Abstract
Purpose
The effectiveness of platelet-rich plasma (PRP), thymoquinone, and zeolite in corrosive esophageal burns was investigated in a rat model.
Methods
Four groups were comprised as containing 10 rats in each group. For group I, oesophagitis was induced and no other procedure was performed (control group). For group II, oesophagitis was induced and thymoquinone was administered for 1 week via oral gavage once a day (thymoquinone group). For group III, oesophagitis was induced for 1 week via oral gavage once a day (PRP group). For group IV, oesophagitis was induced and zeolite was administered for 1 week via oral gavage once a day (zeolite group). On the 10th day, the rats were sacrificed under anaesthesia and venous blood sampling was performed from the vena portae. The oesophaguses were totally excised. Biochemically, interleukin (IL)-1B, IL-6, TNF-α, and MCP-1 were examined from venous blood. Inflammation score was evaluated histopathologically in oesophageal tissue that was collected.
Results
There was a statistically significant difference among groups in terms of IL-1, IL-6, MCP levels, compared to the control group; median IL-1, IL-6, MCP levels of thymoquinone, PRP, and zeolite groups were statistically significantly lower. There was a statistically significant difference among groups in terms of inflammation scores, compared to group I; median inflammation scores of groups II, III and IV were statistically significantly lower thymoquinone.
Conclusion
PRP, and zeolite exhibited positive effect on recovery in oesophagitis by reducing inflammation in the involved segment.
Keywords: Thymoquinone, Platelet-rich plasma, Zeolite, Oesophagitis, Inflammation
INTRODUCTION
The burns developed in oesophagus due to swallowing strong acidic or alkaline substances are called corrosive oesophageal burn and the inflammation developed as a result of these is called corrosive oesophagitis. Ingestion of strong corrosive substances, particularly alkaline substances, may cause acute perforation and death. Corrosive oesophageal stenosis in cases that survive the acute phase is a severe problem.
The most common substances that cause corrosive oesophagitis lesions are sodium hydroxide, potassium carbonate, calcium oxide, and sodium peroxide, which have alkaline properties [1]. Alkaline agents cause injuries that may harm all layers of the organ wall throughout the oesophagus [2]. Strong alkaline substances produce a chemical burn by leading to cellular dehydration and coagulation of collagen and other cellular proteins following their contact with living tissues.
The most commonly injured organ due to caustic ingestion is the oesophagus [3]. Severity of lesions is related to duration of contact with oesophageal mucosa as well as volume and concentration of the substance that is taken.
Classification of corrosive oesophagitis: in first-degree burn, the lesion is superficial and there is hyperaemia and oedema in the mucosa. In second-degree burns, there is an infiltration into the oesophageal wall, bullae, ulceration, exudation, and mucosal loss and damage extending to the muscular layer. In third-degree burns, there is erosion involving all layers of the oesophagus and perforation [4].
During the clinical course of oesophageal burns the acute phase involves an initial 2-week period. The most significant complication is chemical laryngotracheobronchitis, which is caused by aspiration of a corrosive substance and occurs within the initial hours. In cases with burns in the larynx, respiratory distress may develop. Oedema and oesophageal spasm, which excels within initial 48 hours, is another common complication [5].
Platelet-rich plasma (PRP) is an autologous part of plasma that contains 3–5 fold more platelets than basal plasma value. Platelets secrete growth factors such as insulin-like growth factor, TGF-β, platelet-derived growth factor, and vascular endothelial growth factor. PRP and its products are components that have begun to be used in clinical practice due to their promoting effects on wound healing, cellular mitogenesis, osteogenesis and angiogenesis [6,7,8].
Zeolites are aluminosilicate minerals. They exist in nature most commonly in clinoptilolite form. The major property of clinoptilolite is to have the ability of ion exchange. It also has the ability to absorb and catalyze. By means of its light and sponge-like structure, which is formed of small crystals, it is frequently used in agriculture, stockbreeding, contamination control, mining and metallurgy. In medicine, it is used most commonly in prevention of toxicity of aflatoxin, diarrhoea, various malignant tumours and treatment of immune system diseases [9,10,11].
Thymoquinone (TQ) is a plant extract whose antioxidant, anti-inflammatory, antiviral, antihelminthic, antibacterial, gastroprotective, hepatoprotective, antimalarial, antitumour, antifungal and antidiabetic properties have been demonstrated in studies conducted. In conducted studies [12,13,14,15,16], TQ is expressed as a potent inhibitor of thromboxane B2 and leucotriene B4. TQ inhibits metabolism of arachidonic acid in peritoneal leucocytes via cyclooxygenase (COX) and 5-lipooxygenase pathways [12,13,14,15,16].
In the literature, various substances have been used in order to promote recovery in caustic oesophagitis (heparin [17], vitamin E [18], caffeic acid phenethyl ester [19], F mitomycin C [20], etc.) We examined the effectiveness of TQ, zeolite, and PRP whose effectiveness in oesophagitis has not been investigated.
METHODS
This study was conducted in an experimental animal laboratory by courtesy of a university ethical committee of experimental animals (30.05.2014/69). In the study, 45 Wistar Albino rats weighing 220–240 g were used. Rats were kept under a temperature of 20℃–25℃ and 40% humidity and left under sunlight for 12 hours daily. Rats were fed with pelletized animal feed of 2700 ME kcal/kg. Water was freely available to the rats.
PRP preparation
For PRP preparation, five female Wistar Albino rats weighing 220–240 g were used. Cardiac blood from rats narcotised under anaesthesia was taken into 3.2% sodium citrate (Merck, Darmstadt, Germany) tubes with blood/citrate ratio of 9/1. Collected blood was centrifuged for 10 minutes at 400 × g. The upper portion was further centrifuged for 10 minutes at 800 × g. The upper 2/3 portion was thrown away. The lower 1/3 portion was considered to be PRP [21]. PRP was kept in a freezer under –20℃ until it was used.
Study design
Four groups comprised of 10 rats each. Five rats were used in obtaining PRP.
Anaesthesia was achieved with 90 mg/kg intraperitoneal ketamine (Ketalar, 500 mg/10 mL Pfizer, Berlin, Germany) and 10 mg/kg xylazine (Rompun, Bayer, Leverkusen, Germany). For all groups, in order to induce oesophagitis, a midline incision was performed on the day of the procedure, after that the abdominal oesophagus was clamped, an NaOH of 37.5% was administered through a feeding catheter and aspirated after 90 seconds, then the oesophagus was soaked with physiological saline.
For the 1st group, oesophagitis was induced and no other procedure was performed (control group). For the 2nd group, oesophagitis was induced and TQ was administered within 0.5 mL of physiological saline at a dosage of 100 mg/kg for 1 week via oral gavage once a day (TQ group). For the 3rd group, oesophagitis was induced and 0.5 mL of PRP was administered for 1 week via oral gavage once a day (PRP group). For the 4th group, oesophagitis was induced and zeolite was administered within 0.5 mL of physiological saline at a dosage of 5 mg/kg for 1 week via oral gavage once a day (zeolite group).
On the 10th day, rats were sacrificed under anaesthesia and venous blood sampling was performed from the vena portae. The oesophagus was totally excised. Biochemically, IL-1B, IL-6, TNF-α, and MCP-1 were examined from venous blood. Inflammation score was evaluated histopathologically in the oesophageal tissue that was collected.
Histopathological examination
Distal oesophageal segments of all rats were reserved for histopathological examination. Examination was performed blindly by 1 pathologist. Slides were stained with haematoxylineosine with 5-mm slices. Inflammation was defined as infiltration of neutrophils and eosinophils into oesophageal tissue. Stricture was defined as an increased amount of collagen in subepithelial tissue. Classification was as follows: Inflammation score 0 was normal tissue, Inflammation score 1 was presence of inflammation and stricture in mucosa and submucosa, Inflammation score 2 was presence of inflammation and stricture in tunica muscularis, Inflammation score 3 was presence of inflammation and stricture in all 3 layers, and Inflammation score 4 was presence of necrosis [22].
Statistical analysis
Whether distribution of continuous numeric variables was close to normal was evaluated by using Shapiro-Wilk test and homogeneity of variances was evaluated by using Levene test. Descriptive statistics were demonstrated as median (interquartile range).
Significance of the difference among groups in terms of IL-1, TNF, IL-6, MCP, and inflammation scores was evaluated by using Kruskal-Wallis test. In determining the results of the Kruskal-Wallis test as significant, condition(s) leading to a difference was/were determined by using Conover test of multiple comparisons.
Analysis of data was performed on SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) package program. For P < 0.05 results were considered to be statistically significant.
RESULTS
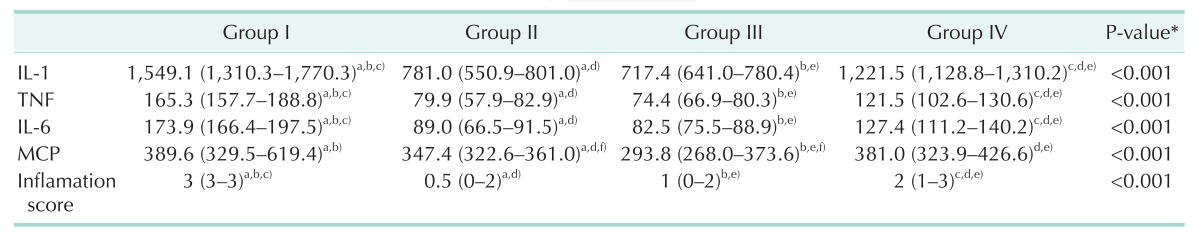
There was a statistically significant difference among groups in terms of IL-1 levels (P < 0.001), compared to group I; median IL-1 levels of groups II, III, and IV were statistically significantly lower (P < 0.001, P < 0.001, and P = 0.007, respectively). Additionally, compared to groups II and III, median IL-1 level of group IV was statistically significantly higher (P < 0.001 and P < 0.001, respectively). There was no statistically significant difference between groups II and III in terms of IL-1 levels (P = 0.248) (Table 1).
Table 1. IL-1, TNF, IL-6, MCP and the inflammation scores of the groups.
Values are presented as median (intrequartile range).
Group I, control; group II, thymoquinone; group III, platelet-rich plasma; group IV, Zeolite.
*Kruskal-Wallis test, a)The difference between group I and group II was statistically significant (P < 0.01), b)The difference between group I and group III was statistically significant (P < 0.001), c)The difference between group I and group IV was statistically significant (P < 0.01), d)The difference between group II and group IV was statistically significant (P < 0.05), e)The difference between group III and group IV was statistically significant (P < 0.05), f)The difference between group II and group III was statistically significant (P = 0.005).
There was a statistically significant difference among groups in terms of TNF levels (P < 0.001), compared to group I; median TNF levels of groups II, III, and IV were statistically significantly lower (P < 0.001, P < 0.001, and P = 0.007, respectively). Additionally, compared to groups II and III, median TNF level of group IV was statistically significantly higher (P < 0.001 and P < 0.001, respectively). There was no statistically significant difference between groups II and III in terms of TNF levels (P = 0.248) (Table 1).
There was a statistically significant difference among groups in terms of IL-6 levels (P < 0.001), compared to group I; median IL-6 levels of groups II, III, and IV were statistically significantly lower (P < 0.001, P < 0.001, and P = 0.007, respectively). Additionally, compared to groups II and III, median IL-6 level of group IV was statistically significantly higher (P < 0.001 and P < 0.001, respectively). There was no statistically significant difference between groups II and III in terms of IL-6 levels (P = 0.327) (Table 1).
There was a statistically significant difference among groups in terms of MCP levels (P < 0.001), compared to group I; median MCP levels of groups II and III were statistically significantly lower (P = 0.005 and P < 0.001, respectively). Additionally, compared to group III, median MCP levels of group II and group IV were statistically significantly higher (P = 0.005 and P < 0.001, respectively). Compared to group II, median MCP level of group IV was statistically significantly higher (P < 0.001). There was no statistically significant difference between groups I and IV in terms of MCP levels (P = 0.560) (Table 1).
There was a statistically significant difference among groups in terms of inflammation scores (P < 0.001), compared to group I; median inflammation scores of groups II, III, and IV were statistically significantly lower (P < 0.001, P < 0.001 and P = 0.005, respectively). Additionally, compared to groups II and III, median inflammation score of group IV was statistically significantly higher (P < 0.001 and P = 0.035, respectively). There was no statistically significant difference between groups II and III in terms of inflammation scores (P = 0.092) (Table 1).
DISCUSSION
Caustic substance ingestion remains an important health problem that leads to high mortality and morbidity in society, particularly in children.
Up to today, the studies conducted, which were oriented to recovery of caustic oesophagitis, focused on the period prior to development of stricture. Various substances have been used in order to promote recovery in this period (heparin [17], F vitamin E [18], F caffeic acid phenethyl ester [19], F mitomycin C [20], etc.). However, unfortunately very few of them exhibited sufficient effectiveness and yet were begun to be used in clinical practice. Substances that are used may exhibit their effects topically or systemically. We prefer to administer this application topically via gavage.
In order to determine the severity of corrosive oesophagitis, various biochemical parameters have been used. However, no parameter that definitely indicates the severity of caustic oesophagitis has been determined. In our study, we examined IL-1, IL-6, TNF, and MCP levels as biochemical parameters and inflammation scores histopathologically in order to measure intensive inflammatory response, which occurs in the early phase.
Nowadays, we know that due to corrosive substance intake, acute inflammatory response increases within the first week and fibroblast proliferation and collagen formation, which participate in development of stricture, occur after the second week [23]. Therefore, treatments started in the early period play an important role in prevention of formation of stricture and other complications. In our study, following completion of the inflammatory period, rats on which oesophagitis models were applied were sacrificed on the 10th day. IL-1, IL-6, TNF-α, and MCP levels, which are inflammatory parameters, were examined. Parallelism was present among all inflammatory parameters, statistically significantly lower levels of inflammatory parameters in TQ, PRP, and zeolite groups compared to the control group indicate that medical treatment given in each group significantly reduce inflammation in induced oesophagitis models. When treatment groups were compared with each other; it was determined that levels of all inflammatory parameters that were examined in TQ and PRP groups were statistically significantly lower compared to those of the zeolite group; this indicates that the intense inflammatory response, which is the major pathogenesis in oesophagitis, is suppressed by medical treatment, particularly by use of TQ and PRP, and these substances contribute to treatment positively.
We considered, especially, the effects of substances that were used in our study on inflammatory cell infiltration and collagen deposition. Lower inflammatory scores, which were calculated with neutrophilic and eosinophilic infiltration in the involved oesophageal segment, especially in PRP and TQ groups, support the fact that anti-inflammatory properties of these substances influence recovery positively.
In a study conducted by Çevik et al., [24] with hyaluronic acid, they revealed the relationship of corrosive oesophageal burns with increased reactive oxygen radicals, free radicals, and reduced antioxidant capacity. Reactive oxygen radicals and free radicals are known to cause many pathological disorders [18,25,26]. Therefore, removal of these free radicals from the environment plays an important role in recovery [27].
In a previously conducted study, the antioxidant and anti-inflammatory properties of TQ, which is one of the substances used in our study, were revealed [28,29]. It was revealed in various studies that PRP, which is another substance used in our study, reduces oxidative injury and has antioxidant properties [30].
In our study, we observed that PRP and TQ groups have positive outcomes on corrosive oesophagitis both biochemically and histopathologically. We believe that this outcome results from both the anti-inflammatory properties and antioxidant properties of these substances.
In conclusion, TQ , PRP, and zeolite exhibited positive effects on recovery in oesophagitis by reducing inflammation in the involved segment. When the groups were examined against each other, it was determined that this effect was highest between TQ and PRP groups.
ACKNOWLEDGEMENTS
This paper supported by Zhejiang Provincial Natural Science Foundation of China (grant number: LY13H030005).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Rigo GP, Camellini L, Azzolini F, Guazzetti S, Bedogni G, Merighi A, et al. What is the utility of selected clinical and endoscopic parameters in predicting the risk of death after caustic ingestion? Endoscopy. 2002;34:304–310. doi: 10.1055/s-2002-23633. [DOI] [PubMed] [Google Scholar]
- 2.Haller JA, Jr, Andrews HG, White JJ, Tamer MA, Cleveland WW. Pathophysiology and management of acute corrosive burns of the esophagus: results of treatment in 285 children. J Pediatr Surg. 1971;6:578–584. doi: 10.1016/0022-3468(71)90382-4. [DOI] [PubMed] [Google Scholar]
- 3.Andreoni B, Farina ML, Biffi R, Crosta C. Esophageal perforation and caustic injury: emergency management of caustic ingestion. Dis Esophagus. 1997;10:95–100. doi: 10.1093/dote/10.2.95. [DOI] [PubMed] [Google Scholar]
- 4.Contardo C. Ingestion of corrosive substances. Rev Gastroenterol Peru. 1998;18:264–272. [PubMed] [Google Scholar]
- 5.Cardona JC, Daly JF. Corrosive esophagitis. Am J Surg. 1957;93:242–247. doi: 10.1016/0002-9610(57)90774-2. [DOI] [PubMed] [Google Scholar]
- 6.Rai B, Ho KH, Lei Y, Si-Hoe KM, Jeremy Teo CM, Yacob KB, et al. Polycaprolactone-20% tricalcium phosphate scaffolds in combination with platelet-rich plasma for the treatment of critical-sized defects of the mandible: a pilot study. J Oral Maxillofac Surg. 2007;65:2195–2205. doi: 10.1016/j.joms.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Dugrillon A, Eichler H, Kern S, Kluter H. Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg. 2002;31:615–619. doi: 10.1054/ijom.2002.0322. [DOI] [PubMed] [Google Scholar]
- 8.Kark LR, Karp JM, Davies JE. Platelet releasate increases the proliferation and migration of bone marrow-derived cells cultured under osteogenic conditions. Clin Oral Implants Res. 2006;17:321–327. doi: 10.1111/j.1600-0501.2005.01189.x. [DOI] [PubMed] [Google Scholar]
- 9.Elmore AR Cosmetic Ingredient Review Expert Panel. Final report on the safety assessment of aluminum silicate, calcium silicate, magnesium aluminum silicate, magnesium silicate, magnesium trisilicate, sodium magnesium silicate, zirconium silicate, attapulgite, bentonite, Fuller's earth, hectorite, kaolin, lithium magnesium silicate, lithium magnesium sodium silicate, montmorillonite, pyrophyllite, and zeolite. Int J Toxicol. 2003;22(Suppl 1):37–102. [PubMed] [Google Scholar]
- 10.Nikawa H, Yamamoto T, Hamada T, Rahardjo MB, Murata H, Nakanoda S. Antifungal effect of zeolite-incorporated tissue conditioner against Candida albicans growth and/or acid production. J Oral Rehabil. 1997;24:350–357. doi: 10.1046/j.1365-2842.1997.d01-297.x. [DOI] [PubMed] [Google Scholar]
- 11.Oguz H, Kurtoglu V, Coskun B. Preventive efficacy of clinoptilolite in broilers during chronic aflatoxin (50 and 100 ppb) exposure. Res Vet Sci. 2000;69:197–201. doi: 10.1053/rvsc.2000.0417. [DOI] [PubMed] [Google Scholar]
- 12.Roepke M, Diestel A, Bajbouj K, Walluscheck D, Schonfeld P, Roessner A, et al. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol Ther. 2007;6:160–169. doi: 10.4161/cbt.6.2.3575. [DOI] [PubMed] [Google Scholar]
- 13.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Shoieb AM, Elgayyar M, Dudrick PS, Bell JL, Tithof PK. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int J Oncol. 2003;22:107–113. [PubMed] [Google Scholar]
- 15.Yi T, Cho SG, Yi Z, Pang X, Rodriguez M, Wang Y, et al. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol Cancer Ther. 2008;7:1789–1796. doi: 10.1158/1535-7163.MCT-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaoui A, Cherrah Y, Alaoui K, Mahassine N, Amarouch H, Hassar M. Effects of Nigella sativa fixed oil on blood homeostasis in rat. J Ethnopharmacol. 2002;79:23–26. doi: 10.1016/s0378-8741(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 17.Senturk E, Pabuscu E, Sen S, Unsal C. Comparison of mitomycin-c and heparin affects in experimental corrosive esophagitis on rats. Int J Pediatr Otorhinolaryngol. 2011;75:785–789. doi: 10.1016/j.ijporl.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Guven A, Demirbag S, Uysal B, Topal T, Erdogan E, Korkmaz A, et al. Effect of 3-amino benzamide, a poly(adenosine diphosphate-ribose) polymerase inhibitor, in experimental caustic esophageal burn. J Pediatr Surg. 2008;43:1474–1479. doi: 10.1016/j.jpedsurg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Ocakci A, Kanter M, Cabuk M, Buyukbas S. Role of caffeic acid phenethyl ester, an active component of propolis, against NAOH-induced esophageal burns in rats. Int J Pediatr Otorhinolaryngol. 2006;70:1731–1739. doi: 10.1016/j.ijporl.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Turkyilmaz Z, Sonmez K, Karabulut R, Gulbahar O, Poyraz A, Sancak B, et al. Mitomycin C decreases the rate of stricture formation in caustic esophageal burns in rats. Surgery. 2009;145:219–225. doi: 10.1016/j.surg.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Franco D, Franco T, Schettino AM, Filho JM, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012;36:1254–1259. doi: 10.1007/s00266-012-9957-3. [DOI] [PubMed] [Google Scholar]
- 22.Topaloglu B, Bicakci U, Tander B, Ariturk E, Kilicoglu-Aydin B, Aydin O, et al. Biochemical and histopathologic effects of omeprazole and vitamin E in rats with corrosive esophageal burns. Pediatr Surg Int. 2008;24:555–560. doi: 10.1007/s00383-008-2126-8. [DOI] [PubMed] [Google Scholar]
- 23.Occleston NL, Daniels JT, Tarnuzzer RW, Sethi KK, Alexander RA, Bhattacharya SS, et al. Single exposures to antiproliferatives: long-term effects on ocular fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci. 1997;38:1998–2007. [PubMed] [Google Scholar]
- 24.Cevik M, Demir T, Karadag CA, Ketani MA, Celik H, Kaplan DS, et al. Preliminary study of efficacy of hyaluronic acid on caustic esophageal burns in an experimental rat model. J Pediatr Surg. 2013;48:716–723. doi: 10.1016/j.jpedsurg.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Kiyan G, Aktas S, Ozel K, Isbilen E, Kotiloglu E, Dagli TE. Effects of hyperbaric oxygen therapy on caustic esophageal injury in rats. J Pediatr Surg. 2004;39:1188–1193. doi: 10.1016/j.jpedsurg.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz F, Terzi A, Coban S, Bitiren M, Celik H, Aksoy N, et al. Purified micronized flavonoid fraction ameliorates the injury of spleen and ileum secondary to hepatic ischemia-reperfusion in rats. Dig Dis Sci. 2010;55:2237–2243. doi: 10.1007/s10620-009-1018-7. [DOI] [PubMed] [Google Scholar]
- 28.Gokce EC, Kahveci R, Gokce A, Cemil B, Aksoy N, Sargon MF, et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine. 2016;24:949–959. doi: 10.3171/2015.10.SPINE15612. [DOI] [PubMed] [Google Scholar]
- 29.Cobourne-Duval MK, Taka E, Mendonca P, Bauer D, Soliman KF. The antioxidant effects of thymoquinone in activated BV-2 murine microglial cells. Neurochem Res. 2016;41:3227–3238. doi: 10.1007/s11064-016-2047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins RP, Hartmann DD, de Moraes JP, Soares FA, Puntel GO. Platelet-rich plasma reduces the oxidative damage determined by a skeletal muscle contusion in rats. Platelets. 2016;27:784–790. doi: 10.1080/09537104.2016.1184752. [DOI] [PubMed] [Google Scholar]