Abstract
Trichoderma spp. are well known biocontrol agents used against phytopathogens. In the present work Trichoderma-mediated Selenium nanoparticles (SeNPs) were synthesized and extent of downy mildew (DM) disease control in pearl millet (PM) was studied. Six species of Trichoderma namely, T. asperellum, T. harzianum, T. atroviride, T. virens, T. longibrachiatum and T. brevicompactum were evaluated in the form of culture filtrate (CF), cell lysate (CL) and crude cell wall (CW) to synthesize SeNPs. All these components produced SeNPs, but CF was significant than CL and CW. The size of SeNPs ranged from 49.5 to 312.5 nm with zeta potential of +3.3 mv to −200 mv. The nanoparticles suppressed the growth, sporulation and zoospore viability of Sclerospora graminicola and these biological activities were inversely proportional to the size of SeNPs. Under greenhouse conditions, application of SeNPs and T. asperellum together enhanced the early plant growth and suppressed DM incidence as compared to their individual application. This study demonstrated the ability of Trichogenic-SeNPs to suppress growth and proliferation of S. graminicola, the incitant of DM of PM and their activity is inversely proportional to size of nanoparticles.
Introduction
Selenium (Se) is a naturally occurring mineral in soil and being absorbed and accumulated by plants thereby entering the food chain. In addition, contaminated water and air also act as sources of Se exposure. Selenium is an important micronutrient required by both plants and animals. It was earlier recognized as toxic until Schwarz and Foltz1 reported its vital function in living organisms. Selenocystin present in the active site of glutathione peroxidase removes free radicals in cells reducing the adverse effect on cell components2, 3. On the other hand, increased concentration of Se in biological system leads to various health disorders4–8.
Selenium toxicity varies depending on its concentration and chemical form and usually present in the order, sodium selenite > selenium sulfide > elemental selenium9. Selenium nanoparticles (SeNPs) are less toxic than Se-methylselenocystenine (SeMSC), but up-regulate phase 2 enzymes as efficiently as SeMSC thereby preventing liver damage10. Because of its semi-conducting property Se and SeNPs are widely used in photovoltaic cells, electric rectifiers, photographic exposure meters and xenography11, 12. SeNPs have excellent bioavailability, unique physicochemical characteristics with high surface to volume ratio and exhibit admirable biological activity such as anti-microbial13–15, anti-oxidant16, anti-cancerous17 and anti-inflammatory18, and exhibit minimum toxicity than other forms of Se10, 19, 20.
By realizing the advantage of SeNPs over their other chemical forms, there is an increasing interest to generate SeNPs with various functionality. Most of the production methods employed for SeNPs involve chemicals such as hydrazine, sodium ascorbate or glycol (reducing agents), oxidation methods and by providing harsh conditions21–23. These methods are expensive, environmentally hazardous and require special equipment24. There is increasing interest in the synthesis of SeNPs using green nanobiotechnology which include microorganisms and plants or their byproducts (Secondary metabolites, proteins/enzymes, and lipids) with assistance of various biotechnological tools25, 26. These methods are ecofriendly, cost effective, overcome toxic and harsh chemicals, and do not need high energy.
Trichoderma spp. are ubiquitous soil fungi grow on a wide range of organic substrates, essentially cellulosic materials and take part in nutrient recycling thereby improving soil health27, 28. Symbiotically/endophytic association of these fungi with plants and protect them from biotic and abiotic stresses29, 30. Trichoderma spp. thrives under varying environmental conditions because of their high adaptability to growth regulation, sporulation and secretion of lytic enzymes31. These fungi are also known to resist/tolerate most of the pesticides used in agriculture32–34. These reasons make this group of fungi useful for industrial applications and as biofertilizer/biopesticide to improve plant health and yield. The genus Trichoderma is also explored for the production of silver nanoparticles (AgNPs) as it produced most positive results35–38.
Even with the advent of superior agro-technologies, plant diseases still poses a major problem in food production and is a threat to future food security. Sclerospora graminicola is a major constraint in pearl millet (PM) [Pennisetum glaucum (L.) R. Br.] production causing downy mildew (DM) disease. Under severe conditions it causes an estimated annual yield loss of <€11 million in India39, 40. The availability of DM resistant PM cultivars are limited and their durability is always questioned because of emergence of virulent pathogens. With increasing area under hybrid cultivation since 1970s, the disease has become more severe by the evolution of new virulent pathotypes in response to new hybrid genotypes41. Substantial work has been reported to manage PM-DM disease using biocontrol agents. Even after screening a large number of biocontrol agents still chemical pesticides are dominating under field conditions. This may cause considerable damage to the environment and lead to pesticide resistance in DM pathogen. Hence, eco-friendly integrated disease management strategies are gaining prominence across the world.
The present study aimed to develop reliable protocol for the synthesis of Trichoderma-assisted SeNPs possessing higher anti-mildew and zoosporicidal activity and evaluate them against DM pathogen. As per our knowledge and according to so far published reports this is the first attempt made to manage oomycetes disease in plants using Trichogenic-SeNPs and Trichoderma spp. together.
Results
Biosynthesis and characterization of SeNPs
The SeNPs were synthesized with 25 mM sodium selenite using culture filtrate (CF), cell lysate (CL) and crude cell wall (CW) of 6 different Trichoderma spp. [T. asperellum (T.as), T. harzianum (T.ha), T. atroviride (T.at), T. virens (T.vi), T. longibrachiatum (T.lo) and T. brevicompactum (T.br)]. Culture filtrate from Trichoderma spp. gave noticeable SeNPs production followed by CL and CW. In CF, formation of nanoparticles by the reduction of selenite ions could be visualized as change in solution color from pale yellow to insoluble orange-red within 12 h after incubation but in CL and CW, it was observed after 24 h (Fig. 1).
Figure 1.
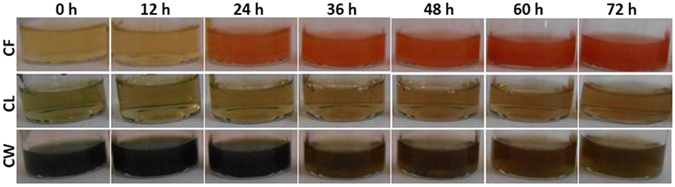
Trichogenesis of selenium nanoparticles (SeNPs) from the T. asperellum at different time intervals (hours). CW: Crude cell wall; CL: Cell lysate; CF: Culture filtrate.
SeNPs characters are presented in Table 1. Detailed characters of SeNPs generated from T.as-CF are shown in Fig. 2. Morphology and size of SeNPs were verified by SEM and TEM. Shapes of SeNPs were observed as hexagonal, near spherical and irregular (Fig. 2a,b,c, Supplementary Figs S1 and S2). X-ray Photoelectron spectroscopic (XPS) analysis of SeNPs was shown in Fig. 2d, an intense peak recorded at 55.6 eV which was corresponding to the binding energy of elemental Se. The characteristic red color of SeNPs observed in all reactions was due to the excitation of the surface plasmon vibrations of the monoclinic Se particles. The solutions of SeNPs when subjected to UV-visible spectral scan showed maximum absorption between 200 to 400 nm, corresponding to surface Plasmon resonance indicating the formation of SeNPs. In most of the CF-SeNPs, absorption peak appeared at 280 nm, a typical spectrum of T.as-CF-SeNPs is shown in Fig. 2e. In CL-SeNPs, an additional peak at 415 nm was present (Supplementary Fig. S3). The size of the nanoparticles generated from different methods varied from 49.5 nm (T.as-CF-SeNPs) to 312.5 nm (T.vi-CW-SeNPs). Zeta potential analysis of SeNPs obtained varied from +3.3 mv (T.at-CL-SeNPs) to −200 mv (T.as-CL-SeNPs) (Table 1).
Table 1.
Summary of Trichogenic-selenium nanoparticles (SeNPs) characters.
| Trichogenic-SeNPs | Size (nm) | Shape | Zeta potential/Polarity |
|---|---|---|---|
| Culture filtrate | |||
| T.as-CF | 49.5 | Irregular | −63.8 mv/negative |
| T.ha-CF | 60.8 | Spherical | −14.4 mv/negative |
| T.vi-CF | 96.2 | Spherical | −28.2 mv/negative |
| T.lo-CF | 87.5 | Spherical | +11.8 mv/positive |
| T.at-CF | 157.9 | Irregular | −7.8 mv/negative |
| T.br-CF | 99.6 | Irregular | +7.3 mv/positive |
| Cell lysate | |||
| T.as-CL | 61.3 | Irregular | −200 mv/negative |
| T.ha-CL | 140.4 | Spherical | +5.7 mv/positive |
| T.vi-CL | 158.8 | Irregular | +6.7 mv/positive |
| T.lo-CL | 256.1 | Irregular | −38.1 mv/negative |
| T.at-CL | 168.4 | Irregular | +3.3 mv/positive |
| T.br-CL | 109.2 | Irregular | +5.3 mv/positive |
| Crude cell wall | |||
| T.as-CW | 130.2 | Spherical | −26.1 mv/negative |
| T.ha-CW | 103.5 | Spherical | −16.7 mv/negative |
| T.vi-CW | 312.5 | Irregular | −28.0 mv/negative |
| T.lo-CW | 158.4 | Irregular | −15.6 mv/negative |
| T.at-CW | 67.9 | Irregular | +6.9 mv/positive |
| T.br-CW | 199.6 | Irregular | +7.3 mv/positive |
T.as – T. asperellum; T.ha – T. harzianum; T.vi – T. virens; T.lo – T. longibrachiatum; T.at – T. atroviride; T.br – T. brevicompactum; CF – Culture filtrate, CL – cell lysate; CW – crude cell wall.
Figure 2.

Characterization of Trichogenic-SeNPs generated using culture filtrate of T. asperellum. (a) Scanning electron microscopic (SEM) view of commercially available sodium selenite compound. (b) Scanning electron microscopic (SEM) view of Trichogenic-SeNPs. (c) Transmission electron microscopic (TEM) view of Trichogenic-SeNPs. (d) Se3d XPS spectrum. (e) UV- Visible spectrum showing the absorption peak at 280 nm. (f) Particle size distribution histogram of Trichogenic-SeNPs. (g) FTIR spectrum showing the reduction process with different % Transmittance. (h) XRD spectrum of the sodium selenite. (i) XRD spectrum showing the presence of Trichogenic-SeNPs.
FTIR analysis of T.as-CF-SeNPs confirmed the presence of elemental SeNPs, an indication of the reduction process (Fig. 2g). It disclosed a broad peak at 3349 cm−1, which is the trait of O-H stretching form and N-H stretch in amine group. The peaks at 2975 and 2891 cm−1 correspond to the asymmetric stretching vibration of –CH3, the asymmetric and symmetric stretching vibrations of –CH2, respectively. The peaks, at 1649 and 1381 cm−1 specify the incidence of interaction between C=O and C–N groups and SeNPs separately. Hence, the association of proteins with SeNPs is confirmed and possibly they prevent agglomeration of particles by stabilizing SeNPs in the medium. The distinctive XRD patterns of commercially available sodium selenite and T.as-CF-SeNPsare presented in Fig. 2h and i, respectively. In Fig. 2i, amorphous/nano-crystalline nature of the synthesized SeNPs is known by noisier feature with wider peaks and each diffraction pattern peaks in the range of 2θ. In SeNPs, there are no clear sharp Bragg reflections which indicate the association of SeNPs with protein.
Anti-mildew and zoosporicidal activity of SeNPs
Selenium nanoparticles suppressed sporulation of S. graminicola when applied on to the surface of infected PM leaf. The extent of suppression varied significantly (P ≤ 0.05) among the SeNPs generated by different methods. CF-SeNPs significantly (P ≤ 0.05) suppressed sporulation when compared to CL-SeNPs and CW-SeNPs (Fig. 3a–d). Maximum suppression of sporulation was recorded with SeNPs generated by T.as-CF followed by T.br-CF as indicated by the low minimum inhibitory concentration (MIC) value of 150 ppm and 250 ppm, respectively (Fig. 4a, Table S1).
Figure 3.

Anti-mildew activity. (a) Set up showing anti-mildew activity assay. (b) T.as-CF-SeNPs treatment suppressed the growth and sporulation of Sclerosporagraminicola. (c) Untreated infected leaf showing profuse sporulation of Sclerospora graminicola. (d) Close up view of infected leaf showing sporangia and sporangiospores, observed under stereo binocular microscope. (e–i) Viable and non-viable zoospores observed under microscope after TTC staining. (e) Empty sporangia after releasing viable zoospores (control), (f). Deeply red colored zoospores in sporangia (control), (g). T.ha-CF-SeNPs treatment showing mixture of live and dead zoospores, (h) and (i) zoospores showing reduction in viability of zoospores in T.br-CF-SeNPs and T.as-CF-SeNPs treatment, respectively.
Figure 4.

Anti-mildew and zoosporicidal activity of Trichogenic-SeNPs. (a) Anti-mildew activity of Trichogenic-SeNPs generated using CF, CL and CW of Trichoderma spp. (b) Correlation between anti-mildew activity and size of SeNPs. (c) Zoosporicidal activity of Trichogenic-SeNPs generated using CF, CL and CW. (d) Correlation between zoosporicidal activity and size of SeNPs. *Minimum concentration SeNPs showing complete inhibition of sporulation. #Concentration of SeNPs corresponding to IC50 value. Valuesare means of three replicates.
This trend was also observed with SeNPs, when subjected to zoosporicidal assay. CF-SeNPs of different Trichoderma showed significantly (P ≤ 0.05) lower IC50 values than SeNPs generated using CL and CW. Least IC50 value of 64 ppm and 109 ppm were recorded for T.as-CF-SeNPs and T.br-CF-SeNPs, respectively (Fig. 4c). Microscopic observation of sporangia revealed that T.as-CF-SeNPs reduced viability of sporangia significantly (P ≤ 0.05), whereas in control the sporangia were red colored due to accumulation of insoluble formazan (Fig. 3e–i).
It was also evident from the assays that the size of SeNPs is inversely proportional to its DM pathogen suppressive ability (Fig. 4b and d).
Effect of seed treatment with combined T.as-CF-SeNPs and Trichoderma on plant growth and disease protection under greenhouse conditions
Seed treatment with SeNPs (100 ppm) promoted all the plant growth parameters under greenhouse conditions as compared to control. All Trichoderma-SeNPs in combination significantly (P ≤ 0.05) increased plant height, dry weight, number of tillers per plant and chlorophyll content over that of control (Table 2). Foliar spray with SeNPs (100 ppm) did not affect any of the plant growth parameters and has not visually shown any phytotoxic effect. Among different treatments, application of T. asperelleum + SeNPs significant (P ≤ 0.05) improved plant height (24.9 cm), number of tillers (3.40 tillers/seedling) and chlorophyll content (3.76 mg/g) of PM seedlings, as compared to control plants (Table 2).
Table 2.
Effect of combined application of Trichoderma and T.as-CF-SeNPs on growth parameters and downy mildew disease incidence in pearl millet.
| Treatments | APH (cm) | Number of tillers/seedling | Dry weight (g/seedling) | Total Chlorophyll (mg/g fresh weight) | Disease Incidence (%) | ||
|---|---|---|---|---|---|---|---|
| Seed treament | SeNPs Foliar Sparay | Challenge inoculation | |||||
| Control | − | − | 20.41 ± 0.55ab | 2.68 ± 0.15defgh | 2.43 ± 0.06fg | 3.63 ± 0.10a | — |
| + | 20.83 ± 0.89ab | 2.60 ± 0.10gh | 2.43 ± 0.07fg | 3.55 ± 0.13a | — | ||
| − | + | 11.50 ± 0.48d | 2.10 ± 0.15i | 1.15 ± 0.03i | 2.28 ± 0.10b | 93 ± 1.45a | |
| + | 15.35 ± 1.18cd | 2.45 ± 0.09hi | 1.65 ± 0.06h | 2.95 ± 0.04ab | 49 ± 1.45b | ||
| T.as + SeNPs | − | − | 24.71 ± 0.62a | 3.22 ± 0.06ab | 3.03 ± 0.06abc | 3.64 ± 0.10a | — |
| + | 24.85 ± 1.41a | 3.40 ± 0.06a | 3.04 ± 0.09ab | 3.76 ± 0.08a | — | ||
| − | + | 21.60 ± 0.72ab | 2.88 ± 0.09bcdefgh | 2.64 ± 0.07defg | 3.53 ± 0.19a | 20 ± 0.88d | |
| + | 23.55 ± 1.19a | 3.14 ± 0.08abc | 2.66 ± 0.08bcedf | 3.64 ± 0.06a | 12 ± 1.15e | ||
| T.ha + SeNPs | − | − | 22.02 ± 0.76ab | 3.13 ± 0.04abcd | 2.93 ± 0.06abcd | 3.62 ± 0.10a | — |
| + | 22.39 ± 0.65ab | 3.23 ± 0.09ab | 2.93 ± 0.06abcde | 3.66 ± 0.12a | — | ||
| − | + | 21.35 ± 0.61ab | 2.90 ± 0.06bcdefg | 2.54 ± 0.07efg | 3.32 ± 0.13a | 29 ± 1.20c | |
| + | 21.54 ± 0.38ab | 3.12 ± 0.01abcd | 2.26 ± 0.04g | 3.51 ± 0.14a | 14 ± 0.88e | ||
| T.at + SeNPs | − | − | 20.95 ± 1.41ab | 3.08 ± 0.06abcde | 2.83 ± 0.07abcde | 3.59 ± 0.26a | — |
| + | 20.57 ± 0.56ab | 3.00 ± 0.06abcdefg | 2.88 ± 0.09abcde | 3.53 ± 0.17a | — | ||
| − | + | 18.72 ± 0.23bc | 2.63 ± 0.08fgh | 2.75 ± 0.09abcdef | 3.16 ± 0.14a | 30 ± 2.40c | |
| + | 20.56 ± 1.18ab | 2.96 ± 0.09abcdefg | 2.86 ± 0.09abcde | 3.50 ± 0.12a | 21 ± 1.53d | ||
| T.vi + SeNPs | − | − | 21.44 ± 0.48ab | 3.03 ± 0.12abcdefg | 2.94 ± 0.07abcd | 3.72 ± 0.06a | — |
| + | 21.05 ± 1.04ab | 3.05 ± 0.09abcdef | 2.93 ± 0.12abcde | 3.66 ± 0.08a | — | ||
| − | + | 18.43 ± 0.54bc | 2.75 ± 0.09cdefgh | 2.82 ± 0.08abcde | 3.27 ± 0.05a | 28 ± 0.58c | |
| + | 20.90 ± 1.42ab | 2.91 ± 0.06bcdefg | 2.89 ± 0.10abcde | 3.52 ± 0.16a | 25 ± 1.45cd | ||
| T.lo + SeNPs | − | − | 22.92 ± 0.57ab | 3.10 ± 0.06abcde | 2.84 ± 0.05abcde | 3.72 ± 0.12a | — |
| + | 23.18 ± 0.98ab | 3.03 ± 0.08abcdefg | 3.05 ± 0.08a | 3.60 ± 0.25a | — | ||
| − | + | 21.66 ± 0.46ab | 2.66 ± 0.03efgh | 2.82 ± 0.07abcdef | 3.46 ± 0.26a | 26 ± 0.88cd | |
| + | 22.57 ± 0.66ab | 2.80 ± 0.06bcdefgh | 2.83 ± 0.07abcde | 3.70 ± 0.10a | 22 ± 1.45d | ||
| T.br + SeNPs | − | − | 21.72 ± 0.49ab | 3.00 ± 0.06abcdefg | 2.82 ± 0.07abcde | 3.73 ± 0.13a | — |
| + | 21.26 ± 1.39ab | 3.03 ± 0.09abcdefg | 2.85 ± 0.05abcde | 3.58 ± 0.21a | — | ||
| − | + | 20.49 ± 0.64ab | 2.81 ± 0.06bcdefgh | 2.65 ± 0.06cdef | 3.43 ± 0.07a | 21 ± 0.58d | |
| + | 21.28 ± 0.90ab | 2.92 ± 0.06bcdefg | 2.82 ± 0.06abcde | 3.70 ± 0.15a | 18 ± 0.88de | ||
| Apron | − | − | 20.27 ± 0.61ab | 2.71 ± 0.06cdefgh | 2.65 ± 0.05cdefg | 3.66 ± 0.13a | — |
| − | + | 20.55 ± 0.59ab | 2.70 ± 0.06cdefgh | 2.68 ± 0.05abcdef | 3.60 ± 0.25a | 9 ± 0.88e | |
APH - average plant height; T.as - T. asperellum; T.ha – T. harzianum; T.vi – T. virens; T.lo – T. longibrachiatum; T.at – T. atroviride; T.br – T. brevicompactum. Values are the mean of three replicates; values within column sharing the same letter(s) are not significantly different according to Tukey’s HSD at P ≤ 0.05.
All combinations of Trichoderma spp. and SeNPs suppressed DM disease of PM under greenhouse conditions as compared to its individual application and control. The extent of disease control varied considerably depending upon combinations. Both individual and combined applications significantly (P ≤ 0.05) reduced DM infected plants as compared with their respective controls. Disease protection ability of all Trichoderma spp. increased in the presence of SeNPs. Further enhancement in disease protection efficacy of the combination was observed, when additional SeNPs was applied as spray treatment. Maximum protection was observed with T. asperellum + SeNPs applied as seed treatment followed by foliar spray with SeNPs, which recorded 12% disease incidence as compared to 87% in control (Table 2). The control challenged plants recorded significant (P ≤ 0.05) reduction in plant height (11.5 cm), number of tillers (2.10 tillers/seedling) and chlorophyll content (2.28 mg/g).
Discussion
In this study, SeNPs generated from CF, CL and CW of six Trichoderma spp. were evaluated to suppress DM disease in PM in combination with Trichoderma spp. The CF of all the Trichoderma spp. were best in producing SeNPs and it was visualized as early as 12 h after incubation in comparison with CL and CW.
Use of a cellular component of microbes is always considered as more advantageous in generating nanoparticles, than using whole microorganism. When live microorganisms are used, control over the size of nanoparticles cannot be achieved as growth stage and incubation period greatly affect the size and characters of nanoparticles42–44. Also,use of culture filtrate makes the downstream process easier. Culture filtrates of Trichoderma spp.35, 38, Fusarium oxysporum 45, Aspergillus flavus 46 and Aspergillus terreus 47 were successfully used earlier for the generation of AgNPs. Similarly, Zare et al.48 by using culture filtrate of A. terreus generated spherical shaped SeNPs with an average size of 47 nm.
Previous studies, have established that biogenesis of NPs depends on several factors including, pH, temperature, raw material form and concentration, incubation period, cocktail of the biochemical present in the biological extracts49–53. Characterization of 18 SeNPs generated from three components of six Trichoderma spp. had common features such as brick red color of solutions and maximum absorbance at 200–400 nm. Results of XPS analysis confirmed the presence of elemental Se. FTIR, XRD pattern, size and shape as observed under TEM and SEM and zeta potential were not same among the SeNPs generated through different methods. Characters SeNPs generated in our studies are in accordance with previous reports15, 53–58. The present results clearly indicate the differential ability of Trichoderma to generate SeNPs. Such observation was also made by Devi et al.35, when they tested 75 isolates of Trichoderma spp. belonging to five different species for AgNPs generation. In our studies, CF of T. asperellum yielded the most favourable results with respect to the incubation period, zeta potential and size of the SeNPs.
In our experiments, the most striking difference observed between the SeNPs was their differential ability to suppress the sporulation of DM pathogen on PM leaves and their zoosporicidal activity. These phenomena of SeNPs may be due to its variable characters as discussed earlier. Recently, Ajitha et al.59 reported the dependency of AgNPs antimicrobial activity on their particle size. They observed that decrease in the size of AgNPs increased the antimicrobial activity against E. coli, Pseudomonas spp. Aspergillus niger and Penicillium spp. Studies on shape dependent activity of AgNPs with E. coli made by Pal et al.60 revealed that, truncated triangular AgNPs were remarkably inhibited bacterial growth as compared to spherical and rod-shaped AgNPs. It was observed that the anti-mildew and zoosporicidal activity of SeNPs was inversely proportional to its size (Fig. 4b and d).
The application of nanometals in plant disease management is promising as an alternative to chemical pesticides. DNA-directed AgNPs grown on graphine oxide (Go) suppresses bacterial spot of tomato caused by Xanthomonas perforans 61 and powdery mildew in cucrubits when applied as foliar spray at 100 ppm concentration62. Jo et al.63 evaluated the antifungal activity of silver ions and silver nanoparticles against two plant pathogenic fungi, Bipolaris sorkiniana and Mangoporthe grisea. Foliar application of AgNPs prior to the application of pathogens suppressed fungal growth and reduced disease incidence in perennial ray grass. But the use of SeNPs for plant disease management is not reported.
Anti-mildew activity revealed that highest concentration (1000 ppm) of SeNPs treatment was not phytotoxic to PM leaves. At 100 ppm concentration SeNPs did not inhibit the growth of Trichoderma spp. and PM seedlings (Supplementary Fig. S4). The sub-inhibitory concentration of SeNPs stimulates the growth of A. niger which could be attributed to the microbial growth promotion by trace elements64. Also low concentrations of Se improve plant growth and yield of Brassica, ryegrass, potato and soybean65–68. In accordance with previous reports, at lower concentration SeNPs applied as seed treatment along with Trichoderma enhances plant growth parameters.
Materials and Methods
Microorganisms and culture conditions
Trichoderma spp
All Trichoderma spp. [T. asperellum (DL-81: KM100835), T. harzianum (HR-73: KM100834), T. atroviride (MH-50: KM100830), T. virens (MP-60: KM100832), T. longibrachiatum (MP-59: KM100831) and T. brevicompactum (UP-91: KM100836)] used in the study were collected from the culture collection of Department of Biotechnology, University of Mysore. These fungi were originally isolated from PM rhizosphere soil samples and were well characterized individually for plant growth promoting and downy mildew (DM) disease suppressing ability (unpublished data). All the fungi were subcultured once in 15 days and maintained on Potato dextrose agar (PDA) at 28 ± 1 °C throughout the experimental period.
For biosynthesis of SeNPs, the test fungi were grown on Potato dextrose broth (PDB) under dark and static conditions at 28 ± 1 °C for 7 days. At the end of incubation period, mycelial mat was collected by filtering through four layers of muslin cloth and washed three times with sterile distilled water. Crude cell wall debris was washed three times with sterile distilled water before further use. The culture filtrates (CF), cell lysate (CL) and crude cell wall (CW) from six Trichoderma spp. were used for SeNPs production. Three g of wet biomass of mycelial mat was ground to fine powder with liquid nitrogen and suspended in 25 ml sterile distilled water, further the cells were disrupted by sonication (8 × 8 s, 18 micron amplitude, 30 s cooling on ice between sonication cycles, Sonic Vibra cell, Sonic and Materials Inc., USA)69. To ensure complete lysis, 5 μl of the solution was spread over Potato dextrose agar (PDA) and analyzed for fungal growth upto five days. No fungal colonies on PDA indicated the complete disruption of the fungal mycelia. The lysed mycelial solution was centrifuged at 12000 rpm for 10 min at 4 °C and crude cell wall debris (pellet) was separated from cell lysate (supernatant).
For greenhouse studies, all Trichoderma spp. were grown on PDA for 12 days on 90 mm Petri plates at 28 ± 1 °C. At the end of incubation period, the conidia were dislodged by adding 5 ml of sterile distilled water and by using a sterile soft brush. The conidial suspension was washed three times with sterile distilled water, the final concentration adjusted to 1 × 108 conidia/ml using hemocytometer and used for further experiments.
Sclerospora graminicola
Downy mildew (DM) pathogen, S. graminicola sick plot is maintained at the Department of Biotechnology, University of Mysore, Mysuru (N24°18′, E79°26′, 903 m altitude) since last three decades under All India co-ordinated pearl millet improvement project (AICPMIP).
The infected leaves showing typical DM disease symptoms were collected from field grown PM cv. 7042S in the evening hours. The collected leaves showing profuse DM growth on the abaxial surface of leaves were washed under running tap water to remove old sporulation and adhering extraneous particles. The leaves were blot dried and incubated overnight in a humid chamber (>70% RH and 20 °C) by keeping abaxial surface upwards. Next day early morning the sporangia grown profusely on abaxial leaf surface were collected in distilled water using a sterile soft brush without damaging sporangia. Immediately, the final concentration was adjusted to 5 × 103 sporangia/ml using hemocytometer and used as inoculum.
Biosynthesis of SeNPs
Culture filtrate, CL and CW components were used for SeNPs biosynthesis. Initiation of SeNPs synthesis was done by adding 20 ml CF or CL or 3 g wet weight of CW to 70 ml of sterile distilled water containing 25 mM sodium selenite made up to 100 ml using sodium selenite solution (25 mM)35. The reaction mixture was kept at 28 ± 1 °C on a shaker at 150 rpm for 6 days. The formation of nanoparticles was first visually examined for change in color of reaction mixture. At different time intervals of the reaction, the reaction mixture was collected and the nanoparticles were precipitated by centrifuging at 10,000 rpm for 10 min. The precipitate was washed with double distilled water and further purified as explained by Zhang et al.70. SeNPs from CW was extracted by the method of Sonkusre et al.71. Throughout the experimental period, appropriate controls were maintained.
Characterization of biosynthesized SeNPs
Ultraviolet-visible spectrum from 200 to 800 nm was recorded at the resolution of 1 nm in HITACHI U-200 Spectrophotometer. X-ray photoelectron spectroscopy (XPS) measurements were carried out on an SPECS system using an Mg Ka x-ray source. The system was calibrated with C1s peak (284.8 eV). The functional association of SeNPs was analyzed by FTIR spectrum (PerkinElmer Spectrum NIOS2) after grinding with KBr. The spectrum was recorded at a resolution of 4 cm−1 at the range of 500–4000 cm−1. X-ray diffraction (XRD) pattern of the samples was generated using εMMA X-Ray Diffractometer (Braeside, Australia) operating at a voltage of 40 kV and current of 20 mA. The scanning was done in the 2θ range of 20° to 80° at 0.02°/min with a time constant of 2 sec. The size and morphology of Trichogenic-SeNPs were assessed by scanning electron microscopy (SEM) (Zeiss EVOLS 15) and Transmission electron microscopy (TEM) by coating them on a thin film of carbon-coated copper grid. Zeta potential and particle distributions of SeNPs were detected using Microtrac SL-PS-25 Rev.
Anti-mildew activity
Leaf disc method was followed to assess the anti-mildew activity of the synthesized SeNPs against DM pathogen of PM following the method of Girijamba et al.72. The diseased leaves were collected from the sick plot and washed with distilled water and blot-dried to remove excess water. Leaf discs of 10 mm were obtained by cutting the infected leaf using sterile cork borer. The discs were treated with 0 to 1000 ppm of SeNPs at an interval of 50 ppm for 5 min. The treated and control leaf discs were placed (adaxial surface downwards) on the moist blotter paper in petridish and the plates were incubated for 12–14 h in moist chambers (>70% RH and 20 °C) under dark condition. The treated leaf discs were observed for sporulation under a stereobinocular microscope. Various sporulation behaviors in leaves were recorded on the basis of inhibition scale: A (100% inhibition), B (<100–75> % inhibition), C (<75–50> % inhibition), D (<50–25> % inhibition) and D (<25–00> % inhibition) (Table S1) and minimum concentration of SeNPs needed for 100% suppression of sporulation was recorded.
Zoosporicidal assay
Fresh sporangia were harvested from the infected leaves as explained earlier. The sporangial concentration was immediately adjusted to 5 × 103/ml using hemocytometer and it was used as inoculum. The reaction mixture containing different concentrations (0 to 1000 ppm at an interval of 50 ppm) of SeNPs (100 µl) and inoculum (100 µl) was incubated in dark for one h. After incubation, 20 µl of Triphenyltetrazolium chloride (TTC) solution were added and further incubated for 30 min. The sporangia were observed under compound microscope for the red colored insoluble formazan. The resultant mixture was centrifuged at 8000 rpm for 8 min and the pellet was washed three times with distilled water. The pellet was mixed with 1 ml of 95% ethanol and incubated in a waterbath at 85 °C for 30 min. The mixture was centrifuged at 8000 rpm for 8 min, 200 µl supernatant was transferred to a microtiter plate and read at 485 nm against respective controls. The percent inhibition was calculated by considering color developed in control as 100% viability. The assay was performed in triplicate for each treatment.
Disease protection studies under greenhouse conditions
Greenhouse study was limited only to T.as-CF-SeNPs, since it showed better anti-mildew and zoosporicidal activity. The seeds of PM (cv. 7042 S), which are highly susceptible to DM disease was used after surface sterilization. A formulation containing different Trichoderma (1 × 108 conidia/ml), T.as-CF-SeNPs (100ppm) and CMC (0.4%, W/V) was prepared in sterile water. The PM Seeds were treated with this formulation on a rotary shaker (200 rpm) for 30 min at 30 ± 1 °C. Treated seeds after separation from the solution by filtration were spread uniformly on three layers of blotter sheet and dried overnight in laminar air flow. The seeds treated with distilled water amended with CMC and Apron 35 SD (6 g/kg seeds) served as negative and positive control, respectively. The seeds were sown in earthen pots filled with sterilized potting mixture (2:1:1, soil:sand:farm yard manure, v/v). The seedlings were raised under greenhouse conditions (25 ± 2 °C, 80% RH, natural sunlight). To one set of experiment, an additional foliar spray with T. as-CF-SeNPs (100 ppm prepared in sterile distilled water) was given 24 h prior to challenge inoculation. Three-day-old seedlings were challenge inoculated whorally with zoospore suspension during early morning for three consecutive days73. Pots containing seedlings raised with different treatments followed by challenge-inoculation were arranged in a randomized complete block design and watered as and when required. Thirty days after sowing (DAS), plant height was measured from the base to the tip of the plant, seedlings were uprooted without damaging the root system washed under running tap water to remove adhering soil particles and blot dried. The dry weight of the seedlings was determined after drying in an oven at 60 °C until the constant weight was achieved. Chlorophyll content in leaves of different treatments was determined following the standard procedures74. Before growth parameter analysis, the plants showing typical DM symptoms such as, stunted growth, chlorosis and sporulation on the abaxial leaf surface were recorded and the percentage disease incidence was calculated. For each treatment, six pots were maintained in triplicates, each pot containing 6–8 seedlings. Each pot was labeled and arranged in a randomized order. The control plants (without challenge inoculation) were maintained separately to avoid cross contamination of pathogen. The whole experiment was replicated thrice and the data expressed as the mean of three experiments.
Statistical analysis
The data collected from laboratory and greenhouse experiments were subjected to analysis of variance using SPSS Inc. 17.0. Significant treatment effects were determined by calculating F values (P ≤ 0.05). Treatment means were compared for significant differences (at P ≤ 0.05) using Tukey’s honest significant differences (HSD) test.
Conclusion
The results suggest that Trichogenic-SeNPs are anti-mildew and zoosporicidal and the activity is inversely proportional to the size of SeNPs. The SeNPs generated using CF of T. asperellum had better anti-mildew and zoosporicidal activity and they are highly effective when applied in combination with different Trichoderma spp. in reducing DM disease in PM. This study points to the potential in integrated approach for management of DM disease in PM. This opens up a new avenue where Trichoderma formulations along with SeNPs can be successfully employed for plant disease management.
Electronic supplementary material
Acknowledgements
The authors are thankful to the Indian Council of Agricultural Research (ICAR), Government of India, New Delhi, for providing field facilities at Department of studies in Biotechnology, University of Mysore and Institution of Excellence (IOE) Project Authorities University of Mysore. The authors also wish to acknowledge Mr. Yogesh, Technical assistant, IOE, University of Mysore for his kind support in SEM studies and Central Research Facility, Indian Institute of Technology Delhi for providing TEM facility.
Author Contributions
B.N., carried out the experimental work and wrote the manuscript. N.G. and P.H., designed the experiment, analyzed data and revised the manuscript. H.S.P. and H.S.S., participated in the design of the study and revised the manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02737-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwarz K, Foltz CM. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957;79:3292–3293. doi: 10.1021/ja01569a087. [DOI] [PubMed] [Google Scholar]
- 2.Baker RD, Baker SS, LaRosa K, Whitney C, Newburger PE. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch. Biochem. Biophys. 1993;304:53–57. doi: 10.1006/abbi.1993.1320. [DOI] [PubMed] [Google Scholar]
- 3.Bakir MA, Yaseene T, Sarheel A, Othman I. The determination of selenium concentration in blood and tumour tissues of breast cancer patients in syria using instrumental neutron activation analysis. J. Radioanal. Nucl.Chem. 2004;260:607. doi: 10.1023/B:JRNC.0000028220.00481.8e. [DOI] [Google Scholar]
- 4.Mac Farquhar JK, et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010;170:256–261. doi: 10.1001/archinternmed.2009.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinceti M, et al. Selenium neurotoxicity in humans: bridging laboratory and epidemiologic studies. Toxicol. Lett. 2014;230:295–303. doi: 10.1016/j.toxlet.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Barceloux DG. Selenium. J. Toxicol. Clin. Toxicol. 1999;37:145–172. doi: 10.1081/CLT-100102417. [DOI] [PubMed] [Google Scholar]
- 7.Brown TA, Shrift A. Selenium: toxicity and tolerance in higher plants. Biol. Rev. Cambr. Philos. Soc. 1982;57:59–84. doi: 10.1111/j.1469-185X.1982.tb00364.x. [DOI] [Google Scholar]
- 8.Pilon-Smits, E. A. H. & Quinn, C. F. Selenium metabolism in plants. Plant Cell Monographs17: Cell Biology of Metals and Nutrients (eds Hell, R. & Mendel, R. R.) 225–241 (Springer, 2010).
- 9.Nuttall KL. Evaluating selenium poisoning. Ann. Clin. Lab. Sci. 2006;36:409–420. [PubMed] [Google Scholar]
- 10.Zhang J, Wang X, Xu T. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008;101:22–31. doi: 10.1093/toxsci/kfm221. [DOI] [PubMed] [Google Scholar]
- 11.Poborchii VV, Kolobov AV, Tanaka K. An in situ Raman study of polarization-dependent photocrystallization in amorphous selenium films. Appl. Phys. Lett. 1998;72:1167–1169. doi: 10.1063/1.121002. [DOI] [Google Scholar]
- 12.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin. Cancer Res. 2004;10:2561–2569. doi: 10.1158/1078-0432.CCR-03-0268. [DOI] [PubMed] [Google Scholar]
- 13.Tran PA, Webster TJ. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomedicine. 2011;6:1553–1558. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava N, Mukhopadhyay M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosys. Eng. 2015;38:1723–1730. doi: 10.1007/s00449-015-1413-8. [DOI] [PubMed] [Google Scholar]
- 15.Khiralla GM, El-Deeb BA. Antimicrobial and antibiofilm effects of selenium nanoparticles on some food borne pathogens. LWT Food Sci. Technol. 2015;63:1001–1007. doi: 10.1016/j.lwt.2015.03.086. [DOI] [Google Scholar]
- 16.Kong H, et al. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014;65:155–162. doi: 10.1016/j.ijbiomac.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Ramamurthy CH, et al. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013;36:1131–1139. doi: 10.1007/s00449-012-0867-1. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, et al. High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS. Toxicol. Lett. 2014;224:16–23. doi: 10.1016/j.toxlet.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Kojouri GA, Jahanabadi S, Shakibaie M, Ahadi AM, Shahverdi AR. Effect of selenium supplementation with sodium selenite and selenium nanoparticles on iron homeostasis and transferrin gene expression in sheep: A preliminary study. Res. Vet. Sci. 2012;93:275–278. doi: 10.1016/j.rvsc.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Shakibaie M, et al. Acute and subacute toxicity of novel biogenic selenium nanoparticles in mice. Pharm. Biol. 2013;51:58–63. doi: 10.3109/13880209.2012.710241. [DOI] [PubMed] [Google Scholar]
- 21.Mishra B, Hassan PA, Priyadarsini KI, Mohan H. Reactions of biological oxidants with selenourea: formation of redox active nanoselenium. J. Phys. Chem B. 2005;109:12718–23. doi: 10.1021/jp051328n. [DOI] [PubMed] [Google Scholar]
- 22.Shah CP, Kumar M, Bajaj PN. Acid-induced synthesis of polyvinyl alcohol-stabilized selenium nanoparticles. Nanotechnology. 2007;18:38. doi: 10.1088/0957-4484/18/38/385607. [DOI] [Google Scholar]
- 23.Stroyuk AL, et al. Structural and optical characterization of colloidal Se nanoparticles prepared via the acidic decomposition of sodium selenosulfate. Colloids Surf. A Physicochem. Eng. Asp. 2008;320:169–174. doi: 10.1016/j.colsurfa.2008.01.055. [DOI] [Google Scholar]
- 24.Langi B, et al. Ionic liquid-induced synthesis of selenium nanoparticles. Mater. Res. Bull. 2010;45:668–671. doi: 10.1016/j.materresbull.2010.03.005. [DOI] [Google Scholar]
- 25.Patra, J. K. & Baek, K. H. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J. Nanomater. 1–12 (2014).
- 26.Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA. Biogenic selenium nanoparticles: current status and future prospects. Appl. Microbiol. Biotechnol. 2016;100:2555–2566. doi: 10.1007/s00253-016-7300-7. [DOI] [PubMed] [Google Scholar]
- 27.Kubicek CP, Komon-Zelazowska M, Druzhinina IS. Fungal genus Hypocrea/Trichoderma: from barcodes to biodiversity. J. Zhejiang Univ. Sci. B. 2008;9:753–763. doi: 10.1631/jzus.B0860015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaklitsch WM. European species of Hypocrea. Part I. The green-spored species. Stud. Mycol. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mastouri F, Bjorkman T, Harman GE. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic and physiological stresses in germinating seeds and seedlings. Phytopathol. 2010;100:1213–1221. doi: 10.1094/PHYTO-03-10-0091. [DOI] [PubMed] [Google Scholar]
- 30.Shoresh M, Harman GE, Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 31.Harman GE, Herrera-Estrella AH, Benjamin A, Matteo L. Special issue: Trichoderma – from basic biology to biotechnology. Microbiology. 2012;58:1–2. doi: 10.1099/mic.0.056424-0. [DOI] [PubMed] [Google Scholar]
- 32.Chaparro AP, Carvajal LH, Orduz S. Fungicide tolerance of Trichoderma asperelloides and T. harzianum strains. Agric. Sci. 2011;2:301–307. [Google Scholar]
- 33.Goldman GH, et al. A nucleotide substitution in one of the beta-tubulin genes of Trichoderma viride confers resistance to the antimitotic drug methyl benzimidazole-2-yl-carbamate. Mol. Gen. Genet. 1993;240:73–80. doi: 10.1007/BF00276886. [DOI] [PubMed] [Google Scholar]
- 34.Mukherjee PK, Sherkhane PD, Murthy NBK. Induction of stable benomyl-tolerant phenotypic mutants of Trichoderma pseudokoningii MTCC 3011 and their evaluation for antagonistic and biocontrol potential. Indian J. Exp. Biol. 1999;37:710–712. [PubMed] [Google Scholar]
- 35.Devi TP, et al. Biosynthesis of silver nanoparticles from Trichoderma species. Indian J. Exp. Biol. 2013;51:543–547. [PubMed] [Google Scholar]
- 36.Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 2014;55:202–206. doi: 10.1016/j.indcrop.2014.01.026. [DOI] [Google Scholar]
- 37.Vahabi K, Mansoori GA, Karimi S. Biosynthesis of silver nanoparticles by fungus Trichoderma reesei (A Route for Large-Scale Production of AgNPs) Insciences J. 2011;1:65–79. doi: 10.5640/insc.010165. [DOI] [Google Scholar]
- 38.Mukherjee P, et al. Green synthesis of highly Stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology. 2008;19:075103. doi: 10.1088/0957-4484/19/7/075103. [DOI] [PubMed] [Google Scholar]
- 39.Hess, D. E., Thakur, R. P., Hash, C. T., Sereme, P. & Magill, C.W. Pearl millet downy mildew: problems and control strategies for the new millennium. Sorghum and Millets Diseases. (ed. Leslie, J. F.) Iowa State Press, 37–41 (2002).
- 40.Thakur RP, Rao VP, Amruthesh KN, Shetty HS, Datar VV. Field surveys of pearl millet downy mildew - effects of hybrids, fungicides and cropping sequence. J. Mycol. Plant Pathol. 2003;33:387–394. [Google Scholar]
- 41.Thakur RP, Rai KN, Khairwal IS, Mahala RS. Strategy for downy mildew resistance breeding in pearl millet in India. J. SAT Agric. Res. 2008;6:1–11. [Google Scholar]
- 42.Tam K, et al. Growth mechanism of amorphous selenium nanoparticles synthesized by Shewanella sp. HN-41. Biosci. Biotechnol. Biochem. 2010;74:696–700. doi: 10.1271/bbb.90454. [DOI] [PubMed] [Google Scholar]
- 43.Torres SK, et al. Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J. Nanopart. Res. 2012;14:1236. doi: 10.1007/s11051-012-1236-3. [DOI] [Google Scholar]
- 44.Fernández-Llamosas H, Castro L, Blazquez ML, Díaz E, Carmona M. Biosynthesis of selenium nanoparticles by Azoarcus sp. CIB. Microb. Cell Fact. 2016;15:109. doi: 10.1186/s12934-016-0510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad A, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll. Surf. B. Biointer. 2003;28:313–318. doi: 10.1016/S0927-7765(02)00174-1. [DOI] [PubMed] [Google Scholar]
- 46.Naqvi SZH, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomedicine. 2013;8:3187–3195. doi: 10.2147/IJN.S49284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, et al. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 2012;13:466–476. doi: 10.3390/ijms13010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zare B, Babaie S, Setayesh N, Shahverdi AR. Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles. Nanomed. J. 2013;1:13–19. [Google Scholar]
- 49.Qin Y, et al. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A Physicochem. Eng. Asp. 2010;372:172–176. doi: 10.1016/j.colsurfa.2010.10.013. [DOI] [Google Scholar]
- 50.Soni N, Prakash S. Factors affecting the geometry of silver nanoparticles synthesis in Chrysosporium tropicum and Fusarium oxysporum. Am. J.Nanotechnol. 2011;2:112–121. doi: 10.3844/ajnsp.2011.112.121. [DOI] [Google Scholar]
- 51.Lynch I, et al. The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century. Adv. Colloid Interface Sci. 2007;134–135:167–174. doi: 10.1016/j.cis.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, et al. Magnetic and fluorescent multifunctional chitosan nanoparticles as a smart drug delivery system. Nanotechnology. 2007;18:40. [Google Scholar]
- 54.Rajasree RSR, Gayathri S. Extracellular biosynthesis of selenium nanoparticles using some species of lactobacillus. Ind. J. geo. Mar. sci. 2015;43:1–10. [Google Scholar]
- 55.Yu S, et al. The inhibitory effect of selenium nanoparticles on protein glycation in vitro. Nanotechnology. 2015;26:14. doi: 10.1088/0957-4484/26/14/145703. [DOI] [PubMed] [Google Scholar]
- 56.Basu B, Swain SK, Sarkar D. Cryogenically cured hydroxyapatite–gelatin nanobiocomposite for bovine serum albumin protein adsorption and release. RSC Adv. 2013;3:14622–14633. doi: 10.1039/c3ra42369g. [DOI] [Google Scholar]
- 57.Dwivedi C, Shah CP, Singh K, Kumar M, Bajaj PN. An organic acid-induced synthesis and characterization of selenium nanoparticles. J. Nanotech. 2011;2011:1–6. doi: 10.1155/2011/651971. [DOI] [Google Scholar]
- 58.Huang P, Li Z, Hu H, Cui D. Synthesis and characterization of bovine serum albumin-conjugated copper sulfide nanocomposites. J.Nanomater. 2010;2010:1–6. [Google Scholar]
- 59.Ajitha B, Reddy YA, Reddy PS. Biosynthesis of silver nanoparticles using Momordica charantia leaf broth: Evaluation of their innate antimicrobial and catalytic activities. J. Photochem. Photobiol. B. 2015;146:1–9. doi: 10.1016/j.jphotobiol.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 60.Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticles? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007;73:1712–1720. doi: 10.1128/AEM.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ocsoy I, et al. Nanotechnology in plant disease management: DNA-directed silver nanoparticles on graphene oxide as an antibacterial against Xanthomonas perforans. ACS Nano. 2013;7:8972–8980. doi: 10.1021/nn4034794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamsal K, et al. Inhibition effects of silver nanoparticles against powdery mildews on cucumber and pumpkin. Mycobiology. 2011;39:26–32. doi: 10.4489/MYCO.2011.39.1.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jo YK, Kim BH, Jung G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009;93:1037–1043. doi: 10.1094/PDIS-93-10-1037. [DOI] [PubMed] [Google Scholar]
- 64.Kazempour ZB, Yazdi MH, Rafii F, Shahverdi AR. Sub-inhibitory concentration of biogenic selenium nanoparticles lacks post antifungal effect for Aspergillus niger and Candida albicans and stimulates the growth of Aspergillus niger. Iran J. Microbiol. 2013;5:81–85. [PMC free article] [PubMed] [Google Scholar]
- 65.Lyons GH, et al. Selenium increases seed production in Brassica. Plant Soil. 2009;318:73–80. doi: 10.1007/s11104-008-9818-7. [DOI] [Google Scholar]
- 66.Hartikainen H, Xue T, Piironen V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil. 2000;225:193–200. doi: 10.1023/A:1026512921026. [DOI] [Google Scholar]
- 67.Turakainen M, Hartikainen H, Seppänen MM. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agr. Food Chem. 2004;52:5378–5382. doi: 10.1021/jf040077x. [DOI] [PubMed] [Google Scholar]
- 68.Djanaguiraman M, Durga DD, Shanker AK, Sheeba JA, Bangarusamy U. Selenium, an antioxidative protectant in soybean during senescence. Plant Soil. 2005;272:77–86. doi: 10.1007/s11104-004-4039-1. [DOI] [Google Scholar]
- 69.Pakula TM, et al. Monitoring the kinetics of glycoprotein synthesis and secretion in the filamentous fungus Trichoderma reesei: cellobiohydrolase I (CBHI) as a model protein. Microbiology. 2000;146:223–232. doi: 10.1099/00221287-146-1-223. [DOI] [PubMed] [Google Scholar]
- 70.Zhang W, et al. Biosynthesis and Structural Characteristics of selenium nanoparticles by Pseudomonas alcaliphila. Colloids Surf. B Biointer. 2011;88:196–201. doi: 10.1016/j.colsurfb.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 71.Sonkusre P, Nanduri R, Gupta P, Cameotra SS. Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention. J. Nanomed. Nanotechnol. 2014;5:194. doi: 10.4172/2157-7439.1000194. [DOI] [Google Scholar]
- 72.Girijamba R, Hariprasad P, Brijeshsingh S, Niranjana SR. Antimildew activity of methanolic extract from selected medicinal plants against Plasmopara halstedii (Farl.) Berl. and De Tonic incitant of sunflower downy mildew disease. Int. J. Pharm. Bio. Sci. 2014;5:1010–1019. [Google Scholar]
- 73.Singh SD, Gopinath R. A seedling inoculation technique for detecting downy mildew resistance in pearl millet. Plant Dis. 1985;69:582–584. [Google Scholar]
- 74.Hiscox JD, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


