Abstract
The origin of the progenitor cell for Barrett’s esophagus remains a major unsolved mystery. Understanding the source of this progenitor may improve strategies to prevent the development of esophageal adenocarcinoma. Esophageal submucosal glands (ESMGs) and ducts may serve as a potential source of progenitor cells that respond to esophageal injury. Through the use of human histologic and molecular analysis, ESMGs and ducts have been described in physical continuity with areas of columnar esophagus, and shared mutations have been described between ESMG ducts and Barrett’s esophagus. Acinar ductal metaplasia, associated with carcinogenesis in other organs, occurs within ESMGs with human esophageal injury and esophageal adenocarcinoma. By using atypical animal models, a squamous epithelial defect well above the gastroesophageal junction healed to columnar epithelium and continuity of ESMG ducts was noted in the new epithelium. Increased proliferation in ESMGs and ducts in response to injury also has been noted in human beings and animals.

The origin of the progenitor cell for Barrett’s esophagus (BE) and the more broadly defined esophageal columnar epithelium remains a major unsolved mystery. Several potential theories suggest how columnar epithelium arises in the esophagus including transdifferentiation of basal cells in the squamous epithelium, extension of a special population of cells from the gastroesophageal junction into the tubular esophagus, and repopulation of the esophagus after injury with cells derived from progenitors in the esophageal submucosal glands (ESMGs) or ducts. These potential sources of esophageal columnar epithelium need not be mutually exclusive. However, understanding which source most often provides the BE progenitor may improve strategies to prevent the development of esophageal adenocarcinoma (EAC).
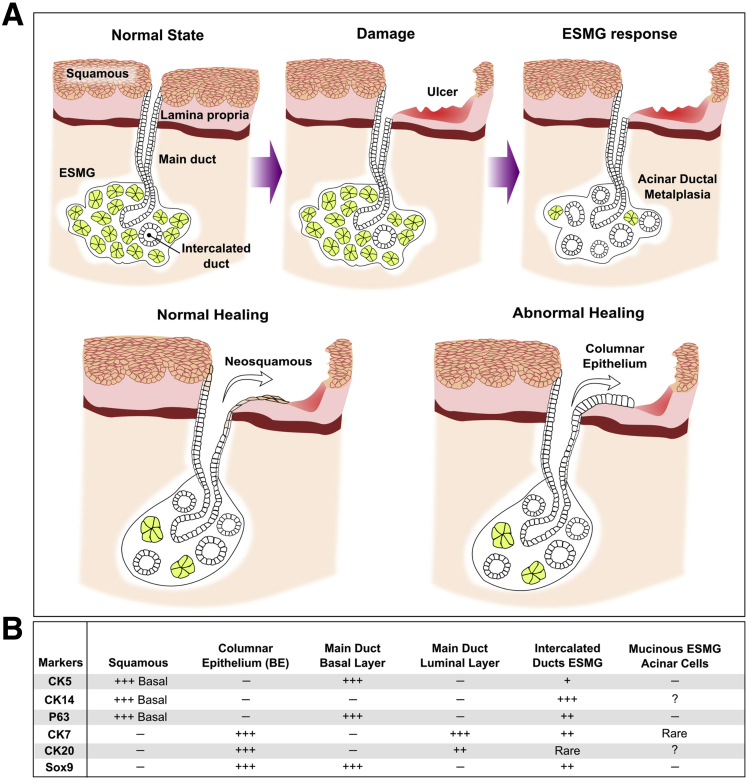
Based on published observational human and experimental animal data, ESMGs and ducts may serve as a potential source of progenitor cells that respond to esophageal injury through either normal repair to squamous epithelium or abnormal repair to columnar epithelium (Figure 1A). In reviewing evidence supporting ESMGs and ducts as a source of BE progenitors, it is important to note that laboratory models using mice and rats cannot be used because these animals lack ESMGs. To address this challenge, atypical animal models have been required.
Figure 1.
Proposed role of ESMGs in normal and abnormal healing of the esophagus, and the markers used to distinguish the cell types/structures involved in both. (A) Top: Schematic of the following: normal state on the left with normal ESMG under healthy squamous epithelium, then damage in the center with significant epithelial damage with ulceration, and ESMG response on the right with acinar ductal metaplasia replacing normal mucinous acini within the ESMG. (A) Bottom: The concept of ESMG and duct response in normal healing to neosquamous epithelium on the left, or, under different conditions, abnormal healing to columnar epithelium (right). (B) Table representing reported markers of different components represented in the figure including squamous epithelium, columnar epithelium, the basal and luminal layers of the main ducts, intercalated ducts within ESMGs, and the mucinous cells of normal ESMGs. Of note, ESMG ducts share markers with both squamous and columnar epithelium.
Basic Biology of ESMG Secretion and Distribution
ESMGs are similar in structure to salivary glands and secrete protective substances such as bicarbonate, mucins, epidermal growth factor, and prostaglandins.1 To produce these protective factors, ESMGs contain secretory acini composed of simple columnar epithelium. Excretory ducts carry secretions from ESMGs to the esophageal lumen. The basal segment of a duct is close to the ESMGs and this area typically is lined by cuboidal cells. This cuboidal ductal lining normally transitions to squamous toward the esophageal lumen.2
In human esophagus, the ESMG distribution is heterogeneous, but, in general, similar concentrations of ESMGs are present in proximal and distal regions.1, 3 However, the incidence varies and the median proportion of esophageal epithelium containing ESMGs ranges from 3.96% to >50% in some individuals.1 Because ESMGs are not visualized during standard endoscopy and they typically are not sampled by endoscopic biopsies, the prognostic significance of ESMG distribution and morphology remains unknown.
ESMGs in Regeneration of Human Esophagus
In evaluation of regenerated esophageal epithelium in esophagectomy specimens, both normal squamous islands and areas of columnar metaplasia have been associated with ESMGs. Although not the main focus of this commentary, it is worth specifically noting that islands of squamous epithelium in a field of columnar esophagus consistently and uniformly have been associated with the squamous lining of underlying ESMG ducts.2 Interestingly, in patients with columnar metaplasia, ESMGs were found to be highly concentrated at junctions found at the border of the columnar metaplasia with areas of squamous epithelium.3
Evidence for the theory that BE may arise from ESMGs or ducts has been derived from careful human histology analysis. Coad et al2 reviewed paraffin blocks to identify ESMG ducts that opened directly onto areas of columnar epithelium. The morphology of cells lining ducts into BE gradually transitioned from cuboidal cells in the basal ducts, to the squamous lining of the distal ducts closer to the lumen, and also to a single layer of columnar cells in the area of metaplastic columnar epithelium at the mucosal surface. Continuity of ESMG ducts with columnar epithelium supports a hypothesis that ESMG ducts could, in the setting of erosive esophagitis, supply progenitors that are the source of the columnar-lined esophagus.
In a follow-up report, Leedham et al4 returned to paraffin-embedded specimens and performed a high-resolution clonal analysis of individual BE crypts, neosquamous islands, and ESMG ducts. A major finding was genetic heterogeneity of BE crypts, arguing against the concept that after esophageal injury, one progenitor sweeps through the esophagus creating a field of BE. Conversely, the BE crypt heterogeneity supports the concept that along the length of injured esophagus, multiple independent clones of BE may arise separately. In tissue blocks, BE crypts were identified as continuous with underlying ducts.4 Thus, throughout a segment of injured esophagus, it is possible that multiple ESMG ducts can give rise to multiple and diverse BE clones. Importantly, in one case, microdissection and clonal analysis showed that both a duct lined by squamous epithelium and the connected BE crypt shared a p16 mutation.4 This evidence of an identical clone in both the squamous tissue of a duct and the adjacent BE supports the concept that ESMGs or ducts contain progenitors for BE. However, the evaluation of clonality was limited to the depth of ESMG ducts and did not include microdissection and clonal analysis of ESMGs themselves.
Experimental Animal Models of BE and ESMG Response to Injury
Given the lack of ESMGs in mice and rats, dog models have been used to study the response of ESMGs to esophageal damage. In the 1980s, a canine model of acid reflux and epithelial damage was created by cardioplasty and fixed hiatal hernias with stripping of the squamous epithelium.5 This model was designed to address the question of columnar epithelialization of the esophagus by direct extension from the gastroesophageal junction vs re-epithelialization from ESMGs and ducts. To study the origin of BE, squamous stripping was performed just above the squamocolumnar junction and a 2-cm area of intact squamous epithelium remained in place while another more proximal area of squamous mucosa was excised. After cardioplasty, squamous stripping, and subcutaneous pentagastrin administration to augment acid production, many animals developed a columnar phenotype in the lower ring.5 Importantly, a third of the animals also developed columnar epithelium above the intact squamous epithelium. This columnar re-epithelialization was separated from the squamocolumnar junction by intact squamous epithelium, indicating a source of progenitors distinct from the gastroesophageal junction. In addition, histology showed ESMG ducts continuous with re-epithelialization, leading Gillen et al5 to conclude that the columnar epithelium arose from ESMG ducts.
Proliferation Within ESMGs and Ducts
Proliferative response after injury provides additional evidence supporting ESMGs and ducts as esophageal progenitors. At baseline, very little proliferation occurs within ESMGs and ducts. To determine the proliferative rate of ESMGs and ducts in human beings, patients with late-stage EAC were administered the thymidine analog, bromodeoxyuridine (BrdU), intravenously to label cells synthesizing new DNA. The labeling index was 0.07% in the esophageal gland ducts; no labeling was noted in the ESMGs.1 To determine how the labeling index changed in the setting of epithelial injury, a dog study of cardioplasty with high acid exposure times was performed.6 Similar to human reports, in the uninjured state, no ESMG labeling was observed; after 2 weeks of acid exposure, even without epithelial ulceration, the labeling index of ESMGs increased from no labeling to 0.35% BrdU-labeled cells, and the labeling index of ESMG ducts increased from 0.11% to 3.37%.6 Although BrdU labeling of squamous epithelium also increased in response to acid reflux (6.03% to 10.56%), the relative proliferative response in ESMGs and ducts was more intense than in the squamous.6
Abnormal ESMG Morphology in Association With Esophageal Disease in Human Beings
Although normal ESMGs contain predominantly mucinous acini and eccentrically located ducts, ESMGs can assume a ductal appearance where normal mucin-producing acini are replaced by duct-like structures within the ESMGs. Our group has identified a ductal phenotype in ESMGs with an increased proportion of duct-like acini characterized by flat or cuboidal epithelium.7 Importantly, the ductal phenotype was associated with active esophageal injury such as epithelial ulcer, as well as with EAC and BE with high-grade dysplasia.7 Interestingly, the ductal phenotype observed in ESMGs is similar to acinar ductal metaplasia found in premalignant lesions in the pancreas. Acinar ductal metaplasia has been characterized as dedifferentiation of acinar cells to a ductal phenotype with progenitor-like markers and increased proliferation.
Marker Proteins in BE and ESMGs
Similarities have been noted between ESMGs, their ducts, and BE, although this is an area in need of further investigation. Classically, cytokeratin (CK)5 and CK14 have been described in basal squamous epithelium and are absent in BE,8 and, likewise, p63 marks basal squamous epithelium but is not present in BE.9 In contrast, CK7, CK20, and SRY-box9 (SOX9) are present in BE but typically are not found in normal squamous epithelium.8 Gonzalez et al10 evaluated ESMGs in human autopsies. Mucous cells within ESMGs had rare CK7 staining without SOX9 or p63 staining.10 Ducts leading from ESMGs to surface epithelium showed strong CK5 staining in basal epithelial cells and strong CK7 staining in luminal epithelial cells. Within these main duct layers, basal epithelial cells expressed strong SOX9 and p63 whereas luminal cells had rare CK7 and p63 staining. Single-layer ductal epithelium connecting acini to the main duct within ESMGs had positive CK7 staining; most of these cells also showed SOX9 and p63. In acinar ductal metaplasia, CK7 staining was quite strong in ESMGs with the ductal phenotype.7 In summary, as tabulated in Figure 1B, ESMGs and ducts express markers found in both squamous epithelium, such as CK5, CK14, and p63, as well as markers found in BE, such as SOX9 and CK7.
American Gastroenterological Association Freston Conference Presentations on Proliferation and Differentiation of ESMGs
At the 2016 American Gastroenterological Association Freston Conference, our group presented data from new in vivo and in vitro porcine models of ESMG response to injury and proliferation. The finding of acinar ductal metaplasia in human ESMGs prompted us to develop prospective models to study ESMGs as a potential progenitor niche in the esophagus. By using a porcine model of radiofrequency ablation–induced epithelial injury, we observed the ductal phenotype within ESMGs after injury. Similar to human and dog studies described earlier,2, 5 in the in vivo porcine radiofrequency ablation model, ESMG ducts appeared in direct continuity with re-epithelializing esophageal lumen. In our 3-dimensional culture system, ESMG-derived cells formed 2 distinct phenotypes of spheroids: a hollow/ductal spheroid expressing the CK7 marker of BE, and a solid spheroid expressing the p63 basal squamous marker. In both the in vivo model and the in vitro models, we observed proliferation in response to injury and culture conditions, a marked contrast to the normally quiescent ESMGs in the uninjured state.1, 6
Conclusions
Support for the role of ESMGs and ducts in BE pathogenesis has emerged from human histologic evidence linking ESMG ducts spatially and clonally with overlying BE.2 In a dog model, columnar epithelium emerged above a strip of squamous epithelium separating injury from the gastroesophageal junction, and re-epithelialization occurred in continuity with ESMG ducts.5 Within ESMGs themselves, a ductal phenotype has been associated with esophageal injury, BE, and EAC.7 This ductal phenotype has been observed in a porcine model of esophageal injury and repair and in continuity with re-epithelialization of injured esophagus. In a 3-dimensional culture model of ESMGs, 2 phenotypes of spheroids emerge from ESMGs, supporting the hypothesis that ESMGs and ducts within may contain progenitors capable of both squamous and columnar repair of damaged esophageal epithelium.
Several critical areas of research still need to be addressed. The exact cell type that may serve as a BE progenitor within ESMGs and ducts remains unknown. Additional clonal analysis of columnar esophagus, ESMG ducts, and ESMGs themselves are needed. Although the pathways associated with BE are well established, the roles of sonic hedgehog/bone morphogenic protein, Notch, Wnts, and retinoic acid on the proliferation and differentiation of cells from ESMGs and ducts remain unknown. Moving forward, use of animal models that contain ESMGs will offer new ways to test the influence of these pathways on progenitors from ESMGs or ducts. In addition, human research using deeper endoscopic imaging of ESMGs or endoscopic mucosal resection specimens may help determine the prognostic significance of persistent acinar ductal metaplasia in ESMGs. Long term, research in the pathogenesis of BE continues to be of importance to develop strategies to support healing to normal healthy squamous epithelium after esophageal injury and to prevent the development of BE and EAC.
Acknowledgments
The author thanks Susan J. Henning and Richard J. von Furstenberg for their intellectual contributions and assistance in preparation of the manuscript.
Footnotes
Conflicts of interest The author discloses no conflicts.
Funding Supported by National Institutes of Health grant K08-DK098528.
References
- 1.van Nieuwenhove Y., Destordeur H., Willems G. Spatial distribution and cell kinetics of the glands in the human esophageal mucosa. Eur J Morphol. 2001;39:163–168. doi: 10.1076/ejom.39.3.163.4674. [DOI] [PubMed] [Google Scholar]
- 2.Coad R.A., Woodman A.C., Warner P.J. On the histogenesis of Barrett's oesophagus and its associated squamous islands: a three-dimensional study of their morphological relationship with native oesophageal gland ducts. J Pathol. 2005;206:388–394. doi: 10.1002/path.1804. [DOI] [PubMed] [Google Scholar]
- 3.Lorinc E., Oberg S. Submucosal glands in the columnar-lined oesophagus: evidence of an association with metaplasia and neosquamous epithelium. Histopathology. 2012;61:53–58. doi: 10.1111/j.1365-2559.2012.04180.x. [DOI] [PubMed] [Google Scholar]
- 4.Leedham S.J., Preston S.L., McDonald S.A. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett's oesophagus. Gut. 2008;57:1041–1048. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillen P., Keeling P., Byrne P.J. Experimental columnar metaplasia in the canine oesophagus. Br J Surg. 1988;75:113–115. doi: 10.1002/bjs.1800750208. [DOI] [PubMed] [Google Scholar]
- 6.Van Nieuwenhove Y., Willems G. Gastroesophageal reflux triggers proliferative activity of the submucosal glands in the canine esophagus. Dis Esophagus. 1998;11:89–93. doi: 10.1093/dote/11.2.89. [DOI] [PubMed] [Google Scholar]
- 7.Garman K.S., Kruger L., Thomas S. Ductal metaplasia in oesophageal submucosal glands is associated with inflammation and oesophageal adenocarcinoma. Histopathology. 2015;67:771–782. doi: 10.1111/his.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glickman J.N., Chen Y.Y., Wang H.H. Phenotypic characteristics of a distinctive multilayered epithelium suggests that it is a precursor in the development of Barrett's esophagus. Am J Surg Pathol. 2001;25:569–578. doi: 10.1097/00000478-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Glickman J.N., Yang A., Shahsafaei A. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol. 2001;32:1157–1165. doi: 10.1053/hupa.2001.28951. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez G., Huang Q., Mashimo H. Characterization of oncocytes in deep esophageal glands. Dis Esophagus. 2016;29:670–680. doi: 10.1111/dote.12382. [DOI] [PubMed] [Google Scholar]