Abstract
Liver fibrosis arises because prolonged injury combined with excessive scar deposition within hepatic parenchyma arising from overactive wound healing response mediated by activated myofibroblasts. Fibrosis is the common end point for any type of chronic liver injury including alcoholic liver disease, nonalcoholic fatty liver disease, viral hepatitis, and cholestatic liver diseases. Although genetic influences are important, it is epigenetic mechanisms that have been shown to orchestrate many aspects of fibrogenesis in the liver. New discoveries in the field are leading toward the development of epigenetic biomarkers and targeted therapies. This review considers epigenetic mechanisms as well as recent advances in epigenetic programming in the context of hepatic fibrosis.
Keywords: Liver Fibrosis, Epigenetics, DNA Methylation, Histone Modifications, Chronic Liver Disease
Abbreviations used in this paper: CLD, chronic liver disease; CpG, cytosine-phospho-guanine; DNMT, DNA methyltransferase; HDAC, histone deacetylase; HSC, hepatic stellate cell; miRNA, microRNA; NAFLD, nonalcoholic fatty liver disease; ncRNA, non-coding RNA; PPAR, peroxisome proliferator activated receptor; TET, Ten Eleven Translocation
Summary.
Liver fibrosis is a common end pathway of any type of chronic hepatic injury. It is now known that epigenetic mechanisms including DNA methylation, histone modifications and non-coding RNAs appear to orchestrate many aspects of liver fibrogenesis. This review considers recent gains in knowledge of epigenetic programming in the context of hepatic fibrosis, which is paving the way to discovery of epigenetic biomarkers as well as long awaited diagnostic and prognostic tools.
Chronic liver disease (CLD) comprises many different etiologies, with fibrosis being the common pathologic outcome of virtually all CLD, usually defined by the excessive accumulation of fibrous connective tissue in and around inflamed or damaged tissue.1, 2, 3
The liver is made up of many cell types whose composition as well as phenotype ultimately changes in CLD. It is now well-documented that cellular phenotype is at least in part under control of chromatin configuration at key regulatory genes; this in turn is governed by epigenetic mechanisms.4 The term epigenetics describes reversible changes in gene expression that can be inherited through cell division that do not involve alterations to the underlying DNA sequence.5 Epigenetic changes occur ubiquitously in all cells and are most readily observed in our bodies where a single genome gives rise to numerous different cell types.4
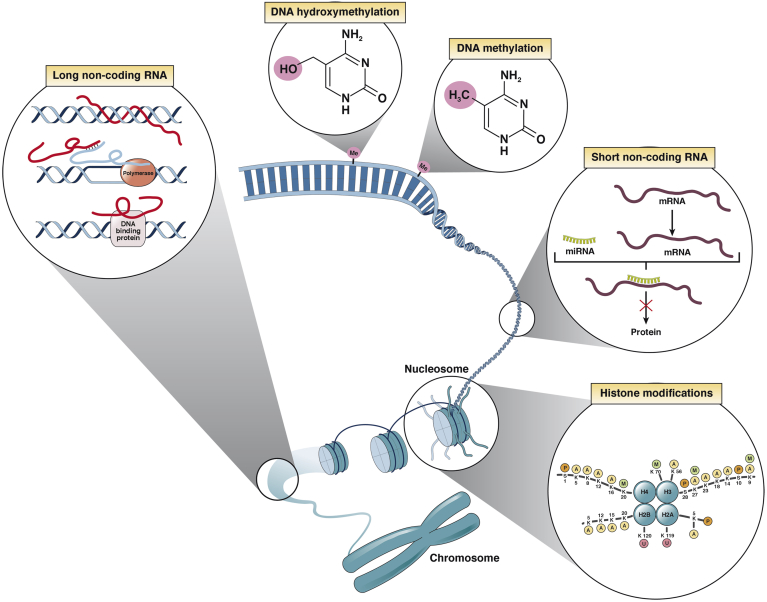
The epigenome is influenced by a number of factors including age, gender, the environment (diet, drug use, smoking), as well as the underlying genome through presence of single nucleotide polymorphisms.6 The epigenome is governed by at least 3 systems, namely DNA methylation, histone modifications, and non-coding RNA (ncRNA) mediated gene silencing7, 8, 9, 10 (Figure 1). These separate but interacting and overlapping epigenetic mechanisms are currently considered to initiate and sustain DNA and chromatin modifications that underpin cellular phenotype by facilitating the control of gene transcription by sequence-specific transcription factors.10, 11, 12, 13 All 3 epigenetic mechanisms regulate the chromatin structure, modifications, and the initiation of transcription in a manner that alters the accessibility of genes to transcription factors and their cofactors that dictate the rate at which a gene is actively transcribed.7, 8, 9, 10 Therefore, it is not surprising that epigenetics has become a research area of much interest, linking changes in chromatin states to the cellular phenotype and, in turn, the functioning of an organ. Large numbers of studies have considered the impact of epigenetic changes on liver function in health as well as in disease states. Here we consider the epigenetic mechanisms involved in the pathogenesis of liver fibrosis as well as examine recent advancements in the field and discuss new epigenetic approaches and strategies for the treatment of liver fibrosis.14
Figure 1.
Epigenetic mechanisms of heritable gene expression regulation. There are several highly interdependent epigenetic mechanisms that are important in the control of gene expression, namely DNA methylation (and hydroxymethylation), histone post-translational modifications, and ncRNA-based pathways, including small and long ncRNA species.
The Epigenetic Code and Mechanisms
Genomic DNA contains all the information that a cell, and indeed the organism, requires for life. The DNA sequence, or the genome, is identical in all cells of a particular organism. However, the epigenome is entirely cell type specific, such that combination of the above-mentioned 3 epigenetic mechanisms is carefully defined and maintained to support the phenotype of that particular cell.4, 10, 12, 13 Therefore, although the genome of every cell in the body is the same, the epigenome will govern the phenotype, such that a hepatocyte will have its own defined epigenetic signature that will differ from that of an adipocyte or a nerve cell.15, 16
DNA in a cell is not naked but rather packaged around histones into a structure known as chromatin. Chromatin is composed of ∼146 base pairs of genomic DNA sequence wrapped around 8 core histones to form the basic unit of chromatin, the nucleosome. The main functions of chromatin are to efficiently package DNA into a reduced volume such that it can fit into the nucleus of a cell, protect the DNA structure and sequence, prevent chromosome breakage, and regulate gene expression as well as DNA replication. Each nucleosome contains a core of 8 histones (2 copies of H2A, H2B, H3, and H4), which are small, globular proteins with a long N-terminal tail that is subject to numerous post-translational modifications including acetylation, methylation, phosphorylation, SUMOylation, ubiquitination, or ADP-ribosylation. A large number of histone-modifying enzymes act to carry out more than 60 different possible modifications within each octamer of histones.
The presence of chemical groups on the histones creates binding sites for specific protein complexes that can promote either activation or silencing of gene transcription.10 As an example, lysine residues within histones can be acetylated, which is mediated by histone acetyltransferases and associated with active gene transcription due to enhanced recruitment of other chromatin remodelling enzymes and prevention of chromatin compaction. Conversely, gene silencing or repression is frequently associated with the removal of acetyl groups by histone deacetylases (HDACs).17 Histone methyltransferases have the ability to add 1, 2, or 3 methyl groups to lysines or arginines within histones H3 or H4. The impact of methylation on gene transcription depends on the specific site of the covalent modification; for instance, histone 3 lysine 4 trimethylation causes transcriptional activity, whereas histone 3 lysine 9 or lysine 27 leads to transcriptionally silent chromatin.10, 18, 19 Combinations of histone marks therefore provide changes in chromatin conformation and confer unique biological functions to the regions of the genome associated with these marks, which is also termed the histone code.
Histone modifications do not operate in isolation. They are tightly interlinked with DNA methylation, the second epigenetic mechanism. DNA methylation takes place on the fifth carbon of pyrimidine ring in cytosine nucleotides and most commonly in cytosine-phospho-guanine (CpG) dinucleotides.20 Long stretches of DNA (longer than 200 base pairs) containing dense (>55%) clusters of CpG sequences are termed CpG islands. Most human gene promoters contain 1 or more clusters of CpG islands, mostly found in unmethylated state, whereas many non-promoter CpGs are methylated throughout the genome.21, 22 Methylation of DNA is carried out by enzymes called DNA methyltransferases (DNMTs), which use S-adenosyl methionine as methyl donor. DNMT family has 5 members, but only 3 of the 5 members have DNMT activity. The DNMT3a and DNMT3b methylate de novo CpG islands generate new epigenetic marks.23 DNMT1 maintains DNA methylation status during the DNA replication, a process that in mammals requires a protein UHRF1, which is thought to recruit DNMT1 to DNA replication forks through a unique hemi-methylated CpG-binding activity.24
DNA methylation is the most studied mechanism of epigenetic programming and is important in the regulation of imprinted gene expression as well as silencing the expression and mobility of transposable elements.25 Genomic methylation patterns are stable, heritable, and crucial to generate cells from somatic differentiated cells.26, 27 Recently the existence of an oxidative form of the cytosine, 5-hydroxymethylcytosine has been reported; 5-hydroxymethylcytosine is generated by the Ten Eleven Translocation (TET) enzyme family that comprises TET1, TET2, and TET3. TETs present multiple modes of action either directly or through partners to initiate DNA demethylation.28
DNA methylation correlates with gene silencing due to several mechanisms. Methylated CpG islands may promote condensation of chromatin to states that are unfavorable for gene expression; they can directly inhibit interaction of DNA binding proteins to their target sites and provide recognition signals for the recruitment of methyl-CpG-binding domain proteins (MeCP2, MBD1-4, and Kaiso) with their associated complexes.21 However, outside of CpG islands it is becoming apparent that CpG methylation can correlate with transcriptionally active genes, particularly when occurring within the gene body; however, the mechanisms responsible for this are as yet poorly defined but may relate to structural requirements for transcription elongation.29
In addition to histone modification and DNA methylation, RNA molecules are also able to define cellular phenotype through their gene regulatory functions. It was originally considered that RNA was a merely intermediary molecule responsible for transmitting coding information from genes to proteins, and it was also thought that regions of the genome that lacked obvious protein-coding sequence were “junk”. However, advances in molecular biology and high-throughput genomic techniques provided comprehensive genomic maps. The ENCODE project revealed that more than 80% of the genome has biological activity, and only 2% is transcribed to protein-coding genes. ncRNAs are functional non–protein-coding RNA molecules that are concentrated within the nucleus and expressed at lower concentrations than coding RNAs.30, 31 Functional mechanisms of ncRNAs involve epigenetic regulation of gene expression. The extensive number of ncRNAs transcribed from the genome and discovered in the last decade has required detailed classification related to their sizes and functional mechanisms, which include small nucleolar RNAs, microRNAs (miRNAs), PIWI-interacting RNAs, small-interfering RNAs, enhancer RNAs, and long non-coding (lncRNAs). In this review we focus our attention toward miRNAs because they have been the most extensively studied of all ncRNAs in the context of liver disease.32, 33, 34 miRNAs are single-stranded short ncRNA molecules (20–24 nucleotides) that post-transcriptionally regulate gene expression by several mechanisms including repression of translation or mRNA cleavage and degradation (by binding to the 3′-untranslated regions of mRNA).35, 36 miRNAs are able to epigenetically silence expression of genes in a wide variety of biological pathways including proliferation, apoptosis, and cellular signalling and thus are thought to play an important role in a variety of human diseases.
Modulation of DNA Methylation After Liver Damage
Aberrant DNA methylation patterns are associated with inappropriate gene repression and human disease processes including fibrosis.37, 38, 39 Komatsu et al40 reported a crucial DNA hypomethylation in fibrogenic genes from the onset of liver fibrosis by using an in vivo early-stage animal model of liver fibrosis. Moreover, an in vitro approach using quiescent and early culture-activated hepatic stellate cells (HSCs) showed a global demethylation during activation of HSCs.41 Genome-wide analysis performed by the van Grunsven group identified an integrative concordance between promoter methylation landscapes and gene expression in culture-activated human HSCs.42
DNA methylation in cells is in large part interpreted via binding of methyl binding proteins, which in turn recruit transcriptional repressor complexes to the sites of DNA methylation. During transdifferentiation, HSCs start to express MECP2, which along with MBD1, MBD2, MBD3, and MBD4 comprises a family of nuclear proteins related by the presence of a methyl-CpG binding domain.43, 44, 45, 46 MECP2 is able to repress transcription from methylated gene promoters, and it is therefore considered to be a transcriptional repressor that has been shown to mediate epigenetic silencing of the peroxisome proliferator activated receptor gamma (PPARγ) gene, a master transcriptional regulator of the adipogenic, quiescent, and non-fibrogenic phenotype of the HSC.43, 44, 47 To confirm functional association of DNA methylation with liver fibrosis, our group reported that transdifferentiation of the HSC to the profibrogenic myofibroblast phenotype is suppressed by the DNMT inhibitor 5′-aza-deoxycytidine.44
In addition to its repressive role, MeCP2 has been reported to also exert transcriptional activation, although the mechanisms behind this effect are not as yet fully elucidated.48 In relation to this function, we have shown that MeCP2 positively regulates expression of the histone methyltransferase ASH1 during HSC transdifferentation.49 ASH1 regulates attachment of methyl group to lysine 4 on histone H3 (H3K4), thus promoting transcriptional activity.50 In activated HSCs, ASH1 is required for expression of classic profibrogenic genes collagen I, tissue inhibitor of metalloproteinase-1, and transforming growth factor β1.49 Taken together, MeCP2 appears to regulate repression of antifibrogenic genes while also inducing expression of positive regulators of profibrogenic genes such as ASH1. Mice lacking MeCP2 are therefore resistant to developing fibrosis in models of chronic lung and liver damage, which coupled with the ability of MeCP2 to stimulate upregulation of multiple fibrogenic genes indicates its potential to function as a bona fide “core” master epigenetic regulator of myofibroblast phenotype and fibrogenesis.43 As such, it will now be important to reveal finer details of MeCP2 mechanism of actions in hepatic myofibroblasts, which may include regulation of gene expression via direct me-CpG-dependent transcriptional processes or by indirect post-transcriptional mechanisms.
Development of fibrosis is associated with changes in the expression of enzymes that regulate DNA methylation and hydroxymethylation.51 We have recently shown that expression of the maintenance DNA methyltransferase DNMT1 and of the de novo methyltransferases DNMT3a and 3b tend to increase in fibrotic liver.51 By contrast, hepatic expression of the TET demethylase enzymes is downregulated in chronic liver disease.51 Associated with these changes in expression of DNA methylation regulators were global changes in 5-methylcytosine and 5-hydroxymethylcytosine that are indicative of genome-wide alterations in gene expression. The mechanisms by which methylome relandscaping promotes genome-wide resetting of gene transcription in fibrosis are yet to be defined but may involve alterations in the recruitment of chromatin remodelling complexes that silence gene expression. Another possibility is that TET-regulated conversion of 5-methylcytosines to 5-hydroxymethylcytosines may result in transcriptional activation or an increase in transcriptional elongation.52, 53 It is anticipated that the recent development of Cas9-targeting protocols for the experimental manipulation of sequence-specific DNA methylation will dramatically improve our understanding of the mechanistic relationships between methylome relandscaping and gene expression.54, 55
Advancing on these studies, we have identified genome-wide sequence-specific changes in 5-methylcytosine and 5-hydroxymethylcytosine marks occurring during HSC transdifferentiation that were accompanied by alterations in the expression of DNMTs and TET enzymes.51 The next challenge will be to establish whether these alterations in methylation at specific CpG sites result in alterations in gene expression that impact on HSC fate and function. Furthermore, experimental manipulation of the activities of DNMTs or TETs at fibrogenic genes may offer new therapeutic avenues. Clearly, such approaches would have to be carried out by using a cell type–specific and targeted approach, because systemic epigenetic therapy is likely to have many unwanted side effects.
The interrogation of DNA methylome signatures in human liver can distinguish patients with different stages of nonalcoholic fatty liver disease (NAFLD) or fibrosis.56, 57, 58 Hence, determining the DNA methylation status of specific CpG sites in patient tissues may provide useful biomarkers to define disease diagnosis and prognosis. However, these approaches have so far been unattractive because they relied on analysis of tissue provided by liver biopsy, an invasive procedure that carries a number of procedure-associated risks.58, 59 Advancing on studies carried out on liver biopsy tissue, we have recently published that quantification of DNA methylation from circulating cell-free DNA isolated from patient plasma has the potential to stratify fibrosis grade with high accuracy.60 Specifically, we demonstrated that differential DNA methylation status at the PPARγ promoter in cell-free DNA in plasma can distinguish between patients with mild versus severe fibrosis in the context of NAFLD.60 In this context, it is thought that dying hepatocytes release degraded genomic DNA into systemic circulation, which can be picked up peripherally by isolation from plasma. The level of DNA methylation at particular CpGs within the PPARγ promoter can therefore reflect the changes in methylation present in hepatocytes; thus, it is a proxy measurement of changes within the liver without the need for a needle biopsy.
Dysregulation of Histone Code in Liver Disease
The number of studies pinpointing the regulatory role of histone modifications in liver injury has increased in the last decade, with most relating to changes in histone acetylation due to pharmacologic use of HDAC inhibitors.61, 62 HDAC inhibitors have been shown to reverse myofibroblast differentiation and exert antifibrogenic effects in fibrosis-related pathologies such as pulmonary, dermal, or renal fibrosis.63, 64, 65, 66 Class I and II HDAC inhibitors have received most of the attention in the liver field, probably owing to their antiproliferative properties and ability to induce cell death via deacetylation of multiple HDAC substrates.17 As an example, the class I inhibitor largazole can induce apoptosis and suppress proliferation of HSC by increasing the acetylation of histones H3 and H4.67 Likewise, valproate sodium, a broad class I and II HDAC inhibitor, exerts its antifibrogenic properties by inhibiting the expression of collagen 1A1 and transforming growth factor β1 without causing cytotoxic damage.68 Valproate sodium has also been shown to block myofibroblast differentiation and fibrogenesis in mouse models of liver fibrosis.69 The mechanism of HDAC1 effects in myofibroblasts at least in part relates to anti-inflammatory and antifibrogenic action via recruitment to genes such as Ccl2, Cxcl10, Gm-csf, and Mmp13.70 However, the majority of HDAC inhibitors lack target or cell-specific activities, with none currently tested in human clinical trials. Consequently, although there is interest in the development of HDAC inhibitors as antifibrogenic agents, further investigation should be carried out to explore side effects and the design of more target-selective inhibitors as well as test their efficacy in patients.
Another means of regulating histone acetylation is via inhibition of bromodomain binding. Bromodomains are 110-kDa domains contained within a number of different proteins that serve as “readers” of lysine acetylation. These domains have been predominantly studied in the context of the bromodomain and extra-terminal domain family, most notably BRD4, which is critical for binding to hundreds of enhancers associated with genes involved in multiple profibrotic pathways in HSCs.71 Targeted inhibition of BRD4 through use of the JQ1 drug has been shown to block HSC activation and proliferative capacity, thus limiting fibrosis in vivo.71
HSC transdifferentiation is in part orchestrated by the histone methyltransferases (EZH2 and ASH1) downstream of the master epigenetic regulator MeCP2. EZH2 is induced at the protein level during the initiation stage of HSC transdifferentiation and is recruited to the PPARγ gene where it promotes accumulation of the repressive chromatin signature H3K27me3; this event is required to reprogram the quiescent HSC transcriptome to myofibroblast phenotype.43 In parallel, ASH1 is recruited to the promoter regions of alpha-smooth muscle actin, collagen 1A1, tissue inhibitor of metalloproteinase-1, and transforming growth factor β1 genes, facilitating a transcriptional active state.49 To increase the knowledge of how these enzymes regulate liver fibrosis, it will be interesting to develop specific conditional knockout mice along with the design of specific drugs that target their activities. For instance, the active compounds of the herbal preparation Yang-Gan-Wan can attenuate liver fibrosis progression and HSC transdifferentiation by repressing the MeCP2-EZH2 axis.72 More recently we have used an HSC-targeted nanoparticle approach for selectively delivering the EZH2 inhibitor 3′deazaneplanocin A to these cells in vivo and shown that this achieves suppression of the progression of pre-established liver fibrosis in mice.
Liver-damaging agents can also dysregulate chromatin structure. Several studies have described the epigenetic mechanisms involved in alcoholic liver injury, which entail action of reactive oxygen species on several histone modifications.73, 74, 75, 76 Alcohol and its metabolites can promote transdifferentiation of HSC either directly or through profibrogenic cytokines expressed by hepatocytes. HDAC6 function is compromised in ethanol-treated HSCs, which leads to modifications in microtubule dynamics.77 A study performed by Kim and Shukla78 reported that alcohol increased acetylation of H3K9 in a dose- and time-dependent basis in rat HSCs. Multiple histone methyltransferases including MLL1 are induced in ethanol-exposed HSCs; these changes are associated with enrichment of the transcriptional stimulatory H3K4 methylation mark at numerous genes.79 Illuminating the mechanisms by which alcohol directly influences the HSC epigenome is important because it will improve our understanding of how alcohol promotes fibrosis and potentially reveal new therapeutic targets. Two recent studies performed on a genome-wide survey basis suggest that genes activated in the livers of patients with NAFLD strongly correlate with histone modification marks.80, 81
Small Non-coding RNAs as Epigenetic Intermediaries
The role of miRNAs in liver pathophysiology has been covered in excellent reviews elsewhere.32, 82 miRNAs contribute to the various pathologic stages of liver disease and also participate in the control of HSC transdifferentiation, making them potential biomarker and therapeutic tools.83 The number of publications describing the role of miRNAs in liver fibrosis has grown exponentially in the last decade, therefore creating a list of liver profibrotic (miR-21, -221/222, -181b, or -150) and antifibrotic (miR-29b, -101, -122, or -214-3p) miRNAs.84 Recent advances in comparative bioinformatics have revealed an extensive list of miRNAs associated with quiescent state and myofibroblastic states of HSC. By using the Mercury array platform, upregulation of 12 miRNAs (miR-874, -29c*, -501, -349, -325-5p, -328, -138, -143, -207, -872, -140, and 193) and downregulation of 9 miRNAs (miR-341, -20b-3p, -15b, -16, -375, -122, -146a, -92b, and -126) have been associated with transdifferentiation of rat HSCs.85 miRNA profiles were also determined in quiescent, partially activated, and activated rat HSCs by using the Agilent microarray platform. Chen et al86 confirmed the increased expression of miR-221, -143, and-145 and the downregulated expression of miR-335 and -150 during the HSC activation. Finally, the latest next-generation sequencing by using Ago2 immunoprecipitation plus use of the deep sequencing Illumina platform has identified novel miRNA targets in human and rat HSCs.87 Neuronal-specific miRNAs were identified in myofibroblastic HSC, with mir-9, -125b, and -128 being upregulated and found to be regulating chemokine networks.87 Moreover, the Sancho-Bru group recently reported an integrative gene expression and miRNA profiling in human HSC.88 By using the miRNA Taqman array, they identified novel miRNA-target/mRNA interaction networks involved in HSC activation and highlighted the downregulation of miR-192 as a key event in the early phase of HSC transdifferentiation.88
Circulating microRNAs may offer a biologically stable blood-based biomarker tool for detection and stratification of liver disease. Circulating miRNAs were discovered as stable miRNAs secreted extracellularly to different biofluids through extracellular boundary to Ago2 or protected against RNase activity when internalized in exosomes (cell-derived extracellular vesicles).89, 90 In relation to this, alterations in the expression of cell-free circulating miRNAs have been associated with progression of disease in NAFLD and alcoholic liver disease.91, 92 Increasing serum levels of miR-196 and the liver regulator miR-122 have been found in NAFLD/nonalcoholic steatohepatitis, as well as in alcoholic liver disease.93, 94 Moreover, upregulation of miR-571 and reduction of miR-652 in serum samples from CLD patients highlighted the cellular-compartment specific roles these circulatory miRNA infer on the fibrogenic and inflammatory processes.95 Therefore, future identification of circulating miRNA profiles could be a potential tool to evaluate liver homeostasis and disease prognosis.
Heritability of Liver Fibrosis
Recent years have seen a major advancement in the field of transgenerational epigenetic inheritance of traits. These advances show existence of phenotypic adaptation of species in response to environmental pressures and cues. Although described studies cover several organ systems ranging from olfactory perception to increased susceptibility to depressive tendencies, some of the reports concern liver disease.
Our lab has shown that ancestral history of liver fibrosis in male rats leads to suppression of wound healing responses in offspring in at least 2 subsequent generations.56 The mechanism of adaptation in this study involves remodelling of DNA methylation in key fibrotic genes in the liver as well as sperm.56 Importantly, similar remodelling has been observed in human NAFLD liver tissues, where DNA methylation signature can discern mild from severe fibrosis, whereas a separate study also confirms existence of altered DNA methylation signatures in human ALD livers.58
Although there is no existing study in humans to confirm the presence of transgenerational epigenetic inheritance, a recent study carried out in monozygotic and dizygotic twins delineated the presence of epigenetic mechanisms that contribute to the heritable component of NAFLD.96, 97 This study interrogates epigenetic mechanisms that could account for discordance in the presence or absence of NAFLD in pairs of individuals who are genetically highly similar. By using liver magnetic resonance imaging proton-density fat fraction to quantify fat content and miR profiling of their serum, the study identifies a panel of 10 miRs that differentiated the twin with NAFLD from the twin without the disease. Of those, miR-331-3p and miR-30c were both highly correlated with each one and found to be heritable, suggesting involvement in a common mechanistic pathway, as shown by interactome analysis that highlights 7 common target genes.98
Evidence that epigenetic mechanisms can be inherited has also come from a study that compares spermatozoa from lean versus obese patients and outlines differences in DNA methylation patterns and small ncRNA content of sperm. Importantly, these epigenetic signatures were remodelled after bariatric surgery and subsequent weight loss in the obese patients, suggesting that mechanisms likely exist to ensure inheritance of metabolic traits by the progeny, which can be passed on in sperm.99 These studies provide exciting novel insights into heritability of traits and their intergenerational plasticity. However, much more work is required to begin to appreciate the extent to which epigenetics can explain complex human disease.
Conclusion
The latest studies highlight the regulatory effect that epigenetic modifications exert in the liver fibrosis process; however despite many published data, epigenetics are far from being elucidated.100, 101 Next-generation sequencing, along with advances in molecular technologies such as CRISPR/Cas9, provide the tools that can, in time, expand our current knowledge of the liver epigenome. Defining epigenetic signatures through the stages of liver disease could provide novel opportunities to develop therapies to specific targets. However, these developments will first have to grasp the complexities of interaction between histone code, DNA methylation, and ncRNA in a single type of liver cell, followed by interactions between numerous cell types within the liver, all of which will have their exquisitely specific epigenome. These epigenomes are highly plastic and responsive to the cues from their microenvironment and macroenvironment. Therefore, the second major task will be to understand which of these epigenetic signatures is “pre-set” and hard-wired into developmental inheritance of a particular phenotype versus epigenetic signatures that evolve because of environmental pressure as well as pressures created by the disease process. Furthermore, the environmental epigenetic signatures need to be distinguished from the epigenetic marks associated with ageing, which may introduce a third layer of complexity because the latter are likely to be predetermined and potentially more difficult to remodel. This is an area that is fast developing, and future studies are eagerly anticipated. The diagnostic, prognostic, and therapeutic possibilities that may emerge from greater understanding of epigenetic mechanisms and their operation in liver disease promise to deliver exciting advances in personalized medicine.
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman S.L. Hepatic fibrosis: overview. Toxicology. 2008;254:120–129. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 5.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dongen J., Nivard M.G., Willemsen G., Hottenga J.J., Helmer Q., Dolan C.V., Ehli E.A., Davies G.E., van Iterson M., Breeze C.E., Beck S., BIOS Consortium. Suchiman H.E., Jansen R., van Meurs J.B., Heijmans B.T., Slagboom P.E., Boomsma D.I. Genetic and environmental influences interact with age and sex in shaping the human methylome. Nat Commun. 2016;7:11115. doi: 10.1038/ncomms11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartke T., Kouzarides T. Decoding the chromatin modification landscape. Cell Cycle. 2011;10:182. doi: 10.4161/cc.10.2.14477. [DOI] [PubMed] [Google Scholar]
- 9.Bartke T., Vermeulen M., Xhemalce B., Robson S.C., Mann M., Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143:470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Jin G., Zhou X. Modeling the relationship of epigenetic modifications to transcription factor binding. Nucleic Acids Res. 2015;43:3873–3885. doi: 10.1093/nar/gkv255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger S.L. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 13.Jenuwein T., Allis C.D. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 14.Zeybel M., Mann D.A., Mann J. Epigenetic modifications as new targets for liver disease therapies. J Hepatol. 2013;59:1349–1353. doi: 10.1016/j.jhep.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 15.Murrell A., Rakyan V.K., Beck S. From genome to epigenome. Hum Mol Genet. 2005;14(Spec No 1):R3–R10. doi: 10.1093/hmg/ddi110. [DOI] [PubMed] [Google Scholar]
- 16.Yuan G.C. Linking genome to epigenome. Wiley Interdiscip Rev Syst Biol Med. 2012;4:297–309. doi: 10.1002/wsbm.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dokmanovic M., Clarke C., Marks P.A. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5:981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 18.Tammen S.A., Friso S., Choi S.W. Epigenetics: the link between nature and nurture. Mol Aspects Med. 2013;34:753–764. doi: 10.1016/j.mam.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suganuma T., Workman J.L. Signals and combinatorial functions of histone modifications. Annu Rev Biochem. 2011;80:473–499. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 20.Reik W., Dean W. DNA methylation and mammalian epigenetics. Electrophoresis. 2001;22:2838–2843. doi: 10.1002/1522-2683(200108)22:14<2838::AID-ELPS2838>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 21.Klose R.J., Bird A.P. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M.M., Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 23.Jones P.A., Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostick M., Kim J.K., Estève P.O., Clark A., Pradhan S., Jacobsen S.E. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 25.Slotkin R.K., Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–285. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 26.Reik W., Dean W., Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 27.Denis H., Ndlovu M.N., Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–656. doi: 10.1038/embor.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delatte B., Deplus R., Fuks F. Playing TETris with DNA modifications. EMBO J. 2014;33:1198–1211. doi: 10.15252/embj.201488290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 30.Mouse ENCODE Consortium, Stamatoyannopoulos J.A., Snyder M., Hardison R., Ren B., Gingeras T., Gilbert D.M., Groudine M., Bender M., Kaul R., Canfield T., Giste E., Johnson A., Zhang M., Balasundaram G., Byron R., Roach V., Sabo P.J., Sandstrom R., Stehling A.S., Thurman R.E., Weissman S.M., Cayting P., Hariharan M., Lian J., Cheng Y., Landt S.G., Ma Z., Wold B.J., Dekker J., Crawford G.E., Keller C.A., Wu W., Morrissey C., Kumar S.A., Mishra T., Jain D., Byrska-Bishop M., Blankenberg D., Lajoie B.R., Jain G., Sanyal A., Chen K.B., Denas O., Taylor J., Blobel G.A., Weiss M.J., Pimkin M., Deng W., Marinov G.K., Williams B.A., Fisher-Aylor K.I., Desalvo G., Kiralusha A., Trout D., Amrhein H., Mortazavi A., Edsall L., McCleary D., Kuan S., Shen Y., Yue F., Ye Z., Davis C.A., Zaleski C., Jha S., Xue C., Dobin A., Lin W., Fastuca M., Wang H., Guigo R., Djebali S., Lagarde J., Ryba T., Sasaki T., Malladi V.S., Cline M.S., Kirkup V.M., Learned K., Rosenbloom K.R., Kent W.J., Feingold E.A., Good P.J., Pazin M., Lowdon R.F., Adams L.B. An encyclopedia of mouse DNA elements (Mouse ENCODE) Genome Biol. 2012;13:418. doi: 10.1186/gb-2012-13-8-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., Dinger M.E., Mattick J.S. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X.W., Heegaard N.H.H., Ørum H. MicroRNAs in liver disease. Gastroenterology. 2012;142:1431–1443. doi: 10.1053/j.gastro.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo G., Bala S. MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol. 2013;10:542–552. doi: 10.1038/nrgastro.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roderburg C., Luedde T. Circulating microRNAs as markers of liver inflammation, fibrosis and cancer. J Hepatol. 2014;61:1434–1437. doi: 10.1016/j.jhep.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Chuang J.C., Jones P.A. Epigenetics and microRNAs. Pediatr Res. 2007;61(Pt 2):24R–29R. doi: 10.1203/pdr.0b013e3180457684. [DOI] [PubMed] [Google Scholar]
- 36.Saetrom P., Snove O., Jr., Rossi J.J. Epigenetics and microRNAs. Pediatr Res. 2007;61(Pt 2):7R–23R. doi: 10.1203/pdr.0b013e318045760e. [DOI] [PubMed] [Google Scholar]
- 37.Gu J., Stevens M., Xing X., Li D., Zhang B., Payton J.E., Oltz E.M., Jarvis J.N., Jiang K., Cicero T., Costello J.F., Wang T. G3: Genes|Genomes|Genetics; Bethesda: 2016. Mapping of variable DNA methylation across multiple cell types defines a dynamic regulatory landscape of the human genome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong J., Agha G., Baccarelli A.A. The role of DNA methylation in cardiovascular risk and disease: methodological aspects, study design, and data analysis for epidemiological studies. Circ Res. 2016;118:119–131. doi: 10.1161/CIRCRESAHA.115.305206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 40.Komatsu Y., Waku T., Iwasaki N., Ono W., Yamaguchi C., Yanagisawa J. Global analysis of DNA methylation in early-stage liver fibrosis. BMC Med Genomics. 2012;5:5. doi: 10.1186/1755-8794-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Götze S., Schumacher E.C., Kordes C., Häussinger D. Epigenetic changes during hepatic stellate cell activation. PLoS One. 2015;10:e0128745. doi: 10.1371/journal.pone.0128745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Taghdouini A., Sørensen A.L., Reiner A.H., Coll M., Verhulst S., Mannaerts I., Øie C.I., Smedsrød B., Najimi M., Sokal E., Luttun A., Sancho-Bru P., Collas P., van Grunsven L.A. Genome-wide analysis of DNA methylation and gene expression patterns in purified, uncultured human liver cells and activated hepatic stellate cells. Oncotarget. 2015;6:26729–26745. doi: 10.18632/oncotarget.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann J., Chu D.C., Maxwell A., Oakley F., Zhu N.L., Tsukamoto H., Mann D.A. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology. 2010;138:705–714. doi: 10.1053/j.gastro.2009.10.002. 714 e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann J., Oakley F., Akiboye F., Elsharkawy A., Thorne A.W., Mann D.A. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14:275–285. doi: 10.1038/sj.cdd.4401979. [DOI] [PubMed] [Google Scholar]
- 45.Song C., Feodorova Y., Guy J., Peichl L., Jost K.L., Kimura H., Cardoso M.C., Bird A., Leonhardt H., Joffe B., Solovei I. DNA methylation reader MECP2: cell type- and differentiation stage-specific protein distribution. Epigenetics & Chromatin. 2014;7:1–16. doi: 10.1186/1756-8935-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meehan R.R., Lewis J.D., Bird A.P. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992;20:5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaludov N.K., Wolffe A.P. MeCP2 driven transcriptional repression in vitro: selectivity for methylated DNA, action at a distance and contacts with the basal transcription machinery. Nucleic Acids Res. 2000;28:1921–1928. doi: 10.1093/nar/28.9.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellen M., Ayata P., Dewell S., Kriaucionis S., Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417–1430. doi: 10.1016/j.cell.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perugorria M.J., Wilson C.L., Zeybel M., Walsh M., Amin S., Robinson S., White S.A., Burt A.D., Oakley F., Tsukamoto H., Mann D.A., Mann J. Histone methyltransferase ASH1 orchestrates fibrogenic gene transcription during myofibroblast transdifferentiation. Hepatology. 2012;56:1129–1139. doi: 10.1002/hep.25754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byrd K.N., Shearn A. ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3. Proc Natl Acad Sci U S A. 2003;100:11535–11540. doi: 10.1073/pnas.1933593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Page A., Paoli P., Moran Salvador E., White S., French J., Mann J. Hepatic stellate cell transdifferentiation involves genome-wide remodeling of the DNA methylation landscape. J Hepatol. 2016;64:661–673. doi: 10.1016/j.jhep.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ji D., Lin K., Song J., Wang Y. Effects of Tet-induced oxidation products of 5-methylcytosine on Dnmt1- and DNMT3a-mediated cytosine methylation. Mol Biosyst. 2014;10:1749–1752. doi: 10.1039/c4mb00150h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tahiliani M., Koh K.P., Shen Y., Pastor W.A., Bandukwala H., Brudno Y., Agarwal S., Iyer L.M., Liu D.R., Aravind L., Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald J.I., Celik H., Rois L.E., Fishberger G., Fowler T., Rees R., Kramer A., Martens A., Edwards J.R., Challen G.A. Reprogrammable CRISPR/Cas9-based system for inducing site-specific DNA methylation. Biol Open. 2016;5:866–874. doi: 10.1242/bio.019067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vojta A., Dobrinić P., Tadić V., Bočkor L., Korać P., Julg B., Klasić M., Zoldoš V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016;44:5615–5628. doi: 10.1093/nar/gkw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeybel M., Hardy T., Wong Y.K., Mathers J.C., Fox C.R., Gackowska A., Oakley F., Burt A.D., Wilson C.L., Anstee Q.M., Barter M.J., Masson S., Elsharkawy A.M., Mann D.A., Mann J. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–1377. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy S.K., Yang H., Moylan C.A., Pang H., Dellinger A., Abdelmalek M.F., Garrett M.E., Ashley-Koch A., Suzuki A., Tillmann H.L., Hauser M.A., Diehl A.M. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076–1087. doi: 10.1053/j.gastro.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeybel M., Hardy T., Robinson S.M., Fox C., Anstee Q.M., Ness T., Masson S., Mathers J.C., French J., White S., Mann J. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin Epigenetics. 2015;7:25. doi: 10.1186/s13148-015-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castera L., Pinzani M. Biopsy and non-invasive methods for the diagnosis of liver fibrosis: does it take two to tango? Gut. 2010;59:861–866. doi: 10.1136/gut.2010.214650. [DOI] [PubMed] [Google Scholar]
- 60.Hardy T., Zeybel M., Day C.P., Dipper C., Masson S., McPherson S., Henderson E., Tiniakos D., White S., French J., Mann D.A., Anstee Q.M., Mann J. Plasma DNA methylation: a potential biomarker for stratification of liver fibrosis in non-alcoholic fatty liver disease. Gut. 2016 Mar 21 doi: 10.1136/gutjnl-2016-311526. pii: gutjnl-2016-311526. http://dx.doi.org/10.1136/gutjnl-2016-311526. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Z., Miller R.A., Patel R.T., Chen J., Dhir R., Wang H., Zhang D., Graham M.J., Unterman T.G., Shulman G.I., Sztalryd C., Bennett M.J., Ahima R.S., Birnbaum M.J., Lazar M.A. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 2012;18:934–942. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C., Chen X., Yang L., Kisseleva T., Brenner D.A., Seki E. Transcriptional repression of the transforming growth factor beta (TGF-beta) pseudoreceptor BMP and activin membrane-bound inhibitor (BAMBI) by nuclear factor kappaB (NF-kappaB) p50 enhances TGF-beta signaling in hepatic stellate cells. J Biol Chem. 2014;289:7082–7091. doi: 10.1074/jbc.M113.543769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang M., Kothapally J., Mao H., Tolbert E., Ponnusamy M., Chin Y.E., Zhuang S. Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2009;297:F996–F1005. doi: 10.1152/ajprenal.00282.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Diao J.S., Xia W.S., Yi C.G., Wang Y.M., Li B., Xia W., Liu B., Guo S.Z., Sun X.D. Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res. 2011;303:573–580. doi: 10.1007/s00403-011-1140-1. [DOI] [PubMed] [Google Scholar]
- 65.Davies E.R., Haitchi H.M., Thatcher T.H., Sime P.J., Kottmann R.M., Ganesan A., Packham G., O'Reilly K.M., Davies D.E. Spiruchostatin A inhibits proliferation and differentiation of fibroblasts from patients with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;46:687–694. doi: 10.1165/rcmb.2011-0040OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirpich I., Zhang J., Gobejishvili L., Kharebava G., Barker D., Ghare S., Joshi-Barve S., McClain C.J., Barve S. Binge ethanol-induced HDAC3 down-regulates Cpt1alpha expression leading to hepatic steatosis and injury. Alcohol Clin Exp Res. 2013;37:1920–1929. doi: 10.1111/acer.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Wang Z., Wang J., Lam W., Kwong S., Li F., Friedman S.L., Zhou S., Ren Q., Xu Z., Wang X., Ji L., Tang S., Zhang H., Lui E.L., Ye T. A histone deacetylase inhibitor, largazole, decreases liver fibrosis and angiogenesis by inhibiting transforming growth factor-beta and vascular endothelial growth factor signalling. Liver Int. 2013;33:504–515. doi: 10.1111/liv.12034. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe T., Tajima H., Hironori H., Nakagawara H., Ohnishi I., Takamura H., Ninomiya I., Kitagawa H., Fushida S., Tani T., Fujimura T., Ota T., Wakayama T., Iseki S., Harada S. Sodium valproate blocks the transforming growth factor (TGF)-beta1 autocrine loop and attenuates the TGF-beta1-induced collagen synthesis in a human hepatic stellate cell line. Int J Mol Med. 2011;28:919–925. doi: 10.3892/ijmm.2011.768. [DOI] [PubMed] [Google Scholar]
- 69.Mannaerts I., Nuytten N.R., Rogiers V., Vanderkerken K., van Grunsven L.A., Geerts A. Chronic administration of valproic acid inhibits activation of mouse hepatic stellate cells in vitro and in vivo. Hepatology. 2010;51:603–614. doi: 10.1002/hep.23334. [DOI] [PubMed] [Google Scholar]
- 70.Elsharkawy A.M., Oakley F., Lin F., Packham G., Mann D.A., Mann J. The NF-kappaB p50:p50:HDAC-1 repressor complex orchestrates transcriptional inhibition of multiple pro-inflammatory genes. J Hepatol. 2010;53:519–527. doi: 10.1016/j.jhep.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding N., Hah N., Yu R.T., Sherman M.H., Benner C., Leblanc M., He M., Liddle C., Downes M., Evans R.M. BRD4 is a novel therapeutic target for liver fibrosis. Proc Natl Acad Sci U S A. 2015;112:15713–15718. doi: 10.1073/pnas.1522163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang M.D., Chiang Y.M., Higashiyama R., Asahina K., Mann D.A., Mann J., Wang C.C., Tsukamoto H. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology. 2012;55:1271–1281. doi: 10.1002/hep.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park P.H., Miller R., Shukla S.D. Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochem Biophys Res Commun. 2003;306:501–504. doi: 10.1016/s0006-291x(03)01040-4. [DOI] [PubMed] [Google Scholar]
- 74.James T.T., Aroor A.R., Lim R.W., Shukla S.D. Histone H3 phosphorylation (Ser10, Ser28) and phosphoacetylation (K9S10) are differentially associated with gene expression in liver of rats treated in vivo with acute ethanol. J Pharmacol Exp Ther. 2012;340:237–247. doi: 10.1124/jpet.111.186775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghezzi A., Krishnan H.R., Lew L., Prado F.J., 3rd, Ong D.S., Atkinson N.S. Alcohol-induced histone acetylation reveals a gene network involved in alcohol tolerance. PLoS Genet. 2013;9:e1003986. doi: 10.1371/journal.pgen.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukla S.D., Lim R.W. Epigenetic effects of ethanol on the liver and gastrointestinal system. Alcohol Research: Current Reviews. 2013;35:47–55. doi: 10.35946/arcr.v35.1.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shepard B.D., Joseph R.A., Kannarkat G.T., Rutledge T.M., Tuma D.J., Tuma P.L. Alcohol-induced alterations in hepatic microtubule dynamics can be explained by impaired histone deacetylase 6 function. Hepatology. 2008;48:1671–1679. doi: 10.1002/hep.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim J.S., Shukla S.D. Histone h3 modifications in rat hepatic stellate cells by ethanol. Alcohol Alcohol. 2005;40:367–372. doi: 10.1093/alcalc/agh170. [DOI] [PubMed] [Google Scholar]
- 79.Page A., Paoli P.P., Hill S.J., Howarth R., Wu R., Kweon S.M., French J., White S., Tsukamoto H., Mann D.A., Mann J. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol. 2015;62:388–397. doi: 10.1016/j.jhep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J., Seok S., Yu P., Kim K., Smith Z., Rivas-Astroza M., Zhong S., Kemper J.K. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology. 2012;56:108–117. doi: 10.1002/hep.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bysani M., Wallerman O., Bornelöv S., Zatloukal K., Komorowski J., Wadelius C. ChIP-seq in steatohepatitis and normal liver tissue identifies candidate disease mechanisms related to progression to cancer. BMC Med Genomics. 2013;6:50. doi: 10.1186/1755-8794-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He Y., Huang C., Zhang S.P., Sun X., Long X.R., Li J. The potential of microRNAs in liver fibrosis. Cellular Signalling. 2012;24:2268–2272. doi: 10.1016/j.cellsig.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 83.Szabo G., Csak T. Role of microRNAs in NAFLD/NASH. Dig Dis Sci. 2016;61:1314–1324. doi: 10.1007/s10620-015-4002-4. [DOI] [PubMed] [Google Scholar]
- 84.Teng K.-Y., Ghoshal K. Role of noncoding RNAs as biomarker and therapeutic targets for liver fibrosis. Gene Expression. 2015;16:155–162. doi: 10.3727/105221615X14399878166078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo C.J., Pan Q., Cheng T., Jiang B., Chen G.Y., Li D.G. Changes in microRNAs associated with hepatic stellate cell activation status identify signaling pathways. FEBS J. 2009;276:5163–5176. doi: 10.1111/j.1742-4658.2009.07213.x. [DOI] [PubMed] [Google Scholar]
- 86.Chen C., Wu C.Q., Zhang Z.Q., Yao D.K., Zhu L. Loss of expression of miR-335 is implicated in hepatic stellate cell migration and activation. Exp Cell Res. 2011;317:1714–1725. doi: 10.1016/j.yexcr.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Noetel A., Elfimova N., Altmüller J., Becker C., Becker D., Lahr W., Nürnberg P., Wasmuth H., Teufel A., Büttner R., Dienes H.P., Odenthal M. Next generation sequencing of the Ago2 interacting transcriptome identified chemokine family members as novel targets of neuronal microRNAs in hepatic stellate cells. J Hepatol. 2013;58:335–341. doi: 10.1016/j.jhep.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 88.Coll M., El Taghdouini A., Perea L., Mannaerts I., Vila-Casadesús M., Blaya D., Rodrigo-Torres D., Affò S., Morales-Ibanez O., Graupera I., Lozano J.J., Najimi M., Sokal E., Lambrecht J., Ginès P., van Grunsven L.A., Sancho-Bru P. Integrative miRNA and gene expression profiling analysis of human quiescent hepatic stellate cells. Sci Rep. 2015;5:11549. doi: 10.1038/srep11549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 90.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 91.Povero D., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Momen-Heravi F., Saha B., Kodys K., Catalano D., Satishchandran A., Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tryndyak V.P., Latendresse J.R., Montgomery B., Ross S.A., Beland F.A., Rusyn I., Pogribny I.P. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol Appl Pharmacol. 2012;262:52–59. doi: 10.1016/j.taap.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bala S., Petrasek J., Mundkur S., Catalano D., Levin I., Ward J., Alao H., Kodys K., Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roderburg C., Mollnow T., Bongaerts B., Elfimova N., Vargas Cardenas D., Berger K., Zimmermann H., Koch A., Vucur M., Luedde M., Hellerbrand C., Odenthal M., Trautwein C., Tacke F., Luedde T. Micro-RNA profiling in human serum reveals compartment-specific roles of miR-571 and miR-652 in liver cirrhosis. PLoS One. 2012;7:e32999. doi: 10.1371/journal.pone.0032999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schwimmer J.B., Celedon M.A., Lavine J.E., Salem R., Campbell N., Schork N.J., Shiehmorteza M., Yokoo T., Chavez A., Middleton M.S., Sirlin C.B. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585–1592. doi: 10.1053/j.gastro.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brouwers M.C., Cantor R.M., Kono N., Yoon J.L., van der Kallen C.J., Bilderbeek-Beckers M.A., van Greevenbroek M.M., Lusis A.J., de Bruin T.W. Heritability and genetic loci of fatty liver in familial combined hyperlipidemia. J Lipid Res. 2006;47:2799–2807. doi: 10.1194/jlr.M600312-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Zarrinpar A., Gupta S., Maurya M.R., Subramaniam S., Loomba R. Serum microRNAs explain discordance of non-alcoholic fatty liver disease in monozygotic and dizygotic twins: a prospective study. Gut. 2016;65:1546–1554. doi: 10.1136/gutjnl-2015-309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donkin I., Versteyhe S., Ingerslev L.R., Qian K., Mechta M., Nordkap L., Mortensen B., Appel E.V., Jørgensen N., Kristiansen V.B., Hansen T., Workman C.T., Zierath J.R., Barrès R. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Heyn H., Esteller M. DNA methylation profiling in the clinic: applications and challenges. Nat Rev Genet. 2012;13:679–692. doi: 10.1038/nrg3270. [DOI] [PubMed] [Google Scholar]
- 101.Smith Z.D., Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]