Abstract
Prostate cancer is a very common malignant disease and a leading cause of death for men in the Western world. Tumorigenesis and progression of prostate cancer involves multiple signaling pathways, including the Hippo pathway. Yes-associated protein (YAP) is the downstream transcriptional co-activator of the Hippo pathway, is overexpressed in prostate cancer, and plays a vital role in the tumorigenesis and progression of prostate cancer. However, the role of the YAP paralog and another downstream effector of the Hippo pathway, transcriptional co-activator with PDZ-binding motif (TAZ), in prostate cancer has not been fully elucidated. Here, we show that TAZ is a basal cell marker for the prostate epithelium. We found that overexpression of TAZ promotes the epithelial-mesenchymal transition (EMT), cell migration, and anchorage-independent growth in the RWPE1 prostate epithelial cells. Of note, knock down of TAZ in the DU145 prostate cancer cells inhibited cell migration and metastasis. We also found that SH3 domain binding protein 1 (SH3BP1), a RhoGAP protein that drives cell motility, is a direct target gene of TAZ in the prostate cancer cells, mediating TAZ function in enhancing cell migration. Moreover, the prostate cancer-related oncogenic E26 transformation-specific (ETS) transcription factors, ETV1, ETV4, and ETV5, were required for TAZ gene transcription in PC3 prostate cancer cells. MAPK inhibitor U0126 treatment decreased TAZ expression in RWPE1 cells, and ETV4 overexpression rescued TAZ expression in RWPE1 cells with U0126 treatment. Our results show a regulatory mechanism of TAZ transcription and suggest a significant role for TAZ in the progression of prostate cancer.
Keywords: cell migration, ETS transcription factor family, Hippo pathway, prostate cancer, tumor metastasis, SH3BP1, TAZ
Introduction
Prostate cancer (PC)5 is one of the most common malignant diseases and a leading cause of death for men in the Western world. The incidence of prostate cancer has increased nearly 3-fold in the first 10 years of the new century in China, which may be because of the similar lifestyle and popularization of PC screening using prostate-specific antigen during the modernization process in China (1). Multiple signaling pathways are involved in the tumorigenesis and progression of prostate cancer, such as the activation of the MAPK pathway and loss of PTEN resulting in the activation of PI3K/AKT pathway (2). ETS transcription factors are important downstream targets phosphorylated and activated by MAPKs. The chromosomal rearrangement of the PC-related ETS TFs, such as ERG, ETV1, ETV4, ETV5, occurs in 50–70% of prostate cancers (3, 4), resulting in the overexpression of ETS transcription factors and activation of ETS downstream target genes, which mimics the activated RAS/MAPK pathway in prostate cancer cells (5).
The Hippo pathway is a conserved signaling pathway involved in organ size control and tissue homeostasis in mammals (6). Dysregulation of the Hippo pathway occurs in multiple cancers, including prostate cancer (7, 8). The dysregulation of the Hippo pathway results in the activation of downstream transcriptional co-activators YAP and TAZ, leading to transcription of downstream target genes, mainly through the complex of YAP/TAZ with the TEAD family transcription factors. The transcriptional activation of these genes will lead to the hyperproliferation, anti-apoptosis, and enhanced cell migration, in association with tumorigenesis and aggressive phenotypes. Overexpression of YAP is observed in prostate cancer (7, 8). In prostate cancer cells, YAP can also interact with the transcription factor EGR1 to promote tumorigenesis by enhancing the expression of downstream anti-apoptosis and pro-proliferation genes (9). Also, YAP can interact with androgen receptor (AR) and promote cell proliferation independent of the androgen (10). Interestingly, the ETS transcription factors ERG and ETV1 can promote YAP gene expression, and ERG also cooperates with YAP to regulate YAP downstream target genes (11, 12). However, the function of TAZ, the YAP paralog in the normal prostate tissue, and the development of prostate cancer have not been studied yet.
TAZ was first identified as a 14-3-3 binding protein and then found to be the downstream transcriptional co-activator of the Hippo pathway in mammals (13). TAZ is overexpressed in many human cancers, including colorectal cancer, ovarian cancer, and breast cancer, which is associated with poor prognosis (14–16). In breast cancer, TAZ is mainly overexpressed in the basal type of breast cancer cells, which may be because of the activation of MRTF-SRF transcriptional complex (17). Consistently, in normal breast tissues, TAZ is preferentially expressed in the basal cells and regulates differentiation of the mammary epithelial cells (15). The similar tissue structure between breast tissues and prostate tissues prompted us to explore the TAZ expression in the prostate. In this study, we show that TAZ is a basal cell marker for prostate tissues, which is lost in most prostate cancers, but re-expression of TAZ may indicate more aggressive malignancy of prostate cancer. Overexpression of TAZ promotes epithelial-mesenchymal transition, cell migration, anchorage-independent growth, and metastasis in prostate cancer. TAZ overexpression in aggressive prostate cancer cells could be because of the transcriptional activation of the TAZ gene by ETS transcription factors. We reveal a novel transcriptional regulation mechanism of TAZ and demonstrate that transcriptional activation of TAZ may promote malignant progression of prostate cancer. We also identified SH3 domain binding protein 1 (SH3BP1) as a novel downstream target gene of TAZ in prostate cancer.
Results
TAZ is a basal cell marker for prostate epithelial cells
We first examined TAZ expression in a commercial tissue array consisting of normal prostate tissue, hyperplasia tissue, and prostate cancer tissues. Interestingly, similar to TAZ expression pattern in the normal mammary tissue, TAZ was expressed in the basal cells but not luminal cells in the normal prostate tissue (Fig. 1A). A similar result was observed in the hyperplasia tissue, that basal cells expressed TAZ but not the hyperproliferated luminal cells (Fig. 1A). Most prostate cancer displays a luminal cell phenotype, and loss of basal cell marker expression, such as p63 and CK5/14, is a diagnostic criterion for prostate cancer (18). Similarly, most of the prostate cancer did not express TAZ protein; only a small portion of prostate cancers (3/17) were positive for the TAZ expression (Fig. 1A). Next, we examined the TAZ expression in a prostate tissue array with a larger sample size and the Gleason score information. Consistently, only a small portion of prostate cancers expressed TAZ protein (16/73). Intriguingly, there was a trend that the expression of TAZ was correlated with higher Gleason score (Fig. 1B).
Figure 1.
TAZ is a basal cell marker for prostate epithelium. A, immunohistochemical analysis of TAZ in normal prostate epithelium, hyperplasia, and prostate cancer tissues. The representative images of (a) TAZ in the normal prostate epithelium, (b) TAZ in the hyperplasia tissue, (c) no expression of TAZ in the prostate cancer tissue, and (d) strong expression of TAZ in some prostate cancer tissues were shown. Scale bar: 50 μm (a, c, d), 100 μm (b). B, immunohistochemical analysis of TAZ expression in a prostate cancer tissue array consisting of 73 prostate cancer tissues with Gleason score. The correlation between TAZ expression and Gleason score was analyzed. X2 test was used for the statistical analysis. The distribution of patients was indicated. C, the protein expression of YAP, TAZ, and AR was analyzed by Western blotting in the AR+ prostate cancer cell lines (LNCaP, 22Rv1) and AR− prostate cancer cell lines (DU145, PC3). D, the mRNA expression of YAP, TAZ, and CTGF in the AR+ prostate cancer cell lines (LNCaP, 22Rv1) and AR− prostate cancer cell lines (DU145, PC3) was analyzed by qPCR. E, the expression level of TAZ is positively associated with basal marker TP63 and negatively correlated with the luminal markers CK8 and CK18. Co-expression data in the prostate cancer TCGA dataset were extracted from the cBioPortal database. The Pearson's correlation coefficient and Spearman's correlation coefficient values were generated by the cBioPortal. TAZ expression in the basal cells is indicated as arrows. Error bars, standard deviation.
Both YAP and TAZ, two Hippo downstream co-activators, were assessed in the four prostate cancer cell lines by an antibody detecting YAP/TAZ. The AR+ PC cells (LNCaP, 22Rv1) expressed lower expression of YAP compared with the AR− PC cells (DU145, PC3) (Fig. 1C). Strikingly, TAZ was not expressed in the AR+ PC cells, but strong expression of TAZ was observed in the AR− PC cells (Fig. 1C). We also examined the YAP/TAZ mRNA levels in the PC cell lines. High expression of CTGF, the YAP/TAZ target gene, was also detected in the AR− PC cells (Fig. 1D), indicating high activity of YAP/TAZ. YAP mRNA level increased 3- to 4-fold in the DU145 and PC3 cells compared with the LNCaP and 22Rv1 cells, whereas TAZ mRNA level increased hundreds-fold in the AR− PC cells (Fig. 1D), indicating that transcriptional activation of TAZ could be the main reason for the high expression of TAZ in the AR− PC cells. Finally, we performed co-expression analysis of TAZ with the basal and luminal cell markers by using the TCGA prostate cancer dataset (19, 20). TAZ was reversely correlated with prostate luminal markers KRT8/18 and positively associated with basal marker p63 (Fig. 1E). Taken together, TAZ is a basal cell marker for normal prostate epithelial cells. TAZ is not expressed in most prostate cancer tissues and re-expression of TAZ in a subpopulation of prostate cancer may indicate more aggressive malignancy.
TAZ overexpression promotes EMT, cell migration, and anchorage-independent growth in prostate epithelial cells
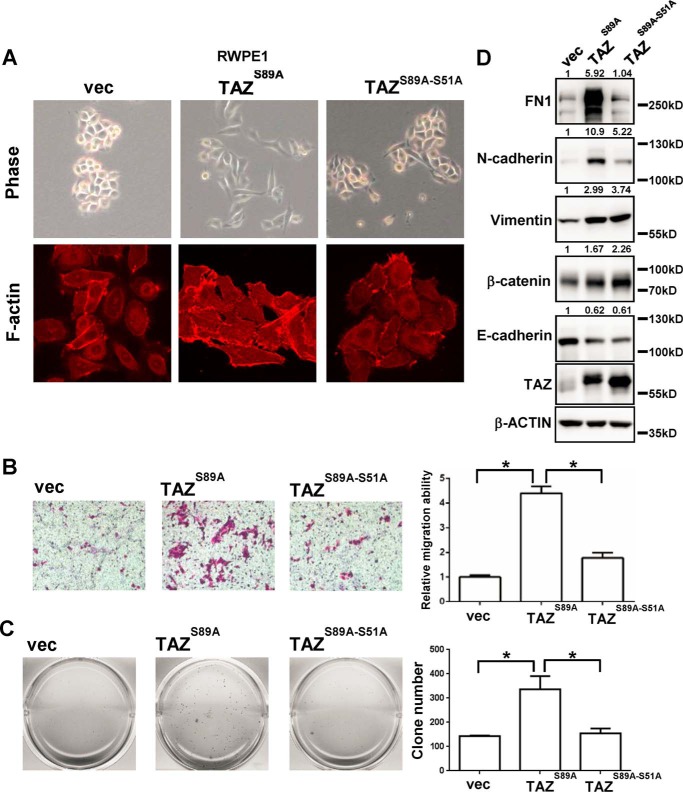
To study the biological function of TAZ in the prostate epithelial cells, we generated TAZ overexpression stable cells by using the normal prostate cell line RWPE1. Similar to the effect of TAZ overexpression in the MCF10A normal mammary epithelial cells (13), TAZS89A (which is no longer regulated by the Hippo pathway for cytoplasmic retention through interaction and represents a constitutively active version of TAZ) expression induced dramatic morphological changes, such as cell scattering and a loss of cell contact (Fig. 2A). The TEAD binding defect mutant TAZS89A-S51A expression still induced some morphological changes but to a lesser extent than the TAZS89A (Fig. 2A). F-actin staining was performed to examine the effect of TAZ overexpression on the cytoskeleton. TAZS89A expression cells displayed more staining of stress fibers and disorganization of cell junction, whereas TAZS89A-S51A expression cells displayed similar F-actin staining pattern to the vector-only RWPE1 cells (Fig. 2A). Consistently, overexpression of TAZS89A enhanced cell migration which was disrupted by the TEAD binding-defective mutation (Fig. 2B). Overexpression of TAZS89A did not promote cell proliferation in normal in vitro culture condition (supplemental Fig. 1A) but did promote colony formation in soft agar assay (Fig. 2C). Then, we assessed the effect of TAZ expression on the EMT-related markers. Overexpression of TAZS89A inhibited the expression of epithelial marker E-cadherin but induced expression of mesenchymal markers such as FN1, N-cadherin, vimentin, and β-catenin (Fig. 2D). Interestingly, expression of only some of the mesenchymal markers depended on the TEAD-binding property of TAZ, which was consistent with some morphological changes induced by the TAZS89A-S51A mutant. Therefore, overexpression of TAZ promotes EMT, cell migration, and anchorage-independent growth in prostate epithelial cells.
Figure 2.
Overexpression of TAZ in the immortalized prostate epithelial cells promotes EMT, cell migration, and anchorage-independent growth. A, the representative phase-contrast images and rhodamine-conjugated phalloidin staining images of vector control RWPE1 cells, TAZS89A overexpressing RWPE1 cells, and TAZS89A-S51A overexpressing RWPE1 cells were shown. B, Transwell assay of the vector control, TAZS89A, and TAZS89A-S51A RWPE1 cells. Representative images of the Transwell migration assays were shown. Relative migration ability was normalized to the vector control group. *, p < 0.05 by the Student's t test. C, soft agar assay of the vector control, TAZS89A, and TAZS89A-S51A RWPE1 cells. Representative images of soft agar colonies were shown. Clone numbers of each well were counted. *, p < 0.05 by the Student's t test. D, Western blotting analysis of the EMT markers (FN1, N-cadherin, vimentin, β-catenin, and E-cadherin) in the vector control, TAZS89A, and TAZS89A-S51A RWPE1 cells were shown. Quantitative analysis of Western blotting was indicated. Error bars, standard deviation.
Loss of TAZ inhibits cell migration and metastasis in prostate cancer cells
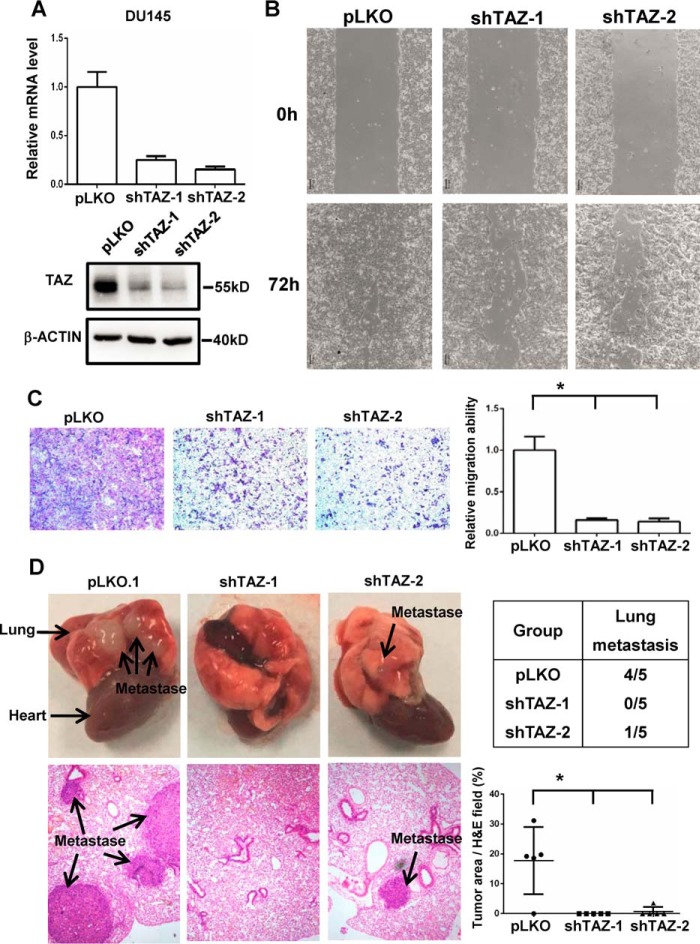
TAZ was overexpressed in the AR− PC cells. To investigate the function of endogenous TAZ in the prostate cancer cells, we generated TAZ knockdown DU145 stable cells by using two independent TAZ shRNA. The knockdown efficiency of TAZ knockdown was confirmed by qPCR and Western blotting (Fig. 3A). Consistent with the conclusion on TAZ overexpression in RWPE1 cells, TAZ knockdown only slightly affected the cell proliferation (supplemental Fig. 1, B and C). However, TAZ knockdown significantly inhibited the cell migration of DU145 prostate cancer cells in the wound healing assay and Transwell assay (Fig. 3, B and C). To assess the TAZ function in vivo, we further examined the role of TAZ in tumor metastasis by injecting DU145 cells into the tail vein of nude mice. Compared with those injected with control DU145 cells, the mice inoculated with TAZ knockdown cells developed dramatically fewer lung metastases (Fig. 3D). These data indicate that TAZ promotes cell migration and metastasis in the prostate cancer cells.
Figure 3.
Knockdown of TAZ in the DU145 prostate cancer cells inhibits cell migration and metastasis. A, generation of TAZ knockdown DU145 cells. The Western blotting analysis and qPCR results of TAZ in the pLKO.1 control DU145 cells and TAZ shRNA knockdown DU145 cells are shown. B, wound-healing assay of pLKO.1 control DU145 cells and TAZ shRNA knockdown DU145 cells. The phase-contrast images after making the wound and 72 h later were shown. C, Transwell assay of pLKO.1 control DU145 cells and TAZ shRNA knockdown DU145 cells. Representative images of the Transwell migration assay were shown. Relative migration ability was normalized to pLKO.1 vector control group. *, p < 0.05 by the Student's t test. D, the metastatic potential of pLKO.1 control DU145 cells and TAZ shRNA knockdown DU145 cells was analyzed in vivo. The images of lung metastases and hematoxylin-eosin stained sections of lung were shown. The lung metastases were marked with the arrow. n = 5 for each group. The incidences of lung metastasis of each group were indicated. Statistical analysis of metastatic potential was performed based on the tumor area/hematoxylin-eosin field. *, p < 0.05 by the Student's t test. Error bars, standard deviation.
SH3BP1 is a direct target gene of TAZ in prostate cancer cells
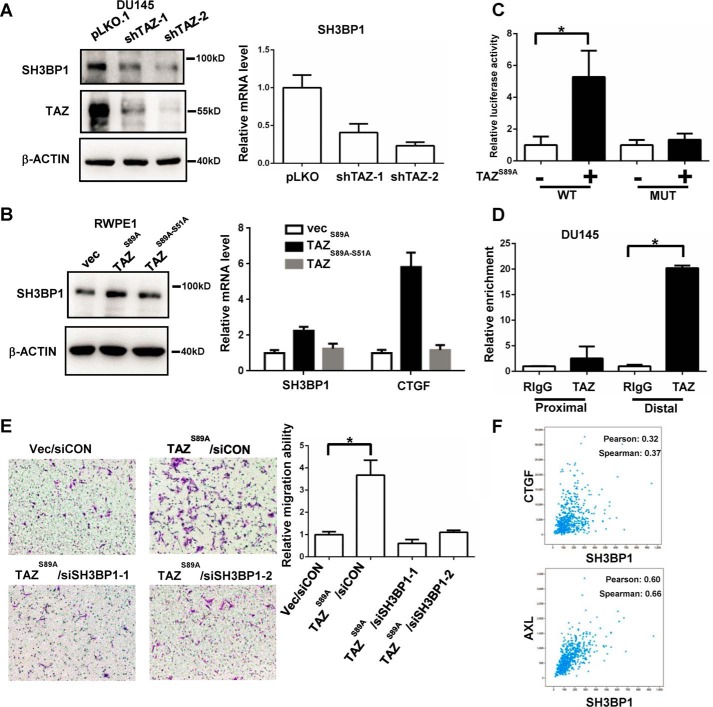
To further elucidate the molecular mechanism of TAZ in promoting the metastasis of prostate cancer cells, we performed RNA-sequence analysis of TAZ knockdown and vector control DU145 cells (supplemental Table 1). SH3BP1 was one of the most differentially expressed genes down-regulated by TAZ knockdown in DU145 cells. SH3BP1 is an exocyst-associated RhoGAP driving cell motility (21). Knockdown of SH3BP1 significantly decreased cell migration (21, 22). Thus, we tested the hypothesis that TAZ could promote cell migration and metastasis through up-regulation of SH3PB1 in prostate cancer cells. Both Western blotting and qPCR analysis confirmed the decreased expression of SH3BP1 in the TAZ knockdown DU145 cells (Fig. 4A). Overexpression of TAZ increased the expression of SH3BP1 in the RWPE1 cells, which was impaired by the TEAD binding defect mutation (Fig. 4B). Bioinformatics analysis of SH3BP1 promoter identified three M-CAT motifs located in the promoter region of SH3BP1 (supplemental Fig. 2, A and B). We generated pGL2-SH3BP1 promoter reporter and the M-CAT motif mutant reporter. Luciferase assay showed that co-expression of TAZ can activate SH3BP1 promoter activity but not the M-CAT motif mutant one (Fig. 4C). ChIP analysis using the ChIP-grade TAZ antibody further showed the direct binding of TAZ to the promoter region of SH3BP1 (Fig. 4D). Next, we investigated whether SH3BP1 mediated the function of TAZ in promoting cell migration. We found that knockdown of SH3BP1 significantly diminished the cell migration induced by TAZ overexpression in the RWPE1 cells (Fig. 4E). The gene co-expression analysis of SH3BP1 in the TCGA prostate cancer dataset further supports that the mRNA expression of SH3BP1 was positively correlated with the classic YAP/TAZ target genes, such as CTGF and AXL, in prostate cancer (Fig. 4F). All these data suggest that SH3BP1 is a direct target gene of TAZ in the prostate cancer, which is activated by TAZ to increase cell motility.
Figure 4.
SH3BP1 is a direct target gene of TAZ, enhancing the cell migration in the prostate cancer cells. A, decreased expression of SH3BP1 in TAZ knockdown DU145 cells. The protein and mRNA levels of SH3BP1 in the TAZ knockdown DU145 cells were analyzed by Western blotting and qPCR, respectively. B, overexpression of TAZ promoted SH3BP1 expression in the RWPE1 cells. The protein and mRNA levels of SH3BP1 in vector control, TAZS89A, and TAZS89A-S51A RWPE1 cells were analyzed by Western blotting and qPCR, respectively. CTGF, a well known downstream target gene of TAZ, was included as the positive control. C, TAZ enhanced the luciferase activity of the SH3BP1 WT promoter but not the M-CAT motif mutant. Luciferase assay of the SH3BP1 WT and M-CAT motif mutant promoters co-transfected with TAZ expression plasmid. *, p < 0.05 by the Student's t test. D, TAZ was enriched in the SH3BP1 promoter region. ChIP analysis of TAZ in the DU145 cells was performed. The enrichment of TAZ in the SH3BP1 promoter was analyzed by qPCR with SH3BP1 promoter-specific primers. *, p < 0.05 by the Student's t test. E, knockdown of SH3BP1 impaired the cell migration induced by overexpression of TAZ in RWPE1 cells. The cells were transfected with NC and SH3BP1 siRNA for 2 days. The cell migration ability of these cells was analyzed by Transwell assay. Representative images of the Transwell migration were shown. Relative migration ability was normalized to the vector/siCON group. *, p < 0.05 by the Student's t test. F, the correlation of SH3BP1 with Hippo target genes (CTGF, AXL) in the TCGA prostate cancer dataset was shown. The Pearson's correlation coefficient and Spearman's correlation coefficient values were generated by the cBioPortal. Error bars, standard deviation.
PC-related ETS transcription factors regulate TAZ gene expression in prostate cancer
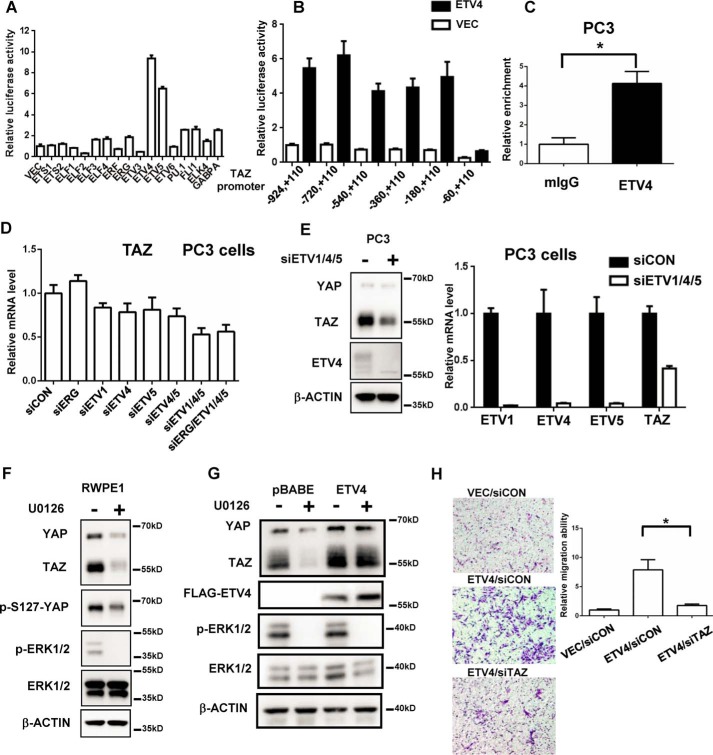
Transcriptional activation of TAZ results in the high expression of TAZ protein in the AR− PC cells. Recently, we identified that MRTF-SRF transcriptional complex activates TAZ transcription in breast cancer cells (17). However, knockdown SRF did not affect TAZ mRNA level in PC3 cells (data not shown), indicating that other transcription factors regulate the transcription of TAZ in prostate cancer. Members of ETS transcription factors (ERG, ETV1, ETV4, and ETV5) are involved in the tumorigenesis and progression of prostate cancer. The analysis of public ChIP-sequence data showed that multiple ETS transcription factors can bind to the promoter region of TAZ gene in the prostate cancer cells (supplemental Fig. 3A) (23, 24), prompting us to explore whether prostate cancer-related ETS TFs can regulate TAZ transcription. We first constructed 17 expression plasmids for ETS TFs and tested the effect of each of these ETS TFs on the TAZ promoter reporter. Interestingly, ETV4/5 dramatically activated the TAZ promoter activity (Fig. 5A). Then we generated several truncated TAZ promoter reporters and found that the proximal promoter region of TAZ (−60, +110 bp) was the main element regulated by ETV4 (Fig. 5B). ChIP analysis also showed that ETV4 transcription factor can directly bind to the promoter region of TAZ, indicating that TAZ could be a direct target gene of ETV4 (Fig. 5C).
Figure 5.
The transcription of TAZ gene is regulated by ETV1, ETV4, and ETV5 PC-related ETS family members in the prostate cancer cells. A, transcription factors ETV4 and ETV5 significantly increased the luciferase activity of the TAZ promoter. TAZ promoter reporter plasmid was co-transfected with individual ETS TFs expression plasmids in 293 cells. The relative luciferase activity was measured after 24 h. B, the ETV4 transcription factor enhanced the luciferase activity of TAZ promoter through the proximal region of TAZ promoter. The truncated TAZ promoters' reporter plasmids were co-transfected with ETV4 expression plasmid in 293 cells. The relative luciferase activity was measured after 24 h. C, ETV4 was enriched in the TAZ promoter region. ChIP analysis of ETV4 in the PC3 cells was performed. The enrichment of TAZ promoter was analyzed by qPCR with TAZ promoter-specific primers. *, p < 0.05 by the Student's t test. D, knockdown of ETV1, ETV4, and ETV5 decreased TAZ mRNA level in the PC3 cells. PC3 cells were transfected with indicated siRNAs for 3 days. The mRNA level of TAZ was analyzed by qPCR. E, knockdown of ETV1, ETV4, and ETV5 decreased TAZ protein level in the PC3 cells. PC3 cells were simultaneously transfected with ETV1, ETV4, and ETV5 siRNA for 3 days. The mRNA and protein level of TAZ and ETV1, ETV4, and ETV5 were analyzed by Western blotting and qPCR. F, inhibition of MAPK activity inhibited TAZ expression in the RWPE1 cells. RWPE1 cells were treated with U0126 (10 μm, 24 h) and the TAZ expression was analyzed by Western blotting. G, overexpression of ETV4 promotes TAZ expression independent on the MAPK activity. The pBABE vector control and FLAG-ETV4 RWPE1 cells were treated with U0126 (10 μm, 24 h), and the TAZ expression was analyzed by Western blotting. H, TAZ knockdown impaired the cell migration induced by overexpression of ETV4 in the RWPE1 cells. The RWPE1 cells were transfected with indicated siRNA for 2 days. The cell migration of these cells was analyzed by Transwell assay. Representative images of the Transwell migration assay were shown. Relative migration ability was normalized to the vector/siCON group. *, p < 0.05 by the Student's t test. Error bars, standard deviation.
Next, we explored the knockdown effect of ETS TFs on the TAZ mRNA level in the PC3 cells. Interestingly, individual knockdown of ETV1, ETV4, and ETV5 mildly decreased the TAZ mRNA level in the PC3 cells (Fig. 5D). No effect of ERG knockdown on the TAZ level was observed, which may be because of low basal expression level of ERG and high expression level of ETV1, ETV4, and ETV5 in the PC3 cells (25). Simultaneous knockdown ETV1, ETV4, and ETV5 mostly decreased the TAZ expression level at both mRNA and protein level (Fig. 5, D and E). In the normal prostate epithelial cells, the activity of ETS TFs requires the activation of the MAPK pathway. Inhibition of MEK1/2 activity by U0126 decreases the expression level of downstream target genes of ETS TFs (5). We found that U0126 treatment largely reduced the protein level of both TAZ and YAP in the RWPE1 cells (Fig. 5F). We also generated ETV4 overexpression RWPE1 stable cells and found that overexpression of ETV4 can promote TAZ expression without the activity of MAPK (Fig. 5G and supplemental Fig. 3B). Interestingly, in RWPE1 cells, the transcription of both YAP and TAZ is re-activated by the overexpression of ETV4 (supplemental Fig. 3B), indicating ETS transcription factors may be the common regulator for both YAP and TAZ. To further investigate the biological function of ETV4 mediated by TAZ, TAZ knockdown was performed in the ETV4 overexpression cells. Consistent to the previous study, the overexpression of ETV4 enhanced the cell motility of the RWPE1 normal prostate epithelial cells, which was significantly diminished by the TAZ knockdown (Fig. 5H). Taken together, our results indicate that prostate cancer-related ETS TFs (ETV1, ETV4, and ETV5) can promote TAZ expression in the prostate cancer.
Discussion
Similar to normal breast tissue, TAZ is also a basal cell marker for normal prostate tissue. About 15–20% of breast cancers are the basal-like type, characterized by the expression of basal cell markers (26). However, in the clinic, most prostate cancers display a luminal cell phenotype, which displays the loss of the basal cell markers such as p63 and CK5/14 (2, 18). Re-expression of basal cell-related genes is associated with malignant transformation, metastasis, and poor prognosis (18, 27). Although our study is based on a relatively small number of patients, we did observe a tendency for TAZ expression to be correlated with a higher Gleason score, indicating that TAZ expression might be involved in the progression of prostate cancer and the indicator of more malignancy. Future study with a larger prostate cancer cohort is needed to conclusively and clinically establish this point. Moreover, we found that TAZ is not expressed in most prostate cancers; in contrast, YAP is overexpressed in prostate cancers (7, 8), indicating the potential different biological function of YAP and TAZ in the context of prostate cancer. Interestingly, overexpression of YAP in the RWPE1 immortalized prostate epithelial cells enhanced cell proliferation, cell migration, and cell invasion but did not induce EMT (7). TAZ overexpression induces strong EMT phenotype and affects cell migration and anchorage-independent growth, but not so much cell proliferation in the RWPE1 cells. In a mice model of YAP GOF in the prostate epithelium, mice with a constitutive active mutant of YAP develop prostate tumors in old age (12). It can be speculated that activation of YAP is involved in the tumorigenesis of prostate cancer, whereas re-expression of TAZ may promote metastasis and indicate an aggressive malignant prostate cancer. It is worthy to explore the in vivo function of TAZ in the context of prostate epithelium in the future study.
The hundreds-fold increase of TAZ mRNA level in the AR− PC cells compared with the AR+ PC cells indicates that activation of TAZ in prostate cancer may mainly be akin to the transcriptional activation. The positive association of TAZ with basal cell markers and the reverse correlation with luminal cell markers further support the vital role of TAZ transcriptional regulation in prostate cancer. Recently, we identified a MRTF-SRF transcriptional regulation mechanism of TAZ in basal-type breast cancer cells (17). However, MRTF-SRF transcriptional complex is not accountable for the activation of TAZ in prostate cancer, indicating a context-dependent transcriptional regulation of TAZ in different tissues. The PC-related ETS TFs promote TAZ gene expression in prostate cancer cells. Interestingly, individually knockdown of ETV1, 4, or 5 only mildly decreased the TAZ expression, indicating that activation of any PC-related ETS TFs can potentially activate TAZ expression. MAPK pathway can regulate activity of the ETS family members. Inhibition of MAPK activity by U0126 decreased TAZ expression level in the RWPE1 cells but not the ETV4 overexpressed cells, demonstrating that overexpression of ETS TFs rendered cell malignancy independent of the activity of MAPK pathway. Previously, several reports found that FGF2 (28), insulin growth factor 1 (IGF1) (29), lysophosphatidic acid (LPA) (30) and phorbaketal A (31) could increase TAZ mRNA expression through activation of MEK-ERK pathway, which was blocked by the treatment of U0126. ETS transcription factors may be involved in these conditions, which need to be explored.
SH3BP1 is an exocyst-associated RhoGAP that inactivates Rac1 to enhance cell motility. Recently, the overexpression of SH3BP1 was found in the hepatocellular carcinoma (32). SH3BP1 overexpression promotes cell invasion and metastasis in hepatocellular carcinoma, which is associated with poor prognosis. Here, we show that SH3BP1 is a direct target of TAZ in prostate cancer. SH3BP1 is an important downstream target of TAZ, promoting cell migration induced by TAZ overexpression, which indicates the potential role of SH3BP1 in the malignant progression of prostate cancer. Above all, we show that TAZ is a basal cell marker for prostate epithelium and absent in most prostate cancer tissue. Overexpression of TAZ in a subset of prostate cancer indicates a more malignant progression, which promotes EMT, cell migration, and metastasis. Although other players are likely be involved, our study highlights the cascade of ETS TFs→TAZ→SH3BP1 in driving more invasive malignancy of prostate cancer cells.
Experimental procedures
Immunohistochemistry and Western blotting
The prostate cancer tissue array consisting of normal prostate epithelium, prostate hyperplasia tissue, and cancer tissues was purchased from Abcam (ab178263). The prostate cancer tissue array with Gleason score information was purchased from Biomax (PR1921a). TAZ antibody (HPA007415) was used for the immunohistochemistry analysis. Immunohistochemistry and Western blotting were performed as described previously (33, 34). The antibodies used for Western blotting were TAZ (HPA007415), YAP/TAZ (Cell Signaling Technology, 8418), SH3BP1 (HPA000757), vimentin (Abcam, ab92547), E-cadherin (BD Biosciences, 610182), N-cadherin (BD Biosciences, 610921), β-catenin (Cell Signaling Technology, 8480), FN1 (BD Biosciences, 555867), ETV4 (Santa Cruz Biotechnology, sc-113), β-actin (Sigma, A3854), and GAPDH (Cell Signaling Technology, 3683).
Cell culture, stable cell establishment, and transfection
Prostate cancer cell lines LNCaP, 22Rv1, DU145, and PC3 and immortalized epithelial cell line RWPE1 were purchased from ATCC and cultured according to ATCC guidelines. Passage 2 liquid nitrogen stocks from the original ATCC cells were used for this study. Cell authentication was performed by the ATCC. The RWPE1 stable cells were established by infection with the retrovirus of pBABE-TAZS89A and pBABE-TAZS89A-S51A and selected for 7 days by puromycin. TAZ knockdown DU145 cells were established by using the pLKO.1 lentivirus system. shTAZ-1: 5′-CGGACTTCATTCAAGAGGAAT-3′; shTAZ-2: 5′-CTGTACGAGCTCATCGAGAAG-3′. Lipofectamine RNAi MAX was used for siRNA transfection according to the manufacture's protocol. The TAZ and SH3BP1 siRNA were synthesized from the Shanghai GenePharma Company. siTAZ-1: 5′-ACGUUGACUUAGGAACUUU-3′ (35); siTAZ-2: 5′-AGGUACUUCCUCAAUCACA-3′ (35); siSH3BP1–1: 5′-GAUGACAGCCACCCACUUC-3′ (22); siSH3BP1–2: 5′-UGGAGAUUCAGGCCGAUUA-3′ (22). The following siRNA were purchased from Dharmacon: ERG, L-003886-00-0005; ETV1, L-003801-00-0005; ETV4, L-004207-00-0005; ETV5, L-008894-00-0005.
qPCR and RNA-sequence assay
RNA was extracted by using RNAiso Plus (Takara Bio Inc.) reagent. cDNA was reverse transcribed by using the PrimeScript RT Master Mix kit (Takara). qPCR was performed by using the SYBR Green reagents. Two pLKO.1 vector control RNA samples with the shTAZ-1 and shTAZ-2 samples were used for RNA-sequence analysis. The RNA-sequence data have been deposited in the Gene Expression Omnibus under accession no. GSE93748.
Luciferase array
SH3BP1 promoter was amplified by PCR and inserted in the pGL2-basic plasmid. The M-CAT motifs were mutated by using the QuikChange Site-Directed Mutagenesis Kit. The construct of pGL2-TAZ reporter was reported previously (17). The truncated TAZ reporters were constructed using different 5′ primers. The primers used in this study have been included in supplemental Table 2.
ChIP assay
Experiments were performed as described previously (17). Briefly, formaldehyde was added directly to cell culture media to a final concentration of 3.7% at room temperature. Thirty min later, glycine was added. The cells were washed with ice-cold PBS, scraped, and collected in cold PBS followed by nuclear extraction and sonication. The antibodies used for immunoprecipitation were ETV4 antibody (Santa Cruz, sc-113) and control mouse IgG (Cell Signaling Technology, 5415), TAZ antibody (HPA007415), and control rabbit IgG (Cell Signaling Technology, 2729). The ChIP-enriched DNA was subjected to qPCR using promoter-specific primers. The primers used in this study have been included in supplemental Table 2.
Cell migration assay
For the wound-healing assay, DU145 cells were seeded and grown to the confluence. The cells were scratched by a sterile tip to make a wound. Then the medium was changed to a 2% FBS culture medium, and the cells were cultured for another 72 h. Images were taken immediately after scratch and 72 h later.
For the Transwell assay of DU145 cells, 5 × 104 DU145 cells were added into the upper chamber (Corning Transwell, 8-μm pore) in serum-free DMEM. 10% FBS DMEM was added into the lower chamber to create the chemotaxis. Cell migration ability was analyzed after 48 h.
For the Transwell assay of RWPE1 cells, 7.5 × 104 cells were added into the upper chamber in the growth supplements deficient medium. Normal culture medium was used to create the chemotaxis. Cell migration ability was analyzed after 56 h.
Xenograft tumor formation and the lung metastasis assay
Nude mice (4–6 weeks old, male) were used for the in vivo mouse model assay. All mouse procedures were approved by the Animal Care and Use Committees of Xinhua Hospital. For xenograft tumors, 5 × 106 DU145 cells were subcutaneously inoculated into the flank of nude mice. After 4 weeks, the mice were sacrificed, and the xenograft tumors were collected for weight and analysis. For the lung metastasis assay (36), 1 × 106 DU145 cells were injected into the tail vein of the mice. After 8 weeks, the whole lungs were resected and photographed. The lung tissues were sectioned and stained with hematoxylin-eosin, and lung metastases were determined under a microscope (double blinded).
Statistics
The Student's t test was used for comparison between two groups. p value <0.05 was considered to be statistically significant.
Author contributions
C.-Y. L, L. C., and W. H. designed the study and wrote the manuscript. C.-Y. L. and T. Y. performed the experiments with the help of Y. H.
Supplementary Material
Acknowledgment
We thank Dr. Jiahuai Han (Xiamen University) for providing the ETS cDNA.
This work was supported by National Natural Science Foundation of China Grants 81672517 and 81372636 and Medical Engineering Interdisciplinary Grant YG2014MS71 of Shanghai Jiao Tong University. This study was also supported by the Agency for Science, Technology and Research (A*STAR) funds (to W. H.). The authors declare that they have no conflicts of interest with the contents of this article.
The RNA-sequence data have been deposited in the Gene Expression Omnibus under accession no. GSE93748.
This article contains supplemental Figs. 1–3 and supplemental Tables 1 and 2.
- PC
- prostate cancer
- YAP
- Yes-associated protein
- TAZ
- transcriptional co-activator with PDZ-binding motif
- EMT
- epithelial-mesenchymal transition
- SH3
- Src homology 3
- ETS
- E26 transformation-specific
- TF
- transcription factor
- AR
- androgen receptor
- TCGA
- The Cancer Genome Atlas
- PC
- prostate cancer
- qPCR
- quantitative PCR.
References
- 1. Chen W., Zheng R., Baade P. D., Zhang S., Zeng H., Bray F., Jemal A., Yu X. Q., and He J. (2016) Cancer statistics in China, 2015. CA–Cancer J. Clin. 66, 115–132 [DOI] [PubMed] [Google Scholar]
- 2. Shen M. M., and Abate-Shen C. (2010) Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 24, 1967–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomlins S. A., Mehra R., Rhodes D. R., Smith L. R., Roulston D., Helgeson B. E., Cao X., Wei J. T., Rubin M. A., Shah R. B., and Chinnaiyan A. M. (2006) TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 66, 3396–3400 [DOI] [PubMed] [Google Scholar]
- 4. Tomlins S. A., Rhodes D. R., Perner S., Dhanasekaran S. M., Mehra R., Sun X. W., Varambally S., Cao X., Tchinda J., Kuefer R., Lee C., Montie J. E., Shah R. B., Pienta K. J., Rubin M. A., and Chinnaiyan A. M. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 [DOI] [PubMed] [Google Scholar]
- 5. Hollenhorst P. C., Ferris M. W., Hull M. A., Chae H., Kim S., and Graves B. J. (2011) Oncogenic ETS proteins mimic activated RAS/MAPK signaling in prostate cells. Genes Dev. 25, 2147–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong A. W., Meng Z., and Guan K. L. (2016) The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 13, 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang L., Yang S., Chen X., Stauffer S., Yu F., Lele S. M., Fu K., Datta K., Palermo N., Chen Y., and Dong J. (2015) The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol. Cell Biol. 35, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., and Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zagurovskaya M., Shareef M. M., Das A., Reeves A., Gupta S., Sudol M., Bedford M. T., Prichard J., Mohiuddin M., and Ahmed M. M. (2009) EGR-1 forms a complex with YAP-1 and upregulates Bax expression in irradiated prostate carcinoma cells. Oncogene 28, 1121–1131 [DOI] [PubMed] [Google Scholar]
- 10. Thoma C. (2015) Prostate cancer: targetable YAP1-AR interaction key to disease progression. Nat. Rev. Urol. 12, 596. [DOI] [PubMed] [Google Scholar]
- 11. Kim T. D., Jin F., Shin S., Oh S., Lightfoot S. A., Grande J. P., Johnson A. J., van Deursen J. M., Wren J. D., and Janknecht R. (2016) Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. J. Clin. Invest. 126, 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen L. T., Tretiakova M. S., Silvis M. R., Lucas J., Klezovitch O., Coleman I., Bolouri H., Kutyavin V. I., Morrissey C., True L. D., Nelson P. S., and Vasioukhin V. (2015) ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell 27, 797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., and Guan K. L. (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuen H. F., McCrudden C. M., Huang Y. H., Tham J. M., Zhang X., Zeng Q., Zhang S. D., and Hong W. (2013) TAZ expression as a prognostic indicator in colorectal cancer. PLoS ONE 8, e54211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skibinski A., Breindel J. L., Prat A., Galván P., Smith E., Rolfs A., Gupta P. B., Labaer J., and Kuperwasser C. (2014) The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep. 6, 1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen G., Xie J., Huang P., and Yang Z. (2016) Overexpression of TAZ promotes cell proliferation, migration and epithelial-mesenchymal transition in ovarian cancer. Oncol. Lett. 12, 1821–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C. Y., Chan S. W., Guo F., Toloczko A., Cui L., and Hong W. (2016) MRTF/SRF dependent transcriptional regulation of TAZ in breast cancer cells. Oncotarget 7, 13706–13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xin L. (2013) Cells of origin for cancer: an updated view from prostate cancer. Oncogene 32, 3655–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., and Schultz N. (2013) Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cancer Genome Atlas Research Network (2015) The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parrini M. C., Sadou-Dubourgnoux A., Aoki K., Kunida K., Biondini M., Hatzoglou A., Poullet P., Formstecher E., Yeaman C., Matsuda M., Rossé C., and Camonis J. (2011) SH3BP1, an exocyst-associated RhoGAP, inactivates Rac1 at the front to drive cell motility. Mol. Cell 42, 650–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elbediwy A., Zihni C., Terry S. J., Clark P., Matter K., and Balda M. S. (2012) Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J. Cell Biol. 198, 677–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y., Chi P., Rockowitz S., Iaquinta P. J., Shamu T., Shukla S., Gao D., Sirota I., Carver B. S., Wongvipat J., Scher H. I., Zheng D., and Sawyers C. L. (2013) ETS factors reprogram the androgen receptor cistrome and prime prostate tumorigenesis in response to PTEN loss. Nat. Med. 19, 1023–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu J., Yu J, Mani R.-S., Cao Q., Brenner C. J., Cao X., Wang X., Wu L., Li J., Hu M., Gong Y., Cheng H., Laxman B., Vellaichamy A., Shankar S., Li Y., Dhanasekaran S. M., Morey R., Barrette T., Lonigro R. J., Tomlins S. A., Varambally S., Qin Z. S., and Chinnaiyan A. M. (2010) An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 17, 443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollenhorst P. C., Paul L., Ferris M. W., and Graves B. J. (2011) The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer 1, 1044–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kohler B. A., Sherman R. L., Howlader N., Jemal A., Ryerson A. B., Henry K. A., Boscoe F. P., Cronin K. A., Lake A., Noone A. M., Henley S. J., Eheman C. R., Anderson R. N., and Penberthy L. (2015) Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 107, djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith B. A., Sokolov A., Uzunangelov V., Baertsch R., Newton Y., Graim K., Mathis C., Cheng D., Stuart J. M., and Witte O. N. (2015) A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc. Natl. Acad. Sci. U.S.A. 112, E6544–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Byun M. R., Kim A. R., Hwang J. H., Kim K. M., Hwang E. S., and Hong J. H. (2014) FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone 58, 72–80 [DOI] [PubMed] [Google Scholar]
- 29. Xue P., Wu X., Zhou L., Ma H., Wang Y., Liu Y., Ma J., and Li Y. (2013) IGF1 promotes osteogenic differentiation of mesenchymal stem cells derived from rat bone marrow by increasing TAZ expression. Biochem. Biophys. Res. Commun. 433, 226–231 [DOI] [PubMed] [Google Scholar]
- 30. Jeong G. O., Shin S. H., Seo E. J., Kwon Y. W., Heo S. C., Kim K. H., Yoon M. S., Suh D. S., and Kim J. H. (2013) TAZ mediates lysophosphatidic acid-induced migration and proliferation of epithelial ovarian cancer cells. Cell Physiol. Biochem. 32, 253–263 [DOI] [PubMed] [Google Scholar]
- 31. Byun M. R., Lee C. H., Hwang J. H., Kim A. R., Moon S. A., Sung M. K., Roh J. R., Hwang E. S., and Hong J. H. (2013) Phorbaketal A inhibits adipogenic differentiation through the suppression of PPARγ-mediated gene transcription by TAZ. Eur. J. Pharmacol. 718, 181–187 [DOI] [PubMed] [Google Scholar]
- 32. Tao Y., Hu K., Tan F., Zhang S., Zhou M., Luo J., and Wang Z. (2016) SH3-domain binding protein 1 in the tumor microenvironment promotes hepatocellular carcinoma metastasis through WAVE2 pathway. Oncotarget 7, 18356–18370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang W., Zhu Y., Gao J., Fu J., Liu C., Liu Y., Song C., Zhu S., Leng Y., Wang G., Chen W., Du P., Huang S., Zhou X., Kang J., and Cui L. (2014) MicroRNA-29a promotes colorectal cancer metastasis by regulating matrix metalloproteinase 2 and E-cadherin via KLF4. Br. J. Cancer 110, 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Y., Wang G., Yang Y., Mei Z., Liang Z., Cui A., Wu T., Liu C. Y., and Cui L. (2016) Increased TEAD4 expression and nuclear localization in colorectal cancer promote epithelial-mesenchymal transition and metastasis in a YAP-independent manner. Oncogene 35, 2789–2800 [DOI] [PubMed] [Google Scholar]
- 35. Zanconato F., Forcato M., Battilana G., Azzolin L., Quaranta E., Bodega B., Rosato A., Bicciato S., Cordenonsi M., and Piccolo S. (2015) Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li N., Dhar S. S., Chen T. Y., Kan P. Y., Wei Y., Kim J. H., Chan C. H., Lin H. K., Hung M. C., and Lee M. G. (2016) JARID1D is a suppressor and prognostic marker of prostate cancer invasion and metastasis. Cancer Res. 76, 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.