Abstract
Introduction
Cerebrospinal fluid collection by lumbar puncture (LP) is performed in the diagnostic workup of several neurological brain diseases. Reluctance to perform the procedure is among others due to a lack of standards and guidelines to minimize the risk of complications, such as post-LP headache or back pain.
Methods
We provide consensus guidelines for the LP procedure to minimize the risk of complications. The recommendations are based on (1) data from a large multicenter LP feasibility study (evidence level II-2), (2) systematic literature review on LP needle characteristics and post-LP complications (evidence level II-2), (3) discussion of best practice within the Joint Programme Neurodegenerative Disease Research Biomarkers for Alzheimer's disease and Parkinson's Disease and Biomarkers for Multiple Sclerosis consortia (evidence level III).
Results
Our consensus guidelines address contraindications, as well as patient-related and procedure-related risk factors that can influence the development of post-LP complications.
Discussion
When an LP is performed correctly, the procedure is well tolerated and accepted with a low complication rate.
Keywords: Lumbar puncture, Cerebrospinal fluid, Post-LP complications, Headache, Back pain, Consensus guidelines, Evidence-based guidelines
1. Introduction
Lumbar puncture (LP) is a technique to sample cerebrospinal fluid (CSF) as a window into brain pathology (Supplemental Data). The procedure involves introducing a needle into the subarachnoid space of the lumbar sac, at a level safely below the spinal cord [1]. Despite modern neuroimaging techniques, LP remains an important diagnostic tool as CSF analysis provides important diagnostic information for many neurological conditions. For example, no procedure can replace the CSF analysis in differential diagnosis of infectious disorders of the central nervous system (e.g., bacterial or viral meningitis, neuroborreliosis). Moreover, CSF analysis is now at the core of the diagnostic criteria for the diagnosis of Alzheimer's disease [2], [3], [4]. In addition, an LP is the easiest procedure to perform a CSF pressure measurement. Given the use of CSF analysis for diagnosis, LPs are currently often performed to perform research to discover novel diagnostic biomarkers and understand brain pathology.
A recent large international, multicenter study on LP feasibility that included 3868 patients in a memory clinic setting showed that LPs can be safely performed [5]. The acceptance rate of an LP was high, especially taking into consideration that there was no acute medical indication.
The most common complications of LP consist of post-LP back pain and post-LP headache (PLPH) [6]. PLPH typically begins within three days after the procedure in most patients [7]. If a patient develops typical PLPH, bed rest, adequate hydration, and simple analgesics should be started [8]. Further review of possible treatments will be given in later sections of this study.
Very rare (prevalence of <0.01%) but potential serious complications consist of post-LP infections, spinal and subdural cerebral hematoma, and cerebral venous thrombosis [1]. In the multicenter LP feasibility study, a substantial proportion (31%) of patients reported post-LP complaints; however, these were mostly mild in nature, and severe complications were very rare [5]. Back pain, headache, and typical PLPH were reported by 17%, 19%, and 9%, respectively [5]. Only 0.3% of the patients needed a blood patch, and in 0.7%, a hospitalization was required [5]. The most important risk factors for post-LP complaints were related to patient characteristics: history of headache and fear of complications. A cutting bevel needle type appeared to be the only procedure-related risk factor for typical PLPH. The number of LP attempts was the only procedure-related risk factor for occurrence of local back pain. A large needle diameter (≤22G, gauge [G]) was a risk factor for severe headache [5].
To date, consensus guidelines and recommendations for the LP procedure to optimize diagnostic yield and to minimize the risk of complications are lacking. We developed consensus guidelines and recommendations for the LP procedure based on the international multicenter LP feasibility study outcomes combined with a literature-based analysis of risk factors. These guidelines will be applicable to neurological brain diseases that require a diagnostic LP. Moreover, these guidelines will serve to reduce complication rates in daily neurological practice, will be a reference for educational purposes, thus be of help for trainees in neurology, and will be a guide to comfort or give adequate information to the patients.
2. Materials and methods
These consensus guidelines originate from two international consortia. The EU Joint Programme Neurodegenerative Disease Research (JPND) consortium “Biomarkers for Alzheimer's disease (AD) and Parkinson's Disease (PD)” (BIOMARKAPD) aims at standardizing and harmonizing existing biomarkers for AD and PD. The Biomarkers for Multiple Sclerosis (BioMS) consortium aims at optimizing all aspects of CSF biomarkers research for multiple sclerosis and related disorders, via collaboration and developing guidelines for relevant procedures. One of the objectives of these consortia is to standardize and harmonize preanalytical procedures for CSF biomarkers that are used for or contribute to diagnosis for neurological diseases. These preanalytical procedures include the LP procedure.
To provide consensus recommendations for the LP procedure to optimize diagnostic yield and minimize the risk of complications, three consecutive steps were used:
-
(1)
data from the large multicenter LP feasibility study [5],
-
(2)
systematic literature review on LP needle characteristics and post-LP complications,
-
(3)
discussion within the JPND BIOMARKAPD (53 member centers) and BioMS (30 member centers) consortia.
Our consensus guidelines address contraindications, as well as patient-related and procedure-related risk factors for the development of post-LP complications. Based on U.S. preventive services task force [9], the level of evidence of these recommendations is indicated. The level of evidence was mostly level II-2 (well-designed cohort preferably with more than one research group or center [e.g., data of large multicenter LP feasibility study]) and level III (consensus evidence based on clinical experience of the two consortia involved [JPND BIOMARKAPD and BioMS]).
2.1. Systematic literature review search strategy
As several studies have been published with regard to the relation of LP needle characteristics and post-LP complications, we performed a systematic literature review, covering relevant articles that have been published between January 1970 and April 2016. Searches were conducted through PubMed and Google Scholar. Only English articles that contained needl* and lumbar punctur* in title and/or abstract were taken into account [1]. In total, 307 articles were found in the databases and screened/selected based on PRISMA (Supplementary Material, Supplementary Fig. 1) [10]. Case reports (n = 22), reviews (n = 29), articles about animal models (n = 6), and articles that not presented a recommendation of a choice of needle were deselected (n = 187). Of the 63 remaining articles, statements (n = 3) and comments (n = 7) about other publications were deselected as well. Three “statement” articles pointed out that atraumatic needles with a small-bore diameter (≥24G) are not often used in practice although literature often recommend using this type of needle [11], [12], [13]. Three of the seven comments had no recommendation [14], [15], [16], two agreed with the original article which recommended atraumatic needles [17], [18], one article agreed on small-bore needles [19], and one article recommended small-bore atraumatic needles [20]. In total, 53 fulfilled our criteria and were subdivided into three groups. Articles that made recommendations on needle design (n = 24), the second group consisted of articles with recommendations on needle diameter (n = 12), and the last group that did both (needle design and diameter, n = 17).
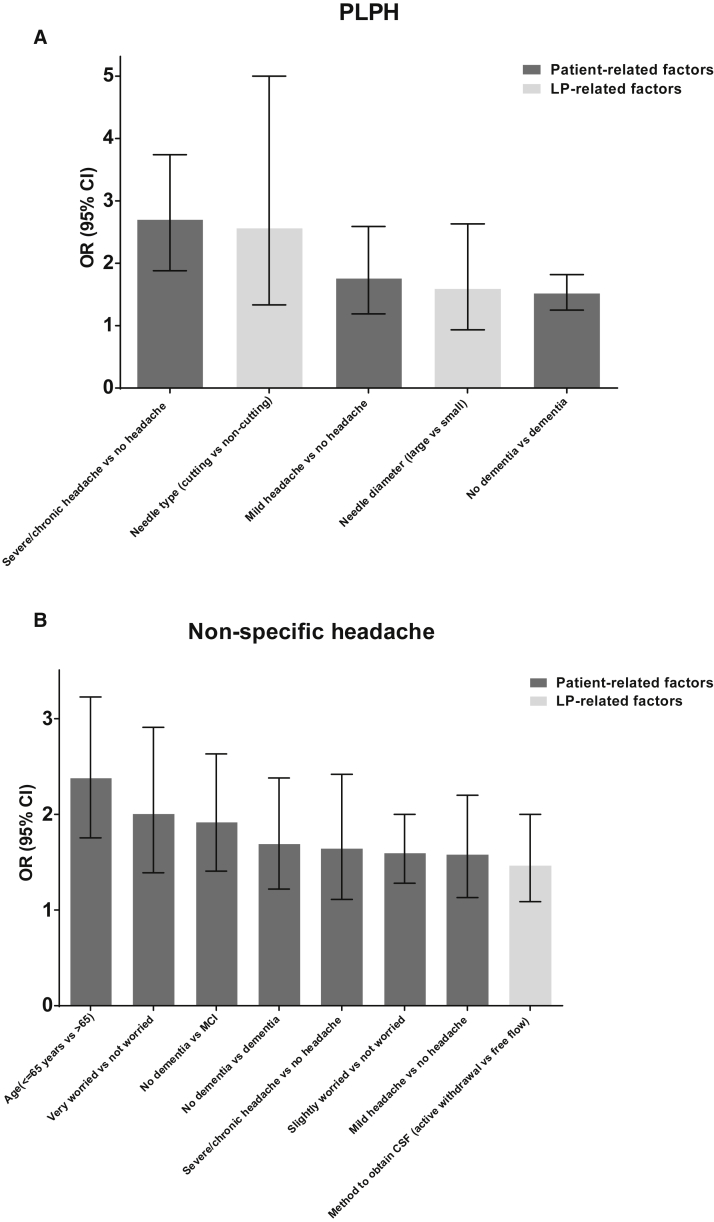
Fig. 1.
Risk factors for PLPH (A) and nonspecific headache (B) ranked by magnitude (defined by odds ratio) as reported by the multicenter LP feasibility study [5]. Abbreviations: CI, confidence interval; LP, lumbar puncture; OR, odds ratio; PLPH, post-LP headache.
The 53 articles that did recommend a specific needle type were graded based on the grade system described by Leone et al. [21] (Supplementary Material, Supplementary Table 1). Articles were classified as “low evidence” if they had a total score (sum = limitations assets) of ≤−5 (n = 5), “medium evidence” articles if they had a score between −4 and +4 (n = 21), and “high evidence” articles if they had a score ≥+5 (n = 27). For an extensive description of the grading approach, we refer to the Supplementary Material.
2.2. Selection of variables to become part of the consensus guidelines
Based on the results of the multicenter LP feasibility study [5] as well as on the systematic literature review [1], the following variables were selected to provide consensus guidelines:
-
•
Conditions representing (potential) contraindications for LP: the risk for cerebral herniation including space-occupying lesion with mass effect, abnormal intracranial pressure due to increased CSF pressure and Arnold-Chiari malformation, increased bleeding risk (thrombocytopenia, coagulopathies, anticoagulant drugs), and local infections at the puncture site.
-
•
Patient-related factors, associated with increased risk for post-LP complications: age, gender, history of headache, and fear of LP.
-
•
Procedure-related factors that were associated with increased risk for post-LP complications such as design and diameter of the needle, number of LP attempts, active versus passive withdrawal of CSF, reinsertion of the stylet, positioning of the patient during LP, use of local anesthetic, volume of CSF that was withdrawn during LP, as well as bed rest after LP.
-
•
Review of the possible treatments of PLPH.
2.3. Strategy to reach consensus
A first set of guidelines was designed based on the results of the international multicenter LP feasibility study [5], available literature [1], and systematic literature review. These were discussed during a JPND “BIOMARKAPD” general assembly, after which the guidelines were further adapted (Mainz, Germany, spring 2014). A second version of the consensus guidelines was drafted by S.E., E.N., H.S., F.D., and C.E.T. This draft was e-mailed (July, 2014) to all participants of the JPND “BIOMARKAPD” task force on standardization of preanalytical procedures, who were invited to provide their comments and feedback. The prefinal draft was subsequently mailed (November, 2014) with an invitation for feedback to BioMS members and neurologists being key opinion leaders in neurological disorders other than neurodegenerative brain diseases. The feedback and comments obtained in this way was subsequently integrated which resulted in the present consensus guidelines. This final version of the consensus guidelines was again sent to all coauthors for final approval.
3. Results
3.1. Identification of LP contraindications
Contraindications were identified from the available literature. The most important contraindication for LP is an intracranial space–occupying lesion with mass effect as well as a posterior fossa mass because it can lead to herniation of the cerebellar tonsils, regardless of the volume of CSF that is sampled [22], [23], [24], [25], [26]. Other contraindications for LP include a risk for cerebral herniation caused by abnormal intracranial pressure due to increased CSF pressure or Arnold-Chiari malformation, as well as anticoagulant medication, coagulopathies and uncorrected bleeding diathesis, congenital spine abnormalities, and local skin infection at the puncture site (Table 1). Based on consensus (evidence level III); a brain computed tomography or magnetic resonance imaging scan should at least be performed before LP if an intracranial lesion with mass effect, abnormal intracranial pressure due to increased CSF pressure, or tonsillar herniation is suspected, or whenever a patient has focal neurological deficits, is immune compromised, has a previous central nervous system disease, recent seizures, impaired consciousness, or papilledema at fundoscopy. The following imaging findings should be considered as a contraindication for LP:
-
•
Evidence for a pressure gradient across the falx cerebri.
Unequal supratentorial pressure gradients can result in a lateral shift of midline structures. Asymmetry of lateral ventricles is frequently a result of congenital brain abnormality or congenital/perinatal stroke, presenting an abnormal anatomical variant. On the other hand, an asymmetry of the lateral ventricles should not be considered to be an accurate sign, as ipsilateral ventricular dilation may be caused by stroke and as coaptation of a frontal horn may represent a normal anatomical variant. Performing an LP in patients with unequal supratentorial pressure gradients can result in compression of the ipsilateral temporal lobe, leading to uncal herniation.
-
•
Evidence for a pressure gradient between the supratentorial and infratentorial compartments.
Such pressure gradient may be caused by elevated pressure above the tentorium cerebelli, and elevated pressure below the tentorium cerebelli. Under these circumstances, LP may lead to a bilateral uncal herniation.
-
•
Arnold-Chiari malformation. Patients with an Arnold-Chiari malformation are prone to develop tonsillar herniation, sometimes even after removal of small volumes of CSF.
Table 1.
Procedures to rule out contraindications for LP
| Contraindication for LP | Procedures to rule out contraindications | Required action |
|---|---|---|
|
Clinical neurological examination Fundoscopy |
Perform brain CT/MRI scan if
|
|
Check medication before LP | Can anticoagulants temporarily be discontinued? |
|
Recent blood analysis: platelet >40 × 109/L; quick > 50%; INR < 1.5 | Correction possible? |
|
Local inspection Imaging in case of suspicion of tethered cord |
Guidance of LP procedure by fluoroscopy, ultrasound, or CT |
|
Local inspection | Treat skin infection |
Abbreviations: LP, lumbar puncture; CSF, cerebrospinal fluid; CT, computed tomography; MRI, magnetic resonance imaging; CNS, central nervous system; INR, international normalized ratio.
NOTE. All recommendations are based on level-III evidence.
Brain and spinal hemorrhage and spinal epidural or subdural hematoma are rare but potentially serious complications of an LP. For example, in the multicenter LP feasibility study, only 1 of 3558 patients who underwent LP experienced this side effect (leading to death after restarting their oral anticoagulant) [5]. Therefore, it is advised (level-III evidence) to have a recent analysis of the platelet count (that should be >40 × 109/L) and coagulation status (quick >50%; international normalized ratio < 1.5). Coagulopathies and uncorrected bleeding diathesis should be absent. It is advised (level-III evidence) to discontinue anticoagulant treatments to minimize hemorrhagic risks. The short-acting direct oral anticoagulants (DOACs) have as advantage that they can be discontinued shortly before the LP and that anticoagulation can be restarted within a few hours (6–8 hours) after the procedure [27]. The decision process should take into account the risk of discontinuation and consequent risk of thrombosis, the possibility of bridging with a low molecular weight or intravenous heparin (which is not advised in case of DOACs), as well as the potential benefit of the LP, and possible alternative examinations (e.g., amyloid positron emission tomography instead of LP to analyze AD biomarkers in CSF) [28]. Antiplatelet drugs are only a relative contraindication, and most centers do not interrupt treatment with antiplatelet drugs before LP. Studies on LP complication risks in cases taking combinations of antiplatelet drugs (e.g., clopidogrel and acetylsalicylic acid) are lacking, but it is considered safer to temporarily withhold one of both before LP (level-III evidence). In case of dual antiplatelet therapy, it is advised to continue acetylsalicylic acid, whereas the intake of thienopyridine derivatives (e.g., clopidogrel, ticlopidine) should be temporally withhold (1 or 2 weeks) before LP unless patients are at high thrombotic risk or unless there is an urgent indication to perform an LP (level-III evidence) [29]. Congenital spine abnormalities and spinal cord abnormalities (e.g., tethered cord), or local skin infections at the LP site can be contraindications to perform an LP. In case of congenital spine abnormalities, the LP procedure can be guided by fluoroscopy, ultrasound, or computed tomography [1], [30], [31], [32], [33]. Performing an LP through infected tissue increases the risk of central nervous system infections and reduces the diagnostic value of the LP in case of a suspected central nervous system infection.
3.2. Patient-related risk factors
Data from the international multicenter LP feasibility study indicate that patient characteristics are equally important as LP procedure–related factors with regard to the risk for post-LP complications (PLPH, post-LP back pain) [5].
Younger age is the most important and well-known risk factor for PLPH and post-LP back pain [5], [34], [35]. Both Monserrate et al. and the multicenter LP feasibility study, including patients age 66 ± 11 years, did not demonstrate a gender difference [5], [36], although it was repeatedly reported that PLPH is more common in women than in men, and especially women aged less than 40 years seem to have a substantially higher risk of PLPH [7], [34], [35], [37], [38], [39]. Patients with history of headache had a higher risk to develop PLPH and post-LP back pain (Fig. 1, Fig. 2) [5]. Although age was accounted for, a diagnosis of mild cognitive impairment or dementia was still associated with a risk reduction of these post-LP complications in a memory clinic setting. Although these patient-related risk factors for post-LP complaints cannot be modified, these variables can be of help to identify those patients that are at high risk to develop post-LP complications. This information can be used by physicians for counseling purposes (level II-2 evidence).
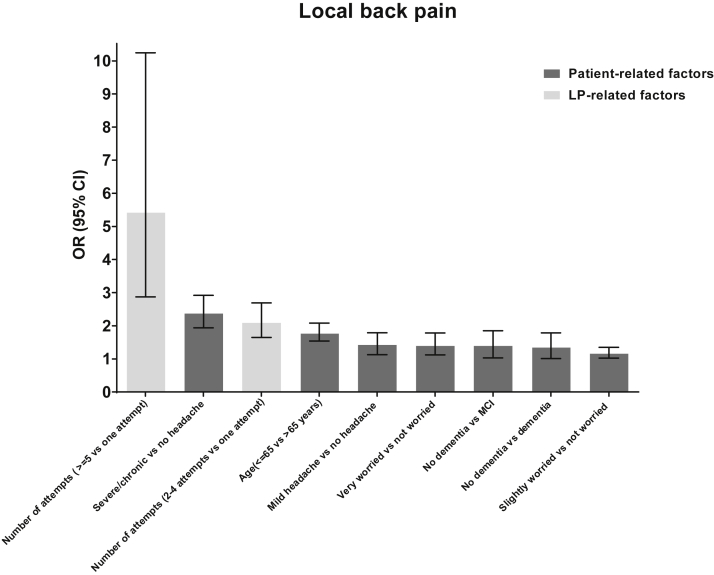
Fig. 2.
Risk factors for back pain ranked by magnitude (defined by odds ratio) as reported by the multicenter LP feasibility study [5]. Abbreviations: CI, confidence interval; LP, lumbar puncture; OR, odds ratio.
In the multicenter LP feasibility study, it was shown that fear of LP was an important independent risk factor for experiencing post-LP complaints (Fig. 1, Fig. 2) [5]. Hence, there seems to be a psychological effect of anxiety relating to more complaints, which might be explained by lack of information beforehand, personality characteristics, or even a nocebo effect of the LP [40]. As fear of the LP and post-LP complications can be influenced by the attitude of the physician and nursing staff and can be decreased by giving reassuring but adequate information, it is of most importance to carefully inform the patient. Moreover, although use of local anesthesia is not related to reduced post-LP complaints, it may help to control the fear of the LP procedure. The physician should explain the procedure and should outline the potential complications as well as the diagnostic benefits from having an LP. Some of the patient-related risk factors for post-LP complications can also be used to reassure patients (e.g., when there is no history of headache, patients are older or optimal needle type is used). During the LP procedure, it is important to explain the different steps of the LP procedure, thereby reducing eventual anxiety and discomfort, meanwhile considering the eventual cognitive deficits that affect memory and language comprehension, which requires a stepwise approach (level II-2 evidence).
3.3. LP procedure–related factors
3.3.1. LP procedure
Infection is a rare but potentially serious complication of an LP. Therefore, also in an outpatient setting, an aseptic technique should be applied using sterile gloves, and a thorough disinfection of the lumbar region of the patient. Furthermore, the physician can use a mask to minimize the risk for infection. Excess of the antiseptic solution should be removed from the skin before needle insertion (level-III evidence). We refer to Fig. 3 for the LP procedure [1], [41], which holds true for both atraumatic and cutting bevel needles [39], [42], [43].
Fig. 3.
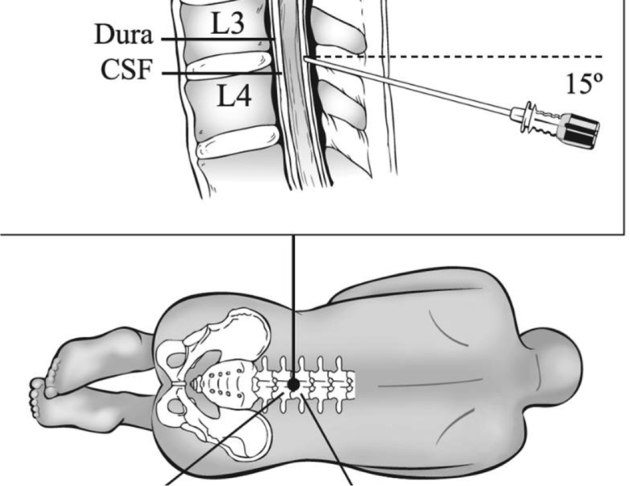
Lumbar puncture procedure and position of the needle during a lumbar puncture. The procedure involves introducing a needle or its respective introducer at the superior aspect of the inferior spinal process into the subarachnoid space of the lumbar sac, at the L4/L5 level or other level safely (L3/L4) below the spinal cord. The technique is for both atraumatic and cutting bevel needles the same, but when using an atraumatic needle, an introducer is inserted into the interspinal ligament first, after which the smaller atraumatic needle is inserted through the introducer. The introducer should be inserted no more than 2/3 – 1/4 of the total length, depending on the adipose tissue availability. During the procedure, the needle stylet is removed every 2-mm interval to check for flow of CSF. In case a nonrecommended cutting bevel needle is used, it is preferred to hold the bevel in the sagittal plane as this diminishes injury to the dura mater by separating its longitudinal fibers rather than cutting through them and reduces the risk of leakage of CSF after the LP. Abbreviations: CSF, cerebrospinal fluid; LP, lumbar puncture.
During a minority of the LP procedures, nerve root irritation may occur [44]. The patient should be informed on forehand that this may occur, albeit not dangerous, nor linked to any complications (level-III evidence).
3.3.2. LP needle
The optimal needle length, diameter, and design should be chosen when an LP is performed. This choice also depends on the medical indication (e.g., LP as urgent procedure in case of acute bacterial meningitis vs. an LP to sample CSF to analyze AD biomarkers). Important variables are as follows: adequate flow rate, fast and accurate transduction of CSF pressure, minimizing traumatic taps, leakages, failures, and post-LP complications. LP needles differ with regard to length, diameter, and design. The choice of the needle depends on a patient's age and weight, the purpose of the LP procedure, and should be focused on minimizing discomfort and risk for complications (Table 2).
Table 2.
Needle characteristics are compared based on needle length, size, and design (systematic literature review 2016 [1])
| Needle | Comparison | Advantages | Disadvantages |
|---|---|---|---|
| Length | Regular (70–90 mm) | Adults | — |
| Long (>90 mm) | Obesity | Challenging approach | |
| Diameter in gauge | Small (≥24G) | Reduced complication rates Reduced pain and discomfort Decreased risk factor: blood contaminations Requires less medical assistance and medications |
Decreased flow rates, increased sampling times More failures Requires training and practice |
| Large (≤22G) | Increased flow rates Decreased sampling times Fewer failures |
Increased complication rates Perforations are larger Increased risk factor: contaminations |
|
| Design | Cutting bevel | Penetration is felt through skin | Increased complication rates. Requires more use of medications and medical assistance, which results in increased costs. |
| Atraumatic | Reduced complication rates Reduced medical health care costs due to less post-LP complications, medical assistance, and medications Decreased traumatic taps |
Decreased flow rates, increased sampling times Increased amount of attempts and failures Penetration through skin is difficult to feel |
Abbreviation: G, gauge.
Interestingly, the multicenter LP feasibility study showed that effects of needle design and diameter were not as important for post-LP complications as suggested by results of previous studies (Fig. 1, Fig. 2) [5]. Nevertheless, a cutting bevel needle is a risk factor for typical PLPH (odds ratio [OR] [95% Cl], 2.6 [1.3–5.0]) and a large-bore needle diameter (≤22G) may be a risk factor for severe PLPH (OR [95% Cl], 1.6 [0.9–2.7]) [5].
3.3.2.1. Length of the needle
In case of a dry tap (punctio sicca), the needle should be advanced further to obtain CSF. Long spinal needles (>90 mm) are used in patients with obesity. Nevertheless, length of the needle is not the only factor that contributes to a possible LP failure, experience of the operator is important as well [45]. The procedure will be more difficult when longer needles are used as these needles will be more flexible and often divert off track during the procedure [46].
3.3.2.2. Diameter of the needle
Many considerations can be taken into account to decide on needle gauge. Twenty-eight studies, published between 1970 and 2016 (references in this paragraph resulted from the systematic literature review, Table 3), compared the performance and complications of different diameters of needles. No differences were detected in six studies comparing different needle diameters [47], [48], [49], [50], [51], [52], whereas one study concluded that needles with a larger diameter (≤22G) had a positive effect on collection time and resulted in less failures [53]. The LP feasibility study performed in memory clinic settings did not show that large-bore diameter needles confer independent risk compared with small bore needle types. However, smaller bore needles (defined as ≥24G) are recommended by most studies [5], [36], [38], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], based on lower incidence of PLPH, back pain, and discomfort. A lower incidence of complications resulted in less medical assistance and less medication after the procedure.
Table 3.
Needle diameters compared through a systematic literature review [1]
| Conclusions | Diameters of needles compared | Motivations | References |
|---|---|---|---|
| No difference: in needle diameters | 20G versus 22G | No difference: PLPH, complaints, traumatic tap incidence, CSF pressure measurement | [47], [48], [49] |
| 22G versus 25G | No difference: PLPH, complaints, attempts | [50], [51] | |
| 23G versus 25G | No difference: PLPH, low back pain, attempts | [52] | |
| Favors: large-bore diameters | 20G versus 22G versus 24G versus 25G | Reduced: collection times Faster: CSF pressure measurement |
[53] |
| Favors: small-diameter bores | 18G versus 20G versus 22G versus 24G versus 25G | Lower frequency: PLPH | [54] |
| 18G versus 20G versus 22G versus 24G versus 25G versus 26G versus 27G | Reduced: collection times Faster: CSF pressure measurement |
[55] | |
| 19G versus 20G versus 22G 22 G versus 25G versus 27G |
Lower frequency: PLPH | [56] | |
| 20G versus 22G | Lower frequency: PLPH, complaints, blood patch rates Increased: collection times |
[57], [58], [59], [60], [61] | |
| 20G versus 22G versus 23G | Lower frequency: PLPH | [62] | |
| 20G versus 22G versus 25G | Lower frequency: PLPH Increased: collection times More: practice, failures |
[63] | |
| 20G versus 24G 20 G versus 23G versus 25G versus 26G |
Lower frequency: PLPH | [36] | |
| 22G versus 24G | Lower frequency: PLPH, complaints | [64] | |
| 22G versus 25G | Lower frequency: PLPH, low back pain, complaints Reduced: costs (health care) Lower frequency: leakage |
[65], [66], [67] | |
| 22G versus 26G | Lower frequency: PLPH, pain More: practice |
[38], [68], [69] | |
| 22G versus 29G | Lower frequency: PLPH, Reduced: failures |
[70] | |
| 25G versus 26G versus 27G | Lower frequency: PLPH Reduced: blood patch rates |
[71] |
Abbreviations: G, gauge; PLPH, postlumbar puncture headache; CSF, cerebrospinal fluid.
Small-bore needles result in less blood contamination (>5/μL red blood cells in the first tube of CSF collected) [34]. Advantages of large-bore spinal needles are a faster CSF flow (e.g., when large volumes of CSF should be collected like in case of an evacuating LP), and a shorter time needed to equilibrate CSF pressure, when a manometer is used. For CSF, pressure measurement needles that are smaller than 22G (thus >22G) are not suitable. Moreover, the smallest needle types (27G-29G) are not recommended because more technical difficulties occur, which might result in more failures, and due to the prolonged duration of the LP procedure [72]. On the other hand, large-bore needles (≤22G) are not recommended as they result in large dural perforations and increased risk of post-LP complications and blood contaminations [56], [62].
On the basis of the systematic literature analysis (level II-2 evidence), a balance between risk of PLPH, procedure duration, and technical failure has to be considered for each individual patient when choosing for a specific needle type. In daily clinical practice, a 22G or larger diameter (<22G) needles are most often used [73], [74]. Moreover, because there was no risk with large needles in the LP feasibility study, this needle can be chosen in memory clinics. Once the practitioner is more confident, by a more frequent usage, small-bore needles should be considered in other populations, which can then be used with a small number of failures [39], [75].
3.3.2.3. Design of the needle
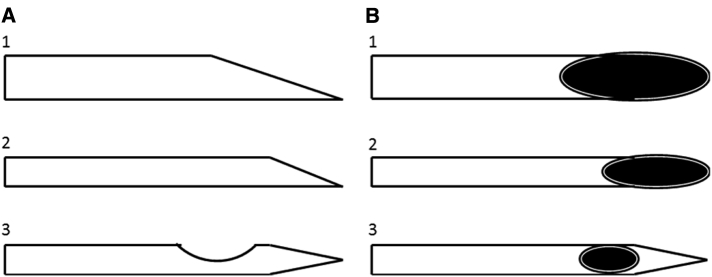
Different needle designs are available. Cutting bevel needles (standard Quincke [76] and Yale) are the standard needles with a medium cutting bevel and an orifice at the needle tip. Atraucan, Pecan, Sprotte, and Whitacre needles are noncutting, pencil-point, and atraumatic needles (Fig. 4). Medium (23G-24G) cutting bevel needles are most frequently used in clinical practice. As most atraumatic needles have smaller bores, these needles are associated with the same disadvantages of small-bore needles described previously [34], [35], [39], [73], [74], [75], [77], [78].
Fig. 4.
Schematic representative of lateral (A) and superior (B) aspects of the tips of spinal needles: 1, standard large-bore beveled needle; 2, standard small-bore beveled needle; and 3, atraumatic small-bore needle.
Thirty-seven studies, published from 1970 till 2016, compared needle designs (all references in the following paragraphs are based on the systematic literature review, Table 4). Four studies did not reveal a difference with regard to incidence of traumatic tap of vascular structures, procedure time and success rate in children, through comparison of transdural fluid leakage and vascular trauma associated with blood contamination in CSF (Pencan vs. Yale, Quincke vs. Yale, Whitacre vs. Quincke) [93], [95], [96], [97]. One study investigated the incidence of PLPH and low back pain and did not reveal any difference (Whitacre vs. Quincke) [94]. Two studies compared the flow rates and reported no significant differences for both needle designs (atraumatic vs. cutting bevel needles, Whitacre vs. Sprotte vs. Quincke) [55], [98].
Table 4.
Needle designs compared through a systematic literature review [1]
| Conclusion | Needle designs compared | Motivations | Comments | References |
|---|---|---|---|---|
| Favors: atraumatic needle | Quincke versus Pencan | Lower frequency: PLPH, low back pain | Reduced: PLPH interval | [66] |
| Quincke versus Pencan | Lower frequency: PLPH, post-LP complaints Reduced: costs |
[67] | ||
| Quincke versus Sprotte | Lower frequency: PLPH, post-LP complaints, nausea/vomiting Reduced: costs (health care) due to less medications and medical assistance Reduced: traumatic taps More: training |
Availability of atraumatic is less Same concentrations of IgM in CSF and serum Training increases the confidence of handling atraumatic needles |
[49], [57], [75], [79], [80], [81], [82], [83], [84], [85] | |
| Quincke versus Sprotte | Lower frequency: PLPH Increased: failure rates |
[86] | ||
| Quincke versus Sprotte versus Whitacre | Lower frequency: PLPH | Fewer cells attached to needle (size of cells are smaller), minor epithelial cells | [63], [87], [88] | |
| Quincke versus Sprotte versus Whitacre | Lower frequency: PLPH | [53] | ||
| Quincke versus Whitacre | Lower frequency: PLPH, post-LP complaints Reduced: blood patch rates Lower frequency: leakage |
[48], [60], [65], [71], [89], [90], [91] | ||
| Questionnaire to medical institutions. Favors: atraumatic needle | Quincke versus Sprotte | Lower frequency: PLPH More: practice, training Increased: costs (needles) |
[73], [75] | |
| Quincke versus Sprotte versus Whitacre | Lower frequency: PLPH Reduced: costs (health care) More: practice, training |
[92] | ||
| No difference: in needle designs | Quincke versus Sprotte versus Whitacre | No difference: flow properties | No influence of the performance of needle designs (on flow properties) | [55] |
| Quincke versus Whitacre | No difference: PLPH, low back pain | Same incidence (of PLPH and low back pain) for both needle designs | [93], [94] | |
| Quincke versus Whitacre | No difference: counts in RBC, leakage | [95] | ||
| Yale versus Pencan | No difference: success rates | No influence of the performance of needle designs (on success rates in children) | [96] | |
| Yale versus Sprotte | No difference: PLPH, traumatic taps | Same incidence (of PLPH and traumatic tap) for both needle designs | [97] |
Abbreviations: PLPH, postlumbar puncture headache; LP, lumbar puncture; CSF, cerebrospinal fluid; RBC, red blood cell.
However, atraumatic needles are recommended by most of the 37 articles as the best needle to perform LPs. Indeed, when comparing different needle designs, atraumatic needles resulted in a lower prevalence of post-LP complaints, including PLPH, low back pain or nausea/vomiting (Table 4) [5], [36], [48], [49], [53], [54], [57], [58], [60], [61], [63], [64], [65], [66], [67], [71], [75], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91]. A lower frequency of complications results in reduced health care costs due to less medication or medical assistance, such as blood patches [48], [49], [53], [54], [57], [61], [64], [66], [75], [79], [80], [81], [82], [83], [84], [86], [87], [88], [89], [90], [91], [93], [96]. A lower incidence of traumatic taps has been reported when using atraumatic needles. However, atraumatic needles result in more attempts and failures [86]. No data are available concerning post-LP complaints other than PLPH, number of traumatic taps, attempts, or failures. The technical drawbacks of atraumatic needles can be overcome with more training. Therefore, we conclude that the disadvantages are less decisive compared with the reduced risk of PLPH, which results in the time and cost savings for the health care system and less discomfort for the patient [11], [81], [82], [92], [99], [100].
In summary, head-to-head studies are in favor of atraumatic type needles with regard to the lower incidence of PLPH (level II-2 evidence).
3.3.3. Number of LP attempts
In case of an unsuccessful attempt, the needle (in case of cutting bevel needle) or the guide (in case of atraumatic needle) has to be withdrawn partially to the subcutaneous tissue and has to be reangled [101]. If the attempt is still unsuccessful, repalpation should reassure that the needle is in the midline. Multiple attempts may lead to local swelling and/or bruising as well as muscle spasms and is therefore associated with a higher risk for back pain [5]. Furthermore, it will obscure surface anatomical landmarks, making future attempts technically more difficult [46].
The number of LP attempts was significantly associated with post-LP back pain (Fig. 2) [5]. In case of 2–4 attempts, the OR [95% CI] was 2.1 [1.7–2.7], whereas in case of ≥5 attempts, the OR [95% Cl] increased to 5.4 [2.9–10.2] [5]. A total of four attempts is regarded as an acceptable maximum (level II-2 evidence).
3.3.4. Active versus passive withdrawal of CSF
Active withdrawal obviously reduces the procedural time, especially with small-bore needles, but this may be less important than the higher risk of PLPH. Passive withdrawal of CSF resulted in a lower risk for nonspecific headache (OR [95% Cl] 0.68 [0.50–0.92]), and a strongly lower risk for severe headache (OR [95% CI] 0.52 [0.33–0.84]) [5]. In addition, using a syringe results in negative pressure and might cause subdural hemorrhage or herniation or might result in paresthesia, pain, or injury in case a spinal nerve root was pulled, although these complications occur rarely. Active CSF withdrawal using a syringe, in combination with an adequate needle diameter, is often performed in the course of collection of large volumes for research purposes, but should only be performed when a long procedure is not appropriate for the patient or clinical setting (level II-2 evidence).
3.3.5. Other LP-related factors
An LP can be performed with the patient in the lateral recumbent position, also known as supine position, or in sitting position. In the lateral recumbent position, the patient should be positioned with knees-to-chest and with the back flexed as far as possible toward the physician performing the LP, and the coronal plane of the trunk should be perpendicular to the floor with one hip exactly above the other. In this position, lumbar lordosis is overcome and the neck is in a neutral position. The needle should stay in the midline of the spine, and therefore, it is placed parallel to the floor. In the sitting position, the patient completely flexes neck and back as this facilitates the course of the needle by widening the gap between adjacent lumbar spinal processes. The preferred position depends on the physician and on the patient's clinical conditions (level-III evidence). In case of immobilized patients and/or patients being unable to sit up, the lateral recumbent position should be applied. This position may be included in the clinical training/practice of residents, to be familiar with the procedure. For CSF pressure measurement, patients need to be in the lateral recumbent position [101], [102]. The sitting position has the advantage of a higher CSF pressure and flow, which reduces the sampling time. In the multicenter LP feasibility study, positioning of the patient was not associated with PLPH or post-LP back pain [5]. However, additional analysis showed that sitting position during LP procedure appeared to be a risk factor for severe headache, not for typical PLPH. Monserrate et al. mentioned also a trend for developing headache after LP in the sitting position [36].
When using an atraumatic needle, it is recommended to reinsert the stylet to the tip of the needle before removing the needle as this was associated with a lower prevalence of PLPH [103].
In the multicenter LP feasibility study, local anesthesia was used in 41% of the patients, mostly when the LP was performed with a large-bore diameter needle [5]. The application of local anesthesia or not was not associated with post-LP complaints. In addition, bed rest after LP and the volume of CSF withdrawn (up to 12 mL and more) were not associated with change in prevalence of PLPH or post-LP back pain [5]. Therefore, there is no recommendation to apply local anesthesia and bed rest after the LP to reduce postpuncture complaints. Collection of up to 30 mL of CSF is well tolerated and safe [36] and is advised as an acceptable maximum. Nevertheless, other LP procedure–related factors should depend on patient's-related, physician-related, and/or clinical setting–related factors (level II-2 evidence). For example, very small needle bores will extend the collection time for this volume.
3.4. Treatment of PLPH
More than 85% of PLPH will resolve without any other specific treatment [104]. The only evidence-based treatment for typical severe PLPH (severe usually frontal headache possibly accompanied by nausea and vomiting which is relieved by recumbent posture) is an epidural blood patch [105]. The most efficient volume of blood appears to be 20–30 mL [104]. This procedure has a success rate of 70%–98% if carried out more than 24 hours after the LP [104]. If a first epidural blood patch fails to resolve the headache, repeating the procedure has a similar success rate [104]. There is a lack of evidence to recommend pharmacological treatments of typical PLPH [106]. The few randomized clinical trials that have been performed included a limited number of patients and half of the participants were postpartum women in their 30s, limiting the generalizability [106]. Taking into account these limitations, caffeine decreased the proportion of subjects with persisting PLPH and the subjects requiring supplementary interventions compared with placebo [106]. Gabapentin, hydrocortisone, and theophylline decreased pain severity scores [106].
4. Conclusions: Recommendations
Based on the results of the international, multicenter LP feasibility study, literature-based analysis of risk factors, and best clinical practices discussed in the previous sections, we formulate the following consensus guidelines and recommendations for the LP procedure in adults. These recommendations should minimize post-LP complications, the most frequent being PLPH and post-LP back pain. The recommendations are summarized in Box 1 and Table 5. As these are guidelines, every professional can adapt the guidelines based on their own experience and based on the patient's comfort and benefits. We refer to the last article in a series of four articles on issues in the development and use of clinical guidelines, which focuses on how clinical guidelines should be used, in health care settings but also by an individual clinician [107].
Box 1. Recommendations to minimize post-LP complaints and complications (All recommendations are at least level II evidence, type 2).
Patient-related characteristics with regard to post-LP complaints and complications:
-
•
Risk factors: younger age, being female <40 years old, previous history of headache, and fear of post-LP complications
-
•Less risk: cognitive deterioration
-
→Serve to identify patients that are at high risk to develop post-LP complaints
-
→Serve to inform and reassure patients, before and during the LP procedure
-
→
LP procedure:
-
•
Use 25G atraumatic needles
-
•
Not more than four LP attempts
-
•
Passive withdrawal of CSF
-
•
Collection of up to 30 mL of CSF is well tolerated and safe
-
•
Lateral recumbent position
No influence of local anesthesia and bed rest after LP.
Abbreviations: CSF, cerebrospinal fluid; G, gauge; LP, lumbar puncture.
Table 5.
Recommendations based on level II-2 and III evidence described by graded risk factors
| Recommended procedure | Risk factors | Grading risk factors | Level of evidence |
|---|---|---|---|
| Rule out contraindications | |||
|
− | III | |
|
Coagulopathy Uncorrected bleeding diathesis |
+/− − |
III III |
|
Anticoagulant medication | +/− | III |
| Patients-related risk factors: | |||
|
|||
| Risk factors | |||
|
PLPH, back pain | ++ | II-2, III |
|
Post-LP complaints | +/− | III |
|
PLPH, back pain | ++ | II-2 |
|
Post-LP complaints | + | II-2 |
| Less risk | |||
|
PLPH, back pain | + | II-2, III |
| Procedure-related risk factors | |||
|
Post-LP complaints PLPH |
+ ++ |
III II-2, III |
|
Back pain | ++ | II-2 |
|
Severe headache | + | II-2, III |
|
Post-LP complaints | +/− | III |
|
Headache | + | II-2, III |
Abbreviations: LP, lumbar puncture; INR, International normalized ratio; PLPH, postlumbar puncture headache; G, gauge.
NOTE. Grading the impact on risk factors: + high (mentioned in multicenter LP feasibility study as one of the most important factors that influences post-LP complications), + high (mentioned in multicenter LP feasibility study as factor that influences post-LP complications or reported in other study with high quality evidence), +/− moderate (not detected as risk factor in multicenter LP feasibility study, based on consensus and literature), − low (not detected as risk factor in multicenter LP feasibility study, based on consensus and sparse literature). Level of evidence: II-2 = well-designed cohorts preferably with more than one research group or center (e.g., data of large multicenter LP feasibility study, systematic review); III = consensus evidence based on clinical experience of the two consortia (JPND BIOMARKAPD and BioMS).
First, contraindications for LP should be ruled out (Table 1). The following recommendations are consensus based (level-III evidence).
-
•
It is advised to perform brain imaging before LP, whenever an intracranial lesion with mass effect, abnormal intracranial pressure due to increased CSF pressure, or tonsillar herniation is suspected based on medical history or neurological examination, and in case of recent seizures, impaired consciousness, or papilledema.
-
•
Coagulation status and platelet count (should be higher than 40 × 109/L) should be checked by (recent) blood analysis before LP.
-
•
Concomitant medication should be checked before LP. In case of intake of anticoagulants, an LP is contraindicated unless the risk of the procedure outweighs the potential benefit. Direct acting anticoagulants can be temporarily interrupted. An LP can be performed without substantial risk when patients take one type of antiplatelet drug.
-
•
Infections at the LP site are relative contraindications.
As patient-related characteristics are among the most important risk factors for PLPH and post-LP back pain, the physician should determine the risk profile of the patient. Although the patient-related risk factors cannot be modified, these can be of help to identify those patients that are at high risk to develop post-LP complaints. Moreover, as fear of complications is an important risk factor for post-LP complaints, the patient should be informed thoroughly and should be reassured, before and during the LP procedure (level-III evidence). Recommended based on level II-2 and III evidence:
-
•
Younger age, being a female aged less than 40 years, previous history of headache, and fear of post-LP complications are risk factors for PLPH and post-LP back pain.
-
•
Post-LP complaints are less prevalent in patients with cognitive deterioration [5].
Recommendations with regard to the LP procedure itself are based on level II-2 and III evidence:
-
•
It is recommended to use 25G atraumatic needles given the reduced incidence of PLPH.
-
•
A total of four attempts may be regarded as an acceptable maximum, as the risk for back pain significantly increased with more than 4 attempts [5].
-
•
Active CSF withdrawal using a syringe should only be performed when a patient cannot tolerate a long procedure. If a large volume of CSF has to be withdrawn (e.g., for research purposes or in case of an evacuating LP), a larger needle diameter is recommended instead of active withdrawal by using a syringe.
-
•
It is recommended to perform an LP in the lateral recumbent position due to the fact that the sitting position was associated with more severe headache [5], [36]. For CSF pressure measurement, patients need to be in the lateral recumbent position.
-
•
The collection of up to 30 mL of CSF is well tolerated and safe [36].
As local anesthesia and bed rest after LP are not associated with increased prevalence of post-LP complications, there are no recommendations to apply (level II-2 and III evidence).
In conclusion, an LP is a common and generally well-tolerated diagnostic procedure with a high diagnostic yield. The application of these evidence-based guidelines will help to reduce complication rates.
Research in Context.
-
1.
Systematic review: Consensus guidelines and recommendations for the lumbar puncture (LP) procedure to obtain cerebrospinal fluid (CSF) to minimize risk of complications and to optimize diagnostic yield are lacking. CSF analysis is increasingly relevant for diagnosis and research for neurological diseases, even at the core criteria for dementia diagnosis.
-
2.
Interpretation: It is recommended to use 25G atraumatic needles. A total of four LP attempts is an acceptable maximum, active CSF withdrawals should be avoided. It is advised to perform LP in the lateral recumbent position, and collection up to 30-mL CSF is well tolerated and safe. There is no recommendation to apply local anesthesia and bed rest after the LP to reduce postpuncture complaints.
-
3.
Future directions: The application of these evidence-based guidelines will help to reduce complication rates. Moreover, these guidelines will be a reference for educational purposes, thus be of help for trainees in neurology, and will be a guide to comfort or give adequate information to the patients.
Acknowledgments
This work was supported by the University of Antwerp Research Fund; the Agency for Innovation by Science and Technology (IWT, www.iwt.be); the Research Foundation Flanders (FWO, www.fwo.be); the Belgian Science Policy Office Interuniversity Attraction Poles (IAP) program (BELSPO, www.belspo.be); the Flemish Government–initiated Methusalem excellence grant (EWI, www.ewi-vlaanderen.be); the Flanders Impulse Program on Networks for Dementia Research (VIND). This work was supported by Alzheimer Nederland (WE.15-2013-08; F.D.). R.B. is a senior clinical investigator of the Research Foundation–Flanders (FWO).
This is an EU Joint Programme–Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organizations under the aegis of JPND—www.jpnd.eu: Ministry of Health (Italy), Fundação para a Ciência e a Tecnologia (Portugal), Instituto de Salud Carlos III (Spain), The Swedish Research Council (Sweden), ZonMw (The Netherlands).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2017.04.007.
Supplementary data
References
- 1.Niemantsverdriet E., Struyfs H., Duits F., Teunissen C., Engelborghs S. Techniques, contraindications and complications of CSF collection procedures. In: Deisenhammer F., Sellebjerg F., Teunissen C.E., Tumani H., editors. Cerebrospinal Fluid in Clinical Neurology. Springer; New York: 2015. pp. 37–57. [Google Scholar]
- 2.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 5.Duits F.H., Martinez-Lage P., Paquet C., Engelborghs S., Lleo A., Hausner L. Performance and complications of lumbar puncture in memory clinics: Results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12:154–163. doi: 10.1016/j.jalz.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 Suppl 1:9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 7.Leibold R.A., Yealy D.M., Coppola M., Cantees K.K. Post-dural-puncture headache: characteristics, management, and prevention. Ann Emerg Med. 1993;22:1863–1870. doi: 10.1016/s0196-0644(05)80416-0. [DOI] [PubMed] [Google Scholar]
- 8.Safa-Tisseront V., Thormann F., Malassine P., Henry M., Riou B., Coriat P. Effectiveness of epidural blood patch in the management of post-dural puncture headache. Anesthesiology. 2001;95:334–339. doi: 10.1097/00000542-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 9.US. Preventive Services Task Force . DIANE Publishing; Darby, PA: 1989. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force; p. 24. [Google Scholar]
- 10.PRISMA. Transparent reporting of systematic reviews and meta-anayses. Available at: http://www.prisma-statement.org/. Accessed October 19, 2016.
- 11.Linker G., Mirza N., Manetti G., Meyer M., Putnam K., Sunderland T. Fine-needle, negative-pressure lumbar puncture: a safe technique for collecting CSF. Neurology. 2002;59:2008–2009. doi: 10.1212/01.wnl.0000038360.01635.39. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt A., Oheim S., Neundorfer B. Post-lumbar puncture headache: experiences with Sprotte's atraumatic needle. Cephalalgia. 1992;12:259. doi: 10.1046/j.1468-2982.1992.1204259.x. [DOI] [PubMed] [Google Scholar]
- 13.Dakka Y., Warra N., Albadareen R.J., Jankowski M., Silver B. Headache rate and cost of care following lumbar puncture at a single tertiary care hospital. Neurology. 2011;77:71–74. doi: 10.1212/WNL.0b013e318220abc0. [DOI] [PubMed] [Google Scholar]
- 14.Turnbull D., McKevitt F. Headache after diagnostic lumbar puncture. Authors should have used smaller gauge needles. BMJ. 2001;322:994. [PubMed] [Google Scholar]
- 15.Davies J.R. Headache after diagnostic lumbar puncture. Smaller is better where needles are concerned. BMJ. 2001;322:993–994. [PubMed] [Google Scholar]
- 16.Reynolds F., O'Sullivan G. Lumbar puncture and headache. “Atraumatic needle” is a better term than “blunt needle”. BMJ. 1998;316:1018. [PubMed] [Google Scholar]
- 17.Chow T.K. Education research: changing practice: residents' adoption of the atraumatic lumbar puncture needle. Neurology. 2014;82:734–735. doi: 10.1212/01.wnl.0000444557.46526.8d. [DOI] [PubMed] [Google Scholar]
- 18.Toyka K.V., Muller B., Reichmann H. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2002;59:1120. doi: 10.1212/wnl.59.7.1120. author reply 1120–1. [DOI] [PubMed] [Google Scholar]
- 19.Kuczkowski K.M. Pneumocephalus following an uneventful lumbar puncture: does the gauge of a spinal needle matter? Anesth Analg. 2004;99:303. doi: 10.1213/01.ANE.0000127908.24929.1F. author reply 303–4. [DOI] [PubMed] [Google Scholar]
- 20.Smeltzer J.S. Headache after diagnostic lumbar puncture. Using 20 gauge needle is blunderbuss technique. BMJ. 2001;322:993. author reply 994. [PMC free article] [PubMed] [Google Scholar]
- 21.Leone M.A., Brainin M., Boon P., Pugliatti M., Keindl M., Bassetti C.L. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces - revised recommendations 2012. Eur J Neurol. 2013;20:410–419. doi: 10.1111/ene.12043. [DOI] [PubMed] [Google Scholar]
- 22.Hasbun R., Abrahams J., Jekel J., Quagliarello V.J. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727–1733. doi: 10.1056/NEJMoa010399. [DOI] [PubMed] [Google Scholar]
- 23.Korein J., Cravioto H., Leicach M. Reevaluation of lumbar puncture; a study of 129 patients with papilledema or intracranial hypertension. Neurology. 1959;9:290–297. doi: 10.1212/wnl.9.4.290. [DOI] [PubMed] [Google Scholar]
- 24.Archer B.D. Computed tomography before lumbar puncture in acute meningitis: a review of the risks and benefits. CMAJ. 1993;148:961–965. [PMC free article] [PubMed] [Google Scholar]
- 25.Practice parameters: lumbar puncture (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1993;43:625–627. [PubMed] [Google Scholar]
- 26.Gower D.J., Baker A.L., Bell W.O., Ball M.R. Contraindications to lumbar puncture as defined by computed cranial tomography. J Neurol Neurosurg Psychiatry. 1987;50:1071–1074. doi: 10.1136/jnnp.50.8.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidbuchel H., Verhamme P., Alings M., Antz M., Hacke W., Oldgren J. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013;34:2094–2106. doi: 10.1093/eurheartj/eht134. [DOI] [PubMed] [Google Scholar]
- 28.Keeling D., Baglin T., Tait C., Watson H., Perry D., Baglin C. Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol. 2011;154:311–324. doi: 10.1111/j.1365-2141.2011.08753.x. [DOI] [PubMed] [Google Scholar]
- 29.Horlocker T.T., Wedel D.J., Schroeder D.R., Rose S.H., Elliott B.A., McGregor D.G. Preoperative antiplatelet therapy does not increase the risk of spinal hematoma associated with regional anesthesia. Anesth Analg. 1995;80:303–309. doi: 10.1097/00000539-199502000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Brook A.D., Burns J., Dauer E., Schoendfeld A.H., Miller T.S. Comparison of CT and fluoroscopic guidance for lumbar puncture in an obese population with prior failed unguided attempt. J Neurointerv Surg. 2014;6:324–328. doi: 10.1136/neurintsurg-2013-010745. [DOI] [PubMed] [Google Scholar]
- 31.Boddu S.R., Corey A., Peterson R., Saindane A.M., Hudgins P.A., Chen Z. Fluoroscopic-guided lumbar puncture: fluoroscopic time and implications of body mass index–a baseline study. AJNR Am J Neuroradiol. 2014;35:1475–1480. doi: 10.3174/ajnr.A3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S., Adler D.K. Ultrasound-assisted lumbar puncture in pediatric emergency medicine. J Emerg Med. 2014;47:59–64. doi: 10.1016/j.jemermed.2012.09.149. [DOI] [PubMed] [Google Scholar]
- 33.Peterson M.A., Pisupati D., Heyming T.W., Abele J.A., Lewis R.J. Ultrasound for routine lumbar puncture. Acad Emerg Med. 2014;21:130–136. doi: 10.1111/acem.12305. [DOI] [PubMed] [Google Scholar]
- 34.Armon C., Evans R.W., Therapeutics, Technology Assessment Subcommittee of the American Academy of Neurology Addendum to assessment: Prevention of post-lumbar puncture headaches: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2005;65:510–512. doi: 10.1212/01.wnl.0000173034.96211.1b. [DOI] [PubMed] [Google Scholar]
- 35.Amorim J.A., Gomes de Barros M.V., Valenca M.M. Post-dural (post-lumbar) puncture headache: risk factors and clinical features. Cephalalgia. 2012;32:916–923. doi: 10.1177/0333102412453951. [DOI] [PubMed] [Google Scholar]
- 36.Monserrate A.E., Ryman D.C., Ma S., Xiong C., Noble J.M., Ringman J.M. Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA Neurol. 2015;72:325–332. doi: 10.1001/jamaneurol.2014.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilming S.T., Schrader H., Monstad I. The significance of age, sex, and cerebrospinal fluid pressure in post-lumbar-puncture headache. Cephalalgia. 1989;9:99–106. doi: 10.1046/j.1468-2982.1989.0902099.x. [DOI] [PubMed] [Google Scholar]
- 38.Tourtellotte W.W., Henderson W.G., Tucker R.P., Gilland O., Walker J.E., Kokman E. A randomized, double-blind clinical trial comparing the 22 versus 26 gauge needle in the production of the post-lumbar puncture syndrome in normal individuals. Headache. 1972;12:73–78. doi: 10.1111/j.1526-4610.1972.hed1202073.x. [DOI] [PubMed] [Google Scholar]
- 39.Evans R.W., Armon C., Frohman E.M., Goodin D.S. Assessment: prevention of post-lumbar puncture headaches: report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2000;55:909–914. doi: 10.1212/wnl.55.7.909. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan G. The psychogenic etiology of headache post lumbar puncture. Psychosom Med. 1967;29:376–379. doi: 10.1097/00006842-196707000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Straus S.E., Thorpe K.E., Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA. 2006;296:2012–2022. doi: 10.1001/jama.296.16.2012. [DOI] [PubMed] [Google Scholar]
- 42.Boon J.M., Abrahams P.H., Meiring J.H., Welch T. Lumbar puncture: anatomical review of a clinical skill. Clin Anat. 2004;17:544–553. doi: 10.1002/ca.10250. [DOI] [PubMed] [Google Scholar]
- 43.Doherty C.M., Forbes R.B. Diagnostic lumbar puncture. Ulster Med J. 2014;83:93–102. [PMC free article] [PubMed] [Google Scholar]
- 44.Evans R.W. Complications of lumbar puncture. Neurol Clin. 1998;16:83–105. doi: 10.1016/s0733-8619(05)70368-6. [DOI] [PubMed] [Google Scholar]
- 45.Sahebkar-Moghaddam F., Adornato B.T. The failed lumbar puncture. Neurology. 2005;64:E24. doi: 10.1212/01.WNL.0000159942.64562.AD. [DOI] [PubMed] [Google Scholar]
- 46.Wright B.L., Lai J.T., Sinclair A.J. Cerebrospinal fluid and lumbar puncture: a practical review. J Neurol. 2012;259:1530–1545. doi: 10.1007/s00415-012-6413-x. [DOI] [PubMed] [Google Scholar]
- 47.Ellis R.W., 3rd, Strauss L.C., Wiley J.M., Killmond T.M., Ellis R.W., Jr. A simple method of estimating cerebrospinal fluid pressure during lumbar puncture. Pediatrics. 1992;89:895–897. [PubMed] [Google Scholar]
- 48.Alcolea D., Martinez-Lage P., Izagirre A., Clerigue M., Carmona-Iragui M., Alvarez R.M. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer's disease: a multicenter study in Spain. J Alzheimers Dis. 2014;39:719–726. doi: 10.3233/JAD-131334. [DOI] [PubMed] [Google Scholar]
- 49.Hammond E.R., Wang Z., Bhulani N., McArthur J.C., Levy M. Needle type and the risk of post-lumbar puncture headache in the outpatient neurology clinic. J Neurol Sci. 2011;306:24–28. doi: 10.1016/j.jns.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez D., Branstetter B.F., Agarwal V., Palfey S., Ching K.C., Bump G.M. JOURNAL CLUB: Incidence of Complications Following Fluoroscopically Guided Lumbar Punctures and Myelograms. AJR Am J Roentgenol. 2016;206:20–25. doi: 10.2214/AJR.15.14664. [DOI] [PubMed] [Google Scholar]
- 51.Crock C., Orsini F., Lee K.J., Phillips R.J. Headache after lumbar puncture: randomised crossover trial of 22-gauge versus 25-gauge needles. Arch Dis Child. 2014;99:203–207. doi: 10.1136/archdischild-2013-305145. [DOI] [PubMed] [Google Scholar]
- 52.Kim M., Yoon H. Comparison of post-dural puncture headache and low back pain between 23 and 25 gauge Quincke spinal needles in patients over 60 years: randomized, double-blind controlled trial. Int J Nurs Stud. 2011;48:1315–1322. doi: 10.1016/j.ijnurstu.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Carson D., Serpell M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47:33–37. doi: 10.1212/wnl.47.1.33. [DOI] [PubMed] [Google Scholar]
- 54.Vidoni E.D., Morris J.K., Raider K., Burns J.M., Alzheimer's Disease Neuroimaging Initiative Reducing post-lumbar puncture headaches with small bore atraumatic needles. J Clin Neurosci. 2014;21:536–537. doi: 10.1016/j.jocn.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ginosar Y., Smith Y., Ben-Hur T., Lovett J.M., Clements T., Ginosar Y.D. Novel pulsatile cerebrospinal fluid model to assess pressure manometry and fluid sampling through spinal needles of different gauge: support for the use of a 22 G spinal needle with a tapered 27 G pencil-point tip. Br J Anaesth. 2012;108:308–315. doi: 10.1093/bja/aer372. [DOI] [PubMed] [Google Scholar]
- 56.Flaatten H., Krakenes J., Vedeler C. Post-dural puncture related complications after diagnostic lumbar puncture, myelography and spinal anaesthesia. Acta Neurol Scand. 1998;98:445–451. doi: 10.1111/j.1600-0404.1998.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 57.Muller B., Adelt K., Reichmann H., Toyka K. Atraumatic needle reduces the incidence of post-lumbar puncture syndrome. J Neurol. 1994;241:376–380. doi: 10.1007/BF02033354. [DOI] [PubMed] [Google Scholar]
- 58.Aamodt A., Vedeler C. Complications after LP related to needle type: pencil-point versus Quincke. Acta Neurol Scand. 2001;103:396–398. doi: 10.1034/j.1600-0404.2001.103006396.x. [DOI] [PubMed] [Google Scholar]
- 59.Vilming S.T., Kloster R., Sandvik L. The importance of sex, age, needle size, height and body mass index in post-lumbar puncture headache. Cephalalgia. 2001;21:738–743. doi: 10.1046/j.1468-2982.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- 60.Hatfield M.K., Handrich S.J., Willis J.A., Beres R.A., Zaleski G.X. Blood patch rates after lumbar puncture with Whitacre versus Quincke 22- and 20-gauge spinal needles. AJR Am J Roentgenol. 2008;190:1686–1689. doi: 10.2214/AJR.07.3351. [DOI] [PubMed] [Google Scholar]
- 61.Kleyweg R.P., Hertzberger L.I., Carbaat P.A. Significant reduction in post-lumbar puncture headache using an atraumatic needle. A double-blind, controlled clinical trial. Cephalalgia. 1998;18:635–637. doi: 10.1111/j.1468-2982.1998.1809635.x. [DOI] [PubMed] [Google Scholar]
- 62.Kovanen J., Sulkava R. Duration of postural headache after lumbar puncture: effect of needle size. Headache. 1986;26:224–226. doi: 10.1111/j.1526-4610.1986.hed2605224.x. [DOI] [PubMed] [Google Scholar]
- 63.Bertolotto A., Malentacchi M., Capobianco M., di Sapio A., Malucchi S., Motuzova Y. The use of the 25 Sprotte needle markedly reduces post-dural puncture headache in routine neurological practice. Cephalalgia. 2016;36:131–138. doi: 10.1177/0333102415583983. [DOI] [PubMed] [Google Scholar]
- 64.Palmers Y., Kuhn F.P., Petersen D., De Greef D. Comparison in myelography between iodixanol 270 and 320 mgI/ml and iotrolan 300 mgI/ml: a multicentre, randomised, parallel-group, double-blind, phase III trial. Eur Radiol. 2002;12:686–691. doi: 10.1007/s003300100881. [DOI] [PubMed] [Google Scholar]
- 65.Ready L.B., Cuplin S., Haschke R.H., Nessly M. Spinal needle determinants of rate of transdural fluid leak. Anesth Analg. 1989;69:457–460. [PubMed] [Google Scholar]
- 66.Lowery S., Oliver A. Incidence of postdural puncture headache and backache following diagnostic/therapeutic lumbar puncture using a 22G cutting spinal needle, and after introduction of a 25G pencil point spinal needle. Paediatr Anaesth. 2008;18:230–234. doi: 10.1111/j.1460-9592.2008.02414.x. [DOI] [PubMed] [Google Scholar]
- 67.Engedal T.S., Ording H., Vilholm O.J. Changing the needle for lumbar punctures: results from a prospective study. Clin Neurol Neurosurg. 2015;130:74–79. doi: 10.1016/j.clineuro.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Kaukinen S., Kaukinen L., Kannisto K., Kataja M. The prevention of headache following spinal anaesthesia. Ann Chir Gynaecol. 1981;70:107–111. [PubMed] [Google Scholar]
- 69.Wilkinson A.G., Sellar R.J. The influence of needle size and other factors on the incidence of adverse effects caused by myelography. Clin Radiol. 1991;44:338–341. doi: 10.1016/s0009-9260(05)81272-3. [DOI] [PubMed] [Google Scholar]
- 70.McConaha C., Bastiani A.M., Kaye W.H. Significant reduction of post-lumbar puncture headaches by the use of a 29-gauge spinal needle. Biol Psychiatry. 1996;39:1058–1060. doi: 10.1016/0006-3223(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 71.Lambert D.H., Hurley R.J., Hertwig L., Datta S. Role of needle gauge and tip configuration in the production of lumbar puncture headache. Reg Anesth. 1997;22:66–72. doi: 10.1016/s1098-7339(06)80058-1. [DOI] [PubMed] [Google Scholar]
- 72.Flaatten H., Rodt S.A., Vamnes J., Rosland J., Wisborg T., Koller M.E. Postdural puncture headache. A comparison between 26- and 29-gauge needles in young patients. Anaesthesia. 1989;44:147–149. doi: 10.1111/j.1365-2044.1989.tb11167.x. [DOI] [PubMed] [Google Scholar]
- 73.Birnbach D.J., Kuroda M.M., Sternman D., Thys D.M. Use of atraumatic spinal needles among neurologists in the United States. Headache. 2001;41:385–390. doi: 10.1046/j.1526-4610.2001.111006385.x. [DOI] [PubMed] [Google Scholar]
- 74.Stendell L., Fomsgaard J.S., Olsen K.S. There is room for improvement in the prevention and treatment of headache after lumbar puncture. Dan Med J. 2012;59:A4483. [PubMed] [Google Scholar]
- 75.Tung C.E. Education research: changing practice. Residents' adoption of the atraumatic lumbar puncture needle. Neurology. 2013;80:e180–e182. doi: 10.1212/WNL.0b013e31828f1866. [DOI] [PubMed] [Google Scholar]
- 76.Quincke H. Die lumbarpunktion des hydrocephalus. Klin Wochenschr. 1891;28:929–933. 65–68. [Google Scholar]
- 77.Moller A., Afshari A., Bjerrum O.W. Diagnostic and therapeutic lumbar puncture performed safely and efficiently with a thin blunt needle. Dan Med J. 2013;60:A4684. [PubMed] [Google Scholar]
- 78.Sharma S., Gambling D., Joshi G., Sidawi J., Herrera E. Comparison of 26-gauge Atraucan® and 25-gauge Whitacre needles: insertion characteristics and complications. Can J Anaesth. 1995;42:706–710. doi: 10.1007/BF03012669. [DOI] [PubMed] [Google Scholar]
- 79.Braune H.J., Huffmann G.A. A prospective double-blind clinical trial, comparing the sharp Quincke needle (22G) with an “atraumatic” needle (22G) in the induction of post-lumbar puncture headache. Acta Neurol Scand. 1992;86:50–54. doi: 10.1111/j.1600-0404.1992.tb08053.x. [DOI] [PubMed] [Google Scholar]
- 80.Ohman S., Ernerudh J., Forsberg P., Roberg M., Vrethem M. Lower values for immunoglobulin M in cerebrospinal fluid when sampled with an atraumatic Sprotte needle compared with conventional lumbar puncture. Ann Clin Biochem. 1995;32:210–212. doi: 10.1177/000456329503200215. [DOI] [PubMed] [Google Scholar]
- 81.Strupp M., Schueler O., Straube A., Von Stuckrad–Barre S., Brandt T. “Atraumatic” Sprotte needle reduces the incidence of post-lumbar puncture headaches. Neurology. 2001;57:2310–2312. doi: 10.1212/wnl.57.12.2310. [DOI] [PubMed] [Google Scholar]
- 82.Tung C.E., So Y.T., Lansberg M.G. Cost comparison between the atraumatic and cutting lumbar puncture needles. Neurology. 2012;78:109–113. doi: 10.1212/WNL.0b013e31823efca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vakharia V.N., Lote H. Introduction of Sprotte needles to a single-centre acute neurology service: before and after study. JRSM Short Rep. 2012;3:82. doi: 10.1258/shorts.2012.012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis A., Dobson R., Kaninia S., Espasandin M., Berg A., Giovannoni G. Change practice now! Using atraumatic needles to prevent post lumbar puncture headache. Eur J Neurol. 2014;21:305–311. doi: 10.1111/ene.12307. [DOI] [PubMed] [Google Scholar]
- 85.Castrillo A., Tabernero C., Garcia-Olmos L.M., Gil C., Gutierrez R., Zamora M.I. Postdural puncture headache: impact of needle type, a randomized trial. Spine J. 2015;15:1571–1576. doi: 10.1016/j.spinee.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Thomas S.R., Jamieson D.R., Muir K.W. Randomised controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture. BMJ. 2000;321:986–990. doi: 10.1136/bmj.321.7267.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puolakka R., Andersson L.C., Rosenberg P.H. Microscopic analysis of three different spinal needle tips after experimental subarachnoid puncture. Reg Anesth Pain Med. 2000;25:163–169. doi: 10.1053/rapm.2000.0250163. [DOI] [PubMed] [Google Scholar]
- 88.Quinn C., Macklin E.A., Atassi N., Bowser R., Boylan K., Cudkowicz M. Post-lumbar puncture headache is reduced with use of atraumatic needles in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:632–634. doi: 10.3109/21678421.2013.808227. [DOI] [PubMed] [Google Scholar]
- 89.Pedersen O.N. Use of a 22-gauge Whitacre needle to reduce the incidence of side effects after lumbar myelography: a prospective randomised study comparing Whitacre and Quincke spinal needles. Eur Radiol. 1996;6:184–187. doi: 10.1007/BF00181141. [DOI] [PubMed] [Google Scholar]
- 90.Lavi R., Rowe J.M., Avivi I. Traumatic vs. atraumatic 22 G needle for therapeutic and diagnostic lumbar puncture in the hematologic patient: a prospective clinical trial. Haematologica. 2007;92:1007–1008. doi: 10.3324/haematol.10883. [DOI] [PubMed] [Google Scholar]
- 91.Lavi R., Yarnitsky D., Rowe J.M., Weissman A., Segal D., Avivi I. Standard vs atraumatic Whitacre needle for diagnostic lumbar puncture: a randomized trial. Neurology. 2006;67:1492–1494. doi: 10.1212/01.wnl.0000240054.40274.8a. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Y.C., Chandler A.J., Kagetsu N.J. Technical compliance to standard guidelines for lumbar puncture and myelography: survey of academic neuroradiology attendings and fellows. Acad Radiol. 2014;21:612–616. doi: 10.1016/j.acra.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 93.Kokki H., Salonvaara M., Herrgard E., Onen P. Postdural puncture headache is not an age-related symptom in children: a prospective, open-randomized, parallel group study comparing a22-gauge Quincke with a 22-gauge Whitacre needle. Paediatr Anaesth. 1999;9:429–434. doi: 10.1046/j.1460-9592.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- 94.Luostarinen L., Heinonen T., Luostarinen M., Salmivaara A. Diagnostic lumbar puncture. Comparative study between 22-gauge pencil point and sharp bevel needle. J Headache Pain. 2005;6:400–404. doi: 10.1007/s10194-005-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knowles P.R., Randall N.P., Lockhart A.S. Vascular trauma associated with routine spinal anaesthesia. Anaesthesia. 1999;54:647–650. doi: 10.1046/j.1365-2044.1999.00957.x. [DOI] [PubMed] [Google Scholar]
- 96.Kokki H., Heikkinen M., Turunen M., Vanamo K., Hendolin H. Needle design does not affect the success rate of spinal anaesthesia or the incidence of postpuncture complications in children. Acta Anaesthesiol Scand. 2000;44:210–213. doi: 10.1034/j.1399-6576.2000.440213.x. [DOI] [PubMed] [Google Scholar]
- 97.Lenaerts M., Pepin J.L., Tombu S., Schoenen J. No significant effect of an “atraumatic” needle on incidence of post-lumbar puncture headache or traumatic tap. Cephalalgia. 1993;13:296–297. doi: 10.1046/j.1468-2982.1993.1304296.x. [DOI] [PubMed] [Google Scholar]
- 98.Pelzer N., Vandersteene J., Bekooij T.J., Schoonman G.G., Wirtz P.W., Vanopdenbosch L.J. Are atraumatic spinal needles as efficient as traumatic needles for lumbar puncture? Neurol Sci. 2014;35:1997–1999. doi: 10.1007/s10072-014-1924-0. [DOI] [PubMed] [Google Scholar]
- 99.Peskind E.R., Riekse R., Quinn J.F., Kaye J., Clark C.M., Farlow M.R. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005;19:220–225. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 100.Arendt K., Demaerschalk B.M., Wingerchuk D.M., Camann W. Atraumatic lumbar puncture needles: after all these years, are we still missing the point? Neurologist. 2009;15:17–20. doi: 10.1097/NRL.0b013e318184f476. [DOI] [PubMed] [Google Scholar]
- 101.Van Dellen J.R., Bill P.L. Lumbar puncture–an innocuous diagnostic procedure? S Afr Med J. 1978;53:666–668. [PubMed] [Google Scholar]
- 102.Adams R.D., Maurice V., Ropper A.H. 6th Ed. McGraw-Hill; New York: 1997. Principles of Neurology; pp. 13–14. [Google Scholar]
- 103.Strupp M., Brandt T., Muller A. Incidence of post-lumbar puncture syndrome reduced by reinserting the stylet: a randomized prospective study of 600 patients. J Neurol. 1998;245:589–592. doi: 10.1007/s004150050250. [DOI] [PubMed] [Google Scholar]
- 104.Turnbull D.K., Shepherd D. Post-dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–729. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 105.Boonmak P., Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database Syst Rev. 2010:CD001791. doi: 10.1002/14651858.CD001791.pub2. [DOI] [PubMed] [Google Scholar]
- 106.Basurto Ona X., Osorio D., Bonfill Cosp X. Drug therapy for treating post-dural puncture headache. Cochrane Database Syst Rev. 2015:CD007887. doi: 10.1002/14651858.CD007887.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feder G., Eccles M., Grol R., Griffiths C., Grimshaw J. Clinical guidelines: using clinical guidelines. BMJ. 1999;318:728–730. doi: 10.1136/bmj.318.7185.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.