Abstract
Peptide-conjugated nanoparticles (NPs) have promising potential for applications in biosensing, diagnosis, and therapeutics because of their appropriate size, unique self-assembly, and specific substrate-binding properties. However, controlled assembly and selective target binding are difficult to achieve with simple peptides on NP surfaces because high surface energy makes NPs prone to self-aggregate and adhere nonspecifically. Here, we report the self-assembly and gelatin binding properties of collagen mimetic peptide (CMP) conjugated gold NPs (CMP-NPs). We show that the orientation of CMPs displayed on the NP surface can control NP assembly either by promoting or hindering triple helical folding between CMPs of neighboring NPs. We also show that CMP-NPs can specifically bind to denatured collagen by forming triple-helical hybrids between denatured collagen strands and CMPs, demonstrating their potential use for detection and selective removal of gelatin from protein mixtures. CMP conjugated NPs offer a simple and effective method for NP assembly and for targeting denatured collagens with high specificity. Therefore, they may lead to new types of functional nanomaterials for detection and study of denatured collagen associated with diseases characterized by high levels of collagen degradation.
Keywords: collagen mimetic peptide, denatured collagen, gold nanoparticle, self-assembly, gelatin, triple helix hybridization
Graphical abstract

1. INTRODUCTION
Conjugation of biomolecules (e.g., DNA, proteins and peptides) to inorganic nanoparticles (NPs) opened up a broad range of exciting research in photonics,1,2 biocatalysis,1,3–5 bioelectronics,1,2,6 biosensors,1–3 drug delivery,7,8 and gene regulation.9–11 NPs used in drug and gene delivery are particularly attractive, since their small size allows for tissue penetration as well as elimination, while still affording a high number of targeting moieties and bioactive molecules on NP surfaces.12 NPs in these studies are often conjugated with molecules that have high affinity to specific biomolecules for the purpose of targeting diseased cells or tissues; however such molecules tend to also exhibit nonspecific affinity to other biomolecules. This poses a significant difficulty for clinical applications that involve the use of NPs in bodily fluid that is comprised of high ionic strengths and numerous surface active biomolecules. Even peptides that are carefully designed to disperse NPs do not protect them from aggregation under a wide range of conditions.13 Practical applications of NP targeting rely on not only NP’s ability to recognize target molecules, but also its inertness toward nonspecific binding.
Self-assembly is a powerful tool in controlling both the physical and biological properties of NPs. Biomolecular recognition, such as DNA hybridization, protein–ligand interactions and peptide–peptide interactions have been widely exploited for controlled assembly of NPs. A NP’s aggregated state is reported to affect their electro-optical properties1,3,10,14–17 and recent studies have shown that the NP assemblies play an important role in delivery and elimination in vivo.18 For example, the use of DNA-modified gold NPs, which assemble into a superstructure, was reported to improve its accumulation in tumors and at the same time facilitate elimination from the body,18 because after the accumulation, the assembly can degrade into individual NPs which are cleared via the kidney. We believe that developing new tools for controlled assembly of NPs will expand the design of new nano- and mesoscale materials for functional application in biotechnology.
Collagen is the most abundant protein in mammals, and controlling the collagen assembly process in the design of new biomaterials has been a long-standing interest for many researchers. In particular, small peptides that mimic the triple helical structure of collagen have been investigated for applications in disease detection and imaging,19,20 drug delivery,16,21 and tissue engineering.22–24
Collagen mimetic peptides (CMPs) are a family of synthetic peptides that mimic the basic structural motif of natural collagen—the triple helix. They are made of the Gly-Xaa-Yaa triplet repeating sequence, where Xaa and Yaa are largely populated by proline and 4(R)-hydroxylproline, respectively.16,19,25,26 CMPs have a strong propensity to self-assemble into a triple helix structure, and have been used as synthetic models to study the structure and folding behaviors of collagens.27 Previously, our research group found that the CMPs with the sequence (GPO)n (n = 6–10, O = hydroxylproline) have a strong propensity to bind to denatured collagens (also known as gelatin) by forming triple helical hybrids.16,19,20,28–30 This hybridization process is similar to small DNA fragments binding to DNA strands having complementary base pairs. We also reported that the gold NPs conjugated with CMPs are colloidally stable in aqueous solution with a wide range of pH (pH 0–14) and even in high salt concentration (5 M NaCl).25 It is believed that the polar, nonionic character, and the extended conformation of the peptides prevent aggregation of the CMP conjugated NPs in aqueous solution. Except for hybridization to gelatin strands, the peptide has low nonspecific binding affinity to other biomolecules. Therefore, CMP conjugated NPs (broadly designated as CMP-NPs) are ideal materials for biological assembly and targeting studies.
There are a number of potential applications for CMP-NPs. Since CMP is known to hybridize with denatured collagen strands, CMP-NP can directly be used as a contrast agent (in TEM or CT) to detect the location of collagen damaged by either proteases or by injury. The aggregation behavior of CMP-NPs can be affected by the presence of collagen fragments, which could be exploited for detection of diseases associated with high collagen remodelling activity.31 CMP-NP can also be used to remove denatured collagen from protein mixtures, since gelatin is a common additive in protein drug formulation that can cause negative host response.32
In this study, we present the self-assembly and gelatin binding properties of CMP-NPs. Here, we show that the triple helical folding between CMPs of neighboring NPs can control the large-scale NP assembly when the peptides are displayed with correct orientation (Figure 1). We also show that the CMP-NPs can bind to denatured collagen strands with high specificity, and demonstrate their use in selective removal of gelatin from protein mixtures. The results suggest that the CMP-NPs can potentially be used to detect diseases that produce high levels of denatured collagen (e.g., arthritis), and to remove gelatin from commercial protein drug formulations for reduced side effects.
Figure 1.
Schematic of CMP-NPs assembly. Two types of gold NPs (10 and 20 nm) conjugated with CMP of opposite polarity assemble into well ordered aggregates by forming triple helical structures. C(GPO)9 (1), colored in green, was conjugated to 10 nm NPs (NP-1); (GPO)9C (2), colored in yellow, was conjugated to 20 nm NPs (NP-2), to make two different size NPs with opposite peptide polarity. On their own, both NPs exhibit high colloidal stability with no sign of aggregation at a pH range 0–14 and high ionic strengths.25 When the two NPs are mixed together, they assemble by triple helical folding of parallelly aligned CMPs.
2. EXPERIMENTAL SECTION
2.1. Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Citrate-capped AuNP solution was purchased from Ted Pella, Inc. (Redding, CA). For peptide synthesis, Fmoc-Gly-OH, Fmoc-Pro-OH, Fmoc-Ahx–OH, and 1-hydroxy-6-chloro-benzotriazole (Cl-HOBt) were purchased from Advanced ChemTech (Louisville, KY). O-(Benzo-triazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) was purchased from AAPPTec (Louisville, KY), N,N-diisopropylethylamine (DIPEA) from Acros (Geel, Belgium), and dimethylformamide (DMF), N-Methyl-2-pyrrolidone (NMP), and trifluoroacetic acid (TFA) were purchased from Fisher (Pittsburgh, PA) and used without further purification. Fmoc-Hyp(tBu)–OH and Fmoc-Cys(trt)–OH were purchased from EMD Millipore (Temecula, CA). TentaGel R RAM resin was purchased from Peptides International (Louisville, KY). FITC labeled gelatin was purchased from Life Technology (Grand Island, NY).
2.2. CMP Synthesis
CMPs were synthesized on the TentaGel R RAM resin via standard F-moc chemistry using Focus XC peptide synthesizer (AAPPTec, Louisville, KY) with coupling cycles based on HBTU/DIEA-mediated (Adv Chemtech, Louisville, KY) activation. 5-fold molar excess of the amino acids and coupling reagents were used in a typical coupling reaction. The peptides were cleaved from the resins by treatment with water/1,2-ethanedithiol/thioanisole/trifluoroacetic acid (2.5/2.5/1/94) for at least 2 h. Peptides were purified by reverse phase high performance liquid chromatography (RP-HPLC) (Agilent, Santa Clara, CA) on a C18 column with a gradient of water–acetonitrile containing 0.1% trifluoroacetic acid (TFA). The mass and purity of the CMPs were analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) (UltrafleXtreme, Bruker Daltonics, Billerica, MA) and HPLC (Agilent, Santa Clara, CA). The concentration of peptide solution was calculated from the weight of the dry peptide powder. We also prepared tyrosine containing CMPs, CYG(GPO)9, and (GPO)9GYC to quantify and calibrate the concentration of CMPs by measuring their UV–vis absorbance at 280 nm (extinction coefficient of 1280 M−1cm−1) using a SpectraMax M-2 microplate reader (Molecular Devices, Sunnyvale, CA). 5(6)-Carboxyfluorescein (CF) conjugated CMPs were synthesized as reported before.19
2.3. Peptide-Au NP Conjugation and NP Assembly
CMPs with a single cysteine residue (Cys-CMPs) at N terminus (compound 1, Table 1) was conjugated to 10 nm gold NP to produce CMP conjugated NP designated as NP-1. Similarly, CMP with a single cysteine residue at C terminus (compound 2, Table 1) was conjugated to 20 nm gold NP to produce NP-2. In a typical experiment, purified CMP was preheated at 80 °C for 5 min to melt the triple helices and added to a citrate-stabilized gold NP solution to give final concentrations of 25 μM of CMP and 5 nM of NPs, followed by incubation at room temperature overnight. The resulting reaction mixture was heated again at 80 °C for 5 min, and excess free CMPs were removed by repeated centrifugation (20,000 rcf) and washing in DI water. The concentrations of CMP-NPs were determined using optical absorbance at 520 nm. For NP assembly, solutions of NP-1 (50 μL, 20 nM) and NP-2 (50 μL, 2 nM) were mixed to yield a final concentration of 10 nM and 1 nM (ratio 10:1), respectively. The solution mixture was left at 4 °C overnight. For the disassociation experiment, free (GPO)9 (12.5 μL, 4 mM) was preheated to 80 °C for 5 min to melt the triple helix, and immediately added to the above NP-1 and NP-2 mixture solution followed by incubation at 4 °C overnight.
Table 1.
CMPs Used for Gold NP Conjugation and NP Assembly
| CMP | sequence | Tm (°C) | calculated [M + Na]+ | MALDI-TOF [M + Na]+ | |
|---|---|---|---|---|---|
| 1 | C(GPO)9 | CGPOGPOGPOGPOGPOGPOGPOGPOGPO | 76 | 2547.1 | 2547.2 |
| 2 | (GPO)9C | GPOGPOGPOGPOGPOGPOGPOGPOGPOC | 76 | 2547.1 | 2547.3 |
| 3 | CG9P9O9 | Ac-C-Ahx-PGOGPGPOPOGOGOPPGOOPGGOOPPG | 2703.1 | 2702.3 | |
| 4 | (GPO)2GCO(GPO)6 | GPOGPOGCOGPOGPOGPOGPOGPOGPO | 55 | 2450.1 | 2450.1 |
| 5 | (GPO)9 | GPOGPOGPOGPOGPOGPOGPOGPOGPO | 76 | 2444.1 | 2444.2 |
| 6 | CF-(GPO)6 | CF-GGG-GPOGPOGPOGPOGPOGPO | 39 | 2171.1 | 2171.9 |
2.4. Hybridization of CF-(GPO)6 with NP-1
CF-(GPO)6 and NP-1 were mixed together to yield a final concentration of 10 μM and 10 nM, respectively. The mixture was first heated at 80 °C for 5 min to melt all the triple helices, followed by incubation at 4 °C overnight. The CF-(GPO)6 that did not bind to NP-1 were removed by first centrifugation of the NP solution to pellet out the NP-1, followed by decanting CF-(GPO)6 containing supernatant. SpectraMax M-2 microplate reader was used to measure the fluorescence (ex: 489 nm, em: 533 nm) at predetermined temperature. Each binding experiment was done in triplicate.
2.5. Transmission Electron Microscopy (TEM)
TEM was performed on FEI Tecnai T12 microscope (FEI, Hillsboro, OR) operated at 120 kV. TEM samples were prepared by applying a drop of NP containing solutions onto a copper grid covered with a thin carbon film (EMS, Hatfield, PA) followed by overnight drying at room temperature. To test the binding of denatured collagen, a drop (10 μL) of a suspension of type I collagen fibers (0.5 mg/mL in phosphate buffer solution) which was denatured by heating to 80 °C for 5 min and cooled to room temperature, was added to a TEM grid. After 1 min, the excess solution was wicked away with a blotting paper, and 10 μL of NP-1 (10 nM) containing 1% BSA was applied. The grid was incubated for 10 min, and washed 3 times with DI water. The final sample was stained with 2% (weight/volume) uranyl acetate by applying 10 μL staining solution onto the grid, and after 1 min, the excess solution was wicked away with a blotting paper. The grid was dried at room temperature overnight. The images were processed using Gatan Digital Micrograph software (Gatan, Pleasanton, CA) and ImageJ (National Institutes of Health, Bethesda, MD).
2.6. Dynamic Light Scattering (DLS)
DLS measurements were performed using Malvern Zetasizer Nano S (Malvern, Worcestershire, UK), equipped with a 50 mW laser beam and a standard 633 nm laser filter. Fifty microliters of sample solution in a disposable micro volume polystyrene cuvette (Malvern, Worcestershire, UK) was used for all the measurements. All samples were subjected to thorough pipetting prior to measurement to ensure that NPs were dispersed in solution.
2.7. Circular Dichroism (CD)
CD spectra were recorded on a JASCO J-1500 CD (JASCO, Tokyo) in 0.10 mm quartz cells. All CMP samples were prepared in water and incubated at 4 °C for at least 24 h prior to CD measurement. Spectra were recorded from 190 to 300 nm at a scanning rate of 100 nm/min at 0.5 nm increment. CD melting experiments were performed in the temperature range from 20 to 90 °C at a heating rate of 1 °C/min. The intensity of the CD signal at 225 nm was monitored as a function of temperature. Melting temperatures were determined from the maximum of the first derivative of the melting curves.33
2.8. Gelatin Removal and Binding Specificity of CMP-NP
A 100 nm gold NP was conjugated with 1 as described above to produce large size CMP-NP (designated as CMP-NP100). CMP-NP100 and FITC labeled gelatin were mixed to a final concentration of 5 pM and 25 μg/mL, respectively. This mixture was incubated at room temperature for 2 h, followed by centrifuging at 2300 rcf for 5 min to separate the gelatin-bound CMP-NP100. The gelatin remaining in solution was quantified by measuring their fluorescent intensity using SpectraMax M-2 microplate reader (ex: 489 nm, em: 533 nm). To prepare samples for UV–vis and SDS-PAGE characterization, a protein mixture containing conalbumin (75 kDa), bovine albumin serum (BSA, 66 kDa), carbonic anhydrase (29 kDa), and ribonuclease A (13.7 kDa), with or without gelatin was mixed with CMP-NP100 to yield final concentrations of 50 μg/mL of each protein and 5 pM of NP. This mixture was incubated at room temperature for at least 2 h prior to UV–vis measurement, and centrifuged at 2,300 rcf for SDS-PAGE. Protein mixture was replaced with mouse serum, and the gelatin removal procedure was performed under the same condition: 25 μL of mouse serum was mixed with 75 μL of CMP-NP100 solution to yield a final concentration of 5 pM CMP-NP100. To prepare gelatin solution, a rat tail tendon (type I collagen) in acidic solution was first neutralized using 1× PBS buffer (pH 7.4), followed by heating at 80 °C for 5 min to induce permanent protein denaturation.
3. RESULTS AND DISCUSSION
3.1. Characterization of CMP-NP
One of the main goals of this work was to develop a NP system that could detect the presence of denatured collagen or collagen-like molecules by changes in the physical properties or aggregation behavior of the NPs. To study the behavior of NP assembly resulting from the orientation of CMP display (which could either inhibit or promote NP assembly), we synthesized two different types of cysteine containing CMPs (Cys-CMPs), one having a single cysteine residue at the N terminus designated as peptide 1 (Table 1), and the other having a single cysteine residue at the C terminus, designated as peptide 2. As a control, we also prepared a peptide (3) which had a scrambled sequence containing nine residues of G, P, and O. Cys-CMPs were used to functionalize the gold NP surfaces via the ligand exchange reaction as reported previously.25 The 10 nm particles were conjugated with 1 (designated as NP-1, Figure 1), and the 20 nm particles were conjugated with 2 (designated as NP-2, Figure 1).
The Cys-CMPs were first heated in order to melt the triple helix before reacting with the NPs to ensure that the CMPs were conjugated to the NP in a single strand form. Because of the opposite location of Cys in the two peptides, NP-1 displays CMPs with its C termini pointing outward, while NP-2 displays CMPs with its N termini pointing outward. The size and optical properties of these particles after Cys-CMPs conjugation were assessed by dynamic light scattering (DLS), transmission electron microscopy (TEM) and UV–vis spectroscopy (Figure 2). The UV–vis spectra were red-shifted by approximately 5 nm after Cys-CMP conjugation for both NP-1 and NP-2 (Figure 2C). The DLS indicated hydrodynamic sizes of 31.0 and 38.3 nm respectively for NP-1 and NP-2 (Figure 2A and B, lower panels), which correspond to a peptide layer thickness of 10.5 and 9.2 nm, respectively. These values are comparable to the estimated value of 8.7 nm calculated for Cys-CMP assuming a polyproline-II helix as previously reported.25 These results indicate that irrespective of CMP attachment direction, the peptides are extended outward from the NP surface. Pseudohexagonal lattice arrangement of NPs in the TEM (Figure 2A and B) suggests that the NPs are behaving similar to hard spheres due to densely passivated Cys-CMP layers, which prevent NP interactions.
Figure 2.
Transmission electron microscopy (TEM), dynamic light scattering (DLS), and UV–vis spectra of 10 nm Au–C(GPO)9 (NP-1) and (GPO)9C–Au 20 nm NPs (NP-2): (A) 10 nm Au–C(GPO)9 and (B) (GPO)9C–Au 20 nm. (C) UV–vis spectra of unconjugated and CMP conjugated gold NPs. The bottom panel in each TEM corresponds to the DLS trace with the number indicating the average size of the NPs in solution. Scale bar represents 50 nm.
3.2. Hybridization of CF-(GPO)6 with CMP-NP
To first demonstrate that the CMPs on NP surface are able to hybridize with other CMPs, we investigated the binding and release characteristics of CF labeled CMP [CF-CMP: CF-(GPO)6] for NP-1. A mixed solution of CF-CMP and NP-1 was heated to 80 °C for 5 min, followed by incubation at 4 °C overnight. NP-1 purified from unbound CF-CMPs by centrifugation showed negligible fluorescence intensity at 25 °C; however when heated to 40 °C, the intensity increased by over 3 orders of magnitude (Figure 3). At 25 °C, CF-CMP is bound to the NP-1 by triple helical folding which puts CF in close proximity to the NP surface resulting in fluorescence quenching.34 At 40 °C, however, the triple helices melt (Figure S1) releasing the CF-CMPs from the NP surface which is seen by a marked increase in fluorescence intensity (Figure 3). Under the same condition, the CMP with a scrambled sequence (CF-G9P9O9) did not hybridize with NP-1 as evidenced by negligible change in fluorescence before and after the heating (Figure 3). The results confirm that the NP-1 is able to interact with CMPs in solution via triple helical folding. We were unable to determine either the CD signature or the CD melting behavior of the triple helix, because light scattering from the NPs impeded the CD measurements.
Figure 3.

Hybridization of CF-(GPO)6 with CMP conjugated gold NP (NP-1). The fluorescent intensity of NP-1 treated with CF-(GPO)6 or scrambled sequence CMP, CF-G9P9O9 is shown for two different temperatures, 25 and 40 °C. CF-G9P9O9 is unable to fold into triple helix, and did not hybridize with NP-1, resulting in negligible fluorescence at two temperatures. Schematic of NP-1 hybridizing and releasing CF-(GPO)6 is also shown.
3.3. NP Assembly
Although the two types of NPs repel on their own, when mixed together, they exhibited strong tendency to aggregate (Figure 4A). When NP-1 and NP-2 were mixed at a ratio of 10:1, respectively, the initial pink color of the mixture faded after overnight incubation at 4 °C. This was corroborated by the UV–vis spectra, which showed a red-shift (from 524 to 550 nm) and significant broadening of the peak (Figure 4B). DLS indicated formation of large clusters, which were about 6 times the size of NP-1 (Figure 4C). Under TEM, the NPs exhibited a well-defined assembly pattern, where nearly all 20 nm NPs (NP-2) were fully surrounded by 7 to 8 of the 10 nm NPs (NP-1) with 5.0 ± 1.0 nm spacing (Figure 4A). This spacing is approximately half the interparticle distance between single type of NPs (11.7 ± 1.0 nm), which suggests that NP-1 and NP-2 are assembled by interdigitation of CMPs. Since TEM is recorded under dry condition which intensifies NP aggregation, it does not reflect the true aggregation behavior of NPs in solution. However, in this case where the interparticle distance is directly related to the length of the CMP, it is reasonable to infer the interparticle distance in solution from the TEM, particularly knowing that there is only about 1.3% length difference in collagen triple helix between dry and wet conditions.35
Figure 4.
TEM, DLS and UV–vis spectra of 10 nm Au–C(GPO)9 (NP-1) and (GPO)9C–Au 20 nm NPs (NP-2) assembly. (A) Structured aggregates formed by mixing NP-1 and NP-2. (B) UV–vis spectra of CMP conjugated gold NP assembly. The black line shows the spectrum of NP-1 and NP-2 assembly. The gray line shows the mixture of 10 nm Au–CG9P9O9 (scramble sequence) (NP-3) and NP-2. (C) DLS of NP-1 and NP-2 assembly. (D) Mixture of NP-3 and NP-2. (E) NP assembly of NP-1 and NP-2 after the addition of 500 μM of single strand (GPO)9. The bottom panels in D and E are the corresponding DLS traces with the numbers indicating the average size of the NPs in solution.
The assembly of NP-1 and NP-2 into large clusters is a result of two key characteristics of CMPs as they are attached to the NP surfaces. First, the CMPs conjugated on NP surfaces do not readily form stable triple helix among the neighboring CMPs. Anchoring the ends of the peptide chains to the surface of the NPs without a flexible linker appears to prohibit the single amino acid axial shift between CMP chains, which is needed for the triple-helical folding.36 Therefore, the triple helix of full length CMP cannot be assembled on the NP surface, although it is possible that a shorter triple helix of much lower stability could be assembled. Inability to form a stable triple helix for CMPs anchored on NPs was discussed in our previous paper.25 Second, the CMPs can trimerize only when they are in a parallel orientation. CMPs on CMP-NPs repel each other when two identical particles come in contact, since CMPs are in an antiparallel orientation. Only when two different NPs, each with opposite CMP polarity (as is the case with NP-1 and NP-2), come in contact, the CMPs are parallel and therefore capable of folding into triple helices. When the 10 nm NPs conjugated with the scrambled sequence (NP-3) were mixed with NP-2, there was neither a spectral shift (Figure 4B) nor the formation of particle assembly (Figure 4D). These results support the process of triple helical folding as the main mechanism of NP-1 and NP-2 assembly.
We questioned if the assembly between NP-1 and NP-2 can be disrupted by addition of free CMPs. Such disruption would lead to a change in color which can be exploited for detection of collagen fragments associated with arthritis and osteoporosis.37 When CMPs with the sequence of (GPO)9, preheated to 80 °C, were added to the solution mixture containing assemblies of NP-1 and NP-2, as can be seen in Figure 4E, the large-scale assemblies broke down into small clusters. Although, the well-defined flower-like structures were not seen, the formation of small extended aggregates was reminiscent of the larger particles. We expected that the free (GPO)9 were able to disrupt NP-1/NP-2 assembly by invading the triple helical connection by strand exchange; however, this did not completely eliminate the large-scale assembly, possibly because the particles are held tightly together by strong (GPO)9 folding. Preincubating the NP-1 and NP-2 with free (GPO)9 separately, prior to mixing, resulted in complete separation and random dispersion of NP-1 and NP-2, as evidenced by DLS and TEM (Figure S2).
We hypothesized that the CMP’s structural orientation on the NP surface could have an effect on the assembly behavior of the NPs. Specifically, we thought that when CMP is conjugated to the NP surface via Cys which is close to the middle of the peptide sequence, the CMP’s orientation could be more parallel to the NP surface (compared to end anchored CMP-NP) and that it would result in formation of a less dense CMP layer. The less dense CMP layer would lead to a more favorable particle assembly because the two CMP layers can penetrate each other more readily. To test this idea, we synthesized (GPO)2GCO-(GPO)6 (4, Table 1), which has Cys at the 1/3 position of the CMP. Due to the shorter consecutive GPO length, the melting temperature of 4 was 55 °C, which is approximately 20 °C below that of the C(GPO)9 (Figure S3). In contrast to NP-1 and NP-2, the 10 nm NPs conjugated with 4 (NP-4) had a propensity to self-assemble into large aggregates with a particle–particle distance of 3.2 ± 0.6 nm (Figure 5A). The 20 nm NPs conjugated with 4 exhibited the same behavior (Figure S4).
Figure 5.
TEM and corresponding DLS of NP systems comprised of 10 nm NP conjugated with (GPO)2GCO(GPO)6 (NP-4) and 20 nm NP conjugated with C(GPO)9. (A) TEM and DLS of NP-4 self-assembled into clusters (circled). (B) Mixing NP-4 and 20 nm Au–C(GPO)9 produced large aggregates. (C) Mixing of NP-4 and 20 nm Au–CG9P9O9 (scrambled CMP sequence) produced no aggregate. (D) Aggregates in (B) are readily disrupted by addition of 500 μM of single strand (GPO)9. (E) Schematic of NP-4 self-assembly. (F) Schematic of NP-4 and 20 nm Au–C(GPO)9 assembly. Each scale bar represents 50 nm.
We believe that the NP-4s self-assemble because the peptide layer is less dense than the NP-1 and NP-2 as we had expected. (Figure 5E). We also believe that the peptides are unorganized and are in random direction on the NP surface, all of which favor the peptide interactions and aggregation of the same type of NPs by triple helical association of parallely orientated CMPs, particularly via the (GPO)6 portion of the peptides.29,38 We determined the number of peptides on NP surface using a titration method (Figure S5):13 NP-4 had 371 ± 8 of peptides, while the same size 10 nm NP-1 had 517 ± 38 peptides per NP. These results indicate that the individual peptides on NP-4 occupy a larger surface area, which results in significant reduction in the number of peptides immobilized on the surface as compared to that of the NP-1. The less dense peptide layer with more open area seems to offer favorable conditions for interparticle interactions. When NP-4 was mixed with 20 nm Au–C(GPO)9 at a molar ratio of 10:1 (small vs large particles), large aggregates were formed as the NP-4s were able to self-assemble, and also assemble with the 20 nm Au–C(GPO)9 (Figure 5B and 5F). No aggregation was observed when the 20 nm NP conjugated with the scrambled peptide sequence (3) was mixed with NP-4 (Figure 5C). These results indicate that the triple helical folding is dictating the aggregation behavior of the two particles in a manner similar to NP-1/NP-2 assembly. In contrast to NP-1/NP-2 assembly, however, addition of excess CMPs directly to the aggregates turned large aggregates into smaller clusters (Figure 5D). Among the combinations of NPs tested, this produced the greatest change in the size of the aggregates, which we believe to be the result of nonoptimal packing of the CMPs, allowing free CMPs to readily exchange with the CMPs on the NP surface and disrupt particle aggregation. We expect that these NP systems, which can be induced to change their aggregation state by triple helical hybridization, could potentially be used for detecting collagen fragments commonly found in diseases associated with high extracellular matrix degradation, such as arthritis and osteoporosis.37
3.4. CMP-NPs binding to denatured type I collagens
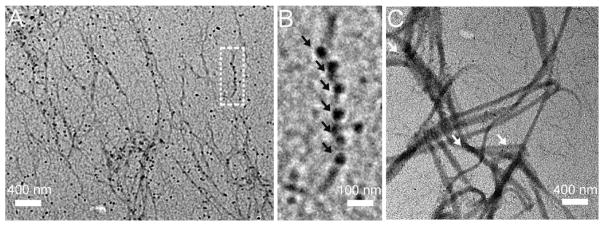
Since CMP has been reported to bind to denatured collagens by forming a triple-helical hybrid with the denatured collagen strands,16,19,20,28,29 we anticipated that CMPs conjugated to gold NPs could also target denatured collagen with high specificity. To investigate this, a denatured type I collagen solution was prepared by heating the solution to 80 °C for 5 min, followed by treatment with NP-1. TEM showed that NP-1 bound to denatured collagen fibers (Figure 6A and B) in high density, whereas, low nonspecific binding was observed for intact collagen fibers treated with NP-1 (Figure 6C). CMP-NPs were previously used to image unstable domains of the collagen triple helix in intact collagen fibers;25 however, this is the first time that its specific affinity to fully denatured collagen strands is presented. The results indicate that CMP-NPs can specifically bind to denatured collagen strands, which can be exploited for detection or even isolation of gelatin from protein mixtures.
Figure 6.
TEM of CMP-NPs binding to denatured type I collagens. (A) Binding of NP-1 to denatured collagen. (B) Magnification of the selected area in (A), black arrows indicate NPs binding along the denatured collagen fibers. (C) Binding of NP-1 to intact type I collagen fibrils. White arrows indicate a few NPs nonspecifically attached to the intact collagen fibrils.
3.5. Gelatin Binding and Removal Mediated by CMP-NPs
In addition to applications in medical device coatings, gelatin is widely used as stabilizers for protein-based pharmaceuticals,39 such as vaccines, antibodies, and therapeutic proteins.39,40 Gelatin in these formulations can interfere with further chemical modification, for example, in antibody conjugation chemistry. Moreover, gelatin-containing vaccines have been found to cause severe, although rare reactions, especially in vaccinating children.41 For these reasons, we investigated the possibility of using CMP-NPs for selective removal of gelatin from protein mixtures. We conjugated C(GPO)9 to a large 100 nm gold NP (designed as CMP-NP100) which can be removed by low speed (2300 rcf) centrifugation, and investigated its specific binding to gelatin as well as its potential to remove gelatin from a protein mixture. The average number of peptides immobilized on the NP100 surface was 52 818 (Figure S5), corresponding to an average surface area of 59 Å2 for a single strand CMP which is comparable to NP-1 (61 Å2) and NP-2 (62 Å2). TEM revealed that CMP-NP100 bound to gelatin fiber networks with high affinity (Figure 7A). Therefore, we prepared a pull-down assay using fluorescently labeled gelatin (Figure 7B). The results showed that approximately 90% of the gelatin in solution was removed by CMP-NP100 (5 pM), while the same NP conjugated with a scrambled sequence was ineffective at gelatin removal with 90% of gelatin remaining in the solution (Figure 7B). Addition of excess amounts of free single strand (GPO)9 to the CMP-NP100 solution prior to mixing with gelatin solution drastically reduced the gelatin removal capacity, to less than 40% (Figure 7B). These results indicate that gelatin can be isolated from solution by the triple helical hybridization between gelatin strands and CMPs displayed on the NP surface.
Figure 7.
Gelatin binding and removal mediated by CMP-NPs. (A) TEM of 100 nm gold NP conjugated with C(GPO)9 (CMP-NP100) bound to gelatin. Scale bar represents 200 nm. (B) Gelatin removal assay. The percentage of gelatin removal from CMP-NP and gelatin solution [CMP-NP100 (5 pM) + gelatin (25 μg/mL)] was determined by the gelatin remaining in the solution compared with the control solution after centrifugation. A FITC-labeled gelatin was used for quantifying the gelatin remaining in solution. (C) UV–vis spectra of the CMP-NP100 (5 pM) solution (black line), the same solution mixed with 50 μg/mL protein mixture (dark gray line) or with 50 μg/mL gelatin (gray line), and 100 nm gold NP conjugated with scrambled sequence CG9P9O9 (5 pM) mixed with 50 μg/mL gelatin (dashed line). Protein mixture (PM): conalbumin (75 kDa), bovine albumin serum (BSA, 66 kDa), carbonic anhydrase (29 kDa), ribonuclease A (13.7 kDa). Each protein concentration was 50 μg/mL. Inset shows the compressed spectrum of CMP-NP100 with 50 μg/mL gelatin (gray line). (D) Shift in SPR peak maximum for CMP-NP100 mixed with varying concentrations of gelatin. The same solution mixed with 50 mg/mL BSA was used as a baseline spectrum. The inset shows the zoom-in data points from 0.0005 μg/mL to 0.5 μg/mL of gelatin. (E) SDS-PAGE for the study of CMP-NP100 mediated gelatin removal from a solution mixture. Mixture solution contained CMP-NP100 (5 pM), protein mixture (25 μg/mL of each protein), and gelatin (25 μg/mL). Lane 1: molecular weight standards; Lane 2: protein mixture (25 μg/mL of each protein) + gelatin (25 μg/mL); Lane 3: supernatant of mixture solution after centrifugation; Lane 4: pellet of mixture solution after centrifugation.
To demonstrate gelatin binding specificity, we prepared a protein solution composed of several common proteins [i.e., conalbumin, bovine serum albumin (BSA), carbonic anhydrase, and rebonuclease A], into which CMP-NP100 were added and incubated. Even in the presence of a protein mixture, the surface plasmon resonance (SPR) of the CMP-NP100 remained nearly identical to the control NPs in a blank solution (Figure 7C). In contrast, a significant red-shift (from 578 to 700 nm) was observed in the SPR signal for NPs added to the solution containing the same amount of gelatin. There was no change in SPR signal when NPs conjugated with the scrambled sequence were used (Figure 7C) under identical conditions. SPR is one of the most sensitive techniques available to detect molecular binding in solution. We were able to detect as low as 0.5 ng/mL of gelatin (Figure 7D and Figure S6).42 The fact that there was almost no change in SPR signal in the common protein solutions but there was over a 100 nm shift in the gelatin solution attests to the NP’s high binding specificity for denatured collagen as well as the potential for sensing collagen denaturation.
CMP-NP100 was incubated with the protein mixture solution containing gelatin, and a pull-down assay was performed to remove the CMP-NP100 by centrifugation. Only gelatin was observed in the pellet, together with the NPs, while all other proteins remained in the supernatant, as evidenced by SDS-PAGE (Figure 7E). Similar results were obtained when the same experiment was performed with a mouse serum (Figure S7). The results show that the CMP conjugated gold NPs can selectively bind to gelatin with little background binding to other proteins, and that the NPs can be used to selectively remove gelatin from protein mixtures. Although CMP-NPs were able to detect as low as 0.5 ng/mL of gelatin using SPR method (Figure 7D), the gelatin removal capacity via gelatin binding and centrifugation of CMP-NP was determined to be 20 μg/mL of gelatin removal per 1 pM of CMP-NP100. Vaccine and antibody formulations typically contain milligrams of gelatin. Therefore, gelatin removal capacity of current system is too low for practical use; however, with the employment of other purification methods, such as affinity column chromatography, we believe that the gelatin removal capacity can be improved significantly.
4. CONCLUSION
CMP conjugated gold NPs can assemble into large aggregates when CMPs displayed on different NPs can combine in a parallel fashion to form triple helices. This NP assembly can be disrupted by addition of free CMPs via strand exchange reaction, suggesting that the resulting shift in UV–vis signal can be used to detect the presence of CMPs and possibly other degradation products derived from collagen. CMP is rigid, highly resistant to degradation, and has little affinity to other biomolecules. Similar to DNA-based NP assembly explored by numerous research groups, we see great potential in CMP-NPs in establishing stable NP assembly with precisely designed structure and interparticle distance.
We demonstrated that, similar to CMP itself, the CMP-conjugated NPs can bind specifically to denatured collagen strands. Previously, we reported that the CMP-NPs are able to bind to periodic positions on intact type I collagen fibrils.25 We speculated that this periodic binding occurs at locations of partially unfolded domains in intact collagen molecules, because it occurred at a relatively narrow temperature window, and also because of the disappearance of periodicity at higher binding temperature.43 In this work, instead of elevating the NP binding temperature, collagen fibers were heated to induce permanent protein denaturation. As we had expected, the denatured collagen attracted high number of CMP-NPs while only low levels of nonspecifically bound NPs were seen on intact collagen fibers. In addition, by employing a large size CMP-NP, gelatin could be isolated from protein mixtures by centrifugation. What is most remarkable about the CMP-NP is its inertness toward nonspecific binding. The particle is highly stable in water and exhibits almost no affinity to common proteins such as albumin, carbonic anhydrase, and RNase. Such low nonspecific binding and selective gelatin affinity is mainly due to the unique binding interaction of CMP-NPs which are mediated by formation of triple helical supersecondary protein structures involving hydrogen bonding of the protein backbones. The same binding mechanism will work for other types of collagen, such as type II and IV, as we have previously demonstrated.20 Since gelatin is commonly used as a stabilizer/additive for protein reagents and therapeutics, the CMP-NPs could be used for removal of gelatin from such formulations to minimize known side effects of gelatin.
Acknowledgments
This work was supported by grants from NIAMS/NIH (R01-AR060484 and R21-AR065124) and DOD (W81XWH-12-1-0555) awarded to S.M.Y.
Footnotes
Notes
The authors declare the following competing financial interest(s): Drs. Yu and Li are founders of 3Helix which commercializes collagen hybridizing peptide.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.6b05707.
Additional TEM and DLS data of CMP-NP assemblies; CD melting curves of CMPs; SDS-PAGE of CMP-NP100 mediated gelatin removal from a mouse serum; and methods for determining the number of CMPs on NP surfaces. (PDF)
References
- 1.Busseron E, Ruff Y, Moulin E, Giuseppone N. Supramolecular Self-Assemblies as Functional Nanomaterials. Nanoscale. 2013;5:7098–7140. doi: 10.1039/c3nr02176a. [DOI] [PubMed] [Google Scholar]
- 2.Nie Z, Petukhova A, Kumacheva E. Properties and Emerging Applications of Self-Assembled Structures Made from Inorganic Nanoparticles. Nat Nanotechnol. 2010;5:15–25. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]
- 3.Daniel MC, Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 4.San BH, Kim S, Moh SH, Lee H, Jung DY, Kim KK. Platinum Nanoparticles Encapsulated by Aminopeptidase: A Multi-functional Bioinorganic Nanohybrid Catalyst. Angew Chem, Int Ed. 2011;50:11924–11929. doi: 10.1002/anie.201101833. [DOI] [PubMed] [Google Scholar]
- 5.San BH, Ha EJ, Paik H-j, Kim KK. Radiofrequency Treatment Enhances the Catalytic Function of an Immobilized Nanobiohybrid Catalyst. Nanoscale. 2014;6:6009–6017. doi: 10.1039/c4nr00407h. [DOI] [PubMed] [Google Scholar]
- 6.San BH, Kim JA, Kulkarni A, Moh SH, Dugasani SR, Subramani VK, Thorat ND, Lee HH, Park SH, Kim T, Kim KK. Combining Protein-Shelled Platinum Nanoparticles with Graphene to Build a Bionanohybrid Capacitor. ACS Nano. 2014;8:12120–12129. doi: 10.1021/nn503178t. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari M. Cancer Nanotechnology: Opportunities and Challenges. Nat Rev Cancer. 2005;5:161–171. doi: 10.1038/nrc1566. [DOI] [PubMed] [Google Scholar]
- 8.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of Drugs and Nanoparticles to Tumors. J Cell Biol. 2010;188:759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young KL, Scott AW, Hao L, Mirkin SE, Liu G, Mirkin CA. Hollow Spherical Nucleic Acids for Intracellular Gene Regulation Based Upon Biocompatible Silica Shells. Nano Lett. 2012;12:3867–3871. doi: 10.1021/nl3020846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams SC. Spherical Nucleic Acids: A Whole New Ball Game. Proc Natl Acad Sci U S A. 2013;110:13231–13233. doi: 10.1073/pnas.1313483110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler JI, Auyeung E, Mirkin CA. Spherical Nucleic Acids. J Am Chem Soc. 2012;134:1376–1391. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 12.Delehanty JB, Boeneman K, Bradburne CE, Robertson K, Bongard JE, Medintz IL. Peptides for Specific Intracellular Delivery and Targeting of Nanoparticles: Implications for Developing Nanoparticle-Mediated Drug Delivery. Ther Delivery. 2010;1:411–433. doi: 10.4155/tde.10.27. [DOI] [PubMed] [Google Scholar]
- 13.Levy R, Thanh NT, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, Brust M, Fernig DG. Rational and Combinatorial Design of Peptide Capping Ligands for Gold Nanoparticles. J Am Chem Soc. 2004;126:10076–10084. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- 14.Chen CL, Rosi NL. Peptide-Based Methods for the Preparation of Nanostructured Inorganic Materials. Angew Chem, Int Ed. 2010;49:1924–1942. doi: 10.1002/anie.200903572. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F, Nangreave J, Liu Y, Yan H. Structural DNA Nanotechnology: State of the Art and Future Perspective. J Am Chem Soc. 2014;136:11198–11211. doi: 10.1021/ja505101a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Yu SM. Targeting and Mimicking Collagens Via Triple Helical Peptide Assembly. Curr Opin Chem Biol. 2013;17:968–975. doi: 10.1016/j.cbpa.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aili D, Stevens MM. Bioresponsive Peptide-Inorganic Hybrid Nanomaterials. Chem Soc Rev. 2010;39:3358–3370. doi: 10.1039/b919461b. [DOI] [PubMed] [Google Scholar]
- 18.Chou LYT, Zagorovsky K, Chan WCW. DNA Assembly of Nanoparticle Superstructures for Controlled Biological Delivery and Elimination. Nat Nanotechnol. 2014;9:148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting Collagen Strands by Photo-Triggered Triple-Helix Hybridization. Proc Natl Acad Sci U S A. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Ho D, Meng H, Chan TR, An B, Yu H, Brodsky B, Jun AS, Michael Yu S. Direct Detection of Collagenous Proteins by Fluorescently Labeled Collagen Mimetic Peptides. Bioconjugate Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima C, Tsumura S, Harada A, Kono K. A Collagen-Mimic Dendrimer Capable of Controlled Release. J Am Chem Soc. 2009;131:6052–6053. doi: 10.1021/ja809639c. [DOI] [PubMed] [Google Scholar]
- 22.Cejas MA, Kinney WA, Chen C, Vinter JG, Almond HR, Balss KM, Maryanoff CA, Schmidt U, Breslav M, Mahan A, Lacy E, Maryanoff BE. Thrombogenic Collagen-Mimetic Peptides: Self-Assembly of Triple Helix-Based Fibrils Driven by Hydrophobic Interactions. Proc Natl Acad Sci U S A. 2008;105:8513–8518. doi: 10.1073/pnas.0800291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung WJ, Kwon KY, Song J, Lee SW. Evolutionary Screening of Collagen-Like Peptides That Nucleate Hydroxyapatite Crystals. Langmuir. 2011;27:7620–7628. doi: 10.1021/la104757g. [DOI] [PubMed] [Google Scholar]
- 24.Jin HE, Jang J, Chung J, Lee HJ, Wang E, Lee SW, Chung WJ. Biomimetic Self-Templated Hierarchical Structures of Collagen-Like Peptide Amphiphiles. Nano Lett. 2015;15:7138–7145. doi: 10.1021/acs.nanolett.5b03313. [DOI] [PubMed] [Google Scholar]
- 25.Mo X, An Y, Yun CS, Yu SM. Nanoparticle-Assisted Visualization of Binding Interactions between Collagen Mimetic Peptide and Collagen Fibers. Angew Chem, Int Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 26.Yu SM, Li Y, Kim D. Collagen Mimetic Peptides: Progress Towards Functional Applications. Soft Matter. 2011;7:7927–7938. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engel J, Bachinger HP. Structure, Stability and Folding of the Collagen Triple Helix. Top Curr Chem. 2005;247:7–33. [Google Scholar]
- 28.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Spatio-Temporal Modification of Collagen Scaffolds Mediated by Triple Helical Propensity. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang AY, Mo X, Chen CS, Yu SM. Facile Modification of Collagen Directed by Collagen Mimetic Peptides. J Am Chem Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, San BH, Kessler JL, Kim JH, Xu Q, Hanes J, Yu SM. Non-Covalent Photo-Patterning of Gelatin Matrices Using Caged Collagen Mimetic Peptides. Macromol Biosci. 2015;15:52–62. doi: 10.1002/mabi.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wahyudi H, Reynolds AA, Li Y, Owen SC, Yu SM. Targeting Collagen for Diagnostic Imaging and Therapeutic Delivery. J Controlled Release. 2016 doi: 10.1016/j.jconrel.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuno-Sakai H, Kimura M. Removal of Gelatin from Live Vaccines and Dtap—an Ultimate Solution for Vaccine-Related Gelatin Allergy. Biologicals. 2003;31:245–249. doi: 10.1016/s1045-1056(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 33.Gauba V, Hartgerink JD. Self-Assembled Heterotrimeric Collagen Triple Helices Directed through Electrostatic Interactions. J Am Chem Soc. 2007;129:2683–2690. doi: 10.1021/ja0683640. [DOI] [PubMed] [Google Scholar]
- 34.Dulkeith E, Morteani AC, Niedereichholz T, Klar TA, Feldmann J, Levi SA, van Veggel FCJM, Reinhoudt DN, Möller M, Gittins DI. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys Rev Lett. 2002;89:203002. doi: 10.1103/PhysRevLett.89.203002. [DOI] [PubMed] [Google Scholar]
- 35.Masic A, Bertinetti L, Schuetz R, Chang SW, Metzger TH, Buehler MJ, Fratzl P. Osmotic Pressure Induced Tensile Forces in Tendon Collagen. Nat Commun. 2015;6:5942. doi: 10.1038/ncomms6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bella J, Eaton M, Brodsky B, Berman HM. Crystal and Molecular Structure of A Collagen-like Peptide at 1. 9 A Resolution. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]
- 37.Myllyharju J, Kivirikko KI. Collagens and Collagen-Related Diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Mo X, Kim D, Yu SM. Template-Tethered Collagen Mimetic Peptides for Studying Heterotrimeric Triple-Helical Interactions. Biopolymers. 2011;95:94–104. doi: 10.1002/bip.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandau DT, Jones LS, Wiethoff CM, Rexroad J, Middaugh CR. Thermal Stability of Vaccines. J Pharm Sci. 2003;92:218–231. doi: 10.1002/jps.10296. [DOI] [PubMed] [Google Scholar]
- 40.Thyagarajapuram N, Olsen D, Middaugh CR. Stabilization of Proteins by Recombinant Human Gelatins. J Pharm Sci. 2007;96:3304–3315. doi: 10.1002/jps.20980. [DOI] [PubMed] [Google Scholar]
- 41.Kelso JM, Jones RT, Yunginger JW. Anaphylaxis to Measles, Mumps, and Rubella Vaccine Mediated by IgE to Gelatin. J Allergy Clin Immunol. 1993;91:867–872. doi: 10.1016/0091-6749(93)90344-f. [DOI] [PubMed] [Google Scholar]
- 42.Vance SA, Sandros MG. Zeptomole Detection of C-Reactive Protein in Serum by a Nanoparticle Amplified Surface Plasmon Resonance Imaging Aptasensor. Sci Rep. 2014;4:5129. doi: 10.1038/srep05129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mo X The Johns Hopkins, U. PhD Thesis. Johns Hopkins University; Maryland, U.S.A: 2008. Design and Synthesis of Collagen Mimetic Peptide Derivatives for Studying Triple Helix Assembly and Collagen Mimetic Peptide-Collagen Binding Interaction. [Google Scholar]