Abstract
Causes of the association between cognitive ability and health remain unknown, but may reflect a shared genetic aetiology. This study examines the causal genetic associations between cognitive ability and physical health. We carried out two-sample Mendelian randomization analyses using the inverse-variance weighted method to test for causality between later life cognitive ability, educational attainment (as a proxy for cognitive ability in youth), BMI, height, systolic blood pressure, coronary artery disease, and type 2 diabetes using data from six independent GWAS consortia and the UK Biobank sample (N = 112 151). BMI, systolic blood pressure, coronary artery disease and type 2 diabetes showed negative associations with cognitive ability; height was positively associated with cognitive ability. The analyses provided no evidence for casual associations from health to cognitive ability. In the other direction, higher educational attainment predicted lower BMI, systolic blood pressure, coronary artery disease, type 2 diabetes, and taller stature. The analyses indicated no causal association from educational attainment to physical health. The lack of evidence for causal associations between cognitive ability, educational attainment, and physical health could be explained by weak instrumental variables, poorly measured outcomes, or the small number of disease cases.
Introduction
Lower cognitive ability, lower educational attainment and greater cognitive decline are all associated with poorer health outcomes1–3. Some of these associations possibly arise because of the effect of lower cognitive ability in childhood on later life health, others because illnesses may lower cognitive ability in later life. The causes of these associations are unclear, but some may reflect, in part, a shared genetic aetiology. Recent papers have reported genetic associations between cognitive ability and educational attainment, and a number of physical and mental health traits and diseases4–6. These4, 6, and other papers7–9, have shown successful use of educational attainment as a proxy for cognitive ability, showing phenotypic correlations between educational attainment and general cognitive ability around 0.509 and a genetic correlation of 0.724.
Some of the reciprocal phenotypic associations between cognitive and physical health variables, and their genetic correlations, are as follows. Short stature has been consistently linked with lower cognitive ability10, 11. Molecular genetic studies have indicated positive genetic correlations between height and cognitive ability4, 12, as well as between height and educational attainment4, 5. Higher polygenic scores for height have been associated with better cognitive ability in adulthood4. A causal association was reported between taller stature and educational attainment (not including individuals with a degree) in UK Biobank using a Mendelian randomization analysis13.
Multiple studies have shown associations between cognitive ability and cardiovascular risk factors. For example, lower childhood cognitive ability is associated with subsequent high blood pressure14 and obesity15. However, higher BMI in mid-life16 and both hypertension and hypotension17 are associated with lower cognitive ability and greater cognitive decline in later life. A negative genetic correlation has been identified between BMI, but not blood pressure, and educational attainment and cognitive ability in mid to late life4, 5, and a polygenic score for higher BMI is associated with lower cognitive ability in mid to late life and lower educational attainment4; however, a polygenic score for higher systolic blood pressure is associated with lower educational attainment, but higher cognitive ability in mid to late life4.
Similarly, associations have been identified between cognitive ability and cardio metabolic diseases. Childhood cognitive ability has been associated with developing diabetes18 and coronary artery disease19 later in life. Diabetes20 and coronary artery disease21, 22 in midlife have been associated with greater cognitive decline later in life. A polygenic risk score for type 2 diabetes is associated with lower educational attainment, but not with cognitive ability in mid to late life4, although one has been associated with reduced cognitive decline23. To date, no genetic correlation between diabetes and cognitive ability has been identified4, 5. A polygenic risk score for coronary artery disease is associated with lower educational attainment and lower mid to late life cognitive ability4, and a negative genetic correlation was identified between coronary artery disease and educational attainment4, 5, but not cognitive ability in mid to late life4.
The question arises as to whether the genetic cognitive-health associations caused by: (1) genes influencing health traits/diseases, and then those health traits/diseases subsequently influencing cognitive ability; (2) genes influencing cognitive ability, and then cognitive ability subsequently influencing health traits/diseases; (3) genes influencing general bodily system integrity24 that influences both cognitive ability and health traits/diseases?
To try to make some progress in understanding causality of the correlation between cognitive ability and a number of physical and mental health traits, in the present report we used a bidirectional, two-sample Mendelian randomization (MR) approach25. MR uses genetic variants as proxies for environmental exposures and is subject to the following assumptions: (1) the genetic variants are associated with the exposure; (2) the genetic variants are only associated with the outcome of interest via their effect on the exposure [i.e., there is no biological pleiotropy (the phenomenon whereby one SNP independently influences multiple traits), also called the exclusion restriction]; and 3) the genetic variants are independent of confounders. Figure 1 shows the Mendelian randomization study model; the instrumental variable, here based on genome-wide significant SNPs from independent studies for the exposure, is used to estimate if the exposure (e.g. BMI) causally influences the outcome (e.g. cognitive ability). Individual single nucleotide polymorphisms (SNPs) are often found to be weak instruments for investigating causality because they often have small effect sizes. Using multiple SNPs can increase the strength of the instrument. However, this increases the chance of violating the MR assumptions, specifically violation of the assumption that the genetic variants affect the outcome only via the exposure. We used multiple genetic variants for a number of health-related traits and diseases, previously identified in genome-wide association studies, as instrumental variables to see if they predicted cognitive ability (verbal-numerical reasoning) in mid to later life in the UK Biobank. We then used genome-wide significant educational attainment SNPs as an instrumental variable to test whether genetic differences associated with educational attainment (a proxy measure of cognitive ability in early life6, 8) predict later life health outcomes in the UK Biobank.
Figure 1.
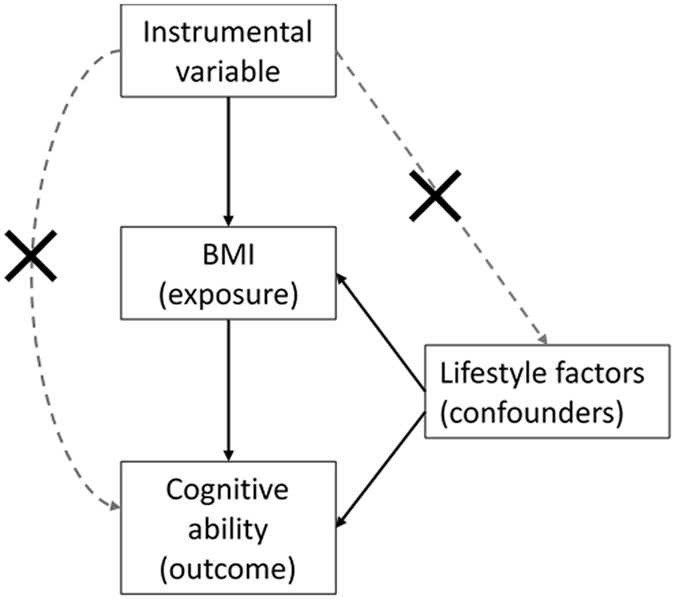
Model for Mendelian randomization study. The instrumental variable, based on genome-wide significant SNPs from independent studies for the exposure, is used to estimate if the exposure (e.g. BMI) causally influences the outcome (e.g. cognitive ability). The instrumental variable should be unrelated to potential confounders of the exposure-outcome association and should only affect the outcome via the exposure.
Methods
Sample
This study uses baseline data from the UK Biobank Study, a large resource for identifying determinants of human diseases in middle aged and older individuals26. Around 500 000 community-dwelling participants aged between 37 and 73 years were recruited and underwent assessments between 2006 and 2010 in the United Kingdom. This included cognitive and physical assessments, providing blood, urine and saliva samples for future analysis, and giving detailed information about their backgrounds and lifestyles, and agreeing to have their health followed longitudinally. For the present study, genome-wide genotyping data were available on 112 151 individuals (58 914 females) aged 40–70 years (mean age = 56.9 years, SD = 7.9) after the quality control process which is described in more detail elsewhere4. The UK Biobank study was approved by the National Health Service (NHS) Research Ethics Service (approval letter dated 17th June 2011, reference: 11/NW/0382). The analyses in the present report were completed under UK Biobank application 10279. All experiments were performed in accordance with guidelines and regulations from these committees. Written informed consent was obtained from each subject.
Measures
Body mass index
Body mass index (BMI) was calculated as weight(kg)/height(m)2, and measured using an impedance measure, i.e. a Tanita BC418MA body composition analyser, to estimate body composition. We used the average of the two methods when both measures were available (r = 0.99); if only one measure was available, that measure was used (N = 1629). 291 individuals did not have information on BMI. One outlier was excluded based on visual inspection of the BMI distribution (BMI > 50). 111 712 individuals had valid BMI and genetic data.
Height
Standing and sitting height (cm) were measured using a Seca 202 device. We used standing height and excluded one individual based on the visual inspection of the height distribution with a standing height <125 cm and a sitting/standing height ratio <0.75. 111 959 had valid height and genetic data.
Systolic blood pressure
Systolic blood pressure was measured twice, a few moments apart, using the Omron Digital blood pressure monitor. A manual sphygmomanometer was used if the digital blood pressure monitor could not be employed (N = 6652). Systolic blood pressure was calculated as the average of measures at the two time points (for either automated or manual readings). Individuals with a history of coronary artery disease were excluded from the analysis (N = 2513). Following the recommendation by Tobin, et al.27, 15 mmHg was added to the average systolic blood pressure of individuals taking antihypertensive medication (N = 10 988). Individuals with a systolic blood pressure (after correcting for medication) more than 4 SD from the mean were excluded from future analyses (N = 75). After all exclusions, 106 759 individuals remained with valid blood pressure and genetic data.
Coronary artery disease
UK Biobank participants completed a touch screen questionnaire on past and current health, which included the question “Has a doctor ever told you that you have had any of the following conditions? heart attack/angina/stroke/high blood pressure/none of the above/prefer not to answer”. This was followed by a verbal interview with a trained nurse who was made aware if the participant had a history of certain illnesses and confirmed these diagnoses with the participant. For the present study, coronary artery disease was defined as a diagnosis of myocardial infarct or angina, reported during both the touchscreen and the verbal interview in individuals with genetic data (N = 5288). The control group (N = 104 784) consisted of participants who reported none of the following diseases (based on the non-cancer illness code provided by UK Biobank): myocardial infarction, angina, heart failure, cerebrovascular disease, stroke, transient ischaemic attack, subdural haemorrhage, cerebral aneurysm, peripheral vascular disease, leg claudication/intermittent claudication, arterial embolism.
Type 2 diabetes
Type 2 diabetes case-control status was created using the same method as described by Wood, et al.28, for all individuals with genetic data based on the interim release of UK Biobank. Cases included participants who reported type 2 diabetes or generic diabetes during the nurse interview, started insulin treatment at least one year after diagnosis, were older than 35 years at the time of diagnosis, and did not receive a diagnosis one year prior to baseline testing (N = 3764). The control group consisted of participants who did not fulfil these criteria, and did not report a diagnosis of type 1 diabetes, diabetes insipidus and gestational diabetes (N = 108 015).
Years of education
As part of the sociodemographic questionnaire in the study, participants were asked, “Which of the following qualifications do you have? (You can select more than one)”. Possible answers were: “College or University Degree/A levels or AS levels or equivalent/O levels or GCSE or equivalent/CSEs or equivalent/NVQ or HND or HNC or equivalent/Other professional qualifications e.g. nursing, teaching/None of the above/Prefer not to answer”. For the present study, a new continuous variable was created measuring ‘years of education completed’. This was based on the ISCED coding, using the 1997 International Standard Classification of Education (ISCED) of the United Nations Educational, Scientific and Cultural Organization29. See the Table 1 for further details. Individuals who reported that they had a NVQ or HND or HNC degree, individuals who reported other qualifications, and individuals who preferred not to answer were excluded from analyses. The reason for these exclusions was as follows: the first two categories would correspond to 15 and 19 years of education according to the ISCED coding; regarding their mean scores on cognitive ability tests, this might not be the right place for these two degree levels in the ordered hierarchy of educational attainments (Supplementary Figure 1). For the current study, years of education was used a proxy phenotype for cognitive ability4, 6, 8. A total of 97,550 individuals had valid data for the years of education variable.
Table 1.
Coding for years of education in UK Biobank based on the ISCED coding29.
| UK Biobank degree level | UK Biobank code | ISCED code | Years of education (based on ISCED code) | N |
|---|---|---|---|---|
| College or university degree | 1 | 5 | 20 (19 + 1) | 33852 |
| A levels/AS levels or equivalent | 2 | 3 | 13 | 12560 |
| O levels/GCSEs or equivalent | 3 | 2 | 10 | 24802 |
| CSEs or equivalent | 4 | 2 | 10 | 6064 |
| NVQ or HND or HNC or equivalent | 5 | NA | NA | 7788 |
| Other professional qualification eg: nursing, teaching | 6 | NA | NA | 5776 |
| None of the above | −7 | 1 | 7 | 20272 |
| Prefer not to answer | −3 | NA | NA | 953 |
ISCED, 1997 International Standard Classification of Education of the United Nations Educational, Scientific and Cultural Organization.
Cognitive ability
Cognitive ability was measured using a 13-item touchscreen computerized verbal-numerical reasoning test. The test included six verbal and seven numerical questions, all with multiple-choice answers, with a two-minute time limit. An example verbal item is: ‘If some flinks are plinks and some plinks are stinks then some flinks are definitely stinks?’ (possible answers: ‘True/False/Neither-true-nor-false/do not know/prefer not to answer’). An example numerical item is: ‘If sixty is more than half of seventy-five, multiply twenty-three by three. If not subtract 15 from eighty-five. Is the answer?’ (possible answers: ‘68/69/70/71/72/do not know/prefer not to answer’). The cognitive ability score was the total score out of 13 (further detail can be found in Hagenaars, et al.4). This test was introduced at a later stage during baseline assessment and only a subset of individuals therefore completed this test. A total of 36 035 had valid cognitive ability and genetic data.
Covariates
All analyses were adjusted for the following covariates: age when attending assessment centre, sex, genetic batch and array, and the first ten genetic principal components for population stratification.
Instrumental variables
SNPs associated with each of the five health outcomes and educational attainment were retrieved from the largest available GWAS in European samples for the variables of interest (BMI30, height28, systolic blood pressure31, coronary artery disease32, type 2 diabetes33, and educational attainment34). For educational attainment, we downloaded the summary statistics based on the discovery GWAS only, which did not include the UK Biobank sample. Corresponding SNPs used in the instrumental variables were then extracted from the imputed UK Biobank’s interim release of genotypes, which amounted to 112 151 individuals of self-reported White British ancestry after quality control. Details on the quality control process have been published previously4. SNPs out of Hardy-Weinberg equilibrium (HWE, p < 1 × 10−6), with an imputation quality below 0.9, or individual genotypes with a genotype probability below 0.9 and strand ambiguous SNPs were excluded from the instrumental variables. The individual variants were recoded as 0, 1 or 2 according to the number of trait increasing alleles. Table 2 includes information on the number of SNPs included, and the reference paper. Supplementary Table 3a–f provides details of the included SNPs.
Table 2.
Information about instrumental variables.
| Phenotype | SNPs included | total SNPs | N | Reference | Unavailable in UK Biobank | HWE p < 1 × 10−6 | imputation <0.9 | AT/CG SNPs |
|---|---|---|---|---|---|---|---|---|
| BMI | 70 | 76 | 236,231 | Locke et al. Nature 2015; 518: 197–206. PMID: 25673413 | 1 | 3 | 0 | 2 |
| Height | 331 | 405 | 253,288 | Wood et al. Nat Genet 2014; 11: 1173–86. PMID: 25282103 | 3 | 5 | 2 | 64 |
| Systolic blood pressure | 20 | 25 | 69,395 | Ehret et al. Nature 2011; 478: 103–109. PMID: 21909115 | 0 | 0 | 0 | 5 |
| Coronary artery disease | 19 | 23 | 22,233 cases; 64,762 controls | Schunkert et al. Nat Genet 2011; 43: 333–338. PMID: 21378990 | 0 | 0 | 0 | 4 |
| Type 2 diabetes | 9 | 9 | 12,171 cases; 56,862 controls | Morris et al. Nat Genet 2012; 44: 981–990. PMID: 22885922 | 0 | 0 | 0 | 0 |
| Educational attainment | 63 | 74 | 293,723 | Okbay et al. Nature 2016; 533: 539–542. PMID: 27225129 http://ssgac.org/documents/EduYears_Discovery_5000.txt | 2 | 4 | 0 | 5 |
Statistical analysis
Phenotypic associations
We performed linear regression analysis using BMI, height, systolic blood pressure, coronary artery disease, and type 2 diabetes to predict cognitive ability. We regressed BMI, height, and systolic blood pressure against educational attainment in a linear regression model; coronary artery disease and type 2 diabetes were regressed against educational attainment in logistic regression models.
Mendelian randomization analysis
The Mendelian randomization analysis was performed using inverse variance weighted regression analysis based on SNP level data, with each instrumental variable (IV) consisting of multiple SNPs25. The inverse variance weighted method is based on a regression of two vectors with the intercept constrained to zero, i.e. the genetic variant with the exposure association, and the genetic variant with the outcome association (Fig. 1). By constraining the intercept to zero, this method assumes that all variants are valid instrumental variables based on the Mendelian randomization assumptions. We performed an association analysis between each SNP in the instrumental variable for the exposure and the exposure itself (IV - exposure), as well as between the instrumental variable for the exposure and the outcome (IV - outcome). We then used the vector of the instrumental variable-outcome association analyses against the vector of the instrumental variable-exposure analyses. This association (vector IV - outcome ~ vector IV - exposure) was weighted by the standard error of the original IV-outcome association, to correct for minor allele frequency, as described by Bowden, et al.25. Power calculations for the MR analyses can be found in Supplementary Table 1. No sensitivity analyses were performed due to the lack of causal associations.
Results
Health outcomes predicting cognitive ability
BMI, height, systolic blood pressure, and coronary artery disease predicted performance on the verbal-numerical reasoning test of cognitive ability (Table 3). A 1 SD higher BMI was associated with a 0.05 SD lower score for cognitive ability (β = −0.05, 95% CI = −0.06, −0.04). A 1 SD greater height was associated with a 0.18 SD higher score for cognitive ability (β = 0.18, 95% CI = 0.17, 0.20). A 1 SD higher systolic blood pressure was associated with a 0.05 SD lower score for cognitive ability (β = −0.05, 95% CI = −0.06, −0.04). Individuals with coronary artery disease had, on average, a 0.27 SD lower score for cognitive ability (β = −0.27, 95% CI = −0.32, −0.21). Individuals with type 2 diabetes had, on average, a 0.06 SD lower score for cognitive ability (β = −0.06, 95% CI = −0.12, 0.01). The Mendelian randomization inverse variance weighted analyses, with the five health outcomes as the exposures, and cognitive ability as the outcome, did not provide any causal evidence for any of these associations.
Table 3.
Phenotypic and genetic associations, using Mendelian randomization analysis, between five health instrumental variables and cognitive ability, using the verbal-numerical reasoning test.
| Cognitive ability | Phenotypic: health outcomes – cognitive ability | MR-IVW: health SNPs – cognitive ability | ||||
|---|---|---|---|---|---|---|
| SNPs (nr) | Beta | 95% CI | p | Beta | 95% CI | p |
| BMI (70) | −0.049 | −0.059, −0.039 | 1.51 × 10 −20 | −0.035 | −0.147, 0.077 | 0.5439 |
| Height (331) | 0.1816 | 0.166, 0.197 | 5.53 × 10 −124 | 0.026 | −0.009, 0.061 | 0.1329 |
| Systolic blood pressure (20) | −0.0492 | −0.061, −0.037 | 2.24 × 10 −17 | −0.002 | −0.010, 0.006 | 0.6355 |
| Coronary artery disease (19) | −0.2651 | −0.316, −0.214 | 4.62 × 10 −25 | −0.018 | −0.045, 0.009 | 0.2343 |
| Type 2 diabetes (9) | −0.0634 | −0.120, −0.007 | 0.0292 | 0.010 | −0.019, 0.039 | 0.5316 |
Associations with a p-value < 0.05 are in bold. OR, odds ratio; MR-IVW, Mendelian randomization - inverse variance weighted method.
Education predicting health outcomes
Educational attainment, as measured by years of education, predicted BMI, height, systolic blood pressure, type 2 diabetes and coronary artery disease (Table 4). The difference between 7 and 20 years of education was associated with a 0.37 SD lower BMI (β = −0.37, 95% CI = −0.39, −0.35), 0.31 SD taller stature (β = 0.31, 95% CI = 0.30, 0.32), 0.20 lower SBP (β = −0.20, 95% CI = −0.22, −0.19), 0.58 lower odds of type 2 diabetes (OR = 0.58, 95% CI = 0.52, 0.64), and 0.40 lower odds of coronary artery disease (OR = 0.40, 95% CI = 0.37, 0.43). The differences between the other groups (7 versus 10 and 13 years of education) can be found in Supplementary Table 2. In every case, the Mendelian randomization inverse variance weighted method did not show a causal effect of educational attainment on the health outcomes. The full results can be found in Table 4.
Table 4.
Phenotypic and genetic associations, using Mendelian randomization analysis, between the educational attainment instrumental variable and five health outcomes.
| Educational attainment – health outcomes | Educational attainment SNPs – health outcomes (63 SNPs) | |||||
|---|---|---|---|---|---|---|
| Phenotypic (7 vs 20 years of education) | MR-IVW | |||||
| Beta | 95% CI | p | Beta | 95% CI | p | |
| BMI | −0.367 | −0.385, −0.349 | <1.00 × 10 −130 | −0.026 | −0.157, 0.105 | 0.6986 |
| Height | 0.312 | 0.300, 0.324 | <1.00 × 10 −130 | 0.021 | −0.106, 0.148 | 0.7479 |
| Systolic blood pressure | −0.204 | −0.222, −0.187 | 5.61 × 10 −118 | −0.003 | −0.097, 0.091 | 0.9493 |
| Type 2 diabetes | OR: 0.575 | 0.522, 0.635 | 2.41 × 10 −28 | 0.022 | −0.703, 0.747 | 0.5642 |
| Coronary artery disease | OR: 0.397 | 0.365, 0.432 | 3.73 × 10 −101 | 0.015 | −0.047, 0.077 | 0.6440 |
Associations with a p-value < 0.05 are in bold. OR, odds ratio; MR-IVW, Mendelian randomization - inverse variance weighted method.
Discussion
This study was designed to investigate causes of the well replicated finding that lower cognitive ability is associated with poorer health outcomes1–3. It used a bidirectional two-sample MR approach to investigate this. We found no evidence for causal association between several health outcomes and cognitive ability, in middle and older age, or between educational attainment and physical health.
Tyrrell, et al.13 showed a causal association between taller stature and time spent in full time education in UK Biobank. They did not find a causal association between taller stature and degree level. The measure of time spent in full time education in UK Biobank excluded individuals who reported having a college degree, which could explain the discrepancy in results. The current study did include individuals who reported having a college degree, however used a categorical measure of four categories, whereas Tyrrell, et al.13 used a continuous measure of time spent in full time education. In a non-peer-reviewed (at the time of writing) study, Tillmann, et al.35 did report a causal association from educational attainment to coronary artery disease and BMI using a two-sample MR approach based on two independent consortia35. They used data from two independent GWAS consortia, including 349,306 individuals for educational attainment, 194,427 (63,746 cases) individuals for coronary artery disease, and 339,224 individuals for BMI. The current study used the same data for educational attainment on a subset of individuals (N = 293,723), and 111,712 individuals with BMI data; however, coronary artery disease was based on self-report diagnosis in UK Biobank, which included 110,072 (5288 cases) individuals. The summary level data for coronary artery disease in the Tillmann, et al.35 report included both European and East-Asian individuals, whereas the current study only includes individuals of White British ancestry. They35 excluded overlapping cohorts between educational attainment and coronary artery disease data; however, it is unclear if overlapping cohorts were excluded for BMI.
Another explanation for the lack of causal associations in the present study could be the high polygenic aetiology of the traits analysed in this study. Instrumental variables for cardiovascular disease, type 2 diabetes, blood pressure, and educational attainment explain a small amount of the variance in the exposure. A better instrumental variable would be expected to explain a substantial amount of the variance of the exposure. As shown by the power calculations (Supplementary Table 1), all instrumental variables (except BMI and systolic blood pressure) had sufficient power to detect the same magnitude of association as the observational estimates. The low power for BMI and systolic blood pressure potentially explains the lack of association with cognitive ability. A previous study by the current authors indicated a degree of genetic overlap between cognitive ability and health across the genome4. The idea of genetic overlap between health and cognitive ability is consistent with the theoretical construct of bodily system integrity24, whereby a latent trait is manifest as individual differences in how effectively people meet cognitive and health challenges from the environment, and which has some genetic aetiology.
Strengths of this study include the large sample size of UK Biobank, the participants of which all took the same cognitive tests, completed the same questionnaires and answered the same interview questions, in contrast to most genetic studies, where assessments across different cohorts often vary. A further strength is the fact that all of the UK Biobank genetic data were processed in a consistent matter, on the same platform and at the same location. The genetic variants on which the instrumental variables originated used the largest available GWAS at moment of testing.
Limitations of this study include the fact that cognitive ability was only measured on a subset of the UK Biobank participants and that it was a bespoke test. A second major limitation was that there is no published large genome-wide association study of cognitive ability in early life from which we could obtain genetic variants to use as an instrumental variable. Therefore, we used genome-wide significant SNPs associated with educational attainment as our early life cognitive ability instrument. A further limitation is the case-control ascertainment in UK Biobank, as the current study based case-control status on self-report measures. This may have led to misclassification of disease status, causing a likely bias towards the null hypothesis36.
Overall, this study found phenotypic cognitive-physical health associations, but did not find evidence for causal associations between cognitive ability and physical health. This may be due to weak instrumental variables, poorly measured outcomes, or the small numbers of disease cases. Future work should therefore focus on stronger instrumental variables, as well as better measurement of the outcome variables.
Electronic supplementary material
Acknowledgements
This work was supported by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (MR/K026992/1). Funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. This research was conducted using the UK Biobank Resource.
Author Contributions
All authors contributed to the design of the study. S.P.H. extracted, pre-processed, and analysed the data. S.P.H. and S.E.H. wrote the main manuscript text. All authors discussed and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-02837-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deary IJ, Weiss A, Batty GD. Intelligence and Personality as Predictors of Illness and Death: How Researchers in Differential Psychology and Chronic Disease Epidemiology Are Collaborating to Understand and Address Health Inequalities. Psychological Science in the Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- 2.Calvin CM, et al. Intelligence in youth and all-cause-mortality: systematic review with meta-analysis. International Journal of Epidemiology. 2011;40:626–644. doi: 10.1093/ije/dyq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wraw C, Deary IJ, Gale CR, Der G. Intelligence in youth and health at age 50. Intelligence. 2015;53:23–32. doi: 10.1016/j.intell.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagenaars, S. P. et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N = 112 151) and 24 GWAS consortia. Mol Psychiatry, doi:10.1038/mp.2015.225 (2016). [DOI] [PMC free article] [PubMed]
- 5.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill WD, Davies G, Liewald DC, McIntosh AM, Deary IJ. Age-Dependent Pleiotropy Between General Cognitive Function and Major Psychiatric Disorders. Biological Psychiatry. 2016;80:266–273. doi: 10.1016/j.biopsych.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trampush JW, et al. Independent evidence for an association between general cognitive ability and a genetic locus for educational attainment. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015;168:363–373. doi: 10.1002/ajmg.b.32319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietveld CA, et al. Common genetic variants associated with cognitive performance identified using the proxy-phenotype method. Proceedings of the National Academy of Sciences. 2014;111:13790–13794. doi: 10.1073/pnas.1404623111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks J, Mazzonna F. The Effect of Education on Old Age Cognitive Abilities: Evidence from a Regression Discontinuity Design*. The Economic Journal. 2012;122:418–448. doi: 10.1111/j.1468-0297.2012.02499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silventoinen K, Posthuma D, Van Beijsterveldt T, Bartels M, Boomsma DI. Genetic contributions to the association between height and intelligence: evidence from Dutch twin data from childhood to middle age. Genes, Brain and Behavior. 2006;5:585–595. doi: 10.1111/j.1601-183X.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 11.Keller MC, et al. The Genetic Correlation between Height and IQ: Shared Genes or Assortative Mating? PLoS Genet. 2013;9:e1003451. doi: 10.1371/journal.pgen.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marioni RE, et al. Common Genetic Variants Explain the Majority of the Correlation Between Height and Intelligence: The Generation Scotland Study. Behavior Genetics. 2014;44:91–96. doi: 10.1007/s10519-014-9644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyrrell, J. et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ352, doi:10.1136/bmj.i582 (2016). [DOI] [PMC free article] [PubMed]
- 14.Starr JM, et al. Childhood mental ability and blood pressure at midlife: linking the Scottish Mental Survey 1932 and the Midspan studies. Journal of Hypertension. 2004;22:893–897. doi: 10.1097/00004872-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Belsky DW, et al. Is Obesity Associated With a Decline in Intelligence Quotient During the First Half of the Life Course? American Journal of Epidemiology. 2013;178:1461–1468. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahl A, et al. Being Overweight in Midlife Is Associated With Lower Cognitive Ability and Steeper Cognitive Decline in Late Life. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65A:57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mõttus R, Luciano M, Starr JM, Deary IJ. Diabetes and life-long cognitive ability. Journal of Psychosomatic Research. 2013;75:275–278. doi: 10.1016/j.jpsychores.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA, David Batty G, Clark H, McIntyre S, Leon DA. Association of childhood intelligence with risk of coronary heart disease and stroke: findings from the Aberdeen Children of the 1950s cohort study. European Journal of Epidemiology. 2008;23:695–706. doi: 10.1007/s10654-008-9281-z. [DOI] [PubMed] [Google Scholar]
- 20.Rawlings AM, et al. Diabetes in Midlife and Cognitive Change Over 20 YearsA Cohort StudyDiabetes in Midlife and Cognitive Change Over 20 Years. Annals of Internal Medicine. 2014;161:785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovacic JC, Castellano JM, Fuster V. The links between complex coronary disease, cerebrovascular disease, and degenerative brain disease. Annals of the New York Academy of Sciences. 2012;1254:99–105. doi: 10.1111/j.1749-6632.2012.06482.x. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont, L. H. P. et al. Cardiac disease and cognitive impairment: a systematic review. Heart, doi:10.1136/heartjnl-2012-301682 (2012). [DOI] [PubMed]
- 23.Luciano M, et al. Predicting cognitive ability in ageing cohorts using Type 2 diabetes genetic risk. Diabetic Medicine. 2014;31:714–720. doi: 10.1111/dme.12389. [DOI] [PubMed] [Google Scholar]
- 24.Deary IJ. Looking for ‘System Integrity’ in Cognitive Epidemiology. Gerontology. 2012;58:545–553. doi: 10.1159/000341157. [DOI] [PubMed] [Google Scholar]
- 25.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. International Journal of Epidemiology. 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudlow C, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Statistics in Medicine. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 28.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.UNESCO. International Standard Classification of Education ISCED 1997, http://www.unesco.org/education/information/nfsunesco/doc/isced_1997.htm Date of Access: 30/09/2016 (2006).
- 30.Locke AE, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature478, 103–109, doi:10.1038/nature10405 (2011). [DOI] [PMC free article] [PubMed]
- 32.Schunkert H, et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris AP, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okbay A, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533:539–542. doi: 10.1038/nature17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tillmann, T. et al. Education and coronary heart disease: a Mendelian randomization study. bioRxiv, doi:10.1101/106237 (2017).
- 36.Zheng L, et al. Coronary Artery Disease Is Associated with Cognitive Decline Independent of Changes on Magnetic Resonance Imaging in Cognitively Normal Elderly Adults. Journal of the American Geriatrics Society. 2012;60:499–504. doi: 10.1111/j.1532-5415.2011.03839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


