Abstract
Metastatic disease involving the spine and pelvis is common, often resulting in significant pain and disability. Several percutaneous interventions have been described, including osteoplasty, ablation, and screw fixation, that when used alone or in combination can significantly reduce pain and disability from metastatic bone disease. Although it is possible to make a significant impact in patient care with basic principles and techniques, certain advanced techniques can extend the application of percutaneous interventions while minimizing morbidity.
Keywords: vertebroplasty, ablation, metastases, bone, interventional radiology
Objectives : Upon completion of this article, the reader will be able to describe the percutaneous treatment of spinal metastatic disease, including procedural indications and limitations, appropriate patient selection, and advanced selection criteria for devices and techniques.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Epidemiology of Skeletal Metastatic Disease
Metastatic involvement of the bones is a common manifestation of malignancy, present in up to 70% of prostate and breast malignancies and up to 30% of common malignancies including colorectal, lung, and renal cancers. The most frequent site of skeletal involvement is the spine followed by the pelvis, shoulder, ribs, and appendicular long bones. 1
Skeletal metastatic disease can lead to a variety of complications including pain, pathologic fracture, deconditioning, declining mobility, neuropathy, weakness, decreased respiratory function, and metabolic disorders including severe hypercalcemia.
Minimally invasive treatment of skeletal metastases, including ablation, osteoplasty, and screw fixation, can achieve, among other objectives, palliative pain control, skeletal stabilization, and tumor control while allowing quick recovery and minimal delay in adjunctive therapies such as initiation of chemotherapy and radiation.
The three principal indications for pursuing percutaneous intervention for spinal metastatic disease include symptom/pain control, tumor control, and structural stabilization. While some interventions address more than one of these objectives, it is important to identify the individual goals of care for each patient within their individual context. Important preprocedural considerations include assessment of structural integrity, fracture risk, patient's age and functional status, overall prognosis, availability of adjunctive treatment options, and a clear determination of curative or palliative intent.
Patient Evaluation for Percutaneous Spine Intervention
Structural Integrity
A thorough assessment of the alignment and structural integrity of the spine should be the first step in choosing a treatment option for spinal metastatic disease. This can sometimes be difficult, and early direct communication with consulting spine surgeons can be very helpful in providing the patient with the best long-term plan. Some specific instances where early surgical evaluation is necessary include aggressive metastatic disease with significant multilevel or multicolumn involvement; significant deformity; significant spinal canal encroachment; and on an emergent basis, clinical symptoms of acute spinal cord syndrome, such as acute changes in bowel or bladder function, lower extremity weakness, and lower extremity sensory deficits.
Besides alignment, an initial assessment should include the identification of bones both with pathologic fractures and those with high risk of future fracture. Contrast-enhanced magnetic resonance imaging (MRI) is the preferred modality to evaluate for spine fractures in the setting of skeletal metastatic disease. Occasionally, additional computed tomographic (CT) imaging can be useful to better define complicated or minimally displaced fractures as well as cortical integrity at critical boundaries. In some limited cases, a bone scan with single-photon emission computed tomography can also be useful in characterizing fractures, most often in patients with extensive myeloma where diffuse abnormal signal intensity can limit the sensitivity and specificity of bone edema or when necessary in patients unable to tolerate an MRI due to their pain.
Although not clearly supported by the literature, prophylactic vertebral stabilization with polymethylmethacrylate (PMMA) may be appropriate for certain carefully selected patients at high risk of pathologic fracture to prevent the morbidities associated with a new fracture or progressive collapse of an existing fracture such as progressive kyphosis and respiratory compromise, especially if the active disease cannot be adequately treated, commonly due to maximum dose radiation limits and/or a desire for hospice care. Determining which vertebrae carry a high risk of future fracture is an imprecise science with limited data-driven recommendations. Perhaps, the best validated and accepted scoring system for assessing instability in spinal metastatic lesions is the Spinal Instability Neoplastic Score (SINS). Published in 2010 by the Spine Oncology Study Group, this scoring system is based on literature review and expert opinion and comprises six clinical and imaging elements including location, pain, degree of osteolysis, alignment, collapse, and involvement of the posterior elements. Lesions are identified as stable, potentially unstable, or unstable. While some aspects of the SINS have been validated as helpful in assessing fracture risk, 2 3 this algorithm has not yet proven to be a definitive predictor.
A special note should be made regarding radiation therapy (RT) and fracture risk. It is clear that there is an increased risk of pathologic fracture following both conformable external beam radiation therapy (cEBRT) and the typically higher dose stereotactic beam radiation therapy (SBRT) or stereotactic radiosurgery (SRS) and that risk appears significantly higher with SBRT/SRS, likely around 15%, but it has been reported as high as 40%. 4 5 6 Postradiation fractures are most likely to occur several weeks after radiation, preferentially involving highly lytic lesions with an elevated SINS. 3 Prophylactic stabilization of these moderate- to high-risk lesions prior to RT may make considerable sense when evaluating the risk/benefit profile. Although at present there is no level 1 evidence to support such practice, we commonly perform vertebral augmentation of both painful and nonpainful high-risk lesions prior to RT to prevent fracture-associated morbidities.
Occasionally, prophylactic vertebral augmentation is employed at levels for pedicle screw fixation prior to corpectomy or other decompression surgery to prevent hardware failure and screw pullout in the setting of poor-quality bone. Furthermore, some preliminary research has been reported on augmentation at levels immediately cranial to fixation to prevent proximal junction failure. 7
Pain
For patients presenting with painful spinal metastases, an initial evaluation should include a baseline assessment of the patient's pain using a Visual Analog Scale (VAS), Numeric Rating Scale (NRS), or Brief Pain Inventory (BPI). A functional assessment and mobility should be documented using the Roland Morris Disability Questionnaire (RMDQ) or Oswestry Disability Index (ODI), as they are more objective than self-reported pain scores and reflect a patient's ability to perform important activities of daily living. Finally, documenting patients' current pain medication regimen, especially their narcotic usage, is critical. Most studies in this space now report patients' morphine equivalent daily dose (MEDD) that converts all common narcotics to a total dose of morphine to allow accurate assessment of daily needs over time.
Pain related to spinal metastatic disease is often multifactorial. The periosteum contains a very high density of sensory nerve endings, and the local inflammatory environment caused by tumor invasion can produce significant pain. In contrast, there is relatively little sensory innervation within the medullary space and tumors confined to this location are rarely in themselves painful. Less commonly, large spinal metastases can cause pain by extending directly into exiting nerve roots within the neural foramen or compressing the dura or spinal cord itself. Associated pathologic fractures are a significant source of metastatic pain and important to identify and stabilize when possible.
Clinical Considerations
Patients with widely metastatic disease are often relatively deconditioned and poorly tolerate the prolonged nature of conservative management, including bed rest, bracing, and oral analgesics. 8 9 As such, these patients benefit greatly from early stabilization of acute and subacute fractures. 10 However, even patients with persistent pain lasting longer than 12 months from unhealed fractures can achieve significant benefit. 11
Additionally, it is important to consider a patient's age, functional status, long-term prognosis, tumor histology, and rate of disease progression, often in a multidisciplinary setting. The NOMS decision framework 12 (neurologic symptoms, oncologic parameters, mechanical instability, and systemic disease/medical comorbidities) highlights the multiple aspects that must be addressed, ideally within a multidisciplinary setting when considering a plan of care.
Surgical decompression in metastatic disease has been shown to provide reasonable long-term ambulatory benefit, and for patients who are young and/or highly functional and have a reasonable long-term prognosis, early evaluation for surgical stabilization is recommended. Several surgical scoring systems have been developed to stratify survival following major spine surgery in the setting of metastatic disease that can aid in this decision. 13 14
Percutaneous Vertebral Augmentation
Vertebral stabilization with PMMA in the setting of a pathologic spine fracture was first described in 1981 by orthopedic surgeon Kevin D. Harrington. 15 In general, vertebral stabilization consists of accessing the vertebral body through a posterior approach, either through or adjacent to one or both pedicles, sometimes followed by creation of a cavity or placement of an implant within the vertebral body, followed finally by injection of PMMA.
There is considerable confusion regarding the description of these vertebral augmentation procedures and accurate documentation is important for effective reimbursement. Vertebroplasty refers to the isolated injection of PMMA into the vertebral body. Balloon kyphoplasty or kyphoplasty refers to initial cavity creation within the vertebral body with a balloon followed by PMMA injection into the cavity. Vertebral augmentation encompasses all procedures consisting of cavity creation or device implantation followed by PMMA injection, including kyphoplasty; however, for the purposes of this article, vertebral augmentation will encompass all of the aforementioned interventions. Sacral vertebroplasty and sacral augmentation with cavity creation are extensions of this nomenclature and therapy when performed in the sacral vertebral elements.
For patients not requiring or appropriate for surgical decompression and/or fixation, there is strong evidence that vertebral augmentation can achieve significant spinal stabilization, pain relief, and functional improvement in both osteoporotic and pathologic fractures, with acceptably low complication rates, quick recovery, the avoidance of general anesthesia in the majority of cases, and minimal disruption to adjunctive therapies including chemotherapy and radiation. 10 16 In general, percutaneous vertebral augmentation is highly effective with low risk; however, these risks and benefits must be weighed individually for each patient while accounting for a physician's procedural comfort, as many oncologic lesions can be technically challenging secondary to extensive erosion of protective cortical bone around the spinal cord and exiting nerve roots.
See Figs. 1 and 2 for examples of vertebral augmentation for painful osseous metastases.
Fig. 1.
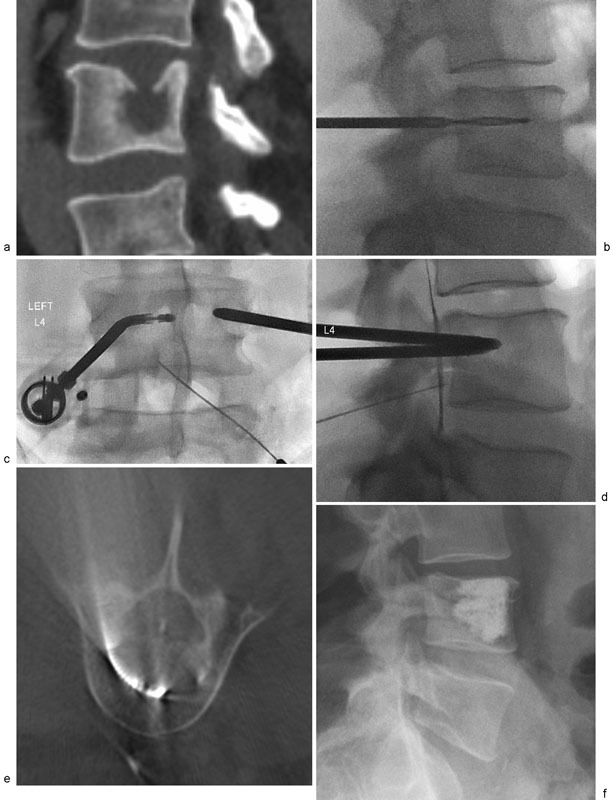
( a ) A 46-year-old man with metastatic lung cancer and painful pathologic fracture of L4 secondary to lytic metastasis. ( b ) Transpedicular access achieved with 10-gauge trocar and hand drill due to bone hardness. ( c ) Bipedicular access combined with articulating RF probe (black arrow) chosen for improved tumor ablation coverage. A 22-gauge spinal needle (white arrow) placed into the epidural space for hydrodissection. ( d ) Contrast injection confirming placement of needle in epidural space (arrow). ( e ) Intraprocedural cone-beam CT confirming position of articulating radiofrequency ablation probe (arrow). ( f ) Final radiograph following bipedicular vertebroplasty.
Fig. 2.

( a ) A 52-year-old woman with metastatic lung cancer and neck pain. Multidisciplinary decision making with spine surgeon determined surgical decompression and fusion would be optimal but complicated by poor bone quality and metastases. ( b, c ) Transoral approach to C2 body using nasal intubation, bite block, and 14-gauge bone trocar (arrow). ( d, e ) Vertebroplasty of C2 using polymethylmethacrylate (PMMA). Additional vertebroplasty was performed at C5 and C7 from an anterolateral approach using ultrasound guidance taking care to avoid critical vascular structures. ( f ) Five days later, the patient underwent surgical decompression and stabilization utilizing previously placed PMMA for screw augmentation.
Spine Ablation
Most data in the Western literature have reported on treatments with radiofrequency ablation (RFA) 17 18 19 and cryoablation 20 with few reports on the use of microwave. 21 22 23 Despite this, all three technologies are being employed in and adjacent to the spine as useful adjunct therapy for local tumor control and pain relief.
Percutaneous thermal ablation of metastatic spine lesions has been shown to provide reasonable rates of curative therapy up to 67% at 1 year 24 for a select subgroup of small, solitary lesions within the vertebral body that are accessible and without significant cortical destruction or involvement of the posterior elements. The difficulty associated with achieving complete ablation, even in solitary lesions, arises from the need to maintain an adequate safety margin regarding adjacent neurologic structures and the challenge of precisely predicting and measuring in real time the zone of ablation. Despite the difficulty in achieving complete tumor control, effective, noncurative cytoreduction of the metastatic lesion can be achieved even in large, locally aggressive metastases and has several suggested benefits despite incomplete tumor control.
Additionally, spine ablation is likely very effective in reducing back pain associated with metastatic disease. Anecdotally, the ablation of osteoblastic spine metastases without fracture, and therefore not receiving cement augmentation, has achieved significant pain relief on its own in our experience. To achieve maximal pain reduction, ablation should be targeted toward the bone/tumor interfaces where there is maximal nerve ending irritation related to a local tumor-induced inflammatory state/cytokine upregulation. Additionally, targeted ablation of the basivertebral nerve plexus in the central vertebral body may also be a reasonable adjunct strategy, as recent randomized data have shown the utility of this technique in reducing pain due to early degenerative endplate disease. 25
One benefit of ablation is the creation of a “cavity” within the central vertebral body that, when performed prior to cement injection, can guide cement deposition and minimize complications, 26 although this has not been rigorously proven. In general, PMMA injection is recommended immediately following ablation of all lytic or partially lytic metastases to prevent postablation structural instability. It is usually not necessary following ablation of blastic lesions unless an associated fracture exists, although there is no high-quality data to support this algorithm.
Specific Ablation Modalities
The decision of which ablation system should be utilized is multifactorial. Experience and comfort level certainly play a large role as with most technologies. There are several device design features and limitations that should be factored into the choice as well as patient-specific tumor features.
Cryoablation is most useful in the setting of a large bulky tumor where ablation size and sculpting are desired. Large ablation zones can be achieved with multiple probes in part due to the unimpeded growth of the ablation zone through intact bone cortex. Furthermore, cryoablation has the unique advantage of ablation zone visualization on CT if there is a significant soft-tissue component. Thermocouple probes are often integrated. Neurologic monitoring is facilitated, as there is no electrical interference and subtle changes in nerve conduction parameters can be identified early, indicating nerve cooling potentially avoiding permanent damage. Some disadvantages include longer ablation times, expensive and cumbersome capital equipment, and the necessary partial melting of the ice ball prior to cement delivery to avoid inadequate or unpredictable cement delivery.
RF ablation in bone has been utilized for over a decade with several mature product lines with integrated access kits and in some cases cement delivery systems. One system has the ability to articulate the ablation probe up to 90 degrees, whereas the remaining ablation systems lack this design feature. Integrated thermocouple monitoring allows for more precise measurement of the ablation zone size despite no real-time visualization of the ablation zone, although in general achievable ablation zones are relatively small. Intact bone cortex reflects RF energy and provides a certain degree of protection from adjacent structures as well as creating an “oven effect” within the vertebral body. 27 Other disadvantages are that RFA is relatively susceptible to the heat-sink effect and tissue charring which in certain cases can prevent adequate ablation. When feasible, highly vascular metastases may benefit from preablation arterial embolization to reduce this heat-sink effect when RFA is planned.
All probes must be used with caution in sclerotic bone with the potential for probe malfunction or fracture leading to treatment failures or complications.
Microwave probes are more fragile given their ceramic tip design, and the presence of sclerotic bone can lead to a risk of probe fracture, potentially requiring more advanced drill access. Despite this, the efficient power and ability to potentially desiccate tumor tissue with microwave are distinct advantages. Additional advantages of microwave include relatively short ablation times, multiple simultaneous ablation probes, less susceptibility to heat-sink and charring effect, and relative insensitivity to the intrinsic high impedance of bone, especially osteosclerotic lesions, allowing for deeper thermal penetration than RF. 28
Like RFA, there is no ability to visualize the ablation zone in real time. With microwave, many manufacturer charts suggest an achieved ablation size in soft tissues with specific combinations of wattage and ablation time; however, heat transmission through cancellous bone is less efficient and more reflected at intact cortical margins, making accurate predictions regarding the size of ablation quite difficult. While similar issues exist with RF ablation, integrated temperature feedback ensures the ablation zone remains within a certain size.
Care should also be taken in the presence of surgical hardware or metallic clips when using RFA and microwave systems. The interventionalist should consult the manufacturer's instructions for use (IFU) for guidance in these situations.
Ablation Techniques
Careful assessment of preoperative imaging, including recent MRI and CT, cannot be overstated. Key factors to identify are the extent of tumor involvement in the posterior vertebrae, evidence of instability, tumor or bone fragment retropulsion, posterior cortical destruction, the presence of tumor in the pedicles, pedicle fracture, and potential for instability. Dural invasion and cord or nerve root compression must also be assessed. Imaging also allows assessment of tumor vascularity, paraspinous soft-tissue component, bone quality, and degree of osteolysis. Lytic lesions are usually most amenable to treatment, allowing for easy access and stabilization with PMMA. Sclerotic and mixed lesions are more challenging, requiring the use of drills for access. In practice, the ability to target regions within the sclerotic vertebral body with predictable instillation of PMMA is challenging. Imaging also determines whether pedicle access can be achieved safely or if a para-pedicular access is preferred. If the pedicle is intact without fracture and there is an intact medial cortex of the pedicle, then this pedicle can be approached in most cases. A para-pedicular approach is required in those cases where the pedicle has tumor involvement.
Preoperative imaging also determines the need for neurologic monitoring, or other protective methods like CO 2 instillation or placement of temperature probes in the epidural space adjacent to critical nerve roots and dura. After standard positioning and preparation, a bipedicular access is most often performed to allow complete targeting of the vertebral body. A unipedicular access will not afford access to the entire vertebral body.
After accessing the pedicle, the needle system is delivered to the anterior portion of the vertebrae. Cavity creation using a mechanical osteotome may be performed to allow manipulation inside the vertebral body.
Transoral Approach
The transoral access technique 29 is necessary for treatment of C2–C3 cancer-related pathologic fractures, impending fractures, and less commonly for benign fractures. At first glance, this technique may be considered as too risky and therefore not reasonable to adopt.
It is in fact the technique used in one of the first vertebroplasties performed by Dr. Herve Deramond for a patient with a benign yet painful C2 hemangioma. The route is direct with only 1 to 2 mm of tissue to pass from the posterior oral pharynx into the anterior vertebral body of C2 or C3. This allows access to the vertebral body in the midline with the ability to target different parts of the vertebra easily based on preoperative imaging. This imaging should be recent, and assessment of the posterior cortex is mandatory to understand the potential for cement leakage and retropulsion of fragments or tumor. This technique requires high-quality fluoroscopy with the capability of achieving lateral and anteroposterior views quickly. A biplane room is ideal room for this procedure, though a single plane system can also be used.
After the patient has been nasally or orally intubated, a tonsillectomy retraction set is placed in the mouth to assist in retracting the lips to allow direct visualization into the posterior oral pharynx. A tongue blade or other means of tongue depression is also required. The technique requires appropriate preparation of the oral cavity with toothbrush and chlorhexidine wash as well as suction to clear the oral cavity. The airway must be protected to prevent aspiration of the preparation.
Once prepared, the 15- to 11-gauge needle can then be advanced directly through the midline tissues into the vertebral body with fluoroscopic confirmation in both projections.
The PMMA is then mixed and slowly injected primarily utilizing the lateral projection. This allows visualization of the posterior cortical line so that control of PMMA leakage can best be avoided. The degree of PMMA fill and the need for targeting cement into the base of the dens are advanced concepts that should be considered prior to the procedure so that curved needle systems can be available to use.
Thermocouple Probes
In complex lesions, the strategic placement of additional thermocouple probes can enhance the safety margin for neurologic injury and allow for more aggressive ablation margins. 30 Thermocouples can be placed within the spinal canal or neuroforamen, positioned directly between the ablation zone and critical structure of interest. Neurologic injury is felt to be unlikely when temperatures are kept between 10 and 45°C. 31
Intraprocedural Neurologic Monitoring
Another adjunctive technique to maximize safety and avoid neurologic injury is intraprocedural neurologic monitoring. 32 In the percutaneous setting, this is most useful during ablation in and around the spinal cord and pelvis. Intraprocedural monitoring is usually performed by a neurophysiologist technologist and can include somatosensory-evoked potentials (SSEP), motor-evoked potentials (MEP), and EMG monitoring. A discussion of the differences of these techniques is beyond the scope of this article, but each has its advantages and can be combined to give early, although not instantaneous, warning of nerve injury. Unfortunately, the reliability of neural monitoring can be quite variable and affected by numerous factors including probe placement and volatile anesthetics.
Radiation Therapy as Adjunct
Radiation therapy has been a mainstay of treatment for spinal metastases and can be a useful adjunct. While RT has clearly demonstrated effective pain palliation in some patients, its benefit is not uniform, with 70 to 80% experiencing some pain relief and only 30% experiencing complete pain relief. 33 Furthermore, this improvement in pain takes several weeks to occur following RT and is frequently temporary, returning in 57% of patients at a median of 15 weeks after RT. 34
Several recent advances in the field of RT have allowed for improved tumor control. Therefore, given the inherent difficulties in achieving complete tumor death with percutaneous ablation, most patients will benefit from additional RT for treatment of the entire lesion, and a cursory understanding of tumor biology and recent RTs is in order.
Retrospective studies have demonstrated that an additive benefit in pain relief with percutaneous ablation is combined with RT. 35 Historically, RT for spinal metastases consisted of cEBRT and with this technology, only certain histologic types were considered radiosensitive (e.g., lymphoma, myeloma, breast, prostate) with others considered radio resistant translating to median durations of improvement of 11 and 3 months, respectively. 36 However, with the advent of SBRT or SRS, much higher doses can be delivered to the target metastases safely even when there is significant breach of the spinal canal and neuroforamen, and nearly all tumor histologies have become radiosensitive with similar achievable local control. While recent studies of SBRT/SRS have shown high response rates with minimal neurologic side effects, there is an increased incidence of post-SBRT/SRS vertebral body fractures of up to 40%, 6 compared with less than 5% with more traditional cEBRT, which may prove to be a compelling space for prophylactic vertebral body augmentation. Regardless, the combination of RT and percutaneous ablation has been shown to be more effective than either in isolation in the treatment of skeletal metastatic disease generally. 37 38 39
Myeloma
Myeloma is a unique systemic disease due to its propensity to produce large lytic lesions through upregulation of osteoclastic activity and plasma cell invasion, which in turn can lead to a high incidence of pathologic fractures, for which vertebroplasty has been recognized as an effective treatment. 40 In short, ablation is probably not necessary for pain control, tumor control, or cavity creation. These lesions are highly radiosensitive and typically have a soft toothpaste-like consistency. PMMA polymerizes in vivo creating transient temperature elevation, and at least one study in breast cancer patients has shown good local tumor control with vertebroplasty alone, 41 independent of RT, suggesting a significant antitumoral effect of PMMA injection alone. While this is likely inadequate for a complete ablation margin, myeloma tends to be a very soft tumor and can “melt away” during cement delivery, perhaps secondary to these local temperature elevations, and complete replacement of focal lytic myeloma lesions without significant cement extravasation is often achievable without initial ablation therapy.
Pain control is often achievable in myeloma with PMMA alone, 42 likely secondary to a combination of reinforced structural stability or fracture fixation when present and possibly thermal ablation from the exothermic process of the peritumoral nerve roots that are chronically inflamed. While there have been some published reports of myeloma tumor embolization following vertebroplasty, in our experience this has not occurred and we believe the practice to be quite safe.
As with all malignancies, myeloma often involves the posterior aspect of the vertebral body and can result in significant posterior cortical destruction, increasing the potential for cement extravasation posteriorly and neurologic impairment. A recent advance is the development of vertebral augmentation implants which consist of initial delivery of a polyether-ether ketone (PEEK) coil or metallic stent implant into the diseased vertebral body followed by PMMA delivery. The KIVA is CE marked, FDA approved, and utilizes a PEEK coil delivered into the vertebral body followed by injection of PMMA through a catheter into the hollow component of the implant channeling and thereby limiting extravasation of PMMA ( Fig. 3 ). The cement is well confined to the immediate area around the coil, which can minimize the risk of cement extravasation while achieving the same benefits for pain and stability. 43
Fig. 3.

( a, b ) A 76-year-old man with multiple myeloma, focal back pain, and dominant plasmacytoma at L2 (open arrow) with destruction of the posterior vertebral body cortex and effacement of the ventral CSF (solid arrow). Patient did not have neurologic symptoms at presentation. ( c ) radiofrequency ablation performed through unilateral pedicle access with articulating probe. ( d ) KIVA device implant placed into inferior aspect of vertebral body. ( e ) Second KIVA device implant placed superiorly through same access followed by polymethylmethacrylate (PMMA) of both. Note relative containment of PMMA within KIVA implant, minimizing risk of cement herniation posteriorly. ( f ) Postprocedural CT demonstrating stacked KIVA implants. Anterior column support achieved.
Pelvis
Similar to lesions in the spine, pelvic metastases can cause severe pain and structural instability. In most cases, a treatment plan for pelvic metastases should begin in a similar fashion, by characterizing the degree of structural instability. Lesions at high risk for pathologic fracture include those with a focal or permeative osteolytic nature, large size, clinical pain with stress, and location.
There are two surgical classifications of pelvic metastatic disease that can be helpful in characterizing a lesion's fracture risk with respect to location. The Enneking classification divides the pelvis into four distinct zones, distinguishing the periacetabular and sacral regions which are highly stressed with weight bearing from the pubic rami and iliac crests which are considered similar to non–weight-bearing bones of the upper and lower extremity. With respect to acetabular (Enneking Class 2) lesions, the Harrington classification provides a more specific severity scale based on whether the medial, superior, or lateral acetabular walls are involved, with the acetabular roof being the most critical, and an evaluation of the underlying subchondral bone to stratify fracture risk. 44 In general, acute exacerbation of pain with weight bearing in the vicinity of a lesion signals a high degree of structural instability and often times can be accompanied by minimally displaced pathologic fractures.
Similar to lesions in the spine, pelvic metastases that result in structural instability generally require some component of stabilization as part of their treatment. For small lesions that are not located in critical, weight-bearing portions of the pelvis, simple osteoplasty with PMMA has been shown to be effective in preventing fracture and relieving pain. 45 46 47 Added structural support can be achieved through minimally invasive screw fixation, most commonly in conjunction with osteoplasty, which has been shown to provide benefit with low complication rates. 48 49 This combination therapy is an especially attractive option for patients with metastatic disease at high risk for fracture or with minimally displaced fractures, especially of the acetabulum and sacroiliac regions, and can often achieve adequate stabilization while avoiding either a catastrophic fracture or a traditional open surgical resection and reconstruction with its high associated morbidity and recovery ( Fig. 4 ).
Fig. 4.

( a ) A 58-year-old man with severe left hip pain and lytic 7-cm supra-acetabular paraganglioma metastasis (arrow) with minimally displaced pathologic fracture. ( b ) A 3.2-mm Steinmann pin was placed along a planned screw access corridor using a power drill. A 10-F peel away sheath was advanced over the guide pin, which was then exchanged for a bone trocar. radiofrequency ablation was then performed in multiple directions using an articulating probe (arrow). ( C ) Additional 3.2-mm guide pins were placed through the ischial corridor and superior and inferior posteroanterior iliac corridors using fluoroscopy and needle guidance software. Next, 8.0-mm cannulated orthopedic screws were then advanced over the guide pins and polymethylmethacrylate (PMMA) was injected through 11-gauge bone trocars placed within partially advanced cannulated screws. ( d ) A curved nitinol needle (arrow) was placed through the cannulated screws and used to direct PMMA deposition in and around the acetabular roof under live fluoroscopy. ( e, f ) Final images demonstrating reinforcement of the acetabulum with three orthopedic screws and PMMA.
Percutaneous stabilization consisting of osteoplasty alone or with additional screw fixation also allows for a much quicker recovery compared with surgical intervention. Patients are typically weight bearing as tolerated by the evening of the procedure (within a few hours after the procedure) and are able to participate in physical therapy the following day to minimize further deconditioning. Because patients recover quickly and do not have large surgical wounds to heal, adjunctive chemotherapy and RT can be quickly initiated or continued with minimal interruption. In many cases, this allows for initial stabilization prior to any cytotoxic chemotherapy or RT that may promote further instability as the metastases regress.
Similar principles of ablation can be applied to pelvic metastases as with the spine, taking care to identify major pelvic nerves and plan for enhanced thermal and neurologic monitoring when in close proximity to ablation zones.
Conclusion
Combinations of percutaneous osteoplasty, ablation, and screw fixation can provide effective symptom and tumor control for skeletal metastatic disease. Evaluation should begin with a complete structural assessment of the spine and pelvis, ideally in a multidisciplinary setting, to determine the degree of stabilization required and whether a surgical or percutaneous approach is more appropriate. Percutaneous ablation, almost always followed by osteoplasty, is beneficial for local tumor control and pain relief in a variety of metastatic diseases, although generally more difficult in osteoblastic lesions and uncertain utility in myeloma. When applied appropriately, percutaneous interventions are well tolerated; minimally invasive, with no significant disruption to ongoing adjunctive chemotherapy and/or RT; and allow for significantly improved patient's quality of life in the difficult setting of symptomatic skeletal metastatic disease.
References
- 1.Piccioli A, Spinelli M S, Maccauro G. Impending fracture: a difficult diagnosis. Injury. 2014;45 06:S138–S141. doi: 10.1016/j.injury.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Thibault I, Al-Omair A, Masucci G L et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. 2014;21(05):711–718. doi: 10.3171/2014.7.SPINE13895. [DOI] [PubMed] [Google Scholar]
- 3.Cunha M VR, Al-Omair A, Atenafu E G et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84(03):e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Rose P S, Laufer I, Boland P J et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27(30):5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jawad M S, Fahim D K, Gerszten P C et al. Vertebral compression fractures after stereotactic body radiation therapy: a large, multi-institutional, multinational evaluation. J Neurosurg Spine. 2016;24(06):928–936. doi: 10.3171/2015.10.SPINE141261. [DOI] [PubMed] [Google Scholar]
- 6.Sahgal A, Whyne C M, Ma L, Larson D A, Fehlings M G. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14(08):e310–e320. doi: 10.1016/S1470-2045(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 7.Martin C T, Skolasky R L, Mohamed A S, Kebaish K M. Preliminary results of the effect of prophylactic vertebroplasty on the incidence of proximal junctional complications after posterior spinal fusion to the low thoracic spine. Spine Deform. 2013;1(02):132–138. doi: 10.1016/j.jspd.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Prather H, Hunt D, Watson J O, Gilula L A.Conservative care for patients with osteoporotic vertebral compression fractures Phys Med Rehabil Clin N Am 20071803577–591., xi [DOI] [PubMed] [Google Scholar]
- 9.Gold D T.The clinical impact of vertebral fractures: quality of life in women with osteoporosis Bone 199618(3, Suppl):185S–189S. [DOI] [PubMed] [Google Scholar]
- 10.Berenson J, Pflugmacher R, Jarzem P et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol. 2011;12(03):225–235. doi: 10.1016/S1470-2045(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 11.Brown D B, Gilula L A, Sehgal M, Shimony J S. Treatment of chronic symptomatic vertebral compression fractures with percutaneous vertebroplasty. AJR Am J Roentgenol. 2004;182(02):319–322. doi: 10.2214/ajr.182.2.1820319. [DOI] [PubMed] [Google Scholar]
- 12.Bilsky M H, Azeem S. The NOMS framework for decision making in metastatic cervical spine tumors. Curr Opin Orthop. 2007;18(03):263–269. [Google Scholar]
- 13.Schoenfeld A J, Le H V, Marjoua Y et al. Assessing the utility of a clinical prediction score regarding 30-day morbidity and mortality following metastatic spinal surgery: the New England Spinal Metastasis Score (NESMS) Spine J. 2016;16(04):482–490. doi: 10.1016/j.spinee.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Leithner A, Radl R, Gruber G et al. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J. 2008;17(11):1488–1495. doi: 10.1007/s00586-008-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrington K D. The use of methylmethacrylate for vertebral-body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(01):36–46. [PubMed] [Google Scholar]
- 16.Clark W, Bird P, Gonski Pet al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): a multicentre, randomised, double-blind, placebo-controlled trial Lancet 2016388(10052):1408–1416. [DOI] [PubMed] [Google Scholar]
- 17.Munk P L, Rashid F, Heran M K et al. Combined cementoplasty and radiofrequency ablation in the treatment of painful neoplastic lesions of bone. J Vasc Interv Radiol. 2009;20(07):903–911. doi: 10.1016/j.jvir.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Nakatsuka A, Yamakado K, Maeda M et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15(07):707–712. doi: 10.1097/01.rvi.0000133507.40193.e4. [DOI] [PubMed] [Google Scholar]
- 19.Lane M D, Le H BQ, Lee S et al. Combination radiofrequency ablation and cementoplasty for palliative treatment of painful neoplastic bone metastasis: experience with 53 treated lesions in 36 patients. Skeletal Radiol. 2011;40(01):25–32. doi: 10.1007/s00256-010-1010-5. [DOI] [PubMed] [Google Scholar]
- 20.Castañeda Rodriguez W R, Callstrom M R. Effective pain palliation and prevention of fracture for axial-loading skeletal metastases using combined cryoablation and cementoplasty. Tech Vasc Interv Radiol. 2011;14(03):160–169. doi: 10.1053/j.tvir.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Pusceddu C, Sotgia B, Fele R M, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24(02):229–233. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25(09):1470–1475. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Pusceddu C, Sotgia B, Fele R M, Ballicu N, Melis L. Combined microwave ablation and cementoplasty in patients with painful bone metastases at high risk of fracture. Cardiovasc Intervent Radiol. 2016;39(01):74–80. doi: 10.1007/s00270-015-1151-y. [DOI] [PubMed] [Google Scholar]
- 24.Deschamps F, Farouil G, Ternes N et al. Thermal ablation techniques: a curative treatment of bone metastases in selected patients? Eur Radiol. 2014;24(08):1971–1980. doi: 10.1007/s00330-014-3202-1. [DOI] [PubMed] [Google Scholar]
- 25.Becker S, Hadjipavlou A, Heggeness M H. Ablation of the basivertebral nerve for treatment of back pain: a clinical study. Spine J. 2017;17(02):218–223. doi: 10.1016/j.spinee.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Georgy B A, Wong W. Plasma-mediated radiofrequency ablation assisted percutaneous cement injection for treating advanced malignant vertebral compression fractures. AJNR Am J Neuroradiol. 2007;28(04):700–705. [PMC free article] [PubMed] [Google Scholar]
- 27.Dupuy D E, Hong R, Oliver B, Goldberg S N. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175(05):1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 28.Lubner M G, Brace C L, Hinshaw J L, Lee F T., JrMicrowave tumor ablation: mechanism of action, clinical results, and devices J Vasc Interv Radiol 201021(8, Suppl):S192–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong F C, Cloft H J, Joseph G J, Rodts G R, Dion J E. Transoral approach to cervical vertebroplasty for multiple myeloma. AJR Am J Roentgenol. 2000;175(05):1322–1324. doi: 10.2214/ajr.175.5.1751322. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsuka A, Yamakado K, Takaki H et al. Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol. 2009;32(01):70–75. doi: 10.1007/s00270-008-9390-9. [DOI] [PubMed] [Google Scholar]
- 31.Deschamps F, Farouil G, de Baere T.Percutaneous ablation of bone tumors Diagn Interv Imaging 201495(7-8):659–663. [DOI] [PubMed] [Google Scholar]
- 32.Kurup A N, Morris J M, Boon A J et al. Motor evoked potential monitoring during cryoablation of musculoskeletal tumors. J Vasc Interv Radiol. 2014;25(11):1657–1664. doi: 10.1016/j.jvir.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Agarawal J P, Swangsilpa T, van der Linden Y, Rades D, Jeremic B, Hoskin P J. The role of external beam radiotherapy in the management of bone metastases. Clin Oncol (R Coll Radiol) 2006;18(10):747–760. doi: 10.1016/j.clon.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Tong D, Gillick L, Hendrickson F R. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncology Group. Cancer. 1982;50(05):893–899. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 35.Grieco C A, Simon C J, Mayo-Smith W W, Dipetrillo T A, Ready N E, Dupuy D E. Image-guided percutaneous thermal ablation for the palliative treatment of chest wall masses. Am J Clin Oncol. 2007;30(04):361–367. doi: 10.1097/COC.0b013e318033e76a. [DOI] [PubMed] [Google Scholar]
- 36.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32(04):959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 37.Di Staso M, Gravina G L, Zugaro L et al. Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PLoS One. 2015;10(06):e0129021. doi: 10.1371/journal.pone.0129021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Staso M, Zugaro L, Gravina G L et al. A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol. 2011;21(09):2004–2010. doi: 10.1007/s00330-011-2133-3. [DOI] [PubMed] [Google Scholar]
- 39.Greenwood T J, Wallace A, Friedman M V, Hillen T J, Robinson C G, Jennings J W. Combined ablation and radiation therapy of spinal metastases: a novel multimodality treatment approach. Pain Physician. 2015;18(06):573–581. [PubMed] [Google Scholar]
- 40.Hussein M A, Vrionis F D, Allison R et al. The role of vertebral augmentation in multiple myeloma: International Myeloma Working Group Consensus Statement. Leukemia. 2008;22(08):1479–1484. doi: 10.1038/leu.2008.127. [DOI] [PubMed] [Google Scholar]
- 41.Roedel B, Clarençon F, Touraine S et al. Has the percutaneous vertebroplasty a role to prevent progression or local recurrence in spinal metastases of breast cancer? J Neuroradiol. 2015;42(04):222–228. doi: 10.1016/j.neurad.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Erdem E, Samant R, Malak S F et al. Vertebral augmentation in the treatment of pathologic compression fractures in 792 patients with multiple myeloma. Leukemia. 2013;27(12):2391–2393. doi: 10.1038/leu.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tutton S M, Pflugmacher R, Davidian M, Beall D P, Facchini F R, Garfin S R. KAST Study: The Kiva system as a vertebral augmentation treatment—a safety and effectiveness trial: a randomized, noninferiority trial comparing the Kiva system with balloon kyphoplasty in treatment of osteoporotic vertebral compression fractures. Spine. 2015;40(12):865–875. doi: 10.1097/BRS.0000000000000906. [DOI] [PubMed] [Google Scholar]
- 44.Müller D A, Capanna R. The surgical treatment of pelvic bone metastases. Adv Orthop. 2015;2015:525363. doi: 10.1155/2015/525363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelekis A, Lovblad K O, Mehdizade A et al. Pelvic osteoplasty in osteolytic metastases: technical approach under fluoroscopic guidance and early clinical results. J Vasc Interv Radiol. 2005;16(01):81–88. doi: 10.1097/01.RVI.0000141717.84515.92. [DOI] [PubMed] [Google Scholar]
- 46.Kurup A N, Morris J M, Schmit G D et al. Balloon-assisted osteoplasty of periacetabular tumors following percutaneous cryoablation. J Vasc Interv Radiol. 2015;26(04):588–594. doi: 10.1016/j.jvir.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Madaelil T P, Wallace A N, Jennings J W. Radiofrequency ablation alone or in combination with cementoplasty for local control and pain palliation of sacral metastases: preliminary results in 11 patients. Skeletal Radiol. 2016;45(09):1213–1219. doi: 10.1007/s00256-016-2404-9. [DOI] [PubMed] [Google Scholar]
- 48.Cazzato R L, Koch G, Buy X et al. Percutaneous image-guided screw fixation of bone lesions in cancer patients: double-centre analysis of outcomes including local evolution of the treated focus. Cardiovasc Intervent Radiol. 2016;39(10):1455–1463. doi: 10.1007/s00270-016-1389-z. [DOI] [PubMed] [Google Scholar]
- 49.Hartung M P, Tutton S M, Hohenwalter E J, King D M, Neilson J C. Safety and efficacy of minimally invasive acetabular stabilization for periacetabular metastatic disease with thermal ablation and augmented screw fixation. J Vasc Interv Radiol. 2016;27(05):682–6880. doi: 10.1016/j.jvir.2016.01.142. [DOI] [PubMed] [Google Scholar]


