III vs IVA-B | 12.26 (0.96-5.29) | .061 |
| Cervical dose, ≤ 64 vs > 64 Gy | 2.00(0.90,4.45) | .088 |
| PFS | ||
| Age, ≤ 50 years vs > 50 years | 1.63 (1.01-2.64) | .045 |
| Clinical stage, II-III vs IVA-B | 11.71 (1.02-2.85) | .040 |
Values in bold are significant (P < .05).
P values were calculated with an adjusted Cox proportional-hazards model.
Subgroup Analysis of NACT Influence on Stage IV Disease
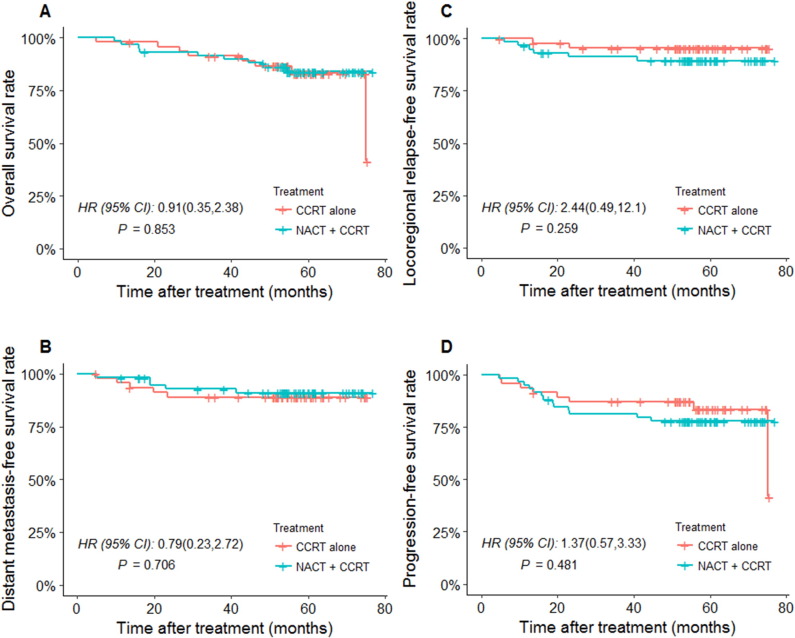
As described above, multivariate analysis showed stage IV disease to be an unfavorable predictor for PFS and OS. For stage IV patients, the 5-year OS for the NACT + CCRT group was 84.7%, and for CCRT alone, this was 83.0% (HR 0.91, 95% CI 0.35-2.38, P = .853; Figure 3A); the 5-year LRFS for the NACR + CCRT group was 89.8%, and for CCRT alone, this was 95.7% (HR 2.44, 95% CI 0.49-12.1, P = .259; Figure 3B); the 5-year DMFS for the NACR + CCRT group was 91.5%, and for CCRT alone, this was 89.4% (HR 0.79, 95% CI 0.23-2.72, P = .706; Figure 3C); and the 5-year PFS for the NACT + CCRT group was 78.0%, and for CCRT alone, this was 83.0% (HR 1.37, 95% CI 0.57-3.33, P = .481; Figure 3D). Therefore, no significant differences were found between the two groups in terms of OS, LRFS, DMFS, or PFS for stage IV patients (all P > .05).
Figure 3.
Kaplan-Meier plot for stage IV patients treated with NACT + CCRT versus CCRT alone for (A) OS, (B) LRFS, (C) DMFS, and (D) PFS.
Acute and Late Toxicity
Treatment-related toxicity was scored according to the Common Terminology Criteria for Adverse Events. During radiotherapy, patients who received NACT + CCRT had a significantly higher incidence of grade 3 to 4 bone marrow suppression than those who received CCRT alone (35.2% vs 27.6%; P = .021), but there was no significant difference in terms of mucositis (18.7% vs 21.3%; P = .167) and skin toxicity (13.3% vs 15.4%; P = .216). Late toxicities were assessed in 589 patients (277 patients in the NACT + CCRT group and 311 patients in the CCRT alone group) with ≥2 years of follow-up. The most common late complications were xerostomia (62%), hearing impairment (47%), and neck fibrosis (35%), which were all grade 1 to 2. Other late complications of at least grade 2 included temporal lobe necrosis in 31 patients (5.3%) and cranial neuropathy in 21 patients (3.6%). There were no significant differences between the two groups in terms of xerostomia, hearing impairment, neck fibrosis, temporal lobe necrosis, and cranial neuropathy. However, the higher radiation dose to the cervical lymph node (i.e., RT > 66 Gy) was associated with higher incidence of neck fibrosis (42.3% vs 23.7%; P < .001).
Discussion
Recently, there has been renewed interest in the use of NACT in locoregionally advanced NPC. This has resulted from two observations. The first is that more effective NACT regimens may well exist. The second is that although high-precision radiation delivery (such as with IMRT), coupled with the wide adoption of concurrent chemotherapy, has improved the local control rate in NPC, distant metastases are now the predominant mode of treatment failures [15], [16]. However, the role of NACT still remains controversial. The current study is the first to our knowledge to provide evidence that NACT before CCRT did not significantly improve survival rates in patients with locoregionally advanced NPC and undetectable pEBV DNA.
A phase II clinical trial by Hui et al. [7] showed a significant improvement of OS with the addition of NACT in patients with locoregionally advanced NPC. Another clinical trial reported by Fountzilas et al. [8] found that NACT when followed by CCRT did not significantly improve response rates and/or survival compared to CCRT alone. Recently, Tan et al. [9] conducted a randomized, phase II/III trial in locally advanced NPC and also found no evidence that NACT before CCRT improved survival. The current study suggests that CCRT is a highly feasible sequential strategy for advanced NPC with undetectable pEBV DNA, but NACT before CCRT did not significantly improve survival. Considering that the main advantage of NACT is to eradicate distant metastases [17], this seems to be reasonable because patients with undetectable pEBV DNA had a relatively low risk of distant metastasis [18].
Recently, the local/regional relapse rate in NPC has significantly decreased with the use of IMRT, and distant metastases have emerged as the predominant reason for treatment failures [16]. However, in the current study, the incidence of locoregional relapse was higher than for distant metastases, with only 5.0% of patients developing distant metastases. This inconsistency might be due to some obvious differences between the patients included in the previous studies [19], [20] and the current study: only treatment-naive patients with no detectable pEBV DNA were eligible for the current study, whereas previous studies did not exclude patients with detectable pEBV DNA. These studies therefore may have included patients with a greater tumor burden which increased the risk of distant metastasis.
Radiation dose is an important prognostic factor for survival, and it is mainly based on experience with conventional radiotherapy. Despite recent advances in radiation technology, the optimal radiation dose is still a point of debate. Ozyar et al. [21] and Guruprasad et al. [22] both reported that a higher radiation dose of >66 Gy was significantly correlated with a better outcome. In contrast, Yan et al. [23] showed no difference in survival between the high–radiation dose group and the low–radiation dose group. The present study also showed that no additional benefit could be achieved by increasing the total radiation dose in either the primary tumor (>68 Gy) or cervical lymph node (>66 Gy). But our research further confirms that a dose to the cervical lymph node of >66 Gy was associated with a higher incidence of neck fibrosis.
As in most solid tumors, the TNM staging system is currently the most reliable method for predicting treatment outcome [24]. The only significant prognostic factor affecting treatment outcome was overall stage in this study. However, we could not find any significantly prognostic factor in determining LRFS, which may be due to the excellent local control offered by IMRT; because this reduces the rate of local failure, it is unsurprising that it weakens the significance of potential prognostic factors. Moreover, N stage in the TNM staging system is a measure of the extent of node involvement and is currently the most reliable tool for assessing metastasis risk in NPC [25], [26]. However, no significant influence of N stage on distant metastasis was observed in the current study, which may have been due to the limited number of patients because only 34/639 (5.3%) patients were staged with N3 disease.
Several studies have demonstrated that age is predictive of prognosis in NPC [2], [27]. Consistent with previous studies, our results also showed that patients under 50 years old appeared to have better OS and PFS. Several mechanisms may explain the observed results. First, patients over 50 were more likely to have comorbidities and a poorer performance status, which may contribute to a low tolerance for intense treatment [28]. Secondly, all patients received CCRT with or without NACT in the current study, and it was possible that older patients may be overtreated, with the consequent adverse events attributed to cancer. Therefore, researchers conducting clinical trials for personalizing cancer therapies should pay attention to the effect of age on treatment.
Conclusion
In summary, CCRT with or without NACT produced a good treatment outcome in patients with locally advanced NPC and undetectable pEBV DNA. However, NACT before CCRT did not significantly improve survival rates in these patients. It is possible that other drug combinations may be more effective in this role. Better pretreatment selection of patients, perhaps using molecular markers, may in the future identify the patients who would most benefit from this treatment approach [29], [30]. The role of adjuvant chemotherapy in addition to CCRT also remains to be defined.
Grants
This work was supported by grants from the National Natural Science Foundation of China (no. 81372409), the Sun Yat-sen University Clinical Research 5010 Program (no. 2012011), and the National Natural Science Foundation of China (no. 81402532).
Conflict of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgement
The authors thank Prof. Jun Ma at Sun Yat-sen University Cancer Center for critical review of this paper.
Contributor Information
Hao-Yuan Mo, Email: mohy@sysucc.org.cn.
Ying Sun, Email: sunying@sysucc.org.cn.
References
- 1.Zhang LF, Li YH, Xie SH, Ling W, Chen SH, Liu Q, Huang QH, Cao SM. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer. 2015;34(8):350–357. doi: 10.1186/s40880-015-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, Xiao JP, Xu GZ. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65(1):161–168. doi: 10.1016/j.ijrobp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX, Hou X, Zhao HY, Huang Y, Fang WF. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2013;24(8):2131–2136. doi: 10.1093/annonc/mdt163. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, Siu L, Ng WT, Sze WK, Au GK. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;102(15):1188–1198. doi: 10.1093/jnci/djq258. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard P., Lee A., Marguet S., Leclercq J., Ng W.T., Ma J., Chan A.T., Huang P.Y., Benhamou E., Zhu G. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, Li WF, Lin AH, Sun Y, Ma J. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2015;26(1):205–211. doi: 10.1093/annonc/mdu507. [DOI] [PubMed] [Google Scholar]
- 7.Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, Yu BK, Chiu SK, Kwan WH, Ho R. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(2):242–249. doi: 10.1200/JCO.2008.18.1545. [DOI] [PubMed] [Google Scholar]
- 8.Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, Zaramboukas T, Nikolaou A, Markou K, Resiga L. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. 2012;23(2):427–435. doi: 10.1093/annonc/mdr116. [DOI] [PubMed] [Google Scholar]
- 9.Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, Ng QS, Tan D, Ong WS, Tan SH. Concurrent chemo-radiation with or without induction gemcitabine, carboplatin, and paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;91(5):952–960. doi: 10.1016/j.ijrobp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Takimoto T, Ogura H, Tanaka J, Hatano M, Glaser R. Heterogeneity of Epstein-Barr virus derived from a nasopharyngeal carcinoma that has transforming and lytic properties. J Natl Cancer Inst. 1986;76(6):1019–1024. [PubMed PMID: 3012175] [PubMed] [Google Scholar]
- 11.Chan SC, Chang KP, Fang YD, Tsang NM, Ng SH, Hsu CL, Liao CT, Yen TC. Tumor heterogeneity measured on F-18 fluorodeoxyglucose positron emission tomography/computed tomography combined with plasma Epstein-Barr virus load predicts prognosis in patients with primary nasopharyngeal carcinoma. Laryngoscope. 2017;127(1):E22–E28. doi: 10.1002/lary.26172. [DOI] [PubMed] [Google Scholar]
- 12.Chen CJ, Liang KY, Chang YS, Wang YF, Hsieh T, Hsu MM, Chen JY, Liu MY. Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res. 1990;10(2B):547–553. [PubMed] [Google Scholar]
- 13.Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, Cheng G, Hu LF, Ernberg I, Zeng YX. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2014;100(6):1162–1170. doi: 10.1002/cncr.20099. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Han F, Lu LX, Huang SM, Lin CG, Deng XW, Lu TX, Cui NJ. Intensity modulated radiotherapy for local-regional advanced nasopharyngeal carcinoma. Ai Zheng. 2004;23(Suppl. 11):1532–1537. [PubMed] [Google Scholar]
- 15.Kam MK, Teo PM, Chau RM, Cheung KY, Choi PH, Kwan WH, Leung SF, Zee B, Chan AT. Treatment of nasopharyngeal carcinoma with intensity-modulated radiotherapy: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2004;60(5):1440–1450. doi: 10.1016/j.ijrobp.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Mo Z, Du W, Wang Y, Liu L, Wei Y. Intensity-modulated radiation therapy versus 2D-RT or 3D-CRT for the treatment of nasopharyngeal carcinoma: a systematic review and meta-analysis. Oral Oncol. 2015;51(11):1041–1046. doi: 10.1016/j.oraloncology.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Chen YP, Guo R, Liu N, Liu X, Mao YP, Tang LL, Zhou GQ, Lin AH, Sun Y, Ma J. Efficacy of the additional neoadjuvant chemotherapy to concurrent chemoradiotherapy for patients with locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis of randomized controlled trials. J Cancer. 2015;6(9):883–892. doi: 10.7150/jca.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Yin L, Wu J, Gu JJ, Jiang XS, Wang DJ, Zong D, Guo C, Zhu HF, Wu JF. Impact of plasma Epstein-Barr virus-DNA and tumor volume on prognosis of locally advanced nasopharyngeal carcinoma. Biomed Res Int. 2015;2015:617949. doi: 10.1155/2015/617949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JC, Chen KY, Wang WY, Jan JS, Liang WM, Tsai CS, Wei YH. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol. 2001;19(10):2607–2615. doi: 10.1200/JCO.2001.19.10.2607. [DOI] [PubMed] [Google Scholar]
- 20.Tang LQ, Li CF, Li J, Chen WH, Chen QY, Yuan LX, Lai XP, He Y, Xu YX, Hu DP. Establishment and validation of prognostic nomograms for endemic nasopharyngeal carcinoma. J Natl Cancer Inst. 2015;108(1) doi: 10.1093/jnci/djv291. [DOI] [PubMed] [Google Scholar]
- 21.Ozyar E, Selek U, Laskar S, Uzel O, Anacak Y, Ben-Arush M, Polychronopoulou S, Akman F, Wolden SL, Sarihan S. Treatment results of 165 pediatric patients with non-metastatic nasopharyngeal carcinoma: a Rare Cancer Network study. Radiother Oncol. 2006;81(1):39–46. doi: 10.1016/j.radonc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Guruprasad B, Tanvir P, Rohan B, Kavitha S, Naik SM, Appaji L. Paediatric nasopharyngeal carcinoma: an 8-year study from a tertiary care cancer centre in South India. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl. 1):131–134. doi: 10.1007/s12070-013-0622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Z, Xia L, Huang Y, Chen P, Jiang L, Zhang B. Nasopharyngeal carcinoma in children and adolescents in an endemic area: a report of 185 cases. Int J Pediatr Otorhinolaryngol. 2013;77:1454–1460. doi: 10.1016/j.ijporl.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lee AW, Ng WT, Chan LK, Chan OS, Hung WM, Chan CC, Cheng PT, Sze H, Lam TS, Yau TK. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol. 2012 Oct;48(10):1007–1013. doi: 10.1016/j.oraloncology.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Mao YP, Xie FY, Liu LZ, Sun Y, Tian L, Tang LL, Lin AH, Li L, Ma J. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol. 2012;104(3):331–337. doi: 10.1016/j.radonc.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61(4):1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 28.Xiao G, Cao Y, Qiu X, Wang W, Wang Y. Influence of gender and age on the survival of patients with nasopharyngeal carcinoma. BMC Cancer. 2013;13:226. doi: 10.1186/1471-2407-13-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ, Lo YM. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003;98(2):288–291. doi: 10.1002/cncr.11496. [DOI] [PubMed] [Google Scholar]
- 30.Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]