Abstract
RIG-I-like receptors (RLRs) are cytosolic innate immune sensors that detect pathogenic RNA and induce a systemic antiviral response. During the last decade, many studies focused on their molecular characterization and the identification of RNA agonists. Therefore, it became more and more clear that RLR activation needs to be carefully regulated, because constitutive signaling or detection of endogenous RNA through loss of specificity is detrimental. Here, we review the current understanding of RLR activation and selectivity. We specifically focus upon recent findings on the function of the helicase domain in discriminating between different RNAs, and whose malfunctioning causes serious autoimmune diseases.
Keywords: ATPase, autoimmune disease, RIG-I-like receptor (RLR), RNA, signaling, ATP hydrolysis, innate immune system, virus sensing
Introduction
Success in evolution not only is the ability to obtain and dissipate enough energy or to escape large predators, it is also the capability to fend off the myriads of co-evolving pathogens. As a consequence, life forms have evolved various capacities to intra- and extracellularly recognize “non-self,” with the mammalian immune system being the most sophisticated. Our immune system consists of an innate and adaptive branch. The innate immune system is based on a limited set of germ line-encoded receptors, signaling proteins, and response factors that detect, signal, and battle infections. It provides a first and rapid response to pathogens and initiates the slower but more powerful adaptive immune system.
The innate branch detects new infections by invading pathogens through pattern recognition receptors that sense specific pathogen-associated molecular patterns (PAMP)2 or microbe-associated molecular patterns. These non-self molecules are absent in the host and originate from viral, bacterial, or fungal sources. Cytosolic viral RNA is a powerful PAMP. However, in contrast to many other PAMPs, it needs to be detected amid a large and diverse pool of related molecules, i.e. the normal host RNA content of the cytosol. Because of this circumstance, it is not surprising that the system responsible for detecting cytosolic RNA is also implicated in several autoimmune diseases where important control mechanisms are defective, and self-molecules are recognized.
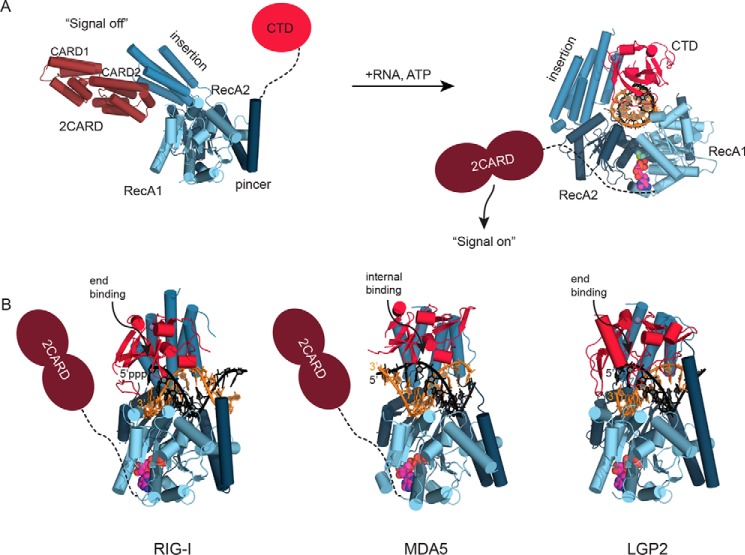
In 2004, retinoic acid-inducible gene I (RIG-I) was identified as a sensor recognizing cytosolic viral RNA (1). Soon it became clear that RNA-sensing involves three RIG-I-like proteins, the other two being melanoma differentiation-associated gene 5 (MDA5) and laboratory of physiology and genetics 2 (LGP2) (2). All three proteins are structurally related and are collectively referred to as RIG-I-like receptors (RLRs) (Fig. 1A).
Figure 1.
Structures of RIG-I-like receptors. A, comparison of RIG-I in open signal-off state (Protein Data Bank code 4A2W) with activated RIG-I bound to RNA (5E3H). The structures are shown as ribbon models with highlighted secondary structure and color-coded functional domains. 2CARD and RNA mutually compete for binding to the superfamily 2 ATPase domain. RNA and ATP binding generates a ring structure that expels the 2CARD signaling module. B, all three RLRs (RIG-I, 5E3H; MDA5, 4GL2; and LGP2, 5JB2) form ring-like structures around RNA. RIG-I and LGP2 have preferences for dsRNA ends, whereas MDA5 binds dsRNA internally.
RLRs are part of the large and diverse superfamily 2 (SF2) of nucleic acid-dependent NTPases. SF2 proteins use the energy of NTP hydrolysis to conduct diverse functions at nucleic acids, and the family includes helicases, motor proteins, and translocases (3). RLRs themselves detect non-self double-stranded (ds) RNA within the cytosol and thereupon initiate a signaling cascade that aims to defend the cell from invading pathogens. ATP turnover is a critical part of sensing and signaling by RLRs, but the function of ATP turnover has been surprisingly unclear for many years. Recent studies and the discovery of RLR-related autoimmune diseases now shed a new light onto the role of ATP turnover by these proteins.
RLR ligands
RIG-I and MDA5 have distinct but overlapping specificity profiles and as a consequence recognize viruses in a differential but partially redundant manner. The optimal in vitro RIG-I ligand is defined as short 5′-tri- or -diphosphorylated dsRNA (7–10 bp) where the phosphate-carrying nucleoside is part of a Watson-Crick base pairing (4–6). More recently, it was shown that dsRNA with CAP-0 (m7-guanosine triphosphate cap) structures are also recognized (40). Physiological RIG-I ligands include the incoming viral genome (7), viral RNA replication intermediates, leader RNAs, or defective interfering particles harboring 5′-triphosphates (8, 9). Although early reports suggested a strict requirement of 5′-triphosphates, evidence also points toward 5′-triphosphate-independent recognition of viral RNA via internal, probably double-stranded mRNA regions and parts of 3′-untranslated regions (UTR) (9–12). Furthermore, also short dsRNAs generated by RNase L through cleavage of U-rich cytosolic RNA have been found to be RIG-I ligands (13, 14).
The optimal MDA5 ligand is long dsRNA (>2000 bp) without bulges (15–17). Accordingly, viral MDA5 agonists have been found to be the replicative form of viruses, for example (18). Furthermore, and similar to RIG-I, several sense or antisense transcripts are recognized in virus-infected cells (6, 9, 12). However, because MDA5 does not bind single-stranded RNA, the identified transcripts might harbor yet unrecognized double-stranded regions.
LGP2, like RIG-I, preferentially binds to dsRNA ends (19, 20), but it does not discriminate between blunt ends with and without triphosphate caps (21). LGP2 does not signal by itself, but several lines of evidence suggest that it augments MDA5-dependent signaling on the one hand and inhibits RIG-I-dependent signaling on the other hand (22, 23). In the case of former, it is suggested that LGP2, through its dsRNA end-binding capabilities, helps load MDA5 near RNA termini (21), and in the case of RIG-I, it may simply compete for RNA ends.
Structural mechanism of RLRs
Key contributions from several laboratories provided structural views of all three RLRs (Fig. 1) and their mode of action (21, 24–27). All RLRs possess an SF2 ATPase domain and a characteristic C-terminal domain (CTD) that together form the nucleic acid recognition module. The SF2 domain itself has four structural submodules. “RecA1” and “RecA2” are the universally conserved tandem RecA-like domains of SF2 enzymes that bind NTPs and nucleic acid ligands via seven characteristic “helicase” motifs (plus other, more recently described motifs) in a sequence-independent manner (3). A characteristic helical insertion domain “Hel2i” in RecA2 is crucial for RNA interaction and signaling, and finally, a “pincer” keeps SF2 in an open state in the absence of RNA, links CTD with RecA1 and -A2, and is critical for ATP-dependent signaling. The SF2 domain of RLRs is most closely related to dicer proteins in antiviral RNAi, showing a common evolutionary origin. RNA substrate specificity of RLRs is provided by their CTDs. The CTDs of RIG-I and LGP2 preferentially bind dsRNA ends, whereas the CTD of MDA5 binds RNA stems. RIG-I's affinity for RNA ends is further increased by 5′-tri- or -diphosphates 5–30-fold (depending on the ATP state of SF2) (21). LGP2, however, does not discriminate between phosphorylated and non-phosphorylated RNA ends and therefore could specifically help prevent RIG-I from recognizing non-phosphorylated dsRNA termini. Interestingly, the CTD does not belong to any of the evolutionary widely used RNA-binding domains but shows structural similarity to cereblon, for instance (28). The latter is a ligand-binding subunit of cullin-4 E3 ubiquitin ligase complexes probably best known as the target of thalidomide (29). Because ubiquitination is an important component of RLR-mediated signaling as well, this structural similarity may point to an origin of RLRs from both RNAi and ubiquitination pathways.
In addition, RIG-I and MDA5, but not LGP2, contain an N-terminal tandem caspase activation and recruitment domain (2CARD), which acts as a signal transducer. CARDs typically signal through the formation of helical filaments, which is a highly cooperative process and triggered by nucleation. Because overexpression of 2CARD in reporter cells is generally enough to induce an immune response (1), both RIG-I and MDA5 require mechanisms to keep 2CARD in a signal off-state or below an oligomerization threshold to prevent an autoactivation. In fact, the signaling mechanism of RIG-I and MDA5 is essentially the formation of 2CARD oligomers through unmasking of 2CARDs and/or their increase in their local concentration by cooperative binding along the RNA. Specifically, in the absence of RNA, the insertion domain of RIG-I masks 2CARD (Fig. 1A) (25), and dsRNA sterically competes with 2CARD for binding to the SF2-Hel2i domain (Fig. 1A). In contrast, MDA5 depends on the formation of oligomers along dsRNA that brings multiple 2CARDs into close proximity such that they can assemble. Thus, the CTD has a dual function in binding dsRNA stems and mediating contacts between MDA5 protomers in the filament. LGP2 is structurally a “hybrid,” harboring a RIG-I-like CTD (RNA end binder) (30) and an MDA5-like SF2, including its oligomerization capabilities (21), an architecture that could explain how LGP2 could augment MDA5 signaling by assisting MDA5 filament formation near dsRNA ends (23). Once bound to SF2 and CTD, all RLRs embrace dsRNA as a ring (Fig. 1B). Subsequently, 2CARDs are ubiquitinated with Lys-63-linked polyubiquitin or interact with unanchored chains (31–33) that stabilize the 2CARD protofilament like brackets (34).
RIG-I and MDA5 signal through their adapter protein mitochondrial antiviral signaling (MAVS, also known as IPS1, VISA, and CARDIF, Fig. 2). MAVS also possesses a CARD at its N terminus, which is fused via an ∼400-residue-long unstructured region to a C-terminal transmembrane domain (35). It resides on the surface of mitochondria and peroxisomes in a diffused state (36), but it polymerizes into filaments upon activation by RIG-I or MDA5. Structural studies suggest that four 2CARDs of RIG-I or MDA5 assemble into a helical protofilament that forms a nucleus from which MAVS filaments can outgrow (37). Polymerized MAVS brings together protein interaction motifs within its unstructured region that lead to the recruitment of multiple E3 ubiquitin ligases for the activation of IκB kinase or TBK1 and the production of type I interferons (IFN) (38).
Figure 2.
RIG-I-like receptor signaling pathway. The three human RIG-I-like receptors RIG-I, MDA5, and LGP2 detect foreign cytosolic double-stranded (ds) RNA, as for example released from entering RNA viruses, and induce an immune response to fight off the infection. In the absence of RNA ligands, signaling by RIG-I and MDA5 is prevented by shielding 2CARD from cytosolic interaction partners. 1, upon RNA detection both RIG-I and MDA5 release their 2CARD signaling module. RIG-I preferentially binds to nonmethylated dsRNA ends containing 5′-tri- or -diphosphates and further needs ATP to eject 2CARD. MDA5, in contrast, cooperatively binds to long dsRNA and forms filaments that are probably stabilized or seeded by LGP2. 2, each four released 2CARD modules of RIG-I or MDA5 subsequently assemble into tetramers and are stabilized by polyubiquitination or ubiquitin binding. 3, RLR 2CARD tetramerization, in turn, triggers the oligomerization of the RLR adapter protein MAVS. MAVS is distributed on the outer mitochondrial membrane but polymerizes into long filaments if seeded by 2CARD protomers. 4, this brings together many interaction sites of downstream factors within the MAVS unstructured region and activates a signaling cascade that leads to the expression of type I interferons or other cytokines and sets the cell into an antiviral state.
The current models imply that MAVS is in a “supersaturated” concentration in uninfected cells and only needs a nucleation trigger, as provided by activated RLRs, to polymerize. This system needs to be tightly controlled because this model suggests an irreversible all-or-none decision for the cell, once triggered. Here, several mechanisms are at play that prevent serendipitous activation of RLRs. Specifically, potential self-RNA ligands are modified to mask epitopes, and the sensors themselves harbor autoinhibition as well as proofreading mechanisms to limit activation by self-RNA. In addition RLRs are subject to post-translational modifications (PTMs) to regulate the sensitivity of the system. The necessity for any of these elements became evident through association of defects in masking of self-RNA epitopes, in autoinhibition or in proofreading capabilities with autoimmune syndromes.
Self-RNAs are masked to prevent RLR activation
Following the idealized notation of RIG-I recognizing phosphorylated RNA ends and MDA5 cooperatively recognizing internal dsRNA elements, the question arose whether potential self-RNAs ligands are prevented from activating RLRs. In the case of RIG-I, initial studies suggested a strict requirement for uncapped 5′-triphosphate RNA, which is absent in the eukaryotic cytosol and thus spatially divided from RIG-I. Interestingly, the RIG-I CTD was recently shown to bind m7-guanosine-capped RNA but to not tolerate 2′-O-methylation of the first 5′-nucleotide (CAP-1) because of a steric clash with a conserved histidine (39, 40). This efficiently prevents binding of all RLRs to host mRNA 5′ ends (Fig. 3A) and thus provides a biological role for the so far unknown purpose of RNA 2′-O-methylation (41). Other potential endogenous RIG-I ligands like tRNAs are cleaved to produce 5′-monophosphate ends, whereas ribosomal RNA is mainly masked by ribonucleoprotein complexes and modified ribonucleotides. Furthermore, miRNAs and siRNAs generated by Dicer have a characteristic two-nucleotide 3′-overhang that is unfavorable for RIG-I end-recognition (42).
Figure 3.
Mechanisms to avoid self-RNA recognition by RLRs and RLR amino acids that are implied in the development of autoimmune diseases. A, recognition of endogenous cytosolic RNA by RLRs is efficiently prevented by modifications within self-RNA. Methylation of the 5′-guanine cap as well as of the first nucleotide ribose mainly block binding of RIG-I. Adenosine-to-inosine editing destabilizes RNA stems and thus especially impedes MDA5 binding and/or oligomerization. Unintended internal binding events are further reduced by ATP hydrolysis of RLRs, which destabilizes the interaction of the SF2 domain with the RNA stem and leads to protein dissociation if no high affinity anchor is present. B, cooperative filament formation of MDA5 and non-cooperative binding of RIG-I on long non-edited dsRNA brings together several 2CARD modules for increased downstream signaling. C, ATP hydrolysis causes translocation of RLRs on dsRNA. D, ATP hydrolysis of RIG-I leads to recycling of the protein on tri- or diphosphorylated dsRNA ends and thus increases the affinity toward non-self RNA. E, several amino acid substitutions, mainly within SF2 domain, of MDA5 and RIG-I have been found to contribute to autoimmune disorders. The highlighted residues either increase (green) or decrease (orange) the risk of the development of the indicated disease. AGS, Aicardi-Goutières syndrome; IgAD, immunoglobulin A deficiency; SLE, systemic lupus erythematosus; SMS, Singleton-Merten syndrome; T1D, type I diabetes.
With respect to MDA5, a lack of characteristic features within its ligands like phosphate-containing ends, renders this RLR perhaps more prone to the recognition of endogenous RNA. Most mRNAs, however, only contain short dsRNA regions within 5′- or 3′-UTRs, and even if longer parts occur, base pairing is reduced by converting adenosine ribonucleotides (A) to inosine (I) by ADAR1 (Fig. 3A) (43, 44). Other potential MDA5 ligands could arise from short interspersed nucleotide elements like repetitive Alu elements that are ∼300 bases long and are, if transcribed from inverted repeats, ideal MDA5, but also ADAR1, substrates (45). A-to-I editing and the subsequent reduction of base-paired RNA stems might thus be one of the major mechanisms preventing MDA5-dependent signaling.
RLR activation is accompanied by a change of their post-translational modifications
In addition to modifications of endogenous RNA, RLRs themselves are found to be intensively modified (Table 1). In fact, several PTMs either increase the threshold for signal induction until ideal RNA ligands are found, enhance signaling by stabilization of the RLR-activated state, or shut down the response by inducing RLR degradation.
Table 1.
Covalent post-translational modifications of RIG-I
| Site | Uninfected cells |
Infected cells |
||
|---|---|---|---|---|
| Modification | Effect | Modification | Effect | |
| Ser-8, Thr-170, Thr-197 | Phosphorylation by PKC-α and PKC-β (46, 47) | Suppression of Lys-63-linked ubiquitination | Dephosphorylation by PP1α and PP1γ (48) | Ubiquitin binding possible |
| Lys-172, Lys-154, Lys-164 | Lys-63-linked ubiquitination by TRIM25 or Riplet (31, 90) | Stabilization of 2CARD tetramers | ||
| ?(2CARD) | Lys-63-linked ubiquitination by TRIM4 and MEX3C (91, 92) | Stabilization of 2CARD tetramers | ||
| ?(2CARD) | SUMOylation by MAPL (93) | Stabilization of 2CARD tetramers | ||
| Thr-770, Ser-854, Ser-855 | Phosphorylation by CKII and IKKϵ (94, 95) | Stabilization of the RNA-free state | Dephosphorylation by an unknown dephosphatase | |
| Lys-788 | Lys-63-linked ubiquitination by Riplet (96) | Stabilization of signaling-competent state | ||
| Lys-858, Lys-909 | Acetylation by unknown acetyltransferase | Reduction of RNA binding | Deacetylation by HDAC6 (50) | Increased recognition of viral RNA |
| ? | Removal of Lys-63-linked polyubiquitin chains by CYLD and USP21 (97, 98) | Prevention of premature signaling | Removal of Lys-63-linked polyubiquitin chains by USP3 and USP21 (99, 100) | Signaling reduction |
Probably the most important and best studied PTMs are located within 2CARD of both RIG-I and MDA5 and complicate their activation in uninfected cells or interfere with shutdown upon infection. Particularly, in uninfected cells, phosphorylation of 2CARD sterically prevents Lys-63-linked ubiquitination (46, 47). During an infection, however, these residues become dephosphorylated (48), thus allowing access of ubiquitin ligases or the direct interaction with polyubiquitin or ubiquitin-like proteins (31–33, 49). This establishes a positive feedback loop by stabilizing RIG-I and MDA5 2CARD tetramers for an increased signaling and prevents refolding of 2CARD to Hel2i.
A similar mechanism was found for two lysines of the RIG-I CTD that are acetylated in uninfected cells and impede RIG-I activation by decreasing RNA stem interactions (50). Upon infection and subsequent deacetylation, however, the increased RNA affinity of RIG-I leads again to an amplification of the immune response. PTMs of RLRs and their exchange on key amino acids thus provide important means to positively or negatively fine-tune the strength of an immune response.
RLR ATPase activity confers specificity toward foreign RNA
One of the most central features that are critical for accurate signaling of RLRs is probably ATP hydrolysis by their SF2 domain (1, 32, 51). The precise function of chemomechanical energy conversion by RLRs, however, has been a mystery for many years and is still not fully resolved. For instance, due do their affiliation to SF2 ATPases, RLRs have been historically classified as ATP-dependent helicases, which usually unwind duplex nucleic acids. The RLR helicase activity, however, is controversial (26, 52), and also mutations that compromise the SF2 domain were shown to have detrimental yet inconsistent effects on their function as innate immune sensors (53, 54).
Collectively, in all three RLRs the SF2 domain provides specificity to signaling on dsRNA. MDA5 for instance cooperatively forms filaments on long dsRNA through SF2-SF2 contacts and thus brings 2CARD from several proteins into spatial proximity (Fig. 3B) (27). In the case of RIG-I, the 2CARD-Hel2i interface ensures that an efficient ejection of 2CARD requires dsRNA (55) even though triphosphorylated single-stranded RNA can bind to the CTD (56). However, it is still unclear how SF2 helps discriminate viral dsRNA from self-RNA-bearing short base-paired regions. Detailed biophysical studies on RIG-I even showed that individual binding energies of CTD and SF2 do not add up and that there is a substantial negative thermodynamic linkage of RNA binding by SF2 onto RNA binding by CTD (57). Hence, SF2 binding to dsRNA may even reduce the affinity of CTD for dsRNA ends, and thus it increases the relative contribution of RNA stem structures, which can be found on self-RNA as well.
Recent results now suggest that the ATPase activity of SF2 helps to increase the selectivity of viral RNA (58–62). The energy released from ATP hydrolysis could be used to either enhance the affinity for viral RNA or alternatively to decrease the affinity for self-RNA. In the case of MDA5, at first, signaling seemed to be independent of ATP because both ATP binding and hydrolysis-deficient mutants were found to induce a constitutive immune response (59). Nevertheless, in vitro filament formation of MDA5 was weakened in the presence of ATP, presumably due to conformational changes induced by cycles of ATP binding and hydrolysis (17). The current model therefore suggests that destabilization of filaments due to ATP hydrolysis is stronger at ends because MDA5 is less stably embedded there, leading to a more rapid disassembly of short filaments (59). In addition, slow nucleation disfavors de novo generation of short filaments, overall shifting the specificity toward longer dsRNA.
In contrast to MDA5, RIG-I can efficiently recognize dsRNA ends in a 1:1 complex and activate type I IFNs in cells in the presence of triphosphorylated dsRNA as short as 8 bp (58). What is the function of RIG-I's ATP hydrolysis? Because RIG-I can form filaments as well (63), one possibility is a similar role than for MDA5, i.e. removing RIG-I from dsRNA. Single molecule studies have indeed indicated that RIG-I translocates on dsRNA (Fig. 3C) (64), an activity that would be well suited to dissipate RIG-I at low affinity RNA ligands. At high affinity sites, however, the CTD might form an anchor that retains RIG-I long enough to be ubiquitinated or to form 2CARD oligomers with other activated RIG-I molecules (Fig. 3D). Translocation might also load multiple molecules onto RNA ends (65) and provide effector-like functions through displacement of viral proteins from viral RNA (66, 67). However, the translocation activity as well as filament formation by RIG-I is still debated. Interestingly, recent structural data showed that dsRNA bound to LGP2 is shifted by 1 bp compared with its complex with RIG-I (21). Although this observation is interpreted as a recognition difference between RIG-I and LGP2, it could also reflect different “translocation” states. Further structural data might shed more light on the nature of these conformational states.
To elucidate the importance of the entire ATP reaction circle on RIG-I signaling, several groups recently dissected ATP binding from ATP hydrolysis and ADP through the use of non-hydrolysable ATP analogs in vitro and tailored mutations in helicase motifs in living cells (55, 58–62). Consent is reached with respect to the role of ATP binding, whereas the role of ATP hydrolysis is still a matter of debate. ATP binding is required for signaling in cellulo, suggesting together with the structural data that both dsRNA and ATP are required to trigger the conformational change that liberates 2CARD from Hel2i (59–61). Consistently, RIG-I tightly binds optimal RNA ligands in the presence of the transition state analog ADP-AlFx (21), albeit other studies showed rather a moderate reduction in affinity in the presence of ATPγS (60). To reassess the purpose of ATP hydrolysis, several groups made use of a mutation in motif II that allows ATP binding and ATP-induced conformational changes but slows down or prevents ATP hydrolysis and thus traps the protein in the ATP-bound state (59, 61). This site in motif II of RIG-I was recently described to be mutated in atypical Singleton-Merten syndrome as well and was suggested to lead to an autoactivated state of RIG-I in uninfected cells (68). Subsequent cell-based studies with RIG-I trapped in the ATP-bound state proposed increased interactions with self-RNA that lead to an elevated signaling as well as activation of type I IFNs even in absence of an infection (59, 61). Potential self-ligands were found to include double-stranded ribosomal expansion segments but may comprise other RNA species as well (61). However, analysis of the same motif by other groups did not lead to increased RIG-I signaling (54, 62), so this matter requires further studies. One possibility for these differences is the cellular background in which these studies were conducted. Autoactivation through self-RNA was especially seen in HUH7.5 or RIG-I KO cells that did not contain a functional endogenous RIG-I gene along with the mutated one. In addition to motif II, mutations in motif III or V were found to lead to activation of RIG-I in uninfected cells (54, 61, 62), suggesting that the chemomechanical coupling of RNA and ATP binding is critical as well.
In summary, based on the available data, one can postulate that ATP hydrolysis of RLRs either leads to proofreading by dynamically enhancing RNA selectivity and/or increased displacement from non-optimal PAMPs (58, 61, 66). In addition it could increase signaling by loading multiple RLR molecules onto a single dsRNA molecule or by promoting RNA filaments (65).
RLR-linked autoimmune diseases
If self-RNAs are inappropriately masked, or SNPs in RLR genes lead to a loss of autoinhibition or gain of RNA recognition, an RLR-triggered immune response can result in autoimmune diseases. Genetic disorders whose pathogenesis is caused by a constitutive up-regulation of type I IFNs are called “type I interferonopathies” (69) and can be, among others, ascribed to SNPs in RLR genes. In most cases, the involved RLR is MDA5, along with only a few known disease-correlated SNPs within the gene of RIG-I and so far none in LGP2. Interestingly, almost all identified pathogenic SNPs locate within the RLR helicase domain (Fig. 3E), emphasizing the importance of a functional SF2 for an adequate immune response.
The Aicardi-Goutières syndrome (AGS) is a rare monogenetic inflammatory disease affecting the skin and brain (70). So far, AGS is known to be induced by mutations in seven genes, including the gene encoding MDA5 (Fig. 3E) (71–73). All MDA5 SNPs affect the SF2 domain and are described as gain-of-function mutations leading to increased RNA-binding properties and thus a higher susceptibility toward self-RNA (71). However, increased levels of potential endogenous RLR ligands can also induce AGS, because loss of RNA editing through SNPs in ADAR1 was shown to generate MDA5 self-ligands (44). Interestingly, in contrast to all other AGS SNPs, mutations within the gene of MDA5 display an autosomal dominant pattern of inheritance and occur exclusively heterozygous (72). This emphasizes the importance of a tightly balanced immune response by RLRs where already minor disturbances can induce autoimmunity.
Systemic lupus erythematosus (SLE) is characterized by a chronic systemic inflammation through an activation of both the innate and adaptive immune system. Here, the damage of multiple organs is induced though an augmented autoantibody production against self-nucleic acids and small nuclear RNA-binding proteins by hyperactive T and B cells (74). So far, more than 40 genetic susceptibility loci for SLE have been identified, mostly within HLA genes and the Fcγ receptor but also gain-of-function mutations within the gene of MDA5 (75–78). The detailed biochemical basis, however, is still missing.
During type 1 diabetes (T1D), autoantibodies are developed against insulin-producing β-cells in pancreatic islets and insulin itself. The trigger for the occurrence of these autoantibodies is still highly controversial, even though more and more evidence points toward a connection between the genetic constitution as well as viral infections (79). Here, the viruses that are mainly implicated with T1D are Coxsackie type B viruses that are sensed by MDA5 (80). The majority of T1D pathogenic SNPs are located within HLA genes, whereas less frequent connections, both disease-promoting as well as protective, have been unveiled for MDA5 as well (81, 82). Here, protective MDA5 SNPs were shown to result in lowered protein expression, domain deletions, or decreased type I IFN production (83, 84).
Recently, two mutations within the gene encoding RIG-I (68) and one within the gene of MDA5 (85) were found to be associated with a rare multisystem disease called Singleton-Merten syndrome (SMS). Even though SMS is so far not recognized as type I interferonopathy, elevated levels of interferon-stimulated gene (ISG) products were found in blood samples of patients (85). All detected RLR mutations involve amino acids of the SF2 domain located within the ATP-binding pocket and lead to a gain-of-function of both proteins in vitro (68, 85). Constitutive activity of both RIG-I mutants was found to be caused by increased RNA-binding properties due to a lack of ATP hydrolysis and the inability to discriminate between self- and non-self-RNA (59, 61). Even though biochemical data for the MDA5 SMS mutant is so far missing, a similar mode of action might be conceivable.
In summary, most diseases show similar genetic features as well as overlapping clinical symptoms rendering a clear distinction solely based on the genotype difficult. It is thus not surprising that the biochemical basis for the development of these diseases often remains elusive. A noteworthy SNP within the gene of MDA5 in that regard is located within the CTD (Fig. 3E) and is implicated in several autoimmune diseases (86). However, so far only controversial results have been found in vitro. Some studies do not see altered RNA-binding properties as well as no changes in type I IFN production upon stimulation compared with the wild-type protein (59, 84, 87) and propose cumulative effects with other SNPs due to a strong linkage disequilibrium (88). In contrast, other studies state a constitutive activity in cells and could not detect any responsiveness to viral infections anymore (89).
Conclusions and future perspectives
It is now well established that for RIG-I and LGP2, the CTD is the main specificity element, providing a high affinity interaction with PAMPs. An ATP-induced structural switch generates a ring around RNA ends with full self versus non-self discriminatory capabilities that also include RNA interactions of SF2 and, in the case of RIG-I, ejection of the 2CARD-signaling module. More work is needed, however, to fully understand the role of free energy released in RLRs through ATP hydrolysis. In the case of MDA5, high affinity retention requires polymerization on long dsRNA, and ATP hydrolysis adds a dynamic to the system that leads to relative stabilization of long versus short filaments. In the case of RIG-I, however, the roles of ATP hydrolysis and the destabilization of RIG-I RNA structures are still debated. The discovery of autoimmune disease-associated mutants that directly affect ATP turnover could be helpful in this regard. These mutations could be interesting for analyzing the postulated RNA translocation activity in the removal of RIG-I from self-RNA using the assays established for effector functions. Whereas mutations that reduce ATP binding clearly prevented RIG-I effector functions, would mutations that reduce ATP hydrolysis and lead to a stable RNA interaction but at the same time stall the postulated translocation do the same? If yes, this may be a hint that translocation is indeed required for effector activities. If not, an alternative explanation such as RIG-I filament formation could be a basis for these activities. A combination of high resolution structural, biochemical, and cell-based studies might help settle this central question of RIG-I mechanism.
This work was supported by German Research Foundation Grant HO2489/8, German Excellence Initiative Grants CIPSM and QBM, and the Bavarian Ministry of Education (BioSysNet). The authors declare that they have no conflicts of interest with the contents of this article.
- PAMP
- pathogen-associated molecular pattern
- RLR
- RIG-I-like receptor
- AGS
- Aicardi-Goutières syndrome
- SMS
- Singleton-Merten syndrome
- T1D
- type 1 diabetes
- SLE
- systemic lupus erythematosus
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- dsRNA
- double-stranded RNA
- CTD
- C-terminal domain
- PTM
- post-translational modification
- CARD
- caspase activation and recruitment domain
- MAVS
- mitochondrial antiviral signaling
- SNP
- single-nucleotide polymorphism.
References
- 1. Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., and Fujita T. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 2. Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y.-M., Gale M. Jr., Akira S., Yonehara S., Kato A., and Fujita T. (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 [DOI] [PubMed] [Google Scholar]
- 3. Singleton M. R., Dillingham M. S., and Wigley D. B. (2007) Structure and mechanism of helicases and nucleic acid translocases. Annu. Rev. Biochem. 76, 23–50 [DOI] [PubMed] [Google Scholar]
- 4. Schlee M., Roth A., Hornung V., Hagmann C. A., Wimmenauer V., Barchet W., Coch C., Janke M., Mihailovic A., Wardle G., Juranek S., Kato H., Kawai T., Poeck H., Fitzgerald K. A., et al. (2009) Recognition of 5′-triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity 31, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt A., Schwerd T., Hamm W., Hellmuth J. C., Cui S., Wenzel M., Hoffmann F. S., Michallet M.-C., Besch R., Hopfner K.-P., Endres S., and Rothenfusser S. (2009) 5′-Triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc. Natl. Acad. Sci. U.S.A. 106, 12067–12072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goubau D., Schlee M., Deddouche S., Pruijssers A. J., Zillinger T., Goldeck M., Schuberth C., Van der Veen A. G., Fujimura T., Rehwinkel J., Iskarpatyoti J. A., Barchet W., Ludwig J., Dermody T. S., Hartmann G., and Reis e Sousa C. (2014) Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514, 372–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber M., Gawanbacht A., Habjan M., Rang A., Borner C., Schmidt A. M., Veitinger S., Jacob R., Devignot S., Kochs G., García-Sastre A., and Weber F. (2013) Incoming RNA virus nucleocapsids containing a 5′-triphosphorylated genome activate RIG-I and antiviral signaling. Cell Host Microbe 13, 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baum A., Sachidanandam R., and García-Sastre A. (2010) Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc. Natl. Acad. Sci. U.S.A. 107, 16303–16308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Runge S., Sparrer K. M., Lässig C., Hembach K., Baum A., García-Sastre A., Söding J., Conzelmann K.-K., and Hopfner K.-P. (2014) In vivo ligands of MDA5 and RIG-I in measles virus-infected cells. PLOS Pathog. 10, e1004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saito T., Owen D. M., Jiang F., Marcotrigiano J., and Gale M. Jr. (2008) Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454, 523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H.-X., Liu Z.-X., Sun Y.-P., Zhu J., Lu S.-Y., Liu X.-S., Huang Q.-H., Xie Y.-Y., Zhu H.-B., Dang S.-Y., Chend H.-F., Zheng G.-Y., Li Y.-X., Kuang Y., Fei J., et al. (2013) Rig-I regulates NF-κB activity through binding to Nf-κB1 3′-UTR mRNA. Proc. Natl. Acad. Sci. U.S.A. 110, 6459–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez David R. Y., Combredet C., Sismeiro O., Dillies M.-A., Jagla B., Coppée J.-Y., Mura M., Guerbois Galla M., Despres P., Tangy F., and Komarova A. V. (2016) Comparative analysis of viral RNA signatures on different RIG-I-like receptors. Elife 5, e11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malathi K., Dong B., Gale M. Jr., and Silverman R. H. (2007) Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448, 816–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malathi K., Saito T., Crochet N., Barton D. J., Gale M. Jr., and Silverman R. H. (2010) RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA 16, 2108–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kato H., Takeuchi O., Mikamo-Satoh E., Hirai R., Kawai T., Matsushita K., Hiiragi A., Dermody T. S., Fujita T., and Akira S. (2008) Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 205, 1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pichlmair A., Schulz O., Tan C.-P., Rehwinkel J., Kato H., Takeuchi O., Akira S., Way M., Schiavo G., and Reis e Sousa C. (2009) Activation of MDA5 requires higher-order RNA structures generated during virus infection. J. Virol. 83, 10761–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peisley A., Lin C., Wu B., Orme-Johnson M., Liu M., Walz T., and Hur S. (2011) Cooperative assembly and dynamic disassembly of MDA5 filaments for viral dsRNA recognition. Proc. Natl. Acad. Sci. U.S.A. 108, 21010–21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng Q., Hato S. V., Langereis M. A., Zoll J., Virgen-Slane R., Peisley A., Hur S., Semler B. L., van Rij R. P., and van Kuppeveld F. J. (2012) MDA5 detects the double-stranded RNA replicative form in picornavirus-infected cells. Cell Rep. 2, 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X., Ranjith-Kumar C. T., Brooks M. T., Dharmaiah S., Herr A. B., Kao C., and Li P. (2009) The RIG-I-like receptor LGP2 recognizes the termini of double-stranded RNA. J. Biol. Chem. 284, 13881–13891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pippig D. A., Hellmuth J. C., Cui S., Kirchhofer A., Lammens K., Lammens A., Schmidt A., Rothenfusser S., and Hopfner K.-P. (2009) The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 37, 2014–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uchikawa E., Lethier M., Malet H., Brunel J., Gerlier D., and Cusack S. (2016) Structural analysis of dsRNA binding to anti-viral pattern recognition receptors LGP2 and MDA5. Mol. Cell 62, 586–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Childs K. S., Randall R. E., and Goodbourn S. (2013) LGP2 plays a critical role in sensitizing mda-5 to activation by double-stranded RNA. PLoS ONE 8, e64202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruns A. M., Leser G. P., Lamb R. A., and Horvath C. M. (2014) The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol. Cell 55, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo D., Ding S. C., Vela A., Kohlway A., Lindenbach B. D., and Pyle A. M. (2011) Structural insights into RNA recognition by RIG-I. Cell 147, 409–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kowalinski E., Lunardi T., McCarthy A. A., Louber J., Brunel J., Grigorov B., Gerlier D., and Cusack S. (2011) Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 [DOI] [PubMed] [Google Scholar]
- 26. Jiang F., Ramanathan A., Miller M. T., Tang G.-Q., Gale M. Jr., Patel S. S., and Marcotrigiano J. (2011) Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature 479, 423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu B., Peisley A., Richards C., Yao H., Zeng X., Lin C., Chu F., Walz T., and Hur S. (2013) Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell 152, 276–289 [DOI] [PubMed] [Google Scholar]
- 28. Chamberlain P. P., Lopez-Girona A., Miller K., Carmel G., Pagarigan B., Chie-Leon B., Rychak E., Corral L. G., Ren Y. J., Wang M., Riley M., Delker S. L., Ito T., Ando H., Mori T., et al. (2014) Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21, 803–809 [DOI] [PubMed] [Google Scholar]
- 29. Ito T., Ando H., Suzuki T., Ogura T., Hotta K., Imamura Y., Yamaguchi Y., and Handa H. (2010) Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 [DOI] [PubMed] [Google Scholar]
- 30. Takahasi K., Kumeta H., Tsuduki N., Narita R., Shigemoto T., Hirai R., Yoneyama M., Horiuchi M., Ogura K., Fujita T., and Inagaki F. (2009) Solution structures of cytosolic RNA sensor MDA5 and LGP2 C-terminal domains identification of the RNA recognition loop in RIG-I-like receptors. J. Biol. Chem. 284, 17465–17474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gack M. U., Shin Y. C., Joo C.-H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S., and Jung J. U. (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 [DOI] [PubMed] [Google Scholar]
- 32. Zeng W., Sun L., Jiang X., Chen X., Hou F., Adhikari A., Xu M., and Chen Z. J. (2010) Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141, 315–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang X., Kinch L. N., Brautigam C. A., Chen X., Du F., Grishin N. V., and Chen Z. J. (2012) Ubiquitin-induced oligomerization of the RNA sensors RIG-I and MDA5 activates antiviral innate immune response. Immunity 36, 959–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peisley A., Wu B., Xu H., Chen Z. J., and Hur S. (2014) Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature 509, 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seth R. B., Sun L., Ea C.-K., and Chen Z. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 36. Dixit E., Boulant S., Zhang Y., Lee A. S., Odendall C., Shum B., Hacohen N., Chen Z. J., Whelan S. P., Fransen M., Nibert M. L., Superti-Furga G., and Kagan J. C. (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu B., Peisley A., Tetrault D., Li Z., Egelman E. H., Magor K. E., Walz T., Penczek P. A., and Hur S. (2014) Molecular imprinting as a signal-activation mechanism of the viral RNA sensor RIG-I. Mol. Cell 55, 511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.-T., Grishin N. V., and Chen Z. J. (2015) Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630. [DOI] [PubMed] [Google Scholar]
- 39. Devarkar S. C., Wang C., Miller M. T., Ramanathan A., Jiang F., Khan A. G., Patel S. S., and Marcotrigiano J. (2016) Structural basis for m7G recognition and 2′-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl. Acad. Sci. U.S.A. 113, 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schuberth-Wagner C., Ludwig J., Bruder A. K., Herzner A.-M., Zillinger T., Goldeck M., Schmidt T., Schmid-Burgk J. L., Kerber R., Wolter S., Stümpel J.-P., Roth A., Bartok E., Drosten C., Coch C., et al. (2015) A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1–2′ O-methylated self-RNA. Immunity 43, 41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B. W., Ziebuhr J., Szretter K. J., Baker S. C., Barchet W., Diamond M. S., Siddell S. G., Ludewig B., and Thiel V. (2011) Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 12, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marques J. T., Devosse T., Wang D., Zamanian-Daryoush M., Serbinowski P., Hartmann R., Fujita T., Behlke M. A., and Williams B. R. (2006) A structural basis for discriminating between self and nonself double-stranded RNAs in mammalian cells. Nat. Biotechnol. 24, 559–565 [DOI] [PubMed] [Google Scholar]
- 43. Mannion N. M., Greenwood S. M., Young R., Cox S., Brindle J., Read D., Nellåker C., Vesely C., Ponting C. P., McLaughlin P. J., Jantsch M. F., Dorin J., Adams I. R., Scadden A. D., Ohman M., Keegan L. P., and O'Connell M. A. (2014) The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liddicoat B. J., Piskol R., Chalk A. M., Ramaswami G., Higuchi M., Hartner J. C., Li J. B., Seeburg P. H., and Walkley C. R. (2015) RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mannion N., Arieti F., Gallo A., Keegan L. P., and O'Connell M. A. (2015) New insights into the biological role of mammalian ADARs; the RNA editing proteins. Biomolecules 5, 2338–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gack M. U., Nistal-Villán E., Inn K.-S., García-Sastre A., and Jung J. U. (2010) Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 84, 3220–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maharaj N. P., Wies E., Stoll A., and Gack M. U. (2012) Conventional protein kinase C-α (PKC-α) and PKC-β negatively regulate RIG-I antiviral signal transduction. J. Virol. 86, 1358–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wies E., Wang M. K., Maharaj N. P., Chen K., Zhou S., Finberg R. W., and Gack M. U. (2013) Dephosphorylation of the RNA sensors RIG-I and MDA5 by the phosphatase PP1 is essential for innate immune signaling. Immunity 38, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ibsen M. S., Gad H. H., Andersen L. L., Hornung V., Julkunen I., Sarkar S. N., and Hartmann R. (2015) Structural and functional analysis reveals that human OASL binds dsRNA to enhance RIG-I signaling. Nucleic Acids Res. 43, 5236–5248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Choi S. J., Lee H. C., Kim J. H., Park S. Y., Kim T. H., Lee W. K., Jang D. J., Yoon J. E., Choi Y. I., Kim S., Ma J., Kim C. J., Yao T. P., Jung J. U., Lee J. Y., and Lee J. S. (2016) HDAC6 regulates cellular viral RNA sensing by deacetylation of RIG-I. EMBO J. 35, 429–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bruns A. M., Pollpeter D., Hadizadeh N., Myong S., Marko J. F., and Horvath C. M. (2013) ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2). J. Biol. Chem. 288, 938–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Takahasi K., Yoneyama M., Nishihori T., Hirai R., Kumeta H., Narita R., Gale M. Jr., Inagaki F., and Fujita T. (2008) Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol. Cell 29, 428–440 [DOI] [PubMed] [Google Scholar]
- 53. Gee P., Chua P. K., Gevorkyan J., Klumpp K., Najera I., Swinney D. C., and Deval J. (2008) Essential role of the N-terminal domain in the regulation of RIG-I ATPase activity. J. Biol. Chem. 283, 9488–9496 [DOI] [PubMed] [Google Scholar]
- 54. Bamming D., and Horvath C. M. (2009) Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J. Biol. Chem. 284, 9700–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ramanathan A., Devarkar S. C., Jiang F., Miller M. T., Khan A. G., Marcotrigiano J., and Patel S. S. (2016) The autoinhibitory CARD2-Hel2i Interface of RIG-I governs RNA selection. Nucleic Acids Res. 44, 896–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang Y., Ludwig J., Schuberth C., Goldeck M., Schlee M., Li H., Juranek S., Sheng G., Micura R., Tuschl T., Hartmann G., and Patel D. J. (2010) Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat. Struct. Mol. Biol. 17, 781–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vela A., Fedorova O., Ding S. C., and Pyle A. M. (2012) The thermodynamic basis for viral RNA detection by the RIG-I innate immune sensor. J. Biol. Chem. 287, 42564–42573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anchisi S., Guerra J., and Garcin D. (2015) RIG-I ATPase activity and discrimination of self-RNA versus non-self-RNA. MBio 6, e02349–02314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Louber J., Brunel J., Uchikawa E., Cusack S., and Gerlier D. (2015) Kinetic discrimination of self/non-self RNA by the ATPase activity of RIG-I and MDA5. BMC Biol. 13, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rawling D. C., Fitzgerald M. E., and Pyle A. M. (2015) Establishing the role of ATP for the function of the RIG-I innate immune sensor. Elife 4, e09391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lässig C., Matheisl S., Sparrer K. M., de Oliveira Mann C. C., Moldt M., Patel J. R., Goldeck M., Hartmann G., García-Sastre A., Hornung V., Conzelmann K.-K., Beckmann R., and Hopfner K.-P. (2015) ATP hydrolysis by the viral RNA sensor RIG-I prevents unintentional recognition of self-RNA. Elife 4, e10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fitzgerald M. E., Rawling D. C., Potapova O., Ren X., Kohlway A., and Pyle A. M. (2017) Selective RNA targeting and regulated signaling by RIG-I is controlled by coordination of RNA and ATP binding. Nucleic Acids Res. 45, 1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peisley A., Wu B., Yao H., Walz T., and Hur S. (2013) RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol. Cell 51, 573–583 [DOI] [PubMed] [Google Scholar]
- 64. Myong S., Cui S., Cornish P. V., Kirchhofer A., Gack M. U., Jung J. U., Hopfner K.-P., and Ha T. (2009) Cytosolic viral sensor RIG-I is a 5′-triphosphate-dependent translocase on double-stranded RNA. Science 323, 1070–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patel J. R., Jain A., Chou Y. Y., Baum A., Ha T., and García-Sastre A. (2013) ATPase-driven oligomerization of RIG-I on RNA allows optimal activation of type-I interferon. EMBO Rep. 14, 780–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yao H., Dittmann M., Peisley A., Hoffmann H.-H., Gilmore R. H., Schmidt T., Schmid-Burgk J. L., Hornung V., Rice C. M., and Hur S. (2015) ATP-dependent effector-like functions of RIG-I-like receptors. Mol. Cell 58, 541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sato S., Li K., Kameyama T., Hayashi T., Ishida Y., Murakami S., Watanabe T., Iijima S., Sakurai Y., Watashi K., Tsutsumi S., Sato Y., Akita H., Wakita T., Rice C. M., et al. (2015) The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity 42, 123–132 [DOI] [PubMed] [Google Scholar]
- 68. Jang M.-A., Kim E. K., Now H., Nguyen N. T., Kim W.-J., Yoo J.-Y., Lee J., Jeong Y.-M., Kim C.-H., Kim O.-H., Sohn S., Nam S.-H., Hong Y., Lee Y. S., Chang S.-A., et al. (2015) Mutations in DDX58, which encodes RIG-I, cause atypical Singleton-Merten syndrome. Am. J. Hum. Genet. 96, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee-Kirsch M. A. (2017) The type I interferonopathies. Annu. Rev. Med. 68, 297–315 [DOI] [PubMed] [Google Scholar]
- 70. Aicardi J., and Goutières F. (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 15, 49–54 [DOI] [PubMed] [Google Scholar]
- 71. Rice G. I., del Toro Duany Y., Jenkinson E. M., Forte G. M., Anderson B. H., Ariaudo G., Bader-Meunier B., Baildam E. M., Battini R., Beresford M. W., Casarano M., Chouchane M., Cimaz R., Collins A. E., Cordeiro N. J., et al. (2014) Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crow Y. J., Chase D. S., Lowenstein Schmidt J., Szynkiewicz M., Forte G., Gornall H. L., Oojageer A., Anderson B., Pizzino A., and Helman G. (2015) Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. 167A, 296–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oda H., Nakagawa K., Abe J., Awaya T., Funabiki M., Hijikata A., Nishikomori R., Funatsuka M., Ohshima Y., Sugawara Y., Yasumi T., Kato H., Shirai T., Ohara O., Fujita T., and Heike T. (2014) Aicardi-Goutieres syndrome is caused by IFIH1 mutations. Am. J. Hum. Genet. 95, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghodke-Puranik Y., and Niewold T. B. (2013) Genetics of the type I interferon pathway in systemic lupus erythematosus. Int. J. Clin. Rheumatol. 8, 657–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gateva V., Sandling J. K., Hom G., Taylor K. E., Chung S. A., Sun X., Ortmann W., Kosoy R., Ferreira R. C., Nordmark G., Svenungsson E., Padyukov L., Sturfelt G., Jönsen A., et al. (2009) A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cunninghame Graham D. S., Morris D. L., Bhangale T. R., Criswell L. A., Syvänen A.-C., Rönnblom L., Behrens T. W., Graham R. R., and Vyse T. J. (2011) Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet. 7, e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Eyck L., De Somer L., Pombal D., Bornschein S., Frans G., Humblet-Baron S., Moens L., de Zegher F., Bossuyt X., Wouters C., and Liston A. (2015) Brief report: IFIH1 mutation causes systemic lupus erythematosus with selective IgA deficiency. Arthritis Rheumatol. 67, 1592–1597 [DOI] [PubMed] [Google Scholar]
- 78. Molineros J. E., Maiti A. K., Sun C., Looger L. L., Han S., Kim-Howard X., Glenn S., Adler A., Kelly J. A., Niewold T. B., Gilkeson G. S,. Brown E. E., Alarcón G. S., Edberg J. C., Petri M., et al. (2013) Admixture mapping in lupus identifies multiple functional variants within IFIH1 associated with apoptosis, inflammation, and autoantibody production. PLoS Genet. 9, e1003222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yeung W.-C., Rawlinson W. D., and Craig M. E. (2011) Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 342, d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang J. P., Cerny A., Asher D. R., Kurt-Jones E. A., Bronson R. T., and Finberg R. W. (2010) MDA5 and MAVS mediate type I interferon responses to coxsackie B virus. J. Virol. 84, 254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Smyth D. J., Cooper J. D., Bailey R., Field S., Burren O., Smink L. J., Guja C., Ionescu-Tirgoviste C., Widmer B., Dunger D. B., Savage D. A., Walker N. M., Clayton D. G., and Todd J. A. (2006) A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat. Genet. 38, 617–619 [DOI] [PubMed] [Google Scholar]
- 82. Nejentsev S., Walker N., Riches D., Egholm M., and Todd J. A. (2009) Rare variants of IFIH1, a gene implicated in antiviral responses, protect against type 1 diabetes. Science 324, 387–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lincez P. J., Shanina I., and Horwitz M. S. (2015) Reduced expression of the MDA5 gene IFIH1 prevents autoimmune diabetes. Diabetes 64, 2184–2193 [DOI] [PubMed] [Google Scholar]
- 84. Shigemoto T., Kageyama M., Hirai R., Zheng J., Yoneyama M., and Fujita T. (2009) Identification of loss of function mutations in human genes encoding RIG-I and MDA5 implications for resistance to type I diabetes. J. Biol. Chem. 284, 13348–13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rutsch F., MacDougall M., Lu C., Buers I., Mamaeva O., Nitschke Y., Rice G. I., Erlandsen H., Kehl H. G., Thiele H., Nürnberg P., Höhne W., Crow Y. J., Feigenbaum A., and Hennekam R. C. (2015) A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am. J. Hum. Genet. 96, 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Looney B. M., Xia C.-Q., Concannon P., Ostrov D. A., and Clare-Salzler M. J. (2015) Effects of type 1 diabetes-associated IFIH1 polymorphisms on MDA5 function and expression. Curr. Diab. Rep. 15, 96. [DOI] [PubMed] [Google Scholar]
- 87. Zouk H., Marchand L., Li Q., and Polychronakos C. (2014) Functional characterization of the Thr946Ala SNP at the type 1 diabetes IFIH1 locus. Autoimmunity 47, 40–45 [DOI] [PubMed] [Google Scholar]
- 88. Zurawek M., Fichna M., Fichna P., Skowronska B., Dzikiewicz-Krawczyk A., Januszkiewicz D., and Nowak J. (2015) Cumulative effect of IFIH1 variants and increased gene expression associated with type 1 diabetes. Diabetes Res. Clin. Pract. 107, 259–266 [DOI] [PubMed] [Google Scholar]
- 89. Funabiki M., Kato H., Miyachi Y., Toki H., Motegi H., Inoue M., Minowa O., Yoshida A., Deguchi K., Sato H., Ito S., Shiroishi T., Takeyasu K., Noda T., and Fujita T. (2014) Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40, 199–212 [DOI] [PubMed] [Google Scholar]
- 90. Gao D., Yang Y.-K., Wang R.-P., Zhou X., Diao F.-C., Li M.-D., Zhai Z.-H., Jiang Z.-F., and Chen D.-Y. (2009) REUL is a novel E3 ubiquitin ligase and stimulator of retinoic-acid-inducible gene-I. PLOS ONE 4, e5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yan J., Li Q., Mao A.-P., Hu M.-M., and Shu H.-B. (2014) TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J. Mol. Cell Biol. 6, 154–163 [DOI] [PubMed] [Google Scholar]
- 92. Kuniyoshi K., Takeuchi O., Pandey S., Satoh T., Iwasaki H., Akira S., and Kawai T. (2014) Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity. Proc. Natl. Acad. Sci. U.S.A. 111, 5646–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mi Z., Fu J., Xiong Y., and Tang H. (2010) SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell 1, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sun Z., Ren H., Liu Y., Teeling J. L., and Gu J. (2011) Phosphorylation of RIG-I by casein kinase II inhibits its antiviral response. J. Virol. 85, 1036–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang X., Yu H., Zhao J., Li X., Li J., He J., Xia Z., and Zhao J. (2016) IKKϵ negatively regulates RIG-I via direct phosphorylation. J. Med. Virol. 88, 712–718 [DOI] [PubMed] [Google Scholar]
- 96. Oshiumi H., Matsumoto M., Hatakeyama S., and Seya T. (2009) Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-β induction during the early phase of viral infection. J. Biol. Chem. 284, 807–817 [DOI] [PubMed] [Google Scholar]
- 97. Zhang M., Wu X., Lee A. J., Jin W., Chang M., Wright A., Imaizumi T., and Sun S.-C. (2008) Regulation of IκB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J. Biol. Chem. 283, 18621–18626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Friedman C. S., O'Donnell M. A., Legarda-Addison D., Ng A., Cárdenas W. B., Yount J. S., Moran T. M., Basler C. F., Komuro A., Horvath C. M., Xavier R., and Ting A. T. (2008) The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 9, 930–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cui J., Song Y., Li Y., Zhu Q., Tan P., Qin Y., Wang H. Y., and Wang R.-F. (2014) USP3 inhibits type I interferon signaling by deubiquitinating RIG-I-like receptors. Cell Res. 24, 400–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fan Y., Mao R., Yu Y., Liu S., Shi Z., Cheng J., Zhang H., An L., Zhao Y., Xu X., Chen Z., Kogiso M., Zhang D., Zhang H., Zhang P., Jung J. U., Li X., Xu G., and Yang J. (2014) USP21 negatively regulates antiviral response by acting as a RIG-I deubiquitinase. J. Exp. Med. 211, 313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]