Abstract
Introduction
Psoriasis patients demonstrate high interest in the role of diet on their skin condition. However, data are lacking to describe dietary interventions among psoriasis patients and associated outcomes. This study aims to identify common dietary habits, interventions and perceptions among patients with psoriasis, and to examine patient-reported skin outcomes in response to these interventions.
Methods
We administered a 61-question survey to the National Psoriasis Foundation membership asking psoriasis patients about dietary habits, modifications, skin responses, and perceptions.
Results
A total of 1206 psoriasis patients responded to the survey. Compared to age- and sex-matched controls, psoriasis patients consumed significantly less sugar, whole grain fiber, dairy, and calcium (p < 0.001), while consuming more fruits, vegetables, and legumes (p < 0.01). Eighty-six percent of respondents reported use of a dietary modification. The percentage of patients reporting skin improvement was greatest after reducing alcohol (53.8%), gluten (53.4%), nightshades (52.1%), and after adding fish oil/omega-3 (44.6%), vegetables (42.5%), and oral vitamin D (41%). Specific diets with the most patients reporting a favorable skin response were Pagano (72.2%), vegan (70%), and Paleolithic (68.9%). Additionally, 41.8% of psoriasis respondents reported that a motivation for attempting dietary changes was to improve overall health.
Conclusion
This national survey is among the first to report the dietary behaviors of patients with psoriasis. The data provided from this large cohort may benefit patients and clinicians as they discuss the role of diet in managing both psoriasis and associated cardiometabolic comorbidities.
Electronic supplementary material
The online version of this article (doi:10.1007/s13555-017-0183-4) contains supplementary material, which is available to authorized users.
Keywords: Diet, Nutrition, Psoriasis, Psoriatic arthritis, Triggers
Introduction
Psoriasis is a chronic immune-mediated disease affecting 3–4% of the world population [1]. The innate and adaptive immune systems are thought to be responsible for psoriasis pathogenesis, while well-recognized environmental factors like smoking and emotional stress can modify disease severity [2].
One environmental factor of high interest to patients is the influence of diet on psoriasis. Although the popular literature contains many dietary recommendations for psoriasis, the scientific literature is limited, especially among randomized controlled trials (RCT). The strongest scientific evidence exists for weight loss, particularly among obese psoriatic patients [3–10], and for gluten-free diets (GFD), which have been reported to improve psoriasis in a subset of patients with celiac-specific antibodies [11–15]. Moreover, there is an absence of studies describing the effects of popular dietary recommendations and regimens, such as a Paleolithic diet or a vegetarian diet. The limited literature on diet and psoriasis represents an important knowledge gap that makes it difficult for patients and clinicians to discuss this topic.
Psoriasis is increasingly being recognized as a systemic inflammatory condition as a result of an emerging and rapidly growing body of evidence supporting an association between psoriasis and cardiometabolic disease as well as various other comorbidities [16]. As a result, a greater need to provide comprehensive care to patients with psoriasis has been recently been proposed and encouraged [17]. Thus, engaging in dietary recommendations and management options with psoriatic patients aligns with this shift towards comprehensive care for not only control and prevention of their skin disease but also for managing their overall and long-term health.
In order to understand the role of dietary modifications in the management of psoriasis, a survey of psoriasis patients with the following objectives was conducted: (1) to quantify nutrient intake in psoriasis patients compared to population controls; (2) to identify popular dietary interventions among psoriasis patients and the influence of those interventions on psoriasis improvement based on patient-reported skin responses; and (3) to understand the attitudes and perceptions of psoriasis patients regarding the role of diet in managing psoriasis.
Methods
Study Design and Subjects
We performed an exploratory survey study of psoriasis patients via email distribution to the National Psoriasis Foundation (NPF) list-serve from August 2014 to January 2015 (survey provided in Appendix 1 within the supplemental material). The NPF was used for recruitment due to their large network and well-engaged, motivated patient community with a convenient means to access this community through a periodic newsletter. A response rate was calculated based on the “click-through rate,” which reflects the number of recipients who opened the survey. The survey contained 61 questions, was voluntary and no incentives were offered. The first 30 questions were from the 2009–2010 National Health and Nutrition Examination Survey (NHANES) dietary screening questionnaire, which assesses nutrient intake in several food categories and has undergone validation using 24-h dietary recall forms [18]. The last 31 questions focused on patient-reported skin responses to dietary changes, patients’ attitudes regarding diet as a management strategy for their psoriasis, and participant demographics. The latter 31 questions were developed based on topics of interest found in NPF discussion forums, popular literature, patient discussion and a review of the scientific literature. The Institutional Review Board (IRB) at the University of California, San Francisco (UCSF), approved this study. All procedures followed were in accordance with the ethical standards of the UCSF IRB and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study. Study data were collected and managed using REDcap (Research Electronic Data Capture) electronic data capture tools hosted at UCSF [19]. Data were exported from REDcap and numerically coded for statistical analysis.
Statistical Analysis
All dietary changes, attitude/perception, and demographic data were compiled and frequencies reported. A geographical information system plot of patients overlaid over the contiguous United States (U.S.) was created with the self-reported zip codes from each participant and created using the maps package in R 3.2.2 [20, 21]. Data processing and scoring of nutrient intake was performed using NHANES guidelines [22]. Population control data from the NHANES 2009–2010 dataset was matched to our psoriasis cohort based on age and gender. Differences in relative daily consumption of key dietary components were identified using a Mann–Whitney U Test in SAS, with statistical significance set to p = 0.05.
Patient-perceived skin responses to dietary changes were rated on a Likert scale consisting of: Worsened, No Change, Improved, and Fully Clear. A positive response was defined as participants reporting Fully Clear or Improved, while a negative response was defined as No Change or Worsened. We performed association testing between demographic variables (race, sex, age, age of onset, education, income level, self-reported psoriasis severity off treatment, self-reported BSA off treatment, psoriatic arthritis status, family history, BMI, celiac disease status, urban/rural living environment) and patient-perceived favorable responses to dietary changes. After performing univariate logistic regression (see Appendix 2 in the supplementary material) to identify individual demographic variables associated with a patient-reported favorable response to dietary changes (p < 0.05), we then used multivariate logistic regression to evaluate for independent effects. We report variables from multivariate analysis with p < 0.05. Odds ratios (OR) and their corresponding 95% confidence intervals were calculated. A similar analysis was conducted to evaluate the association of demographic variables with dietary perceptions and attitudes. A two-step cluster analysis using SPSS v.23.0 (IBM, Armonk, NY, USA) was also performed, grouping respondents based on dietary behavior/experience and comparing the influence of various demographic factors and dietary perceptions. One-way analysis of variance was performed to compare the continuous variables of age, weight, and age of onset by the two clusters. The remaining analyses used Chi-squared tests (cluster analysis data can be found in Appendix 3 within the supplementary material).
Results
Demographics
Overall, 1206 psoriasis patients responded to the survey with a 60% response rate. The demographic characteristics of the patient population are shown in Table 1. The mean age of the sample population was 50.4, and 73% of respondents were female. The sample population represented all levels of psoriasis severity based on self-reported severity scores: 20.9% with mild disease, 42.2% with moderate disease, and 36.9% with severe disease. Self-reported body surface area (BSA) involvement without treatment was well distributed, with 7.8% reporting barely any or very little BSA, 29.6% reporting <5% BSA, 24.9% reporting 5–10% BSA, 19.1% reporting 11–20% BSA and 18.6% reporting >20% BSA. Concomitant psoriatic arthritis was self-reported in 43.9% of respondents. The sample population was predominantly white (87.2%) and lived in an urban setting (79%). A geographic map showing the location of respondents demonstrates representation across the U.S. (Fig. 1).
Table 1.
Demographic characteristics of survey respondents
| Variable | Value |
|---|---|
| Age, mean (SD) | 50.4 (14.3) |
| Sex, n (%) | |
| Male | 322 (26.7) |
| Female | 884 (73.3) |
| Average age at onset of psoriasis, mean (SD) | 27.2 (17.2) |
| Severity of skin condition (without treatment), n (%) | |
| Mild | 252 (20.9) |
| Moderate | 509 (42.2) |
| Severe | 444 (36.9) |
| Body surface area (without treatment), n (%) | |
| Barely any or very little | 94 (7.8) |
| <5% body surface | 357 (29.6) |
| 5–10% body surface | 300 (24.9) |
| 11–20% body surface | 230 (19.1) |
| >20% body surface | 224 (18.6) |
| Presence of psoriatic arthritis | 529 (43.9) |
| Family history of skin condition, n (%) | 581 (48.3) |
| Average BMI, mean (SD) | 28.4 (7.4) |
| Underweight (<18.5), n (%) | 35 (2.9) |
| Normal (18.5–24.9), n (%) | 403 (33.6) |
| Overweight (25–29.9), n (%) | 360 (30.3) |
| Obese 30+, n (%) | 396 (32.8) |
| Race, n (%) | |
| White | 1066 (87.2) |
| Asian/Pacific Islander | 60 (5) |
| Hispanic | 47 (3.9) |
| Native American | 16 (1.3) |
| African American | 8 (0.7) |
| Other | 25 (2.1) |
| Highest level of education, n (%) | |
| Less than high school | 14 (1.2) |
| High school graduate | 207 (17.3) |
| Undergraduate | 371 (30.9) |
| Graduate/professional degree | 608 (50.7) |
| Area in which you live, n (%) | |
| Urban/suburban | 945 (79) |
| Rural | 251 (21) |
| Average annual household income, n (%) | |
| <$20,000 | 88 (7.4) |
| $20,000–$40,000 | 151 (12.7) |
| $40,001–$60,000 | 168 (14.1) |
| $60,001–$100,000 | 242 (20.4) |
| >$100,000 | 298 (25.1) |
| Prefer not to say | 242 (20.4) |
Fig. 1.
Geographic location of psoriasis survey respondents
Daily Consumption in Psoriasis versus Control Group
Compared to the 2009–2010 NHANES controls, psoriasis patients in this study demonstrated a significant difference in daily consumption of specific nutrients based on the food frequency questionnaire (Table 2). On average, respondents reported less daily intake of sugar, whole grain fiber, dairy products, and calcium (p < 0.001), and higher daily intake of fruits/vegetables/legumes (p = 0.007).
Table 2.
Relative daily consumption of key dietary components in psoriasis versus control groups
| Dietary intake | Psoriasis data (n = 1017) Mean ± SD |
NHANES data Mean ± SD (n) |
p value |
|---|---|---|---|
| Daily added sugar (tsp.) | 10.6 ± 7.64 | 15.3 ± 10.7 (2815) | <0.0001 |
| Daily whole grain (oz.) | 0.704 ± 1.17 | 0.846 ± 1.35 (2842) | <0.0001 |
| Daily fiber (g) | 13.7 ± 5.69 | 14.5 ± 5.64 (2609) | 0.0002 |
| Daily dairy (cup) | 1.25 ± 0.891 | 1.45 ± 0.91 (2847) | <0.0001 |
| Daily calcium (mg) | 741 ± 397 | 827 ± 423 (2609) | <0.0001 |
| Daily fruit/vegetable/legume (cup) | 2.58 ± 0.957 | 2.51 ± 1.08 (2724) | 0.0070 |
Perceived Dietary Triggers and Helpful Additives
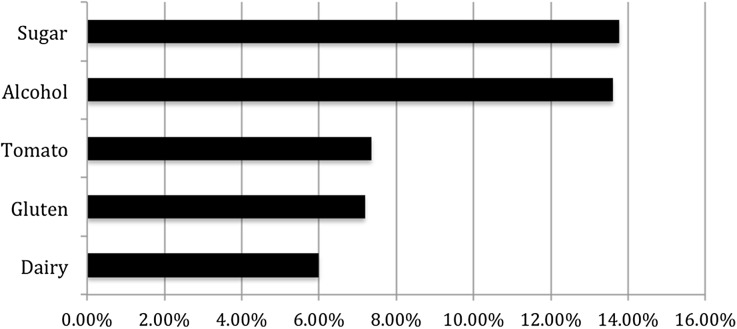
About 37% of respondents reported that they did not recognize any dietary triggers which may worsen their psoriasis or left the survey field blank (Fig. 2). Among respondents, the most common reported triggers were sugar (13.8%), alcohol (13.6%), tomato (7.4%), gluten (7.2%), and dairy (6%). Less commonly reported triggers (2–5% of reported triggers) included meat, processed foods, soda, bread, beer, wine, eggs, and spicy foods.
Fig. 2.
Reported dietary triggers that worsen psoriasis. Only responses >5% listed. Less commonly reported triggers (2–5%) included meat, processed foods, soda, bread, beer, wine, eggs, and spicy foods
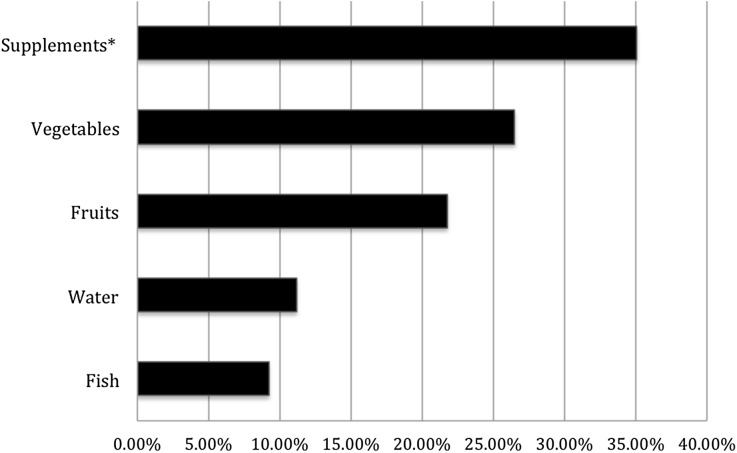
Respondents also reported dietary items that may improve psoriasis. This included consumption of dietary supplements (35.1%), vegetables (26.5%), fruits (21.8%), water (11.2%), and fish (9.2%) (Fig. 3).
Fig. 3.
Reported dietary additions that Improve psoriasis. Only responses >5% listed. *Common dietary supplements reported include: vitamin D, fish oil/omega-3, probiotics, vitamin B, vitamin E, vitamin C, vitamin A, and turmeric capsules
Dietary Modifications and Reported Outcomes
Among respondents, 1037 reported a trial of avoiding or reducing specific foods and 988 noted a trial of adding certain foods; a complete list is shown in Table 3. The most common dietary reductions associated with patient-reported positive skin response were alcohol (53.8%), gluten (53.4%), nightshades (52.1%), junk foods (50%), and white flour products (49.9%). A positive skin response was also reported by respondents when adding fish oil/omega-3 (44.6%), vegetables (42.5%), oral vitamin D (41%), probiotics (40.6%), organic foods (38.4%), and fruits (34.6%) (Table 4).
Table 3.
Reported dietary modifications in psoriasis patients
| Dietary removals | Dietary additions |
|---|---|
| % of respondents reporting trial of dietary item removal (n = 1037) | % of respondents reporting dietary item addition (n = 988) |
|
Junk foodsa: 66.7% White flour products: 55.7% High fat foods: 50.4% Red meat: 49.5% Alcohol: 45% Gluten: 44.6% Dairy: 41.3% Tobacco: 36.1% Sodium/salt: 34.5% Nightshadesb: 28.8% Caffeine: 27% Pork: 26.8% Shellfish: 18% Other: 9.2% |
Vegetables: 58.8% Fish oil/omega-3: 56.8% Oral vitamin D: 55.6% Fruits: 54.7% Probiotics: 44.4% Organic foods: 39.6% Other: 9.2% |
aJunk foods: candy and pastries, chocolate, french fries, potato chips, sweets
bNightshades: tomatoes, eggplant, peppers, paprika, white potatoes
Table 4.
Outcomes to dietary interventions in psoriasis patients
| Dietary removals | Dietary additions |
|---|---|
| Respondents reporting full clearance or improvement of psoriasis with removal of the following from their diet (%) | Respondents reporting full clearance or improvement of psoriasis after addition of the following to their diet (%) |
|
Alcohol: 251 of 462(53.8%) Gluten: 247 of 459 (53.4%) Nightshadesa: 156 of 297 (52.1%) Junk foodsb: 346 of 687 (50%) White flour products: 288 of 573 (49.9%) Dairy: 204 of 424 (47.7%) Shellfish: 73 of 186 (39%) High fat foods: 193 of 519 (36.9%) Caffeine: 102 of 275 (36.4%) Pork: 99 of 276 (35.6%) Tobacco: 131 of 370 (35%) Red meat: 156 of 509 (30.4%) Sodium/salt: 76 of 356 (21.2%) |
Fish oil/omega-3: 250 of 556 (44.6%) Vegetables: 247 of 575 (42.5%) Oral vitamin D: 216 of 545 (41%) Probiotics: 178 of 434 (40.6%) Organic foods: 150 of 388 (38.4%) Fruits: 187 of 534 (34.6%) |
aNightshades: tomatoes, eggplant, peppers, paprika, white potatoes
bJunk Foods: candy and pastries, chocolate, french fries, potato chips, and sweets
Among all respondents, 481 respondents (40%) reported trying a special diet for their psoriasis, the most common being gluten-free (35.6%), low carbohydrate–high protein (16.6%), and Paleolithic (11.6%) diets (Table 5). The three diets with the highest patient-reported positive response were the Pagano, vegan, and Paleolithic diets. Other diets that patients reported to improve their psoriasis included gluten-free, low carbohydrate–high protein, Mediterranean and vegetarian diets. Across all special diets, 69% of patients reported weight loss.
Table 5.
Frequencies of special diets used for psoriasis and outcomes
| Special diets | |
|---|---|
| % of respondents reporting trial of special diet (n = 481) | Respondents reporting full clearance or improvement of psoriasis after special diet (%) |
|
Gluten-free: 35.6% Low carbohydrate–high protein: 16.6% Paleolithic: 11.6% Vegetarian: 9.7% Mediterranean: 5.8% Vegan: 5.4% Other: 9.7% |
Pagano diet (13 of 18, 72.2%) Vegan diet (20 of 29, 70%) Paleolithic diet (42 of 62, 68.9%) Gluten-free diet (101 of 191, 52.9%) Low carbohydrate–high protein diet (45 of 89, 51.7%) Mediterranean diet (15 of 31, 48.4%) Vegetarian diet (21 of 52, 40.4%). |
Demographic Factors Associated with Favorable Dietary Outcomes
We examined whether demographic factors were associated with patient-reported favorable dietary responses (Table 6). We found that younger age was associated with greater reported positive response to avoidance of red meat, high fat foods, sodium, white flour, and alcohol, with an effect size of 1–3% decrease in odds per additional year of age. Non-white race was associated with a greater patient-reported favorable response to avoidance of red meat, pork, high fat foods, sodium, and addition of fruits, with non-white race increasing the odds of patient-reported positive response by approximately two- to fourfold. Patients who reported having severe psoriasis reported responding better to avoidance of caffeine (OR = 2.3), while those with celiac disease reported faring better with avoidance of white flour (OR = 4.3).
Table 6.
Demographic factors associated with response to dietary change
| Intervention | Multivariate analysis | ||
|---|---|---|---|
| Variable | p value | OR (95% CI) | |
| Avoidance of red meat | Age (increasing) | 0.04 | 0.98 (0.97–0.99)a |
| Race (white) | 0.0084 | 0.46 (0.26–0.82)b | |
| Avoidance of pork | Race (white) | 0.03 | 0.45 (0.22–0.92) |
| Avoidance of high fat foods | Age (increasing) | 0.03 | 0.99 (0.97–1.00) |
| Race (white) | 0.03 | 0.53 (0.30–0.93) | |
| Avoidance of sodium | Age (increasing) | 0.0008 | 0.97 (0.95–0.99) |
| Race (white) | 0.03 | 0.45 (0.22–0.93) | |
| Avoidance of white flour | Age (increasing) | 0.03 | 0.98 (0.96–0.998) |
| Positive of celiac disease | 0.005 | 4.3 (1.5–11.9)c | |
| Avoidance of caffeine | Severe psoriasis | 0.004 | 2.30 (1.30–4.00)d |
| Avoidance of alcohol | Age | 0.0001 | 0.97 (0.95–0.98) |
| Addition of fruits | Race (white) | <0.0001 | 0.22 (0.11–0.45) |
| Positive family history of psoriasis | 0.04 | 1.76 (1.03–3.0)e | |
aOR of patient-perceived favorable response with increasing age
bOR of patient-perceived favorable response in white race vs. non-white race
cOR of patient-perceived favorable response in individuals with celiac disease vs. individuals without celiac disease
dOR of patient-perceived favorable response in severe psoriasis vs. mild/moderate psoriasis
eOR of patient-perceived favorable response in patients with positive family history vs. negative family history of psoriasis
Attitudes and Perceptions About Diet
Attitudes surrounding diet as a management strategy for their disease are reported in Table 7. The majority of respondents were not sure how diet affected their skin (43.2%); however, 16.7% felt diet was significantly helping their skin and 17.4% felt diet was slightly helping their skin.
Table 7.
Attitudes surrounding diet as management strategy for psoriasis
| Questions and responses | n (%) |
|---|---|
| Currently, what role is diet playing in managing your skin condition? | |
| Skin condition completely controlled by diet | 27 (2.2) |
| Diet is helping significantly with skin condition | 201 (16.7) |
| Diet is helping slightly with skin condition | 210 (17.4) |
| Diet has no effect on skin condition | 200 (16.6) |
| Not sure how diet affects skin condition | 521 (43.2) |
| Other | 47 (3.9) |
| How difficult/burdensome is it to follow a special diet? | |
| Very difficult | 226 (18.7) |
| Somewhat difficult | 473 (39.2) |
| Not difficult | 324 (26.9) |
| Not applicable | 183 (15.2) |
| What difficulties did you encounter in modifying your diet? | |
| Will power/too limiting | 237 (36.5) |
| Time/inconvenience | 113 (17.4) |
| Family/social pressures | 88 (13.6) |
| Dining out/travel | 88 (13.6) |
| Affordability | 74 (11.4) |
| Access | 48 (7.4) |
| How important is it that physicians discuss with patients the role of diet in managing skin disease? | |
| Very important | 781 (64.8) |
| Somewhat important | 290 (24) |
| Minimally important | 102 (8.5) |
| Not important at all | 33 (2.7) |
Of note, 57.9% of patients found it very or somewhat difficult to follow a special diet, 17.4% of patients found it very time consuming, and 11.4% found it very expensive to follow a special diet.
Motivation for using diet to improve psoriasis varied among patients. The most commonly reported reasons include: diet may improve other health problems (41.8%), diet is a natural method (32.2%), and previous treatments failed (26.5%). Although 86% of respondents had tried a dietary modification, and 88.8% found it very or somewhat important that physicians discuss with patients the role of diet in managing skin disease, only 30.7% had actually discussed dietary changes with a dermatologist.
Patients were asked to rate the importance of diet compared to other interventions or treatments for management of their psoriasis: 41.5% of respondents rated diet to be more important than over-the-counter medications; diet was also rated as more important than complementary medications in 31.9%, prescription medications in 31%, exercise in 21.3%, and stress reduction in 8.9%.
Demographic Factors Associated with Perceptions and Attitudes About Diet
Similarly, we performed an analysis examining demographic predictors for perceptions regarding dietary management of psoriasis (Supplementary Table 1). The belief that “diet plays an important role in managing psoriasis” was associated with younger age and patients reporting less severe psoriasis. The belief that “following a specific diet is burdensome” was associated with younger age, female gender, and those with psoriatic arthritis. Interestingly, respondents who reported discussing dietary changes with a dermatologist were more likely to report having severe psoriasis or higher BSA involvement.
Cluster Analysis
Cluster analysis based on dietary behavior identified two groups: Cluster 1 was highly active in their dietary modifications and frequently engaged in avoiding red meat, pork, sodium, high fat foods, caffeine, alcohol and tobacco and were more likely to add fruits, vegetables, organic foods, probiotics, fish and vitamin D; Cluster 2 was a less active dietary-modifying group and instead selectively avoided shellfish, gluten, white flour and junk food. Examination of variations in demographic factors between the two groups found significant differences, whereby Cluster 2 was younger (p < 0.001), had an earlier mean age of onset (p = 0.012), weighed less (p = 0.011), had a higher prevalence of celiac disease (p = 0.017), and had greater racial diversity (p < 0.001). Cluster 2 was also significantly more likely to discuss with their dermatologist regarding dietary modifications (p < 0.001), and believed that speaking with a dermatologist regarding diet is particularly important (p < 0.001). The groups did not significantly differ in sex, education level, living environment, income level, and prevalence of family history of psoriasis.
Discussion
Relative Daily Consumption in Psoriasis vs. Control Group
Compared to the NHANES 2009–2010 control dataset, the psoriasis cohort presented here consumed less sugar, whole grain fiber, dairy, and calcium, while consuming more fruits and vegetables. These differences may be attributable to psoriasis patients following recommendations in the popular literature, which suggests that sugar, gluten, and dairy may serve as triggers, while fruits and vegetables have health benefits. Individuals participating in our survey may have had greater interest in the topic of diet and therefore engaged in these dietary changes. This is supported by 86% of respondents reporting a history of using some form of dietary modification, and almost half of respondents reported reducing gluten. The lower sugar intake observed in our cohort is consistent with findings of another study using the NHANES data where psoriasis patients reported decreased sugar intake compared to healthy controls [23].
Reported Triggers and Helpful Additives
The most common psoriasis triggers reported by respondents were sugar, alcohol, nightshades, and gluten. The mechanism of how each of these triggers may induce or exacerbate psoriasis is unclear. However, prior studies have implicated these dietary components in causing alterations in the intestinal microbiome composition, irritating the intestinal lining, and upregulation of the immune system. Evidence suggests that dietary simple sugars lead to dysbiosis of the gut microbiome favoring injurious bacterial taxa and an increase in inflammatory cytokines [24–35]. Alternatively, complex carbohydrates with high fiber, such as those found in fruits and vegetables, have been found to have an opposite effect on the gut microbiome and reduce inflammation [36–43].
An increase in inflammatory cytokines may also explain the link between alcohol and psoriasis. Excessive alcohol intake has been associated with the development of psoriasis and is correlated with psoriasis severity [44, 45]. Proposed mechanisms for the interaction of alcohol in psoriasis include enhancement of mitogen-driven lymphocyte proliferation and upregulation of pro-inflammatory cytokines [46, 47].
In addition to upregulation of inflammatory cytokines, factors proposed to disturb the intestinal lining, such as nightshades, may also contribute to the exacerbation of immune-related disorders. Nightshades are part of a plant family called Solanaceae, which includes tomato, potato, eggplant, tobacco, pepper, and petunia, and have been found to affect digestion and absorption of nutrients in humans and animals [48]. Nightshades produce alkaloids, which have been shown to adversely affect the mammalian intestine and to aggravate inflammatory bowel disease (a common comorbidity in psoriasis patients) in the murine model [49].
Several studies have documented celiac disease and psoriasis coexistence and the improvement of psoriatic lesions after starting a GFD [11, 12, 50]. The benefit of GFD has been shown to extend to patients who are antigliadin antibody-positive but without clinical and histological markers of celiac disease [13–15]. These findings explain both a common trigger among respondents in this study being gluten and a patient-reported improvement of their psoriasis following a GFD.
While some dietary elements are posited to trigger psoriasis, other foods have been suggested to improve disease symptoms. The most commonly reported include fish oil/omega-3 polyunsaturated fatty acids (PUFAs), fruits and vegetables, vitamin D supplementation, and probiotics. A systematic review identified 15 trials evaluating fish oil supplementation and found an overall moderate reduction in psoriasis symptoms [51]. There was moderate evidence of benefit for the use of fish oil supplements in psoriasis among twelve trials (six controlled, six uncontrolled) [52–63] and three trials (two controlled, one uncontrolled) showing no benefit [64–66]. PUFAs are thought to reduce the conversion of free arachadonic acids to leukotriene B4, which is elevated in psoriatic lesions [67, 68]. Additionally, PUFAs reduce the production of tumor necrosis factor (TNF)-alpha, interleukin (IL)-1β, and IL-1α in healthy adults and rheumatoid arthritis patients [69–72].
Previous cross-sectional, case–control, and case studies have suggested an inverse relationship between psoriasis and fruits and vegetable intake [73–76]. Fruits and vegetables provide a wealth of antioxidants such as carotenoids, flavenoids, vitamins, and minerals that have been inversely correlated with TNF-alpha, C-reactive protein (CRP), and IL-6 [77–79]. Vitamin D may have antiproliferative and immunomodulatory effects. Among clinical studies, data on vitamin D supplementation have been equivocal. A systematic review identified significant improvements in psoriasis area severity index (PASI) score following oral vitamin D supplementation in eight uncontrolled studies; however, one RCT demonstrated only a slight, statistically insignificant improvement [51, 80–87]. Probiotics aim to restore balance to the host gut microbiome; however, current evidence regarding the role of probiotics in psoriasis is limited. An RCT of oral probiotics in spondyloarthritis patients, including those with psoriasis and psoriatic arthritis, found no significant change in disease severity [88]. The true effect of probiotics may be influenced by factors such as probiotic composition, dose, route of administration and interaction with dietary habits.
Special Diets
Survey respondents self-reported appreciable symptom improvement with Mediterranean, Pagano, Paleolithic, vegan, gluten-free, and low carbohydrate–high protein diets. A case report documented significant improvement in psoriasis lesions in all five patients following a 6-month diet regimen analogous to the Pagano diet, which entails an increase in fruits and vegetables and a decrease in nightshades and junk food among various other recommendations [75, 89]. An observational study found improvement in a subset of psoriasis patients following a 2-week fast and a subsequent 3-week vegetarian diet [90]. Additionally, in patients with rheumatoid arthritis and atopic dermatitis, vegan and vegetarian diets have been shown to alleviate symptoms and promote weight loss, which may decrease adipocyte-mediated inflammation [91, 92]. Similarly, many respondents reported concomitant weight loss with a trial of special diets, possibly contributing to the reported benefits. A case–control study of mild-to-severe psoriasis found extra virgin olive oil and fish oil, typical components of a Mediterranean diet, to decrease PASI scores and CRP levels [93]. In addition to improvement in psoriatic lesions, many of these diets are also advantageous in improving the cardiometabolic profile of patients with psoriasis, as many respondents reported a benefit of overall health as the motivation behind attempting dietary changes. Evidence suggests that the Mediterranean and Paleolithic diets can reduce the risk of cardiometabolic comorbidities in psoriasis, which are a predominant cause of reduced life expectancy and an important aspect of disease management [94–97].
Demographic Variables Associated with Dietary Changes
Favorable dietary changes were more commonly reported in respondents who were younger, non-white, and positive for celiac disease. Chronic inflammation has been shown to be a characteristic of an aging immune system; specifically, evidence has shown an increase in cytokines and inflammatory markers in older compared to younger adults [98–101]. One can speculate that the possible increase in baseline inflammation in older subjects could dampen the anti-inflammatory influences of dietary changes.
The association of non-white race with a favorable response to dietary changes was intriguing. The effect of race could relate to genetics or the environment or both. Several studies have reported racial and ethnic variations in genes implicated in the pathogenesis of psoriasis [102]. These differences in immunological-related genes may result in variations in response to environmental insults such as diet.
Removal of gluten from the diet in patients with celiac disease had a beneficial effect in our survey respondents, which parallels findings from other studies [11, 50]. Interestingly, celiac patients in our study tended to rank diet as more important for controlling their psoriasis than prescription, over-the-counter, and complementary medications.
Demographic Variables Associated with Attitudes and Perceptions
Managing psoriasis using dietary interventions was viewed as more important by respondents who were younger and those self-reporting mild/moderate disease activity. However, respondents who report having severe psoriasis were more prone to discuss dietary changes with their dermatologist, likely reflecting information-seeking behavior due to the severity of their disease. Difficulty with following a diet was more likely to be reported by respondents who were younger, female and with psoriatic arthritis. Factors underlying this association include the cost and labor involved in purchasing and preparing healthier meals, as well as the physical burden of cooking among those with psoriatic arthritis.
Cluster Analysis
Results from the cluster analysis identified two distinct clusters of patients based on dietary behavior. Cluster 1 was composed of older patients with higher BMI who engaged in broad dietary changes aligned with achieving global health and minimizing cardiovascular and/or cancer risk (e.g., avoidance of red meat, pork, sodium, high fat foods, caffeine, alcohol and tobacco and the addition of fruits, vegetables organic foods, probiotics, fish and vitamin D). Cluster 2 was composed of younger patients who selectively avoided or minimized specific foods from their diet (e.g., gluten, white flour, junk food, and shellfish). These patients may approach dietary change with the intention of reducing dietary triggers of their psoriasis and may be less concerned with diets involving improved global health and cardiovascular risk. Given the increased cardiovascular risk in patients with psoriasis, all patients should be counseled on the importance of sustaining a healthy overall diet in light of their increased risk for cardiovascular disease, while also considering dietary habits that may improve their skin condition.
Limitations
Study limitations include the potential for responder bias and a predominance of female gender, white race, respondents with moderate to severe disease, and urban-living respondents, restricting generalizability. Time lapse may be a concern in that the NPF psoriasis data were collected in 2014 while control data were collected in 2010. Substantial changes in diet have not been reported from the 2010 to 2014 NHANES dataset, suggesting a valid comparison of the two nutrition datasets. An article search also did not identify any dietary trends or changes based on NHANES datasets from 2010 to 2014.
Additionally, limitations of the survey instrument include lack of validation for part two of the survey. Duration of dietary modifications was not assessed, neither were the quantitative reduction nor addition of the specific dietary modification, all of which may be contributing factors to the variation in skin responses reported among respondents. However, the study purpose was not to provide evidence-based guidelines, rather the information presented is primarily to understand the role of diet in managing psoriasis among a large cohort of psoriasis patients.
Conclusions
This is the first study to better understand the patient experience regarding the role of diet in the management of psoriasis. Importantly, the majority of respondents report the motivation for trialing dietary modifications is to improve their overall health. Therefore, when discussing with patients interested in dietary management of their psoriasis, physicians can use this opportunity to encourage dietary changes with the intention of benefiting both psoriatic lesions and the cardiometabolic risk factors associated with psoriasis. Future prospective RCTs are needed to identify and validate optimal dietary interventions for psoriasis as well as the length of intervention needed to yield results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Wilson Liao is supported in part by grants from the National Institute of Health (NIH) (R01AR065174, U01AI119125). Richard Ahn acknowledges support from the National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) postdoctoral training grant to the Department of Dermatology at UCSF (T32 AR007175-38). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1TR000004. No sponsorship was received for this study or publication of this article. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. We are grateful to the Dinsmore family for their support of this project. We also thank the National Psoriasis Foundation (NPF) for facilitating the contact of survey respondents and for the patients for their participation. We also would like to thank Elaine Allen Ph.D., Barbara Grimes Ph.D., and Jennifer Creasman from CTSI who assisted with statistical analyses for the study.
Disclosures
Ladan Afifi, Melissa Danesh, Kristina Lee, Kevin Beroukhim, Benjamin Farahnik, Richard Ahn, Di Yan, Rasnik Singh, Mio Nakamura, John Koo, and Wilson Liao have nothing to disclose.
Compliance with Ethics Guidelines
All procedures followed were in accordance with the ethical standards of University of California, San Francisco Institutional Review Board, and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Data Availability
Some data generated or analyzed during this study are included in this published article/as supplementary information files. All other datasets generated during and/or analyzed during the current study are available from the corresponding author on request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/E128F060166C6BD7.
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic S, Raznatovic M, Marinkovic J, Jankovic J, Maksimovic N. Risk factors for psoriasis: a case-control study. J Dermatol. 2009;36(6):328–334. doi: 10.1111/j.1346-8138.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- 3.Bryld LE, Sorensen TI, Andersen KK, Jemec GB, Baker JL. High body mass index in adolescent girls precedes psoriasis hospitalization. Acta Derm Venereol. 2010;90(5):488–493. doi: 10.2340/00015555-0931. [DOI] [PubMed] [Google Scholar]
- 4.Debbaneh M, Millsop JW, Bhatia BK, Koo J, Liao W. Diet and psoriasis, part I: impact of weight loss interventions. J Am Acad Dermatol. 2014;71(1):133–140. doi: 10.1016/j.jaad.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149(7):795–801. doi: 10.1001/jamadermatol.2013.722. [DOI] [PubMed] [Google Scholar]
- 6.Murray ML, Bergstresser PR, Adams-Huet B, Cohen JB. Relationship of psoriasis severity to obesity using same-gender siblings as controls for obesity. Clin Exp Dermatol. 2009;34(2):140–144. doi: 10.1111/j.1365-2230.2008.02791.x. [DOI] [PubMed] [Google Scholar]
- 7.Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125(1):61–67. doi: 10.1111/j.0022-202X.2005.23681.x. [DOI] [PubMed] [Google Scholar]
- 8.Naldi L, Conti A, Cazzaniga S, et al. Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol. 2014;170(3):634–642. doi: 10.1111/bjd.12735. [DOI] [PubMed] [Google Scholar]
- 9.Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch Intern Med. 2007;167(15):1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 10.Wolk K, Mallbris L, Larsson P, et al. Excessive body weight and smoking associates with a high risk of onset of plaque psoriasis. Acta Derm Venereol. 2009;89(5):492–497. doi: 10.2340/00015555-0711. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71(2):350–358. doi: 10.1016/j.jaad.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Erme AM, Kovacikova Curkova A, Agnoletti AF, et al. Gluten-free diet as a therapeutic approach in psoriatic patients: if yes, when. G Ital Dermatol Venereol. 2015;150(3):317–320. [PubMed] [Google Scholar]
- 13.Michaelsson G, Ahs S, Hammarstrom I, Lundin IP, Hagforsen E. Gluten-free diet in psoriasis patients with antibodies to gliadin results in decreased expression of tissue transglutaminase and fewer Ki67+ cells in the dermis. Acta Derm Venereol. 2003;83(6):425–429. doi: 10.1080/00015550310015022. [DOI] [PubMed] [Google Scholar]
- 14.Michaelsson G, Gerden B, Hagforsen E, et al. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br J Dermatol. 2000;142(1):44–51. doi: 10.1046/j.1365-2133.2000.03240.x. [DOI] [PubMed] [Google Scholar]
- 15.Michaelsson G, Kristjansson G, Pihl Lundin I, Hagforsen E. Palmoplantar pustulosis and gluten sensitivity: a study of serum antibodies against gliadin and tissue transglutaminase, the duodenal mucosa and effects of gluten-free diet. Br J Dermatol. 2007;156(4):659–666. doi: 10.1111/j.1365-2133.2006.07725.x. [DOI] [PubMed] [Google Scholar]
- 16.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: implications for management. J Am Acad Dermatol. 2017;76(3):393–403. doi: 10.1016/j.jaad.2016.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National-Cancer-Institute. Dietary Screener Questionnaire (DSQ) in the NHANES 2009–10: dietary factors, food items asked, and testing status for DSQ. 2015 Jan 2016 June 2014. http://epi.grants.cancer.gov/nhanes/dietscreen/evaluation.html.
- 19.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker Wilks ARW (2015) maps: Draw Geographical Maps.(R version by Ray Brownrigg. Enhancements by Thomas P. Minka. Original S code by Richard Ahn).
- 21.R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- 22.National-Cancer-Institute. Dietary Screener Questionnaire (DSQ) in the NHANES 2009–10: Data Processing & Scoring Procedures. Aug 2016 [cited June 2015; Available from: http://epi.grants.cancer.gov/nhanes/dietscreen/scoring/.
- 23.Johnson JA, Ma C, Kanada KN, Armstrong AW. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28(3):327–332. doi: 10.1111/jdv.12105. [DOI] [PubMed] [Google Scholar]
- 24.Sorensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005;82(2):421–427. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]
- 25.Abell GC, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008;66(3):505–515. doi: 10.1111/j.1574-6941.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- 26.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006;72(3):1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belenguer A, Duncan SH, Calder AG, et al. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72(5):3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19(1):194–204. doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- 29.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chassard C, Lacroix C. Carbohydrates and the human gut microbiota. Curr Opin Clin Nutr Metab Care. 2013;16(4):453–460. doi: 10.1097/MCO.0b013e3283619e63. [DOI] [PubMed] [Google Scholar]
- 31.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Vuyst L, Leroy F. Cross-feeding between bifidobacteria and butyrate-producing colon bacteria explains bifdobacterial competitiveness, butyrate production, and gas production. Int J Food Microbiol. 2011;149(1):73–80. doi: 10.1016/j.ijfoodmicro.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Duncan SH, Belenguer A, Holtrop G, et al. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73(4):1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10(5):323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spreadbury I. Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. Diabetes Metab Syndr Obes. 2012;5:175–189. doi: 10.2147/DMSO.S33473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ajani UA, Ford ES, Mokdad AH. Dietary fiber and C-reactive protein: findings from national health and nutrition examination survey data. J Nutr. 2004;134(5):1181–1185. doi: 10.1093/jn/134.5.1181. [DOI] [PubMed] [Google Scholar]
- 37.Estruch R, Martinez-Gonzalez MA, Corella D, et al. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J Epidemiol Community Health. 2009;63(7):582–588. doi: 10.1136/jech.2008.082214. [DOI] [PubMed] [Google Scholar]
- 38.Herder C, Peltonen M, Koenig W, et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia. 2009;52(3):433–442. doi: 10.1007/s00125-008-1243-1. [DOI] [PubMed] [Google Scholar]
- 39.Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr. 2013;4(1):16–28. doi: 10.3945/an.112.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83(4):760–766. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi L, van Dam RM, Liu S, et al. Whole-grain, bran, and cereal fiber intakes and markers of systemic inflammation in diabetic women. Diabetes Care. 2006;29(2):207–211. doi: 10.2337/diacare.29.02.06.dc05-1903. [DOI] [PubMed] [Google Scholar]
- 42.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14(1):82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Suter PM. Carbohydrates and dietary fiber. Handb Exp Pharmacol. 2005;170:231–261. doi: 10.1007/3-540-27661-0_8. [DOI] [PubMed] [Google Scholar]
- 44.Kirby B, Richards HL, Mason DL, et al. Alcohol consumption and psychological distress in patients with psoriasis. Br J Dermatol. 2008;158(1):138–140. doi: 10.1111/j.1365-2133.2007.08299.x. [DOI] [PubMed] [Google Scholar]
- 45.Poikolainen K, Reunala T, Karvonen J. Smoking, alcohol and life events related to psoriasis among women. Br J Dermatol. 1994;130(4):473–477. doi: 10.1111/j.1365-2133.1994.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 46.Ockenfels HM, Keim-Maas C, Funk R, Nussbaum G, Goos M. Ethanol enhances the IFN-gamma, TGF-alpha and IL-6 secretion in psoriatic co-cultures. Br J Dermatol. 1996;135(5):746–751. doi: 10.1111/j.1365-2133.1996.tb03884.x. [DOI] [PubMed] [Google Scholar]
- 47.Schopf RE, Ockenfels HM, Morsches B. Ethanol enhances the mitogen-driven lymphocyte proliferation in patients with psoriasis. Acta Derm Venereol. 1996;76(4):260–263. doi: 10.2340/0001555576260263. [DOI] [PubMed] [Google Scholar]
- 48.Cardenas PD, Sonawane PD, Heinig U, et al. The bitter side of the nightshades: genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry. 2015;113:24–32. doi: 10.1016/j.phytochem.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 49.Patel B, Schutte R, Sporns P, et al. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm Bowel Dis. 2002;8(5):340–346. doi: 10.1097/00054725-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 50.De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230(2):156–160. doi: 10.1159/000369615. [DOI] [PubMed] [Google Scholar]
- 51.Millsop JW, Bhatia BK, Debbaneh M, Koo J, Liao W. Diet and psoriasis, part III: role of nutritional supplements. J Am Acad Dermatol. 2014;71(3):561–569. doi: 10.1016/j.jaad.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balbas GM, Regana MS, Millet PU. Study on the use of omega-3 fatty acids as a therapeutic supplement in treatment of psoriasis. Clin Cosmet Investig Dermatol. 2011;4:73–77. doi: 10.2147/CCID.S17220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bittiner SB, Tucker WF, Cartwright I, Bleehen SS. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1(8582):378–380. doi: 10.1016/S0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- 54.Danno K, Sugie N. Combination therapy with low-dose etretinate and eicosapentaenoic acid for psoriasis vulgaris. J Dermatol. 1998;25(11):703–705. doi: 10.1111/j.1346-8138.1998.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 55.Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis. Rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71(8):634–643. doi: 10.1007/BF00184491. [DOI] [PubMed] [Google Scholar]
- 56.Gupta AK, Ellis CN, Tellner DC, Anderson TF, Voorhees JJ. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120(6):801–807. doi: 10.1111/j.1365-2133.1989.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 57.Kojima T, Terano T, Tanabe E, et al. Effect of highly purified eicosapentaenoic acid on psoriasis. J Am Acad Dermatol. 1989;21(1):150–151. doi: 10.1016/S0190-9622(89)80363-9. [DOI] [PubMed] [Google Scholar]
- 58.Kragballe K. Dietary supplementation with a combination of n-3 and n-6 fatty acids (super gamma-oil marine) improves psoriasis. Acta Derm Venereol. 1989;69(3):265–268. [PubMed] [Google Scholar]
- 59.Kragballe K, Fogh K. A low-fat diet supplemented with dietary fish oil (Max-EPA) results in improvement of psoriasis and in formation of leukotriene B5. Acta Derm Venereol. 1989;69(1):23–28. [PubMed] [Google Scholar]
- 60.Lassus A, Dahlgren AL, Halpern MJ, Santalahti J, Happonen HP. Effects of dietary supplementation with polyunsaturated ethyl ester lipids (Angiosan) in patients with psoriasis and psoriatic arthritis. J Int Med Res. 1990;18(1):68–73. doi: 10.1177/030006059001800109. [DOI] [PubMed] [Google Scholar]
- 61.Maurice PD, Allen BR, Barkley AS, et al. The effects of dietary supplementation with fish oil in patients with psoriasis. Br J Dermatol. 1987;117(5):599–606. doi: 10.1111/j.1365-2133.1987.tb07492.x. [DOI] [PubMed] [Google Scholar]
- 62.Mayser P, Mrowietz U, Arenberger P, et al. Omega-3 fatty acid-based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38(4):539–547. doi: 10.1016/S0190-9622(98)70114-8. [DOI] [PubMed] [Google Scholar]
- 63.Ziboh VA, Cohen KA, Ellis CN, et al. Effects of dietary supplementation of fish oil on neutrophil and epidermal fatty acids. Modulation of clinical course of psoriatic subjects. Arch Dermatol. 1986;122(11):1277–1282. doi: 10.1001/archderm.1986.01660230069013. [DOI] [PubMed] [Google Scholar]
- 64.Bjorneboe A, Smith AK, Bjorneboe GE, Thune PO, Drevon CA. Effect of dietary supplementation with n-3 fatty acids on clinical manifestations of psoriasis. Br J Dermatol. 1988;118(1):77–83. doi: 10.1111/j.1365-2133.1988.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 65.Kettler AH, Baughn RE, Orengo IF, Black H, Wolf JE., Jr The effect of dietary fish oil supplementation on psoriasis. Improvement in a patient with pustular psoriasis. J Am Acad Dermatol. 1988;18(6):1267–1273. doi: 10.1016/S0190-9622(88)70133-4. [DOI] [PubMed] [Google Scholar]
- 66.Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328(25):1812–1816. doi: 10.1056/NEJM199306243282504. [DOI] [PubMed] [Google Scholar]
- 67.Jiang J, Li K, Wang F, et al. Effect of marine-derived n-3 polyunsaturated fatty acids on major eicosanoids: a systematic review and meta-analysis from 18 randomized controlled trials. PLoS ONE. 2016;11(1):e0147351. doi: 10.1371/journal.pone.0147351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turini ME, Powell WS, Behr SR, Holub BJ. Effects of a fish-oil and vegetable-oil formula on aggregation and ethanolamine-containing lysophospholipid generation in activated human platelets and on leukotriene production in stimulated neutrophils. Am J Clin Nutr. 1994;60(5):717–724. doi: 10.1093/ajcn/60.5.717. [DOI] [PubMed] [Google Scholar]
- 69.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63(1):116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 70.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320(5):265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 71.Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121(4):547–555. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 72.Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33(6):810–820. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- 73.Kavli G, Forde OH, Arnesen E, Stenvold SE. Psoriasis: familial predisposition and environmental factors. Br Med J (Clin Res Ed) 1985;291(6501):999–1000. doi: 10.1136/bmj.291.6501.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naldi L, Parazzini F, Peli L, Chatenoud L, Cainelli T. Dietary factors and the risk of psoriasis. Results of an Italian case-control study. Br J Dermatol. 1996;134(1):101–106. doi: 10.1111/j.1365-2133.1996.tb07846.x. [DOI] [PubMed] [Google Scholar]
- 75.Brown AC, Hairfield M, Richards DG, et al. Medical nutrition therapy as a potential complementary treatment for psoriasis–five case reports. Altern Med Rev. 2004;9(3):297–307. [PubMed] [Google Scholar]
- 76.Wong AP, Kalinovsky T, Niedzwiecki A, Rath M. Efficacy of nutritional treatment in patients with psoriasis: a case report. Exp Ther Med. 2015;10(3):1071–1073. doi: 10.3892/etm.2015.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holt EM, Steffen LM, Moran A, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109(3):414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walston J, Xue Q, Semba RD, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163(1):18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 79.Watzl B, Kulling SE, Moseneder J, Barth SW, Bub A. A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr. 2005;82(5):1052–1058. doi: 10.1093/ajcn/82.5.1052. [DOI] [PubMed] [Google Scholar]
- 80.El-Azhary RA, Peters MS, Pittelkow MR, Kao PC, Muller SA. Efficacy of vitamin D3 derivatives in the treatment of psoriasis vulgaris: a preliminary report. Mayo Clin Proc. 1993;68(9):835–841. doi: 10.1016/S0025-6196(12)60690-9. [DOI] [PubMed] [Google Scholar]
- 81.Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5(1):222–234. doi: 10.4161/derm.24808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaal J, Lakos G, Szodoray P, et al. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: results of an open, follow-up pilot study. Acta Derm Venereol. 2009;89(2):140–144. doi: 10.2340/00015555-0555. [DOI] [PubMed] [Google Scholar]
- 83.Huckins D, Felson DT, Holick M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study. Arthritis Rheum. 1990;33(11):1723–1727. doi: 10.1002/art.1780331117. [DOI] [PubMed] [Google Scholar]
- 84.Morimoto S, Yoshikawa K, Kozuka T, et al. Treatment of psoriasis vulgaris by oral administration of 1 alpha-hydroxyvitamin D3—open-design study. Calcif Tissue Int. 1986;39(3):209–212. doi: 10.1007/BF02555120. [DOI] [PubMed] [Google Scholar]
- 85.Perez A, Raab R, Chen TC, Turner A, Holick MF. Safety and efficacy of oral calcitriol (1,25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134(6):1070–1078. doi: 10.1111/j.1365-2133.1996.tb07945.x. [DOI] [PubMed] [Google Scholar]
- 86.Smith EL, Pincus SH, Donovan L, Holick MF. A novel approach for the evaluation and treatment of psoriasis. Oral or topical use of 1,25-dihydroxyvitamin D3 can be a safe and effective therapy for psoriasis. J Am Acad Dermatol. 1988;19(3):516–528. doi: 10.1016/S0190-9622(88)70207-8. [DOI] [PubMed] [Google Scholar]
- 87.Takamoto S, Onishi T, Morimoto S, et al. Effect of 1 alpha-hydroxycholecalciferol on psoriasis vulgaris: a pilot study. Calcif Tissue Int. 1986;39(6):360–364. doi: 10.1007/BF02555172. [DOI] [PubMed] [Google Scholar]
- 88.Jenks K, Stebbings S, Burton J, et al. Probiotic therapy for the treatment of spondyloarthritis: a randomized controlled trial. J Rheumatol. 2010;37(10):2118–2125. doi: 10.3899/jrheum.100193. [DOI] [PubMed] [Google Scholar]
- 89.Pagano J. Psoriasis: the natural alternative. Englewood Cliffs: The Pagano Organization; 1991. [Google Scholar]
- 90.Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63(5):397–403. [PubMed] [Google Scholar]
- 91.McDougall J, Bruce B, Spiller G, Westerdahl J, McDougall M. Effects of a very low-fat, vegan diet in subjects with rheumatoid arthritis. J Altern Complement Med. 2002;8(1):71–75. doi: 10.1089/107555302753507195. [DOI] [PubMed] [Google Scholar]
- 92.Tanaka T, Kouda K, Kotani M, et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J Physiol Anthropol Appl Human Sci. 2001;20(6):353–361. doi: 10.2114/jpa.20.353. [DOI] [PubMed] [Google Scholar]
- 93.Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18. doi: 10.1186/s12967-014-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Azzini E, Polito A, Fumagalli A, et al. Mediterranean diet effect: an Italian picture. Nutr J. 2011;10:125. doi: 10.1186/1475-2891-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esposito K, Maiorino MI, Bellastella G, Panagiotakos DB, Giugliano D. Mediterranean diet for type 2 diabetes: cardiometabolic benefits. Endocrine. 2017;56(1):27–32. doi: 10.1007/s12020-016-1018-2. [DOI] [PubMed] [Google Scholar]
- 96.Frassetto LA, Schloetter M, Mietus-Synder M, Morris RC, Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63(8):947–955. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- 97.Jonsson T, Granfeldt Y, Ahren B, et al. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross-over pilot study. Cardiovasc Diabetol. 2009;8:35. doi: 10.1186/1475-2840-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fagiolo U, Cossarizza A, Scala E, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23(9):2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 99.Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A. 2010;65(4):429–433. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51(25):1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 101.Roubenoff R, Harris TB, Abad LW, et al. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A. 1998;53(1):M20–M26. doi: 10.1093/gerona/53A.1.M20. [DOI] [PubMed] [Google Scholar]
- 102.Yin X, Low HQ, Wang L, et al. Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat Commun. 2015;6:6916. doi: 10.1038/ncomms7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some data generated or analyzed during this study are included in this published article/as supplementary information files. All other datasets generated during and/or analyzed during the current study are available from the corresponding author on request.