Abstract
Background & Aims
Mesalamine is a first-line drug for treatment of inflammatory bowel diseases (IBD). However, its mechanisms are not fully understood. CD4+ Foxp3+ regulatory T cells (Tregs) play a potential role in suppressing IBD. This study determined whether the anti-inflammatory activity of mesalamine is related to Treg induction in the colon.
Methods
We examined the frequencies of Tregs in the colons of wild-type mice, mice deficient for aryl hydrocarbon receptor (AhR-/- mice), and bone marrow–chimeric mice lacking AhR in hematopoietic cells (BM-AhR-/- mice), following oral treatment with mesalamine. We also examined the effects of mesalamine on transforming growth factor (TGF)-β expression in the colon.
Results
Treatment of wild-type mice with mesalamine increased the accumulation of Tregs in the colon and up-regulated the AhR target gene Cyp1A1, but this effect was not observed in AhR-/- or BM-AhR-/- mice. In addition, mesalamine promoted in vitro differentiation of naive T cells to Tregs, concomitant with AhR activation. Mice treated with mesalamine exhibited increased levels of the active form of TGF-β in the colon in an AhR-dependent manner and blockade of TGF-β signaling suppressed induction of Tregs by mesalamine in the colon. Furthermore, mice pretreated with mesalamine acquired resistance to dextran sodium sulfate–induced colitis.
Conclusions
We propose a novel anti-inflammatory mechanism of mesalamine for colitis: induction of Tregs in the colon via the AhR pathway, followed by TGF-β activation.
Keywords: Mesalamine, Aryl Hydrocarbon Receptor, TGF-β, Regulatory T Cells
Abbreviations used in this paper: AhR, aryl hydrocarbon receptor; BM, bone marrow; DSS, dextran sodium sulfate; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; IBD, inflammatory bowel disease; IFN, interferon; IL, interleukin; LPL, lamina propria lymphocytes; mAb, monoclonal antibody; MLN, mesenteric lymph nodes; PBS, phosphate-buffered saline; Q-PCR, quantitative polymerase chain reaction; RPMI, Roswell Park Memorial Institute; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TGF, transforming growth factor; TNF, tumor necrosis factor; Tregs, regulatory T cells; WT, wild-type; XRE, xenobiotic responsive element
Graphical abstract
Summary.
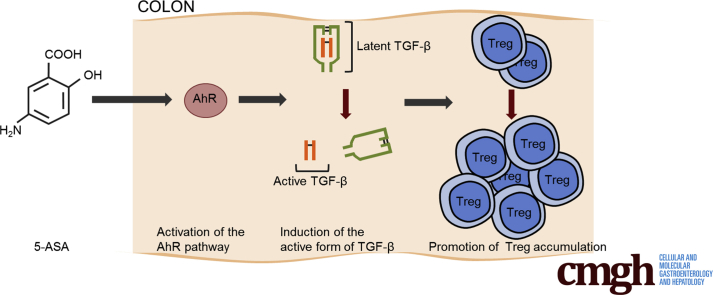
We propose a novel anti-inflammatory mechanism of mesalamine for colitis: induction of regulatory T cells in the colon via the aryl hydrocarbon receptor pathway, followed by transforming growth factor-β activation.
Mesalamine (or mesalazine or 5-aminosalicylic acid [5-ASA]) is an anti-inflammatory drug indicated for the treatment of inflammatory bowel disease (IBD).1, 2, 3 In particular, mesalamine is the core therapy for both induction and maintenance of remission in patients with mild-to-moderate ulcerative colitis. Mesalamine has pleiotropic effects on various signaling pathways, including the NF-kB, Wnt/β-catenin, PPAR-γ, MAPK, and PI3K/Akt axes,4 which are thought to be responsible for inhibition of colonic inflammation. However, the key mechanisms underlying mesalamine-mediated suppression of colitis remain unclear.
CD4+ Foxp3+ regulatory T cells (Tregs) contribute to the maintenance of immune homeostasis and may play a role in suppressing inflammatory diseases.5 At steady state, Tregs are abundant in the colonic mucosa in mice, constitute the most dominant CD4+ population in the colon, and suppress excessive immune responses to commensal bacteria.6 Bacterial colonization immediately after birth, especially by the spore-forming component of the indigenous intestinal microbiota (eg, clusters IV and XIVa of genus Clostridium), plays a pivotal role in the colon Treg accumulation by generating a transforming growth factor (TGF)-β-rich microenvironment.7 In IBD, the ability of transferred Treg to control inflammatory lesions has been demonstrated in rodent models of IBD.8, 9, 10 In addition, the Treg frequency in the peripheral blood was significantly lower in patients with active IBD than in healthy control subjects, suggesting disrupted Treg homeostasis in IBD.11, 12 Thus, Tregs may play a role in suppression of IBD.13
This study tested the hypothesis that mesalamine induces Tregs in the colon, and that these cells mediate the drug’s anti-inflammatory effects on colitis. We also investigated how mesalamine affected the accumulation of colon Tregs and whether this mechanism was associated with prevention of colitis.
Materials and Methods
Oral Administration of Mesalamine
Male or female 6-to-8-week-old C57BL/6J mice, male mice aged 30 ± 4 weeks (purchased from Japan SLC, Tokyo, Japan), mice bearing a homozygous deletion of the AhR gene (AhR-/- mice),14 and xenobiotic responsive element (XRE)-based sensing via SEAP (DRESSA) mice15 were bred under specific pathogen-free conditions. Mice were orally treated with phosphate-buffered saline (PBS) or mesalamine (5 or 50 mg/kg in 0.05 N HCl, Sigma-Aldrich Inc, St Louis, MO) once per day for 1 day, 3 days, or 2 weeks. Mice were orally treated with either dimethyl sulfoxide or 5 μg/kg 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; dioxin, Wako Pure Chemical Co, Osaka, Japan). Mice were orally treated with acetylsalicylic acid (aspirin) added to drinking water (0.6 mg/mL) for 2 weeks. Aspirin was added in drinking water because high doses of 1-shot oral feeding of aspirin induced severe injury of the small intestine as previously described.16 Based on the amounts of drinking water that the aspirin-treated mice took per day, we estimated that they received ∼50 mg/kg of aspirin per day. For some experiments, the TGF-β type I receptor kinase inhibitor GW788388 (R&D Systems, Minneapolis, MN) was added in drinking water (8 mg/mL) as previously described.17 All animal experiments were approved by the institutional review board for animal experiments at the University of Yamanashi and were carried out under the board’s guidelines.
Generation of Bone Marrow Chimeric Mice
Six-week-old recipient wild-type (WT) mice were exposed to X-irradiation (2 doses of 500 rad, 4 hours apart), and then injected intravenously 6 hours later with 1 × 107 bone marrow (BM) cells derived from the femurs of WT or AhR-/- donor mice. These chimeric mice were used for experiments at 14 weeks old. Two sets of BM chimeric mice were generated: WT mice were reconstituted either with WT mice BM (BM-AhR+/+) or with AhR-/- mice BM (BM-AhR-/-). The chimerism was confirmed by examining AhR mRNA expression levels in the mesenteric lymph nodes (MLNs) of BM chimeric mice by quantitative polymerase chain reaction (Q-PCR).
Preparation of Lamina Propria Lymphocytes
Lamina propria lymphocytes (LPLs) were isolated as described previously.7 Briefly, intestines were opened longitudinally, washed, and shaken for 45 minutes at 37°C in Hank’s balanced salt solution containing 5 mM EDTA. After removal of epithelial cells, the intestines were cut into small pieces and shaken for 40 minutes at 37°C in RPMI 1640 supplemented with 2% fetal bovine serum (FBS) and 1 mg/mL collagenase D (Wako Pure Chemical Co). The digested tissues were washed and resuspended in 100% Percoll (GE Healthcare, Little Chalfont, UK) with an underlayer of 40% Percoll followed by centrifugation at 850 × g for 20 minutes. LPLs were collected at the interface of the gradient and washed with RPMI 1640/10% FBS. For some experiments, CD4+ T cells from LPLs were purified by incubating LPLs with antibodies against CD4 coupled to magnetic beads, following manufacturer's instructions (MACS, Miltenyi Biotec, Auburn, CA). CD4+ T-cell population was sorted to a purity of 60%−70%.
Preparation of Mesenteric Lymph Nodes and Spleen
Single-cell suspensions were prepared from the MLNs and spleen, by removing fat and connective tissue and smashing between glass slides. Leukocyte suspensions were then washed with RPMI 1640/10% FBS. Additionally, splenic erythrocytes were then lysed with ammonium, chloride solution and the remaining leukocytes washed twice with RPMI 1640/10% FBS.
Flow Cytometry
For analyses of Tregs, the LPLs were stained with fluorescein isothiocyanate (FITC)-CD3 monoclonal antibody (mAb; clone 145-2C11, eBioscience, San Diego, CA) and APC-CD4 mAb (clone GK1.5, eBioscience), fixed, permeabilized, and stained with PE-Foxp3 mAb (clone FJK-16s, eBioscience) and PerCP-Cy 5.5-Ki67 mAb (clone B56, BD Pharmingen, San Diego, CA) using the Foxp3 staining buffer set (eBioscience). For the analyses of Th1 and Th17 cells, LPLs were stimulated at 37°C for 4 hours with 50 ng/mL phorbol myristate acetate (Sigma-Aldrich Inc), 750 ng/mL ionomycin (Sigma-Aldrich Inc) in the presence of 10 μg/mL brefeldin A (Sigma-Aldrich Inc). The cells were stained with PE or FITC-CD3 mAb and APC-CD4 mAb, fixed, permeabilized, and stained with FITC-interferon (IFN)-γ mAb (clone XMG1.2, eBioscience) or PE-IL-17 mAb (clone Bio17B7, eBioscience) using the fixation and permeabilization (eBioscience). All data were analyzed on a BD ACCURI C6 (BD Biosciences, San Jose, CA). Single-color compensation controls were prepared from cells treated in the same fashion as the test samples.
Immunofluorescence Study
Colon tissues were isolated from 5 mice treated orally either with PBS or mesalamine, washed, and then embedded in Tissue-Tek OCT compound (Sakura Fintek USA, Inc, Torrance, CA). Cryostat sections of 3 μm were fixed with 4% paraformaldehyde (Merck, Darmstadt, Germany) in 0.1 M phosphate buffer (pH 7.4) for 30 minutes at 4°C, followed by incubation with FITC-conjugated rat antimouse CD4 mAb (Clone RM4-5, BD Pharmingen) or isotype-matched control FITC-conjugated rat IgG2a (Clone R35-95, BD Pharmingen) for 1 hour at 37°C. Endogenous biotin was blocked using avidin-biotin blocking kit (Vector Laboratories, Burlingame, CA), according to the manufacturer’s instructions. After blocking, the sections were incubated with biotin-conjugated rat antimouse Foxp3 mAb (Clone FJK-16S, eBioscience) or isotype-matched control biotin-conjugated rat IgG2a (Clone R35-95, BD Pharmingen) for 1 hour at 37°C, and then labeled with Alexa Fluor 594-conjugated streptavidin (Invitrogen, Eugene, OR). The fluorescence images were captured with AxioPlan2 (Zeiss, Jena, Germany). The number of infiltrating CD4+ Foxp3+ cells in mucosa in each section was analyzed using KS400 Image analysis system (Zeiss). A total of 5–7 typical areas were examined in each specimen, and positively stained cell density was calculated per square millimeter.
Quantitative Real-Time Polymerase Chain Reaction
Q-PCR using cDNA from mouse tissue specimens was performed using the StepOne real-time PCR system (Applied Biosystems, Foster City, CA), using primers and probes for mouse Foxp3, T-bet, RorC (retinoic acid–related orphan receptor C), Cyp1A1, ALDH1A2 (aldehyde dehydrogenase 1A2), RAR-α (retinoic acid receptor), IL33, ST2, P2X7 (purinergic receptor P2X7), integrin αV, TSP1 (thrombospondin 1), AhR, TGF-β1, HO-1 (heme oxigenase-1), IL1β, IL6, MPO (myeloperoxidase), TNF-α (tumor necrosis factor), and 18S ribosomal RNA (rRNA) (Applied Biosystems). Each mRNA level was normalized against 18S rRNA, and the relative expression levels are shown.
Enzyme-Linked Immunosorbent Assay
The concentrations of IFN-γ and interleukin (IL) 17A in supernatants of cell cultures were measured using specific enzyme-linked immunosorbent assay (ELISA) kits (eBioscience). For quantitation of TGF-β1, colon tissue samples (0.4 g) were mixed with 1 mL of PBS containing 1% Triton X-100 and protease inhibitor cocktail (CALBIOCHEM, Cambridge, MA) and homogenized using a bead shocker. After centrifugation (15,000 rpm, 15 minutes), supernatants were collected, and the concentrations of TGF-β1 were measured with or without treatment by 0.2 N HCl using the TGF-β1 ELISA kit (R&D Systems).
T-Cell Differentiation In Vitro
Naïve CD4+ T cells were isolated from the spleens of WT mice or AhR-/- mice using the Naive CD4+ T-cell isolation kit (Miltenyi Biotech, Bergisch Gladbach, Germany). For Treg cell polarization, naive CD4+ T cells were stimulated with immobilized anti-CD3 mAb (2 μg/mL, clone145-2G1, eBioscience) and soluble anti-CD28 mAb (1 μg/mL, clone37.51, eBioscience) supplemented with human TGF-β1 (2.5 ng/mL, R&D Systems) and mouse IL2 (10 ng/mL, R&D Systems) with the indicated concentration of mesalamine for 3 days. For Th1 or Th17 cell polarization, naive CD4+ T cells were stimulated with immobilized anti-CD3 mAb and soluble anti-CD28 mAb for Th0; with recombinant mouse IL12 (10 ng/mL, R&D Systems) plus anti-IL4 mAb (10 μg/mL, clone11B11, eBioscience) for Th1; and with human TGF-β1, mouse IL6 (30 ng/mL, R&D Systems), anti-IL4 mAb, and anti-IFN-γ mAb (10 μg/mL, cloneXMG1.2, eBioscience) for Th17, with the indicated concentration of mesalamine, for 3 days.
Measurement of Serum SEAP Levels
Activity of SEAP in serum of DRESSA mice was evaluated by a chemiluminescent method using the Great EscAPe SEAP detection kit (BD Biosciences) as previously described.15
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation assay was performed using SimpleChIP Enzymatic Chromatin IP Kit (Agarose Beads, Cell Signaling, Tokyo, Japan, #9002). Naive CD4+ T cells were isolated from the spleens of WT mice and cultured for 2 hours in Treg differentiation media with or without mesalamine (1 μM) or TCDD (1 nM), and then cross-linked in 1% formaldehyde, followed by chromatin immunoprecipitation assay. Rabbit polyclonal antibody against AhR was purchased from Biomol (SA-210, Plymouth Meeting, PA). DNA was purified and Q-PCR was performed using the SYBR Green reagent (QIAGEN, Hilden, Germany). Q-PCR reactions were carried out using the following primer pairs: Cyp1A1-845, forward 5′-AGGCTCTTCTCACGCAACTC-3′ and reverse 5′-CTGGGGCTACAAAGGGTGAT-3′; Foxp3 (NCAB-2)-1596, forward 5′-GCCTTGTCAGGAAAAACTCTG-3′ and reverse 5′-GTCCTCGATTTGGCACAGAC-3′; Foxp3 (CAB) +13343, forward 5′-GCTTTGTGCGAGTGGAGAG-3′ and reverse 5′-AGGGATTGGAGCACTTGTTG-3′.
Measurement of Transforming Growth Factor-β Activity
TGF-β activity in colon samples was measured using clone MFB-F11, embryonic fibroblasts from TGF-β1-/- mice stably transfected with a reporter plasmid consisting of TGF-β-responsive Smad-binding elements coupled to a secreted alkaline phosphatase reporter gene (SBE-SEAP), as previously described.18
Briefly, colon tissue samples were prepared as described previously in the Methods section (ELISA) from WT mice or AhR-/- mice orally treated with mesalamine for 2 weeks. A total of 2 μL (1.6 mg) of colon tissue samples were added into the culture of MFB-F11 (1 × 104 cells/100 μL) for 24 hours and activities of SEAP were evaluated. To block TGF-β signaling, 10 μM of TGF-β type I receptor kinase inhibitor HTS466284 (Calbiochem, San Diego, CA) was added into the cell culture.
Fecal IgA Levels
For quantitation of IgA, stool pellets (0.25 g) were mixed with 1 mL of PBS and incubated at 4°C for 2 hours. Then, the pellet was vortexed for 5 minutes. After centrifugation (4,000 × g, 20 minutes), supernatants were collected and IgA concentrations were measured using the IgA ELISA kit (ICL Lab, Portland, OR).
Induction of Dextran Sodium Sulfate–Induced Colitis
Colitis was induced by feeding 3% dextran sodium sulfate (DSS) dissolved in distilled drinking water (molecular weight 5000; Wako Pure Chemical Co) for 8 days to WT mice or 5 days to AhR-/- mice.
Stool Scores
Stool scores were calculated as the sum of the diarrheal score and the bloody stool score. Briefly, stool consistency was graded as 0 = firm, 1 = loose, 2 = diarrhea, and 3 = severe diarrhea. Blood in the stool was also evaluated as 0 = no blood, 1 = occult blood, and 2 = gross rectal bleeding.
Histopathologic Scoring
The entire colon was removed and divided into 4 equal segments and fixed with 4% paraformaldehyde. The 2-μm sectioned colon was stained with hematoxylin and eosin and examined at 40 × magnification by light microscopy (Olympus BX50, Tokyo, Japan). Histopathologic evaluation was graded using a previously validated scoring system as previously described19: (a) inflammation severity: none = 0, slight = 1, moderate = 2, severe = 3; (b) depth of injury: none = 0, mucosal = 1, mucosal and submucosal = 2, transmural = 3; (c) crypt damage: none = 0, basal one-third damaged = 1, basal two-thirds damaged = 2, only surface epithelium intact = 3, entire crypt and epithelium lost = 4; and (d) percentage of area involved: none = 0, 1%–25% = 1, 26%–50% = 2, 51%–75% = 3, 76%–100% = 4. The final scores are the averages of all individual scores of 4 pieces per colon.
Statistical Analysis
Values represent the mean ± SEM. Statistical analysis was performed using the unpaired Student’s t test or the 1-way analysis of variance with the Tukey post hoc test. The 1-way analysis of variance with the Tukey post hoc test was used for multiple comparisons of the Q-PCR data (Table 1). A value of P < .05 was considered to be significant.
Table 1.
Changes of mRNA Levels of Genes Related to Treg Differentiation/Proliferation in the Colon of Mice Treated With Mesalamine
| Genes | Ratio of change ± SEMa | |
|---|---|---|
| AhR pathway | AhR | 1.55 ± 0.22 |
| Retinoic acid pathway | ALDH1A2 | 0.96 ± 0.14 |
| RAR-α | 1.40 ± 0.30 | |
| IL33/ST2 pathway | IL33 | 1.13 ± 0.13 |
| ST2 | 0.93 ± 0.29 | |
| ATP receptor | P2X7 | 0.96 ± 0.14 |
| Inflammatory cytokine | IL6 | 0.46 ± 0.07 |
| TNF-α | 0.91 ± 0.22 |
NOTE. Each mRNA level was normalized against 18S rRNA. The relative expression levels (mesalamine-treated mouse colon/PBS-treated mouse colon) are shown. The values are mean ± SEM.
P > .05; significance was calculated using 1-way analysis of variance with the Tukey post hoc test.
Results
Mesalamine Promotes Accumulation of Regulatory T Cells in the Lamina Propria of the Colon
To determine whether mesalamine affects the accumulation of Tregs in the large intestine, we measured the proportion of Tregs in the LP of the colon in WT mice treated with or without mesalamine. Patients with IBD generally take 25–50 mg/kg of mesalamine per day (1.5–3.0 g/day).20 Accordingly, in these experiments, we orally treated male 6-to-8-week-old WT mice with 50 mg/kg mesalamine per day for 2 weeks.
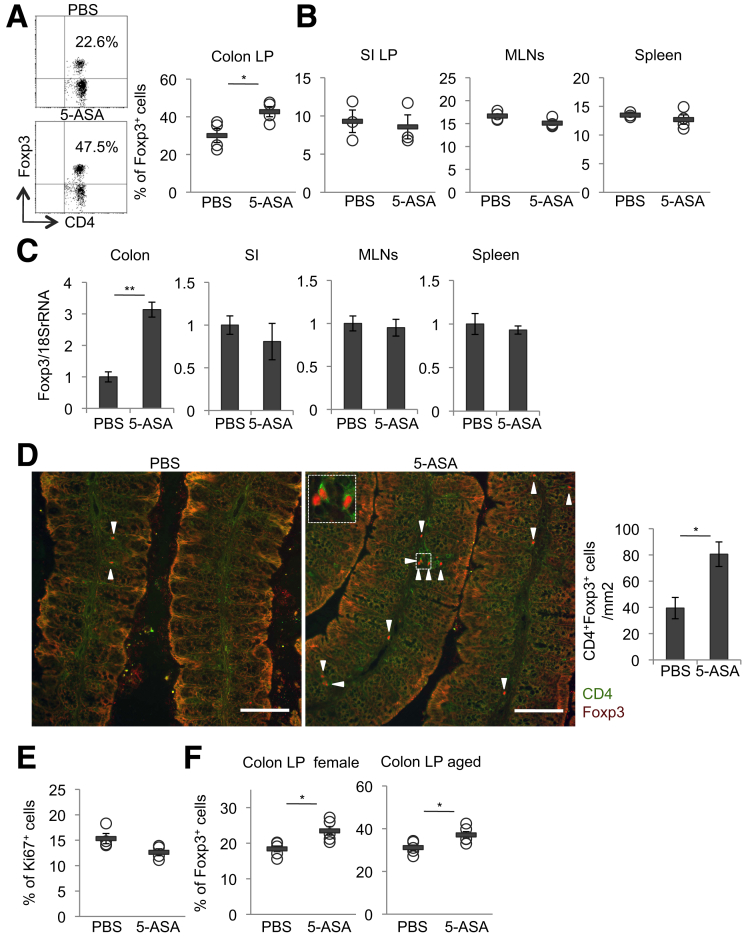
Flow cytometry analysis showed that treatment of mice with mesalamine significantly increased the proportion of CD4+ Foxp3+ Tregs in the LP of the colon, but not in the small intestine, MLNs, or spleen (Figures 1A and B), although the numbers of colon LPLs were comparable between control mice and mesalamine-treated mice (PBS-treated mice: 1.955 × 106/mouse vs mesalamine-treated mice: 1.86 × 106/mouse; n = 5; P = .8). Consistent with this, Foxp3 mRNA expression in the colon, but not in the small intestine, MLNs, or spleen, was significantly higher in mesalamine-treated mice than in control mice (Figure 1C). Furthermore, immunofluorescence analysis showed that treatment of mice with mesalamine significantly increased the numbers of Tregs in the LP of the colon (Figure 1D). In addition, treatment of mice with mesalamine did not affect the proportions of Ki67+ cells of Tregs in the LP of the colon (Figure 1E) and there were no differences in IL6 and TNF-α mRNA expression levels between control mice and mesalamine-treated mice (Table 1). We also observed that treatment of female 6-to-8-week-old WT mice or male mice aged 30 ± 4 weeks with 50 mg/kg mesalamine per day for 2 weeks significantly increased the proportion of Tregs in the LP of the colon (Figure 1F).
Figure 1.
Mesalamine increases the accumulation of Tregs in the LP of the colon. (A–E) Male 6-week-old WT mice were orally treated with mesalamine for 2 weeks. (A) Representative plots (left) and quantitative data (right) of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP. n = 4 mice per group. (B) Percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the LP of small intestine (SI) (n = 3 mice per group), MLNs (n = 4 mice per group), and spleen (n = 4 mice per group). (C) Foxp3 mRNA levels in the colon (n = 8 mice per group), SI (n = 8 mice per group), MLNs (n = 4 mice per group), and spleen (n = 4 mice per group). (D) Representative images (left) and the numbers of CD4+Foxp3+ cells in square millimeter in the lamina propria (right). Scale bar, 100 μm. Arrowheads indicate CD4+Foxp3+ cells (n = 5 mice per group). (E) The percentages of Ki67+ cells within the CD3+CD4+ Foxp3+ cell population isolated from the colon LP (n = 4 mice per group). (F) Female 6-week-old WT mice (left, n = 5 mice per group) or male aged (30 ± 4 week-old) mice (right, n = 5 mice per group) were orally treated with mesalamine for 2 weeks. Percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP. Error bars represent mean ± SEM. *P < .05, ∗∗P < .01. 5-ASA, mesalamine.
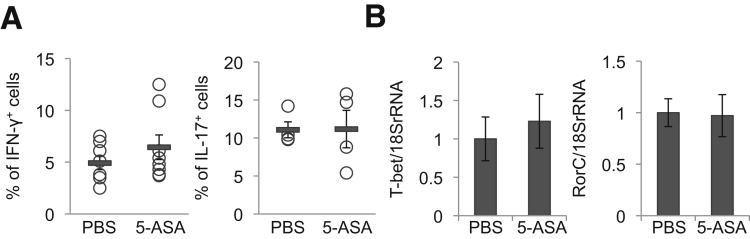
By contrast, 2-week mesalamine treatment of male 6-to-8-week-old WT mice did not affect the numbers of CD4+ IFN-γ+ cells (Th1-type cells) or CD4+IL17+ cells (Th17-type cells) in the colon LP or the levels of T-bet or RorC mRNAs in the colon (Figures 2A and B).
Figure 2.
Mesalamine does not increase the accumulation of Th1 cells and Th17 cells in the LP of the colon. Wild-type mice were orally treated with 50 mg/kg of mesalamine for 2 weeks. (A) Percentages of IFN-γ+ cells (n = 8 mice per group) or IL17+ cells (n = 4 mice per group) within the CD3+CD4+ cell population isolated from the colon LP. (B) T-bet and RorC mRNA levels in the colon (n = 4 mice per group). Error bars represent mean ± SEM. 5-ASA, mesalamine.
Collectively, these findings suggest that treatment of WT mice with mesalamine at a dose equivalent to its medical use for IBD treatment can promote the accumulation of Tregs in the colon without affecting other helper T-cell subsets. It seems that the accumulation of colon LP Tregs due to mesalamine treatment is not caused by elevated proliferation of Tregs or by subclinical inflammation induced by the drug.
Treatment of Mice With Mesalamine for Inflammatory Bowel Disease Treatment for at Least 3 Consecutive Days Can Promote the Accumulation of Regulatory T Cells in the Colon
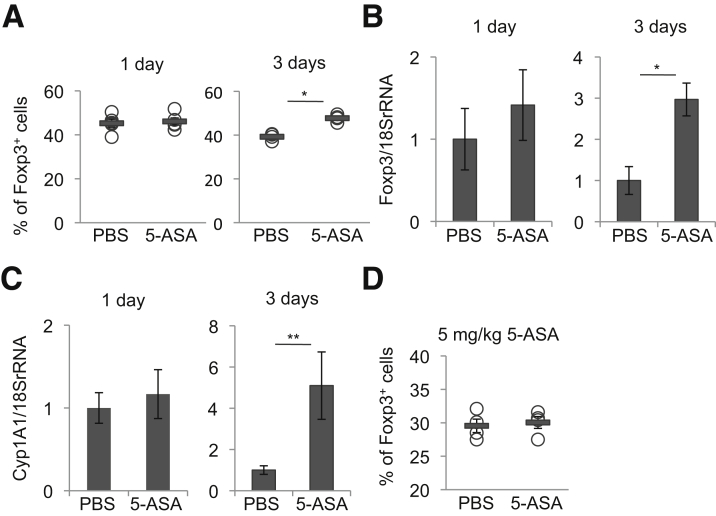
We next examined the time- and dose-dependent effects of mesalamine on the colonic Treg accumulation in mice. Male 6-to-8-week-old WT mice treated with mesalamine for 3 days, but not 1 day, significantly increased the proportion of colon LP Tregs and Foxp3 mRNA expression (and Cyp1A1 mRNA expression; see later) in the colon (Figures 3A–C). In addition, treatment of mice with 5 mg/kg/day, but not 50 mg/kg/day, of mesalamine for 2 weeks did not increase the proportion of colon LP Tregs (Figure 3D). Collectively, these findings suggest that treatment of mice with mesalamine at a dose equivalent to its medical use for IBD treatment (50 mg/kg) for at least 3 consecutive days can promote the accumulation of Tregs in the colon.
Figure 3.
Treatment of mice with mesalamine for at least 3 consecutive days can promote the accumulation of Tregs in the colon. (A–C) WT mice were orally treated with 50 mg/kg of mesalamine for 1 (n = 5 mice per group) or 3 days (n = 4 mice per group). The percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from colon LP (A) and Foxp3 (B) or Cyp1A1 (C) mRNA levels in the colons. (D) WT mice were orally treated with 5 mg/kg of mesalamine for 2 weeks, and the percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP are shown (n = 4 mice per group). Error bars represent mean ± SEM. *P < .05, **P < .01. 5-ASA, mesalamine.
Mesalamine Activates the Aryl Hydrocarbon Receptor Pathway, Which Is Necessary for Regulatory T Cell Induction
To obtain mechanistic insight into how mesalamine induces colon LP Tregs, we examined mRNA levels of genes related to Treg differentiation/proliferation in the mouse colon following treatment with 50 mg/kg of mesalamine per day for 2 weeks (Table 1).
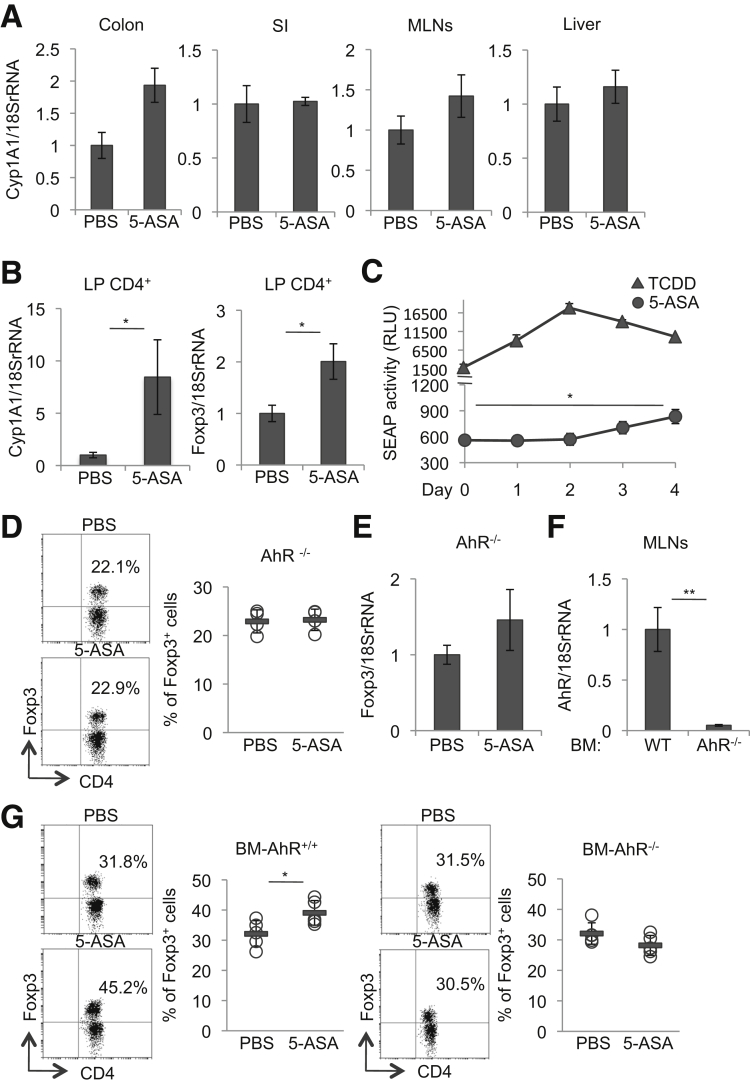
Among the genes examined, we observed an increased trend in mRNA levels of cytochrome P-450 family 1A1 (Cyp1A1), a major target gene of the aryl hydrocarbon receptor (AhR), in the colon, but not the small intestine, MLNs, and liver, of mice treated with mesalamine for 2 weeks (Figure 4A). Notably, we found a significant increase in mRNA levels of Cyp1A1 and Foxp3 in the colon LP CD4+ T cells of mice treated with mesalamine for 2 weeks (Figure 4B). In addition, we found a significant increase in Cyp1A1 mRNA expression levels in the colon of mice treated with 50 mg/kg of mesalamine for 3 days, but not for 1 day, (Figure 3C). Thus, the AhR pathway seemed to be activated by mesalamine in the colon, particularly in the colon LP CD4+ T cells.
Figure 4.
Mesalamine activates the AhR pathway, thereby inducing colon LP Tregs. (A) Cyp1A1 mRNA levels in the colon, small intestine (SI), MLNs, and liver isolated from WT mice orally treated with 50 mg/kg of mesalamine for 2 weeks (n = 8 mice per group). (B) Cyp1A1 and Foxp3 mRNA levels of the CD4+ cells in the LP of the colon isolated from WT mice orally treated with 50 mg/kg of mesalamine for 3 days (n = 5 mice per group). (C) Kinetics of serum SEAP in AhR-reporter DRESSA mice following orally treated once with 5 μg/kg TCDD (n = 3 mice per group) or orally treated with 50 mg/kg mesalamine for 4 days (n = 8 mice per group). (D, E) Percentages of Foxp3+ cells within the CD3+CD4+ cell population in the colon LP (D) (representative plots [left] and quantitative data [right]) and Foxp3 mRNA levels in the colon (E) isolated from AhR-/- mice orally treated with mesalamine for 2 weeks (n = 4 mice per group). (F) AhR mRNA levels in the MLNs from WT mice reconstituted with WT mice BM (BM-AhR+/+) or with AhR-/- mice BM (BM-AhR-/-) (n = 5 mice per group). (G) The percentages of Foxp3+ cells within the CD3+CD4+ cell populations in the colon LP isolated from WT mice reconstituted with WT mice BM (BM-AhR+/+) or WT mice reconstituted with AhR-/- mice BM (BM- AhR-/-) orally treated with mesalamine for 2 weeks (n = 5 mice per group). Representative plots (left) and quantitative data (right). Error bars represent mean ± SEM. *P < .05, **P < .01. 5-ASA, mesalamine.
The AhR is a ligand-activated transcriptional factor that is ubiquitously expressed in vertebrate cells.21, 22 Upon a ligand binding, AhR translocates to the nucleus and initiates the transcription of the target genes with promoters containing an XRE consensus sequence. Using AhR reporter mice in which the XRE consensus sequence is fused to the serum alkaline phosphatase (SEAP) gene,15 we detected activation of the AhR pathway after oral treatment with mesalamine for 3 days, although the AhR-activating capacity was much weaker than that of TCDD (dioxin), a strong synthetic AhR ligand (Figure 4C).
To determine whether activation of the AhR pathway by mesalamine is required for induction of Tregs in the colon LP, we examined the effects of AhR deficiency on Treg induction by mesalamine. Treatment of mice deficient for AhR (AhR-/- mice)14 with mesalamine did not affect the proportion of Tregs in the colon LP or Foxp3 mRNA expression in the colon (Figure 4D and E). In addition, we generated BM-chimeric mice reconstituted with BM cells derived from AhR-/- mice (BM-AhR-/- mice) or from WT mice (BM-AhR+/+ mice). We confirmed the engraftment of the donor cells in the chimeric experiments by analyzing AhR mRNA levels in the MLNs (Figure 4F). Treatment of BM-AhR-/- mice with mesalamine did not affect the frequencies of Tregs in the colon LP, in contrast to BM-AhR+/+ mice (Figure 4G).
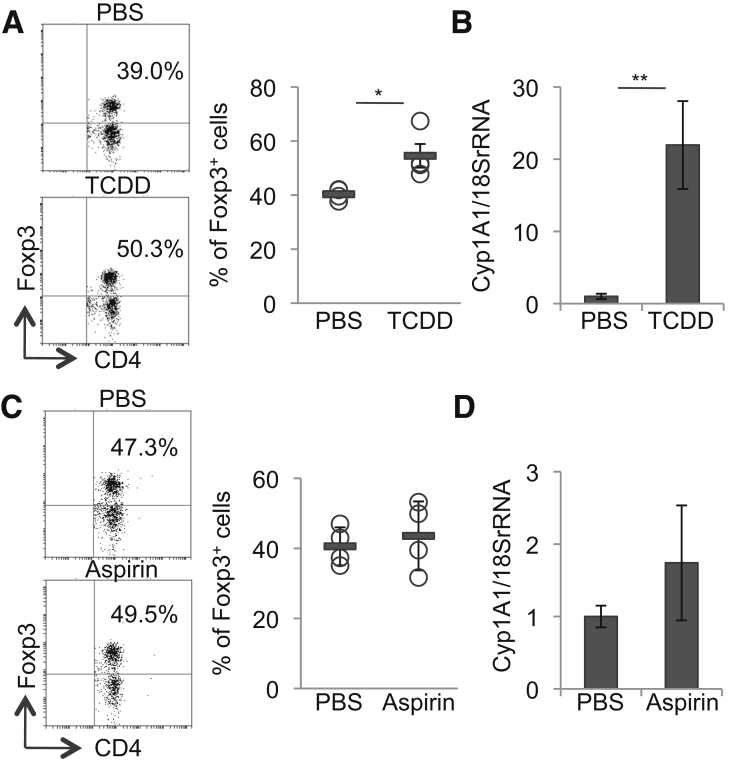
As previously described,23 TCDD increased the proportion of Tregs in the colon LP in association with Cyp1A1 mRNA induction in the colon (Figures 5A and B). By contrast, although mesalamine has a structure similar to that of acetylsalicylic acid (aspirin), treatment of mice with ∼50 mg/kg of aspirin per day for 2 weeks did not affect the proportion of Tregs in the colon LP and failed to induce Cyp1A1 mRNA expression in the colon (Figures 5C and D).
Figure 5.
TCDD, but not aspirin induces Tregs in the LP of the colon, concomitant with AhR activation. (A, B) WT mice were orally treated once with 5 μg/kg of TCDD. Three days later, the percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP of mice were analyzed by flow cytometry. (A) Representative plots (left) and quantitative data (right). (B) Cyp1A1 mRNA levels in the colon. (C, D) WT mice were orally treated with aspirin added to drinking water for 2 weeks, the percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP of mice were analyzed by flow cytometry. (C) Representative plots (left) and quantitative data (right). (D) Cyp1A1 mRNA levels in the colon. Error bars represent mean ± SEM, n = 4 mice per group. *P < .05, **P < .01.
Collectively, these findings suggest that mesalamine at a dose equivalent to its medical use for IBD treatment for at least 3 consecutive days can activate the AhR pathway in the colon, and that AhR expressed in hematopoietic cells is critical for the induction of colon LP Tregs by mesalamine.
Mesalamine Promotes Regulatory T-Cell Differentiation In Vitro, Concomitant With Aryl Hydrocarbon Receptor Activation
To obtain mechanistic insight into how mesalamine affects Treg accumulation in the mouse colon, we examined the effects of mesalamine on the in vitro differentiation of naive CD4+ T cells to Tregs. Under Treg differentiation conditions, mesalamine promoted differentiation from naive CD4+ T cells to Tregs in a dose-dependent manner, but little effects were observed in naive CD4+ T cells isolated from AhR-/- mice (Figure 6A). Mesalamine did not affect differentiation from naive CD4+ T cells into Th1 and Th17 cells under the corresponding differentiation conditions (Figure 6B).
Figure 6.
Mesalamine promotes Treg differentiation, concomitant with AhR activation. (A) Naive CD4+ T cells isolated from the spleen of WT mice or AhR-/- mice were cultured in Treg differentiation conditions with the indicated concentrations of mesalamine for 3 days, and the percentages of Foxp3+ cells within CD4+ cells were analyzed by flow cytometry (n = 3). (B) Naive CD4+ T cells isolated from the spleen in WT mice were stimulated in Th0, Th1, or Th17 differentiation conditions with the indicated concentrations of mesalamine for 3 days, and the supernatants of the cultures were collected. The concentrations of IFN-γ or IL17A were measured by ELISA (n = 2). (C) Chromatin immunoprecipitation assay analysis of the interaction of AHR with the nonconserved AhR binding site (NCABS) and conserved AhR binding site (CABS) in Foxp3 or XRE sequence of Cyp1A1 in naive CD4+ T cells under Treg differentiation conditions supplemented with PBS, mesalamine (1 μM), or TCDD (1 nM) (n = 3). Error bars represent mean ± SEM. *P < .05, **P < .01. Similar results were obtained from at least 3 independent experiments. 5-ASA, mesalamine.
There are 4 XRE sequence elements (nonconserved AhR binding sites 1, 2, and 3, and conserved AhR binding site) in the promoter region of the mouse Foxp3 gene.23 Chromatin immunoprecipitation assay assays revealed that mesalamine, like TCDD,24 induced AhR binding to the nonconserved AhR binding site 2 and conserved AhR binding site XRE in T cells under Treg differentiation conditions (Figure 6C). We also confirmed that mesalamine induced AhR binding to the XRE in the Cyp1A1 promoter, similar to TCDD in T cells under Treg differentiation conditions (Figure 6C). Collectively, these findings suggest that mesalamine can directly promote Treg differentiation in vitro, concomitant with AhR activation.
Mesalamine Increases the Level of the Active Form of TGF-β1, Which Is Necessary for Regulatory T Cell Induction
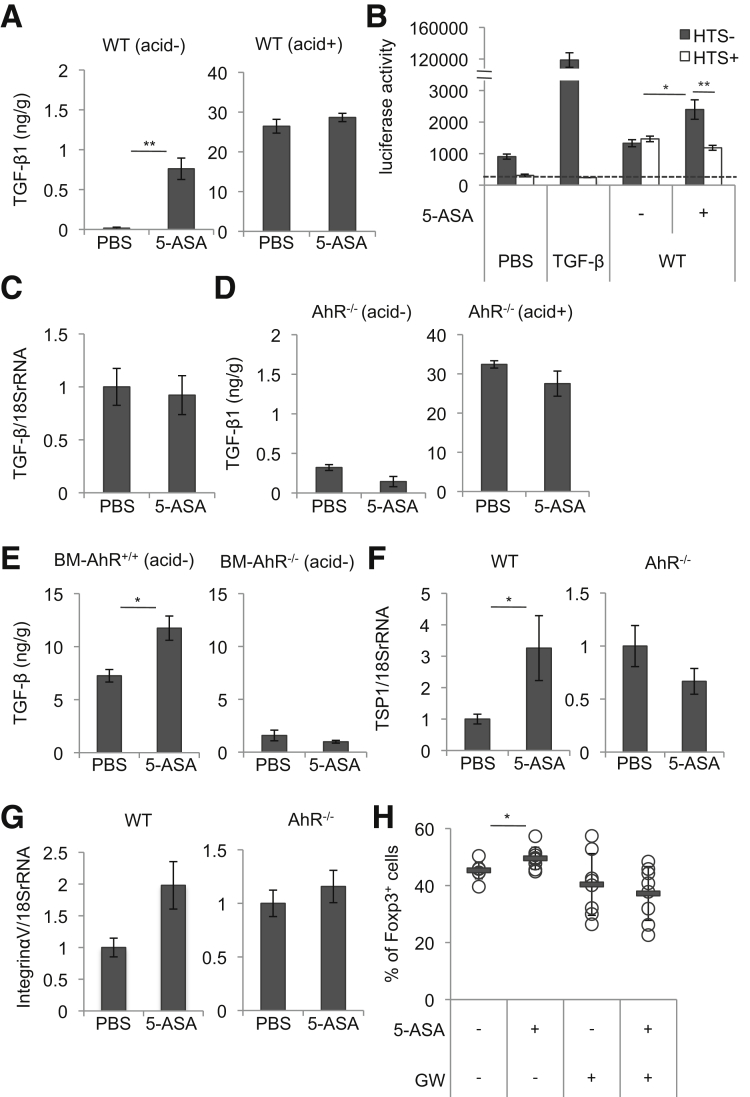
TGF-β is a critical regulator of Treg differentiation. Therefore, to further investigate how mesalamine induces Tregs via AhR activation, we examined the effects of mesalamine on TGF-β1 expression and activity in the colon. TGF-β is secreted by cells as an inactive precursor (the latent form) that must be activated by several factors including pH change, integrins, and proteases to exert its biologicaeffects.25 Interestingly, mesalamine significantly increased the amount of the active form of TGF-β1, but not the total amount of TGF-β1 (measured after acid treatment of samples), in the colons of WT mice (Figure 7A). By using reporter cells (MFB-F11 cells) that can measure as little as 1 pg/mL of active TGF-β1 in vitro,18 we confirmed that TGF-β1 activity significantly increased in the colons of mesalamine-treated mice, but not control mice (Figure 7B). TGF-β1 mRNA expression did not increase in the colon following mesalamine treatment (Figure 7C). The increase in the amount of the active form of TGF-β1 in the colon following mesalamine treatment was not observed in AhR-/- or BM-AhR-/- mice, but was observed in BM-AhR+/+ mice (Figures 7D and E). Consistent with the increase in the level of the active form of TGF-β1, we observed a significant increase and an increased trend in mRNA levels of thrombspondin-1 (TSP-1)26 and integrin αV,27 genes related to the conversion of the latent form of TGF-β to the active form, respectively, in the colons of mesalamine-treated WT mice, but not AhR-/- mice (Figures 7F and G).
Figure 7.
Mesalamine increases the level of the active form of TGF-β1, which is necessary for Treg induction. (A) Levels of the active form of TGF-β1 (acid-) or total TGF-β1 (acid+) in colons isolated from WT mice orally treated with mesalamine for 2 weeks (n = 5 mice per group). (B) Colon tissue samples (1.6 mg) from WT mice orally treated with mesalamine for 2 weeks were added into the culture of TGF-β reporter cells (MFB-F11) for 24 hours with or without HTS466284 (a chemical inhibitor of TGF-β signaling); then, SEAP activities of the supernatants were measured. 10 ng/mL human TGF-β1 was used as a positive control (n = 5). (C) TGF-β1 mRNA levels in the colon isolated from WT mice orally treated with mesalamine for 2 weeks (n = 8 mice per group). (D) Levels of active TGF-β1 (acid-) or of total TGF-β1 (acid+) in the colon isolated from AhR-/- mice treated orally with mesalamine for 2 weeks (n = 4 mice per group). (E) Levels of active TGF-β1 (acid-) or of total TGF-β1 (acid+) in the colon isolated from bone marrow chimeric mice (BM-AhR+/+ mice and BM-AhR-/- mice) treated orally with mesalamine for 2 weeks (n = 5 mice per group). (F, G) TSP-1 and Integrin αV mRNA levels in the colon isolated from WT mice or AhR-/- mice orally treated with mesalamine for 2 weeks (n = 8 mice per group). (H) WT mice were orally treated with mesalamine with or without GW788388, an inhibitor of TGF-β type I receptor kinase, in drinking water for 2 weeks, and the percentages of Foxp3+ cells within the CD3+CD4+ cell population isolated from the colon LP were determined (n = 8 mice per group). Error bars represent mean ± SEM. *P < .05, **P < .01. 5-ASA, mesalamine.
Importantly, pharmacologic blockade of TGF-β signaling in WT mice using GW788388, an inhibitor of TGF-β type I receptor kinase,17 suppressed induction of colonic LP Tregs by mesalamine (Figure 7H). These findings suggest that treatment of mice with mesalamine increased the active form of TGF-β1 in the colon, depending on AhR expressed in hematopoietic cells, which is required for induction of colon Tregs by mesalamine.
Mesalamine Increases Fecal IgA Production
Tregs and TGF-β contribute to IgA production in the mouse intestine.28, 29 If mesalamine indeed increases the numbers of Tregs and the active form of TGF-β1 in the colon, it should increase IgA production. Accordingly, we examined the production of IgA in the stools of mice treated with 50 mg/kg mesalamine per day for 2 weeks. The amount of IgA in stool samples was significantly higher in mesalamine-treated mice than in control mice and AhR-/- mice (Figure 8A). In addition, no increase in the level of stool IgA was observed in BM-AhR-/- mice, but an increase was observed in BM-AhR+/+ mice (Figure 8B). These findings suggest that mesalamine can increase intestinal IgA production in an AhR-dependent manner, which may be associated with increased numbers of Tregs and the active form of TGF-β1 in the colon.
Figure 8.
Mesalamine increases fecal IgA production. (A) WT (n = 6 mice per group) and AhR-/- mice (n = 4 mice per group), or (B) bone marrow chimeric mice (BM-AhR+/+ mice and BM-AhR-/- mice) (n = 10 mice per group) were orally treated with 50 mg/kg of mesalamine for 2 weeks. Concentrations of IgA of stool samples were measured by ELISA. Error bars represent mean ± SEM. *P < .05. 5-ASA, mesalamine.
Mice Pretreated With Mesalamine Are Resistant to Colitis
Colon Tregs induced by a defined mix of Clostridium strains confer resistance to DSS-induced colitis, a model of ulcerative colitis.30 To evaluate the potential benefits of mesalamine-induced colon Tregs, we examined the effects of mesalamine, under colon Treg-inducing conditions, on DSS-induced colitis.
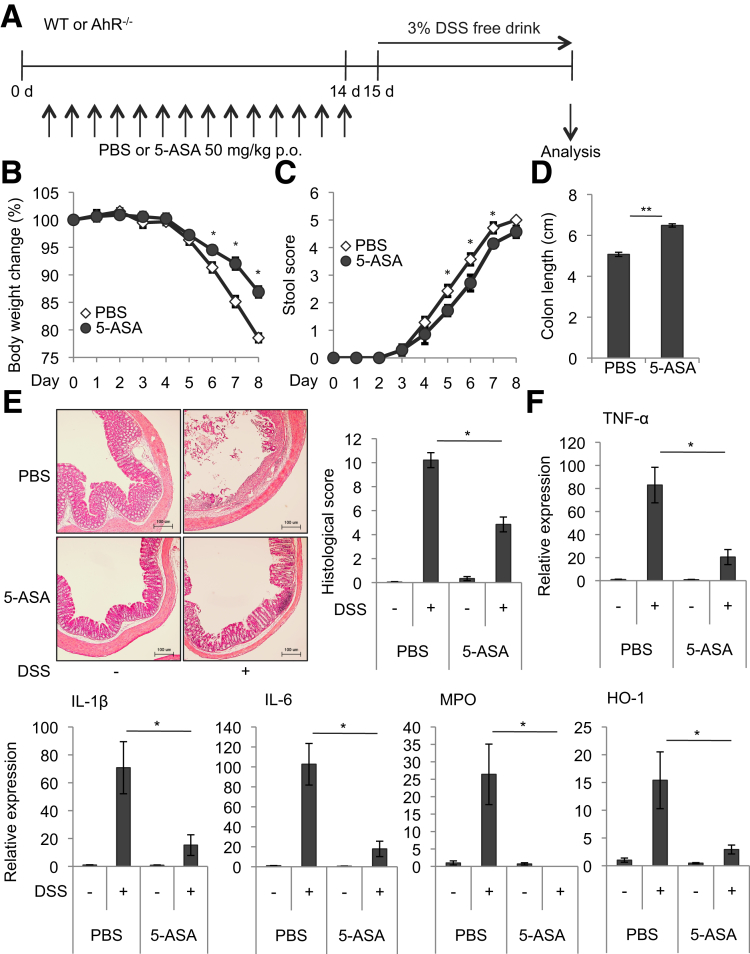
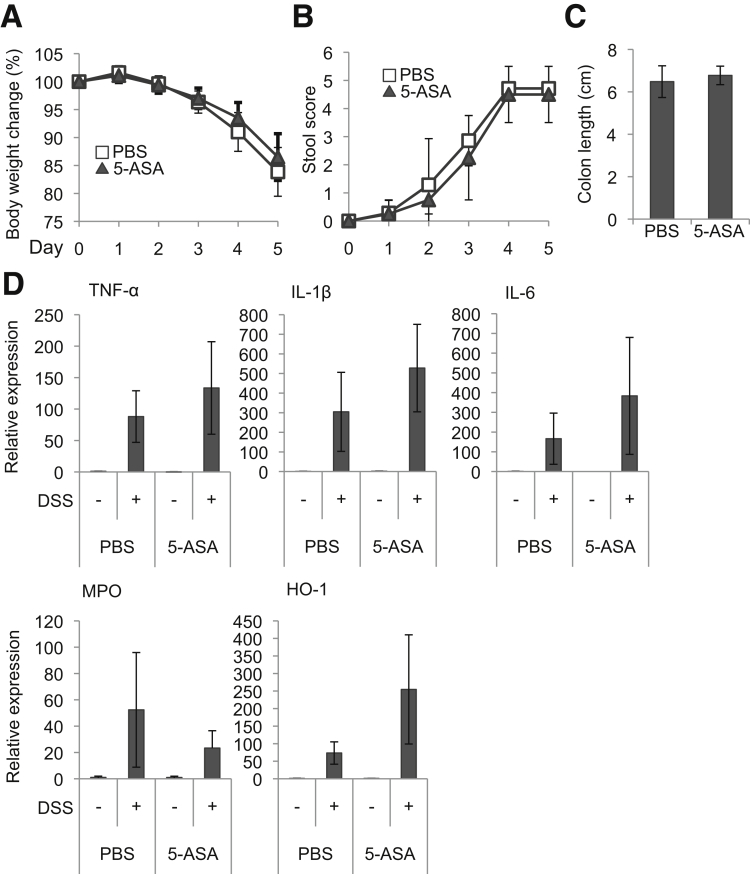
We treated mice orally with 50 mg/kg of mesalamine per day for 2 weeks (Days 1–14) and, in light of the ∼5-hour biologic half-life of mesalamine,31 24 hours after the last mesalamine administration (Day 15) subjected the mice to DSS treatment to induce colitis (Figure 9A). Following the induction of DSS-induced colitis, mesalamine-treated mice exhibited less weight change, stool consistency and bleeding, colon shortening, histologic changes, and colonic expression of inflammatory markers (TNF-α, IL1β, IL6, MPO, and HO-1) than control mice (Figures 9B–F). Similarly, AhR-/- mice were treated with 50 mg/kg of mesalamine per day for 2 weeks, and 24 hours later the mice were treated with DSS to induce colitis. However, although AhR-/- mice showed severe phenotypes of DSS-induced colitis compared with WT mice, AhR-/- mice treated with mesalamine exhibited weight changes, stool consistency and bleeding, colon shortening, and expression of inflammatory markers (TNF-α, IL1β, IL6, MPO, and HO-1) comparable with those in AhR-/- mice treated with vehicle following DSS treatment (Figures 10A–D). Collectively, although additional mechanisms and cell types could be involved, these findings suggest that mesalamine-mediated induction of colon Tregs via AhR may be responsible for inhibition of DSS-induced colitis by mesalamine.
Figure 9.
Mice pretreated with mesalamine acquire the resistance to DSS-induced colitis. (A) Experimental protocol. WT or AhR-/- mice (see Figure 10) were orally treated with mesalamine for 2 weeks; 24 hours after the last administration of mesalamine, the mice were treated with 3% DSS to induce colitis. (B) Body weight changes in mice after administration of DSS (n = 7 mice per group). (C) Stool score changes in mice after administration of DSS (n = 7 mice per group). (D) Colon length in mice after administration of DSS (n = 7 mice per group). (E) Colon histopathology. Representative pictures (left) and quantitative data (right). Scale bar indicates 100 μm (n = 3 per group). (F) TNF-α, IL1β, IL6, MPO, and HO-1 mRNA levels in the colon after administration of DSS were analyzed by Q-PCR (n = 7 mice per group). Error bars represent mean ± SEM. *P < .05, **P < .01. 5-ASA, mesalamine.
Figure 10.
AhR-/-mice pretreated with mesalamine do not acquire the resistance to DSS-induced colitis. AhR-/- mice were orally treated with mesalamine for 2 weeks; 24 hours after the last administration of mesalamine, the mice were treated with 3% DSS to induce colitis. (A) Body weight changes in mice after administration of DSS (n = 5 mice per group). (B) Stool score changes in mice after administration of DSS (n = 5 mice per group). (C) Colon length in mice after administration of DSS (n = 5 per group). (D) TNF-α, IL1β, IL6, MPO, and HO-1 mRNA levels in the colon after administration of DSS were analyzed by Q-PCR (n = 5 per group). Error bars represent mean ± SEM. 5-ASA, mesalamine.
Discussion
Mesalamine plays a well-established role in the management of IBD, but its anti-inflammatory mechanisms are not fully understood. The results of this study may reveal a previously unknown anti-inflammatory mechanism of mesalamine: induction of colon Tregs. Mesalamine induced activation of the AhR pathway and increased the level of the active form of TGF-β1 in the colon, which is critical for the accumulation of colon LP Tregs. In addition, pretreatment of mice with mesalamine inhibited DSS-induced colitis, concomitant with induction of Tregs. Given that mesalamine can induce Cyp1A1 expression in colon LP CD4+ T cells, AhR expressed in hematopoietic cells is required for the mesalamine-mediated induction of Tregs, mesalamine promotes Treg differentiation via AhR in vitro, and mesalamine does not increase the proportion of Ki67+ Tregs in the colon, mesalamine may increase the accumulation of colon Tregs by directly promoting Treg differentiation, rather than proliferation.
Mesalamine promotes the accumulation of Tregs in the colon LP via AhR, without affecting the Th1 and Th17 subsets. AhR is expressed in Tregs and Th17 cells, but not in Th1 or Th2 cells.24 In contrast to Tregs, we did not observe a significant increase in the proportion of colon Th17 cells following the treatment with mesalamine (Figure 2). Previous studies showed that AhR regulates both Treg and Th17 cell differentiation in a ligand-specific fashion.24, 32 Although TCDD and 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester are potent inducers of Treg differentiation and suppress EAE,24 6-formylindolo[3,2-b] carbazole enhances Th17 differentiation and increases severity of the disease. 24, 32 These findings pointed out that AHR ligands of different origin and, most likely, different affinities differentially impact the outcome of Treg/Th17 cell differentiation. Thus, it will be an important issue to determine direct binding ability of mesalamine to AhR and, if any, its affinity to AhR for understanding how mesalamine preferentially induces Tregs.
Mesalamine is rapidly adsorbed, extensively acetylated to N-acetyl-mesalamine by the N-acetyltransferase 1 enzyme in colonic mucosa, and supposed to lose its activity.33 This metabolic pathway of mesalamine may explain the lack of its systemic effects and also a requirement for a high dose (50 mg/kg) of mesalamine on the colon Tregs accumulation. Given that 5 mg/kg of mesalamine did not affect colon Treg accumulation, we will determine precisely how much dose of mesalamine is a minimum required for the colon Treg induction in our future studies.
It remains to be determined how mesalamine promoted the accumulation of Tregs selectively in the colon LP, concomitant with induction of Cyp1A1 mRNA in the colon, but not in the small intestine. We speculate that unique microenvironment of the large intestine might play a role in promoting colon Treg accumulation by mesalamine. Interactions between indigenous intestinal miroflora and the host play a critical role in the accumulation of colonic, but not small intestinal, LP Tregs.7 The commensals stimulate the differentiation, migration, and proliferation of Tregs in the colon LP,34, 35, 36 which may be independent on the MLNs.37 In addition, colonic Tregs arise by means of commensal bacterial antigen-driven peripheral Treg development, thereby providing tolerance to commensal microbiota.38 Thus, it is conceivable that colon LP is a unique site where Tregs are being constantly developed under the strong influence of commensal bacteria at steady state.36, 37 This unique Treg-developing microenvironment in the colon, but not in the small intestine, might somehow facilitate the promotion of colon LP Treg accumulation in response to mesalamine.
Given that mesalamine-induced activation of AhR in hematopoietic cells is required for colon Treg induction and mesalamine can enhance Treg differentiation in vitro, concomitant with AhR activation under Treg differentiation conditions, we assume that mesalamine induces the accumulation of colon Tregs by directly promoting Treg differentiation in the colon. The findings that mesalamine can induce Cyp1A1 expression in the colon LP CD4+ T cells may also support this notion. However, it is also possible that mesalamine induces colon Tregs by acting on AhR expressed in dendritic cells and thereby promoting the development of tolerogenic dendritic cells that support Treg differentiation, as previously described.39, 40 Other possible mechanisms underlying mesalamine-induced colonic Treg accumulation independent of AhR are discussed later.
The precise mechanisms underlying the increase in the level of the active form of TGF-β1 in the colon by mesalamine via AhR remain to be determined. Given that mesalamine did not affect TGF-β1 mRNA levels and total amounts of TGF-β1 protein, whereas mesalamine increased expression of TSP-1 and integrin αV, genes related to conversion of the latent form of TGF-β1 to the active form, mesalamine might facilitate TGF-β1 activation in the colon via AhR–mediated control of those genes. Consistent with this notion, previous studies suggest that AhR initiates transcription of TSP-1 via activation of the promoter41 and AhR mediates integrin control of TGF-β signaling42. Similarly, a defined mix of Clostridium strains induces colon Tregs, concomitant with increased expression of metalloproteinase 2, 9, and 13,7 which have been reported to be involved in the activation of TGF-β.25
We and others showed that activation of the AhR pathway can suppress rodent models of IBD.43, 44, 45, 46 The current findings suggest that the beneficial effect of mesalamine on IBD is linked to its potential to activate AhR in the colon. Because mesalamine is a first-line drug for IBD, our results provide additional strong support for the protective role of AhR against IBD and a rationale for targeting AhR for treatment of IBD. In support of this notion, Chinese herbal medicine Qing-Dai (also known as indigo naturalis), which has been used to treat patients with ulcerative colitis, is recently shown to contain indole compounds that act as AhR ligands.47 Furthermore, we recently report that 1,4-dihydroxy-2-naphthoic acid, a precursor of menaquinone (vitamin K2) abundantly produced by Propionibacterium freudenreichii ET-3 isolated from Swiss-type cheese, acts as an AhR activator and inhibits DSS-induced colitis.48 Because 1,4-dihydroxy-2-naphthoic acid is commercially available in Japan as a prebiotic supplement without severe adverse effects, 1,4-dihydroxy-2-naphthoic acid might become a promising drug candidate for IBD via AhR activation.
Although we suggest that mesalamine induces colon LP Tregs via AhR, other changes in host responses induced by mesalamine could mediate the colon LP Treg induction independent of AhR. For instance, mesalamine increases PPAR-γ expression and activates its target genes,49 which can lead to the generation of Tregs.50, 51 In addition, salicylate or its derivative, such as acetylsalicylic acid (aspirin), induces AMP-activated protein kinase activation,52 which can lead to the generation of Tregs.53 Because mesalamine has a similar structure to salicylate or aspirin, this mechanism may be also involved in mesalamine-mediated colon Treg induction. Therefore, it is highly likely that mesalamine can induce Tregs through multiple mechanisms, including AhR. Because salicylate can also activate AhR,54 these mechanisms could operate in ways that are not mutually exclusive.
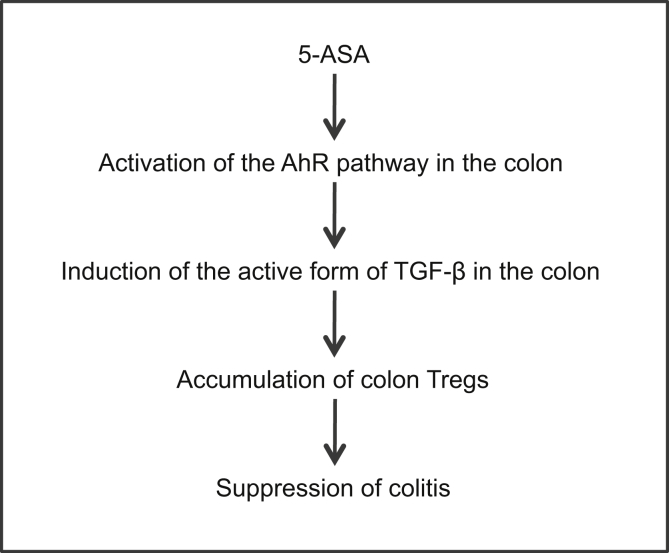
In summary, we propose a novel anti-inflammatory mechanism of mesalamine for colitis: induction of Tregs in the colon via the AhR pathway, followed by TGF-β activation (Figure 11).
Figure 11.
Proposed model. 5-ASA, mesalamine.
Acknowledgements
The authors thank Ms Tomoko Tohno and Ms Mutsuko Hara for their valuable general assistance.
Footnotes
Author contributions Kyoko Oh-oka, Yuko Kojima, Koichiro Uchida, Kimiko Yoda, Shotaro Nakajima, and Jun Hemmi performed the experiments and analyzed the data. Hiroshi Kano, Yoshiaki Fujii-Kuriyama, Ryohei Katoh, and Hiroyuki Ito contributed the reagents. Kyoko Oh-oka, Hiroshi Kano, Hiroyuki Ito, and Atsuhito Nakao conceived the study, designed the experiments, analyzed the data, and wrote the manuscript.
Conflicts of interest These authors disclose the following: Atsuhito Nakao has received grant support from Meiji Co, Ltd. Jun Hemmi, Hiroshi Kano, and Hiroyuki Ito are employees of Meiji Co, Ltd. The remaining authors disclose no conflicts.
Funding This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and from Meiji Co, Ltd.
References
- 1.Hauso Ø., Martinsen T.C., Waldum H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand J Gastroenterology. 2015;50:933–941. doi: 10.3109/00365521.2015.1018937. [DOI] [PubMed] [Google Scholar]
- 2.Perrotta C., Pellegrino P., Moroni E., De Palma C., Cervio D., Daneli P., Clementi E. Five-aminosalicylic acid: an update for the reappraisal of an old drug. Gastroenterol Res Pract. 2015;2015:456895. doi: 10.1155/2015/456895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohm S.K., Kruis W. Long-term efficacy and safety of once-daily mesalazine granules for the treatment of active ulcerative colitis. Clin Exp Gastroenterol. 2014;7:369–383. doi: 10.2147/CEG.S35691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stolfi C., De Simone V., Pallone F., Monteleone G. Mechanisms of action of non-steroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci. 2013;14:17972–17985. doi: 10.3390/ijms140917972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohkura N., Kitagawa Y., Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Tanoue T., Atarashi K., Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol. 2016;16:295–309. doi: 10.1038/nri.2016.36. [DOI] [PubMed] [Google Scholar]
- 7.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H., Hu B., Xu D., Liew F.Y. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-β, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 9.Mottet C., Uhlig H.H., Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 10.Foussat A., Cottrez F., Brun V., Fournier N., Breittmayer J.P., Groux H. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J Immunol. 2003;171:5018–5026. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- 11.Eastaff-Leung N., Mabarrack N., Barbour A., Cummins A., Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–89. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
- 12.Pedros C., Duguet F., Saoudi A., Chabod M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J Gastroenterol. 2016;22:974–995. doi: 10.3748/wjg.v22.i3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shale M., Schiering C., Powrie F. CD4+ T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252:164–182. doi: 10.1111/imr.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mimura J., Yamashita K., Nakamura K., Morita M., Tkagi T.N., Nakao K., Ema M., Sogawa K., Yasuda M., katsuki M., Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- 15.Kasai A., Hiramatsu N., Hayakawa K., Yao J., Maeda S., Kitamura M. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006;66:7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- 16.Cipriani S., Mencarelli A., Bruno A., Renga B., Distrutti E., Santucci L., Baldelli F., Fiorucci S. Activation of the bile acid receptor GPBAR1 protects against gastrointestinal injury caused by non-steroidal anti-inflammatory drugs and aspirin in mice. Br J Pharmacol. 2013;168:225–237. doi: 10.1111/j.1476-5381.2012.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen M., Thorikay M., Deckers M., van Dinther M., Grygielko E.T., Gellibert F., de Gouville A.C., Huetten Dijke P., Laping N.J. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705–715. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 18.Tesseur I., Zou K., Berber E., Zhang H., Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-β. BMC Cell Biol. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 20.Wang Y., Parker C.E., Feagan B.G., MacDonald J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016;5:CD000544. doi: 10.1002/14651858.CD000544.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 22.Mulero-Navarro S., Fernandez-Salguero P.M. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N.P., Singh U.P., Singh B., Price R.L., Nagarkatti M., Nagarkatti P.S. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintana F.J., Basso A.S., Iglesias A.H., Korn T., Faraz M.F., Bettelli E., Caccamo M., Oukka M., Weiner H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 25.Annes J.P., Munger J.S., Rifkin D.B. Making sense of latent TGF-β activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 26.Crawford S.E., Stellmach V., Murphy-Ullrich J.E., Ribeiro S.M., Lawler J., Hynes R.O., Boivin G.P., Bouck N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 27.Travis M.A., Reizis B., Melton A.C., Masteller E., Tang Q., Proctor J.M., Wang Y., Bernstein X., Huang X., Reichardt L.F., Bluestone J.A., Sheppard D. Loss of integrin α(v)β8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cong Y., Feng T., Fujihashi K., Schoeb T.R., Elson C.O. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohtsuka Y., Sanderson I.R. Transforming growth factor-β: an important cytokine in the mucosal immune response. Curr Opin Gastroenterol. 2000;16:541–545. doi: 10.1097/00001574-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Kiesler P., Fuss I.J., Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aumais G., Lefebvre M., Tremblay C., Bitton A., Martin F., Giard A., Madi M., Spenard J. Rectal tissue, plasma and urine concentrations of mesalazine after single and multiple administrations of 500 mg suppositories to healthy volunteers and ulcerative proctitis patients. Aliment Pharmacol Ther. 2003;17:93–97. doi: 10.1046/j.1365-2036.2003.01409.x. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M., Hirota K., Westendorf A.M., Buer J., Dumoutier L., Renauld J.C., Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 33.Rijk M.C.M., van Schaik A., van Tongeren J.H.M. Disposition of 5-aminosalicylic acid by 5-aminosalicylic acid-delivering compounds. Scand J Gastroenterol. 1988;23:107–712. doi: 10.3109/00365528809093858. [DOI] [PubMed] [Google Scholar]
- 34.Arpaia N., Campbell C., Fan X., Dikiy S., van der Veeken J., deRoos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., Rudensky A.Y. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly-YM, Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obata Y., Furusawa Y., Endo T.A., Sharif J., Takahashi D., Atarashi K., Nakayama M., Onawa S., Fujimura Y., Takahashi M., Ikawa T., Otsubo T., Kawamura Y.I., Dohi T., Tajima S., Masumoto H., Ohara O., Honda K., Hori S., Ohno H., Koseki H., Hase K. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat Immunol. 2014;15:571–579. doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]
- 37.Geem D., Ngo V., Harusato A., Chassaing B., Gewirtz A.T., Newberry R.D., Denning T.L. Contribution of mesenteric lymph nodes and GALT to the intestinal Foxp3+ regulatory T-Cell compartment. Cell Mol Gastroenterol Hepatol. 2016;2:274–280. doi: 10.1016/j.jcmgh.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lathrop S.K., Bloom S.M., Rao S.M., Nutsch K., Lio C.W., Santacruz N., Peterson D.A., Stappenbeck T.S., Hsieh C.S. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quintana F.J., Murugaiyan G., Farez M.F., Mitsdoerffer M., Tukpah A.M., Burns E.J., Weiner H.L. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010;107:20768–20773. doi: 10.1073/pnas.1009201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen N.T., Kimura A., Nakahama T., Chinen I., Masuda K., Nohara K., Fujii-Kuriyama Y., Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107:19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dabir P., Marinic T.E., Krukovets I., Stenina O.I. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silginer M., Burghardt I., Gramatzki D., Bunse L., Leske H., Rushing E.J., Hao N., Platten M., Weller M., Roth P. The aryl hydrocarbon receptor links integrin signaling to the TGF-β pathway. Oncogene. 2016;35:3260–3271. doi: 10.1038/onc.2015.387. [DOI] [PubMed] [Google Scholar]
- 43.Monteleone I., Macdonald T.T., Pallone F., Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28:310–313. doi: 10.1097/MOG.0b013e328352ad69. [DOI] [PubMed] [Google Scholar]
- 44.Takamura T., Harama D., Matsuoka S., Shimokawa N., Nakamura Y., Okumura K., Ogawa H., Kitamura M., Nakao A. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol. Cell Biol. 2010;88:685–689. doi: 10.1038/icb.2010.35. [DOI] [PubMed] [Google Scholar]
- 45.Benson J.M., Shepherd D.M. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn's disease. Toxicol Sci. 2011;120:68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furumatsu K., Nishiumi S., Kawano Y., Ooi M., Yoshie T., Shiomi Y., Kutsumi H., Ashida H., Fujii-Kuriyama Y., Azuma T., Yoshida M. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci. 2011;56:2532–2544. doi: 10.1007/s10620-011-1643-9. [DOI] [PubMed] [Google Scholar]
- 47.Sugimoto S., Naganuma M., Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51:853–861. doi: 10.1007/s00535-016-1220-2. [DOI] [PubMed] [Google Scholar]
- 48.Fukumoto S., Toshimitsu T., Matsuoka S., Maruyama A., Oh-oka K., Takamura T., Nakamura Y., Ishimaru K., Fujii-Kuriyaman Y., Ikegami S., Itou H., Nakao A. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol Cell Biol. 2014;92:460–465. doi: 10.1038/icb.2014.2. [DOI] [PubMed] [Google Scholar]
- 49.Rousseaux C., Lefebvre B., Dubuquoy L., Lefebvre P., Romano O., Auwerx J., Metzger D., Wahli W., Desvergne B., Naccari G.C., Chavatte P., Farce A., Bulois P., Cortot A., Colombel J.F., Desreumaux P. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wohlfert E.A., Nichols F.C., Nevius E., Clark R.B. Peroxisome proliferator-activated receptor-γ (PPAR-γ) and immunoregulation: enhancement of regulatory T cells through PPAR-γ-dependent and -independent mechanisms. J Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 51.Hontecillas R., Bassaganya-Riera J. Peroxisome proliferator-activated receptor-γ is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 52.Hawley S.A., Fullerton M.D., Ross F.A., Schertzer J.D., Chevtzoff C., Walker K.J., Peggie M.W., Zibrova D., Green K.A., Mustard K.J., Kemp B.E., Sakamoto K., Steinberg G.R., Hardie D.G. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–922. doi: 10.1126/science.1215327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michalek R.D., Gerriets V.A., Jacobs S.R., Macintyre A.N., Maclver N.J., Mason E.F., Sullivan S.A., Nichols A.G., Rathmell J.C. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sridharan G.V., Choi K., Klemashevich C., Wu C., Prabakeran D., Pan L.B., Steinmeyer S., Mueller C., Yousofshahi M., Alaniz R.C., Lee K., Jayaraman A. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat Commun. 2014;5:5492. doi: 10.1038/ncomms6492. [DOI] [PubMed] [Google Scholar]